- Department of Radiation Oncology, General Hospital of Northern Theater Command, Shenyang, China
Background: The prevalence of venous thromboembolism (VTE) is high in patients with cancer and can often present as the first symptom of malignancy. Cancer-associated VTE is one of the most important risk factors contributing to cancer mortality, making its prevention and treatment critical for patients with lung cancer.
Methods: We systematically searched for observational studies that estimated the prevalence of VTE in patients with lung cancer. A comprehensive search of electronic databases, including PubMed, EMBASE and Cochrane Library, was systematically conducted from database inception through January 21, 2022. The qualities of included studies were assessed in three domains, including patient selection, comparison, and results. Random effects meta-analyses of the prevalence of VTE in lung cancer were conducted using the metaprop procedure. Chi-square test and I2 value were used to evaluate study heterogeneity.
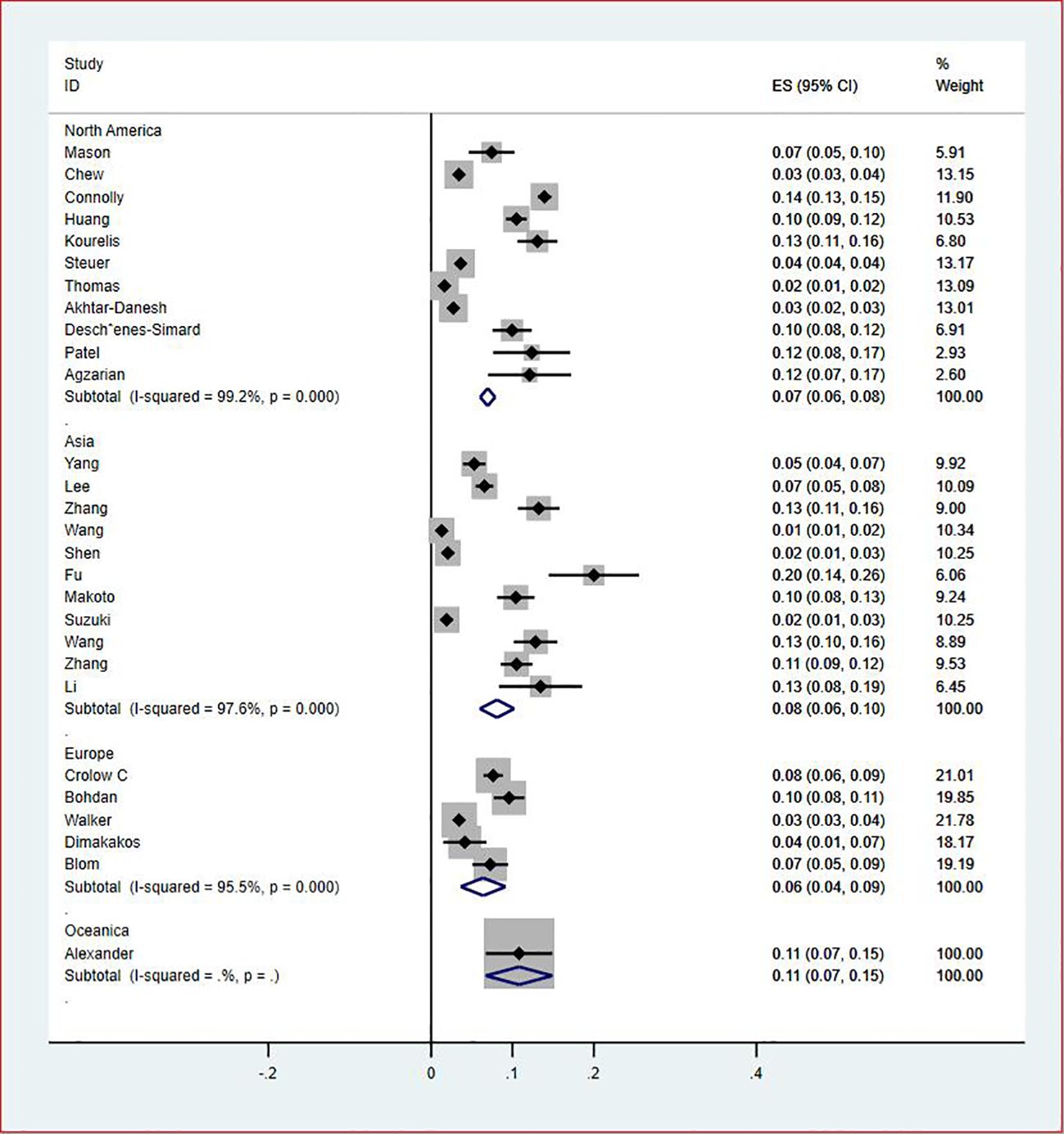
Results: Thirty-five studies involving 742,156 patients were considered eligible for this study. The pooled prevalence of VTE among patients with lung cancer was 5% (95% CI: 0.043–0.056, P = 0.000). The regional prevalence of VTE was 7% (95% CI: 0.06–0.08; I2 = 99.2%) in North America, 8% (95% CI: 0.06–0.10; I2 = 97.6%) in Asia, 6% (95% CI: 0.04–0.09; I2 = 95.9%) in Europe and 11% (95% CI: 0.07–0.15) in Australasia.
Conclusions: The prevalence of lung cancer-related VTE is high and region-specific. These results of this review emphasize the importance of understanding the incidence of lung cancer-related VTE and provide argue for VTE screening of patients with lung cancer.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier PROSPERO (CRD42022306400).
Introduction
Primary lung cancer is the second most prevalent malignancy worldwide but has the highest rate of mortality (1). Although the overall survival of patients with lung cancer substantially improved in the past decade with comprehensive and updated treatment strategies, including surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy, the risk of venous thromboembolism (VTE) in this class of patients has further increased recently (2). Venous thromboembolism can occur at any time in the course of the disease, and may at times constitute the presenting symptoms, triggering further investigations that lead to an eventual cancer diagnosis. Venous thromboembolism is the second most important risk factor contributing to mortality, after cancer itself (3). As expected, a similar trend is also observed in lung cancer (4, 5).
Venous thromboembolism can be classified into two major clinical conditions: deep vein thrombosis (DVT) and pulmonary embolism (PE). Large studies have shown that VTE is associated with worse outcomes in patients with cancer, including increased mortality. For instance, in one study, the one-year overall rate of survival in patients with lung cancer, who simultaneously had VTE, was 12%, compared to 36% in those without VTE (P < 0.001) (5). The mortality of those with VTE was 2.2-fold higher (95% confidence interval (CI) 2.05 to 2.40), while patients diagnosed with cancer within twelve months following a diagnosis of VTE had a one-year survival rate of 38%, compared to 47% percent in the control group without VTE (P <0.001) (6). The prevalence of cancer-related VTE varies according to patient-related factors, such as additional thrombogenic risk factors, including degree of mobility, performance status, presence of other comorbidities, past history of VTE, cancer-related factors, such as cancer location, stage, and grade, and treatment-related factors, such as whether the patient received surgery, chemotherapy, anti-angiogenic therapy, hormonal, and supportive therapy (7–9). Understanding and addressing the factors contributing to lung cancer-related VTE can significantly aid the prevention of VTE, which in turn reduces patient comorbidities and prevents reductions in performance status. This, in turn, helps to optimize the treatment schedules of cancer patients and is expected to benefit patient survival. Therefore, it is important to systematically analyze the characteristics of patients with VTE so that primary thromboprophylaxis with anticoagulant therapy and non-pharmacological interventions can be constituted appropriately.
At present, domestic and international guidelines for the prevention and management of VTE in cancer patients do not specifically outline the management of different VTE risk factors, nor do they suggest risk-factor specific or individualized management for VTE in patients suffering from different types of cancer. Therefore, assessment of the prevalence and characteristics of VTE in patients with lung cancer is of value toward optimizing the prevention and treatment of VTE in lung cancer. This may additionally aid in support of drafting updated guidelines to support these measures. The aim of this systematic review and meta-analysis is to determine the prevalence and patient-specific characteristics of VTE grouped by gender and regional characteristics of patients with lung cancer.
Methods
This review was conducted in accordance with the PRISMA 2020 statement for systematic reviews (9). The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), registration number: CRD42022306400.
Search strategy
Literature from the databases of PubMed, EMBASE, and the Cochrane Library were systematically searched without restrictions on language or geographical region, from the inception of the databases through January 21, 2022. Medical Subject Headings (MESH) terms and keywords used in the search included “venous thromboembolism” OR “VTE” OR “thromboembolism” OR “venous thrombosis” OR “pulmonary thrombosis” OR “pulmonary embolism” OR “pulmonary thromboembolism” OR “deep vein thrombosis”, AND “lung cancer” OR “pulmonary cancer” OR “lung tumor” OR “pulmonary tumor” OR “lung neoplasm*” OR “lung carcinoma” OR “pulmonary neoplasm*”. References of potential reviews were manually evaluated to identify additional studies relevant to these subjects. A full search strategy can be found in Appendix 1.
Eligibility criteria
Eligible studies included observational studies reporting the prevalence of VTE among patients with lung cancer as the primary malignancy.
Exclusion criteria
Studies excluded from this review included conference abstracts, duplicate studies, and publications with incomplete results.
Study selection and data extraction
Two independent reviewers screened for all included studies following the primary database search. First, duplicate studies and irrelevant literature were excluded. Each reviewer independently reviewed the full texts of each potentially eligible study, and included studies based on the aforementioned inclusion and exclusion criteria. In cases of disagreement between the reviewers, discussions around the potential study eligibility were carried out with a third reviewer until consensus was drawn.
Extraction and analysis of data were carried out by the two reviewers independently using predesigned forms based on the guideline for data extraction for systematic reviews and meta-analysis (10). The following information was extracted: the names of authors, year of publication, country of study, types of observational study carried out, sample size of the studied population, study duration, patient age, VTE subclass diagnoses (PE or DVT), locations of VTE occlusions, and the number of VTE events.
Quality assessment
The quality of the included studies was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) (11) in three domains, including patient selection, comparison, and results. The NOS scores of observational studies are graded from 0 to 9, where higher scores indicate higher study qualities. Specifically, we grouped these scores into 3 categories: ≥7, 4–6 and 0–3, to represent studies of high, medium and low qualities, respectively.
Statistical analysis
The metaprop procedure (12) was used to perform a meta-analysis of proportions, using Stata software (version 15.1). This approach was suitable for the analysis of binomial data and permitted the evaluation of the exact binomial and test score-based confidence intervals (CI). The meta-prop procedure provided suitable strategies for dealing with proportions close to or at the margins, where the normal approximation procedures often break down. It uses a binomial distribution to model the within-study variability and allows the Freeman-Tukey double arcsine transformation to stabilize the variances. Study heterogeneity was assessed using chi-square test and I2 values, P < 0.1 or I2>50% was considered to be clinically significant, and a random-effect model was adopted. Sensitivity analyses were performed to verify the robustness of the overall results and to explore sources of heterogeneity. Subgroup analyses were conducted for lung cancer-related VTE in different genders and regions. Finally, we used funnel plots and Egger’s regression test to identify publication bias.
Results
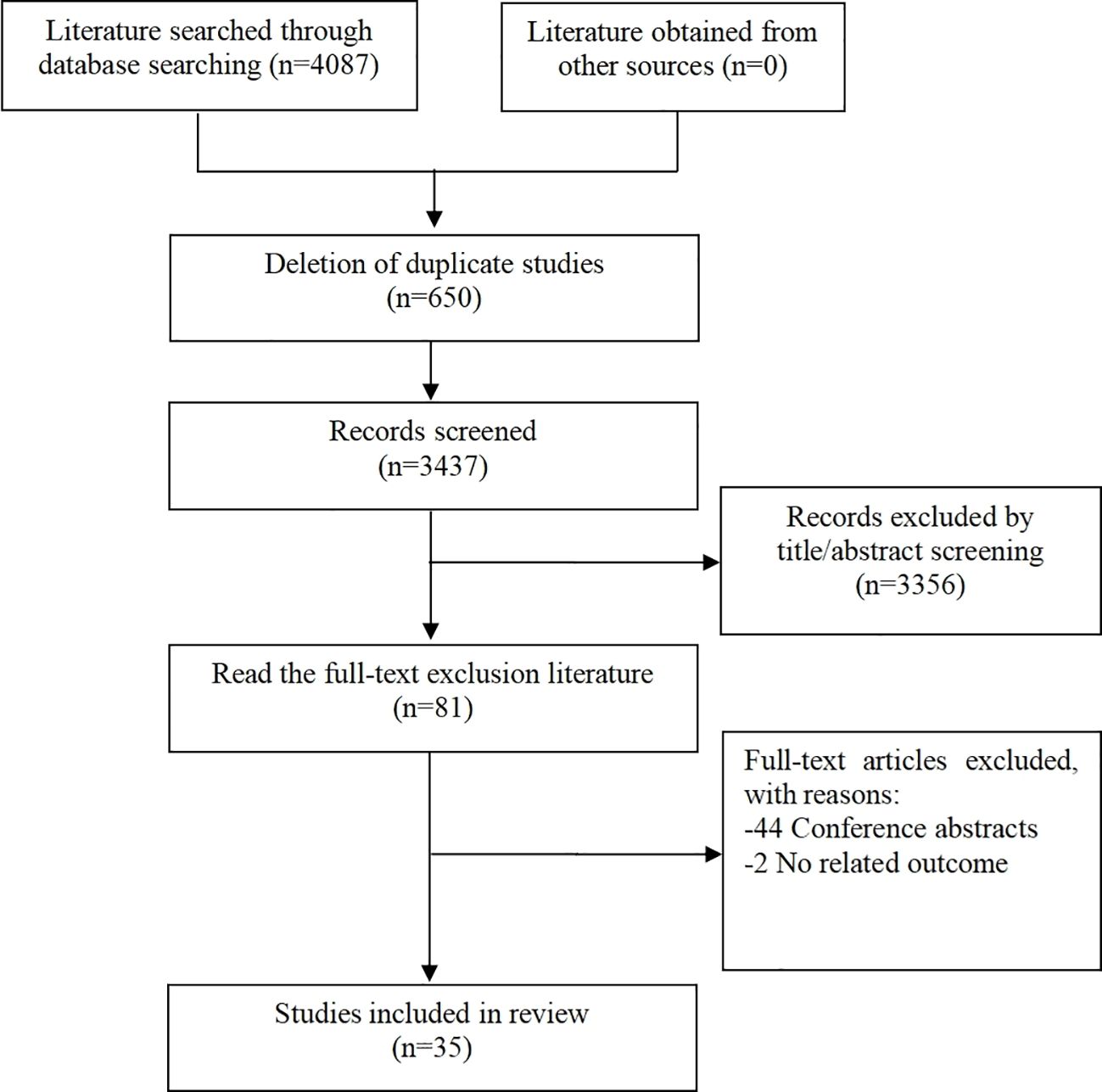
Overall, a total of 4087 articles were found through the initial literature search based on the aforementioned search criteria. Of these, 650 duplicate articles and 3356 irrelevant articles were excluded after initial abstract screening. Eighty-one additional articles were excluded after full text screening. Out of the literature excluded, 44 were conference abstracts and 2 were trials with non-related outcomes. Altogether, 35 studies (13–47) were found to be eligible for this systematic review. The literature selection process is outlined in Figure 1.
Study characteristics
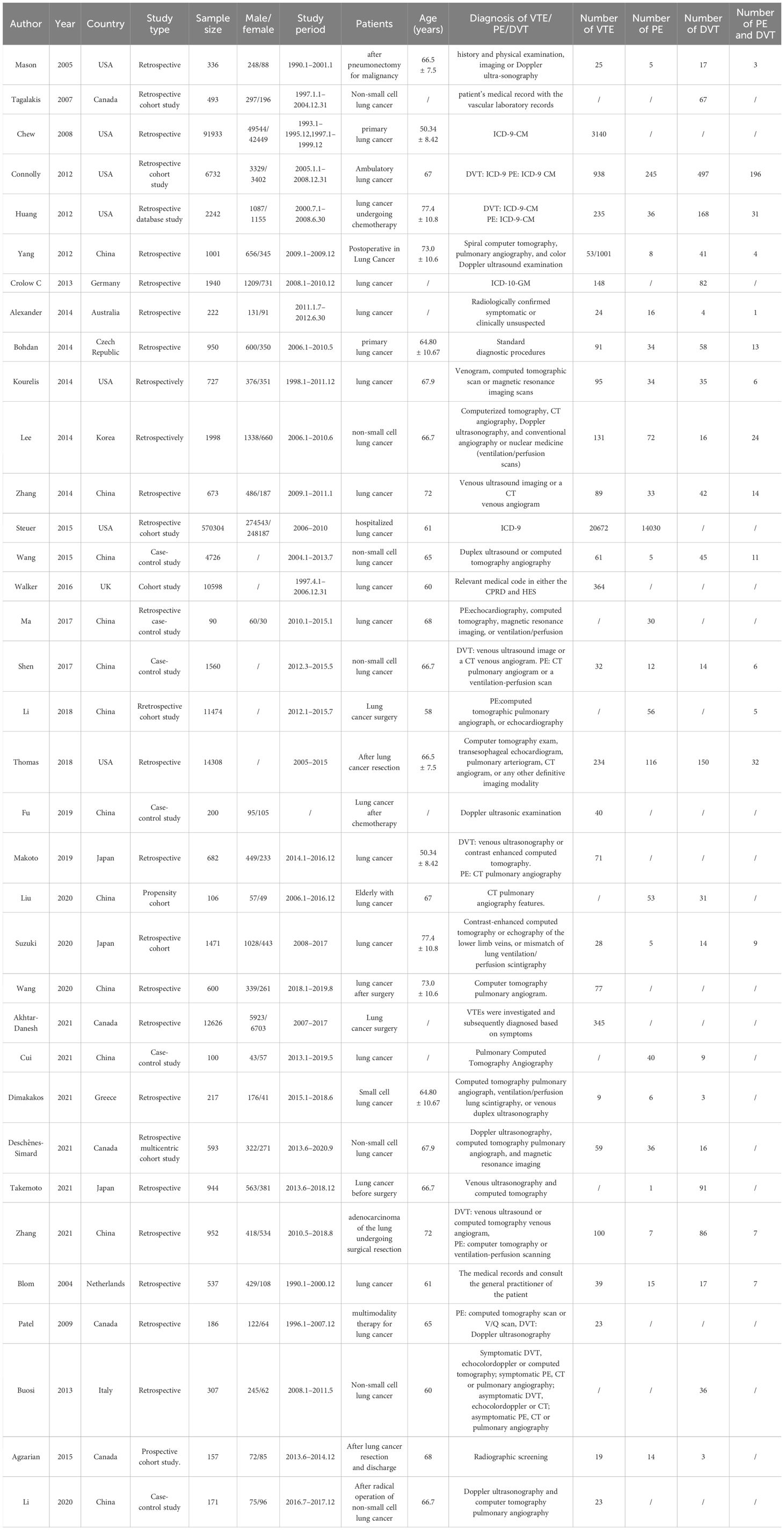
Among the 35 studies included with VTE listed as the primary outcome (13–47), 20 were retrospective studies (13, 14, 16, 17, 19–27, 35, 37, 41, 42, 45–47), nine were cohort studies (15, 18, 28, 30–32, 38, 40, 44) and six were case-control studies (29, 32, 33, 36, 39, 43). All studies included were published from 2005 to 2021. The number of study participants varied from a minimum of 90 to a maximum of 570,304. The studies were conducted in 12 countries, including China, the United States, Canada, Japan, Germany, Australia, Czech Republic, Korea, Greece, the Netherlands, Italy, and Great Britain, which are grouped into their respective continents for ease of categorization. Specifically, there were two (16.67%) countries categorized into the North American group, three (25%) countries in Asia, six (50%) countries in Europe, and one (8.33%) country in Australasia. Thirty studies (13–19, 21–28, 30–34, 36–40, 43–47) reported the mean age of the participants, ranging from 50.34 to 72 years. International Classification of Diseases (ICD)-9 or ICD-10 codes were used to label the diagnoses of VTE in five studies (16, 18, 19, 22, 28). Most of the other 30 studies combined medical history, physical examination and imaging to aid in the diagnosis of VTE. Out of all studies included, eight studies (16, 17, 31, 36, 37, 39, 41, 42) reported on VTE, two (32, 34) reported PE only, two reported DVT only (15, 21) and 23 (13, 14, 18–20, 22–30, 33, 35, 38, 40, 43–47) simultaneously reported VTE, PE, or DVT. General characteristics of the included studies are shown in Table 1.
Quality bias assessment
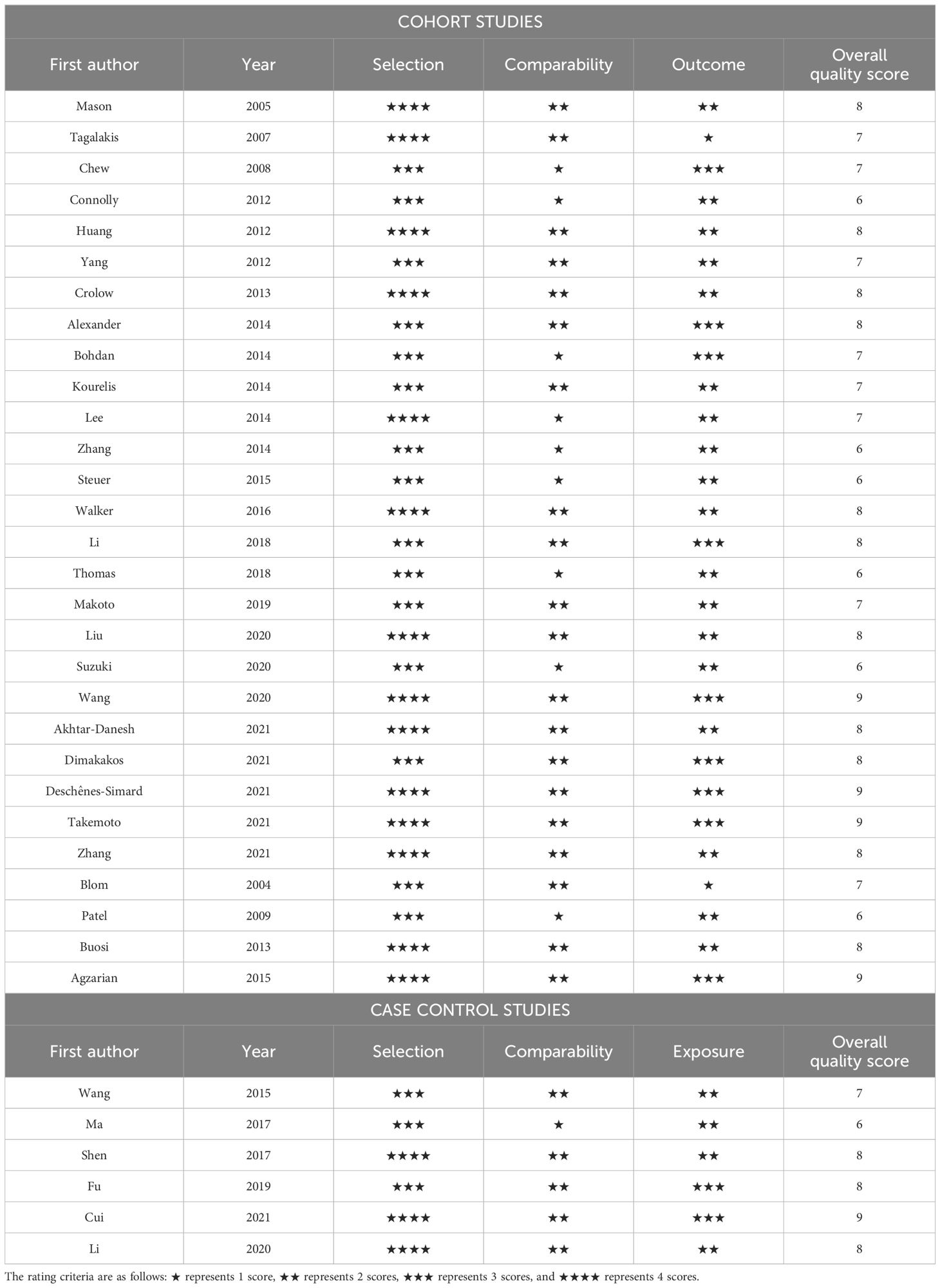
The qualities of all eligible studies in our meta-analysis, evaluated using the NOS scale, are outlined in Table 2. Twenty-eight (80%) studies (13–16, 19–26, 29–31, 33, 34, 36–39, 41–47) scored seven or higher, which were classified as high quality. Seven (20%) studies (17, 18, 27, 28, 32, 35, 40) scored between four and six inclusive and were classified as medium quality. There were no low-quality studies scoring three or below. Our 35 studies had a mean NOS score of 7.486, indicating high quality of the studies included in this series of studies reviewed.
Prevalence of VTE in patients with lung cancer
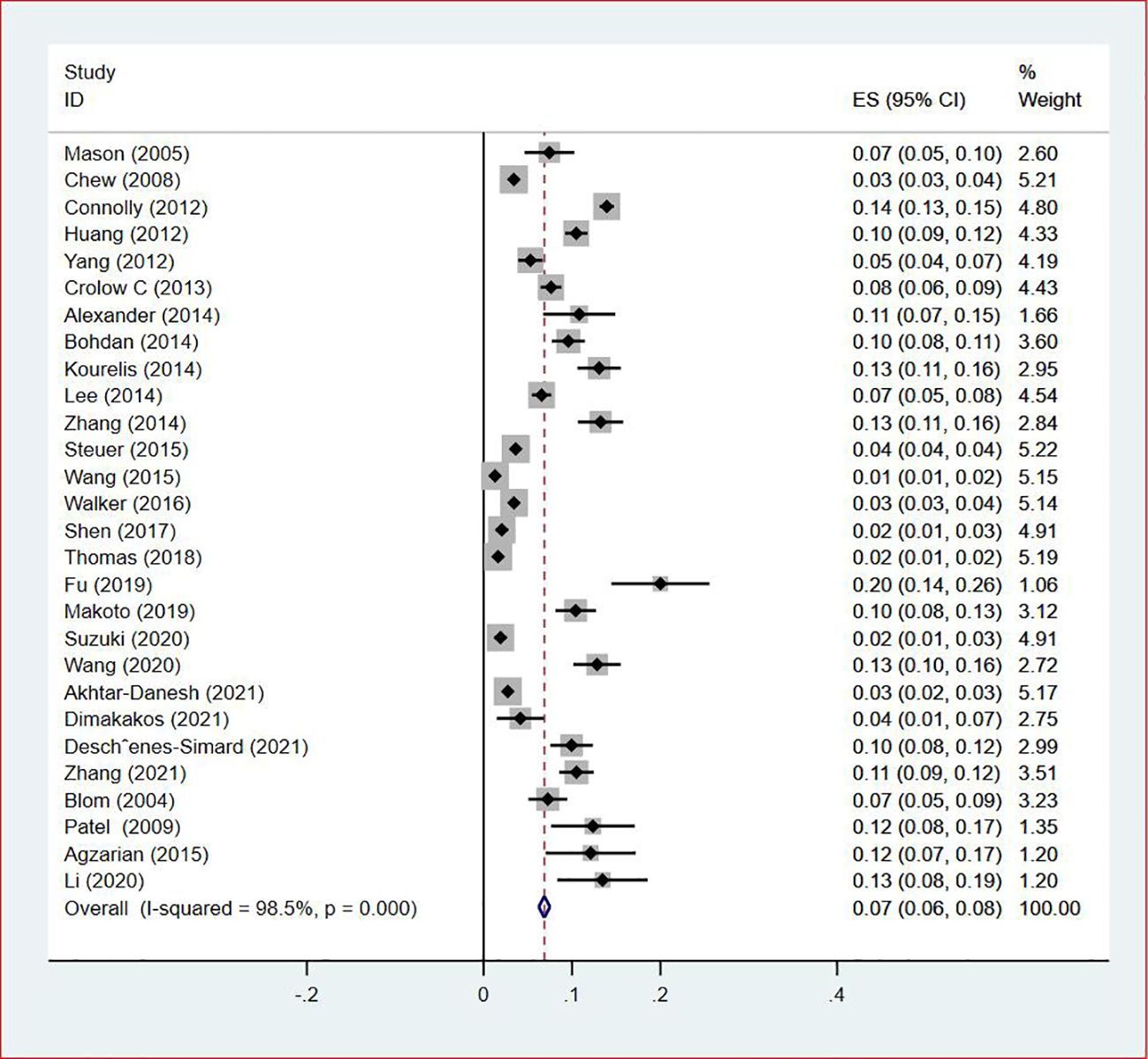
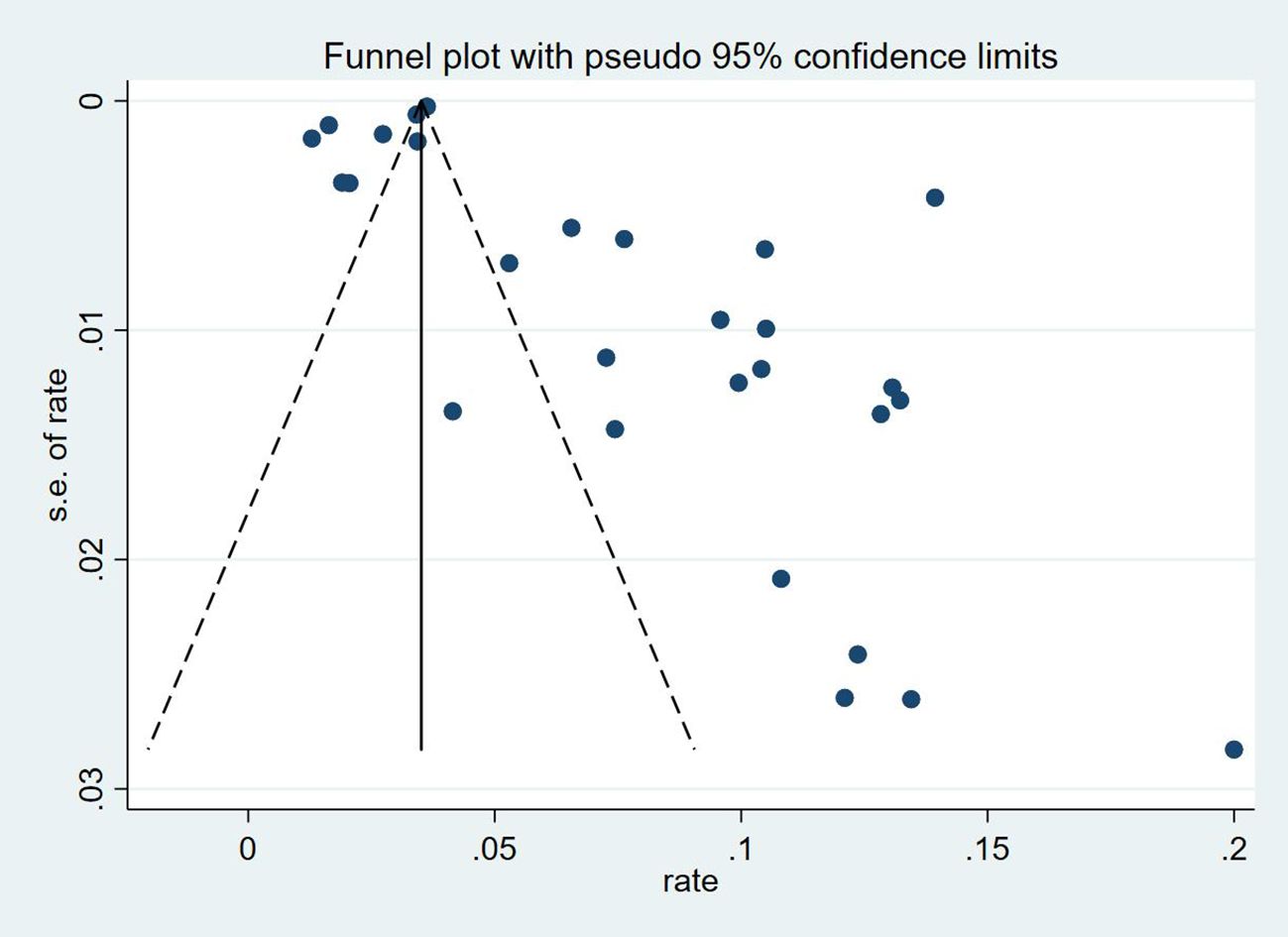
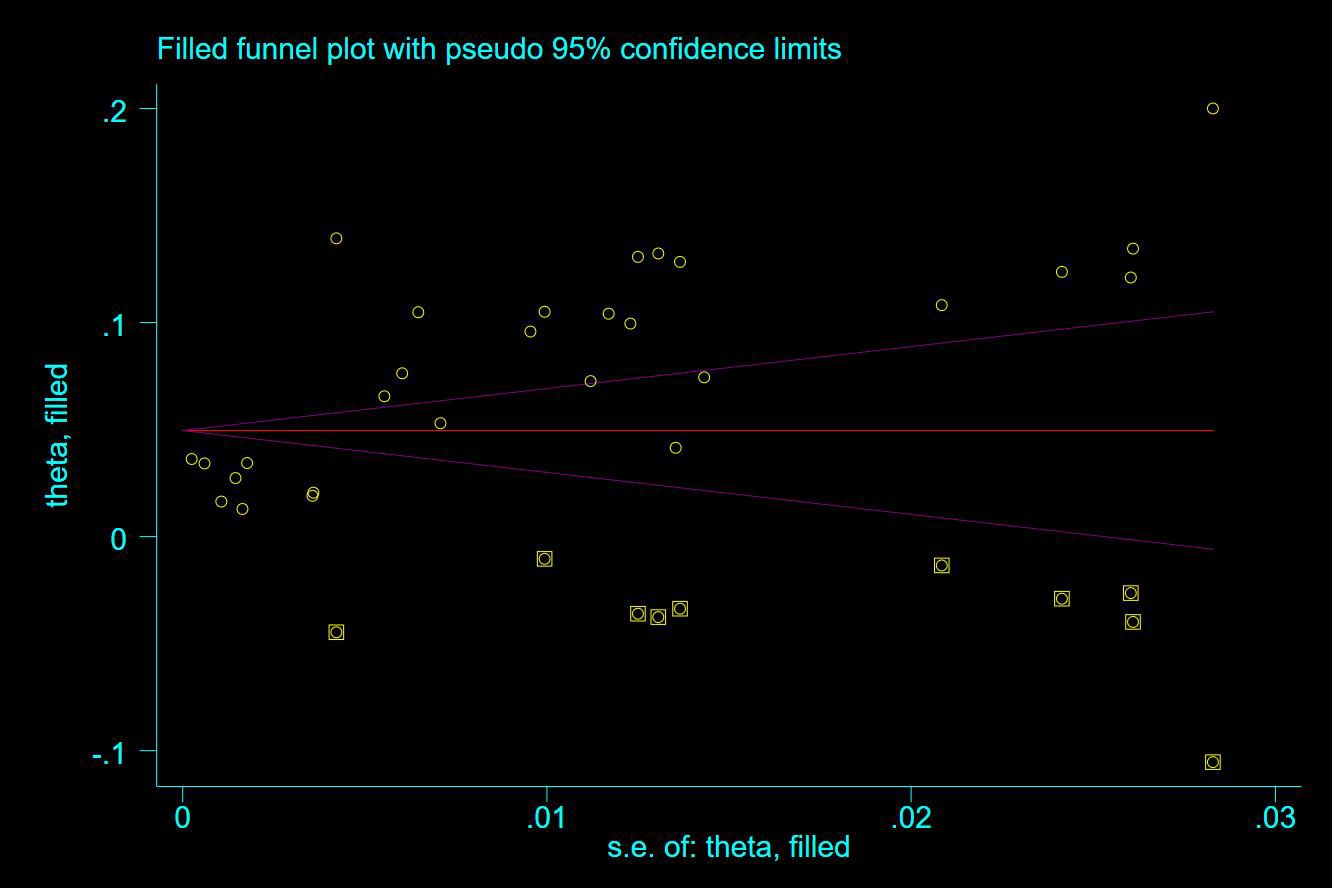
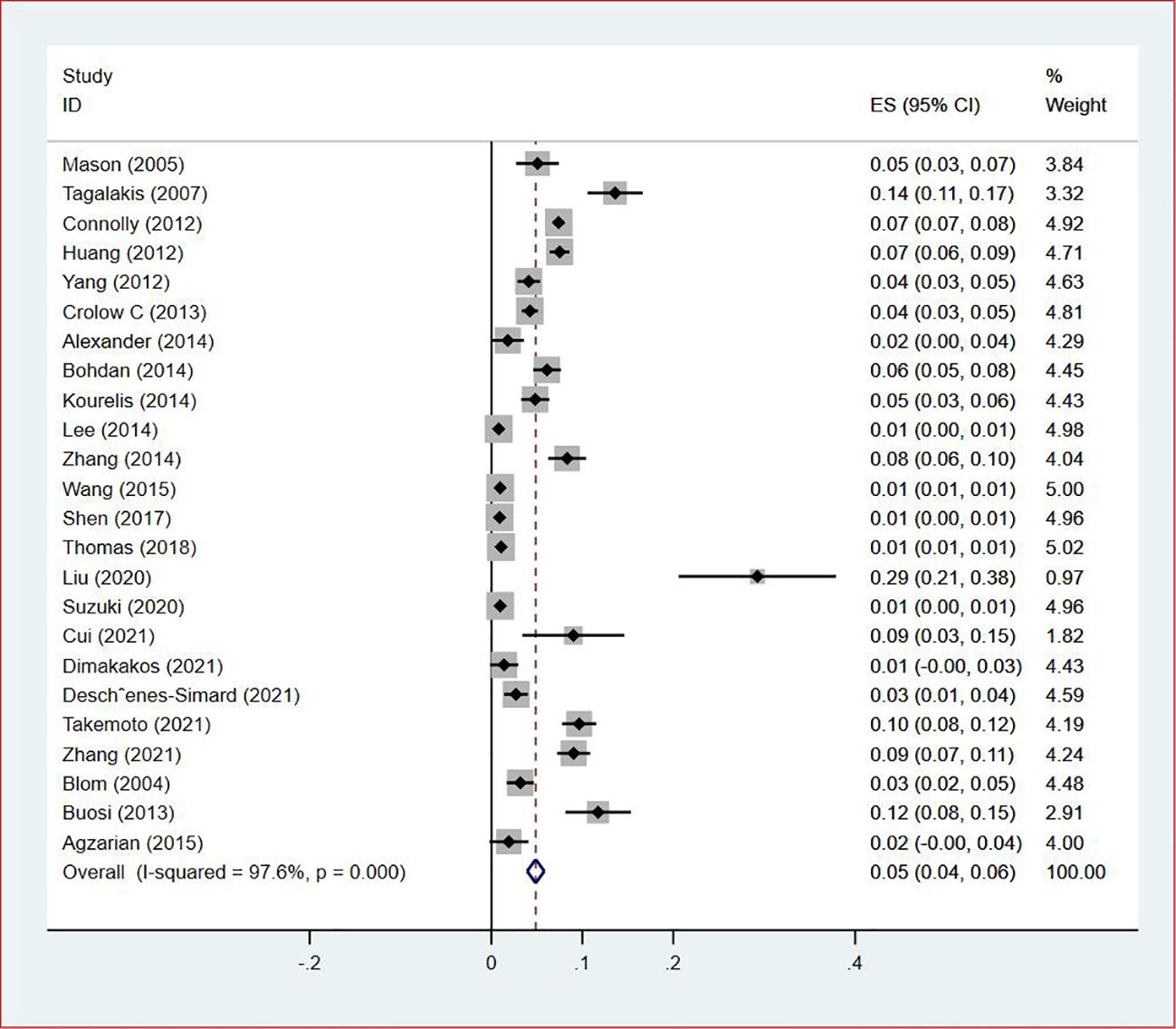
Thirty-five studies (13–47), 29 (82.86%) (13, 14, 16–31, 33, 35–37, 39–42, 44, 45, 47) reported the prevalence of VTE among patients with lung cancer, ranging from 1% to 20%, with an estimated pooled prevalence of 7% (95% CI: 0.06–0.08; I2 = 98.5%), as shown in Figure 2. Owing to the significant heterogeneity between different study designs (I2 = 98.5%, P = 0.0002), a sensitivity analysis was performed by omitting each study individually to explore the sources of heterogeneity. The effect of lung cancer on the overall prevalence of VTE was relatively robust after removing three studies (16, 18, 28) with high risks of bias (Appendix 2). A funnel plot on this revealed significant publication bias (Figure 3). Publication bias was found to be high in this meta-analysis, as demonstrated by Egger’s regression tests (P=0.034). Using the clipping method, we found the adjusted prevalence of VTE in patients with lung cancer to be 5% (95% CI: 0.043–0.056, P < 0.001). This was not significantly different from the pre-adjusted prevalence of 7% (95% CI: 0.062–0.075, < 0.001), indicating the prevalence of VTE was not significantly affected by publication bias (Figure 4).
Sub-group analysis of VTE in patients with lung cancer
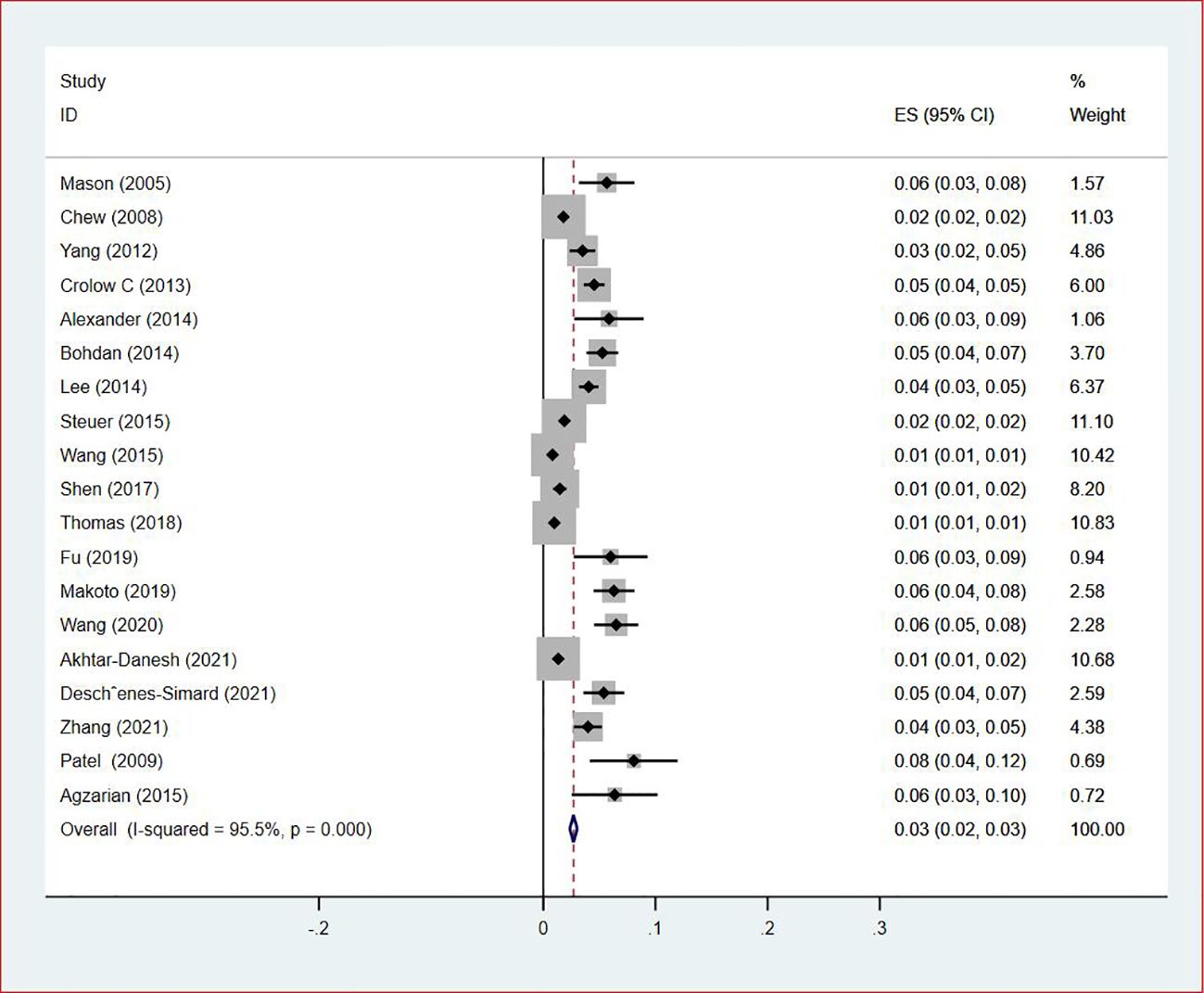
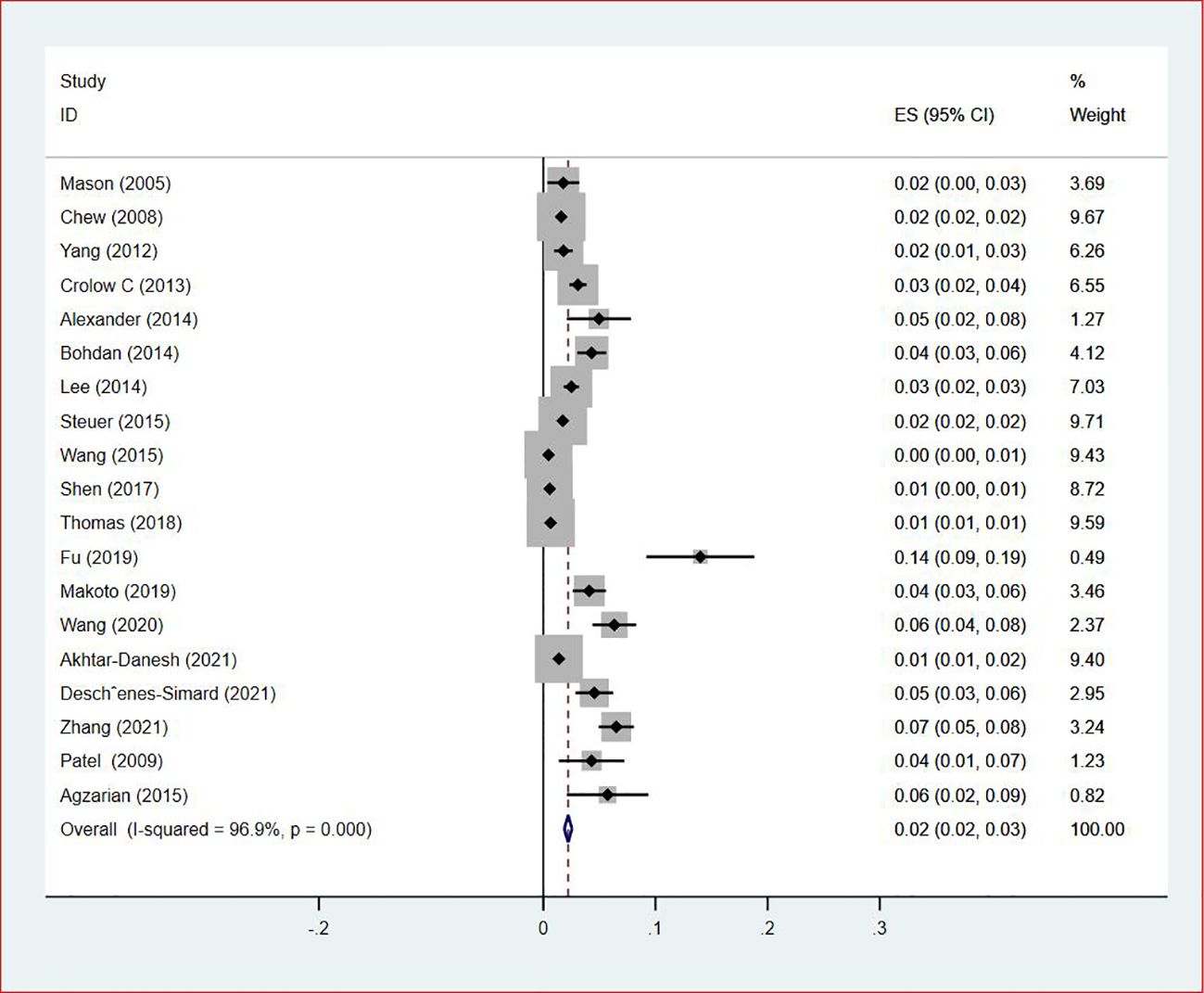
Of all included studies, 19 studies (14, 16, 17, 20, 22–24, 26, 28–30, 33, 35–37, 41, 42, 44, 47) reported the prevalence of VTE by gender. The pooled prevalences of VTE in male and female patients were 3% (95% CI: 0.02–0.03; I2 = 95.5%) and 2% (95% CI: 0.02–0.03; I2 = 96.9%), respectively (Figures 5, 6). Eleven studies were performed in North America and Asia each, five were conducted in Europe and one in Australasia. The regional prevalence of VTE in patients with lung cancer was 7% (95% CI: 0.06–0.08; I2 = 99.2%) in North America (15–19, 25, 28, 30, 35, 42, 44), 8% (95% CI: 0.06–0.10; I2 = 97.6%) in Asia (20, 26, 27, 29, 33, 36, 37, 39–41, 47), 6% (95% CI: 0.04–0.09; I2 = 95.9%) in Europe (13, 22, 24, 31, 45) and 11% (95% CI: 0.07–0.15) in Australasia (23), respectively (Figure 7).
Prevalence of DVT in patients with lung cancer
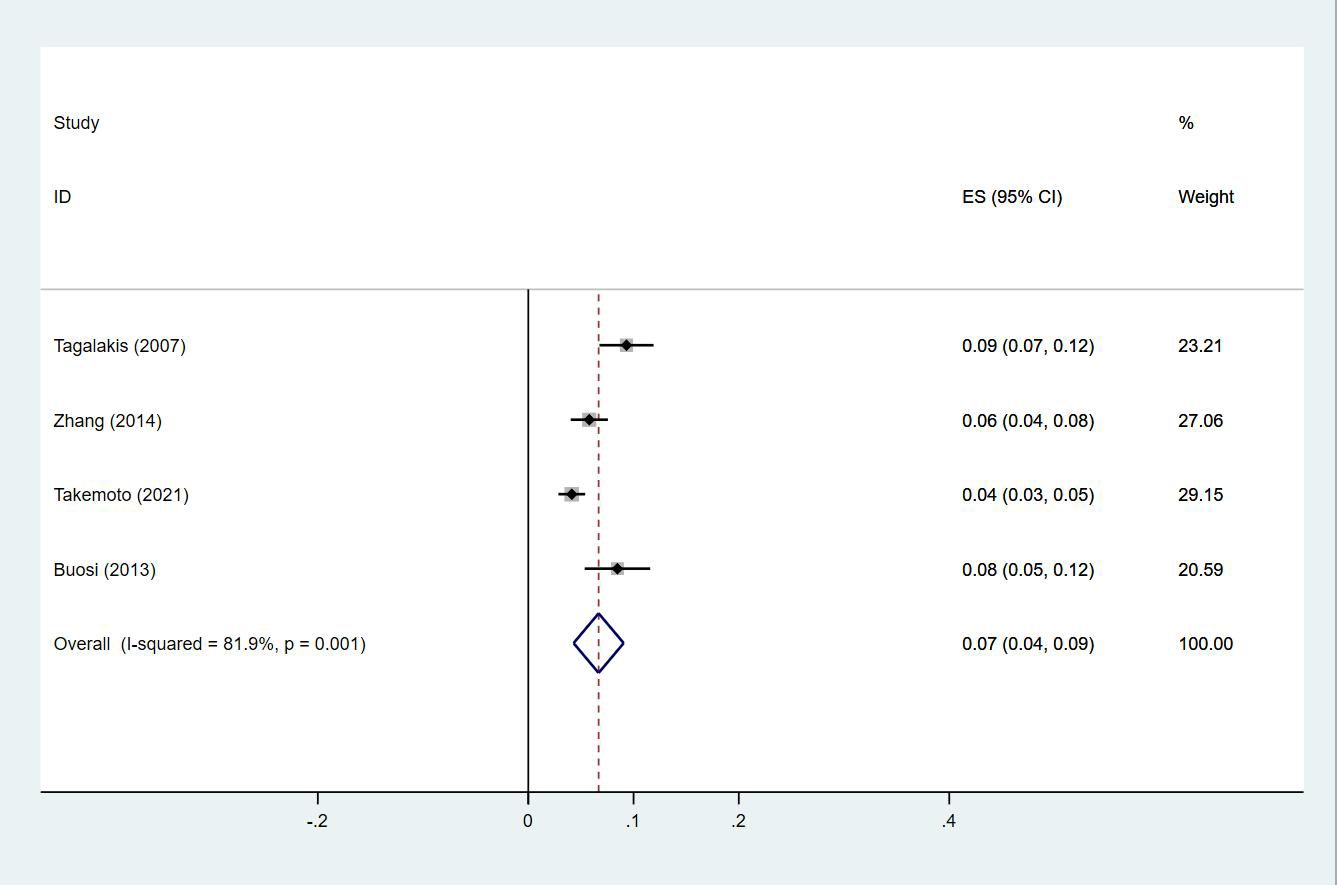
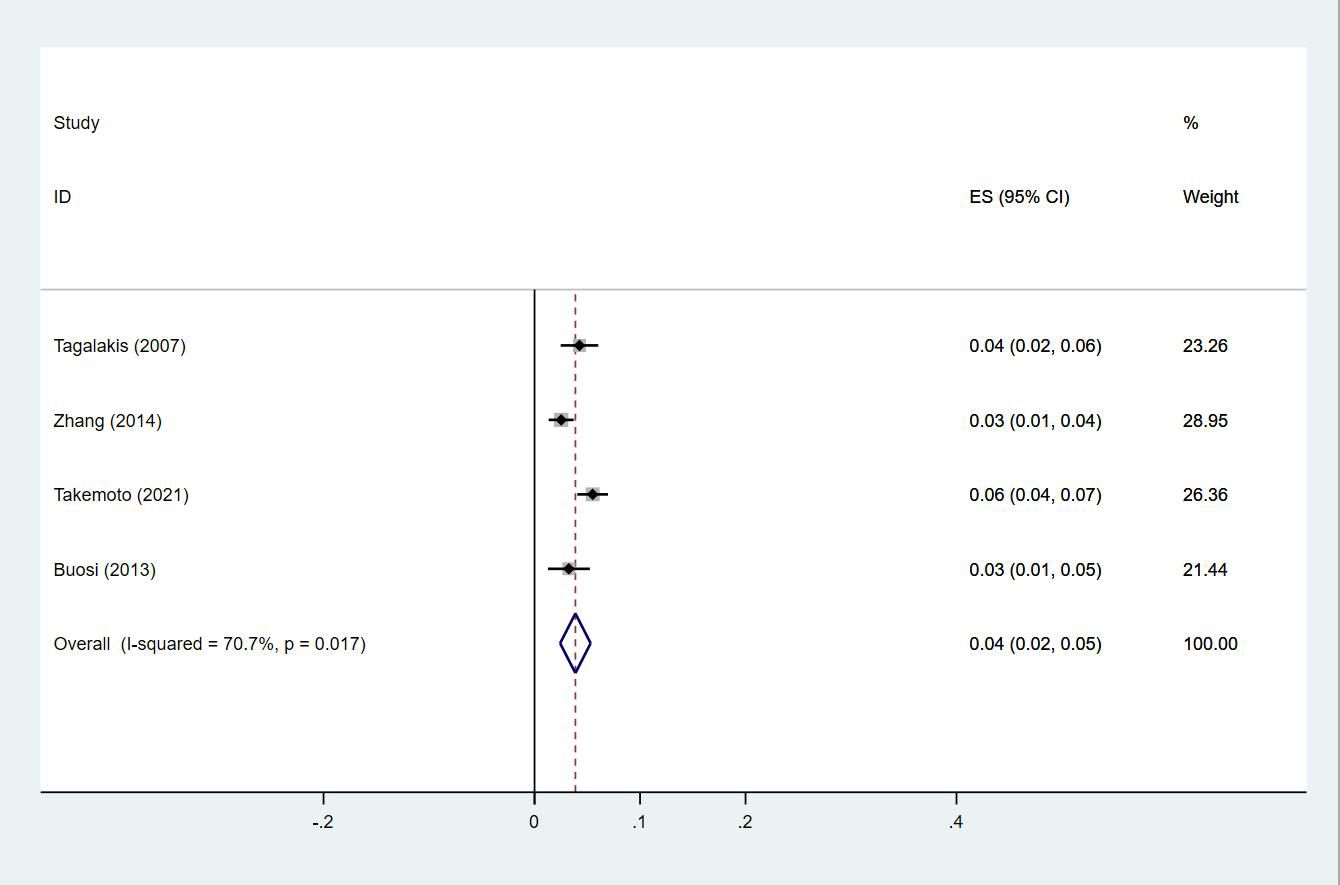
Twenty-four studies (13–15, 18–27, 29, 30, 33, 35, 38, 40, 43–47) reported the prevalence of DVT in patients with primary lung cancer, which ranged from 1% to 29%. The pooled prevalence was 5% (95% CI: 0.04–0.06; I2 = 97.6%) (Figure 8). Four studies (15, 21, 27, 46) simultaneously reported the proportions of DVT grouped by gender, with the pooled prevalences in men and women being 7% (95% CI: 0.04–0.09; I2 = 81.9%) and 4% (95% CI: 0.02–0.05; I2 = 70.7%), respectively (Figures 9, 10).
Prevalence of PE in patients with lung cancer
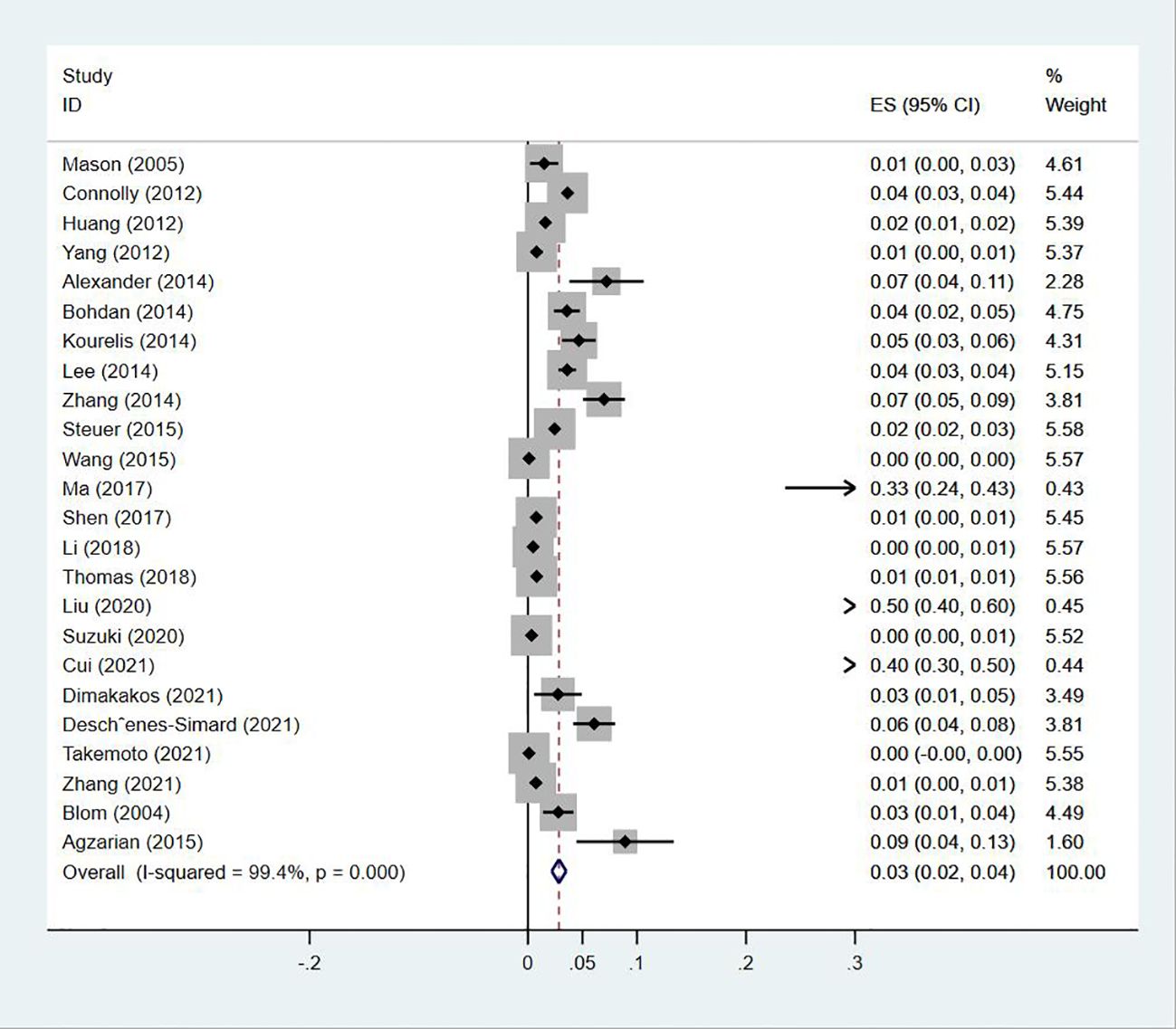
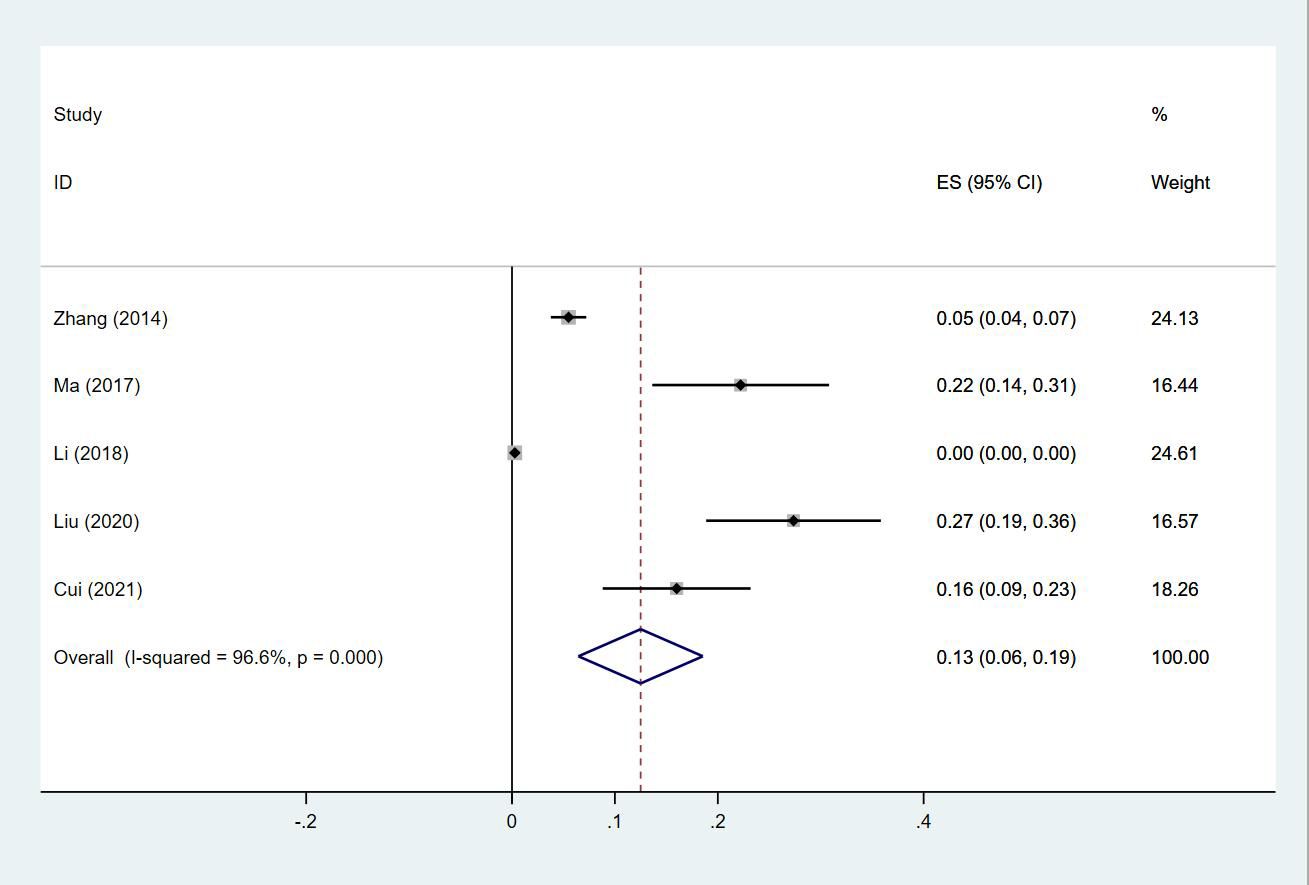
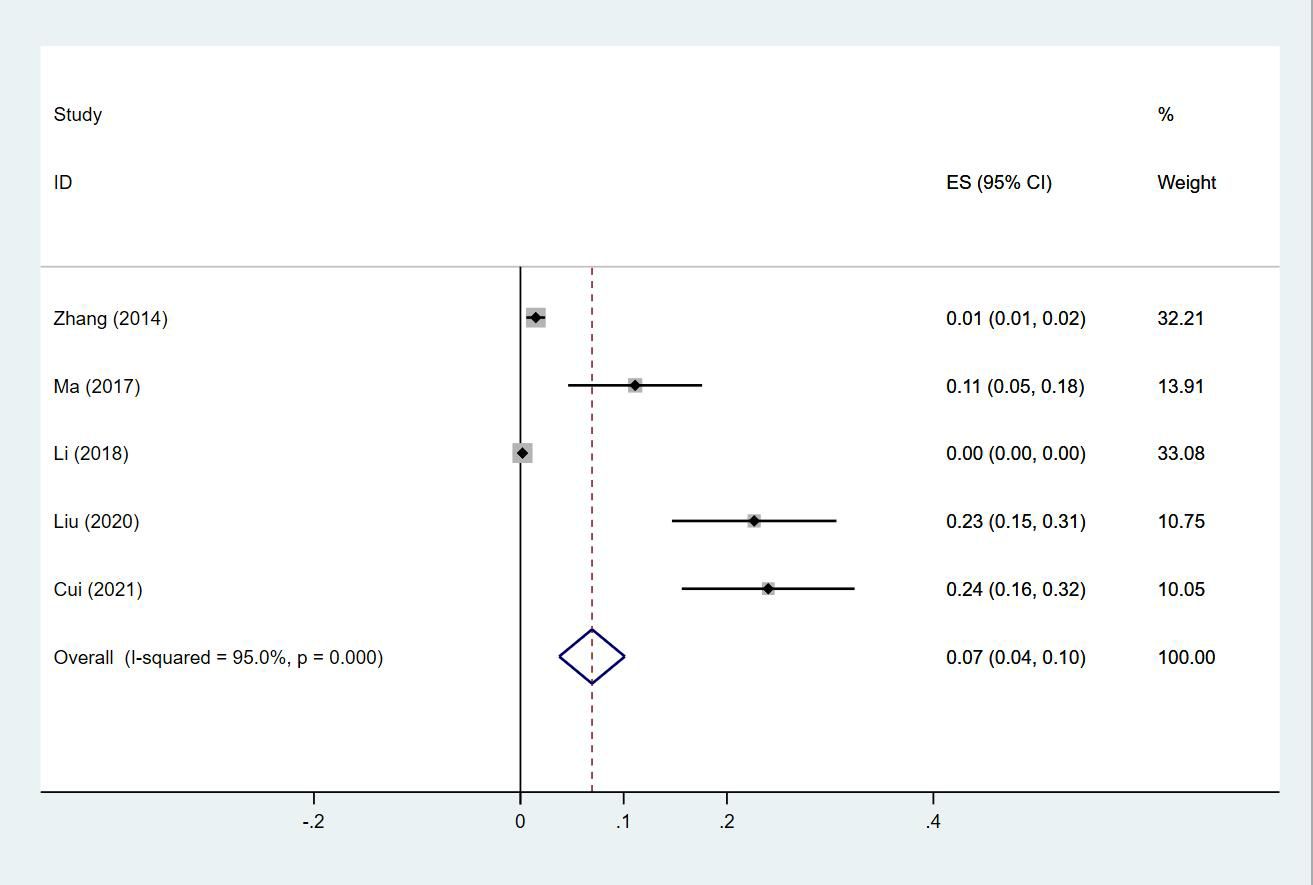
Twenty-four (13, 14, 18–20, 23–30, 32–35, 38, 40, 43–47) studies reported the prevalence of PE in patients with lung cancer, which ranged from 0% to 50%. The pooled prevalence was 3% (95% CI: 0.02–0.04; I2 = 99.4%) (Figure 11). Five studies (27, 32, 34, 38, 43) reported the prevalence of VTE in men and women, with the pooled prevalences being 13% (95% CI: 0.06–0.19; I2 = 96.6%) and 7% (95% CI: 0.04–0.10; I2 = 95.0%) in men and women, respectively (Figures 12, 13).
Prevalence of DVT and PE in patients with lung cancer
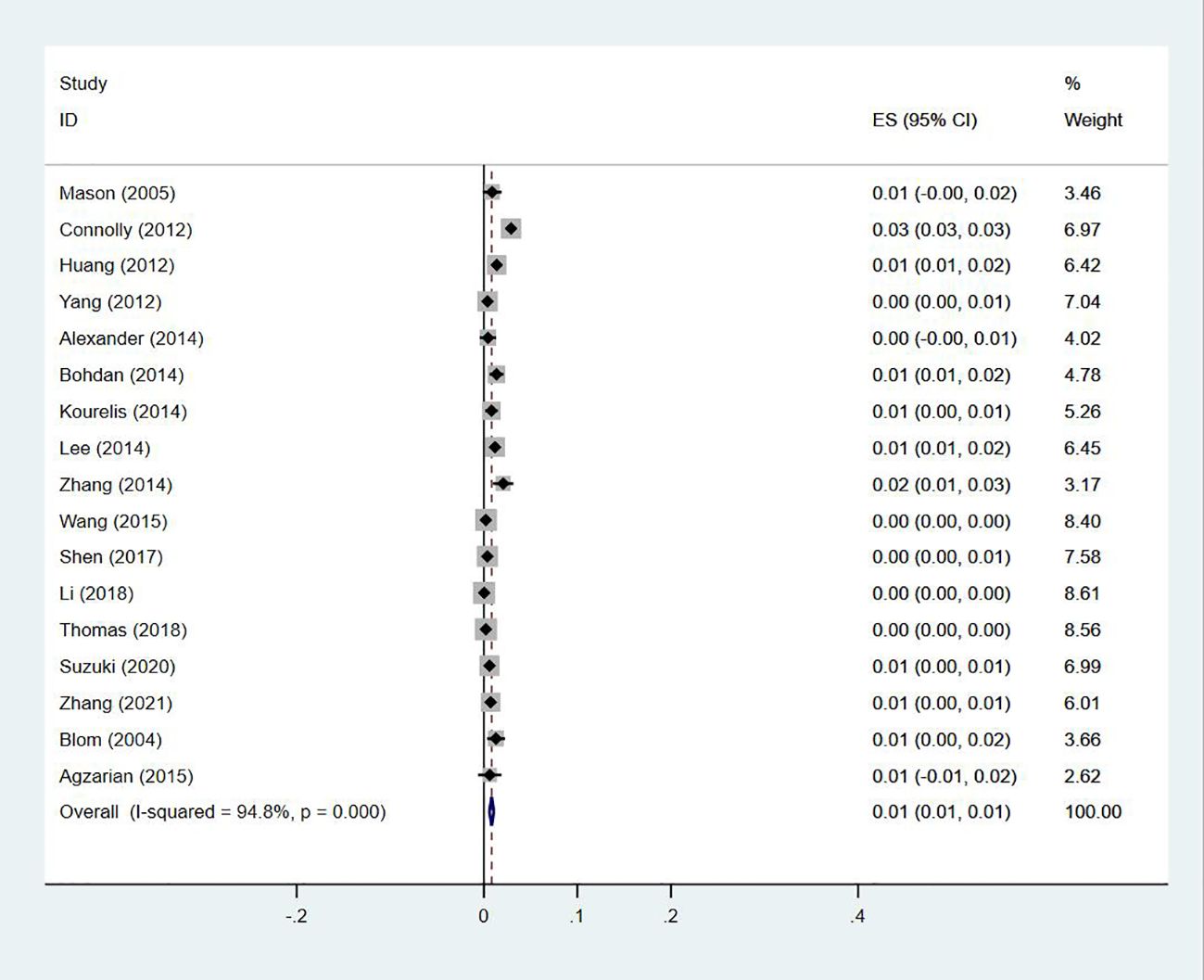
Seventeen studies (13, 14, 18–20, 23–27, 29, 30, 33–35, 40, 47) reported the prevalence of the simultaneous occurrence of DVT and PE in lung cancer, ranging from 0% to 3%, with a pooled prevalence of 1% (95% CI: 0.01–0.01; I2 = 94.8%) (Figure 14).
Sites of occlusion of VTE in acute exacerbations of chronic obstructive pulmonary disease (AECOPD)
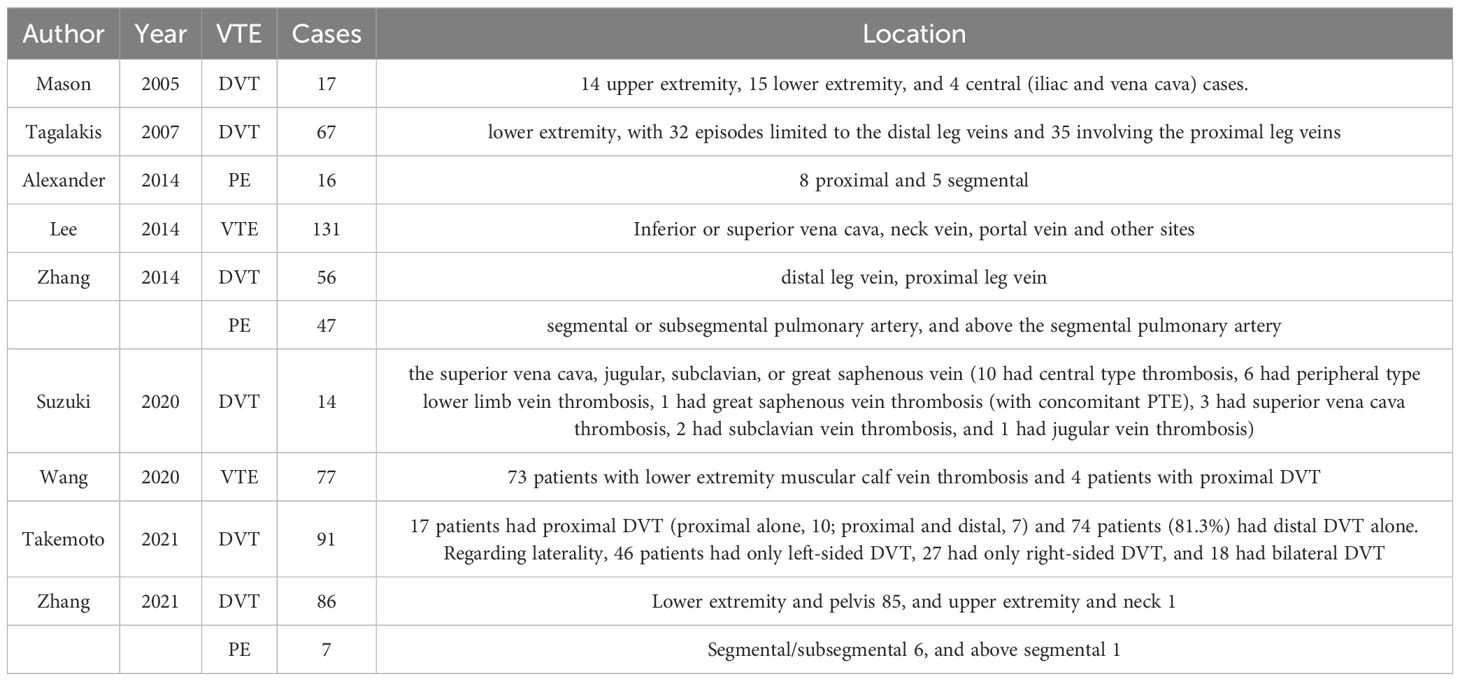
Nine studies (14, 15, 23, 25, 27, 30, 41, 46, 47) reported on the specific sites of occlusion for VTE events, in which two (13, 33) reported that of both PE and DVT, whereas others (14, 15, 23, 25, 40, 41, 46) focused on either PE, DVT or VTE. Details on the sites of occlusion for the various VTE events are shown in Table 3.
Discussion
This systematic review included 35 studies with 742,156 individuals. It demonstrated that the number of patients with VTE, as previously reported, is substantial in those suffering from lung cancer, with gender and regional variances. In evaluating the prevalence and characteristics of all VTE, DVT, and PE, we found the prevalence of VTE was the highest in Australasia and lowest in Europe. The prevalence of VTE was higher in male patients with lung cancer. DVT predominantly occurred in the distal lower extremities, while PE mostly occurred in segmental or subsegmental pulmonary arteries. This systematic review summarized the prevalence of VTE in patients with lung cancer, which provides a theoretical basis in constructing guidelines for the screening, prevention and management of thromboembolism in lung cancer.
VTE is one of the most important comorbidities leading to increased morbidity and mortality in cancer patients (7). Approximately 15% of patients are diagnosed with at least one VTE event in the course of their cancer (48). Primary lung cancer remains the leading cause of cancer death worldwide, which is further compounded by the high prevalence of VTE that both worsens the performance status of affected patients and complicates their cancer management. Understanding the characteristics of lung cancer-associated VTE is therefore of the utmost importance.
The association of race with VTE may reflect the different regional rates of VTE. It is widely understood that patients of Asian ethnicity have lower risks of VTE, where several ethnic comparative studies reported lower rates of thromboembolism affecting the venous system in patients of Asian descent in the United States when compared to Caucasians and African Americans (49). A study found at most a modest association of race with risk of VTE, particularly once comorbid conditions and socioeconomic status were accounted for in three large US cohort studies including 51,149 individuals and 916 VTE events. VTE rates were higher among black persons than white persons in the United States (risk ratio, 1.81; 95% confidence interval, 1.20–2.73) and significantly higher among black persons in the Southeast than black persons in the rest of the United States (risk ratio, 1.63; 95% confidence interval, 1.08–2.48), whereas they were not higher among white persons (risk ratio, 0.83; 95% confidence interval, 0.61–1.14) (50). A recent investigation found that the incidence of VTE varied across ethnic groups, with the highest incidence identified among African Americans (4.02%), followed by non-Hispanic Caucasians (2.98%), Hispanics (2.08%) and Chinese (0.79%) participants (51). The association between BMI and venous thromboembolism was strongest among non-White women with the higher incidence for obese compared with non-obese patients. Risk of venous thromboembolism increased with age for all race/ethnicities. Most of the above studies of geographic or racial incidence of VTE have used the Atherosclerosis Study Cohort, the Cardiovascular Health Study Cohort, and the Causes of Geographic and Racial Differences in Stroke Study Cohort, among others. Fewer lung cancer study cohorts are currently available. We used the existing literature to summarize the geographic and racial incidence of VTE associated with lung cancer. Our meta-analysis found that the overall prevalence of VTE in patients with lung was 5%, with a male predominance. The incidence of VTE varied significantly across regions and appeared to be the highest in Australasia, though only one study from Australia was included. Patients in North America and Asia had similar incidences of VTE, while Europeans had the lowest incidence of VTE. Interestingly, previous studies have not shown these meaningful conclusions. Reasons for such potential differences between different ethnicities are unclear. A few potential confounding factors may be the varying risk factors occurring in patients of different ethnicities, with obesity, diabetes mellitus, and elevated factor VIII being more common in African Americans. Similarly, genetic polymorphisms, such as factor V Leiden and mutations in the prothrombin gene 20210A are more common in Caucasians (52), which may explain the high incidence of VTE in Australia, with Caucasians being the largest ethnicity in this study. These findings may promote widespread adoption of risk assessment tools, and target prophylactic anticoagulation. Regional differences suggest the need to adapt prevention strategies to local epidemiological data and to increase medical resources, such as specialized training, equipment, and anticoagulants, in high-risk areas. In addition, raising patients’ awareness of VTE risk can help improve adherence to preventive measures.
DVT and PE are the most common types of VTE, with PE closely associated and often occurring secondary to DVT. In our study, 17 studies reported a low combined prevalence of 1% of DVT and PE, which was consistent with previous reports. DVT mostly occurred in the distal lower extremities, while PE mostly occurred in segmental or subsegmental pulmonary arteries. In one of our studies (41), 28 of 1471 patients with lung cancer were diagnosed with VTE, including five patients with PE alone (0.34%), nine with PE diagnosed following DVT (0.61%), and 14 patients with DVT alone (0.95%). The incidence of PE occurring following diagnoses of DVT was 44.7%, and patients with risk factors accounted for 88.3%. The biggest risk factors for VTE were prolonged bed rest, surgery, hyperlipidemia, hypertension, malignancy, increased hemoglobin, heart disease, trauma, and femoral vein puncture. The number of risk factors increases with age and patient comorbidities. The most common clinical manifestations of DVT were lower limb swelling (91.3%) and pain (58.3%). The femoral vein is the most commonly involved site of occlusion for DVT in lung cancer, which also has the highest association with secondary PE. Therefore, for this group of high-risk patients, we need to closely monitor these risk factors, implement stratified management, and take appropriate preventive and therapeutic measures. This will help reduce the risk of DVT and PE, which are of critical clinical importance.
The incidence of DVT in cancer ranges from 1% to 11%, and is commonly indicative of poor prognosis (53). Among cancer patients presenting with DVT, cancers of the lung, prostate, breast, and colorectum are the most common, which comprise approximately 10% to 15% of all patients diagnosed with DVT (54). In our study, the prevalence of DVT was 5%, with a prevalence of 7% and 4% in men and women, respectively. The prevalence of DVT is higher in men, with male gender being an independent risk factor for DVT (17). As demonstrated in another study, the cumulative incidence of PE was 2.2% among 8014 patients with lung cancer (55). PE is associated with increased mortality in lung cancer (5). In a large registry study, 3% of deaths in cancer patients were PE-related, versus 1% of deaths in patients not suffering from cancers (56). In our study, the prevalence of PE was 3%, with a higher prevalence in men than in women similar to that for DVT, which were 13% and 7%, respectively. Being male is an independent risk factor for PE, which has been found consistently in a number of observational studies (57). Increased hemoglobin is a known risk factor for VTE, with generally higher hemoglobin levels in men potentially being one of the reasons why men are more likely to develop VTE. In summary, VTE, encompassing DVT and PE, is a critical complication in cancer patients, especially lung cancer patients. Therefore, close clinical monitoring, accurate risk assessment, and appropriate prophylactic measures need to be taken in such patients. In addition, gender and hemoglobin level, as important risk factors, should not be ignored in clinical management.
This study has a number of limitations that need to be acknowledged. Although the qualities of our included studies were examined using the NOS scale, with an average score of 7.486 being obtained, indicating a high overall quality, the use of ICD-9 and ICD-10 codes were only used in five studies for diagnosing VTE. As most of the other 30 studies combined medical history, physical examination and imaging, the inconsistency observed in diagnosing VTE between different studies may have introduced significant bias. In addition, some of the studies dated back to the mid-2000s. As such, the observed prevalence of VTE in patients with lung cancer may not apply to patients undergoing more up-to-date contemporary individualized treatments, including targeted therapy and immunotherapy from the early 2010s onwards. Additionally, since the literature we included did not provide sufficient data to support an analysis of the incidence of VTE associated with different types of lung cancer, we did not proceed with graphical representation or further analysis of this aspect.
In conclusion, our meta-analysis demonstrates that the prevalence of lung cancer-related VTE is high and is gender- and region-specific. This study emphasizes the need to further explore the causes of regional differences and develop effective preventive strategies, provides an important basis for improving the recognition and management of VTE in lung cancer patients, and may lead to changes in clinical practice to reduce the risk of this serious complication.
Limitations
In some of the literature we included, there may be instances where lung cancer patients have undergone surgery, radiotherapy, or chemotherapy, leading to a certain degree of heterogeneity in our analysis of the literature. Unfortunately, these studies did not provide sufficient data to allow for in-depth subgroup analysis, which is indeed regrettable. Therefore, risk factors related to VTE, such as surgery, radiotherapy, and chemotherapy, were not systematically analyzed in this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YX: Conceptualization, Data curation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. TW: Writing – review & editing. XR: Supervision, Writing – review & editing. JL: Writing – original draft. HZ: Supervision, Writing – review & editing. DY: Supervision, Writing – review & editing. YY: Conceptualization, Writing – original draft, Writing – review & editing. DL: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the funding program of Science and Technology Project of Liaoning Province (2023JH2/101700111).
Acknowledgments
We would like to thank the authors of all studies included in this review who provided additional data. The corresponding author had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of our data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1405147/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Gervaso L, Dave H, Khorana AA. Venous and arterial thromboembolism in patients with cancer: jacc: cardiooncology state-of-the-art review. JACC CardioOncol. (2021) 3:173–90. doi: 10.1016/j.jaccao.2021.03.001
3. Streiff MB, Holmstrom B, Angelini D, Ashrani A, Elshoury A, Fanikos J, et al. Cancer-associated venous thromboembolic disease, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1181–201. doi: 10.6004/jnccn.2021.0047
4. Ay C, Ünal UK. Epidemiology and risk factors for venous thromboembolism in lung cancer. Curr Opin Oncol. (2016) 28:145–9. doi: 10.1097/CCO.0000000000000262
5. Howlett J, Benzenine E, Cottenet J, Foucher P, Fagnoni P, Quantin C. Could venous thromboembolism and major bleeding be indicators of lung cancer mortality? A nationwide database study. BMC Cancer. (2020) 20:461. doi: 10.1186/s12885-020-06930-1
6. Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. (2000) 343:1846–50. doi: 10.1056/NEJM200012213432504
7. Giustozzi M, Curcio A, Weijs B, Field TS, Sudikas S, Katholing A, et al. Variation in the association between antineoplastic therapies and venous thromboembolism in patients with active cancer. Thromb Haemost. (2020) 120:847–56. doi: 10.1055/s-0040-1709527
8. Gangireddy C, Rectenwald JR, Upchurch GR, Wakefield TW, Khuri S, Henderson WG, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. (2007) 45:335–41; discussion 41-2. doi: 10.1016/j.jvs.2006.10.034
9. Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. (2017) 117:219–30. doi: 10.1160/TH16-08-0615
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
11. Taylor KS, Mahtani KR, Aronson JK. Summarising good practice guidelines for data extraction for systematic reviews and meta-analysis. BMJ Evid Based Med. (2021) 26:88–90. doi: 10.1136/bmjebm-2020-111651
12. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
13. Nyaga VN, Arbyn M, Aerts M. Metaprop: A stata command to perform meta-analysis of binomial data. Arch Public Health. (2014) 72:39. doi: 10.1186/2049-3258-72-39
14. Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost. (2004) 2:1760–5. doi: 10.1111/j.1538-7836.2004.00928.x
15. Mason DP, Quader MA, Blackstone EH, Rajeswaran J, DeCamp MM, Murthy SC, et al. Thromboembolism after pneumonectomy for Malignancy: an independent marker of poor outcome. J Thorac Cardiovasc Surg. (2006) 131:711–8. doi: 10.1016/j.jtcvs.2005.10.027
16. Tagalakis V, Levi D, Agulnik JS, Cohen V, Kasymjanova G, Small D. High risk of deep vein thrombosis in patients with non-small cell lung cancer: A cohort study of 493 patients. J Thorac Oncol. (2007) 2:729–34. doi: 10.1097/JTO.0b013e31811ea275
17. Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. (2008) 6:601–8. doi: 10.1111/j.1538-7836.2008.02908.x
18. Patel A, Anraku M, Darling GE, Shepherd FA, Pierre AF, Waddell TK, et al. Venous thromboembolism in patients receiving multimodality therapy for thoracic Malignancies. J Thorac Cardiovasc Surg. (2009) 138:843–8. doi: 10.1016/j.jtcvs.2009.02.028
19. Connolly GC, Dalal M, Lin J, Khorana AA. Incidence and predictors of venous thromboembolism (Vte) among ambulatory patients with lung cancer. Lung Cancer. (2012) 78:253–8. doi: 10.1016/j.lungcan.2012.09.007
20. Huang H, Korn JR, Mallick R, Friedman M, Nichols C, Menzin J. Incidence of venous thromboembolism among chemotherapy-treated patients with lung cancer and its association with mortality: A retrospective database study. J Thromb Thrombolysis. (2012) 34:446–56. doi: 10.1007/s11239-012-0741-7
21. Yang Y, Zhou Z, Niu XM, Li ZM, Chen ZW, Jian H, et al. Clinical analysis of postoperative venous thromboembolism risk factors in lung cancer patients. J Surg Oncol. (2012) 106:736–41. doi: 10.1002/jso.23190
22. Buosi R, Borra G, Alabiso O, Galetto A, Pappagallo G, Campanini M. Venous thromboembolism in non-small cell lung cancer patients: retrospective analysis of cases treated at the oncology day hospital of novara, Italy. Ital J Med. (2013) 2):113–7. doi: 10.4081/itjm.2013.113
23. Crolow C, Samulowski M, Blum T, Kollmeier J, Schönfeld N, Bittner RC, et al. Frequency of thromboembolic complications in patients with lung cancer. Pneumologie. (2013) 67:442–7. doi: 10.1055/s-0033-1344341
24. Alexander M, Kirsa S, Wolfe R, MacManus M, Ball D, Solomon B, et al. Thromboembolism in lung cancer - an area of urgent unmet need. Lung Cancer. (2014) 84:275–80. doi: 10.1016/j.lungcan.2014.02.009
25. Kadlec B, Skrickova J, Merta Z, Dusek L, Jarkovsky J. The incidence and predictors of thromboembolic events in patients with lung cancer. ScientificWorldJournal. (2014) 2014:125706. doi: 10.1155/2014/125706
26. Kourelis TV, Wysokinska EM, Wang Y, Yang P, Mansfield AS, Tafur AJ. Early venous thromboembolic events are associated with worse prognosis in patients with lung cancer. Lung Cancer. (2014) 86:358–62. doi: 10.1016/j.lungcan.2014.10.003
27. Lee YG, Kim I, Lee E, Bang SM, Kang CH, Kim YT, et al. Risk factors and prognostic impact of venous thromboembolism in asian patients with non-small cell lung cancer. Thromb Haemost. (2014) 111:1112–20. doi: 10.1160/TH13-11-0956
28. Zhang Y, Yang Y, Chen W, Guo L, Liang L, Zhai Z, et al. Prevalence and associations of vte in patients with newly diagnosed lung cancer. Chest. (2014) 146:650–8. doi: 10.1378/chest.13-2379
29. Steuer CE, Behera M, Kim S, Patel N, Chen Z, Pillai R, et al. Predictors and outcomes of venous thromboembolism in hospitalized lung cancer patients: A nationwide inpatient sample database analysis. Lung Cancer. (2015) 88:80–4. doi: 10.1016/j.lungcan.2015.01.022
30. Wang Z, Yan HH, Yang JJ, Wang BC, Chen HJ, Zhou Q, et al. Venous thromboembolism risk factors in chinese non-small cell lung cancer patients. Support Care Cancer. (2015) 23:635–41. doi: 10.1007/s00520-014-2405-y
31. Agzarian J, Hanna WC, Schneider L, Schieman C, Finley CJ, Peysakhovich Y, et al. Postdischarge venous thromboembolic complications following pulmonary oncologic resection: an underdetected problem. J Thorac Cardiovasc Surg. (2016) 151:992–9. doi: 10.1016/j.jtcvs.2015.11.038
32. Walker AJ, Baldwin DR, Card TR, Powell HA, Hubbard RB, Grainge MJ. Risk of venous thromboembolism in people with lung cancer: A cohort study using linked uk healthcare data. Br J Cancer. (2016) 115:115–21. doi: 10.1038/bjc.2016.143
33. Ma L, Wen Z. Risk factors and prognosis of pulmonary embolism in patients with lung cancer. Med (Baltimore). (2017) 96:e6638. doi: 10.1097/MD.0000000000006638
34. Shen Q, Dong X, Tang X, Zhou J. Risk factors and prognosis value of venous thromboembolism in patients with advanced non-small cell lung cancer: A case-control study. J Thorac Dis. (2017) 9:5068–74. doi: 10.21037/jtd.2017.11.116
35. Li YP, Shen L, Huang W, Hu XF, Xie D, Yang J, et al. Prevalence and risk factors of acute pulmonary embolism in patients with lung cancer surgery. Semin Thromb Hemost. (2018) 44:334–40. doi: 10.1055/s-0037-1612625
36. Thomas DC, Arnold BN, Hoag JR, Salazar MC, Detterbeck FC, Boffa DJ, et al. Timing and risk factors associated with venous thromboembolism after lung cancer resection. Ann Thorac Surg. (2018) 105:1469–75. doi: 10.1016/j.athoracsur.2018.01.072
37. Fu X, Ping L, Ye G. Analysis of the risk factors of peripherally inserted central catheter-associated venous thrombosis after chemotherapy in patients with lung cancer. Int J Clin Exp Med. (2019) 12:5852–9.
38. Hiraide M, Shiga T, Minowa Y, Nakano Y, Yoshioka H, Suzuki K, et al. Identification of risk factors for venous thromboembolism and evaluation of khorana venous thromboembolism risk assessment in Japanese lung cancer patients. J Cardiol. (2020) 75:110–4. doi: 10.1016/j.jjcc.2019.06.013
39. Junjun L, Pei W, Ying Y, Kui S. Prognosis and risk factors in older patients with lung cancer and pulmonary embolism: A propensity score matching analysis. Sci Rep. (2020) 10:1272. doi: 10.1038/s41598-020-58345-4
40. Ke L, Cui S, Chen S, Hu B, Li H. Dynamics of D-dimer in non-small cell lung cancer patients receiving radical surgery and its association with postoperative venous thromboembolism. Thorac Cancer. (2020) 11:2483–92. doi: 10.1111/1759-7714.13559
41. Suzuki T, Fujino S, Inaba S, Yamamura R, Katoh H, Noji Y, et al. Venous thromboembolism in patents with lung cancer. Clin Appl Thromb Hemost. (2020) 26:1076029620977910. doi: 10.1177/1076029620977910
42. Wang P, Zhao H, Zhao Q, Ren F, Liu H, Chen G, et al. Risk factors and clinical significance of D-dimer in the development of postoperative venous thrombosis in patients with lung tumor. Cancer Manag Res. (2020) 12:5169–79. doi: 10.2147/CMAR.S256484
43. Akhtar-Danesh GG, Akhtar-Danesh N, Shargall Y. Venous thromboembolism in surgical lung cancer patients: A provincial population-based study. Ann Thorac Surg. (2022) 114:890–7. doi: 10.1016/j.athoracsur.2021.10.018
44. Cui YQ, Tan XM, Liu B, Zheng Y, Zhang LY, Chen ZA, et al. Analysis on risk factors of lung cancer complicated with pulmonary embolism. Clin Respir J. (2021) 15:65–73. doi: 10.1111/crj.13270
45. Deschênes-Simard X, Richard C, Galland L, Blais F, Desilets A, Malo J, et al. Venous thrombotic events in patients treated with immune checkpoint inhibitors for non-small cell lung cancer: A retrospective multicentric cohort study. Thromb Res. (2021) 205:29–39. doi: 10.1016/j.thromres.2021.06.018
46. Dimakakos E, Livanios K, Vathiotis I, Gomatou G, Gkiozos I, Kotteas E, et al. Risk factors for venous thromboembolism in patients with small cell lung cancer. Anticancer Res. (2021) 41:1523–8. doi: 10.21873/anticanres.14911
47. Takemoto T, Soh J, Ohara S, Fujino T, Koga T, Nishino M, et al. The prevalence and risk factors associated with preoperative deep venous thrombosis in lung cancer surgery. Surg Today. (2021) 51:1480–7. doi: 10.1007/s00595-021-02243-3
48. Eichinger S. Cancer associated thrombosis: risk factors and outcomes. Thromb Res. (2016) 140 Suppl 1:S12–7. doi: 10.1016/S0049-3848(16)30092-5
49. White RH. The epidemiology of venous thromboembolism. Circulation. (2003) 107:I4–8. doi: 10.1161/01.CIR.0000078468.11849.66
50. Zakai NA, McClure LA, Judd SE, Safford MM, Folsom AR, Lutsey PL, et al. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. (2014) 129:1502–9. doi: 10.1161/CIRCULATIONAHA.113.006472
51. Weze KO, Obisesan OH, Dardari ZA, Cainzos-Achirica M, Dzaye O, Graham G, et al. The interplay of race/ethnicity and obesity on the incidence of venous thromboembolism. Am J Prev Med. (2022) 63:e11–20. doi: 10.1016/j.amepre.2021.12.023
52. Zakai NA, McClure LA. Racial differences in venous thromboembolism. J Thromb Haemost. (2011) 9:1877–82. doi: 10.1111/j.1538-7836.2011.04443.x
53. Falanga A, Russo L, Milesi V, Vignoli A. Mechanisms and risk factors of thrombosis in cancer. Crit Rev Oncol Hematol. (2017) 118:79–83. doi: 10.1016/j.critrevonc.2017.08.003
54. Heit JA, O’Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population-based study. Arch Intern Med. (2002) 162:1245–8. doi: 10.1001/archinte.162.11.1245
55. Sun JM, Kim TS, Lee J, Park YH, Ahn JS, Kim H, et al. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer. (2010) 69:330–6. doi: 10.1016/j.lungcan.2009.11.015
56. Gussoni G, Frasson S, La Regina M, Di Micco P, Monreal M. Three-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the riete registry. Thromb Res. (2013) 131:24–30. doi: 10.1016/j.thromres.2012.10.007
Keywords: lung cancer, venous thromboembolism, deep vein thrombosis, pulmonary embolism, meta-analysis
Citation: Xu Y, Wu T, Ren X, Liu J, Zhang H, Yang D, Yan Y and Lv D (2024) Prevalence and clinical characteristics of venous thromboembolism in patients with lung cancer: a systematic review and meta-analysis. Front. Oncol. 14:1405147. doi: 10.3389/fonc.2024.1405147
Received: 22 March 2024; Accepted: 23 July 2024;
Published: 14 August 2024.
Edited by:
Carmine Siniscalchi, University of Parma, ItalyReviewed by:
Elisa Roca, Casa di cura Pederzoli, ItalySerafeim Chlapoutakis, Hospital Agios Savvas, Greece
Copyright © 2024 Xu, Wu, Ren, Liu, Zhang, Yang, Yan and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Yan, eWFueWluZ2RvY3RvckBzaW5hLmNvbQ==; Dongyang Lv, MTMzMDk4ODg1NzNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ying Xu
Ying Xu Tong Wu†
Tong Wu† Jing Liu
Jing Liu