
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 03 June 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1403120
This article is part of the Research Topic The Role of Immunotherapy in Urothelial Cancer View all 8 articles
 Se Hoon Park1*
Se Hoon Park1* Sang Joon Shin2
Sang Joon Shin2 Sun Young Rha2
Sun Young Rha2 Seung-Hoon Beom2
Seung-Hoon Beom2 Ho Kyung Seo3
Ho Kyung Seo3 Bhumsuk Keam4
Bhumsuk Keam4 Miso Kim4
Miso Kim4 Yoon-Hee Hong5
Yoon-Hee Hong5 Shinkyo Yoon6
Shinkyo Yoon6 Jae-Lyun Lee6
Jae-Lyun Lee6Background: The JAVELIN Bladder 100 phase 3 trial demonstrated the efficacy and safety of avelumab administered as first-line (1L) maintenance treatment in patients with advanced urothelial carcinoma (UC) without disease progression after 1L platinum-based chemotherapy. This study provides the first real-world data from Korea regarding avelumab 1L maintenance treatment, comprising data obtained from a nationwide expanded access program (EAP).
Methods: This open-label EAP was conducted at five centers from September 2021 until June 2023. Eligible patients had unresectable locally advanced or metastatic UC and were progression free after 1L platinum-based chemotherapy. Patients received avelumab 10 mg/kg intravenously every 2 weeks per local prescribing information. Safety and effectiveness were assessed by treating physicians according to routine practice.
Results: Overall, 30 patients were enrolled. At initial UC diagnosis, 20 patients (66.7%) had stage 4 disease and 12 (40.0%) had visceral metastases. The most common 1L chemotherapy regimen was gemcitabine + cisplatin (21 patients; 70.0%). All but one patient (96.7%) had received 4-6 cycles of 1L chemotherapy. The median interval from end of 1L chemotherapy to start of avelumab was 4.4 weeks. Median duration of avelumab treatment was 6.2 months (range, 0.9-20.7); nine patients (30.0%) received >12 months of treatment. Adverse events related to avelumab occurred in 21 patients (70.0%) and were grade ≥3 or classified as serious in three patients (10.0%). Median progression-free survival was 7.9 months (95% CI, 4.3-13.1). Overall survival was not analyzed because only one patient died.
Conclusion: Results from this EAP demonstrated the clinical activity and acceptable safety of avelumab 1L maintenance treatment in Korean patients with advanced UC, consistent with previous studies.
Urothelial carcinoma (UC) is a cancer that arises from the cells lining the urinary tract (1, 2). At least 90% of cases of UC originate in the bladder, with other cases originating in the ureter, urethra, or renal pelvis (1–3). Globally in 2020, bladder cancer was the tenth most common cancer and the thirteenth leading cause of death (4). The incidence of bladder cancer in Korea in 2020 (age-standardized rates per 100,000) according to the Korea National Cancer Incidence Database (KNCI DB) and International Agency for Research on Cancer (IARC), respectively, was 7.5 and 7.9 in men and 1.4 and 1.5 women. In comparison, global incidence rates (per IARC data) were 9.5 in men and 2.4 in women (5, 6). In Korea (per KNCI DB data), the annual incidence of bladder cancer increased nearly three-fold from 2000 to 2020 (from 1744 to 4753 cases). The incidence was five times higher in men than in women. During the same period, bladder cancer–related deaths per year in Korea doubled (from 778 to 1593) (7). The 5-year survival rate for patients diagnosed with bladder cancer in Korea from 2016 to 2020 was 76.5% (5).
Clinical stages of bladder cancer include nonmuscle-invasive, muscle-invasive, and locally advanced/metastatic (generally called advanced) disease, each of which exhibits distinct clinical characteristics and prognoses and requires different treatment approaches (2, 8). Although only ≈5% of patients are initially diagnosed with metastatic disease (2, 3), a notable proportion of patients diagnosed with earlier-stage disease will eventually progress to advanced disease. For example, ≈15% to 20% of nonmuscle-invasive tumors progress to muscle-invasive tumors, and even with radical cystectomy and pelvic lymph-node dissection, ≈50% of patients with muscle-invasive tumors will develop advanced disease (8). Metastatic bladder cancer has a poor prognosis, including a historical median overall survival (OS) of 13 to 15 months (2). In men in Korea diagnosed with bladder cancer with distant metastatic disease, the 5-year survival rate was 13.0% (data in women were not reported) (5).
For decades, platinum-based chemotherapy has been a standard first-line (1L) treatment for patients with advanced UC (2, 3, 8–10). In the early 1990s, the combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) demonstrated increased response rates and longer OS compared with single-agent cisplatin, albeit with increased toxicity (11). Subsequently, cisplatin + gemcitabine became widely used following a randomized phase 3 trial showing similar effectiveness to MVAC but with improved tolerability (12, 13). Additionally, standard-dose MVAC was superseded by a dose-dense regimen that showed improved efficacy and tolerability (14, 15). Carboplatin + gemcitabine is the recommended 1L platinum regimen for patients unfit for cisplatin-based regimens (3, 9, 10, 16, 17).
In patients without progressive disease (PD) following platinum-based chemotherapy, avelumab (an immune checkpoint inhibitor) administered as 1L maintenance treatment is the recommended standard of care in international guidelines based on results from the pivotal JAVELIN Bladder 100 phase 3 trial (NCT02603432) (3, 9, 10, 18, 19). In this trial, 700 patients with advanced UC who were without PD following 4 to 6 cycles of 1L cisplatin + gemcitabine or carboplatin + gemcitabine were randomized to receive avelumab 1L maintenance + best supportive care (BSC) or BSC alone. After ≥2 years of follow-up, median OS was 23.8 vs 15.0 months (hazard ratio [HR], 0.76 [95% CI, 0.63-0.91]; p=0.0036) and median progression-free survival (PFS) was 5.5 vs 2.1 months (HR, 0.54 [95% CI, 0.46-0.64]; p<0.0001), respectively. The long-term safety of avelumab 1L maintenance treatment was also demonstrated and was consistent with previous analyses (19). In a post hoc analysis, median OS with avelumab 1L maintenance treatment measured from the start of 1L platinum-based chemotherapy in this population without PD was 29.7 months (20). In a subgroup analysis of patients enrolled in Asian countries, efficacy and safety findings were generally consistent with those observed in the overall population (21). Furthermore, prospective and retrospective real-world studies conducted in several countries have confirmed the favorable clinical activity and acceptable safety profile of avelumab 1L maintenance treatment reported in the JAVELIN Bladder 100 trial (22–25).
In Korea, avelumab was initially approved by the Ministry of Food and Drug Safety for the treatment of metastatic Merkel cell carcinoma on March 22, 2019, and it was subsequently approved for 1L maintenance treatment of patients with advanced UC on August 5, 2021. Here, we report the first real-world data from Korea in patients with advanced UC receiving avelumab 1L maintenance treatment, comprising data obtained from an expanded access program (EAP).
The protocol for this nationwide, open-label EAP was approved on July 2, 2021, before the Korean approval for avelumab 1L maintenance treatment for patients with advanced UC on August 5, 2021. The EAP began on September 2, 2021, and continued until the final visit of the last patient on June 16, 2023. The purpose of the EAP was to offer access to avelumab 1L maintenance treatment before it was reimbursed in Korea. All patients were enrolled prospectively. Safety and effectiveness data were collected. The EAP was performed in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Council for Harmonisation and Good Clinical Practice and Ministry of Food and Drug Safety requirements. The protocol, amendments, and informed consent form were approved by institutional review boards at each site. Written informed consent was obtained from all patients.
Eligible patients were aged ≥18 years; had histologically confirmed, unresectable locally advanced or metastatic UC; and were progression free following 1L platinum-based chemotherapy. Patients were required to have adequate bone marrow function (absolute neutrophil count, ≥1500/mm3 or ≥1.5×109/L; platelets, ≥100,000/mm3 or ≥100×109/L; and hemoglobin, ≥9 g/dL); adequate renal function (estimated creatinine clearance, ≥30 mL/min); and adequate liver function (total serum bilirubin, ≤1.5× upper limit of normal [ULN]; aspartate aminotransferase and alanine aminotransferase, ≤2.5×ULN or, for patients with metastatic disease in the liver, ≤5×ULN). Patients were ineligible if they had hypersensitivity to avelumab or any of its excipients; had participated in clinical trials with avelumab; or had any severe acute or chronic medical condition inappropriate for entry into EAP, as determined by the treating physician.
Patients were treated with avelumab 10 mg/kg intravenously every 2 weeks per local prescribing information. Patients received premedication with an antihistamine and acetaminophen before the first 4 infusions of avelumab. After completing the fourth infusion without an infusion-related reaction, premedication for subsequent doses was administered at the discretion of the treating physician. Treatment continued until PD, unacceptable toxicity, or reimbursement approval or for 18 months from the last patient’s first visit, whichever occurred first.
Baseline data, including demographics, medical and disease history, and prior treatment, were collected upon program enrollment (baseline). During avelumab treatment, clinical information, including data related to safety and effectiveness, was collected by treating physicians according to local routine practice. Data on avelumab exposure, safety, and effectiveness were obtained from the patient’s medical records from enrollment until last follow-up or data cutoff. Information on subsequent treatment was also collected.
Safety was monitored closely and recorded by the treating physician per local practice. Although data on all adverse events (AEs) were collected, only treatment-emergent AEs (TEAEs), i.e. those that initially occurred or worsened in severity or seriousness during the on-treatment period, were analyzed. The on-treatment period was defined as the time from the start date of avelumab treatment until the date of any of the following, whichever occurred first: final avelumab administration plus 30 days, termination of or discontinuation from the EAP, death, or administration of first subsequent anticancer therapy was received minus 1 day. Treatment-related adverse events (TRAEs) were defined as TEAEs that were classified by the treating physician as causally related to avelumab. TEAEs were coded using the Medical Dictionary for Regulatory Activities version 26.0, and severity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Effectiveness data collected included: survival status; PFS (defined as time from start of avelumab until the first documentation of PD or death from any cause, whichever occurred first); best overall response (confirmed complete response [CR], partial response [PR], stable disease [SD], PD, or not evaluable [NE]); objective response (confirmed CR or PR); disease control (confirmed CR, PR, or SD); time to response (defined as time from first avelumab administration until the first documentation of objective response); and duration of response (defined as time from the first documentation of objective response to the first documentation of PD or death from any cause, whichever occurred first). Tumor assessments were performed per local practice according to Response Evaluation Criteria in Solid Tumors version 1.1; the protocol did not include any specifications regarding the imaging modality to be used. Best response was determined using the following definitions: CR was defined as ≥2 determinations of CR recorded ≥4 weeks apart and before the first documentation of PD; PR was defined as ≥2 determinations of PR or better (PR followed by PR or PR followed by CR) recorded ≥4 weeks apart and before the first documentation of PD (and not qualifying as CR); SD was defined as ≥1 determination of SD (or better) ≥6 weeks after the start of first date of study drug administration and before the first documentation of PD (and not qualifying as CR or PR); PD was defined as the first documentation of PD ≤12 weeks after the first date of study drug administration (and not qualifying as CR, PR, or SD); all other cases were defined as NE.
For continuous variables, mean, standard deviation, median, minimum, and maximum were summarized in patients with nonmissing data. Categorical variables were summarized as number and percentage in patients with nonmissing data. PFS and duration of response were estimated using the Kaplan-Meier method, and corresponding 95% CIs for the median were calculated using the Brookmeyer-Crowley method with log-log transformation. Subgroup analyses of outcomes are not reported because of the small population size.
Between September 28, 2021, and June 16, 2023, 31 patients provided informed consent and 30 patients were treated with avelumab at five centers in Korea (Supplementary Table S1); one patient did not receive avelumab treatment because they did not meet the eligibility criterion requiring lack of PD after 1L platinum-based chemotherapy. Baseline characteristics are summarized in Table 1.
The median time between initial UC diagnosis and enrollment was 1.3 years (range, 0.3-13.7). At initial UC diagnosis, the primary tumor location was bladder in 21 patients (70.0%), upper tract in 7 patients (23.3%), both bladder and upper tract in 1 patient (3.3%), and not reported in 1 patient (3.3%); disease stage was 1, 2, 3, or 4 in two patients (6.7%), one patient (3.3%), two patients (6.7%), and 20 patients (66.7%), respectively, and not reported in 5 patients (16.7%). Also at initial diagnosis, 10 patients (33.3%) were eligible for curative surgery, one patient (3.3%) had unresectable locally advanced disease, and 19 patients (63.3%) had distant metastases, including visceral and nonvisceral metastases in 12 (40.0%) and seven (23.3%), respectively. Seven patients (23.3%) had received chemotherapy for earlier stages of disease, including neoadjuvant chemotherapy in five patients (16.7%) and adjuvant chemotherapy in two patients (6.7%). In addition, seven patients (23.3%) had received radiation therapy, and 24 patients (80.0%) had undergone surgery, most commonly transurethral bladder resection (Supplementary Table S2).
At the start of avelumab 1L maintenance treatment, all patients had advanced UC, had received 1L platinum-based chemotherapy, and were progression free. Median age at the start of avelumab treatment was 65.5 years (range, 44-86), and Eastern Cooperative Oncology Group performance status was 0 in 18 patients (60.0%) and 1 in 12 patients (40.0%). Most patients were male (n=26 [86.7%]). The 1L chemotherapy regimen was cisplatin + gemcitabine in 21 patients (70.0%), carboplatin + gemcitabine in eight patients (26.7%), and MVAC in one patient (3.3%; Table 2). The number of cycles of 1L chemotherapy received was 4 in nine patients (30.0%), 5 in seven patients (23.3%), 6 in 13 patients (43.3%), and 8 in one patient (3.3%). Best overall response to 1L platinum-based chemotherapy was CR in six patients (20.0%), PR in 17 patients (56.7%), and SD in seven patients (23.3%). Response status at the start of avelumab treatment was CR in five patients (16.7%), PR in 18 patients (60.0%), and SD in seven patients (23.3%). The median treatment-free interval (TFI) between end of 1L chemotherapy and start of avelumab was 4.4 weeks (range, 1.0-21.0).
Of 30 patients treated with avelumab, seven (23.3%) were still receiving avelumab despite the end of the EAP. The median duration of avelumab treatment was 6.2 months (range, 0.9-20.7), representing a median of 13.5 cycles (Supplementary Table S3). Nine patients received avelumab for >12 months; in this subgroup, the median treatment duration was 19.8 months (median, 42 cycles). Among patients who discontinued avelumab treatment (n=23 [76.7%]), reasons were PD (n=17 [73.9%]), AE (n=3 [13.0%]), and withdrawal of consent (n=3 [13.0%]; Supplementary Table S1). Fifteen patients received a subsequent anticancer therapy (50.0% of all patients; 65.0% of those who discontinued), most commonly rechallenge with platinum-based chemotherapy (Supplementary Table S4). The median time from the last avelumab administration to the start of subsequent anticancer therapy was 22 days (range, 15.0-97.0).
During avelumab treatment, TEAEs of any grade occurred in 27 patients (90.0%), including grade ≥3 TEAEs in eight (26.7%; Table 3; Supplementary Table S5). TRAEs were reported in 21 patients (70.0%), including grade ≥3 TRAEs in three patients (10.0%). The most common TRAEs of any grade were rash in four patients (13.3%) and pruritus in four patients (13.3%) (Supplementary Table S6). Infusion-related reactions occurred in three patients (10.0%) and none were grade ≥3. Serious TEAEs occurred in seven patients (23.3%) and serious TRAEs occurred in three patients (10.0%; comprising dyspnea, acute kidney injury, and deep vein thrombosis in one patient each; Supplementary Table S7). AEs led to interruption of avelumab treatment in 14 patients (46.7%) and discontinuation in three patients (10.0%). One patient died, which was due to PD and disease-related factors.
Median PFS from start of avelumab treatment was 7.9 months (95% CI, 4.3-13.1; Figure 1). OS was not analyzed because only one patient died. In the nine patients who received >12 months of avelumab treatment, median PFS was NE (95% CI, 1.3-NE; range, 13.1-20.1). During avelumab treatment, best overall response was CR in six patients (20.0%), PR in three patients (10.0%), SD in 14 patients (46.7%), PD in one patient (3.3%), and NE in six patients (20.0%; Table 4). The objective response rate was 30.0% (95% CI, 13.6-46.4). Among responding patients (CR or PR), median time to response was 2.7 months (range, 1.5-9.2) and median duration of response was 17.8 months (95% CI, 5.2-NE; range, 5.2-17.8).
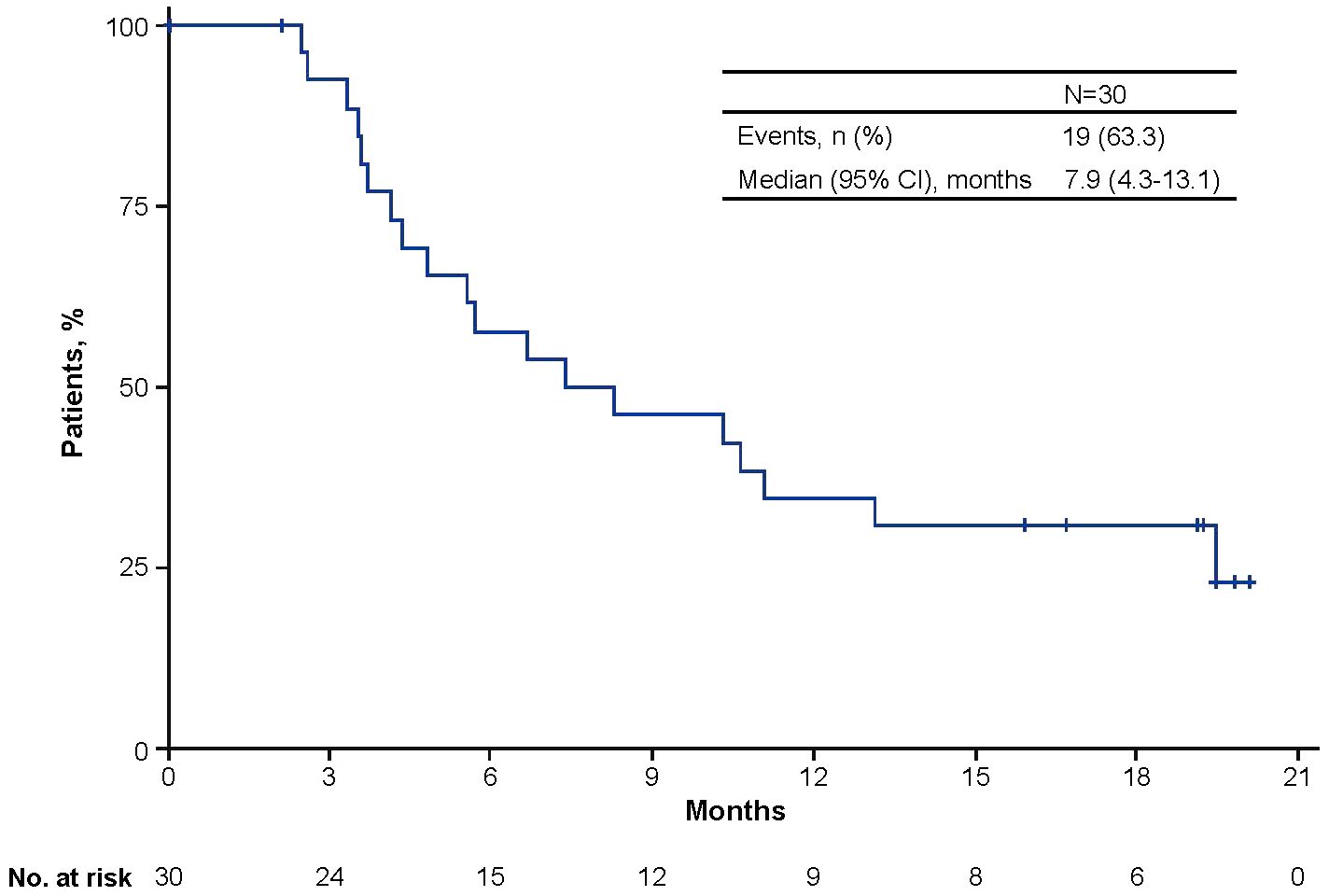
Figure 1 Kaplan-Meier analysis of progression-free survival from the start of avelumab maintenance treatment.
In the JAVELIN Bladder 100 phase 3 trial, avelumab 1L maintenance + BSC significantly prolonged both OS and PFS compared with BSC alone in patients with advanced UC who were progression free after 1L platinum-based chemotherapy, with long-term follow-up yielding consistent findings (18, 19). Comparable clinical outcomes and safety were reported in subsequent real-world studies performed in several countries; however, real-world data from Asian countries are limited (22–25). Here, we report the first real-world evidence for avelumab 1L maintenance treatment in Korean patients, including safety and effectiveness data, which was obtained from an EAP that enrolled patients prospectively.
In total, 30 patients were treated with avelumab. Although the number of patients was limited, baseline characteristics were mostly consistent with those seen in JAVELIN Bladder 100 and other real-world studies. For example, shared characteristics across all studies included a median age of >65 years, a higher proportion of men vs women, and a higher proportion of patients with bladder vs upper tract primary tumors (18, 22–26). Visceral metastases, which are an established negative prognostic factor in advanced UC (27), were present at diagnosis in 40% of patients in the EAP population. Nevertheless, in the JAVELIN Bladder 100 trial, HRs for OS and PFS favored avelumab 1L maintenance + BSC vs BSC alone in patients with visceral metastases (18, 19, 28). The most common 1L chemotherapy regimen in the EAP population was cisplatin + gemcitabine, consistent with the Asian subgroup of JAVELIN Bladder 100 (21). The number of cycles of 1L platinum-based chemotherapy received in the EAP population ranged from 4 to 6 in 97% of patients, which aligns with international guidelines and the design of the JAVELIN Bladder 100 study (3, 9, 10, 18). Because the optimal duration of prior 1L platinum-based chemotherapy is not yet defined, a phase 2 study assessing avelumab 1L maintenance treatment after 3 vs 6 cycles (DISCUS) is in progress (29).
Best response to 1L platinum-based chemotherapy in the EAP population was an objective response (CR or PR) in 77% and SD in 23%. In JAVELIN Bladder 100, OS and PFS benefits were demonstrated with avelumab 1L maintenance treatment in patients with CR, PR, or SD (19, 28). Patients enrolled in JAVELIN Bladder 100 were required to have a TFI of 4 to 10 weeks between end of chemotherapy and start of avelumab, which allowed any AEs to resolve and for tumor status to be evaluated (30). In the EAP population, TFIs were more heterogeneous (<4 weeks in 40%, 4 to 10 weeks in 47%, and >10 weeks in 13%). It should be noted that a TFI >10 weeks after completing 1L chemotherapy may increase the risk of disease progression (30). Overall, patient characteristics in this population are reflective of a real-world population of patients eligible for avelumab 1L maintenance treatment.
The median duration of avelumab treatment was 6.2 months. Seven patients (23%) continued treatment until the end of the EAP and nine patients (30%) received avelumab 1L maintenance treatment for >12 months. After initiating avelumab 1L maintenance treatment, grade ≥3 TRAEs and serious TRAEs occurred in 10% of patients, similar to previous studies (18, 19, 31). Overall, 70% of patients had a TRAE of any grade, most commonly low-grade skin disorders (23% of patients). No new safety signals were observed.
Median PFS in this population was 7.9 months, which is numerically higher than the PFS of 5.5 months reported after long-term follow-up in the JAVELIN Bladder 100 trial (19). In other real-world studies of avelumab 1L maintenance treatment, median PFS ranged from 5.7 to 9.6 months (22–24). In this EAP population, subgroup analyses of outcomes were not performed because of the small number of patients. In the JAVELIN Bladder 100 trial, OS and PFS benefits were seen with avelumab 1L maintenance treatment across a wide range of subgroups, including those defined by patient or disease characteristics, 1L chemotherapy regimen, response to 1L chemotherapy, duration of 1L chemotherapy, and TFI (18, 19, 28, 30). These data support the use of avelumab 1L maintenance treatment as the standard of care for platinum-treated patients without PD, irrespective of patient, disease, or prior treatment characteristics. An ongoing real-world study being conducted in the Asia-Pacific region (SPADE) will prospectively assess treatment outcomes with avelumab 1L maintenance, including 6- and 12-month OS and health-related quality of life (32).
Our study has several limitations, which should be considered when interpreting its findings. Firstly, the population size was relatively small (30 patients), and all patients received treatment at 5 institutions within a single country (Korea), which may limit the generalizability of the data reported. Study data only included information collected as part of routine clinical practice, which is less extensive than would have been collected in a clinical trial. Subgroup analyses could not be performed because of the small population size. Lastly, the duration of follow-up was too short to analyze overall survival.
In conclusion, our data provide the first real-world evidence of the clinical benefits and tolerable safety of avelumab 1L maintenance treatment in patients with advanced UC in Korea. Findings are comparable to those of the JAVELIN Bladder 100 trial and other real-world studies and further support the recommendation of avelumab 1L maintenance treatment as the standard of care for patients with advanced UC without PD after 1L platinum-based chemotherapy.
The datasets presented in this article are not readily available because of the need to maintain patient confidentiality. Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data sharing portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
This expanded access program was performed in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Council for Harmonisation and Good Clinical Practice and Ministry of Food and Drug Safety requirements. The protocol, amendments, and informed consent form were approved by institutional review boards at each site. Written informed consent was obtained from all patients.
SHP: Investigation, Resources, Writing – review & editing. SJS: Investigation, Resources, Writing – review & editing. SYR: Investigation, Resources, Writing – review & editing. S-HB: Investigation, Resources, Writing – review & editing. HKS: Investigation, Resources, Writing – review & editing. BK: Investigation, Resources, Writing – review & editing. MK: Investigation, Resources, Writing – review & editing. Y-HH: Data curation, Formal Analysis, Project administration, Writing – original draft, Writing – review & editing. SY: Investigation, Resources, Writing – review & editing. J-LL: Investigation, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This expanded access program was sponsored by Merck Ltd., Seoul, South Korea, an affiliate of Merck KGaA (CrossRef Funder ID: 10.13039/100009945).
The clinical institutions that participated in the EAP and supported generation of these real-world data are as follows: National Cancer Center, Samsung Medical Center, Severance Hospital, Seoul National University Hospital, and Asan Medical Center. The authors would like to thank all patients, investigators, and study teams at each institution. Editorial support was provided by Nucleus Global and was funded by Merck.
SHP has served in consulting or advisory roles for Janssen; reports honoraria from Merck, Ono Pharmaceutical, and Pfizer; and reports research funding from Ono Pharmaceutical and Sanofi. SYR reports advisory roles and speaker services for and has received research funding from Merck. HKS has received research funding from Astellas, AstraZeneca, Basilea, Janssen, Merck, MSD, Ono-BMS, and Roche; and reports consulting, advisory roles, and speaker services for Astellas, Boehringer Ingelheim, Janssen, MSD, Ono-BMS, and Roche. BK has served in advisory roles for ABL Bio, AstraZeneca, CbsBioscience, Cellid, Genexine, Handok, Merck, MSD, and Trial Informatics; reports speaker services from AstraZeneca, Merck, and MSD; and served as coordinating principal investigator for AstraZeneca, MSD, and Ono Pharmaceutical. MK has served in consulting or advisory roles for Eisai, Ipsen, MSD, Ono-BMS, and Yuhan. Y-HH reports employment at Merck Ltd., Seoul, Republic of Korea, an affiliate of Merck KGaA. J-LL reports stock and other ownership interests in Amgen, Black Diamond Therapeutics, Innovent Biologics, Johnson & Johnson/Janssen, Karyopharm Therapeutics, Merck, and Zymeworks; reports honoraria from Astellas, AstraZeneca, BMS, MSD, and Pfizer; has served in consulting or advisory roles for AstraZeneca, BMS, GI Innovation, Merck, MSD, Oscotec, Pfizer, and Sanofi; and has received institutional research funding from Amgen, AstraZeneca/MedImmune, Bayer Schering Pharma, BMS, GI Innovation, Janssen, MSD, Novartis, Pfizer, Roche/Genentech, and Seagen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Merck Ltd. The funder had the following involvement in the study: providing administrative roles for the present study.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1403120/full#supplementary-material
1. Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel). (2020) 8:15. doi: 10.3390/medsci8010015
2. Lenis AT, Lec PM and Chamie K. Bladder cancer: a review. JAMA. (2020) 324:1980–91. doi: 10.1001/jama.2020.17598
3. NCCN Clinical Practice Guidelines in Oncology. Bladder Cancer. v1.2024. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
4. Zhang Y, Rumgay H, Li M, Yu H, Pan H and Ni J. The global landscape of bladder cancer incidence and mortality in 2020 and projections to 2040. J Glob Health. (2023) 13:4109. doi: 10.7189/jogh.13.04109
5. Kang MJ, Jung KW, Bang SH, Choi SH, Park EH, Yun EH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2020. Cancer Res Treat. (2023) 55:385–99. doi: 10.4143/crt.2023.447
6. International Agency for Research on Cancer. Global Cancer Observatory. Cancer today. Available online at: https://gco.iarc.fr/ (Accessed February 1, 2024).
7. Han SH, Yuk HD. Epidemiology of urologic cancer in Korea: nationwide trends in the last 2 decades. J Urol Oncol. (2023) 21:32–44. doi: 10.22465/juo.234600080004
8. Patel VG, Oh WK and Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. (2020) 70:404–23. doi: 10.3322/caac.21631
9. Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, et al. Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:244–58. doi: 10.1016/j.annonc.2021.11.012
10. Cathomas R, Lorch A, Bruins HM, Compérat EM, Cowan NC, Efstathiou JA, et al. The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur Urol. (2021) 81:95–103. doi: 10.1016/j.eururo.2021.09.026
11. Loehrer P, Einhorn LH, Elson PJ, Crawford ED, Kuebler P, Tannock I, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. (1992) 10:1066–73. doi: 10.1200/JCO.1992.10.7.1066
12. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. (2000) 18:3068–77. doi: 10.1200/JCO.2000.18.17.3068
13. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. (2005) 23:4602–8. doi: 10.1200/JCO.2005.07.757
14. Sternberg CN, de Mulder PH, Schornagel JH, Théodore C, Fossa SD, van Oosterom AT, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. (2001) 19:2638–46. doi: 10.1200/jco.2001.19.10.2638
15. Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. (2006) 42:50–4. doi: 10.1016/j.ejca.2005.08.032
16. De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. (2012) 30:191–9. doi: 10.1200/JCO.2011.37.3571
17. Linardou H, Aravantinos G, Efstathiou E, Kalofonos C, Anagnostopoulos A, Deliveliotis C, et al. Gemcitabine and carboplatin combination as first-line treatment in elderly patients and those unfit for cisplatin-based chemotherapy with advanced bladder carcinoma: phase II study of the Hellenic Co-operative Oncology Group. Urology. (2004) 64:479–84. doi: 10.1016/j.urology.2004.04.024
18. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. (2020) 383:1218–30. doi: 10.1056/NEJMoa2002788
19. Powles T, Park SH, Caserta C, Valderrama BP, Gurney H, Ullen A, et al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 trial after ≥2 years of follow-up. J Clin Oncol. (2023) 41:3486–92. doi: 10.1200/JCO.22.01792
20. Sridhar SS, Powles T, Gupta S, Climent MÁ, Aragon-Ching JB, Sternberg CN, et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): Long-term follow-up from the JAVELIN Bladder 100 trial in subgroups defined by 1L chemotherapy regimen and analysis of overall survival (OS) from start of 1L chemotherapy. J Clin Oncol. (2023) 41:Abstract 508. doi: 10.1200/JCO.2023.41.6_suppl.508
21. Lee J-L, Desai C, Park SH, Tsuchiya N, Su P-J, Wai Chan TT, et al. Avelumab first-line maintenance plus best supportive care (BSC) vs. BSC alone for advanced urothelial carcinoma: JAVELIN Bladder 100 Asian subgroup analysis. Urol Oncol. (2023) 41:256.e17–.e25. doi: 10.1016/j.urolonc.2023.02.002
22. Barthelemy P, Loriot Y, Voog E, Eymard JC, Ravaud A, Flechon A, et al. Full analysis from AVENANCE: a real-world study of avelumab first-line (1L) maintenance treatment in patients (pts) with advanced urothelial carcinoma (aUC). J Clin Oncol. (2023) 41:Abstract 471. doi: 10.1200/JCO.2023.41.6_suppl.471
23. Antonuzzo L, Maruzzo M, De Giorgi U, Santini D, Tambaro R, Buti S, et al. READY: real-world data from an Italian compassionate use program of avelumab first-line maintenance (1LM) treatment for locally advanced or metastatic urothelial carcinoma (la/mUC). J Clin Oncol. (2023) 41:Abstract 469. doi: 10.1200/JCO.2023.41.6_suppl.469
24. Bakaloudi DR, Talukder R, Lin GI, Makrakis D, Diamantopoulos LN, Tripathi N, et al. Response and outcomes of maintenance avelumab after platinum-based chemotherapy (PBC) in patients with advanced urothelial carcinoma (aUC): “real world” experience. Clin Genitourin Cancer. (2023) 21:584–93. doi: 10.1016/j.clgc.2023.06.008
25. Miyake M, Shimizu T, Oda Y, Tachibana A, Ohmori C, Itami Y, et al. Switch-maintenance avelumab immunotherapy following first-line chemotherapy for patients with advanced, unresectable or metastatic urothelial carcinoma: the first Japanese real-world evidence from a multicenter study. Jpn J Clin Oncol. (2023) 53:253–62. doi: 10.1093/jjco/hyac186
26. Grivas P, Barata PC, Moon H, Hutson TE, Gupta S, Sternberg CN, et al. characteristics from a retrospective, observational, US-based, multicenter, real-world (RW) study of avelumab first-line maintenance (1LM) in locally advanced/metastatic urothelial carcinoma (la/mUC) (PATRIOT-II). J Clin Oncol. (2023) 41:Abstract 465. doi: 10.1200/JCO.2023.41.6_suppl.465
27. Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, Scher HI, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. (1999) 17:3173–81. doi: 10.1200/jco.1999.17.10.3173
28. Grivas P, Park SH, Voog E, Caserta C, Gurney H, Bellmunt J, et al. Avelumab first-line maintenance therapy for advanced urothelial carcinoma: comprehensive clinical subgroup analyses from the JAVELIN Bladder 100 phase 3 trial. Eur Urol. (2023) 84:95–108. doi: 10.1016/j.eururo.2023.03.030
29. Jackson-Spence F, Pinto Marin A, Puente J, Molina-Cerrillo J, Hussain SA, Ackerman C, et al. DISCUS: a randomised phase II study of 3 vs 6 cycles of chemotherapy followed by maintenance avelumab in advanced urothelial cancer - wearable devices and circulating biomarkers assessing quality of life and surrogate efficacy endpoints. Ann Oncol. (2023) 34:S1224–5(Abstract 2408TiP). doi: 10.1016/j.annonc.2023.09.2826
30. Sridhar SS, Powles T, Climent Duran MA, Park SH, Massari F, Thiery-Vuillemin A, et al. Avelumab first-line maintenance for advanced urothelial carcinoma: analysis from JAVELIN Bladder 100 by duration of first-line chemotherapy and interval before maintenance. Eur Urol. (2024) 85:154–63. doi: 10.1016/j.eururo.2023.08.001
31. Kelly K, Infante JR, Taylor MH, Patel MR, Wong DJ, Iannotti N, et al. Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN Solid Tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer. (2018) 124:2010–7. doi: 10.1002/cncr.31293
32. Su P-J, Park SH, Tsai YC, Kim M, Wu W-J, Gao S, et al. SPADE: design of a real-world observational study of avelumab first-line (1L) maintenance in advanced urothelial carcinoma (UC) in the Asia-Pacific (APAC) region. J Clin Oncol. (2023) 41:Abstract TPS577. doi: 10.1200/JCO.2023.41.6_suppl.TPS577
Keywords: urothelial carcinoma, avelumab, maintenance treatment, platinum-based chemotherapy, real-world
Citation: Park SH, Shin SJ, Rha SY, Beom S-H, Seo HK, Keam B, Kim M, Hong Y-H, Yoon S and Lee J-L (2024) Avelumab first-line maintenance treatment in patients with locally advanced or metastatic urothelial carcinoma: real-world results from a Korean expanded access program. Front. Oncol. 14:1403120. doi: 10.3389/fonc.2024.1403120
Received: 25 March 2024; Accepted: 22 April 2024;
Published: 03 June 2024.
Edited by:
Marco Maruzzo, Veneto Institute of Oncology (IRCCS), ItalyReviewed by:
Francesco Pierantoni, Veneto Institute of Oncology (IRCCS), ItalyCopyright © 2024 Park, Shin, Rha, Beom, Seo, Keam, Kim, Hong, Yoon and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Se Hoon Park, aGVtYXRvbWFAc2trdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.