- 1Department of Gastrointestinal Surgery, Weihai Central Hospital, Qingdao University, Weihai, Shandong, China
- 2Department of Pediatric Surgery, Weihai Central Hospital, Qingdao University, Weihai, Shandong, China
- 3Department of Anesthesiology, Weihai Central Hospital, Qingdao University, Weihai, Shandong, China
- 4Department of Gastrointestinal Surgery, the Affiliated Hospital of Qingdao University, Pingdu, Shandong, China
- 5Center of Colon and Rectum, Qingdao Municipal Hospital, Qingdao University, Qingdao, Shandong, China
Background: The HALP score, comprising hemoglobin, albumin, lymphocyte, and platelet levels, serves as an indicator of both nutritional and inflammatory status. However, its correlation with all-cause and cause-specific mortality among cancer survivors remains unclear. Therefore, this study aims to investigate the relationship between HALP scores and mortality outcomes in this population.
Method: We extracted cohort data spanning ten cycles (1999-2018) from the U.S. National Health and Nutrition Examination Survey (NHANES). Mortality rates, determined using the National Death Index (NDI) as of December 31, 2019, were assessed. Weighted multivariate logistic regression analyzed the association between HALP scores and cancer prevalence. Kaplan-Meier analyses and weighted multivariate-adjusted Cox analyses investigated the link between HALP scores and all-cause and cause-specific mortality in cancer survivors. Restricted cubic spline (RCS) analysis was employed to assess nonlinear relationships. Furthermore, multi-parametric subgroup analyses were conducted to ensure the robustness of the results.
Results: Our study included 41,231 participants, of whom 3,786 were cancer survivors (prevalence: 9.5%). Over a median follow-up of 91 months (range: 51-136), we observed 1,339 deaths, including 397 from cancer, 368 from cardio-cerebrovascular disease, and 105 from respiratory disease. Elevated HALP scores showed a consistent association with reduced cancer incidence (P for trend <0.001). In multivariable-adjusted Cox regression analyses, HALP scores were significantly inversely associated with all-cause mortality, cancer mortality, cardio-cerebrovascular disease mortality, and respiratory disease mortality in cancer survivors (P for trend < 0.05). Nonlinear relationships between HALP scores and all-cause and cause-specific mortality in cancer survivors were evident through RCS regression modeling (P for nonlinearity < 0.01). Kaplan-Meier analyses demonstrated that higher HALP scores were indicative of a poorer prognosis.
Conclusion: Our findings indicate a notable inverse correlation between HALP scores and both all-cause and cause-specific mortality among cancer survivors.
1 Introduction
Cancer remains the primary cause of mortality worldwide, particularly prevalent in low- and middle-income nations (1). According to the World Health Organization’s (WHO) 2019 statistics, cancer ranks as the primary or secondary cause of death before age 70 in 112 out of 183 countries, and as the third or fourth leading cause in 23 countries (2). Despite a decline in cancer mortality rates from 1991 to 2021, credited to reduced tobacco use, enhanced cancer detection, and advancements in therapies, the global cancer burden is projected to escalate due to demographic shifts and changes in risk factors such as obesity, sedentary lifestyles, and altered fertility rates (3–8). The increasing number of cancer survivors underscores the pressing need for a robust predictive indicator to monitor and enhance their long-term health outcomes.
The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score, introduced by Chen et al. in 2015, initially predicted prognosis in gastric cancer patients (9). Its potential as a biomarker for various illnesses has since garnered attention. Mounting evidence links nutrition, inflammation, and cancer outcomes (10–13). Matsushita et al. suggested a potential link between diet, nutrition, and prostate cancer, possibly mediated by the gut microbiota (14). Dietary-induced inflammation has been implicated in various cancers, such as CRC, liver cancer, prostate cancer, and kidney cancer (15–17). Recent research underscores the significant impact of diet on both mucosal and systemic immune systems, influencing inflammation in tumor cells and their response to cancer therapy (18).
Given HALP score’s ability to assess immune and nutritional status, it can reflect tumor tolerance by integrating immune markers, nutrition, and inflammation. While studies have explored its predictive significance in different cancers, findings vary (19–21). In a multicenter study, lower HALP values correlated with increased risks of mortality and cancer-related deaths in patients with locally advanced colorectal cancer (19). However, a separate study found no significant association between HALP scores and long-term survival in patients with retroperitoneal soft tissue sarcoma (21). Therefore, a comprehensive investigation into HALP score’s association with all-cause and cause-specific mortality in cancer survivors is crucial.
Drawing on NHANES data spanning 1999 to 2018, our cohort study delves into the correlation between the HALP score and all-cause and cause-specific mortality in cancer survivors and to assess the impact of the HALP score on cancer survivors. Our study will provide valuable reference metrics for optimizing treatment and clinical management of cancer survivors.
2 Methods
2.1 Study population
The NHANES, conducted by the National Center for Health Statistics (NCHS), is a nationally representative cross-sectional study employing a stratified multistage random sample design to assess the health and nutritional status of the US population (22). Before implementation, NHANES surveys undergo thorough review and approval by the Disclosure Review Board of the NCHS. Comprehensive details regarding ethical approval and informed consent procedures are available through the NCHS (23). This study employed a nationwide cross-sectional design, utilizing secondary analyses of publicly accessible and deidentified NHANES data. As such, additional institutional review board approval or informed consent was not required. Additional details can be found at http://www.cdc.gov/nchs/nhanes.
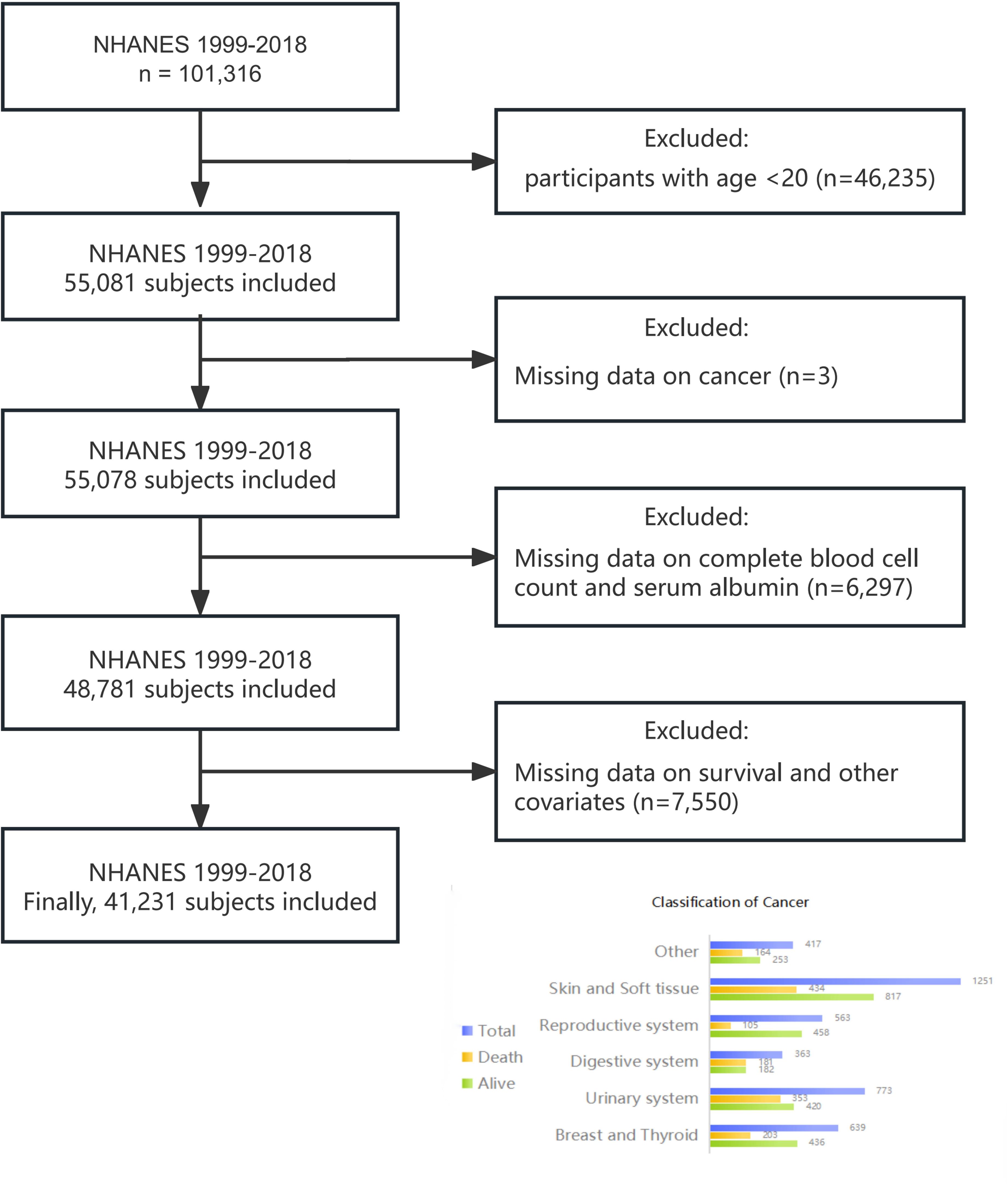
For our cohort study, we included 101,316 participants from ten NHANES cycles spanning 1999 to 2018. Exclusion criteria encompassed individuals under 20 years old, pregnant individuals, those with missing cancer or HALP score data, and participants with incomplete follow-up or covariate information. Ultimately, 41,231 participants were included in the study (Figure 1).
2.2 Definition of hemoglobin, albumin, lymphocyte, platelet score
Blood samples were collected during examinations at Mobile Examination Centers (MECs) and subsequently analyzed in the laboratory. The HALP score comprises serum levels of hemoglobin, albumin, lymphocytes, and platelets. Hemoglobin, lymphocyte, and platelet levels are measured using a hematology-analyzing device (UniCel DxH 800 analyzer), while serum albumin levels are determined using Roche Modular P and Roche Cobas 6000 chemistry analyzers. The HALP score is calculated using the formula: hemoglobin (g/L) × albumin (g/L) × lymphocytes (109/L)/platelets (109/L) (24, 25).
2.3 Definition of cancer survivor
Self-reported cancer history data were sourced from the “Medical Conditions” section of NHANES, gathered via professionally self-administered questionnaires (26). Cancer survivors were identified by their response to the question: “Have you ever been told by a doctor or other health professional that you had cancer or malignancy of any kind?” Positive responses categorized individuals as cancer survivors, while negative responses classified them as non-cancer individuals. Cancer survivors were categorized based on their responses to the question “What kind of cancer?” The classification comprised six categories, as illustrated in Figure 1.
2.4 Mortality outcomes
Mortality data were obtained from NHANES Public-Use Linked Mortality Files, available until December 31, 2019. Causes of death were documented using ICD-10 (International Statistical Classification of Diseases, 10th version) codes (27). Our analysis focused on all-cause and cause-specific deaths, including malignant neoplasms (ICD-10: C00-C97), cardio−cerebrovascular disease (ICD-10: I00-I09, I11, I13, I20-I51, I60-I69), and respiratory diseases (ICD-10: J40-J47, J09-J18). The follow-up period for the study extended from the date of initial diagnosis until the date of death or December 31, 2019, whichever came first.
2.5 Definitions of covariates
Covariates in this cohort study included age (years), gender (male or female), ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, or other race), educational attainment (below high school, high school, or above high school), marital status (married/cohabiting, widowed/divorced/separated, or never married), and body mass index (BMI) categories (<18.5, 18.5-25.0, 25.0-29.9, or >29.9 kg/m²) (28). Income was assessed using the Poverty Income Ratio (PIR), classified as ≤1.0, 1.1-3.0, and >3.0 based on US Department of Health and Human Services guidelines. Never smokers were individuals who had smoked fewer than 100 cigarettes in their lifetime. Those who had smoked more than 100 cigarettes and were currently smoking were classified as current smokers, while those who had smoked more than 100 cigarettes but had quit were classified as former smokers (29). Alcohol consumption was dichotomized into non-drinker or drinker (≥12 drinks in a year). Physical activity was quantified as metabolic equivalent (MET) minutes of moderate to vigorous exercise per week according to World Health Organization guidelines (30). Diabetes mellitus was determined by self-report, glycated hemoglobin ≥6.5%, or fasting blood glucose ≥126 mg/dL (7.0 mmol/L). Hypertension was defined by medication use or self-reported diagnosis. Complete blood count parameters, serum albumin levels, high-density lipoprotein cholesterol (HDL-C), and total cholesterol (TG) levels were also collected from the database.
2.6 Statistical analysis
We utilized NHANES-recommended weights, specifically the 2-year cycle of Mobile Examination Center (MEC) exam weights (wtmec2yr), for statistical analyses, given that HALP scores were derived from four laboratory measurements. Continuous data were presented as medians [first quantile (P25) and third quantile (P75)] and compared using the nonparametric Wilcoxon rank sum test or independent samples t-test, as applicable. Categorical variables were reported as percentages (%) and assessed using the chi-squared test or Fisher’s exact test, if appropriate.
In this cohort analysis, three logistic regression models were employed to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the association between HALP scores and cancer prevalence. Similarly, three Cox regression models were utilized to calculate adjusted hazard ratios (HRs) and 95% CIs for all-cause mortality, cancer mortality, cardio-cerebrovascular disease mortality, and respiratory disease mortality among cancer survivors. Model 1 was unadjusted, while Model 2 was adjusted for age, sex, and race/ethnicity. Model 3 included additional adjustments for education level, family poverty income ratio, MET minutes per week, drinking status, smoking status, BMI, self-reported diabetes, and self-reported hypertension.
Restricted cubic spline regression analyses were conducted to explore dose-response relationships between HALP scores and all-cause and cause-specific mortality among cancer survivors, with knots placed at the 5th, 35th, 65th, and 95th percentiles of each exposure variable. Kaplan-Meier analyses were utilized to evaluate the association between HALP scores and long-term mortality in cancer survivors. Additionally, subgroup analyses were performed to investigate the relationship between HALP scores and mortality outcomes based on age, sex, BMI, smoking status, self-reported hypertension, self-reported diabetes, and different types of cancer.
3 Results
3.1 Baseline characteristics
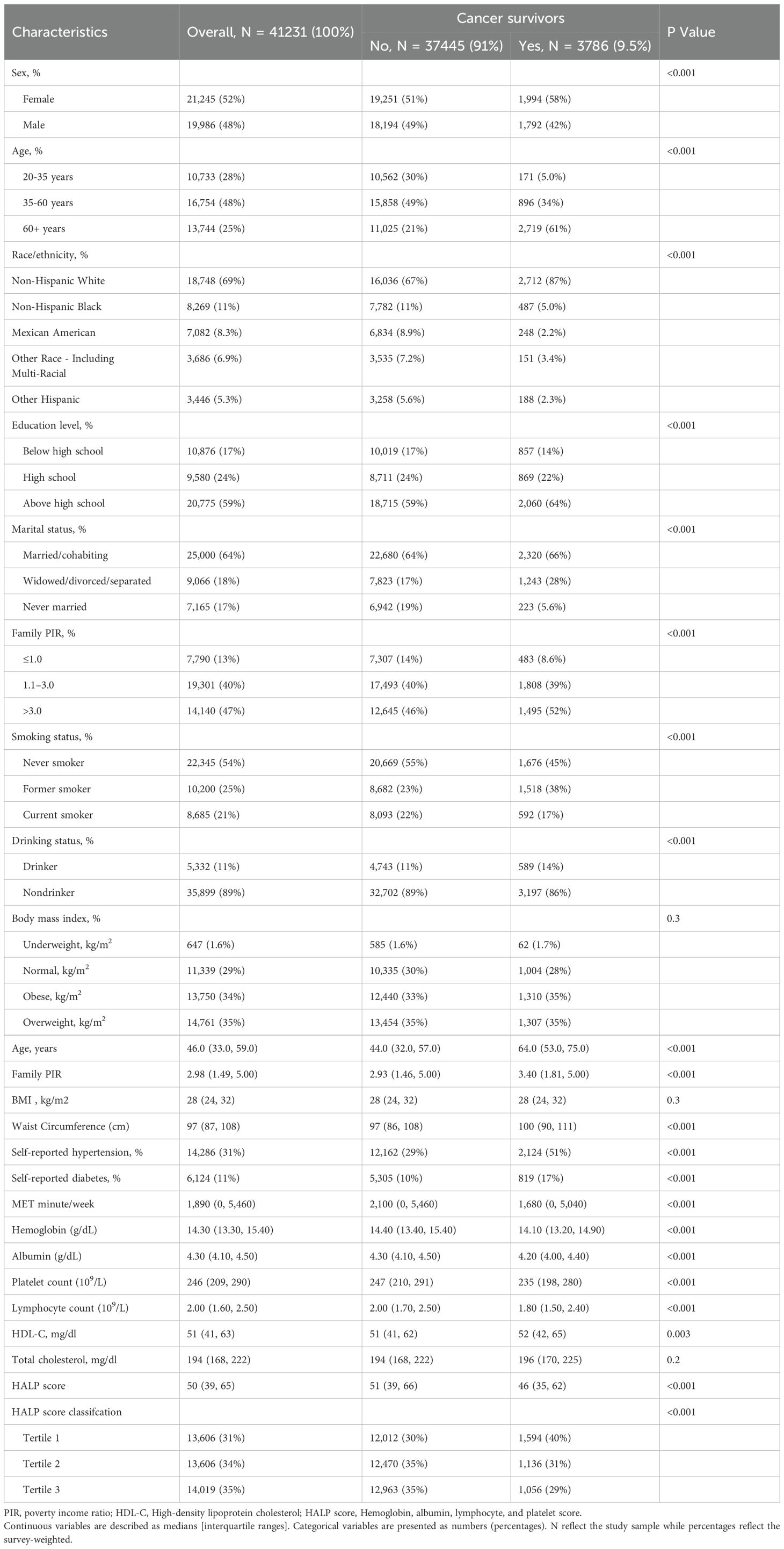
Table 1 presents baseline characteristics and weighted estimates of the study population. Our analysis included 41,231 individuals, representing 17.04 million noninstitutionalized US residents. Among them, 3,786 were cancer survivors, aged 20 to 85 years, with a mean age of 49.47 ± 18.14 years. Race distribution was as follows: Mexican Americans: 2.2%, Other Hispanics: 2.3%, Non-Hispanic Whites people: 87%, Non-Hispanic Blacks people: 5.0%, and Others: 3.4%. Cancer survivors were more likely to be older women, highly educated, higher-income earners, divorced, smokers, and drinkers. Additionally, they exhibited higher waist circumferences, lower physical activity levels, and a higher prevalence of comorbid hypertension or diabetes. Median (P25, P75) values of hemoglobin, serum albumin, lymphocyte count, platelet count, and HALP scores in cancer survivors were 14.10 (13.20, 14.90) g/dL, 4.20 (4.00, 4.40) g/dL, 1.80 (1.50, 2.40)×109/L, 235 (198, 280)×109/L, and 46 (35, 62), respectively, significantly lower than non-cancer participants. Significant differences in HALP scores and HALP-related parameters were observed between individuals with and without cancer (P< 0.05).
3.2 HALP score and cancer prevalence
Table 2 illustrates the relationship between HALP scores and cancer prevalence using weighted multivariate regression models. All three logistic regression models revealed a negative association between HALP score and cancer incidence. The ORs and 95% CIs for the highest tertile compared to the lowest tertile were as follows: OR=0.61 (0.57-0.67), p for trend<0.001; OR=0.83 (0.75-0.89), p for trend<0.001; and OR=0.61 (0.75-0.89), p for trend<0.001, respectively.
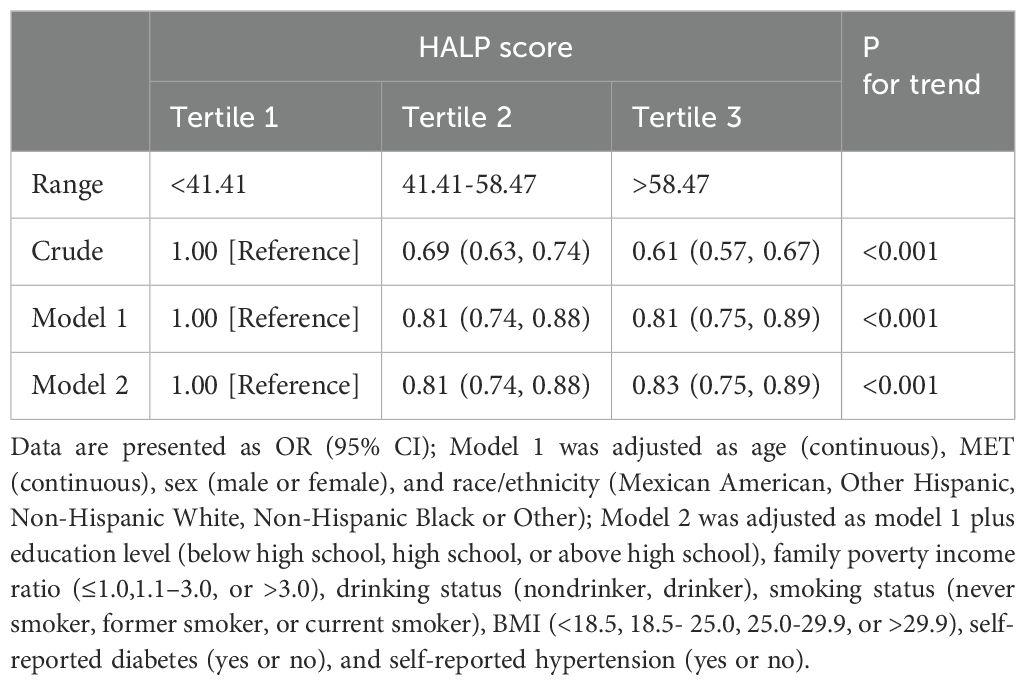
Table 2. Logistic regression analysis between HALP score and prevalence of cancer among adults in NHANES 1999–2018.
3.3 HALP score and mortality
Over a median follow-up of 91 (51, 136) months, 1,339 (35.37%) out of 3,786 cancer survivors succumbed to all-cause mortality, with 397 (10.49%) attributed to cancer, 367 (9.70%) to cardio-cerebrovascular disease, and 105 (2.77%) to respiratory disease. As shown in Table 3, higher HALP scores were significantly associated with a reduced risk of all-cause mortality and cause-specific mortality among survivors, evident in both crude and multivariable-adjusted Models 1 and 2 (all p for trend <0.05). The multivariable-adjusted HRs and 95% CIs for the highest tertile compared with the lowest tertile for all-cause mortality, cancer mortality, cardio-cerebrovascular disease mortality, and respiratory disease mortality among cancer survivors were 0.61 (0.49-0.76), 0.91 (0.70-1.17), 0.67 (0.49-0.91), and 0.60 (0.35-0.97), respectively.
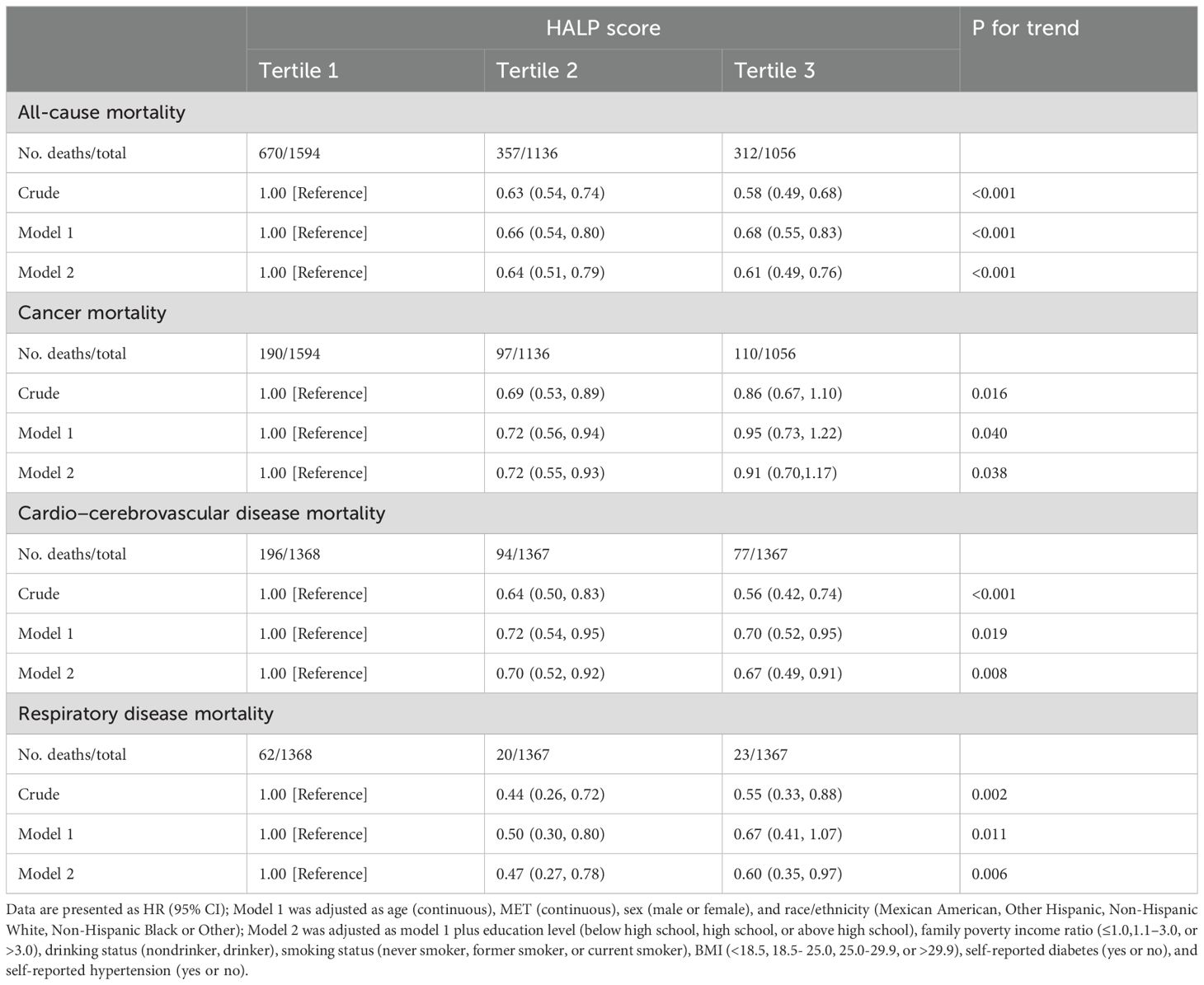
Table 3. Cox regression analysis between HALP score and long-term mortality among cancer survivor in NHANES 1999–2018.
Furthermore, Kaplan-Meier analysis revealed a comprehensive association between HALP scores and both cause-specific and all-cause mortality in cancer survivors. Higher HALP scores were correlated with reduced all-cause mortality (p=0.0033), decreased cancer mortality (p<0.0001), lowered cardio-cerebrovascular disease mortality (p<0.0001), and lessened respiratory disease mortality (p=0.00054) among cancer survivors (Figure 2).
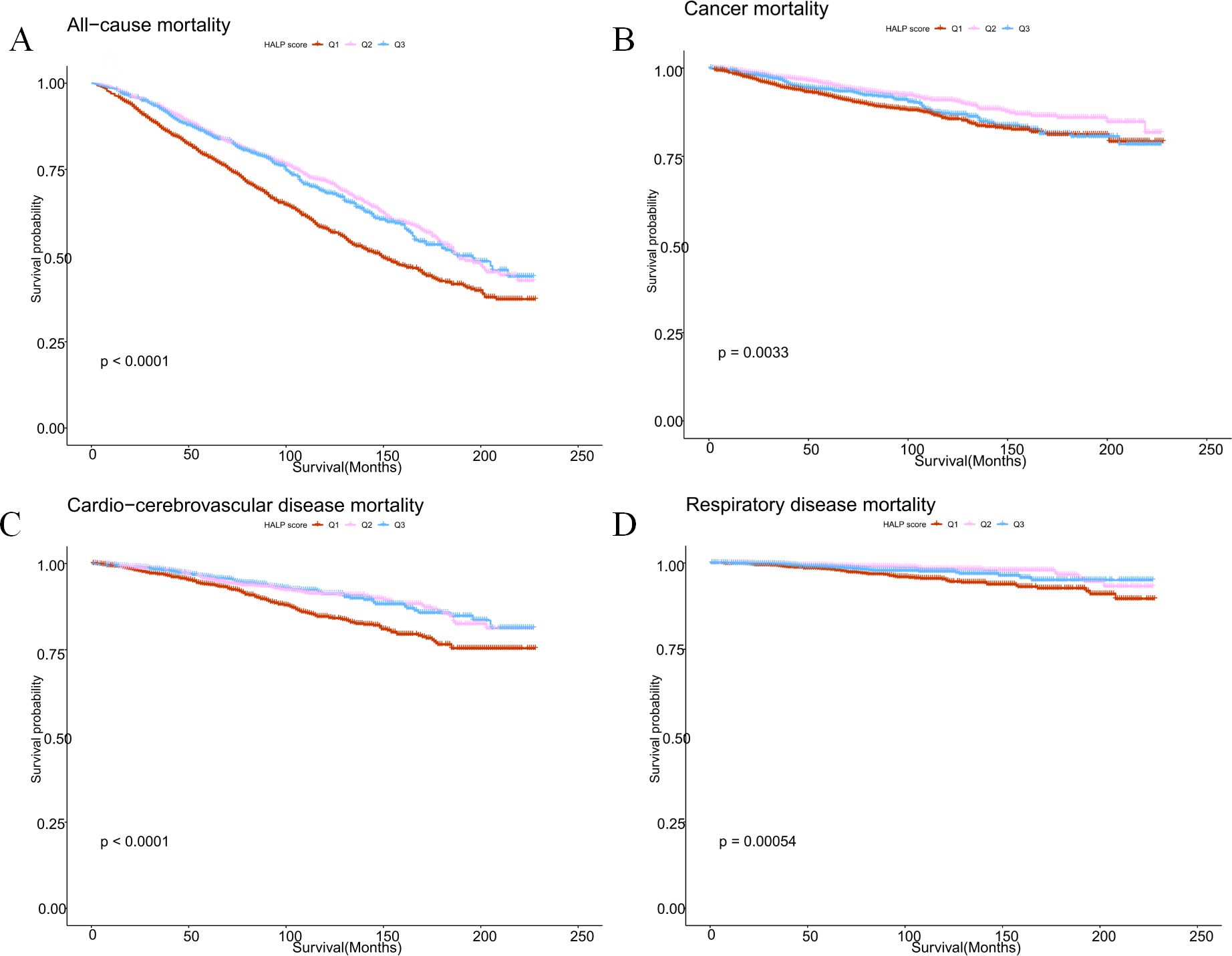
Figure 2. Kaplan-Meier survival survival estimates between HALP scores and all-cause mortality (A), cancer mortality (B), cardio-cerebrovascular disease mortality (C), and respiratory disease mortality (D) in cancer survivors.
3.4 Restricted cubic spline analysis
We employed weighted restricted cubic spline curves to explore the nonlinear relationship between HALP scores and all-cause, as well as cause-specific mortality, while controlling for potential confounders. Illustrated in Figure 3, the restricted cubic spline analysis unveiled a nonlinear association between HALP scores and cardio-cerebrovascular disease mortality in cancer survivors (nonlinear P=0.0015). Notably, lower HALP scores were linked to an elevated risk of cardio-cerebrovascular disease mortality in this population.
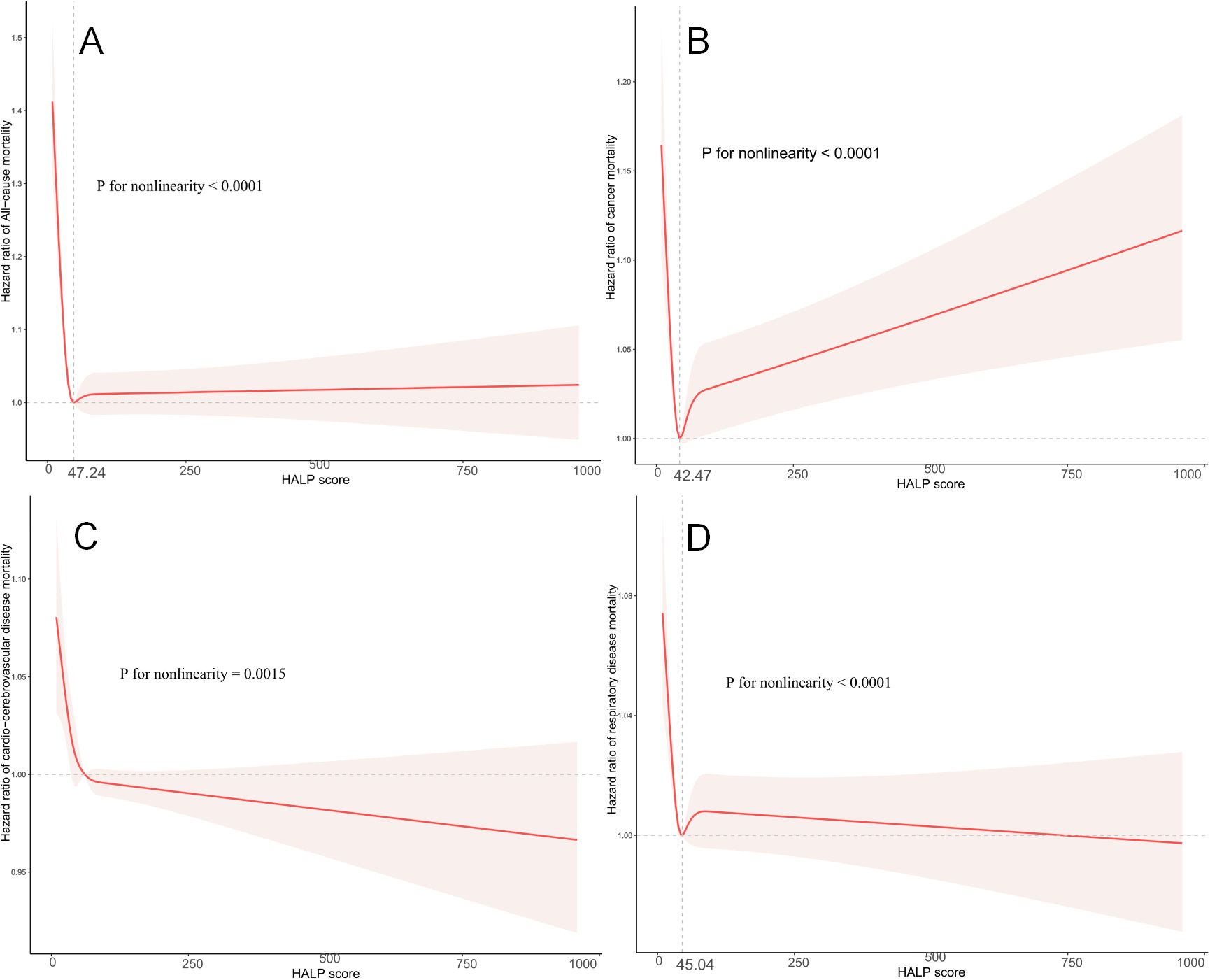
Figure 3. Restricted cubic spline analysis to assess the association between HALP score and all-cause mortality (A), cancer mortality (B), cardio-cerebrovascular disease mortality (C), and respiratory disease mortality (D) in cancer survivors. Adjusted for age (continuous), MET (continuous), sex (male or female), ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black or Other), education level (below high school, high school, or above high school), family poverty income ratio (≤1.0, 1.1–3.0, or >3.0), drinking status (nondrinker, drinker), smoking status (never smoker, former smoker, or current smoker), BMI (<18.5, 18.5- 25.0, 25.0-29.9, or >29.9), self-reported diabetes (yes or no), and self-reported hypertension (yes or no).
Furthermore, nonlinear associations were also observed between HALP scores and all-cause mortality (nonlinear P<0.0001), cancer mortality (nonlinear P<0.0001), and respiratory disease mortality (nonlinear P<0.0001) in cancer survivors. Importantly, the results of the two linear regressions indicated that the probability of all-cause mortality, cancer mortality, and respiratory disease mortality progressively decreased to the lowest point at HALP scores of 47.24, 42.47, and 45.04, respectively, before increasing with rising HALP scores.
3.5 Subgroup analyses
To further evaluate the robustness of the relationship between HALP scores and all-cause, as well as cause-specific mortality in cancer survivors, subgroup analyses were conducted based on sex, age, BMI, smoking, hypertension status, and diabetes status. The findings indicated a largely consistent dose-response relationship between HALP scores and all-cause and cause-specific mortality across subgroups, particularly in all-cause mortality. However, the association between HALP scores and cancer mortality in the subgroup analyses was less pronounced (Table 4). Moreover, no statistically significant interaction p-values were detected (Table 4).
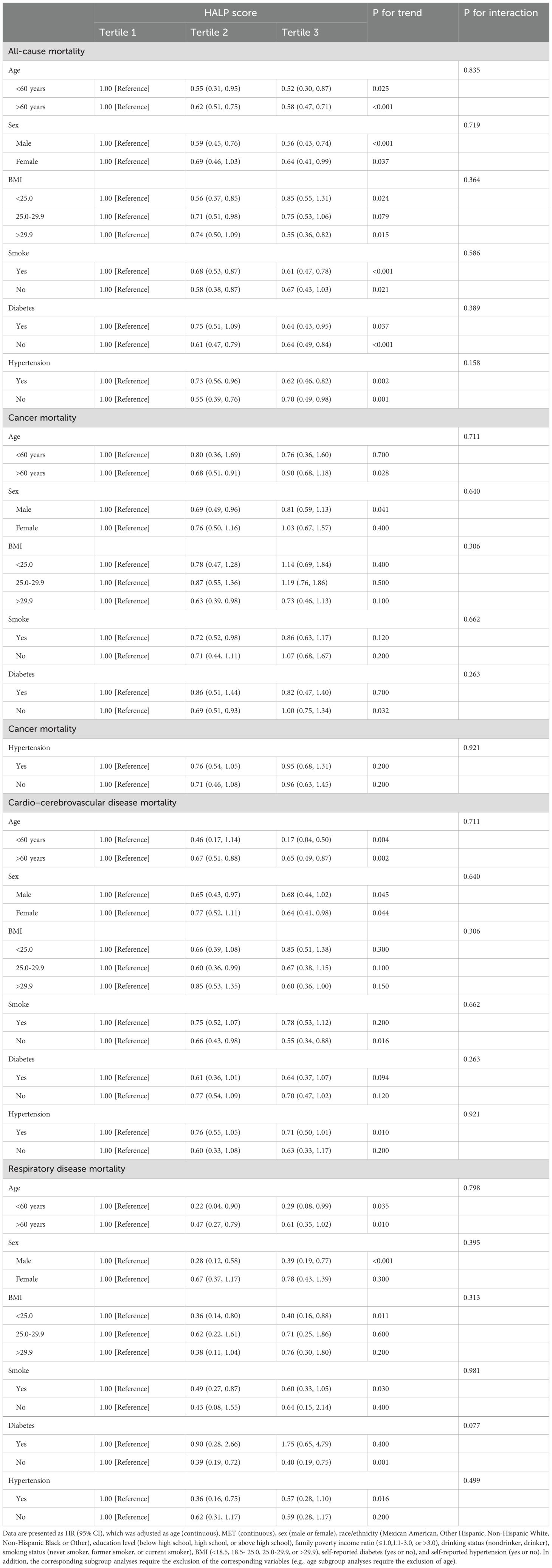
Table 4. Subgroup analysis of HALP score and long-term mortality among cancer survivor in NHANES 1999–2018.
To delve deeper into the influence of HALP scores across different tumor types, subgroup analyses were conducted according to tumor classification. Results presented in Table 5 indicate a notable impact of HALP scores on all-cause mortality among patients with thyroid and breast cancers, as well as those with tumors of the digestive system, and skin and soft tissue tumors. This observation suggests a tumor-specific effect of HALP scores on cancer survivors.
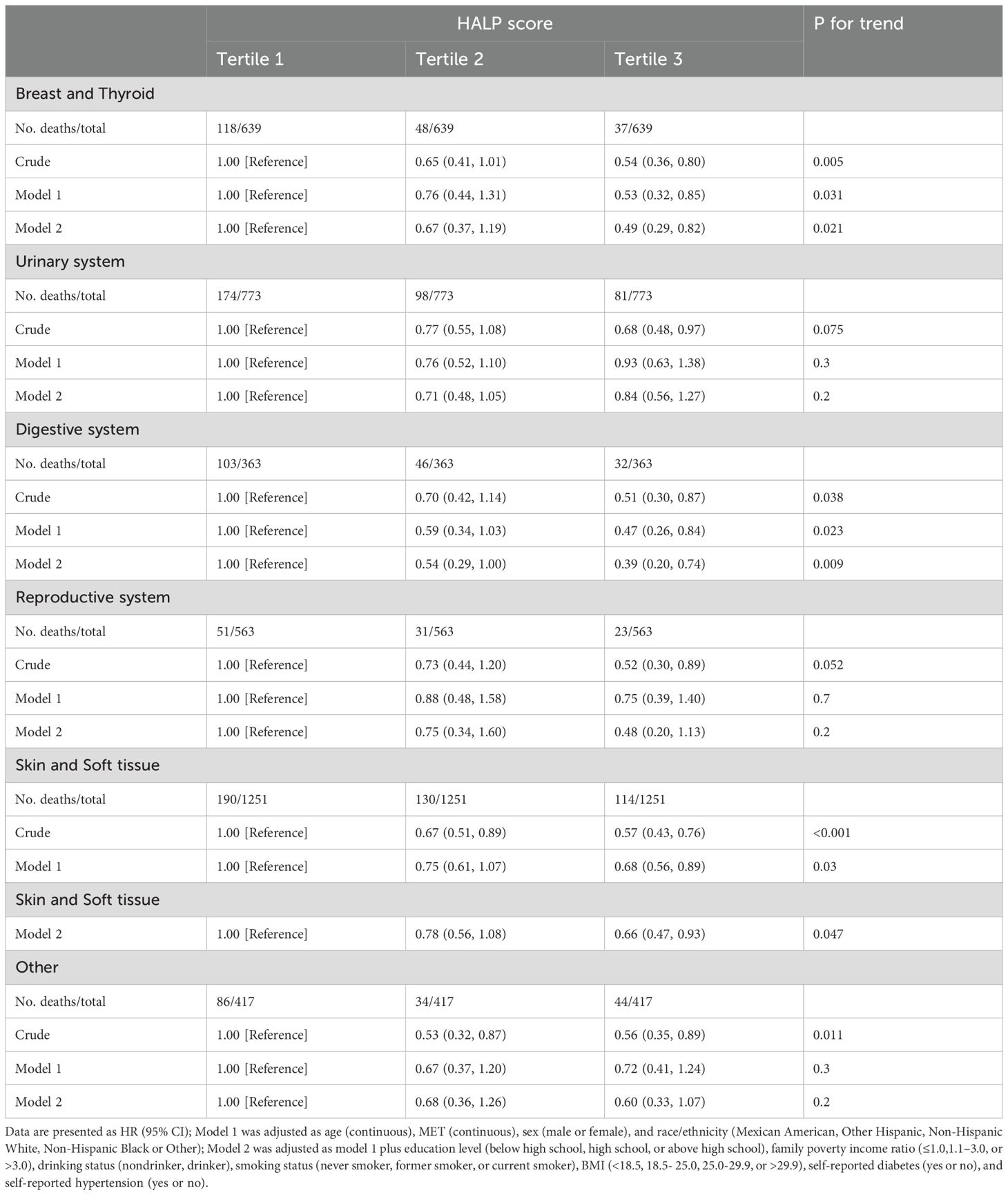
Table 5. Subgroup analysis by cancer of HALP score and all-cause mortality among cancer survivor in NHANES 1999–2018.
4 Discussion
In our cross-sectional study spanning 1999 to 2018, we investigated the association between HALP scores and cancer prevalence, as well as long-term mortality in the US population. Following adjustment for multiple variables, a significant negative relationship emerged between HALP score and cancer incidence. Among cancer survivors, we observed a notable nonlinear association between HALP score and all-cause, as well as cause-specific mortality (including cancer, cardio-cerebrovascular disease, and respiratory disease mortality), with higher HALP scores correlating with reduced mortality rates. Notably, HALP scores of 47.24, 42.47, and 45.04 corresponded to the lowest all-cause, cancer, and respiratory disease mortality, respectively. Kaplan-Meier analysis further revealed that lower HALP scores were associated with shorter survival times among cancer survivors. Importantly, these findings remained robust across multiple subgroup analyses. In addition, subgroup analyses based on different types of cancer revealed that the effect of HALP scores on all-cause mortality in cancer survivors may be tumor-specific. Overall, our results suggest that HALP score holds significant potential as a valuable predictor of outcomes for cancer survivors.
Immunity and nutrition play pivotal roles in cancer development and progression. Chronic inflammation is known to be carcinogenic, contributing to increased stem cell proliferation and local mutagenic effects through long-term cell turnover stimulation (10, 31, 32). The relationship between neoplasia and the immune system is encapsulated in the ‘3E hypothesis,’ which delineates the stages of initial elimination of transformed cells by immunological effector cells, followed by equilibrium between malignant cells and the immune response within a smoldering neoplastic lesion, and ultimately, the escape of cancer cells from immunological control (33). Systemic inflammation, a hallmark of the tumor microenvironment, significantly influences disease progression and prognosis in cancer survivors (34, 35). Numerous studies have investigated various systemic inflammatory biomarkers and have demonstrated their high predictive value for the prognosis of different cancer types (36–40).
Malnutrition is often linked to tumor progression, stemming from inadequate nutritional intake, increased tumor consumption, or the effects of anticancer therapy. International point prevalence studies report malnutrition rates ranging from 31% to 39% among lower gastrointestinal cancer patients (41, 42). Malnutrition can compromise immunity, trigger metabolic disturbances, and diminish treatment tolerance in cancer survivors, all of which can influence the efficacy of oncological treatment and patient prognosis (43, 44). A study investigating penile cancer patients undergoing inguinal lymph node dissection (ILND) revealed that the preoperative albumin alkaline phosphatase ratio (AAPR) reliably predicts pathologic lymph node-positive (pN+) status (45). Similarly, in bladder cancer patients, an elevated preoperative fibrinogen-to-albumin ratio (FAR) has been identified as a potential predictor of malignancy and advanced grade (46). These findings underscore the significant predictive role of nutritional status in cancer patients.
The significance of inflammatory response and nutritional status in cancer prognosis is increasingly recognized. Nøst et al. (47) analyzed the UK Biobank data and found that the systemic immune-inflammatory index (SII), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) correlated positively with the risk of 7 out of 17 cancers, while the lymphocyte-to-monocyte ratio (LMR) correlated negatively. Ouyang et al. (48) identified preoperative SII as an independent prognostic marker in pediatric osteosarcoma. Regarding nutrition, Kheirouri et al. (49) linked high preoperative Controlling Nutritional Status (CONUT) scores to lower overall survival (OS) and cancer-specific survival (CSS) across various cancers. Another study validated the Cholesterol-modified Prognostic Nutritional Index (CPNI) for predicting breast cancer prognosis (50). These findings highlight the combined impact of inflammation and nutrition on cancer outcomes, suggesting that effective prognostic models should integrate both factors.
HALP scores, derived from hemoglobin, albumin, lymphocyte, and platelet levels, serve as indicators of the host’s inflammatory and nutritional status. Hemoglobin, a pivotal factor in tumor progression, is frequently depleted in cancer survivors, contributing to hypoxia (51), a driver of tumor advancement and treatment resistance (52). Numerous studies have established a correlation between hemoglobin levels in cancer patients and both survival outcomes and disease progression (53–55).
Serum albumin, synthesized by the liver, constitutes a crucial component of total serum protein and reflects the host’s inflammatory and nutritional profile. Hypoalbuminemia, stemming from malnutrition, hypermetabolism, systemic inflammation, or heightened cytokine release, compromises the immune response to cancer cells (56). Extensive research has underscored the association between hypoalbuminemia and poor survival across various cancer types (57, 58).
Moreover, lymphocytes play a pivotal role in the host’s anti-cancer defense mechanisms. They secrete cytokines such as interferon-γ and tumor necrosis factor-α (TNF-α), which enhance prognosis by inducing apoptosis and impeding cancer cell proliferation, invasion, and migration (59, 60). Consequently, a decline in lymphocyte levels correlates with a poorer prognosis for cancer survivors.
Additionally, recent research suggests that platelets participate in various signaling pathways implicated in tumor immunity and progression (61). Several studies have demonstrated that elevated pretreatment platelet counts are associated with reduced survival rates in cancer patients (62–64). Taken together, these findings underscore the potential of the HALP score as a valuable prognostic tool for assessing cancer survivors.
The HALP score has demonstrated promising prognostic value across various cancer types, including gastric cancer (9), esophageal squamous cell carcinoma (65), colorectal cancer (19), renal cell carcinoma (66), bladder cancer (20), and small cell lung cancer (67). However, the association between HALP scores and the risk of all-cause and cause-specific mortality across all cancer types remains unexplored. Using NHANES 1999-2018 data, our study unveiled a significant nonlinear relationship between HALP scores and all-cause and cause-specific mortality. Higher HALP scores were consistently associated with reduced all-cause and cause-specific mortality. Notably, HALP scores of 47.24, 42.47, and 45.04 were linked to the lowest levels of all-cause mortality, cancer mortality, and respiratory disease mortality, respectively. Our study bridges this gap in research and provides an invaluable tool for the treatment and management of cancer survivors.
Although survival rates for cancer survivors remain poor, improvements in treatment strategies and care have positively impacted the prognosis of cancer survivors. With increased survival, non-cancer causes of death have become more and more important. In Japan, the reported number of deaths from cancer in 2021 was 381,505 (26.5%) among cancer survivals, and cancer was still the leading cause of death, however, heart disease was the second highest cause of death, accounting for as high as 214,710 (14.91%) recorded deaths, followed by cerebrovascular disease (104,595 deaths, 7.26%) (68). As a result, the proportion of non-cancer mortality among cancer survivors will increase in the future. Thus, a valuable predictive parameter must properly predict not only the risk of all-cause and cancer mortality in cancer survivors but also the risk of non-cancer mortality. To our knowledge, our study represents the first comprehensive investigation into the relationship between HALP scores and all-cause and cause-specific mortality among cancer survivors. Our findings demonstrate significant associations between HALP scores and all-cause mortality, cancer mortality, cardio-cerebrovascular disease mortality, and respiratory disease mortality in this population. Our study introduces a reliable monitoring metric for the management of cancer survivors, facilitating accurate prognosis prediction and enabling timely interventions that can markedly enhance their outcomes.
An interesting observation in our results is that we found a significant effect of the HALP score on all-cause mortality in patients with breast cancer, thyroid cancer, digestive system tumors, and skin and soft-tissue tumors, suggesting that its effect varies across tumor types. Consistent with prior research, Zhao et al. demonstrated the HALP score’s independent prognostic value in early-stage breast cancer, correlating with poorer recurrence-free survival (69). Similarly, Duzkopru et al. identified the HALP score as a prognostic indicator in patients with metastatic gastric cancer (70). These findings underscore the tumor-specific impact of the HALP score on cancer survivors, warranting further investigation into its underlying mechanisms. In addition, in our subgroup analysis, we found a consistently robust correlation between HALP score and all-cause mortality in cancer survivors. However, certain specific mortality results did not reach significance, likely due to the limited data available. Therefore, additional validation through large-scale studies is necessary.
Our study demonstrates several strengths. Firstly, our cohort analysis draws from ten NHANES cycles spanning 1999 to 2018, ensuring robustness with ample data and a lengthy investigative period. Secondly, our choice of the HALP score as a parameter offers a comprehensive assessment of both inflammation and nutritional status, surpassing single inflammation metrics. This approach enriches the evaluation of cancer survivors’ physical well-being and enhances outcome prediction reliability. Thirdly, our study pioneers the comprehensive examination of HALP scores’ association with all-cause and specific mortalities in cancer survivors, including cancer, cardio-cerebrovascular disease, and respiratory disease mortalities. These findings provide invaluable insights for prognostic management in this population. Lastly, employing diverse analytical methodologies such as RCS nonlinear analysis, subgroup scrutiny, and Kaplan-Meier analysis further consolidates the HALP score’s validity as a reliable mortality risk indicator for cancer survivors.
Several limitations must be acknowledged in our study. Firstly, NHANES data relies on self-reports from patients, which could introduce recall bias. Secondly, despite our efforts to adjust for known confounding factors such as age, gender, and smoking status, there may still be unidentified confounders affecting our results. Thirdly, the HALP score utilized in our study was derived from a single complete blood count parameter and serum albumin measurement, which may not fully capture individuals’ overall health status, potentially leading to bias.
5 Conclusions
By conducting a comprehensive survey of cancer survivors across the United States, our study unveiled a significant nonlinear relationship between HALP scores and both all-cause and cause-specific mortality. Elevated HALP scores were consistently associated with lower long-term mortality rates among cancer survivors. Therefore, the HALP score emerges as a practical and cost-effective tool for identifying high-risk groups within this population. Our findings underscore the promising potential of HALP scores in prognosticating outcomes for cancer survivors, offering valuable insights for clinical decision-making in this demographic.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the NCHS Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. XY: Formal analysis, Methodology, Validation, Writing – original draft. YZ: Methodology, Validation, Writing – original draft. JZ: Supervision, Validation, Writing – review & editing. XW: Conceptualization, Methodology, Supervision, Writing – review & editing. DZ: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Project of medical and health technology development program of Shandong province (202204080217).
Acknowledgments
We appreciate the people who contributed to the NHANES data we studied.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. (2021) 127:3029–30. doi: 10.1002/cncr.33587
2. Sedeta E, Sung H, Laversanne M, Bray F, Jemal A. Recent mortality patterns and time trends for the major cancers in 47 countries worldwide. Cancer Epidemiol Biomarkers Prev. (2023) 32:894–905. doi: 10.1158/1055-9965.Epi-22-1133
3. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
4. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. (2017) 66:683–91. doi: 10.1136/gutjnl-2015-310912
5. Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. (2012) 61:1079–92. doi: 10.1016/j.eururo.2012.02.054
6. Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. (2020) 77:38–52. doi: 10.1016/j.eururo.2019.08.005
7. DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. (2015) 24:1495–506. doi: 10.1158/1055-9965.Epi-15-0535
8. Torre LA, Siegel RL, Ward EM, Jemal A. International variation in lung cancer mortality rates and trends among women. Cancer Epidemiol Biomarkers Prev. (2014) 23:1025–36. doi: 10.1158/1055-9965.Epi-13-1220
9. Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. (2015) 6:41370–82. doi: 10.18632/oncotarget.5629
10. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. (2017) 18:843–50. doi: 10.1038/ni.3754
11. Coussens LM, Werb Z. Inflammation and cancer. Nature. (2002) 420:860–7. doi: 10.1038/nature01322
13. Blasiak J, Chojnacki J, Pawlowska E, Szczepanska J, Chojnacki C. Nutrition in cancer therapy in the elderly-an epigenetic connection? Nutrients. (2020) 12:3366. doi: 10.3390/nu12113366
14. Matsushita M, Fujita K, Nonomura N. Influence of diet and nutrition on prostate cancer. Int J Mol Sci. (2020) 21:1447. doi: 10.3390/ijms21041447
15. Shateri Z, Makhtoomi M, Mansouri F, Rajabzadeh-Dehkordi M, Nouri M, Rashidkhani B. The association between empirical dietary inflammatory pattern and colorectal cancer risk: a case-control study. BMC Nutr. (2023) 9:136. doi: 10.1186/s40795-023-00797-8
16. Chen K, Yang F, Zhu X, Qiao G, Zhang C, Tao J, et al. Association between pro-inflammatory diet and liver cancer risk: a systematic review and meta-analysis. Public Health Nutr. (2023) 26:2780–9. doi: 10.1017/s1368980023002574
17. Dai YN, Yi-Wen Yu E, Zeegers MP, Wesselius A. The association between dietary inflammatory potential and urologic cancers: A meta-analysis. Adv Nutr. (2024) 15:100124. doi: 10.1016/j.advnut.2023.09.012
18. Siracusa F, Tintelnot J, Cortesi F, Gagliani N. Diet and immune response: how today’s plate shapes tomorrow’s health. Trends Immunol. (2024) 45:4–10. doi: 10.1016/j.it.2023.10.010
19. Jiang H, Li H, Li A, Tang E, Xu D, Chen Y, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget. (2016) 7:72076–83. doi: 10.18632/oncotarget.12271
20. Peng D, Zhang CJ, Gong YQ, Hao H, Guan B, Li XS, et al. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. (2018) 8:794. doi: 10.1038/s41598-018-19146-y
21. Matsui Y, Matsuda A, Maejima A, Shinoda Y, Nakamura E, Komiyama M, et al. The clinical significance of perioperative inflammatory index as a prognostic factor for patients with retroperitoneal soft tissue sarcoma. Int J Clin Oncol. (2022) 27:1093–100. doi: 10.1007/s10147-022-02150-8
22. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat 1. (2013) 56):1–37.
23. Shen R, Zou T. The association between cardiovascular health and depression: Results from the 2007-2020 NHANES. Psychiatry Res. (2024) 331:115663. doi: 10.1016/j.psychres.2023.115663
24. Tian M, Li Y, Wang X, Tian X, Pei LL, Wang X, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is associated with poor outcome of acute ischemic stroke. Front Neurol. (2020) 11:610318. doi: 10.3389/fneur.2020.610318
25. Pan H, Lin S. Association of hemoglobin, albumin, lymphocyte, and platelet score with risk of cerebrovascular, cardiovascular, and all-cause mortality in the general population: results from the NHANES 1999-2018. Front Endocrinol (Lausanne). (2023) 14:1173399. doi: 10.3389/fendo.2023.1173399
26. Medina HN, Liu Q, Cao C, Yang L. Balance and vestibular function and survival in US cancer survivors. Cancer. (2021) 127:4022–9. doi: 10.1002/cncr.33787
27. Outland B, Newman MM, William MJ. Health policy basics: implementation of the international classification of disease, 10th revision. Ann Intern Med. (2015) 163:554–6. doi: 10.7326/m15-1933
28. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:1–253.
29. Qiu Z, Chen X, Geng T, Wan Z, Lu Q, Li L, et al. Associations of serum carotenoids with risk of cardiovascular mortality among individuals with type 2 diabetes: results from NHANES. Diabetes Care. (2022) 45:1453–61. doi: 10.2337/dc21-2371
30. Liang J, Huang S, Jiang N, Kakaer A, Chen Y, Liu M, et al. Association between joint physical activity and dietary quality and lower risk of depression symptoms in US adults: cross-sectional NHANES study. JMIR Public Health Surveill. (2023) 9:e45776. doi: 10.2196/45776
31. Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. (2008) 9:628–38. doi: 10.1038/nrm2455
32. Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. (2013) 13:349–61. doi: 10.1038/nri3423
33. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. (2014) 27:16–25. doi: 10.1016/j.coi.2014.01.004
34. Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. (2010) 29:243–8. doi: 10.1007/s10555-010-9227-2
35. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
36. Xie H, Ruan G, Ge Y, Zhang Q, Zhang H, Lin S, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. (2022) 41:1236–43. doi: 10.1016/j.clnu.2022.04.019
37. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
38. Matsubara T, Takamori S, Haratake N, Fujishita T, Toyozawa R, Ito K, et al. Identification of the best prognostic marker among immunonutritional parameters using serum C-reactive protein and albumin in non-small cell lung cancer. Ann Surg Oncol. (2021) 28:3046–54. doi: 10.1245/s10434-020-09230-x
39. Wei L, Xie H, Yan P. Prognostic value of the systemic inflammation response index in human Malignancy: A meta-analysis. Med (Baltimore). (2020) 99:e23486. doi: 10.1097/md.0000000000023486
40. Efil SC, Guner G, Guven DC, Celikten B, Celebiyev E, Taban H, et al. Prognostic and predictive value of tumor infiltrating lymphocytes in combination with systemic inflammatory markers in colon cancer. Clin Res Hepatol Gastroenterol. (2023) 47:102171. doi: 10.1016/j.clinre.2023.102171
41. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. (2014) 38:196–204. doi: 10.1177/0148607113502674
42. Pressoir M, Desné S, Berchery D, Rossignol G, Poiree B, Meslier M, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. (2010) 102:966–71. doi: 10.1038/sj.bjc.6605578
43. Yang M, Zhang Q, Ge Y, Tang M, Hu C, Wang Z, et al. Prognostic roles of inflammation- and nutrition-based indicators for female patients with cancer. J Inflamm Res. (2022) 15:3573–86. doi: 10.2147/jir.S361300
44. Maumy L, Harrissart G, Dewaele P, Aljaber A, Bonneau C, Rouzier R, et al. Impact of nutrition on breast cancer mortality and risk of recurrence, a review of the evidence. Bull Cancer. (2020) 107:61–71. doi: 10.1016/j.bulcan.2019.08.009
45. Tufano A, Napolitano L, Barone B, Pezone G, Alvino P, Cilio S, et al. Preoperative albumin-to-alkaline phosphatase ratio as an independent predictor of lymph node involvement in penile cancer. Medicina (Kaunas). (2024) 60:414. doi: 10.3390/medicina60030414
46. Barone B, Napolitano L, Reccia P, De Luca L, Morra S, Turco C, et al. Preoperative fibrinogen-to-albumin ratio as potential predictor of bladder cancer: A monocentric retrospective study. Medicina (Kaunas). (2022) 58(10):1490. doi: 10.3390/medicina58101490
47. Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. (2021) 36:841–8. doi: 10.1007/s10654-021-00752-6
48. Ouyang H, Wang Z. Predictive value of the systemic immune-inflammation index for cancer-specific survival of osteosarcoma in children. Front Public Health. (2022) 10:879523. doi: 10.3389/fpubh.2022.879523
49. Kheirouri S, Alizadeh M. Prognostic potential of the preoperative controlling nutritional status (CONUT) score in predicting survival of patients with cancer: A systematic review. Adv Nutr. (2021) 12:234–50. doi: 10.1093/advances/nmaa102
50. Shi J, Liu T, Ge Y, Liu C, Zhang Q, Xie H, et al. Cholesterol-modified prognostic nutritional index (CPNI) as an effective tool for assessing the nutrition status and predicting survival in patients with breast cancer. BMC Med. (2023) 21:512. doi: 10.1186/s12916-023-03225-7
51. Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys. (2005) 63:25–36. doi: 10.1016/j.ijrobp.2005.04.049
52. Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. (2019) 18:157. doi: 10.1186/s12943-019-1089-9
53. Belcher DA, Ju JA, Baek JH, Yalamanoglu A, Buehler PW, Gilkes DM, et al. The quaternary state of polymerized human hemoglobin regulates oxygenation of breast cancer solid tumors: A theoretical and experimental study. PloS One. (2018) 13:e0191275. doi: 10.1371/journal.pone.0191275
54. Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. (2001) 91:2214–21. doi: 10.1002/1097-0142(20010615)91:12<2214::AID-CNCR1251>3.0.CO;2-P
55. Xia L, Hu G, Guzzo TJ. Prognostic significance of preoperative anemia in patients undergoing surgery for renal cell carcinoma: A meta-analysis. Anticancer Res. (2017) 37:3175–81. doi: 10.21873/anticanres.11677
56. Lucijanic M, Veletic I, Rahelic D, Pejsa V, Cicic D, Skelin M, et al. Assessing serum albumin concentration, lymphocyte count and prognostic nutritional index might improve prognostication in patients with myelofibrosis. Wien Klin Wochenschr. (2018) 130:126–33. doi: 10.1007/s00508-018-1318-z
57. Liu X, Meng QH, Ye Y, Hildebrandt MA, Gu J, Wu X. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis. (2015) 36:243–8. doi: 10.1093/carcin/bgu247
58. Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. (2007) 14:381–9. doi: 10.1245/s10434-006-9093-x
59. Lim JA, Oh CS, Yoon TG, Lee JY, Lee SH, Yoo YB, et al. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: an in vitro analysis. BMC Cancer. (2018) 18:159. doi: 10.1186/s12885-018-4064-8
60. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
61. Lyu J, Yang EJ, Head SA, Ai N, Zhang B, Wu C, et al. Pharmacological blockade of cholesterol trafficking by cepharanthine in endothelial cells suppresses angiogenesis and tumor growth. Cancer Lett. (2017) 409:91–103. doi: 10.1016/j.canlet.2017.09.009
62. Rao XD, Zhang H, Xu ZS, Cheng H, Shen W, Wang XP. Poor prognostic role of the pretreatment platelet counts in colorectal cancer: A meta-analysis. Med (Baltimore). (2018) 97:e10831. doi: 10.1097/md.0000000000010831
63. Yuan Y, Zhong H, Ye L, Li Q, Fang S, Gu W, et al. Prognostic value of pretreatment platelet counts in lung cancer: a systematic review and meta-analysis. BMC Pulm Med. (2020) 20:96. doi: 10.1186/s12890-020-1139-5
64. Xin-Ji Z, Yong-Gang L, Xiao-Jun S, Xiao-Wu C, Dong Z, Da-Jian Z. The prognostic role of neutrophils to lymphocytes ratio and platelet count in gastric cancer: A meta-analysis. Int J Surg. (2015) 21:84–91. doi: 10.1016/j.ijsu.2015.07.681
65. Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol. (2017) 46:75–9. doi: 10.1016/j.intimp.2017.02.027
66. Peng D, Zhang CJ, Tang Q, Zhang L, Yang KW, Yu XT, et al. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol. (2018) 18:20. doi: 10.1186/s12894-018-0333-8
67. Shen XB, Zhang YX, Wang W, Pan YY. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score in patients with small cell lung cancer before first-line treatment with etoposide and progression-free survival. Med Sci Monit. (2019) 25:5630–9. doi: 10.12659/msm.917968
68. Fujii M, Morishima T, Nagayasu M, Kudo H, Ohno Y, Sobue T, et al. Cause of death among long-term cancer survivors: the NANDE study. Healthcare (Basel). (2023) 11(6):835. doi: 10.3390/healthcare11060835
69. Zhao Z, Xu L. Prognostic significance of HALP score and combination of peripheral blood multiple indicators in patients with early breast cancer. Front Oncol. (2023) 13:1253895. doi: 10.3389/fonc.2023.1253895
Keywords: cancer survivors, HALP score, all-cause mortality, cause-specific mortality, national health and nutrition examination survey
Citation: Fu J, Yue X, Zou Y, Zhang J, Wang X and Zhang D (2024) Association of hemoglobin, albumin, lymphocyte, and platelet score with risk of all-cause and cause-specific mortality among cancer survivors: NHANES 1999-2018. Front. Oncol. 14:1402217. doi: 10.3389/fonc.2024.1402217
Received: 17 March 2024; Accepted: 30 August 2024;
Published: 18 September 2024.
Edited by:
Adriano Sabino, Federal University of Minas Gerais, BrazilReviewed by:
Biagio Barone, Azienda Ospedaliera di Caserta, ItalyDina Keumala Sari, Universitas Sumatera Utara, Indonesia
Xiangliang Liu, The First Hospital of Jilin University, China
Copyright © 2024 Fu, Yue, Zou, Zhang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dianliang Zhang, qymr2014@163.com; Xinjian Wang, whchwangxj@126.com
 Jixin Fu1
Jixin Fu1