
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol. , 10 July 2024
Sec. Pediatric Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1401761
This article is part of the Research Topic Recent Advances in Diagnosis and Treatment of Brain Tumors: From Pediatrics to Adults View all 23 articles
 Cora Hedrich1†
Cora Hedrich1† Priya Patel2†
Priya Patel2† Lukas Haider3,4
Lukas Haider3,4 Tracey Taylor2
Tracey Taylor2 Elaine Lau2
Elaine Lau2 Roxanne Hook2
Roxanne Hook2 Christian Dorfer5
Christian Dorfer5 Karl Roessler5
Karl Roessler5 Natalia Stepien1
Natalia Stepien1 Maria Aliotti Lippolis1
Maria Aliotti Lippolis1 Hannah Schned1
Hannah Schned1 Clara Koeller1
Clara Koeller1 Lisa Mayr1
Lisa Mayr1 Amedeo A. Azizi1
Amedeo A. Azizi1 Andreas Peyrl1
Andreas Peyrl1 Bienvenido Ros Lopez6
Bienvenido Ros Lopez6 Alvaro Lassaletta7,8
Alvaro Lassaletta7,8 Julie Bennett9,10
Julie Bennett9,10 Johannes Gojo1*‡
Johannes Gojo1*‡ Ute Bartels9*‡
Ute Bartels9*‡Background: Children with craniopharyngiomas (CPs) typically suffer from a life-long chronic disease. The younger the child, the more vulnerable the maturing brain is to invasive therapies such as surgery or radiotherapy. Therefore, treatment modalities facilitating avoidance or delay of invasive therapies are beneficial for these patients. In the last decade, intracystic injection of interferon alfa-2a or alfa-2b evolved as a treatment of choice based on efficacy and minor toxicity. However, the drug is no longer available internationally. After an extensive pharmacological review, peginterferon alfa-2a was identified as the agent with closest similarity.
Methods: A retrospective case series is described, including five patients treated with intracystic peginterferon alfa-2a for cystic CP according to an innovative care protocol. After initial CP cyst aspiration, peginterferon alfa-2a was injected once per week via an Ommaya reservoir for 6 weeks followed by response assessment with MRI.
Results: Patients’ age ranged from 4 to 54 years (four patients <12 years, one adult patient). Intracystic therapy with peginterferon alfa-2a was tolerated well by all five individuals without any major toxicities and resulted in cyst shrinkage in all of the five patients. The importance of a permeability study prior to commencing intracystic therapy became apparent in one patient who suffered from cyst leakage.
Conclusions: Intracystic treatment with peginterferon alfa-2a was found to be a tolerable and efficacious treatment modality in patients with cystic CP. This experience warrants further research with a larger number of patients with measurement of long-term efficacy and safety outcomes.
Adamantinomatous craniopharyngioma (CP) accounts for approximately 5%–10% of pediatric brain tumors. Histologically, they are characterized as benign tumors (1). Gross total resection would represent a cure, but due to spatial proximity or invasion of the tumor to vital anatomical structures, such as the pituitary gland, the optic pathway, the circle of Willis, and the hypothalamus, radical resection could cause serious harm to these structures and, thus, is not generally considered of clinical benefit (2–8). Typical sequelae of surgery include visual impairment, stroke, loss of endocrine function with life-long dependency on hormonal substitution, and life-threatening situations including adrenal crisis or complex electrolyte imbalances and hypothalamic dysfunction, which can result in morbid obesity (3, 4, 8, 9). Another effective method of craniopharyngioma treatment is the use of radiotherapy with good response rates across previous studies (10–13). However, the proximity to the vital structures listed above may cause the same irreversible complications as surgery along with the characteristic long-term complications of radiotherapy, namely, decreased cognitive function, vasculopathies, e.g., Moya Moya, and increased risk of secondary neoplasms (14–20).
Craniopharyngiomas typically consist of solid, calcified, and cystic components (21). Cysts occur in more than 90% of the tumors, often encompassing a major part of the tumor bulk, causing impairment of important structures such as the optic chiasm or obstruction of cerebrospinal fluid circulation (22, 23). This feature is the basis for intracystic therapy, where the neurosurgeon inserts a catheter into the cyst and attaches it to a subgaleal Ommaya reservoir. Via Ommaya reservoir access, drugs can be directly administered into the cyst. In the last decade, interferon alfa-2a and alfa-2b evolved as the treatment of choice due to persuasive efficacy and minor toxicity (22, 24–28).
Interferon alfa-2a and alfa-2b was introduced in the 1950s as an antiviral therapy and has been found to have anti-proliferative activity through inhibition of the JAK/STAT/MAPK pathways and apoptosis through the FAS pathway (29–32).
Typical internationally accepted standard of care schemes such as the São Paulo series or the Toronto protocol consist of the administration of 3 million IU interferon alfa-2a or alfa-2b three times a week (Monday, Wednesday, and Friday) for 4 weeks that were designed as a cycle (24, 33).
Two different forms of interferon alfa, namely, interferon alfa-2a (Roferon®-A) and interferon alfa-2b (Intron® A), were previously available but discontinued due to availability of pegylated forms of interferon alfa for the licensed indications. Consequently, the drugs are no longer available.
Being used to the drawbacks of treating rare but very severe diseases with orphan therapies, we set out to find an alternative drug for intracystic therapy to treat children with craniopharyngioma appropriately.
In brief, a prefilled syringe of 180 mcg (1 ml) peginterferon alfa-2a is injected once weekly via an intracystically placed Ommaya reservoir for 6 weeks (one treatment cycle). At the start of treatment course (day 1), the maximum possible amount of cystic fluid (as patient tolerates) was slowly removed. At the following administrations, the maximal possible amount of fluid (at least 1.5–2 ml) was aspirated. More cycles can be added to obtain a maximal response.
Depending on the availability of MRI slots in the institution and the need of sedation, fast MRI sequences (see below) every 3 weeks can be performed as optional diagnostic follow-up (Figure 1).
A complete MRI examination (34) for the evaluation of solid tumor components and surrounding key structures was performed at least every 3 months. Notably, a contrast-enhanced MRI is necessary at initial diagnosis or if a solid component progressed, whereas for the evaluation of cyst size, the contrast agent can be dismissed.
The optional fast MRI sequences for response assessment were performed according to the recommendations of the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group and consist of at least three orthogonal T2-weighted sequences and conceivably additional sequences at the radiologist’s discretion for optimal assessment (35). Whenever applicable, cyst size was evaluated by the local radiologist by measurement of cyst size in three dimensions.
Regular neurological exams, vision exams, testing for the pituitary function, and anthropometric measures were performed at least every 3 months for comprehensive evaluation of response assessment.
Five patients from four different institutions (Medical University of Vienna, The Hospital for Sick Children, Princess Margaret Cancer Centre, and Hospital Infantil Universitario Niño Jesús) treated with intracystic peginterferon alfa-2a are included in this report. Data including patient demographics, symptoms at diagnosis or progression, side effects, MRI findings, and response to treatment were collected as standard of care.
In three of the five patients (patient 1, patient 3, and patient 5), the diagnosis of craniopharyngioma was confirmed by histopathology. The other two patients met classical radiological criteria, and the cyst aspiration demonstrated pathognomonic engine oil-like fluid.
Based on thorough reviews of each patients’ case within respective institutional interdisciplinary tumor boards, a suggestion of intracystic treatment was made, and informed consent was given by patient or patient’s legal guardian prior to commencing treatment with peginterferon alfa-2a.
A literature search was conducted in October 2020 in Ovid MEDLINE and Embase to determine if there was any published literature with intracystic administration of other interferon products for the treatment of craniopharyngioma. Search terms included interferon and craniopharyngioma, and studies published from 1980 and onwards were included in the search. In addition, a review of all available interferon products was undertaken to better understand the pharmaceutical differences and to make a recommendation for off-label intracystic administration. Based on this review, it was determined that the closest interferon product marketed in Canada to interferon-alfa2 was pegylated interferon (peginterferon)-alfa2a (Pegasys®). It was important to note that peginterferon alfa-2a and peginterferon alfa-2b were not interchangeable products as the polyethylene glycol (PEG) chain and bond to the interferon-alfa molecule differed (36). PEGylation is the process of attachment of PEG polymer chains to a molecule and therefore increasing its molecular weight and improving pharmaceutical properties such as the extension of therapy effect. The systemic clearance of peginterferon alfa-2a is approximately 100-fold lower in comparison to interferon alfa-2a. The terminal half-life of peginterferon is approximately 60–80 h after intravenous and 160 h in subcutaneous administration. The dosing that was suggested for peginterferon-alpha2a for craniopharyngioma (one treatment course encompasses once weekly cystic aspirations followed by injection of 180 mcg peginterferon alfa-2a via an Ommaya reservoir for 6 weeks) was extrapolated based on the conversion of subcutaneous hepatitis C dosing of non-pegylated interferon-alfa2a to peginterferon-alpha2a (i.e., peginterferon alfa2a 180 mcg/dose once weekly = interferon alfa2a 3 million units/dose three times per week). The pharmacy team also reviewed the formulation for compatibility with intracystic administration. Typically, formulations administered via an Ommaya reservoir or intrathecally are preservative-free and isotonic. The osmolality of peginterferon alpha-2a is 375–415 mOsmol/kg (slightly hypertonic) (personal communication with Roche, February 2021), and the product also contains benzyl alcohol (37) (preservative), which can cause transient paraplegia or neurotoxicity and polysorbate 80 (38) (solubilizing agent), which can cause allergic reactions. These ingredients would not typically make for an ideal formulation for intracystic administration. However, both of these ingredients are also found in interferon-alfa2a (39), which has been used intracystically across multiple patient series. Thus, we decided to offer intracystic administration with the peginterferon-alfa-2a product disclosing the potential risks to the patient and family. First experiences in five patients treated in exact accordance to the innovative care protocol at four different institutions are presented in this article.
Within an international collaboration, we compiled the first five cases treated with peginterferon alfa-2a (Table 1; Figures 2, 3) according to the same innovative care protocol for treatment guidance (Supplementary Material 1).
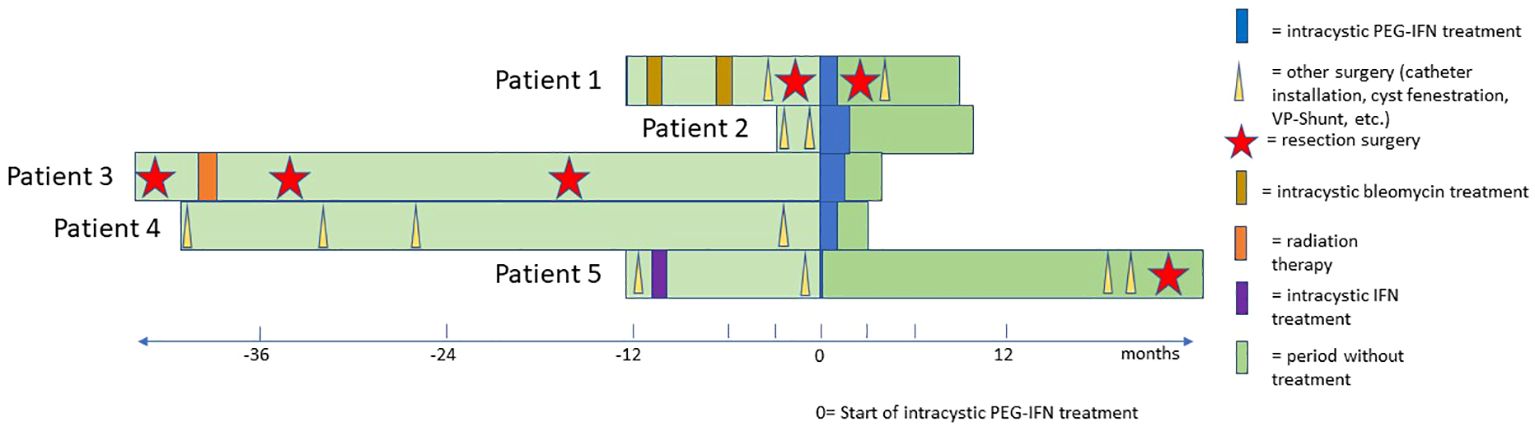
Figure 2 Swimmer’s plot that shows the individual treatment course of each patient from the initial diagnosis until the preparation of the manuscript. The vertical purple line represents the start of cystic Peg Interferon alfa treatment in each patient.
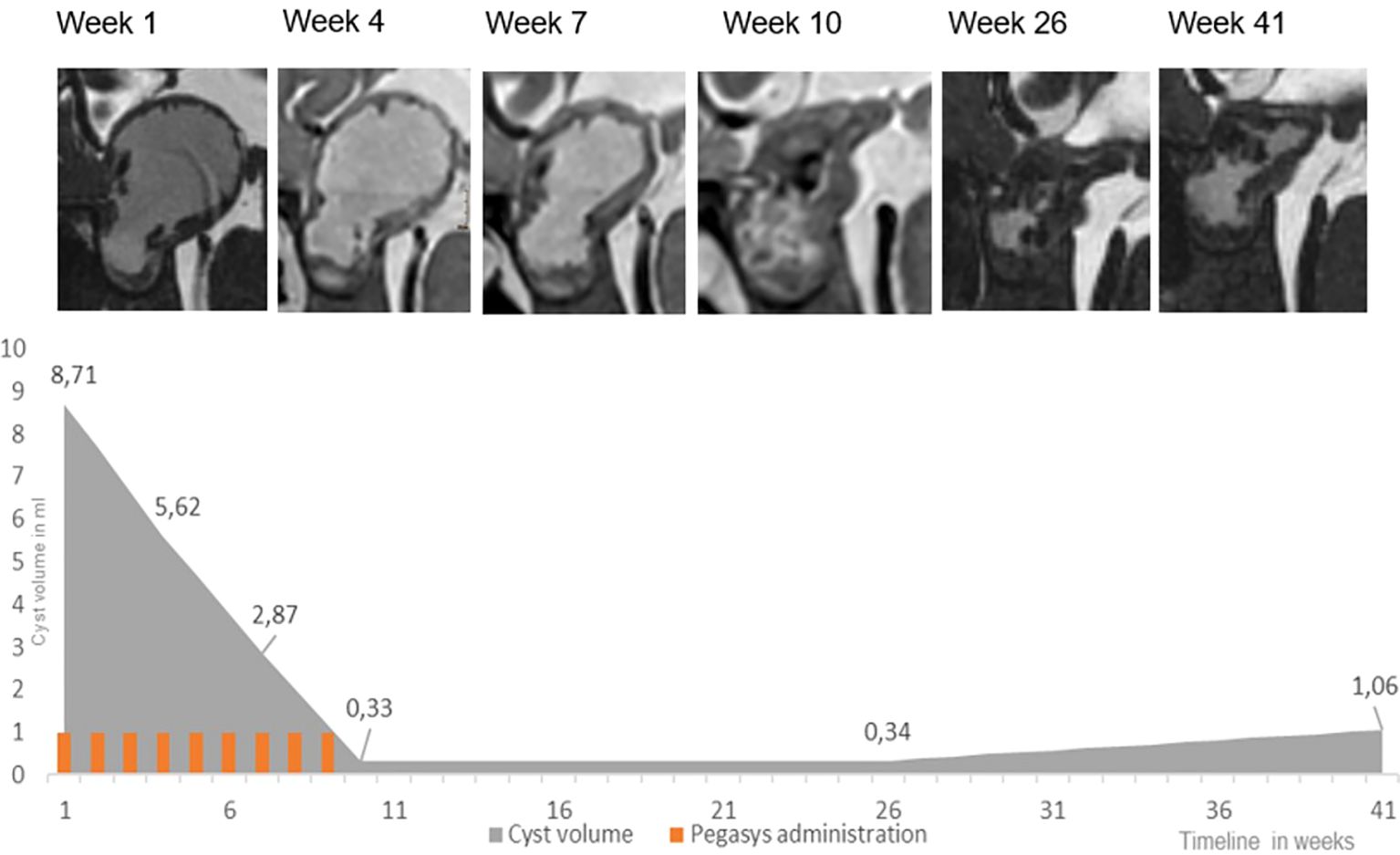
Figure 3 Peginterferon alfa-2a treatment in patient 2: high resolution, isotropic, strongly T2-weighted sequences, with 0.7 mm voxel size were acquired at 1.5 T using a Siemens Aera (CISS) and Philips Ingenia (BTFE). The lower panel indicates development of the cyst volume and treatment.
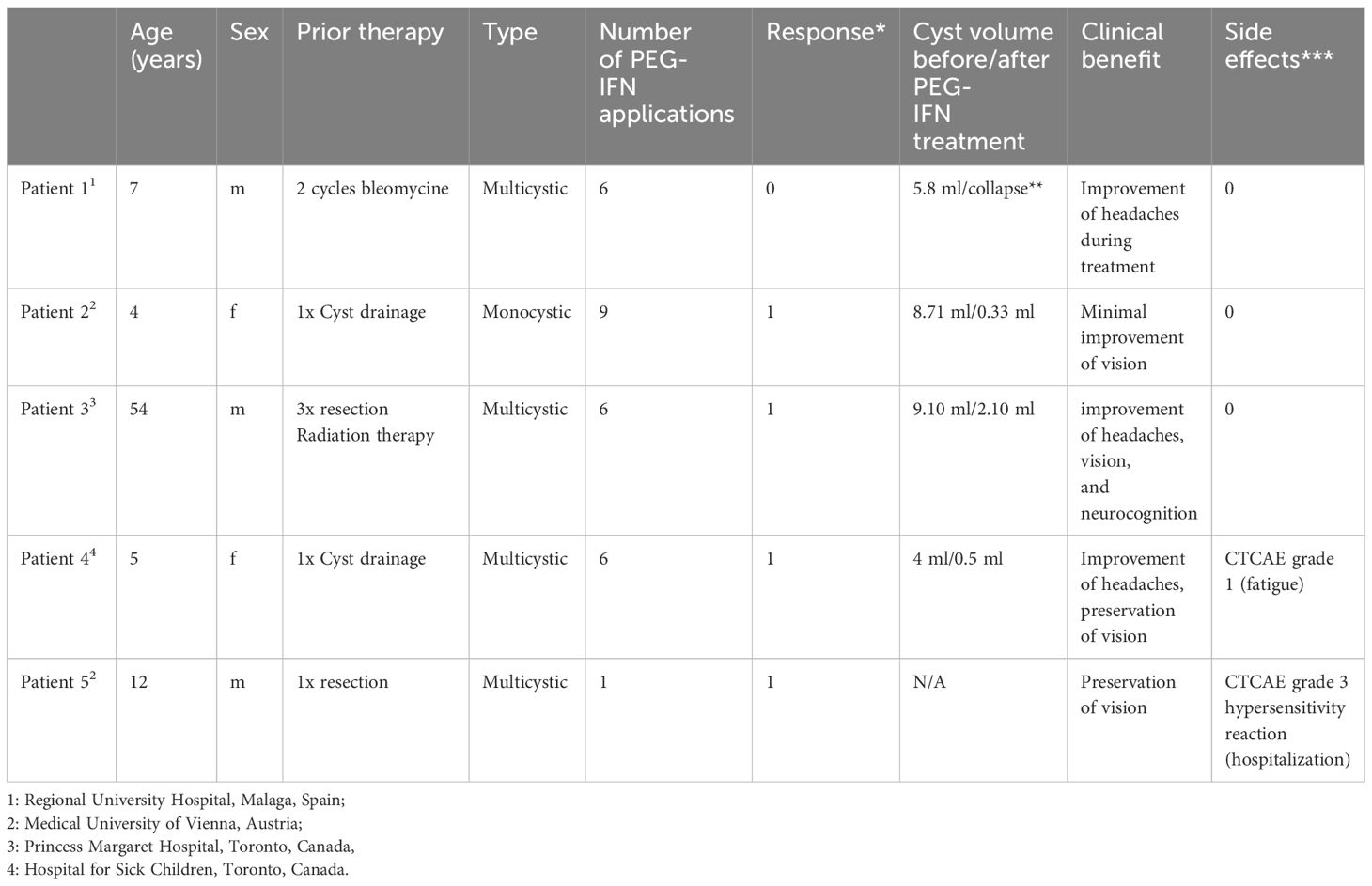
Table 1 * 0= stable disease or mixed response, 1= cyst shrinkage, 2= progression during therapy; **collapse of central multiloculated cyst but growth of small peripheral isolated cysts; ***0= no relevant side effects, Common Terminology Criteria for Adverse Events (CTCAE) Grade.
Patient 1 was a 7-year old boy with a CP who was previously treated with intracystic bleomycin consisting of two cycles in an interval of 6 months. Subsequently, a VP-shunt insertion and a pterional partial resection took place. Due to further cystic progression only 2 months later, he received intracystic peginterferon alfa-2a, which he tolerated well without any side effects. As a response to treatment, the central multiloculated cyst collapsed, but some small peripheral isolated cysts progressed.
Patient 2 was a 4-year-old patient who was newly diagnosed with a monocystic craniopharyngioma. A catheter was inserted to allow for aspiration of cyst fluid without the instillation of medication. This therapeutic effect was only short-lived, as the cyst returned to its original size after a month. In addition, visual acuity decreased during this interval (Table 1). At that time, no cyst fluid could be aspirated, leading to a catheter revision. Subsequently, treatment with intracystic peginterferon alfa-2a was given. The patient tolerated the therapy well, and only reported local skin pain in the area of the Ommaya reservoir and headache during the aspiration of the cyst. The residual cyst volume continuously decreased during treatment and shrunk to such a low volume, that we considered the therapy successfully completed after 9 injections (Figure 1). Four weeks after the first administration of peginterferon alfa-2a, the vision improved to a small extent and remained stable at last follow-up. The CP cyst remained stable in size for 41 weeks after the completion of the peginteferon alfa-2a therapy (Figure 3).
Patient 3 is a 54-year-old man who was histologically diagnosed with adamantinomatous CP. Initially, he underwent a tumor resection followed by 54 Gy in 30 fractions radiation therapy. He suffered from a local recurrence twice and received debulking surgery at 1 and 3 years after the initial diagnosis. The patient suffered a stroke after the third surgery, which led to right-sided paralysis, and he experienced another cystic progression within the same year. Despite cyst aspirations, the patient had nightly headaches and visual disturbance and demonstrated difficulties with memory and slow speech due to fast refilling of the cyst. Hence, a course of intracystic peginteferon alfa-2a was suggested. After the second injection, he experienced nausea. There were no further toxicities, and he tolerated all the other procedures well. Due to a vacation, he interrupted the therapy for 2 weeks during the 6-week cycle. He subjectively noted an improvement of the headaches and the vision. Neuropsychological testing showed amelioration of memory and speech function. The assessment at the treating institution reported a decrease in the cyst volume from 9.1 ml to 2.1 ml (total shrinkage of 77%). One month after completion of treatment, the MRI scan showed a re-accumulation of fluid in the cyst. The ophthalmic examination showed a significant worsening of the vision (right eye, 20/25; left eye, 20/200). He had temporary relief of symptoms with a cyst aspiration, and another course of intracystic peginterferon alfa-2a therapy has been initiated for this patient. In this patient, some clinical improvement may be attributed to the cyst aspirations alone. However, the interval between the interventions and relapse increased after peginterferon application.
Patient 4 is a 5-year-old girl who was treated with cyst drainage and later catheter insertion and catheter revision at the first months of diagnosis of her multicystic craniopharyngioma. When a new posterior cyst showed a progression after 3 years, a new catheter was inserted medial to the pre-existing frontal one. The patient started treatment with peginterferon alfa-2a 3 years after the initial diagnosis and received one cycle. Prior to treatment, the patient reported multiple severe episodes of headaches daily. In the course of the treatment, they improved significantly and decreased in frequency to only one to two times per week. During the treatment, the patient had some flu-like symptoms and fatigue. Noteworthy, the patient did not take her prescribed hydrocortisone substitution for several days due to the taste of the pill, which may have contributed to her fatigue.
Patient 5 was a 12-year-old boy who had previously received treatment with intracystic Interferon alfa-2a at initial diagnosis (three times per week for 4 weeks) with good response. One year later, the patient suffered cystic progression, and due to catheter dysfunction, an operative revision was performed to facilitate intracystic treatment. Two days after the first administration of peginteferon alfa-2a, the patient experienced a distinct systemic reaction with general skin rash and edema. The patient was immediately admitted to his local hospital where he was treated with corticosteroids and antihistamines and showed fast remission within hours. The intracystic therapy was discontinued in this patient. Retrospectively, the catheter was dislocated outside of the cyst due to intraoperative collapse resulting in the administration of the substance in the adjacent tissue inducing a systemic reaction. Subsequently, the CP remained stable for 19 months before a new cyst emerged again, necessitating surgical treatment and placement of a new catheter. This time, a permeability study was performed, indicating leakage of contrast media. Consequently, no intracystic treatment was administered, and a partial resection followed by proton beam therapy was performed.
Intracystic therapy with interferon alfa-2a has been used as an established modality in several institutions in the therapy of children with CP. Analysis of previous retrospective studies demonstrated an advantage in effectiveness and tolerability for intracystic interferon compared to other established therapies (22, 24–26, 31, 33). The largest trial on intratumoral interferon alfa-2a was published by the São Paulo team, who are also the pioneers in the development of intratumoral Interferon alfa-2a treatment (24). Clinical and radiological improvement was achieved in 76% of the 60 patients included (25). However, this review has no sufficient long-term follow-up data yet to inform on the duration of the cyst reduction and the time to subsequent progression.
A global, multicenter assessment on behalf of SIOPE and ISPN represents another broad clinical experience on intracystic therapy with Interferon-alfa including 56 children in this retrospective study (26). While treatment with the previously used (unpegylated) intracystic interferon had shown to delay the need for surgical resection or radiotherapy for a median time of 5.8 years, it is important to note that the long-term efficacy of treatment with intracystic peginterferon still needs to be proven within future clinical trials. The authors proposed a global, prospective randomized clinical trial of intracystic interferon in childhood CP, but the randomized trial concept was considered too expensive and prone to fail in this rare disease by funding resources. Hence, this case series fills an important gap and demonstrates the feasibility of an intracystic peginterferon alfa-2a regimen. It is relevant to address several limitations inherent in this study. First, the series includes only five patients. However, since craniopharyngioma is a rare disease and not all craniopharyngioma patients are suitable for intracystic treatment, we consider our experience of high importance to the neuro-oncology community. Second, the data exhibit a certain heterogeneity such as differences in the decision process on the indication for the treatment, the selection of suitable patients, the previous treatments of the patients, and the follow-up due to the nature of a retrospective study. Additionally, this report does not inform on the neurosurgical aspects to fulfill the requirement of a functional intracystic catheter, which may also limit applicability of intracystic treatment. Case 5 demonstrated the importance of pursuing a permeability study at least 2 weeks postoperatively and prior to the start of intracystic treatment to rule out leakage, most likely the cause of patient’s systemic toxicities (40). One case report of a 13-year-old patient treated with intracystic plus concomitantly subcutaneous pegylated interferon alfa-2b (41) described irreversible visual field loss and confirmed leakage from the intracystic catheter via computed tomographic imaging as the cause.
Herein, we present the first series of five patients treated with intracystic peginterferon alfa-2a. All patients showed prior progressive disease according to the new consensus guidelines of the Response Assessment in Pediatric Neuro-Oncology (RAPNO) committee indicated by change of cyst size and deterioration of a clinical parameter such as a new functional impairment or the need of surgical intervention (35). In general, intracystic treatment is considered particularly effective in monocystic CP. In multicystic CP, peripherical cysts may not communicate with the drained main cyst and are therefore not accessible to the intracystic treatment. This was demonstrated in patient 1 in whom the central multiloculated cyst collapsed, but a growth of small peripherical cysts was noted at the end of the treatment. A proactive approach towards intracystic therapy may postpone morbidities associated with surgical resection and irradiation as the insertion of a catheter is a low-risk operation (9), and there is a certain risk that newly developed functional impairments are not reversible once they occur. There is no doubt that delaying more aggressive surgical interventions and/or radiotherapy will be of significant benefit to young children and their maturing brains. This is in line with the main goal in treatment of CP as a chronic disease with a paradigm to maintain good quality of life (QoL) with minimally invasive intervention given the potential for substantial morbidity in the long-term outcomes of CP patients (21, 42–44).
While our findings prove the feasibility of intracystic peginterferon alfa-2a, no definite conclusions on the future significance can be made. To address these gaps, collaborative efforts across a large number of brain tumor centers must be made to design clinical trials with adherence to well-defined entry criteria, standardized treatment protocols, and interpretation of results by a reference center. With the recent advances in precision medicine, some potential targets such as IL-6, PD1/PD-L1, MEK, IDO-1, and others have been identified for the treatment of craniopharyngioma (45–47). For example, the CONNECT 1905 phase 2 study analyzes the effects in craniopharyngioma patients treated with systemic Tocilizumab, an IL-6 receptor antagonist that is approved for the treatment of arthritis. Additionally, bevacizumab has been shown to effectively reduce cyst size in selected cases (48). The local installation of medication as with intracystic peginteferon alfa-2a appears as an attractive alternative to avoid systemic, potentially persisting side effects, in young children.
An important advantage of the pegylated formula is the possibility of a weekly administration, which represents less applications compared to the previous non-pegylated interferon therapy. This factor represents an essential benefit for the child and its caregivers, as fewer procedures are required. The administration can be done in an outpatient setting. As a consequence, the child has fewer hospital visits and less interruptions in everyday life for a whole family. Lastly, if response is present but insufficient, additional cycles can be added as needed.
In this case series experience using peginterferon alfa-2a for intracystic treatment, its feasibility, tolerability, and response measured in cyst shrinkage were demonstrated. The results of this case series are encouraging that peginterferon could replace previous interferon formulations with the added benefit of less frequent administrations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Local ethical approval and informed consent was available at the respective institutions in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
CH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. PP: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. LH: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization. TT: Conceptualization, Investigation, Writing – review & editing. EL: Conceptualization, Investigation, Validation, Writing – review & editing. RH: Conceptualization, Investigation, Writing – review & editing. CD: Conceptualization, Formal analysis, Methodology, Validation, Writing – review & editing. KR: Validation, Writing – review & editing. NS: Data curation, Investigation, Software, Validation, Visualization, Writing – review & editing. ML: Data curation, Investigation, Visualization, Writing – review & editing. HS: Data curation, Investigation, Project administration, Writing – review & editing. CK: Data curation, Investigation, Writing – review & editing. LM: Data curation, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. AA: Data curation, Investigation, Methodology, Validation, Writing – review & editing. AP: Data curation, Investigation, Methodology, Validation, Writing – review & editing. BR: Data curation, Investigation, Writing – review & editing. AL: Data curation, Investigation, Writing – review & editing. JB: Data curation, Investigation, Writing – review & editing. JG: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. UB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the “Verein unser_kind” (JG) and the “Forschungsgesellschaft für Cerebrale Tumore”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1401761/full#supplementary-material
1. Magill ST, Jane JA, Prevedello DM. Editorial. Craniopharyngioma classification. J Neurosurg. (2021) 135:1293–5. doi: 10.3171/2020.8.JNS202666
2. Caldarelli M, Massimi L, Tamburrini G, Cappa M, Di Rocco C. Long-term results of the surgical treatment of craniopharyngioma: the experience at the Policlinico Gemelli, Catholic University, Rome. Childs Nerv Syst. (2005) 21:747–57. doi: 10.1007/s00381-005-1186-5
3. Cheng J, Shao Q, Pan Z, You J. Analysis and long-term follow-up of the surgical treatment of children with craniopharyngioma. J Craniofac Surg. (2016) 27:e763–6. doi: 10.1097/SCS.0000000000003176
4. Di Rocco C, Caldarelli M, Tamburrini G, Massimi L. Surgical management of craniopharyngiomas–experience with a pediatric series. J Pediatr Endocrinol Metab. (2006) 19 Suppl 1:355–66.
5. Komotar RJ, Roguski M, Bruce JN. Surgical management of craniopharyngiomas. J Neurooncol. (2009) 92:283–96. doi: 10.1007/s11060-009-9841-4
6. Müller HL. Childhood craniopharyngioma: current controversies on management in diagnostics, treatment and follow-up. Expert Rev Neurother. (2010) 10:515–24. doi: 10.1586/ern.10.15
7. Prieto R, Rosdolsky M, Hofecker V, Barrios L, Pascual JM. Craniopharyngioma treatment: an updated summary of important clinicopathological concepts. Expert Rev Endocrinol Metab. (2020) 15:261–82. doi: 10.1080/17446651.2020.1770081
8. Webb KL, Pruter WW, Hinkle ML, Walsh MT. Comparing surgical approaches for craniopharyngioma resection among adults and children: a meta-analysis and systematic review. World Neurosurg. (2023) 175:e876–e896. doi: 10.1016/j.wneu.2023.04.037
9. Lohkamp LN, Kulkarni AV, Drake JM, Rutka JT, Dirks PB, Taylor M, et al. Preservation of endocrine function after Ommaya reservoir insertion in children with cystic craniopharyngioma. J Neurooncol. (2022) 159:597–607. doi: 10.1007/s11060-022-04099-0
10. Clark AJ, Cage TA, Aranda D, Parsa AT, Sun PP, Auguste KI, et al. A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst. (2013) 29:231–8. doi: 10.1007/s00381-012-1926-2
11. Lo AC, Howard AF, Nichol A, Sidhu K, Abdulsatar F, Hasan H, et al. Long-term outcomes and complications in patients with craniopharyngioma: the British Columbia Cancer Agency experience. Int J Radiat Oncol Biol Phys. (2014) 88:1011–8. doi: 10.1016/j.ijrobp.2014.01.019
12. Minniti G, Esposito V, Amichetti M, Enrici RM. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg Rev. (2009) 32:125–32. doi: 10.1007/s10143-009-0186-4
13. Schoenfeld A, Pekmezci M, Barnes MJ, Tihan T, Gupta N, Lamborn KR, et al. The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J Neurooncol. (2012) 108:133–9. doi: 10.1007/s11060-012-0806-7
14. Hess CB, Thompson HM, Benedict SH, Seibert JA, Wong K, Vaughan AT, et al. Exposure risks among children undergoing radiation therapy: considerations in the era of image guided radiation therapy. Int J Radiat Oncol Biol Phys. (2016) 94:978–92. doi: 10.1016/j.ijrobp.2015.12.372
15. Karapinar E, Varkal MA, Saka N. LONG-TERM THYROID DISORDERS IN CHILDREN RECEIVING ONCOLOGIC TREATMENT AND RADIOTHERAPY. Acta Endocrinol (Buchar). (2022) 18:429–35. doi: 10.4183/aeb.2022.429
16. Lassaletta Á, Morales JS, Valenzuela PL, Esteso B, Kahalley LS, Mabbott DJ, et al. Neurocognitive outcomes in pediatric brain tumors after treatment with proton versus photon radiation: a systematic review and meta-analysis. World J Pediatr. (2023) 19:727–40. doi: 10.1007/s12519-023-00726-6
17. Leary JB, Anderson-Mellies A, Green AL. Population-based analysis of radiation-induced gliomas after cranial radiotherapy for childhood cancers. Neurooncol Adv. (2022) 4:vdac159. doi: 10.1093/noajnl/vdac159
18. Merchant TE, Hoehn ME, Khan RB, Sabin ND, Klimo P, Boop FA, et al. Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): a single-arm, phase 2 study. Lancet Oncol. (2023) 24:523–34. doi: 10.1016/S1470-2045(23)00146-8
19. Steinbok P. Craniopharyngioma in children: long-term outcomes. Neurol Med Chir (Tokyo). (2015) 55:722–6. doi: 10.2176/nmc.ra.2015-0099
20. Visser J, Hukin J, Sargent M, Steinbok P, Goddard K, Fryer C. Late mortality in pediatric patients with craniopharyngioma. J Neurooncol. (2010) 100:105–11. doi: 10.1007/s11060-010-0145-5
21. Otte A, Müller HL. Childhood-onset craniopharyngioma. J Clin Endocrinol Metab. (2021) 106:e3820–36. doi: 10.1210/clinem/dgab397
22. Steinbok P, Hukin J. Intracystic treatments for craniopharyngioma. Neurosurg Focus. (2010) 28:E13. doi: 10.3171/2010.1.FOCUS09315
23. Backlund EO. Treatment of craniopharyngiomas: the multimodality approach. Pediatr Neurosurg. (1994) 21 Suppl 1:82–9. doi: 10.1159/000120867
24. Cavalheiro S, Dastoli PA, Silva NS, Toledo S, Lederman H, da Silva MC. Use of interferon alpha in intratumoral chemotherapy for cystic craniopharyngioma. Childs Nerv Syst. (2005) 21:719–24. doi: 10.1007/s00381-005-1226-1
25. Cavalheiro S, Di Rocco C, Valenzuela S, Dastoli PA, Tamburrini G, Massimi L, et al. Craniopharyngiomas: intratumoral chemotherapy with interferon-alpha: a multicenter preliminary study with 60 cases. Neurosurg Focus. (2010) 28:E12. doi: 10.3171/2010.1.FOCUS09310
26. Kilday JP, Caldarelli M, Massimi L, Chen RHH, Lee YY, Liang ML, et al. Intracystic interferon-alpha in pediatric craniopharyngioma patients: an international multicenter assessment on behalf of SIOPE and ISPN. Neuro Oncol. (2017) 19:1398–407. doi: 10.1093/neuonc/nox056
27. Lohkamp LN, Kasper EM, Pousa AE, Bartels UK. An update on multimodal management of craniopharyngioma in children. Front Oncol. (2023) 13:1149428. doi: 10.3389/fonc.2023.1149428
28. Pancucci G, Massimi L, Caldarelli M, D’Angelo L, Sturiale C, Tamburrini G, et al. [Pediatric craniopharyngioma: long-term results in 61 cases]. Minerva Pediatr. (2007) 59:219–31.
29. Asmana Ningrum R. Human interferon alpha-2b: A therapeutic protein for cancer treatment. Scientifica. (2014) 2014:e970315. doi: 10.1155/2014/970315
30. Ierardi DF, Fernandes MJS, Silva IR, Thomazini-Gouveia J, Silva NS, Dastoli P, et al. Apoptosis in alpha interferon (IFN-α) intratumoral chemotherapy for cystic craniopharyngiomas. Childs Nerv Syst. (2007) 23:1041–6. doi: 10.1007/s00381-007-0409-3
31. Mrowczynski OD, Langan ST, Rizk EB. Craniopharyngiomas: A systematic review and evaluation of the current intratumoral treatment landscape. Clin Neurol Neurosurg. (2018) 166:124–30. doi: 10.1016/j.clineuro.2018.01.039
32. Pettorini BL, Inzitari R, Massimi L, Tamburrini G, Caldarelli M, Fanali C, et al. The role of inflammation in the genesis of the cystic component of craniopharyngiomas. Childs Nerv Syst. (2010) 26:1779–84. doi: 10.1007/s00381-010-1245-4
33. Bartels U. Intracystic therapies for cystic craniopharyngioma in childhood. Front Endocrinol. (2012) 3:39. doi: 10.3389/fendo.2012.00039
34. Avula S, Peet A, Morana G, Morgan P, Warmuth-Metz M, Jaspan T, et al. European Society for Paediatric Oncology (SIOPE) MRI guidelines for imaging patients with central nervous system tumours. Childs Nerv Syst. (2021) 37:2497–508. doi: 10.1007/s00381-021-05199-4
35. Hoffman LM, Jaimes C, Mankad K, Mirsky DM, Tamrazi B, Tinkle CL, et al. Response assessment in pediatric craniopharyngioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Neuro Oncol. (2023) 25:224–33. doi: 10.1093/neuonc/noac221
36. Foster GR. Review article: pegylated interferons: chemical and clinical differences. Aliment Pharmacol Ther. (2004) 20:825–30. doi: 10.1111/j.1365-2036.2004.02170.x
37. Felix J. PRODUCT MONOGRAPH. Pegasys®. Health Canada Product Monograph. Hoffmann-La Roche Limited. Date of Revision: November 18 2015. Accessed January 10 2021.
38. Roche | Pegasys (peginterferon α) (2023). Available online at: https://www.roche.com/solutions/pharma/productid-692190d1-d5ba-4400-9b42-ae76668123ca.
39. Drugs.com (2023). Available online at: https://www.drugs.com/pro/roferon-a.html.
40. Sharma J, Bonfield CM, Singhal A, Hukin J, Steinbok P. Intracystic interferon-α treatment leads to neurotoxicity in craniopharyngioma: case report. J Neurosurg: Pediatr. (2015) 16:301–4. doi: 10.3171/2015.2.PEDS14656
41. Tiedemann LM, Manley P, Smith ER, Dagi LR. Visual field loss in a case of recurrent cystic craniopharyngioma during concomitant treatment with pegylated interferon α-2b. J Pediatr Hematology/Oncol. (2016) 38:e26. doi: 10.1097/MPH.0000000000000468
42. Heinks K, Boekhoff S, Hoffmann A, Warmuth-Metz M, Eveslage M, Peng J, et al. Quality of life and growth after childhood craniopharyngioma: results of the multinational trial KRANIOPHARYNGEOM 2007. Endocrine. (2018) 59:364–72. doi: 10.1007/s12020-017-1489-9
43. Klages KL, Berlin KS, Cook JL, Merchant TE, Wise MS, Mandrell BN, et al. Health-related quality of life, obesity, fragmented sleep, fatigue, and psychosocial problems among youth with craniopharyngioma. Psychooncology. (2022) 31:779–87. doi: 10.1002/pon.5862
44. Laffond C, Dellatolas G, Alapetite C, Puget S, Grill J, Habrand JL, et al. Quality-of-life, mood and executive functioning after childhood craniopharyngioma treated with surgery and proton beam therapy. Brain Inj. (2012) 26:270–81. doi: 10.3109/02699052.2011.648709
45. Grob S, Mirsky DM, Donson AM, Dahl N, Foreman NK, Hoffman LM, et al. Targeting IL-6 is a potential treatment for primary cystic craniopharyngioma. Front Oncol. (2019) 9:791. doi: 10.3389/fonc.2019.00791
46. Whelan R, Hengartner A, Folzenlogen Z, Prince E, Hankinson TC. Adamantinomatous craniopharyngioma in the molecular age and the potential of targeted therapies: a review. Childs Nerv Syst. (2020) 36:1635–42. doi: 10.1007/s00381-020-04677-5
47. Study Details | Nivolumab and Tovorafenib for Treatment of Craniopharyngioma in Children and Young Adults. ClinicalTrials.gov (202). Available at: https://www.clinicaltrials.gov/study/NCT05465174?cond=Craniopharyngioma&rank=1#locations.
Keywords: craniopharyngioma, intracystic treatment, peginterferon alfa-2a, pediatric neurooncology, pediatric neurosurgery
Citation: Hedrich C, Patel P, Haider L, Taylor T, Lau E, Hook R, Dorfer C, Roessler K, Stepien N, Lippolis MA, Schned H, Koeller C, Mayr L, Azizi AA, Peyrl A, Lopez BR, Lassaletta A, Bennett J, Gojo J and Bartels U (2024) Feasibility, tolerability, and first experience of intracystic treatment with peginterferon alfa-2a in patients with cystic craniopharyngioma. Front. Oncol. 14:1401761. doi: 10.3389/fonc.2024.1401761
Received: 15 March 2024; Accepted: 17 June 2024;
Published: 10 July 2024.
Edited by:
John Bianco, Princess Maxima Center for Pediatric Oncology, NetherlandsReviewed by:
Emmanuel Jouanneau, Hospices Civils de Lyon, FranceCopyright © 2024 Hedrich, Patel, Haider, Taylor, Lau, Hook, Dorfer, Roessler, Stepien, Lippolis, Schned, Koeller, Mayr, Azizi, Peyrl, Lopez, Lassaletta, Bennett, Gojo and Bartels. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes Gojo, am9oYW5uZXMuZ29qb0BtZWR1bml3aWVuLmFjLmF0; Ute Bartels, dXRlLmJhcnRlbHNAc2lja2tpZHMuY2E=
†These authors share first authorship
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.