- 1Division of Pediatric Hematology-Oncology, Cancer Care Manitoba, Winnipeg, MB, Canada
- 2Department of Pediatrics and Child Health, University of Manitoba, Winnipeg, MB, Canada
- 3Division of Nursing, Cancer Care Manitoba, Winnipeg, MB, Canada
- 4Division of Pharmacy, Cancer Care Manitoba, Winnipeg, MB, Canada
- 5Section of Neuro-Surgery, University of Manitoba, Winnipeg, MB, Canada
- 6Department of Radiology, University of Manitoba, Winnipeg, MB, Canada
- 7Section of Orthopedic Surgery, University of Manitoba, Winnipeg, MB, Canada
- 8Department of Pathology, University of Manitoba, Winnipeg, MB, Canada
- 9Paul Albrechtsen Research Institute, Cancer Care Manitoba, Winnipeg, MB, Canada
We report a case of slipped capital femoral epiphysis (SCFE), an on target skeletal toxicity of a pan-FGFR TKI inhibitor, erdafitinib. A 13-year-old boy was diagnosed to have an optic pathway/hypothalamic glioma with signs of increased intracranial pressure and obstructive hydrocephalus requiring placement of ventriculo-peritoneal (VP) shunt. Sequencing of the tumor showed FGFR1-tyrosine kinase domain internal tandem duplication (FGFR1-KD-ITD). He developed hypothalamic obesity with rapid weight gain and BMI >30. At 12 weeks of treatment with erdafitinib, he developed persistent knee pain. X-ray of the right hip showed SCFE. Erdafitinib was discontinued, and he underwent surgical pinning of the right hip. MRI at discontinuation of erdafitinib showed a 30% decrease in the size of the tumor, which has remained stable at 6 months follow-up. Our experience and literature review suggest that pediatric patients who are treated with pan-FGFR TKIs should be regularly monitored for skeletal side effects.
Introduction
The cell surface receptor tyrosine kinases (RTKs) regulate fundamental cellular processes like cellular proliferation, differentiation, and survival through their signaling (1). Fibroblast growth factor receptor (FGFR) tyrosine kinase pathway signaling plays an important role in normal growth and development including metabolism and skeletal homeostasis (2). FGFR1–4 pathway alterations are rare in pediatric cancers (1%–3%) but are enriched in sarcomas and CNS tumors (3) where they account for 10% of pediatric low-grade gliomas and 4% of pediatric high-grade gliomas (4). FGFR tyrosine kinase inhibitors (FGFR-TKIs) are being increasingly used off-label in children with hard-to-treat tumors harboring specific FGFR alterations. This case report illustrates a rare but significant skeletal side effect of erdafitinib—a pan-FGFR inhibitor (2). Our case is an important addition to the recent literature (5) and informs pediatric oncologists to be monitoring for skeletal side effects when treating with FGFR-TKIs.
Case summary
A previously healthy 13-year-old boy presented to the emergency department (ED) with 1 day history of emesis and headaches. His initial neurological exam was unremarkable. He progressively became unresponsive in the ED, showing signs of increased intracranial pressure with bradycardia, non-reactive pupils, and urinary incontinence. An urgent computerized tomography (CT) scan of the head showed a large suprasellar mass with mass effect on foramen of Munro bilaterally with obstructive hydrocephalus. The mass extended into the region of the Sylvian aqueduct resulting in CSF obstruction, which was relieved by placement of bilateral external ventricular drains (EVDs). On further query, the father reported that the patient has been symptomatic with intermittent headaches associated with nausea and vomiting for the past 6 months.
After stabilization, baseline MRI showed rim-enhancing mass arising from the optic chiasm with mass effect on the pituitary infundibulum and perilesional edema involving the splayed cerebral peduncles and bilateral hypothalamus (Figures 1A–C). He was treated with Dexamethasone (1 mg, IV, QID) for increased intracranial pressure over 6 days and subsequently weaned off steroids over a week. Baseline investigations including renal/liver functions and endocrine tests including pituitary and thyroid functions tests were normal except for TSH of 0.3 mU/L (0.7–5.7 mU/L) with normal Free T3 and Free T4 values. The TSH values normalized in the subsequent follow-up visits. A ventriculo-peritoneal shunt was placed in the right lateral ventricle. Biopsy showed a histologically low-grade astrocytoma (WHO, Grade 1). TruSight RNA Pan-Cancer NGS panel showed FGFR1-tyrosine kinase domain internal tandem duplication (FGFR1-KD-ITD) (chromosome 8, exon 18–exon 10). The tumor was negative for BRAF mutation/fusion. Two weeks after biopsy, he presented to the ED with 1-week history of abdominal pain and was diagnosed with abdominal abscess due to VP shunt infection. The shunt was removed. CSF cultures grew Staphylococcus aureus (methicillin sensitive, MSSA). He was subsequently treated with IV antibiotics for 6 weeks.
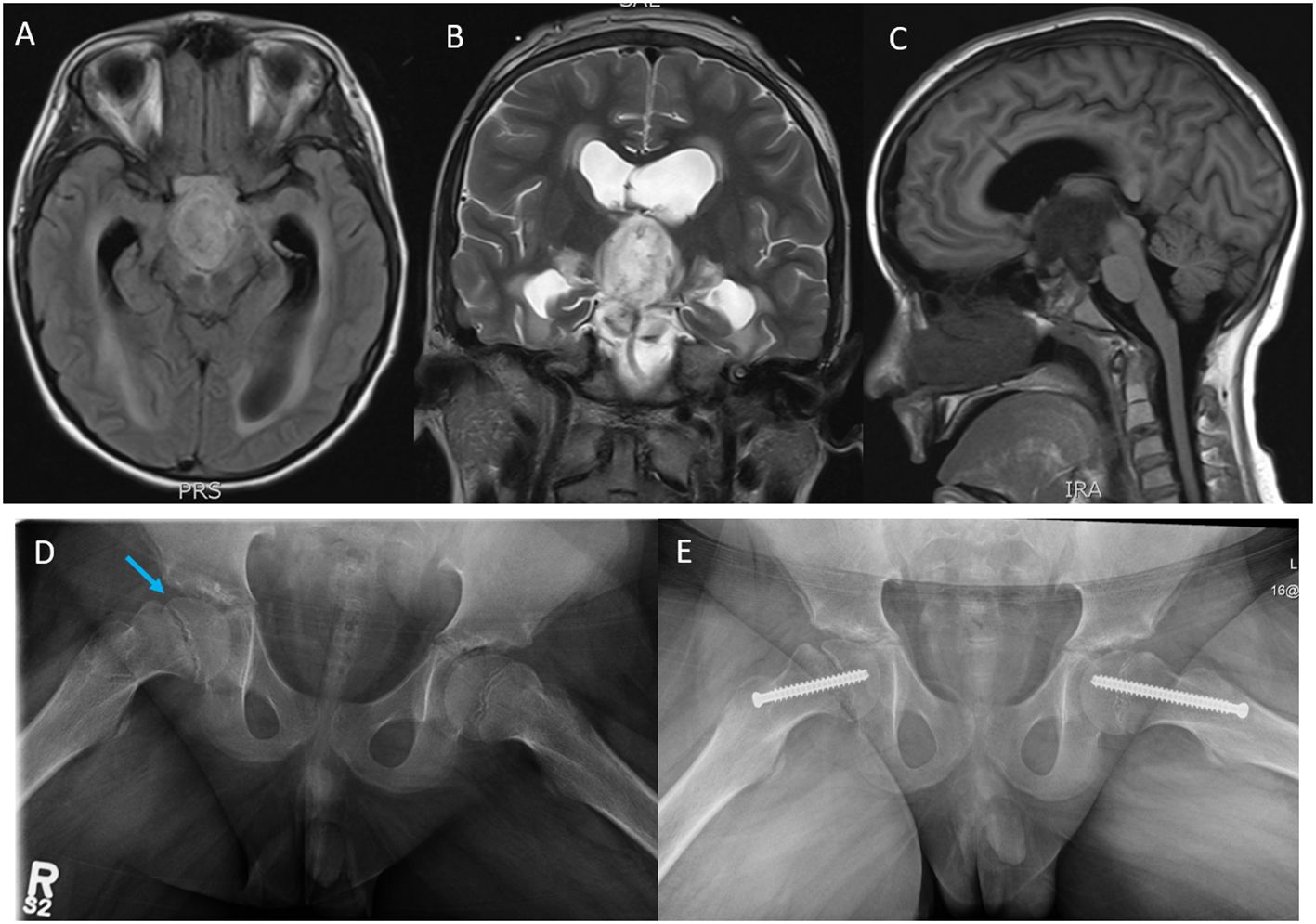
Figure 1 Magnetic resonance imaging (MRI); (A) axial T1 flair, (B) coronal T2, and (C) sagittal T1 flair images showing a rim-enhancing tumor mass likely arising from the Optic chiasm and extending into the third ventricle, the prepontine cistern, the interpeduncular cistern intermittent to the optic chiasm measuring 3.0 cm (AP) × 2.7 cm (CC) ×1.8 cm (T). (D) Plain X-ray films, open frog leg view showing inferior displacement of the right femoral capital epiphysis (blue arrow) relative to the femoral neck associated with widening of the proximal femoral growth plate and minimal joint effusion. (E) Surgical pinning to stabilize the capital epiphysis on the right hip and prophylactic pinning to prevent SCFE on the left hip.
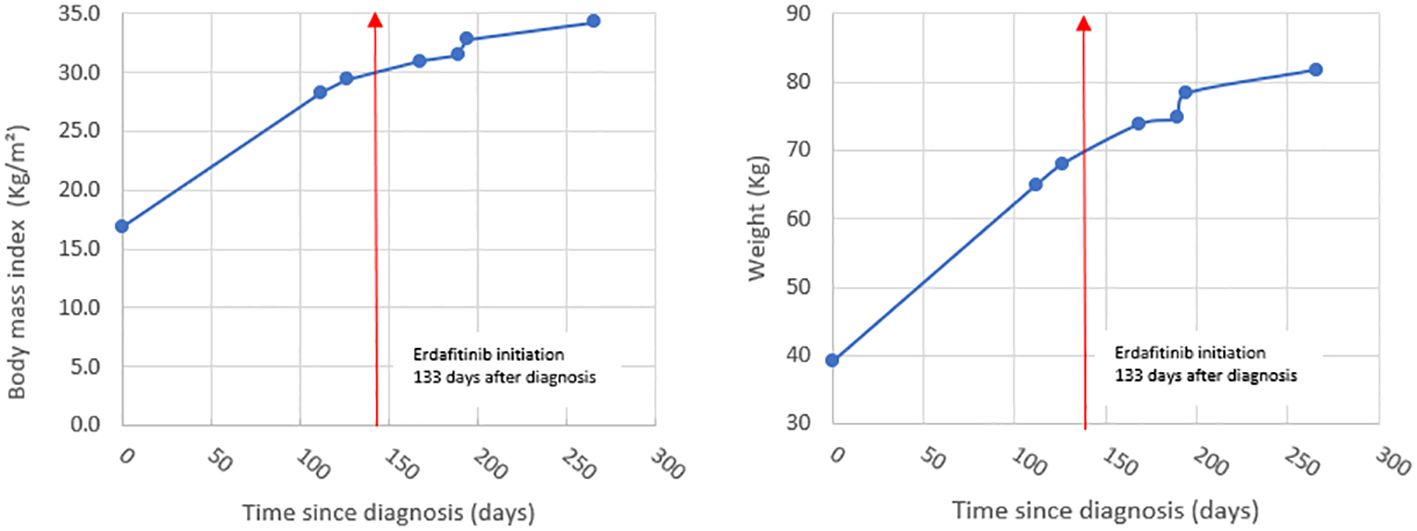
Our patient developed hypothalamic obesity due to the location of the tumor and the perilesional edema involving the hypothalamus at presentation (Figure 1C). This was clinically manifested by a voracious insatiable appetite and accelerated rate of weight gain (82.1 kg->97% percentile for age and sex) (Figure 2). After a 4-month delay from the initial diagnosis, he was started on erdafitinib 4.7mg/m2/day (Janssen BioAdvance), a pan-FGFR1–4 inhibitor, through a special access program. Four weeks later, he developed nail side effects (discoloration and brittleness) and hyperphosphatemia requiring chelation. Erdafitinib was withheld for a week and restarted as per protocol. At approximately 7 weeks into therapy, he started complaining of intermittent pain in his right leg (knee and ankles), progressing to limping, difficult weight bearing, and complete cessation of ambulation. There was no history of trauma or past history of knee, leg, or hip pain. X-rays of the knee/ankles were reported as normal. At approximately 12 weeks of treatment, the persistent knee pain was suspected to be due to pathology of the hip with referred pain to the knee. X-ray of the hips (Figure 1D) showed slipped capital femoral epiphysis (SCFE) of the right hip. Erdafitinib was discontinued, and he underwent surgery with in situ pinning of the right hip and prophylactic pinning of the left hip (to prevent SCFE) (Figure 1E). MRI of the brain at the time of discontinuation of the medication showed a 30% decrease in the size of the tumor, which has remained stable on two subsequent follow-up MRIs.
Discussion
FGFR1–4 inhibitors are being frequently used for hard-to-treat pediatric cancers with FGFR alterations in both ongoing clinical trials (NCI-COG-Pediatric MATCH trial, Phase II, NCT03155620) (6) and off-label (3, 7). FGFR1 and FGFR3 signaling negatively regulates endochondral bone growth by inhibiting growth plate chondrocytes by suppressing mitogenic activity (8, 9). Inhibition of these receptors causes increased linear growth velocity in growing/immature skeleton and predisposes to bone- and joint-related complications (5). These complications are rare in adults, as their growth plates are fused resulting in minimal FGFR signaling in growth plate chondrocytes.
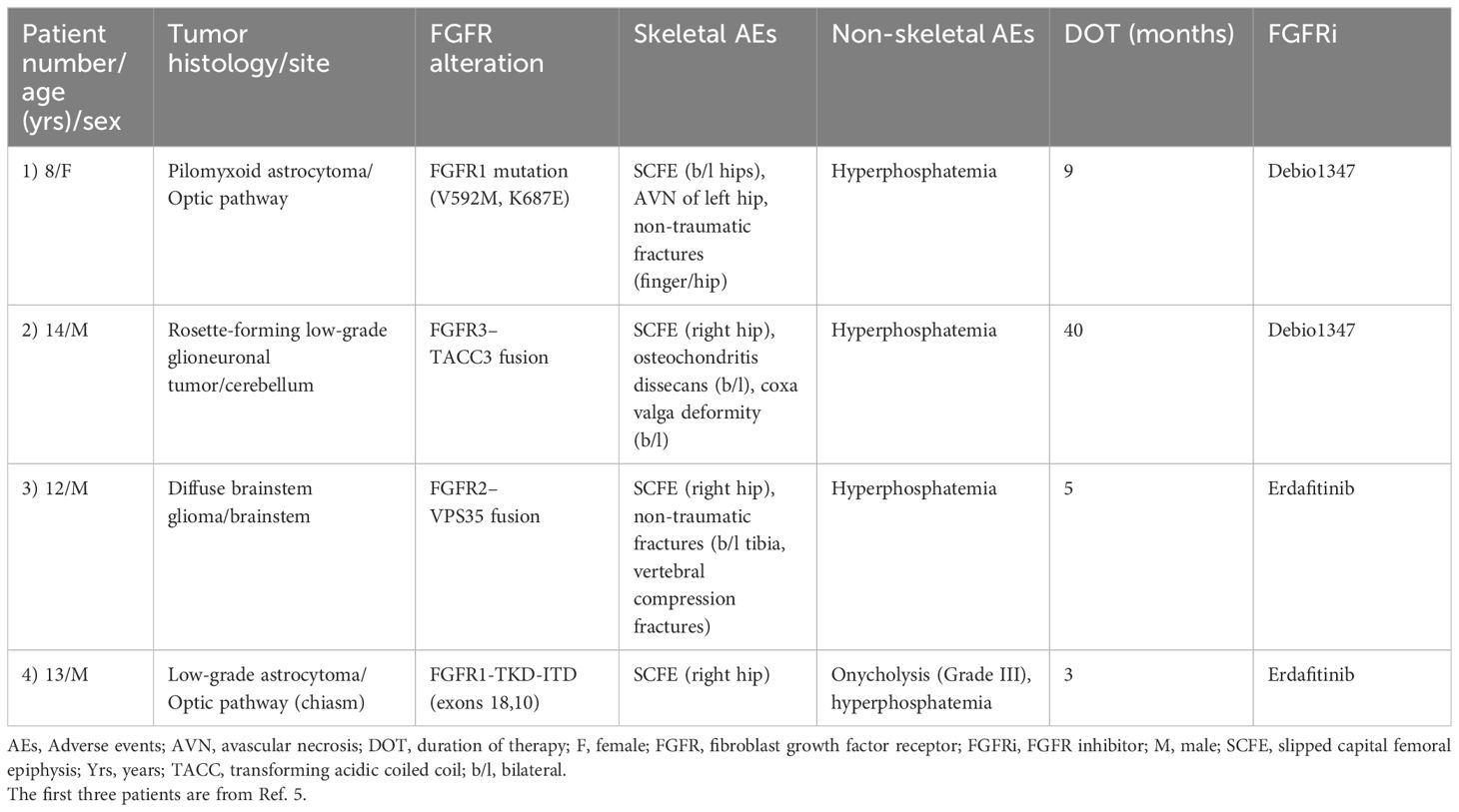
Tyrosine kinase inhibitors are increasingly being used in the treatment of pediatric cancers, and TKI-induced adverse effects have been frequently reported involving multiple organs, most commonly skin, gastrointestinal tract, blood, and cardiac side effects (10). Skeletal side effects of targeted therapies (TKIs, biological therapies) like osteonecrosis and fractures are summarized in a recent review by Konarski et al. (11). SCFE and other skeletal side effects as a complication of FGFR-selective inhibitors was recently reported by Sait et al. (5). In their series, all the three pediatric patients with SCFE had increased growth velocity (Table 1). Two of the three patients were also reported to have obesity. We did not see increased growth velocity in our patient (height between 25th and 50th percentile for age and sex). This could be probably explained by the short duration of erdafitinib treatment in our patient (12 weeks). The unfortunate delay in starting erdafitinib due to MSSA infection resulted in our patient being obese (BMI> 30, >99th percentile for age and sex) at the start of the drug. Obesity has been shown to be a strong and validated risk factor for SCFE (12, 13). Body Mass Index (BMI) >95th percentile for age and sex has a strong correlation with SCFE when compared to children with BMI <85th percentile for age and sex (14). Based on the rate of weight gain and the BMI, we decided to do prophylactic in situ pinning for the contralateral (left) hip to prevent SCFE (14).
Pan-FGFR1–4 inhibitors (like erdafitinib) are pharmacologically more potent when compared to FGFR1/2/3 inhibitors (like Debio1347): IC50 values (in nanomolar, nM) for erdafitinib and Debio1347 are as follows: FGFR1 (2.0 nM vs. 9.3 nM), FGFR2 (2.0 nM vs. 7.6 nM), and FGFR3 (4.0 nM vs. 22 nM) (15). The increased potency of erdafitinib in combination with other risk factors like obesity and hyperphosphatemia could probably explain the relatively early onset of SCFE in patients treated with Pan-FGFR1–4 inhibitor erdafitinib when compared to FGFR1/2/3 inhibitor Debio1347 (Table 1). The dose of erdafitinib for both patients in this series was based on the recommendations of the NCI-COG Pediatric MATCH, APEC1621B protocol, which is 4.7 mg/m2/day (8 mg/1.7 m2) up to a maximum daily dose of 8 mg, orally, once daily (adult recommended phase II dose-RP2D adjusted for BSA).
Hyperphosphatemia, another on-target toxicity specific to FGFR TKIs, was seen in all the patients with SCFE (Table 1). Hyperphosphatemia can potentially contribute to the SCFE, as it can lead to increased bone turnover and bone fragility (16). Our patient did not receive any chemotherapy or radiation prior to starting erdafitinib. Patient 1 in the series was previously treated with chemotherapy (7), and this could have contributed to the SCFE in this patient (17).
Summary
We report a case of SCFE, an on-target skeletal toxicity of a pan-FGFR TKI inhibitor, erdafitinib. Central hypothalamic obesity, secondary to the location of the tumor along with hyperphosphatemia due to drug side effect, contributed to the accelerated onset of SCFE in our patient. Based on our experience and literature review (5) of pediatric patients who are treated with pan-FGFR TKIs, we recommend the following: a) monitor both rate of weight gain/BMI for obesity (especially in suprasellar tumors) and growth velocity while on therapy; b) promptly rule out on-target skeletal toxicities (fractures, SCFE, and osteochondritis) at the onset of clinical symptoms (knee, thigh, or groin pain) with appropriate diagnostic imaging (AP/frog leg lateral views of hip and knee X-rays); c) regular surveillance imaging of the hips to monitor for SCFE especially in pediatric patients with increased weight gain (suprasellar tumors) and/or growth velocity; and d) update the drug toxicity profile of FGFR TKIs to include skeletal toxicities and inform parents/patients of these toxicities at the time of consent for therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MB: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. TD: Investigation, Resources, Writing – review & editing. CB: Investigation, Resources, Writing – review & editing. JS: Investigation, Resources, Writing – review & editing. AD: Conceptualization, Data curation, Investigation, Resources, Validation, Writing – review & editing. JW: Data curation, Resources, Validation, Writing – review & editing. LA: Data curation, Investigation, Resources, Validation, Writing – review & editing. MD: Data curation, Investigation, Resources, Validation, Writing – review & editing. MV: Conceptualization, Data curation, Investigation, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Ms. Jessica Lafreniere form Janssen BioAdvance for helping us to obtain erdafitinib on a compassionate basis for our patient.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. (2000) 103:211–25. doi: 10.1016/S0092-8674(00)00114-8
2. Brown LM, Ekert PG, Fleuren EDG. Biological and clinical implications of FGFR aberrations in paediatric and young adult cancers. Oncogene. (2023) 42:1875–88. doi: 10.1038/s41388-023-02705-7
3. De La Vega LL, Comeau H, Sallan S, Al-Ibraheemi A, Gupta H, Li YY, et al. Rare FGFR Oncogenic Alterations in sequenced Pediatric solid and brain tumors suggest FGFR is a relevant molecular target in childhood cancer. JCO Precis Oncol. (2022) 6:e2200390. doi: 10.1200/PO.22.00390
4. Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD. Tang B et al: Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. (2013) 45:602–12. doi: 10.1038/ng.2611
5. Farouk Sait S, Fischer C, Antal Z, Spatz K, Prince DE, Ibanez K, et al. Slipped capital femoral epiphyses: A major on-target adverse event associated with FGFR tyrosine kinase inhibitors in pediatric patients. Pediatr Blood Cancer. (2023) 70:e30410. doi: 10.1002/pbc.30410
6. Parsons DW, Janeway KA, Patton DR, Winter CL, Coffey B, Williams PM, et al. Actionable tumor alterations and treatment protocol enrollment of pediatric and young adult patients with refractory cancers in the national cancer institute-children’s oncology group pediatric MATCH trial. J Clin Oncol. (2022) 40:2224. doi: 10.1200/JCO.21.02838
7. Farouk Sait S, Gilheeney SW, Bale TA, Haque S, Dinkin MJ, Vitolono S, et al. Debio 1347, an oral FGFR inhibitor: results from a single-center study in pediatric patients with current or refractory FGFR-altered Gliomas. JCO Precis Oncol. (2021) 5:876–83. doi: 10.1200/PO.20.00444
8. Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast Growth Factor Receptor 3 is a negative regulator of bone growth. Cell. (1996) 84:911–21. doi: 10.1016/S0092-8674(00)81069-7
9. Wang Q, Green RP, Zhao G, Ornitz DM. Differential regulation of endochondral bone growth and joint development by FGFR1 and FGFR3 tyrosine kinase domains. Development. (2001) 128:3867–76. doi: 10.1242/dev.128.19.3867
10. Sunder SS, Sharma UC, Pokharel S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal Transduct Target Ther. (2023) 8:262. doi: 10.1038/s41392-023-01469-6
11. Konarski W, Pobozy T, Konarska K, Sliwczynski A, Kotela I, Krakowiak J. Exploring the impact of novel anti-cancer therapies on jaw osteonecrosis and other bones: A comprehensive review. J Clin Med. (2024) 13:1889. doi: 10.3390/jcm13071889
12. Loder RT. The demographics of slipped capital femoral epiphysis. Clin Orthop Relat Res. (1996) 322:8–27. doi: 10.1097/00003086-199601000-00003
13. Restrepo R, Reed MH. Impact of obesity in the diagnosis of SCFE and knee problems in obese children. Pediatr Radiol. (2009) 39:S220–225. doi: 10.1007/s00247-008-1123-3
14. Manoff EM, Banfy MB, Winell JJ. Relationship between Body Mass Index and slipped capital femoral epiphysis. J Pediatr Orthop. (2005) 25:744–6. doi: 10.1097/01.bpo.0000184651.34475.8e
15. Roskoski R. The role of fibroblast growth factor receptor (FGFR) protein-tyrosine kinase inhibitors in the treatment of cancers including those of the urinary bladder. Pharmacol Res. (2020) 151:104567. doi: 10.1016/j.phrs.2019.104567
16. Goyal R, Jialal I. Hyperphosphatemia. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2023).
Keywords: fibroblast growth factor receptor inhibitors, Slipped capital femoral epiphysis, On-target skeletal toxicity, Erdafitinib, hypothalamic obesity
Citation: Brizini M, Drimes T, Bourne C, Streilein J, Drapeau A, Wrogemann J, Archer LA, Del Bigio M and Vanan MI (2024) Case report: Slipped capital femoral epiphysis: a rare adverse event associated with FGFR tyrosine kinase inhibitor therapy in a child. Front. Oncol. 14:1399356. doi: 10.3389/fonc.2024.1399356
Received: 11 March 2024; Accepted: 07 May 2024;
Published: 24 May 2024.
Edited by:
Bo yuan Huang, Capital Medical University, ChinaReviewed by:
Sandeep Batra, Riley Hospital for Children, United StatesShannon Fortin Ensign, Mayo Clinic Arizona, United States
Copyright © 2024 Brizini, Drimes, Bourne, Streilein, Drapeau, Wrogemann, Archer, Del Bigio and Vanan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Magimairajan Issai Vanan, bWl2YW5hbkBjYW5jZXJjYXJlLm1iLmNh
 Meziane Brizini1,2
Meziane Brizini1,2 Marc Del Bigio
Marc Del Bigio Magimairajan Issai Vanan
Magimairajan Issai Vanan