- 1Department of Surgery, Institute of Clinical Sciences, Sahlgrenska Center for Cancer Research, University of Gothenburg, Gothenburg, Sweden
- 2Wallenberg Centre for Molecular and Translational Medicine, University of Gothenburg, Gothenburg, Sweden
- 3Department of Oncology, Sahlgrenska University Hospital, Gothenburg, Sweden
- 4Department of Laboratory Medicine, Institute of Biomedicine, University of Gothenburg, Gothenburg, Sweden
- 5Department of Clinical Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden
- 6Department of Oncology, Institute of Clinical Sciences, University of Gothenburg, Gothenburg, Sweden
Background: KRAS mutation status is a well-established independent prognostic factor in advanced non-small cell lung cancer (NSCLC), yet its role in early-stage disease is unclear. Here, we investigate the prognostic value of combining survival data on KRAS mutation status and tumor size in stage I-II NSCLC.
Methods: We studied the combined impact of KRAS mutational status and tumor size on overall survival (OS) in patients with stage I-II NSCLC. We performed a retrospective study including 310 diagnosed patients with early (stage I-II) NSCLCs. All molecularly assessed patients diagnosed with stage I-II NSCLC between 2016–2018 in the Västra Götaland Region of western Sweden were screened in this multi-center retrospective study. The primary study outcome was overall survival.
Results: Out of 310 patients with stage I-II NSCLC, 37% harbored an activating mutation in the KRAS gene. Our study confirmed staging and tumor size as prognostic factors. However, KRAS mutational status was not found to impact OS and there was no difference in the risk of death when combining KRAS mutational status and primary tumor size.
Conclusions: In our patient cohort, KRAS mutations in combination with primary tumor size did not impact prognosis in stage I-II NSCLC.
Introduction
Non-small cell lung cancer (NSCLC) is the second most common cancer worldwide with 2.1 million new cases annually and the highest mortality rate with 1.8 million deaths (1). Staging is a crucial aspect of NSCLC management, as it is one of the most important predictors of survival. The TNM staging system describes key tumor characteristics such as size, location, and whether the disease has spread to lymph nodes and/or distant organs (2–5). There are four main stages in NSCLC (stage I-IV), with stage IV having the worst prognosis. Pathological stage is considered the most important prognostic factor for resected patients, with 5-year survival rates, gradually decreasing across stages, of 83% for stage IA, 71% for IB, 57% for IIA, 49% for IIB, 36% for IIIA, and 23% for IIIB (4).
The most frequent oncogenic driver in NSCLC is the Kirsten rat sarcoma viral oncogene (KRAS), which is present in up to 40% of all cases, with the most common mutations being G12C, G12V, and G12D (6). KRAS mutations are associated with worse outcomes after chemotherapy and radiotherapy, with shorter OS in stage III and IV patients (7–14). In early-stage NSCLC, however, while several studies have shown that KRAS mutations negatively influence the prognosis (15–17), others have shown no significant effect (18–20). Most recently, it was reported that KRAS G12C mutation (but not other KRAS mutations or with no mutation in KRAS) significantly increased risk of disease recurrence in stage I surgically resected lung adenocarcinomas (21). However, while the study found this in two distinct local cohorts of IRE-LUAD (Rome, Italy) and MSK-LUAD (New York, USA), data extracted from The Cancer Genome Atlas (TCGA) showed no significant difference. Another recent study reported that while STK11 mutation decreased survival probability in stage I lung adenocarcinoma, KRAS mutation showed no significant impact (22). Hence, the debate about the prognostic value of KRAS mutational status in early NSCLC is ongoing (23, 24). In fact, given the lack of consensus regarding its effects on prognosis, testing for KRAS mutations for resectable stage I and II tumors is currently not recommended in clinical guidelines (25). In addition, several inhibitors that specifically bind KRAS-G12C have been investigated in clinical trials, with sotorasib becoming the first treatment to gain approval for adults with stage IV NSCLC harboring a KRAS-G12C mutation as second-line therapy (26–30). However, treatment with sotorasib is not currently recommended for patients with early-stage NSCLC due to lack of evidence showing positive outcomes of treatment in this group.
Therefore, further investigations are warranted to identify potential subgroups in Stage I-III disease who may still have to gain from effective and well-established treatments, and to add to the pool of clinical data required to study this further. One strategy is to stratify patients according to KRAS mutational status together with other key prognostic factors, such as tumor size. Primary tumor size is an established prognostic factor in NSCLC, with larger tumors being associated with poorer survival (24, 31–34). The reason for this association is not yet fully understood but larger tumors may be more resistant to therapy due to having poorer blood supply, differential metabolism, and potentially a higher likelihood of micrometastatic disease compared to smaller tumors (35). Further research is needed to elucidate the underlying mechanisms. However, when considering primary tumor size, the grouping as early (I-II), advanced (III), and metastasized (IV) NSCLC can be argued to be more clinically relevant due to that stage I-II is primarily based on tumor size whereas a spread to the lymph nodes, a negative prognostic factor, is more common in stage III (3, 24).
To our knowledge, no one has investigated the combined impact of primary tumor size and KRAS mutational status on OS and risk of death in stage I-II NSCLC. However, in Sweden, reflex testing for targetable alterations in NSCLC, including KRAS mutational status, has been widely implemented since 2015 for all stages. By screening all consecutive patients diagnosed with stage I-II NSCLC and molecularly assessed between 2016–2018 in Västra Götaland, the second largest county in Sweden with a population of 1.7 million, the current retrospective cohort study provides a unique real-world dataset for assessing the impact of combining KRAS mutations with primary tumor size.
To summarize, primary tumor size is a key determinant of prognosis especially in the early stages of NSCLC. At the same time, the prognostic value of KRAS mutational status in early disease stages remains unclear. Hence patients diagnosed at an early stage are not automatically tested for KRAS mutations and recommended treatment with KRAS-targeted therapy. Here, we investigate whether there is prognostic value in combining KRAS mutational status with tumor size to aid in clinical stratification of potentially treatment-responsive subgroups in early-stage NSCLC.
Materials and methods
Patient population
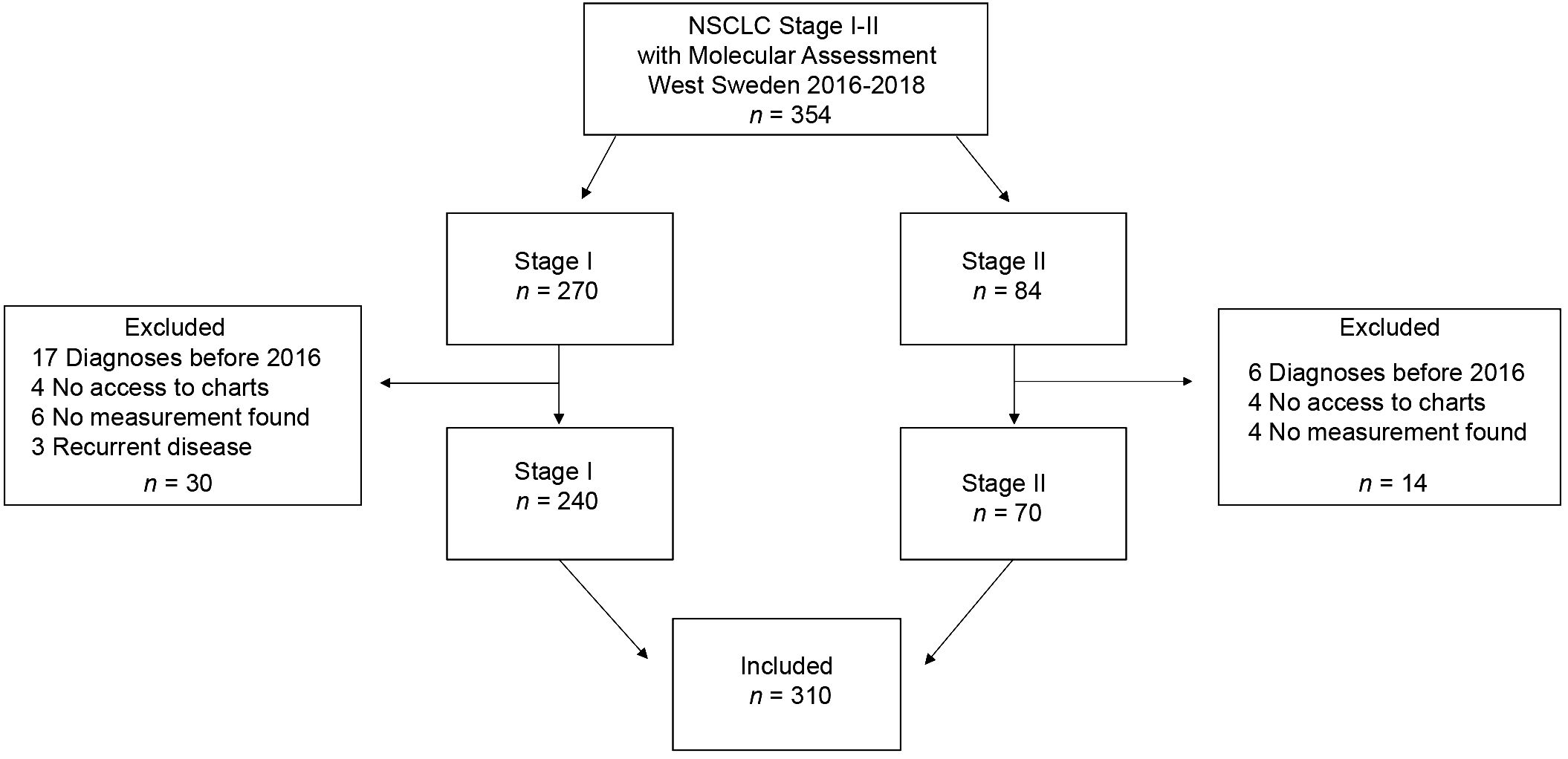
We conducted a multi-center retrospective study screening all consecutive NSCLC patients diagnosed with stage I-II NSCLC and molecular assessment performed between 2016–2018 in Västra Götaland, Sweden (n = 354). Further inclusion criteria included the availability of tumor size from CT scanning or a pathology report as well as follow-up data. Patients were excluded if diagnosed before 2016, had no digitally accessible patient charts, no tumor measurements noted in the patient charts, or had recurrent disease (study cohort n = 310).
Patient demographics (age, sex, Eastern Cooperative Oncology Group [ECOG] performance status [PS], and smoking history), cancer stage, pathological details (histology, mutational status including KRAS mutational status and subtype), first-line treatment and outcome data were retrospectively collected from patient charts and the Swedish Lung Cancer Registry. Clinical staging was based on TNM staging guidelines 7th edition (4). TNM staging 8th edition released in 2017 was introduced in Swedish guidelines in 2018, and full implementation was reached in 2019. Ethical approval was obtained from the Swedish Ethical Review Authority prior to study commencement (Dnr 2019–04771 and 2021–04987). No informed consent was required due to all data presented in a de-identified form according to the Swedish Ethical Review Authority.
Mutational status
Patients were assessed with next-generation sequencing (NGS) for mutational status on DNA from formalin-fixed paraffin-embedded (FFPE) blocks or cytological smears using the Ion AmpliSeq™ Colon and Lung Cancer Panel v2 from Thermo Fisher Scientific as part of the diagnostic workup process at the Department of Clinical Pathology at Sahlgrenska University Hospital, assessing hotspot mutations in EGFR, BRAF, KRAS, and NRAS. Until June 2017, ALK-fusions were assessed with immunohistochemistry (IHC), and with fluorescence in situ hybridization (FISH) if positive or inconclusive IHC. ROS1 was analyzed upon request with FISH. Thereafter, ALK, ROS1, and RET fusions were assessed on RNA using the Oncomine Solid Tumor Fusion Panel from Thermo Fisher Scientific.
Tumor size
To obtain the most recent and accurate untreated primary tumor size, measurements were collected from the radiology report of computed tomography (CT) performed before a final diagnosis of NSCLC was established; this is referred as clinical staging. In patients who underwent surgical resection, the actual primary tumor size was also collected from the pathology report, also referred as pathological staging (PAD). The largest tumor diameter was collected and reported in millimeters.
Study objectives
The primary outcome of this study was OS, defined as the interval between the date of first treatment and the date of death from any cause. Patients alive or lost to follow-up at the cut-off date were censored at last contact. Median follow-up time was estimated using the reverse Kaplan-Meier method. We compared OS and risk of death stratified by KRAS mutational status, i.e., with no mutation in KRAS (wildtype, KRASWT), all KRAS mutations (KRASMUT), KRAS G12C mutations (KRASMUT G12C) and all KRAS mutations other than G12C (KRASMUT not G12C).
Statistical analysis
Clinical characteristics were summarized using descriptive statistics and analysis of associations between KRAS mutational status and clinicopathological parameters was performed using Pearson´s X2 test or T-test. Survival was estimated using the Kaplan-Meier method, visualized at 5-year follow up. The log-rank test was used to assess significant differences in OS between KRASWT and KRASMUT groups. To evaluate if there was a significant difference in primary tumor size between KRASMUT and KRASWT, the Mann-Whitney U test was used. We defined an interaction term between tumor size (largest diameter in mm) and KRAS mutational status to assess the combined impact on the risk of death (HR). First, the mean size of all primary tumors was calculated. Thereafter a dummy variable was calculated by subtracting the mean size from all individual measurements following multiplication with 1 if KRAS was mutated and 0 if KRAS was WT. The interaction term was included in Multivariable Cox regression analysis, also correcting for sex, age, tumor size in mm and KRAS mutational status. Statistical significance was set at p < 0.05 and no adjustments were made for multiple comparisons. Data analysis was conducted using IBM SPSS Statistics version 27 and GraphPad Prism version 9.
Results
Patients and tumor characteristics
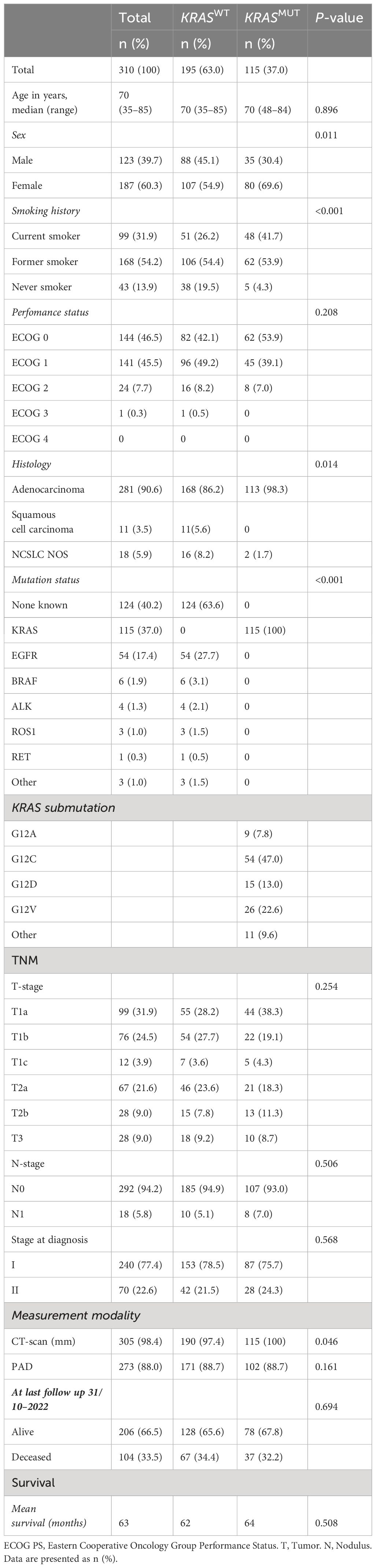
A total of 310 patients, who were diagnosed with stage I-II NSCLC during 2016–2018 in Västra Götaland, Sweden and for whom genetic data was available, were included in this retrospective cohort study (Figure 1). In the total population, majority of patients were female (187, 60.3%), with a median age of 70 years, and most were current or former smokers (267, 86%) (Table 1). Most patients had good PS with ECOG 0–1 at diagnosis (285, 92%) and the proportion of N1 was low (18, 5.8%). NSCLC was predominantly adenocarcinoma of the lung (281, 90.6%), while squamous cell carcinoma incidence was relatively low (11, 3.5%), which was expected due to the selection of histological type for NGS assessment. Of included patients, over a third (115, 37%) had a KRAS mutation (Table 1). This percentage matches what has been previously reported (9), showing good representativeness of the patient group studied here. When comparing the baseline characteristics of KRASWT with KRASMUT patients, a greater proportion of those with KRASMUT were female and current or former smokers. There were no cases of squamous cell carcinoma in the KRASMUT group. The most common KRAS mutation was G12C (47%). In the total population, majority of patients underwent surgical resection (273, 88%; Table 2). Three patients did not receive any treatment and were excluded from further survival analyses. Median follow-up time was 63 months (95% CI, 59.7–68.3) and the data cut-off date was 31 October 2022.
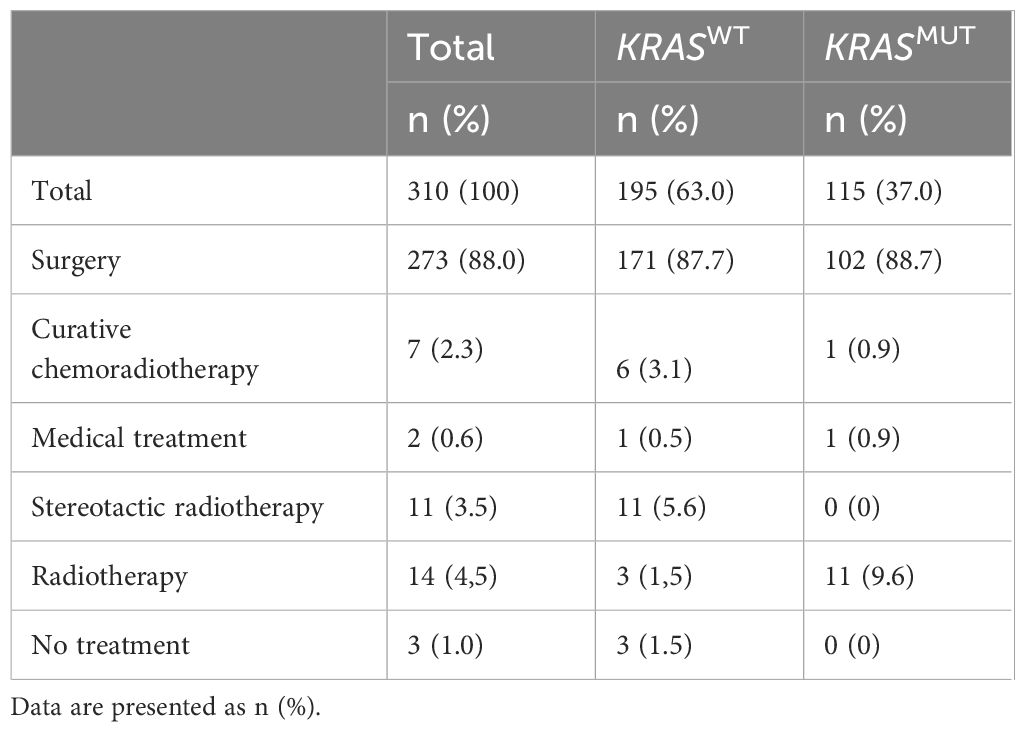
Table 2 Summary of first-line treatments in the total cohort as well as stratified by KRASWT and KRASMUT.
No significant difference in survival for all patients stratified by KRAS mutations
When comparing OS for all (stage I-II) patients stratified by KRAS mutational status, no significant difference was detected with a mean OS (median not reached) of 74 months for KRASWT vs 63 months for KRASMUT (p = 0.847; Figure 2A). Further stratification of the KRAS mutated group by the G12C mutation also did not significantly change survival: 74 months for KRASWT, 61 months for KRASMUT not G12C and 63 months for KRASMUT G12C (p = 0.834; Figure 2B).
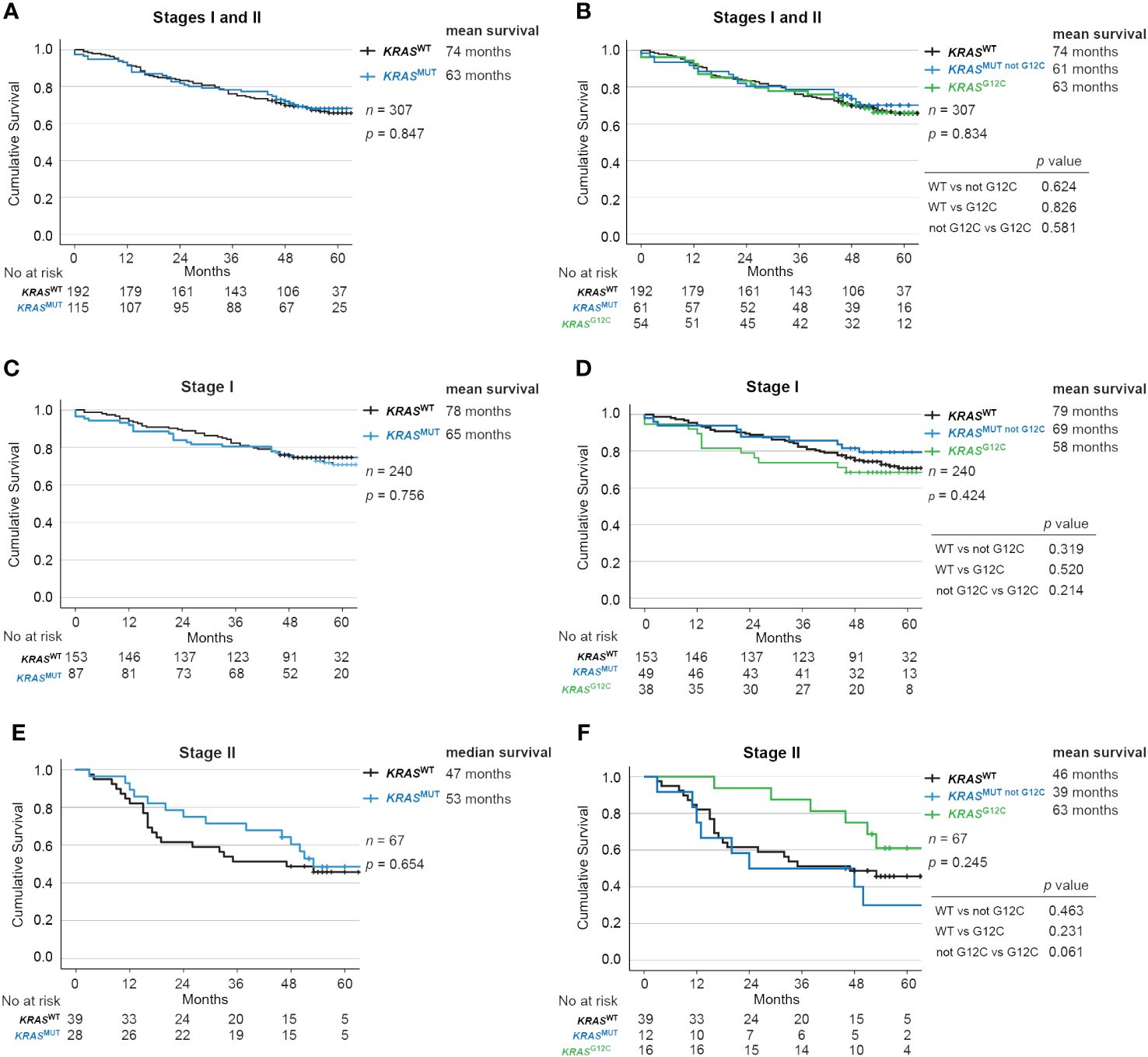
Figure 2 Impact of KRAS mutational status on overall survival in Stage I and II NSCLC. Kaplan-Meier estimates comparing overall survival between (A, B) all patients, (C, D) Stage I and (E, F) Stage II patients with no mutation in KRAS (wildtype, KRASWT), with all KRAS mutations (KRASMUT), only KRAS-G12C mutations (KRASMUT G12C) and KRAS mutations other than G12C (KRASMUT not G12C).
No significant difference in survival for patients in stage I or stage II disease combined with KRAS mutations
There were also no significant differences according to KRAS mutational status in stage I (Figures 2C, D) or Stage II (Figures 2E, F). Similarly in resected patients, no significant difference was observed with a mean OS (median not reached) of 78 months for KRASWT vs 65 months for KRASMUT (p = 0.856; Supplementary Figure 1A), or between the subgroups of KRASMUT (p = 0.471; Supplementary Figure 1B).
Next, we stratified by stage and found mean OS (median not reached) of 79 months for stage I vs 50 months for stage II (Supplementary Figure 2A). We then conducted the analysis separately according to KRAS mutational status. For KRASWT, the mean OS (median not reached) was 78 months for stage I vs 46 months for stage II (Supplementary Figure 2B), and for KRASMUT, 65 months for stage I vs 53 months for stage II (Supplementary Figure 2C).
No significant difference in survival for patients with TNM-stage T1, T2 or T3 disease combined with KRAS mutations
Next, we stratified patients using T-staging and studied OS according to KRAS mutational status. Among those with T1 disease, KRASWT had 83 months while KRASMUT had 66 months OS (p = 0.751; Figure 3A). Further, KRASMUT not G12C patients had survival of 70 months and KRASMUT G12C had 61 months (p = 0.344; Figure 3B). In the T2 group, KRASWT had 53 months while KRASMUT had 59 months OS (p = 0.495; Figure 3C). KRASMUT not G12C patients had survival of 51 months and KRASMUT G12C had 66 months (p = 0.389; Figure 3D). Similarly, in the T3 group, KRASWT had 47 months while KRASMUT had 50 months OS (p = 0.966; Figure 3E). KRASMUT not G12C patients had survival of 50 months and KRASMUT G12C had 53 months (p = 0.984; Figure 3F).
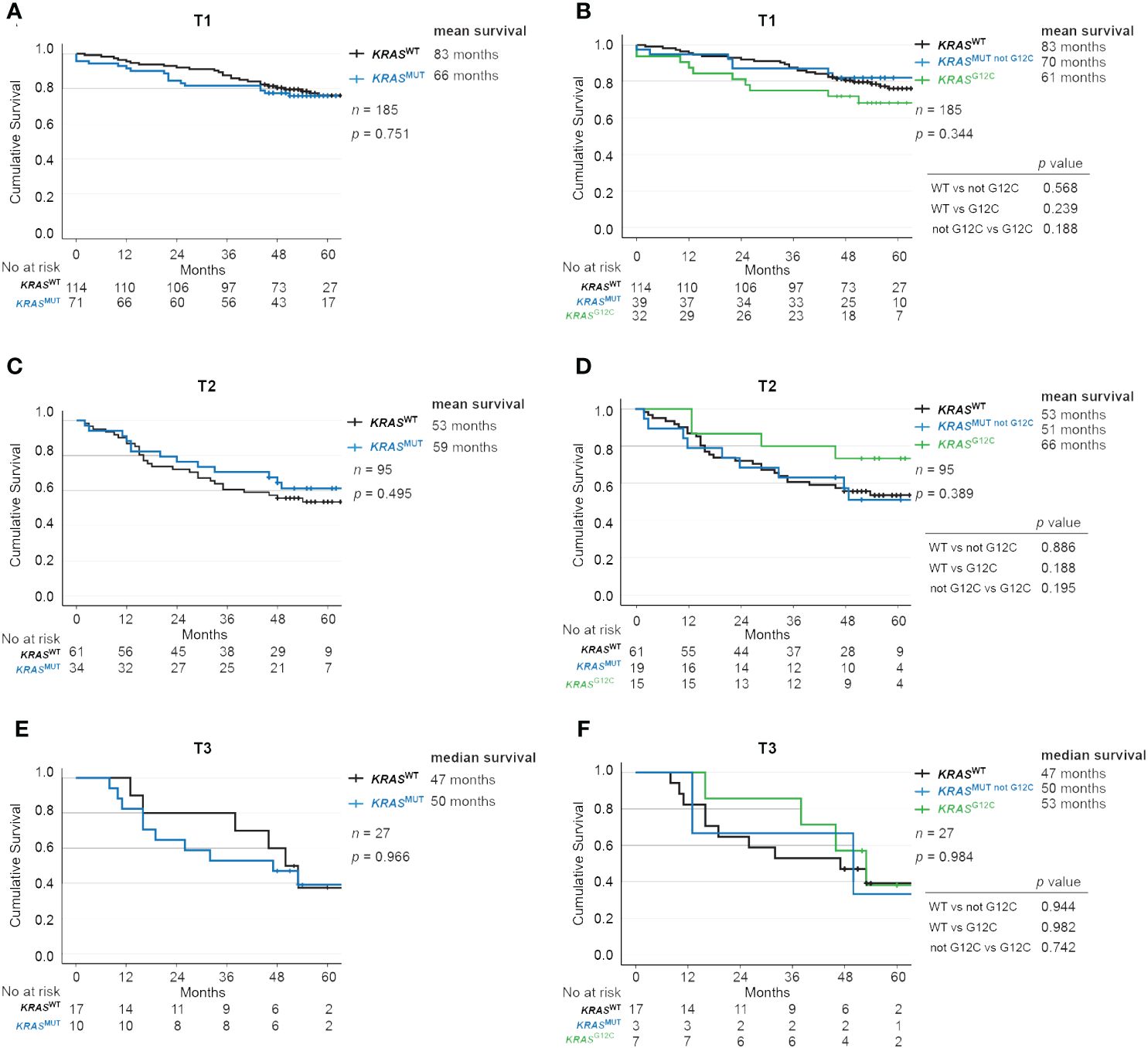
Figure 3 Impact of KRAS mutational status on overall survival across TNM-stages in NSCLC. Kaplan-Meier estimates comparing overall survival between (A, B) T1, (C, D) T2 and (E, F) T3 patients with no mutation in KRAS (wildtype, KRASWT), with all KRAS mutations (KRASMUT), only KRAS-G12C mutations (KRASMUT G12C) and KRAS mutations other than G12C (KRASMUT not G12C).
We further analyzed the impact of T stage on survival and found that it correlated as expected with mean OS of 82 months for T1, 55 months for T2, and 46 months for T3 (p < 0.001; Supplementary Figure 3A). The same trend was observed when separately analyzing KRASWT with a mean OS (median not reached) of 83 months for T1, 53 months for T2, and 45 months for T2 (p < 0.001; Supplementary Figure 3B), and KRASMUT with a mean OS of 65 months for T1, 58 months for T2, and 48 months for T3 (p < 0.023; Supplementary Figure 3C).
KRAS mutations are associated with smaller tumor size measured from CT scans, but not resection specimens
To evaluate differences between primary tumor size from CT scans at diagnosis stratified by KRAS mutational status, we used the Mann-Whitney U test. The test revealed that KRASMUT primary tumors were significantly smaller at diagnosis, with a median size of 20 mm (n = 115) vs KRASWT primary tumors with a median size of 25 mm (n = 190) (p = 0.043; Figure 4A). However, when looking at tumor size as assessed in resected specimens, there were no differences; KRASWT median size 22 mm (n = 171) vs KRASMUT median size 21 mm (n = 102) (p = 0.16; Figure 4B).

Figure 4 KRAS mutations are associated with smaller tumor size at diagnosis. Primary tumor size from (A) CT scans and (B) resection specimens (PAD) in patients with no mutation in KRAS (wildtype, KRASWT) or with KRAS mutations (KRASMUT). Forest plot of multivariable COX regression analysis for patients with tumor size from (C) CT scan and (D) resection specimens (PAD).
Larger tumor size measured from resection specimens, but not CT scans, is associated with a higher risk of death
We found that increase in primary tumor size determined from CT scans did not have a significant effect on risk of death (HR, 1.006; 95% CI, 0.922–1.021; p > 0.5) (Figure 4C). However, when testing the correlation between primary tumor size as assessed in resection specimens, we found a significantly increased risk of death (HR, 1.029; 95% CI, 1.012–1.046; p < 0.001) (Figure 4D). The risk of death increases with 2.9% for every mm increase of size.
When analyzing stage I and stage II patients separately we found that the primary tumor size, as assessed in resection specimens, correlated to a significantly increased risk of death (HR, 1.051; 95% CI, 1.026–1.077; p < 0.001) (Supplementary Table 1) for stage I patients, with 5.1% for every mm increase in tumor size. However, for stage II patients, no correlation was found between tumor size and risk of death. Furthermore, the primary tumor size determined by CT did not impact the risk of death in either stage. Along these lines, we analyzed the risk of death separately for T1, T2 and T3 groups regarding tumor size from CT and resected specimens and no correlation was found (Supplementary Table 1).
The combination of KRAS mutational status and tumor size does not impact the risk of death
To test if the combination of tumor size and KRAS mutational status impacts the risk of death, we defined an interaction term including both variables. For primary tumor size from CT scans and KRAS mutational status, no significant difference in the risk of death was detected (HR, 1.008; 95% CI, 0.988–1.030; p > 0.5) (Figure 4C). Similarly, there were no significant differences for primary tumor size and KRAS mutational status when measured in resection specimens (HR, 1.002; 95% CI, 0.978–1.027; p = 0.807) (Figure 4D). Along these lines, we analyzed the risk of death separately for stage I and II, as well as for T1, T2 and T3 groups regarding the interaction term combining tumor size and KRAS mutational status and no correlation was found (Supplementary Table 1).
Discussion
In this study, we assessed the prognostic value of combining KRAS mutational status with tumor size in early-stage NSCLC. We found that combining these variables had no significant effect on overall survival or the risk of death.
In alignment with previous findings, we found in our patient cohort that later disease stage and larger primary tumor size is associated with worse survival. Interestingly, we found that these correlations are sustained independent of KRAS mutational status. Importantly, the established literature on how KRAS mutations affect outcomes in early-stage NSCLC is varying between worse survival and no significant difference. We find that KRAS mutational status alone does not significantly impact OS or risk of death in patients with stage I-II NSCLC. Taken together, these findings show good representativeness of this well-defined patient cohort.
Our study included only patients with stage I-II disease due to the focus on primary tumor size and to limit the prognostic impact of local invasion and regional lymph node involvement. Only 5.8% of the patients had N1 disease that could affect the prognosis. The major portion of the patients had tumor resection and more than 90% of tumors were adenocarcinoma. During this period, patients diagnosed with squamous cell carcinoma were molecularly assessed to a lesser extent, thus our study is more representative of adenocarcinoma. Even though most tumors were classified according the TNM staging guidelines 7th edition, changes included in the 8th edition, mainly covering substages that were not analyzed in this study, do not alter our findings (4).
No significant differences were observed when comparing OS for all stage I-II patients stratified by KRAS mutational status. However, the mean OS was 11 months shorter for KRASMUT patients. The same trend was observed when looking at resected patients with a 13-month shorter mean survival for KRASMUT patients. Although trends toward a poorer prognosis were present, the KRASMUT subgroup consisted of a relatively small number of patients, potentially necessitating larger cohorts to achieve statistical significance. This observation aligns with recent findings in a similar cohort of stage I LUAD with relatively small sample size in the KRASMUT group (22). Within the KRAS mutational subgroups in our cohort, contrary to prior reports (36, 37), KRASG12C mutation in stage I disease did not indicate a worse prognosis. However, as noted in another study (38), there was a tendency toward improved survival among KRASG12C patients with stage II disease and T2/T3 tumors, although these differences did not reach statistical significance.
Outcome variables other than survival such as recurrence rates and progression-free survival were not examined here. In addition, there remain confounders that were not include in the analyses such as the effect of different treatment methods on survival. Further, we use the T descriptor of the TNM staging system for tumor size but the descriptor also includes invasion status and or intrapulmonary metastasis. In addition, we found that larger tumor size measured from resection specimens, but not CT scans, is associated with a higher risk of death. However, one confounder here is that non-resected patients are included in the CT group but not in the PAD group, which biases toward worse prognosis.
Going forward, much remains to be explored on the role of KRAS mutation in early NSCLC. In the age of precision medicine, our study contributes toward the detailed level clinical data that is required for future pooled analysis of prognosis assessments that can help guide clinical decisions.
In conclusion, we confirm the importance of primary tumor size and stage as a prognostic factor for survival in stage I-II NSCLC. KRAS mutations were not found to impact OS and no difference in the risk of death was observed when combining KRAS mutations and primary tumor size.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Swedish Ethical Review Authority. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because all data are presented in a de-identified form according to the Swedish Ethical Review Authority, no informed consent is required.
Author contributions
EE: Methodology, Data curation, Visualization, Formal analysis, Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization. AM: Writing – original draft, Formal Analysis, Data curation. CW: Formal analysis, Visualization, Writing – review & editing, Funding acquisition. SS: Writing – original draft, Writing – review & editing, Visualization. HF: Writing – review & editing, Resources, Funding acquisition, Data curation, Conceptualization. AH: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. VS: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Swedish Research Council (2018–02318 and 2022–00971 to VS, 2021–03138 to CW), the Swedish Cancer Society (23–3062 to VS, 22–0612FE to CW), the Gothenburg Society of Medicine (2019; 19/889991 to EE), Assar Gabrielsson Research Foundation (to EE, CW, and VS), the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (to HF), Department of Oncology, Sahlgrenska University Hospital (to EE and AH), the Swedish Society for Medical Research (2018; S18–034 to VS), the Knut and Alice Wallenberg Foundation, and the Wallenberg Centre for Molecular and Translational Medicine (to VS).
Acknowledgments
We thank Sayin lab members and Nesrin Vurgun, Scientific Editor at the Institute of Clinical Sciences, University of Gothenburg, for a critical review of the manuscript. We would also like to thank “Akademistatistik - consulting services in statistics and health economics” at the University of Gothenburg for their support with defining the interaction term. In addition, we thank members of the Swedish Lung Cancer Registry and the continuous reporting by Swedish healthcare employees.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1396285/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier estimates of overall survival for resected Stage I-II NSCLC patients stratified by KRAS mutational status. (A) No mutation in KRAS (wildtype, KRASWT), with all KRAS mutations (KRASMUT). (B) Only KRAS-G12C mutations (KRASMUT G12C), KRAS mutations other than G12C (KRASMUT not G12C).
Supplementary Figure 2 | Kaplan-Meier estimates comparing overall survival for (A) all patients, (B) patients with no mutation in KRAS (wildtype, KRASWT), and (C) with all KRAS mutations (KRASMUT), stratified by Stages I and II.
Supplementary Figure 3 | Kaplan-Meier estimates comparing overall survival for (A) all patients, (B) patients with no mutation in KRAS (wildtype, KRASWT), and (C) with all KRAS mutations (KRASMUT), stratified by TNM-stages T1, T2 and T3.
Footnotes
- ^ CT, Computed Tomography; ECOG, Eastern Cooperative Oncology Group; HR, Hazard Ratio; NSCLC, Non-Small Cell Lung Cancer; NGS, Next Generation Sequencing; PS, Performance Status; OS, Overall Survival.
References
1. World Health Organization, International Agency for Research on Cancer. Globocan 2020: Lung Cancer. International Agency for Research on Cancer. Available at: http://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (Accessed March 2, 2021).
2. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67:93–9. doi: 10.3322/caac.21388
3. Detterbeck FC. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J Thorac Cardiovasc Surg. (2018) 155:356–9. doi: 10.1016/j.jtcvs.2017.08.138
4. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
5. Wankhede D. Evaluation of eighth AJCC TNM sage for lung cancer NSCLC: A meta-analysis. Ann Surg Oncol. (2021) 28:142–7. doi: 10.1245/s10434-020-09151-9
6. Tartarone A, Lapadula V, Di Micco C, Rossi G, Ottanelli C, Marini A, et al. Beyond conventional: the new horizon of targeted therapy for the treatment of advanced non small cell lung cancer. Front Oncol. (2021) 11:632256. doi: 10.3389/fonc.2021.632256
7. Brady AK, McNeill JD, Judy B, Bauml J, Evans TL, Cohen RB, et al. Survival outcome according to KRAS mutation status in newly diagnosed patients with stage IV non-small cell lung cancer treated with platinum doublet chemotherapy. Oncotarget. (2015) 6:30287–94. doi: 10.18632/oncotarget.v6i30
8. Eklund EA, Wiel C, Fagman H, Akyürek LM, Raghavan S, Nyman J, et al. KRAS mutations impact clinical outcome in metastatic non-small cell lung cancer. Cancers (Basel). (2022) 14:2063. doi: 10.3390/cancers14092063
9. Goulding RE, Chenoweth M, Carter GC, Boye ME, Sheffield KM, John WJ, et al. KRAS mutation as a prognostic factor and predictive factor in advanced/metastatic non-small cell lung cancer: A systematic literature review and meta-analysis. Cancer Treat Res Commun. (2020) 24:100200. doi: 10.1016/j.ctarc.2020.100200
10. Hallqvist A, Enlund F, Andersson C, Sjögren H, Hussein A, Holmberg E, et al. Mutated KRAS is an independent negative prognostic factor for survival in NSCLC stage III disease treated with high-dose radiotherapy. Lung Cancer Int. (2012) 2012:587424. doi: 10.1155/2012/587424
11. Hames ML, Chen H, Iams W, Aston J, Lovly CM, Horn L, et al. Correlation between KRAS mutation status and response to chemotherapy in patients with advanced non-small cell lung cancer☆. Lung Cancer. (2016) 92:29–34. doi: 10.1016/j.lungcan.2015.11.004
12. Marabese M, Ganzinelli M, Garassino MC, Shepherd FA, Piva S, Caiola E, et al. KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy. Oncotarget. (2015) 6:34014–22. doi: 10.18632/oncotarget.v6i32
13. Mellema WW, Dingemans AM, Thunnissen E, Snijders PJ, Derks J, Heideman DA, et al. KRAS mutations in advanced nonsquamous non-small-cell lung cancer patients treated with first-line platinum-based chemotherapy have no predictive value. J Thorac Oncol. (2013) 8:1190–5. doi: 10.1097/JTO.0b013e318298764e
14. Rodenhuis S, Boerrigter L, Top B, Slebos RJ, Mooi WJ, van't Veer L, et al. Mutational activation of the K-ras oncogene and the effect of chemotherapy in advanced adenocarcinoma of the lung: a prospective study. J Clin Oncol. (1997) 15:285–91. doi: 10.1200/JCO.1997.15.1.285
15. Meng D, Yuan M, Li X, Chen L, Yang J, Zhao X, et al. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: a systematic review with meta-analysis. Lung Cancer. (2013) 81:1–10. doi: 10.1016/j.lungcan.2013.03.019
16. Kadota K, Sima CS, Arcila ME, Hedvat C, Kris MG, Jones DR, et al. KRAS mutation is a significant prognostic factor in early-stage lung adenocarcinoma. Am J Surg Pathol. (2016) 40:1579–90. doi: 10.1097/PAS.0000000000000744
17. Izar B, Zhou H, Heist RS, Azzoli CG, Muzikansky A, Scribner EE, et al. The prognostic impact of KRAS, its codon and amino acid specific mutations, on survival in resected stage I lung adenocarcinoma. J Thorac Oncol. (2014) 9:1363–9. doi: 10.1097/JTO.0000000000000266
18. D’Angelo SP, Janjigian YY, Ahye N, Riely GJ, Chaft JE, Sima CS, et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol. (2012) 7:1815–22. doi: 10.1097/JTO.0b013e31826bb7b2
19. Dalvi T, Nørgaard M, Fryzek JP, Movva N, Pedersen L, Pham Hansen H, et al. Biomarker expression and survival in patients with non-small cell lung cancer receiving adjuvant chemotherapy in Denmark. PloS One. (2023) 18:e0284037. doi: 10.1371/journal.pone.0284037
20. Wahl SGF, Dai HY, Emdal EF, Berg T, Halvorsen TO, Ottestad AL, et al. The prognostic effect of KRAS mutations in non-small cell lung carcinoma revisited: A Norwegian multicentre study. Cancers (Basel). (2021) 13:4294. doi: 10.3390/cancers13174294
21. Gallina FT, Marinelli D, Melis E, Forcella D, Taje R, Buglioni S, et al. KRAS G12C mutation and risk of disease recurrence in stage I surgically resected lung adenocarcinoma. Lung Cancer. (2023) 181:107254. doi: 10.1016/j.lungcan.2023.107254
22. Dolgalev I, Zhou H, Murrell N, Le H, Sakellaropoulos T, Coudray N, et al. Inflammation in the tumor-adjacent lung as a predictor of clinical outcome in lung adenocarcinoma. Nat Commun. (2023) 14:6764. doi: 10.1038/s41467-023-42327-x
23. Ihle NT, Byers LA, Kim ES, Saintigny P, Lee JJ, Blumenschein GR, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. (2012) 104:228–39. doi: 10.1093/jnci/djr523
24. Garinet S, Wang P, Mansuet-Lupo A, Fournel L, Wislez M, Blons H. Updated prognostic factors in localized NSCLC. Cancers (Basel). (2022) 14:1400. doi: 10.3390/cancers14061400
25. Remon J, Soria JC, Peters S. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. (2021) 32:1637–42. doi: 10.1016/j.annonc.2021.08.1994
26. Addeo A, Banna GL, Friedlaender A. KRAS G12C mutations in NSCLC: from target to resistance. Cancers (Basel). (2021) 13:2541. doi: 10.20944/preprints202105.0471.v1
27. Burns TF, Borghaei H, Ramalingam SS, Mok TS, Peters S. Targeting KRAS-mutant non-small-cell lung cancer: one mutation at a time, with a focus on KRAS G12C mutations. J Clin Oncol. (2020) 38:4208–18. doi: 10.1200/JCO.20.00744
28. Indini A, Rijavec E, Ghidini M, Cortellini A, Grossi F. Targeting KRAS in solid tumors: current challenges and future opportunities of novel KRAS inhibitors. Pharmaceutics. (2021) 13:653. doi: 10.3390/pharmaceutics13050653
29. Mathieu M, Steier V, Fassy F, Delorme C, Papin D, Genet B, et al. KRAS G12C fragment screening renders new binding pockets. Small GTPases. (2022) p:1–14. doi: 10.1080/21541248.2021.1979360
30. Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. (2021) 384:2371–81. doi: 10.1056/NEJMoa2103695
31. Chen Y, Zhang Q, Lv Y, Li N, Xu G, Ruan T. Prognostic factors of survival in patients with non-small cell lung cancer: a competing risk model using the SEER database. Transl Cancer Res. (2022) 11:3974–85. doi: 10.21037/tcr
32. Gerber DE, Dahlberg SE, Sandler AB, Ahn DH, Schiller JH, Brahmer JR, et al. Baseline tumour measurements predict survival in advanced non-small cell lung cancer. Br J Cancer. (2013) 109:1476–81. doi: 10.1038/bjc.2013.472
33. Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg. (2005) 129:87–93. doi: 10.1016/j.jtcvs.2004.04.030
34. Zhang J, Gold KA, Lin HY, Swisher SG, Xing Y, Lee JJ, et al. Relationship between tumor size and survival in non-small-cell lung cancer (NSCLC): an analysis of the surveillance, epidemiology, and end results (SEER) registry. J Thorac Oncol. (2015) 10:682–90. doi: 10.1097/JTO.0000000000000456
35. Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. (2007) 99:1441–54. doi: 10.1093/jnci/djm135
36. Cao H, Ma Z, Li Y, Zhang Y, Chen H. Prognostic value of KRAS G12C mutation in lung adenocarcinoma stratified by stages and radiological features. J Thorac Cardiovasc Surg. (2023) 166:e479–99. doi: 10.1016/j.jtcvs.2023.04.037
37. Nadal E, Chen G, Prensner JR, Shiratsuchi H, Sam C, Zhao L, et al. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol. (2014) 9:1513–22. doi: 10.1097/JTO.0000000000000305
38. Isaksson J, Berglund A, Louie K, Willén L, Hamidian A, Edsjö A, et al. KRAS G12C mutant non-small cell lung cancer linked to female sex and high risk of CNS metastasis: population-based demographics and survival data from the national Swedish lung cancer registry. Clin Lung Cancer. (2023) 24:507–18. doi: 10.1016/j.cllc.2023.05.002
Keywords: lung cancer, KRAS, tumor size, stage I and II, clinical outcome
Citation: Eklund EA, Mourad A, Wiel C, Sayin SI, Fagman H, Hallqvist A and Sayin VI (2024) Assessing the prognostic value of KRAS mutation combined with tumor size in stage I-II non-small cell lung cancer: a retrospective analysis. Front. Oncol. 14:1396285. doi: 10.3389/fonc.2024.1396285
Received: 05 March 2024; Accepted: 17 May 2024;
Published: 31 May 2024.
Edited by:
Wouter H. Van Geffen, Medical Center Leeuwarden, NetherlandsReviewed by:
Chao Li, Tongji University, ChinaCheng-Yao Chiang, The Ohio State University, United States
Copyright © 2024 Eklund, Mourad, Wiel, Sayin, Fagman, Hallqvist and Sayin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Volkan I. Sayin, dm9sa2FuLnNheWluQGd1LnNl
 Ella A. Eklund
Ella A. Eklund Ali Mourad1,2
Ali Mourad1,2 Henrik Fagman
Henrik Fagman Volkan I. Sayin
Volkan I. Sayin