- 1Department of Urology, First Hospital of Shanxi Medical University, Taiyuan, ;China
- 2First Clinical Medical College, Shanxi Medical University, Taiyuan, ;China
- 3Department of Neurosurgery, First Hospital of Shanxi Medical University, Taiyuan, ;China
- 4Department of Orthopedics, First Hospital of Shanxi Medical University, Taiyuan, ;China
Fluorescence imaging is a relatively new imaging method used to visualize different tissue structures to help guide intraoperative operations, which has potential advantages with high sensitivity and contrast compared to conventional imaging. In this work, we review fluorescent contrast agents and devices used for lymphatic system imaging. Indocyanine green is the most widely utilized due to its high sensitivity, specificity, low background fluorescence, and safety profile. In prostate and bladder cancer lymph node dissection, the complex lymphatic drainage can result in missed metastatic nodes and extensive dissection increases the risk of complications like lymphocele, presenting a significant challenge for urologists. Fluorescence-guided sentinel lymph node dissection facilitates precise tumor staging. The combination of fluorescence and radiographic imaging improves the accuracy of lymph node staging. Multimodal imaging presents new potential for precisely identifying metastatic pelvic lymph nodes.
1 Introduction
Fluorescence imaging is a relatively new imaging method to visualize different types of tissues by fluorescence (1). It has many advantages over conventional imaging, such as real-time intraoperative imaging, enhanced contrast between tumor and surrounding tissues, identification of tiny occult lesions, and reduction of positive margins (2, 3). The key to fluorescence imaging lies in selecting appropriate fluorescent contrast agents and imaging systems (4). There is increasing interest in developing novel fluorescent contrast agents and fluorescent imaging systems and their use in imaging the lymphatic system (5). As early as 1999, Reynolds JS et al. demonstrated the potential of the fluorescent dye indocyanine green (ICG) for detecting spontaneous breast cancer and regional lymph nodes through animal experiments (6). In 2004, Nimura H et al. used ICG for sentinel lymph node (SLN) detection in gastric cancer and combined it with infrared ray electronic endoscopy to avoid missing metastatic lymph nodes (7). For lung cancer patients, fluorescence-guided SLN resection is also feasible (8). In 2007, a Japanese research team reported for the first time the use of ICG fluorescence lymphography for the diagnosis of lower limb lymphedema as an alternative to conventional lymphography (9). Additionally, ICG fluorescence imaging can be used to quantify lymphatic flow rate and pulse frequency, allowing for real-time, non-invasive monitoring of lymphatic function (10). We focus on the application of fluorescence imaging in lymph node dissection surgery for prostate and bladder cancer. Fluorescence imaging is used to visualize the SLN to improve traditional lymph node dissection procedures, enable more accurate surgical guidance, reduce surgical complications, and potentially improve patient prognosis.
Prostate cancer is the most common malignant tumor of the urinary system, with approximately 1.4 million new cases and 375,000 deaths per year worldwide. Bladder cancer is the second most common malignant tumor in the urinary system, with approximately 573,000 new cases and 213,000 deaths annually (11). Tumor metastasis is the main cause of patient death, and lymph node metastasis is an important route of tumor metastasis (12). About one-third of metastases in male malignant tumors first occur in the lymph nodes and are strongly associated with poor prognosis (13). The incidence of lymph node metastasis ranges from 5% to 70% in localized prostate cancer and from 16% to 35% in bladder cancer (14–16). For patients with bladder cancer in the presence of lymph node metastases, the 5-year survival rate is significantly lower, at about 35%. Surgical removal of tumor-associated lymph nodes can help to improve the prognosis, so pelvic lymph node dissection (PLND) is essential for patients with prostate and bladder cancers at risk of metastasis (17). However, PLND is a complex procedure and is prone to miss metastatic lymph nodes. It is not clear how many and how extensive lymph nodes should be removed for optimal patient benefit. Fluoroscopic SLN-guided lymph node dissection may reduce unnecessary extended dissection while avoiding missing metastatic lymph nodes beyond the extent of the dissection template. However, fluorescence imaging has a limited depth of detection, and combined with radionuclide imaging may overcome this drawback. In addition, various lymph node imaging techniques continue to advance, and the application of multimodal imaging with two or more imaging modalities allows for a more accurate assessment of lymph node status.
In this review, we first provide an overview of fluorescent contrast agents approved for clinical use or effective in clinical trials for bladder or prostate cancer. We summarize the current status of lymph node dissection for these cancers and point out the problems. Second, using the SLN technique, we analyze the current status of fluorescence imaging-guided lymph node dissection for prostate and bladder cancer. Third, other pelvic lymph node imaging techniques are summarized, including fluorescence combined with radionuclide imaging and multimodal imaging. Finally, the limitations of fluorescence imaging are summarized and its future development is prospected.
2 Fluorescence imaging
To obtain clear, high-resolution images for intraoperative guidance, researchers have introduced fluorescence imaging. Fluorescence imaging is an intraoperative optical imaging technique in which the operator uses an external light source to illuminate the tissue of interest, which excites a fluorescent contrast agent and identifies the target tissue by the fluorescence it emits. The technique is based on the Stokes shift principle: there is an energy difference between the absorption and emission spectra of the contrast agent, which is excited by an external light source and captured by a sensor, and displayed on a monitor. Compared with conventional imaging, fluorescence imaging enhances tumor-surrounding tissue contrast, reduces positive margins, identifies occult lesions, and improves routine surgical accuracy (2, 18). For prostate and bladder cancers, fluorescence imaging can assist lymph node dissection by obtaining high-resolution images of the lymph nodes, and the key to achieving good imaging lies in selecting appropriate fluorescent contrast agents and imaging systems. We provide experience in the further development and clinical translation of this technique by reviewing the fluorescent imaging agents and imaging systems.
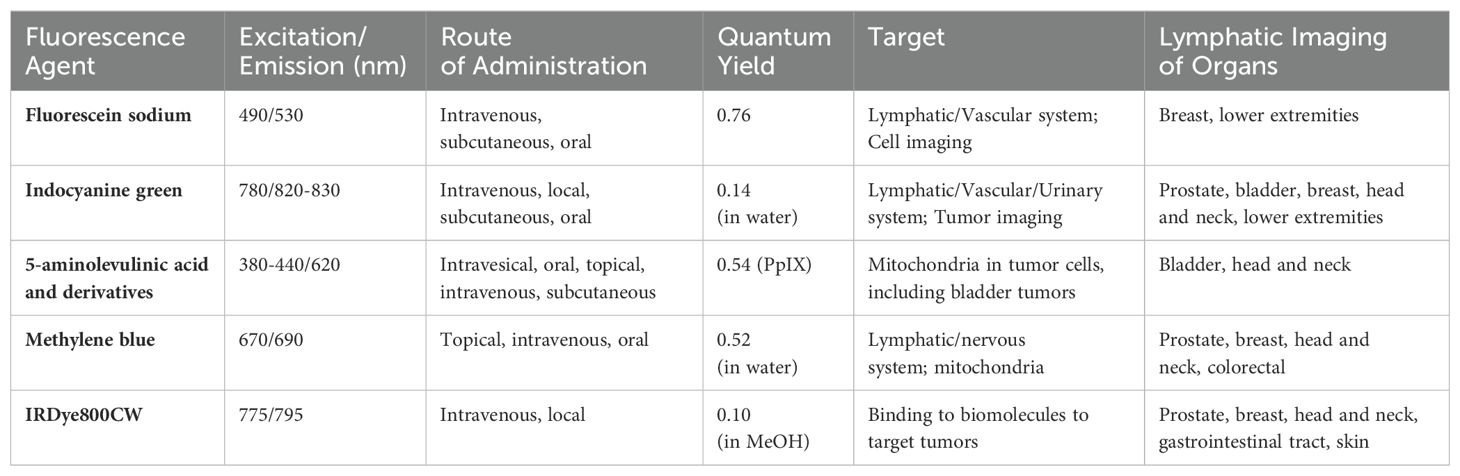
Currently, the fluorescent imaging drugs that have obtained clinical approval in China include fluorescein sodium, methylene blue, ICG, and 5-aminolevulinic acid (5-ALA) (19). Among them, methylene blue, ICG, and 5-ALA can be utilized in surgical procedures for prostate or bladder cancer. Fluorescent probes used in clinical trials for prostate or bladder cancer also include hexaminolevulinate (HAL), hypericin, and IRDye800CW (Table 1) (1, 20). In addition, there are some natural fluorophores present in the human body that produce autofluorescence when excited by visible light, such as aromatic amino acids, riboflavin, and porphyrin (21).
Fluorescein sodium, one of the earliest fluorescent dyes discovered in the mid-19th century, is now widely used for glioma identification and ophthalmic angiography (Approved for use in most countries) (22). It can also be used for lymphatic imaging to show lymphatic drainage pathways (Off-label use) (23). An in vivo imaging study using the Firefly Fluorescence Imaging System showed that sodium fluorescein could be visualized alongside ICG in the same image (24). In lymphatic supermicrosurgery, sodium fluorescein assesses lymphatic drainage patency (25). In resource-limited settings, sodium fluorescein is an effective low-cost alternative to ICG (26). However, its emission spectrum overlaps with natural fluorophores, limiting its use.
The ideal fluorescence wavelength for surgery is located in the Near-infrared (NIR) region (650-900 nm), where light is less likely to be absorbed by tissues, penetrates deeper into tissues and is less affected by scattering effects and autofluorescence (27). A commonly used fluorescent dye in the NIR region is ICG (28). It is a water-soluble tricarbocyanine dye with a half-life of 3-4 minutes after intravenous injection, and it mainly binds to serum albumin and α-1 lipoprotein, is metabolized by the liver, and excreted through the bile (21). Intravenous injection of ICG can be used for breast cancer detection (29). Additionally, ICG combined with albumin in an appropriate ratio can enhance the fluorescence intensity and help in lymph node detection (30). Because of its high contrast and good resolution in fluorescence imaging, ICG is widely used in clinical surgery (Approved for use in most countries). In non-tumor surgery, it can be used for vascular imaging of the retina and liver function assessment. In the field of oncologic surgery, it can be used for real-time fluorescence angiography in laparoscopic colorectal cancer surgery to assess proximal and distal intestinal perfusion, thus reducing the occurrence of anastomotic fistula (31, 32). In addition, ICG allows rapid tracing of lymphatic drainage by intradermal, subcutaneous, or peritumoral injection. It identifies lymphatic flow from the breast to the axilla in real-time in breast cancer surgery, facilitating precise SLN resection. In prostatectomy, ICG can be used for visualizing prostate lymphatic drainage pathways (33, 34). For penile cancer, metastasis occurs first in the inguinal lymph nodes and then spreads to the pelvic lymph nodes, making inguinal lymphatic metastasis the most important prognostic indicator. The use of ICG fluorescence imaging improves the accuracy of lymph node identification in the inguinal region without increasing complications (35, 36). In 2017, ICG was demonstrated to be capable of emitting in the near-infrared region II window (NIR-II, 1000-1700 nm) fluorescence (37). In 2020, Hu et al. further validated the feasibility of intraoperative fluorescence imaging in the NIR-II region in a study of 23 patients with hepatocellular carcinoma (38). Fluorescence imaging in the NIR-II region has further improved in terms of spatial resolution, imaging contrast, and penetration depth (39–41).
5-ALA is a precursor substance of protoporphyrin IX (PpIX), which is processed in the heme synthesis pathway to produce PpIX with strong photosensitizing activity (42). Exogenous 5-ALA entering the human body can be selectively absorbed by tumor cells, accumulating excess PpIX within the cells, which fluoresces red and leads to tumor cell necrosis and apoptosis under light irradiation at specific wavelengths. Although 5-ALA does not belong to near-infrared light, 5-ALA and PpIX can naturally exist in the human body and thus have relatively low toxicity. Currently, 5-ALA is used for the photodynamic diagnosis of bladder cancer, improving the detection rate of carcinoma in situ (Approved for use in most countries) (43, 44). Some studies have also explored its use in determining surgical margins in prostate cancer (Off-label use) (45, 46). HAL, an ester derivative of 5-ALA, was approved by the Food and Drug Administration (FDA) in 2010 for use with blue-light cystoscopy to detect a wider range of Ta or T1 stage bladder lesions (47, 48).
Methylene blue is used for SLN localization in various cancers, including breast, cervical, penile, and malignant melanoma (Off-label use) (49). Its sensitivity for SLN detection in penile cancer was 56%, lower than ICG, and particularly poor in obese patients (50, 51). It can also be used as a photosensitizer in photodynamic therapy (Experimental use). It reduces the survival of prostate cancer cells (PC3) by inducing oxidative stress and autophagy (52). Additionally, it has significant cytotoxic effects on bladder cancer cells (MBT-2 cell line), mainly through damage to cell membranes and mitochondria (53). Its clinical potential is limited by low fluorescence yield and lack of functional groups for ligand addition (54). Additionally, methylene blue may cause severe allergic reactions, hypertension, skin necrosis, and rashes (55–57).
Hypericin acts as a fluorescent dye that induces apoptosis in cancer cells by generating reactive oxygen species through light excitation at specific wavelengths (58, 59). It has shown antitumor effects in several tumor models, especially bladder cancer (Experimental use) (60). The main drawback is its insolubility in water; it is only soluble in ethanol and 1% plasma protein solution. D’Hallewin et al. demonstrated that hypericin can be used for fluorescence detection of bladder cancer, with papillary tumors displaying a red fluorescence and a sensitivity of 93% for detecting carcinoma in situ (61). In a larger study of 87 patients, the sensitivity and specificity for detecting carcinoma in situ were 94% and 95%, respectively (62). Compared with 5-ALA, hypericin has a higher specificity for detecting carcinoma in situ and may reduce the number of biopsies. Therefore, hypericin plays an important role in the early diagnosis and surgical navigation of bladder cancer.
The wavelength of IRDye800CW is located in the NIR region, and its advantage lies in its ability to bind to a wide range of biomolecules such as peptides, antibodies, and other targeting vectors to form specifically targeted fluorescent contrast agents. Combining monoclonal antibodies targeting the epidermal growth factor receptor (cetuximab or panitumumab) with IRDye800CW enables SLN-specific visualization and precise identification of metastatic lymph nodes (63). This drug is FDA-approved for use in clinical trials only. Rosenthal et al.’s study of head and neck tumors showed that cetuximab-IRDye800CW compared to standard histopathological examination of 35 pathologically positive lymph nodes 34 (sensitivity 97.2%) were correctly identified and 401 of 435 negative lymph nodes (specificity 92.7%) were correctly assessed by fluoroscopic imaging, which could avoid unnecessary neck lymph node dissection (64). Panitumumab is the first fully humanized monoclonal antibody, and Panitumumab-IRDye800CW has high sensitivity (84.6%) and specificity (94.0%) in identifying metastatic lymph nodes and distinguishing metastatic lymph nodes of different sizes from benign lymph nodes (65). Prostate-specific membrane antigen (PSMA) is highly expressed in most prostate cancer cells and is an ideal target for therapy. A recent U.S. clinical study testing human imaging results of a humanized PSMA minibody (IAB2M) conjugated to IRDye800CW-NHS ester fluorescent dye was able to detect PSMA-positive primary tumors, tumor-infiltrating lymph nodes (64% sensitivity and specificity), and metastatic lesions (100% sensitivity and 65% specificity) by intraoperative fluorescent imaging in 23 patients, improving the thoroughness of tumor resection (66).
In addition, the accuracy of fluorescence imaging is closely related to the imaging device (67). In 2005, the Novadaq SPY system became the first ICG imaging system approved for use by the FDA, and since then new systems have continued to be developed and introduced (54). A variety of high-definition laparoscopic and full-color surgical cameras are now commercially available on the market, and multispectral imaging devices that recognize multiple fluorescence emission wavelengths have also been introduced (68). In addition, some systems utilize hybrid modalities combining fluorescence imaging and radiotracing (69). The application of these advanced imaging devices may further improve the accuracy and visualization of fluorescence-guided procedures.
2.1 Applications of fluorescence imaging
We mainly focus on the application of fluorescence imaging in the lymphatic system. It is relatively difficult to localize lymph nodes during surgery due to the complex connections between lymphatic vessels, whereas fluorescence imaging can successfully show the draining lymph nodes, and precise intraoperative resection of the associated lymph nodes can significantly improve the patient’s prognosis (70, 71). Huang et al. demonstrated that, in ICG-guided laparoscopic lymph node dissection for gastric cancer, the ICG group detected 50.5 ± 15.9 lymph nodes compared to 42.0 ± 10.3 in the control group. ICG fluorescence imaging can effectively improve the prognosis of D2 lymph node dissection in gastric cancer (72). In esophageal cancer surgery, the mean number of regional lymph nodes dissected in 84 patients under ICG guidance was 25.68 ± 12.00, with stained images of the draining lymph nodes clearly shown endoscopically (73). In prostatectomy, ICG fluorescence imaging allows real-time visualization of draining lymph nodes, successfully displaying internal iliac, external iliac, and obturator nodes (74, 75). In SLN detection of bladder cancer, fluorescence imaging successfully displays lymphatic pathways along the inferior vesical vessels to the internal iliac lymph nodes for bladder drainage detection (76). Overall, the evidence level for fluorescence imaging-guided lymph node dissection is favorable.
3 Status of PLND
Prognostic factors affecting patients with bladder cancer include pT, pN, lymphovascular invasion, surgical margin status, and adjuvant chemotherapy, among which pT and pN are the most important predictors of survival (77, 78). Ku et al. demonstrated that lymph node invasion was present in as high as 25% of the patients, which is one of the major risk factors for poor prognosis (79). Zehnder et al. showed that the incidence of lymph node invasion in bladder cancer ranged from 16%-35% and that the five-year recurrence-free survival and overall survival of patients with positive lymph node pathology (21%-42% and 25%-38%) were significantly worse than those of patients with negative lymph node pathology (56%-78% and 49%-69%) (15). The results of the studies of Bruins et al. and Bi et al. showed that patients with the practice of PLND had better survival outcomes than patients without PLND (80, 81). For prostate cancer, lymph node invasion is likewise a key predictor of disease progression, with a five-year recurrence-free survival rate of 50% for pN1 patients compared to 85% for pN0 patients (82). PLND is essential in intermediate- and high-risk prostate cancer.
Histologic evaluation of PLND specimens provides information on patient prognosis, with the number of metastatic lymph nodes reflecting the severity of disease progression; the more metastatic lymph nodes, the worse the patient’s prognosis. Lymph node density (number of positive nodes/number of nodes removed) has been used previously to reflect disease status, extent, and quality of surgery (83). Kassouf et al. reported five-year tumor-specific survival rates of 15.3% and 54.6% for patients with lymph node densities of >20% and <20%, respectively, which were significantly different (84). However, the number of lymph node dissection is not only affected by the number of metastatic lymph nodes and the quality of surgery, but also by a variety of other factors, such as individual differences, surgical technique, submission method, and pathologic processing methods (85). Davies et al. performed super-expanded lymph node dissection on 26 cadavers and showed that lymph node counts in the same dissection template ranged from 10-53 (86). Chen et al. conducted an autopsy on 30 autopsies to verify the clinical significance of PLND in patients with prostate cancer and similarly found significant individual differences in lymph node counts (87).
Lymph node metastasis usually occurs in the fossa occulta, but can also occur in the region of the abdominal aorta and inferior vena cava. The optimal extent of PLND remains controversial due to the inconsistency and bias of current research findings. The terms “limited PLND, standard PLND, extended PLND (ePLND) and super-extended PLND” are commonly used to denote different degrees of PLND, but the boundaries of the lymph node dissection template are not clearly defined, which makes it difficult to compare the results of different studies. While it is generally accepted that only the obturator lymph nodes are resected is referred to as limited PLND, other studies have suggested that limited PLND includes bilateral obturator, internal iliac, and external iliac lymph nodes (88). To avoid controversy, some studies have advocated the use of “level I, II, and III dissections” as an alternative to different degrees of lymph node dissection (89, 90). level I dissection includes pelvic lymph nodes below the bifurcation of the common iliac artery, mainly internal, external iliac, and obturator lymph nodes; level II dissection includes lymph nodes below the bifurcation of the aorta, including common iliac and presacral lymph nodes; and level III dissection includes para-aortic, para-inferior, and intercavernous lymph nodes upward to the origin of the inferior mesenteric artery.
Currently, PLND is the most precise staging procedure for determining lymph node metastasis, effectively preventing understaging that may occur due to the inability to detect microscopic lesions on preoperative imaging (91, 92). ePLND provides a more accurate assessment of tumor spread to the lymphatic system by examining and resecting more lymph nodes. If cancerous infiltration of lymph nodes is detected during ePLND, patients may require more aggressive treatments, such as adjuvant radiotherapy or chemotherapy. Therefore, ePLND plays a pivotal role in the diagnostic and prognostic assessment of prostate and bladder cancer (93, 94). Leissner et al. described in detail the location of metastatic lymph nodes in bladder cancer and reported the results of a prospective multicenter study at the level of level III dissection, in which metastatic lymph nodes were present in 81 out of 290 patients (27.9%), with the most common metastatic area located in the obturator fossa (25.8%), followed by the common iliac (19.0%), external iliac (15.8%), and presacral regions (8.2%), and 10% of the patients with pN1 are misdiagnosed as pN0 by level I dissection (95). The results of a randomized controlled trial by Gschwend et al. that included 401 patients comparing level I and level III dissection showed that level III dissection did not reduce the rate of tumor recurrence (88). The current consensus is that level I dissection is insufficient to remove all metastatic lymph nodes (96). Outcomes for prostate cancer are similar to those for bladder cancer, with at least 10% of metastatic lymph nodes missed by level I dissection including the obturator, internal iliac, and external iliac lymph nodes, and the oncologic outcomes of ePLND have not been shown to have a clear advantage (97–99). A study by Lestingi et al. comparing limited PLND and ePLND in 291 patients with intermediate- to high-risk prostate cancer showed no significant difference between the two in terms of biochemical recurrence-free survival, bone metastases, and mortality (100). A multi-institutional study emphasized the controversial therapeutic role of PLND, and a systematic review showed no oncologic benefit (97, 101).
Studies have shown that the extent of PLND is strongly associated with complications (91, 102, 103). Briganti et al. demonstrated that ePLND is associated with a higher incidence of lymphocele (91, 104). A systematic review and meta-analysis assessed the perioperative morbidity of PLND, with 1.8% of patients experiencing intraoperative complications such as rectal, ureteral, obturator nerve, internal and external iliac vascular injuries, and intraoperative bleeds. Postoperative complications were seen in 14.1% of patients, with lymphocele being the most common (105). Limited PLND was associated with a lower risk of complications compared to ePLND. To address the complications of ePLND and reduce negative lymph node dissection, Martini et al. used predictive modeling to omit contralateral PLND in a specific patient population (106). This model requires further external validation. Therefore, although greater PLND may improve lymph node staging, its harms and benefits need to be considered together. Surgeons should cautiously decide on the extent of PLND based on metastatic risk and clinical experience.
Overall, PLND is essential for bladder and prostate cancers at risk of lymph node metastasis. It provides a basis for staging and predicts prognosis by removing lymph nodes. The therapeutic role of PLND remains controversial. Limited PLND may miss metastatic lymph nodes, but there is no consensus on whether more extensive dissection improves survival benefits.
3.1 Problems with PLND
First, PLND removes lymph nodes in both tumor-associated drainage areas and non-associated drainage areas in a non-specific manner, resulting in a significant portion of unaffected lymphatic tissue being removed out of an abundance of caution (107). Second, ePLND includes bilateral obturator, internal iliac, external iliac, presacral, common iliac, and lower abdominal lymph nodes, causing extensive disruption of pelvic lymphatic anatomy. Lymphocele and lymphedema are the most common complications of PLND (108). Third, PLND does not take into account individual patient differences, and the site of lymph node metastasis can change, such as the spread of the presacral region (109, 110). To address the problem of PLND, SLN technology was introduced to target the resection of tumor-related lymph nodes by fluorescence imaging. This may reduce the number of unnecessary lymph nodes to be cleared and decrease the complication rate while detecting more metastatic lymph nodes.
4 Fluorescence imaging-guided SLN resection
The SLN is the first group of lymph nodes into which the metastatic tumor cells enter. Identifying and removing the first group of draining lymph nodes allows for accurate tumor staging. The results of a PLND study based on SLN targeting showed that 35% of prostate-associated lymphatic drainage was located outside the ePLND (94).
Cabanas et al. first introduced the concept of sentinel lymph node biopsy in 1977 (111). SLN resection is now a routine screening technique in breast cancer, penile cancer, and melanoma. However, in the field of prostate and bladder cancers, the SLN technique is still in the experimental stage, and the continuous advancement of new tracers, imaging modalities, and surgical techniques may increase its potential for application. Grivas et al. conducted a controlled study of patients with prostate cancer who underwent SLN resection combined with ePLND and those who underwent ePLND only, and the results showed that 5-year biochemical recurrence-free survival rate of the patients who underwent SLN resection was significantly higher than those of the control group (80.5% vs. 69.9%, P=0.03), and the 5-year biochemical recurrence-free survival rate was increased by 29.4% in the subgroup that had more than 14 lymph nodes removed (112). This demonstrates that SLN-oriented lymph node dissection may have a positive impact on tumor outcome.
4.1 Prostate cancer
ePLND remains the gold standard for lymph node staging of prostate cancer (113). Patients with >5% risk of lymph node metastasis as predicted by nomograms are required to undergo ePLND (114). However, ePLND is accompanied by a series of complications, such as lymphocysts, occlusive nerve injury, and lymphedema (97, 105). In addition, the poor oncologic outcome of PLND may be related to lymph node metastasis occurring outside the PLND area. Fluorescence-guided SLN resection is expected to improve this situation.
In 2011, Inoue et al. first described the use of ICG to visualize the lymphatic system during open prostatectomy (74). The study by Claps et al. demonstrated that ICG-guided lymph node dissection combined with ePLND resulted in superior staging outcomes than ePLND alone, with a higher positivity rate of fluorescence-guided dissection of lymph nodes (65.9% vs. 34.1%, P=0.01), and a higher 5-year biochemical recurrence-free survival rate in pN+ patients (54.1% vs. 24.9%, P=0.023) (115). This confirms the feasibility of fluorescence-guided lymph node dissection. In a study by Jeschke et al., that included 582 lymph nodes in 26 patients with prostate cancer, fluorescence guidance revealed an additional 120 lymph nodes (20%) compared to radiation-guided lymph node dissection (116). Subsequently, using a combination of preoperative lymphoscintigraphy and intraoperative fluorescence-guided imaging, Hruby et al. showed fluorescent staining in 75% of 38 prostate cancer patients within the expanded PLND template, with 17.5% of fluorescent lymph nodes and 16.7% of metastatic lymph nodes located outside the expanded PLND template (117). Fluorescence guidance allows complete removal of the prostatic lymphatic drainage system, with fewer lymph nodes removed compared to the PLND template, and higher diagnostic accuracy. Fluorescence-guided imaging may help physicians to accurately identify lymph nodes intraoperatively and improve the accuracy of tumor staging.
ICG has become the most commonly used fluorescent dye for visualizing lymph nodes because of its good staining effect and high contrast. However, it also has some disadvantages, free ICG forms complexes with natural proteins such as human serum albumin and rapidly migrates through tissues and the lymphatic system. This means that ICG is less specific for SLN and stains a wider range. In terms of injection methods, the most common method is the transrectal or transperineal route, where an ultrasound-guided tracer is injected into each lobe of the prostate. Manny et al.’s study evaluated different routes of administration, concluding that robotically guided intraoperative ICG injections were more effective than the cystoscopic or transrectal routes (118). De Korne et al.’s study explored the relationship between the location of tracer injections, the location of primary tumors, and the visualization of SLN association and showed that tracer injection in the paraneoplastic and intracancerous areas was more favorable for detecting metastatic lymph nodes (119). The most effective tracer injection protocol has not yet been determined, and more relevant studies are needed.
4.2 Bladder cancer
Fluorescence-guided techniques in bladder cancer have great potential. Its application is more difficult due to the complex anatomy of the bladder lymphatic drainage system and the complicated mechanisms of lymph node metastasis (120). Lymphatic drainage from the bladder flows from the bladder to multiple lymph nodes in the pelvis, and the SLN may extend beyond the peripheral bladder region. Leadbetter et al. summarized six pathways of normal bladder lymphatic vessels that flow into the peripheral bladder network: (1) the intramural vesical plexus (2) the insertion of lymph nodes (3) the pelvic collecting trunk (4) local pelvic nodes (comprising the external iliac, obturator, and presacral nodes) (5) lymphatics from local pelvic nodes and (6) common iliac nodes (121). In addition, in the presence of multiple tumors in the bladder, it is difficult to determine the correct drainage path for each bladder region, and there may be a crossover of drainage to the contralateral lymph nodes. Polom et al. noted that crossover was present in 45% of cases, which confirms the necessity of bilateral lymph node dissection for muscle-invasive bladder cancer (122).
In 2001, Sherif et al. published the first study of visualized SLN in patients with bladder cancer, where lymphatic visualization was performed by preoperative injection of radiotracer and blue dye around the tumor, and SLN was detected in 11 out of 13 patients (85%) (123). In 2013, Inoue et al. described for the first time the use of ICG for lymphangiography during cystectomy, and the lymphatic pathways were successfully observed in 7 out of 12 patients (76). Unlike blue dyes such as methylene blue, ICG visualizes for a long time during SLN examination by binding to macromolecules in the lymphatic vessels. However, the current problem is that ICG is not yet specific for the identification of metastatic lymph nodes. Liedberg et al. reported a false-negative rate of 19% for the identification of SLN by ICG in 75 patients, which they attributed to the diversion of afferent lymphatic drainage due to complete obstruction of the pathologic SLN by the cancer cells, which prevents the visualization of metastatic lymph nodes (124). There are few studies on specific visualization of metastatic lymph nodes in bladder cancer, and further exploration of fluorescence-targeted probes for visualization of metastatic lymph nodes is needed in the future.
In terms of factors influencing the fluorescence effect, Knapp et al. found that intravesical pressure in the bladder space was a key parameter for fluorescence visualization of lymph nodes in an animal model study of the bladder (125). Similar results were found in a human study by Shaafsma et al. in which ICG was injected with human serum albumin around the bladder tumors and the intravesical pressure was increased by filling the bladder for 15 minutes, while the control group was not filled (126). The results suggested that only 1 patient (33%) showed fluorescent SLN when the bladder was unfilled, whereas 11 out of 12 patients (92%) showed fluorescent SLN when the bladder was filled. In addition, the effect of fluorescence varied with different sites of administration, with fluorescent lymph nodes observed with submucosal injections, and not observed with plasma membrane administration. In contrast, the injection of fluorescent dye into the tumor was unable to visualize lymph nodes due to the lack of lymphatic drainage channels. Manny et al. demonstrated for the first time fluorescence-guided SLN resection in robotic-assisted surgery, and 90% of the patients were able to visualize the draining nodes, only in patients with thick bladder walls (>2 cm), where submucosal and superficial injection of ICG into the peri-tumor periphery and peristomal muscle could lead to labeling failure, implying the technique’s for thick-walled bladders limitations (127). Does preoperative neoadjuvant chemotherapy affect fluorescence-guided SLN resection? A study by Rosenblatt et al. noted that SLN detection is completely feasible in patients with muscle-invasive bladder cancer after receiving neoadjuvant chemotherapy regardless of the pT stage (128). A study by Alvaeus et al. found that the number of SLNs was significantly lower in patients with muscle-invasive bladder cancer than in patients with progressive disease after receiving neoadjuvant chemotherapy (129).
5 Other lymph node imaging techniques
Computed tomography (CT) and magnetic resonance imaging (MRI) are the most commonly used imaging methods for diagnosis and staging of malignant tumors. CT assessment of lymph node metastasis relies mainly on lymph node size, shape, and internal structural changes. However, metastases may be present in normal-sized lymph nodes, and enlarged lymph nodes may also be infectious or inflammatory (130, 131). Most studies have concluded that CT has a sensitivity of 30%-75% and a specificity of 56%-100% for the diagnosis of lymph node metastasis (132). Similar to CT, MRI also assesses lymph node size and morphological changes by tomographic dissection. The difference is that MRI has a better resolution of soft tissues than CT, allowing more detailed visualization of the lymph node margins and whether the interior contains fatty structures, etc.
Preclinical studies are currently developing a variety of novel MRI contrast agents, such as superparamagnetic nanoparticles, transition metal manganese-containing compounds, and superparamagnetic ionic compounds, for detecting lymph node metastasis (133–135). Superparamagnetic iron oxide nanoparticles (USPIO) are a representative class of agents used for MRI lymph node imaging (136). USPIO is lymphophilic and is phagocytosed by macrophages after intravenous injection and accumulates in lymph nodes over a long time. Normal lymph nodes contain a large number of macrophages that phagocytose USPIO, and the signal is significantly reduced on T2WI images. In metastatic lymph nodes, macrophages are replaced by tumor cells, and the reduction in T2WI signal is insignificant or non-decreasing, and benign and malignant lymph nodes can be differentiated by this feature (137). Intraoperatively, the accumulation of USPIO in the tissue can be detected using a handheld magnetometer (A special device, which is Conformité Européenne (CE) certified as a class IIa medical device) (138, 139). A prospective study in Europe reported a 100% SLN detection rate in 50 patients with intermediate- to high-risk prostate cancer, with a sensitivity and specificity of 100% and 97%, respectively, and only 3% of metastatic lymph nodes were not detected because they were outside the expanded PLND template (140). The study also noted that the application of the procedure was limited to a small part of the developed world because of the reliance on radioisotopes and nuclear medicine facilities. Another European study concluded that intraoperative magnetically guided SLN resection in patients with a 5%-20% risk of lymph node invasion could avoid ePLND because SLN resection is sufficient to detect almost all lymph node metastases (141). However, the long scanning time due to the need for patients to undergo two MRIs (pre- and post-injection) and the fact that iron oxide-based contrast agents are not readily available and not approved for use in most countries, limit the clinical application of this procedure (142).
Radionuclide imaging provides images of lymph nodes by detecting gamma rays emitted by radionuclides such as 68Ga, 99mTc, and 131I (143–145). The most commonly used radionuclide is 99mTc, with a half-life of 6 hours. Its labeled colloid has a diameter of 10nm-200nm, which facilitates rapid passage through the lymphatic vessels and retention in the lymph nodes (146, 147). Currently, commonly used nuclide markers include 99mTc-sulfur-colloids and 99mTc-Dextrans. After injection, SLN can be identified intraoperatively by the operator using a hand-held γ-detector. The advantages of radiotracer visualization of lymph nodes include good tissue penetration for detection of deep lymph nodes, preoperative radiological examination to obtain information about lymph nodes, and intraoperative radiation-guided excision of SLN. The disadvantages of this method are mainly the risk of radiation exposure, and the surgeon needs to take protective measures to complete the surgery. In addition, the risk of allergic reactions needs to be evaluated before application (148).
With the increasing importance of PSMA in the diagnosis of prostate cancer, positron emission computed tomography (PET) combined with PSMA has become a new technique for the evaluation of SLN in prostate cancer. Klingenberg et al. detected 31.4% of metastatic lymph nodes in 691 patients with prostate cancer using 68Ga-PSMA PET/CT (149). Sprute et al. detected metastatic lymph nodes using the 18F-PSMA-1007 tracer, and 84 out of 92 positive lymph nodes were determined to be true positives by pathology (150). A meta-analysis including 10 studies using 68Ga-PSMA PET/CT to assess lymph node metastasis showed a combined sensitivity of 61% and specificity of 96% per patient. For patients at low risk of lymph node metastasis, PSMA PET/CT-negative patients may reduce unnecessary lymph node dissection procedures (151). More relevant prospective large-scale cohort studies are needed to prove its accuracy.
6 Combined fluorescence and radionuclide imaging to guide PLND
Fluorescence imaging is less time-consuming, easy to perform, safe, and convenient. The disadvantage is that fluorescence signal penetration is limited, making it difficult to detect deep lesions. In contrast, γ-rays hardly attenuate in tissues, and radionuclide-labeled fluorescent substances can provide in-depth lymph node information by single-photon emission computed tomography (SPECT) or PET examination (152). Combining fluorescence imaging and radionuclide imaging can effectively solve the problem of insufficient fluorescence penetration (153).
In prostate cancer, fluorescence combined with radionuclide imaging or biomarker PSMA aims to improve the accuracy of identifying lymph node metastases, avoiding the omission of potentially metastatic lymph nodes, and reducing complications associated with ePLND. ICG-99mTc nanocolloids were the first hybrid tracer used to improve lymph node dissection procedures (154). Labeling methods using hybridization of 99mTc and ICG reduce their respective limitations and offer the advantages of both. Van der Poel et al. injected a hybrid tracer into the prostate, which allowed for preoperative SLN localization and intraoperative detection of resection (155). Jeschke et al. described an alternative approach with sequential injection of the radiocolloid 99mTc and free ICG, which also allowed for preoperative and intraoperative lymph node detection (116). Wit et al. compared the two methods and showed that the hybridization approach (ICG-99mTc) appeared to be more effective than the sequential use of 99mTc nanocolloid and ICG, with a higher incidence of metastatic fluorescent lymph nodes in the hybridization group (7.4%) than in the sequential group (2.6%; p = 0.002) (156). Hinsenveld et al. evaluated the use of fluorescence imaging in combination with PSMA PET/CT, injecting ICG-99mTc nanocolloids or sequentially applying 99mTc and free ICG in patients who had a negative preoperative PSMA PET/CT, and accurate lymph node staging was possible in 50 out of 53 patients (94% diagnostic accuracy), and all pN+ patients were correctly identified (157).
Radionuclide imaging in bladder cancer can accurately determine the location of lymph nodes, but cannot identify tiny lymphatic vessels. In contrast, fluorescence imaging can accurately observe the flow route of lymphatic fluid in lymphatic vessels. Therefore, the combination of fluorescence and radiotracer shows good application prospects. Studies have demonstrated that the combination of fluorescence and radiotracer can detect more than 80% of the patient’s anterior region, including the SLN outside the PLND template. In 2022, Rietbergen et al. performed the first study of SLN resection for bladder cancer using ICG-99mTc nanocolloid and demonstrated that 83% of SLNs detected by preoperative imaging could be used for intraoperative SLN resection, which was safe and feasible in three patients whose SLNs were located outside of the ePLND (158).
7 Multimodal imaging
The simultaneous use of two or more imaging modalities has triggered the emergence of the concept of multimodal imaging for better characterization of lymphatic vessels and lymph nodes (159, 160). Imaging techniques such as CT, MRI, SPECT, and PET, in combination with fluorescence imaging, have been used for lymph node identification and real-time image-guided resection in preclinical applications (161–164). In addition, the combination of imaging techniques with tumor biomarkers such as PSMA helps to achieve accurate detection of metastatic lymph nodes. Discussions on the biological mechanisms of tumorigenesis, progression, metastasis, and heterogeneity have provided a basis for selecting appropriate biomarkers as targeted imaging drugs (165–167). Nevertheless, drugs ultimately need to be rigorously evaluated to ensure safety and feasibility in the human body. The evolving variety of imaging modalities has collectively contributed to the advancement of multimodal imaging, providing a more precise and reliable solution to the problem of lymph node metastasis in prostate and bladder cancer (163, 168).
8 Limitations and future perspectives of fluorescence imaging
Fluorescence imaging has great potential for lymph node detection in prostate and bladder cancer, providing an easy method to recognize and identify lymph nodes (169, 170). However, its accuracy still needs to be combined with the operator’s clinical experience and preoperative examination. Currently, postoperative pathology is still the gold standard for determining lymph node metastasis, and fluorescence imaging has not yet been able to achieve intraoperative identification of metastatic lymph nodes (171). Currently, it is difficult to directly compare the results of clinical studies from different research centers for reasons that include the lack of consensus on the assessment of clinical outcomes of fluorescence imaging, as well as the inconsistency of various parameters, observer differences, and the learning curve of the technique (172). The development and application of novel fluorescent probes are challenged by the variety of parameters that affect the effectiveness of fluorescent imaging (173). Continued development and precise matching of imaging systems and fluorescent contrast agents may further improve detection.
In addition, understanding the effects of different routes of administration of fluorescent contrast agents, such as intravenous, intratumor, paratumor, subcutaneous, and intradermal injections, on the imaging effect is also extremely important for reducing drug toxicity, decreasing the incidence of adverse reactions and complications, and thus facilitating their clinical translation (174). Fluorescence imaging-guided lymph node dissection in patients with prostate and bladder cancer is still in its infancy, and the goal of clinical studies is to evaluate the safety, feasibility, and optimal dose of fluorescent contrast agents. In the future, multimodal imaging combining multiple imaging modalities and tumor markers may allow for a more comprehensive evaluation of pelvic lymph nodes.
9 Conclusion
For prostate and bladder cancers, pelvic lymphatic drainage is complex and variable, making metastatic lymph nodes easily missed. Determining the number and extent of PLND is a challenge for urologists. Fluorescence-guided SLN resection is an important tool to solve this challenge and contributes to accurate tumor staging. Due to the limited penetration depth of fluorescence imaging, radionuclide imaging compensates for this deficiency. The combined fluorescence and radionuclide-guided approach allows for more accurate lymph node staging. The concept of multimodal imaging brings new hope for identifying pelvic metastatic lymph nodes. In the future, an expert consensus on the results of fluorescence imaging of lymph nodes should be formulated as soon as possible, and comprehensive evaluation through high-quality clinical studies is needed.
Author contributions
MX: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Methodology. PL: Conceptualization, Writing – original draft, Writing – review & editing. JW: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. PY: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. XG: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. CL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. XY: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research has been supported by the Central Guidance on the Local Science and Technology Development Fund of Shanxi Province (No. YDZJSX2021C010), the Patent Conversion Project Fund of Shanxi Province (No. 202304008), and the Natural Science Foundation of Shanxi Province (No. 2021210302124590).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PLND, pelvic lymph node dissection; ePLND, extended pelvic lymph node dissection; ICG, indocyanine green; SLN, sentinel lymph node; CT, computed tomography; MRI, magnetic resonance imaging; USPIO, ultrasmall superparamagnetic iron oxide nanoparticles; SPECT, single-photon emission computed tomography; PSMA, prostate-specific membrane antigen; PET, positron emission tomography; FDA, Food and Drug Administration; NIR, near-infrared; 5-ALA, 5-aminolevulinic acid; HAL, hexaminolevulinate; PpIX, protoporphyrin IX.
References
1. Ito R, Kamiya M, Urano Y. Molecular probes for fluorescence image-guided cancer surgery. Curr Opin Chem Biol. (2022) 67:102112. doi: 10.1016/j.cbpa.2021.102112
2. Lauwerends LJ, van Driel P, Baatenburg de Jong RJ, Hardillo JAU, Koljenovic S, Puppels G, et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. (2021) 22:e186–e95. doi: 10.1016/S1470-2045(20)30600-8
3. Wang C, Wang Z, Zhao T, Li Y, Huang G, Sumer BD, et al. Optical molecular imaging for tumor detection and image-guided surgery. Biomaterials. (2018) 157:62–75. doi: 10.1016/j.biomaterials.2017.12.002
4. Hernot S, van Manen L, Debie P, Mieog JSD, Vahrmeijer AL. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. (2019) 20:e354–e67. doi: 10.1016/S1470-2045(19)30317-1
5. Lee ES, Kim TS, Kim SK. Current status of optical imaging for evaluating lymph nodes and lymphatic system. Korean J radiology. (2015) 16:21–31. doi: 10.3348/kjr.2015.16.1.21
6. Reynolds JS, Troy TL, Mayer RH, Thompson AB, Waters DJ, Cornell KK, et al. Imaging of spontaneous canine mammary tumors using fluorescent contrast agents. Photochem photobiology. (1999) 70:87–94. doi: 10.1111/j.1751-1097.1999.tb01953.x
7. Nimura H, Narimiya N, Mitsumori N, Yamazaki Y, Yanaga K, Urashima M. Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J surgery. (2004) 91:575–9. doi: 10.1002/bjs.4470
8. Ito N, Fukuta M, Tokushima T, Nakai K, Ohgi S. Sentinel node navigation surgery using indocyanine green in patients with lung cancer. Surg Today. (2004) 34:581–5. doi: 10.1007/s00595-004-2780-y
9. Unno N, Inuzuka K, Suzuki M, Yamamoto N, Sagara D, Nishiyama M, et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc surgery. (2007) 45:1016–21. doi: 10.1016/j.jvs.2007.01.023
10. Sharma R, Wang W, Rasmussen JC, Joshi A, Houston JP, Adams KE, et al. Quantitative imaging of lymph function. Am J Physiol Heart Circulatory Physiol. (2007) 292:H3109–18. doi: 10.1152/ajpheart.01223.2006
11. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
12. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodeling in cancer. Nat Rev Cancer. (2014) 14:159–72. doi: 10.1038/nrc3677
13. Seisen T, Vetterlein MW, Karabon P, Jindal T, Sood A, Nocera L, et al. Efficacy of local treatment in prostate cancer patients with clinically pelvic lymph node-positive disease at initial diagnosis. Eur urology. (2018) 73:452–61. doi: 10.1016/j.eururo.2017.08.011
14. Gandaglia G, Fossati N, Zaffuto E, Bandini M, Dell’Oglio P, Bravi CA, et al. Development and internal validation of a novel model to identify the candidates for extended pelvic lymph node dissection in prostate cancer. Eur urology. (2017) 72:632–40. doi: 10.1016/j.eururo.2017.03.049
15. Zehnder P, Studer UE, Skinner EC, Dorin RP, Cai J, Roth B, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J urology. (2011) 186:1261–8. doi: 10.1016/j.juro.2011.06.004
16. Briganti A, Chun FK, Salonia A, Gallina A, Farina E, Da Pozzo LF, et al. Validation of a nomogram predicting the probability of lymph node invasion based on the extent of pelvic lymphadenectomy in patients with clinically localized prostate cancer. BJU Int. (2006) 98:788–93. doi: 10.1111/j.1464-410X.2006.06318.x
17. Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Brunckhorst O, Darraugh J, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer-2024 update. Part I: screening, diagnosis, and local treatment with curative intent. Eur Urol. (2024) 86:148–63. doi: 10.1016/j.eururo.2024.03.027
18. Alam IS, Steinberg I, Vermesh O, van den Berg NS, Rosenthal EL, van Dam GM, et al. Emerging intraoperative imaging modalities to improve surgical precision. Mol Imaging Biol. (2018) 20:705–15. doi: 10.1007/s11307-018-1227-6
19. Tipirneni KE, Rosenthal EL, Moore LS, Haskins AD, Udayakumar N, Jani AH, et al. Fluorescence imaging for cancer screening and surveillance. Mol Imaging Biol. (2017) 19:645–55. doi: 10.1007/s11307-017-1050-5
20. Van Keulen S, Hom M, White H, Rosenthal EL, Baik FM. The evolution of fluorescence-guided surgery. Mol Imaging Biol. (2023) 25:36–45. doi: 10.1007/s11307-022-01772-8
21. Mieog JSD, Achterberg FB, Zlitni A, Hutteman M, Burggraaf J, Swijnenburg RJ, et al. Fundamentals and developments in fluorescence-guided cancer surgery. Nat Rev Clin Oncol. (2022) 19:9–22. doi: 10.1038/s41571-021-00548-3
22. Xi C, Jinli S, Jianyao M, Yan C, Huijuan L, Zhongjie S, et al. Fluorescein-guided surgery for high-grade glioma resection: a five-year-long retrospective study at our institute. Front Oncol. (2023) 13:1191470. doi: 10.3389/fonc.2023.1191470
23. Meershoek P, KleinJan GH, van Oosterom MN, Wit EMK, van Willigen DM, Bauwens KP, et al. Multispectral-fluorescence imaging as a tool to separate healthy from disease-related lymphatic anatomy during robot-assisted laparoscopy. J Nucl medicine: Off publication Soc Nucl Med. (2018) 59:1757–60. doi: 10.2967/jnumed.118.211888
24. Meershoek P, KleinJan GH, van Willigen DM, Bauwens KP, Spa SJ, van Beurden F, et al. Multi-wavelength fluorescence imaging with a da Vinci Firefly-a technical look behind the scenes. J robotic surgery. (2021) 15:751–60. doi: 10.1007/s11701-020-01170-8
25. Ayestaray B, Bekara F. Fluorescein sodium fluorescence microscope-integrated lymphangiography for lymphatic supermicrosurgery. Microsurgery. (2015) 35:407–10. doi: 10.1002/micr.22368
26. Yadav SK, Bharath S, Sharma D, Srivastava A, Jha CK, Agarwal G, et al. A systematic review and meta-analysis of diagnostic performance of fluorescein-guided sentinel lymph node biopsy in early breast cancer. Breast Cancer Res Treat. (2024) 206:19–30. doi: 10.1007/s10549-024-07310-0
27. Frangioni JV. New technologies for human cancer imaging. J Clin oncology: Off J Am Soc Clin Oncol. (2008) 26:4012–21. doi: 10.1200/JCO.2007.14.3065
28. Barth CW, Gibbs SL. Fluorescence image-guided surgery - a perspective on contrast agent development. Proc SPIE–the Int Soc Optical Eng. (2020) 11222. doi: 10.1117/12.2545292
29. Bourgeois P, Veys I, Noterman D, De Neubourg F, Chintinne M, Vankerckhove S, et al. Near-infrared fluorescence imaging of breast cancer and axillary lymph nodes after intravenous injection of free indocyanine green. Front Oncol. (2021) 11:602906. doi: 10.3389/fonc.2021.602906
30. Markuszewski M, Buszewska-Forajta M, Artymowicz M, Połom W, Roslan M, Markuszewski M. Binding indocyanine green to human serum albumin potentially enhances the detection of sentinel lymph nodes. An initial step for facilitating the detection of first-station nodes in penile and other urological cancers. Arch Med science: AMS. (2022) 18:719–25. doi: 10.5114/aoms/113237
31. Renna MS, Grzeda MT, Bailey J, Hainsworth A, Ourselin S, Ebner M, et al. Intraoperative bowel perfusion assessment methods and their effects on anastomotic leak rates: meta-analysis. Br J surgery. (2023) 110:1131–42. doi: 10.1093/bjs/znad154
32. Alius C, Tudor C, Badiu CD, Dascalu AM, Smarandache CG, Sabau AD, et al. Indocyanine green-enhanced colorectal surgery-between being superfluous and being a game-changer. Diagnostics (Basel Switzerland). (2020) 10. doi: 10.3390/diagnostics10100742
33. Ramírez-Backhaus M, Mira Moreno A, Gómez Ferrer A, Calatrava Fons A, Casanova J, Solsona Narbón E, et al. Indocyanine green guided pelvic lymph node dissection: an efficient technique to classify the lymph node status of patients with prostate cancer who underwent radical prostatectomy. J urology. (2016) 196:1429–35. doi: 10.1016/j.juro.2016.05.087
34. Nguyen DP, Huber PM, Metzger TA, Genitsch V, Schudel HH, Thalmann GN. A specific mapping study using fluorescence sentinel lymph node detection in patients with intermediate- and high-risk prostate cancer undergoing extended pelvic lymph node dissection. Eur urology. (2016) 70:734–7. doi: 10.1016/j.eururo.2016.01.034
35. Yan X, Liu C, Cui L, Yan P, Fu X, Chen W, et al. Near-infrared fluorescence-assisted superficial inguinal lymph-node excision for low-risk penile cancer. World J urology. (2024) 42:206. doi: 10.1007/s00345-024-04877-7
36. Yuan P, Xie Y, Xu R, Li Y, Yao K, Liu J, et al. Efficacy of indocyanine green fluorescence-guided inguinal lymph node dissection for penile cancer: a randomized trial. BJU Int. (2024) 133:442–50. doi: 10.1111/bju.v133.4
37. Starosolski Z, Bhavane R, Ghaghada KB, Vasudevan SA, Kaay A, Annapragada A. Indocyanine green fluorescence in second near-infrared (NIR-II) window. PloS One. (2017) 12:e0187563. doi: 10.1371/journal.pone.0187563
38. Hu Z, Fang C, Li B, Zhang Z, Cao C, Cai M, et al. First-in-human liver-tumor surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat Biomed engineering. (2020) 4:259–71. doi: 10.1038/s41551-019-0494-0
39. Li C, Chen G, Zhang Y, Wu F, Wang Q. Advanced fluorescence imaging technology in the near-infrared-II window for biomedical applications. J Am Chem Society. (2020) 142:14789–804. doi: 10.1021/jacs.0c07022
40. Zhu S, Tian R, Antaris AL, Chen X, Dai H. Near-infrared-II molecular dyes for cancer imaging and surgery. Advanced materials (Deerfield Beach Fla). (2019) 31:e1900321. doi: 10.1002/adma.201900321
41. Ishizawa T, McCulloch P, Stassen L, van den Bos J, Regimbeau JM, Dembinski J, et al. Assessing the development status of intraoperative fluorescence imaging for anatomy visualization, using the IDEAL framework. BMJ surgery interventions Health technologies. (2022) 4:e000156. doi: 10.1136/bmjsit-2022-000156
42. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of Malignant glioma: a randomized controlled multicenter phase III trial. Lancet Oncol. (2006) 7:392–401. doi: 10.1016/S1470-2045(06)70665-9
43. Hagimoto H, Makita N, Mine Y, Kokubun H, Murata S, Abe Y, et al. Comparison between 5-aminolevulinic acid photodynamic diagnosis and narrow-band imaging for bladder cancer detection. BMC urology. (2021) 21:180. doi: 10.1186/s12894-021-00946-w
44. Inoue K, Matsuyama H, Fujimoto K, Hirao Y, Watanabe H, Ozono S, et al. The clinical trial on the safety and effectiveness of the photodynamic diagnosis of non-muscle-invasive bladder cancer using fluorescent light-guided cystoscopy after oral administration of 5-aminolevulinic acid (5-ALA). Photodiagnosis Photodyn Ther. (2016) 13:91–6. doi: 10.1016/j.pdpdt.2015.12.011
45. Ganzer R, Blana A, Denzinger S, Wieland WF, Adam C, Becker A, et al. Intraoperative photodynamic evaluation of surgical margins during endoscopic extraperitoneal radical prostatectomy with the use of 5-aminolevulinic acid. J endourology. (2009) 23:1387–94. doi: 10.1089/end.2009.0374
46. Fukuhara H, Inoue K, Kurabayashi A, Furihata M, Shuin T. Performance of 5-aminolevulinic-acid-based photodynamic diagnosis for radical prostatectomy. BMC urology. (2015) 15:78. doi: 10.1186/s12894-015-0073-y
47. Rink M, Babjuk M, Catto JW, Jichlinski P, Shariat SF, Stenzl A, et al. Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: a critical review of the current literature. Eur urology. (2013) 64:624–38. doi: 10.1016/j.eururo.2013.07.007
48. Lotan Y, Bivalacqua TJ, Downs T, Huang W, Jones J, Kamat AM, et al. Blue light flexible cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer: review of the clinical evidence and consensus statement on optimal use in the USA - update 2018. Nat Rev Urology. (2019) 16:377–86. doi: 10.1038/s41585-019-0184-4
49. Skanjeti A, Dhomps A, Paschetta C, Tordo J, Giammarile F. Sentinel node mapping in gynecologic cancers: A comprehensive review. Semin Nucl Med. (2019) 49:521–33. doi: 10.1053/j.semnuclmed.2019.06.012
50. Spiess PE, Izawa JI, Bassett R, Kedar D, Busby JE, Wong F, et al. Preoperative lymphoscintigraphy and dynamic sentinel node biopsy for staging penile cancer: results with pathological correlation. J urology. (2007) 177:2157–61. doi: 10.1016/j.juro.2007.01.125
51. Dell’Oglio P, de Vries HM, Mazzone E, KleinJan GH, Donswijk ML, van der Poel HG, et al. Hybrid indocyanine green-(99m)Tc-nanocolloid for single-photon emission computed tomography and combined radio- and fluorescence-guided sentinel node biopsy in penile cancer: results of 740 inguinal basins assessed at a single institution. Eur urology. (2020) 78:865–72. doi: 10.1016/j.eururo.2020.09.007
52. de Melo Gomes LC, de Oliveira Cunha AB, Peixoto LFF, Zanon RG, Botelho FV, Silva MJB, et al. Photodynamic therapy reduces cell viability, migration and triggers necroptosis in prostate tumor cells. Photochemical photobiological Sci. (2023) 22:1341–56. doi: 10.1007/s43630-023-00382-9
53. Yu DS, Chang SY, Ma CP. Ultrastructural changes of bladder cancer cells following methylene blue-sensitized photodynamic treatment. Eur urology. (1991) 19:322–6. doi: 10.1159/000473652
54. DS AV, Lin H, Henderson ER, Samkoe KS, Pogue BW. Review of fluorescence guided surgery systems: identification of key performance capabilities beyond indocyanine green imaging. J Biomed optics. (2016) 21:80901. doi: 10.1117/1.JBO.21.8.080901
55. Masannat Y, Shenoy H, Speirs V, Hanby A, Horgan K. Properties and characteristics of the dyes injected to assist axillary sentinel node localization in breast surgery. Eur J Surg Oncol. (2006) 32:381–4. doi: 10.1016/j.ejso.2006.01.010
56. Cwalinski T, Polom W, Marano L, Roviello G, D’Angelo A, Cwalina N, et al. Methylene blue-current knowledge, fluorescent properties, and its future use. J Clin Med. (2020) 9. doi: 10.3390/jcm9113538
57. Bézu C, Coutant C, Salengro A, Daraï E, Rouzier R, Uzan S. Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg Oncol. (2011) 20:e55–9. doi: 10.1016/j.suronc.2010.10.002
58. Kochergin M, Fahmy O, Asimakopoulos A, Theil G, Zietz K, Bialek J, et al. Photodynamic therapy: current trends and potential future role in the treatment of bladder cancer. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25020960
59. Buytaert E, Matroule JY, Durinck S, Close P, Kocanova S, Vandenheede JR, et al. Molecular effectors and modulators of hypericin-mediated cell death in bladder cancer cells. Oncogene. (2008) 27:1916–29. doi: 10.1038/sj.onc.1210825
60. Choudhary N, Collignon TE, Tewari D, Bishayee A. Hypericin and its anticancer effects: From mechanism of action to potential therapeutic application. Phytomedicine: Int J phytotherapy phytopharmacology. (2022) 105:154356. doi: 10.1016/j.phymed.2022.154356
61. D’Hallewin MA, De Witte PA, Waelkens E, Merlevede W, Baert L. Fluorescence detection of flat bladder carcinoma in situ after intravesical instillation of hypericin. J urology. (2000) 164:349–51. doi: 10.1016/S0022-5347(05)67357-0
62. D’Hallewin MA, Kamuhabwa AR, Roskams T, De Witte PA, Baert L. Hypericin-based fluorescence diagnosis of bladder carcinoma. BJU Int. (2002) 89:760–3. doi: 10.1046/j.1464-410x.2002.02690.x
63. Gao RW, Teraphongphom N, de Boer E, van den Berg NS, Divi V, Kaplan MJ, et al. Safety of panitumumab-IRDye800CW and cetuximab-IRDye800CW for fluorescence-guided surgical navigation in head and neck cancers. Theranostics. (2018) 8:2488–95. doi: 10.7150/thno.24487
64. Rosenthal EL, Moore LS, Tipirneni K, de Boer E, Stevens TM, Hartman YE, et al. Sensitivity and specificity of cetuximab-IRDye800CW to identify regional metastatic disease in head and neck cancer. Clin Cancer Res. (2017) 23:4744–52. doi: 10.1158/1078-0432.CCR-16-2968
65. Nishio N, van den Berg NS, van Keulen S, Martin BA, Fakurnejad S, Teraphongphom N, et al. Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat Commun. (2019) 10:5044. doi: 10.1038/s41467-019-13076-7
66. Hamdy FC, Lamb AD, Tullis IDC, Verrill C, Rombach I, Rao SR, et al. First-in-man study of the PSMA Minibody IR800-IAB2M for molecularly targeted intraoperative fluorescence guidance during radical prostatectomy. Eur J Nucl Med Mol imaging. (2024) 51:3009–25. doi: 10.1007/s00259-024-06713-x
67. Pogue BW, Rosenthal EL. Review of successful pathways for regulatory approvals in open-field fluorescence-guided surgery. J Biomed optics. (2021) 26. doi: 10.1117/1.JBO.26.3.030901
68. van den Berg NS, Buckle T, KleinJan GH, van der Poel HG, van Leeuwen FWB. Multispectral fluorescence imaging during robot-assisted laparoscopic sentinel node biopsy: A first step towards a fluorescence-based anatomic roadmap. Eur urology. (2017) 72:110–7. doi: 10.1016/j.eururo.2016.06.012
69. Dell’Oglio P, Meershoek P, Maurer T, Wit EMK, van Leeuwen PJ, van der Poel HG, et al. A DROP-IN gamma probe for robot-assisted radioguided surgery of lymph nodes during radical prostatectomy. Eur urology. (2021) 79:124–32. doi: 10.1016/j.eururo.2020.10.031
70. Roh CK, Choi S, Seo WJ, Cho M, Son T, Kim HI, et al. Indocyanine green fluorescence lymphography during gastrectomy after initial endoscopic submucosal dissection for early gastric cancer. Br J surgery. (2020) 107:712–9. doi: 10.1002/bjs.11438
71. Zapardiel I, Alvarez J, Barahona M, Barri P, Boldo A, Bresco P, et al. Utility of intraoperative fluorescence imaging in gynecologic surgery: systematic review and consensus statement. Ann Surg Oncol. (2021) 28:3266–78. doi: 10.1245/s10434-020-09222-x
72. Chen QY, Xie JW, Zhong Q, Wang JB, Lin JX, Lu J, et al. Safety and efficacy of indocyanine green tracer-guided lymph node dissection during laparoscopic radical gastrectomy in patients with gastric cancer: A randomized clinical trial. JAMA surgery. (2020) 155:300–11. doi: 10.1001/jamasurg.2019.6033
73. Wang X, Hu Y, Wu X, Liang M, Hu Z, Gan X, et al. Near-infrared fluorescence imaging-guided lymphatic mapping in thoracic esophageal cancer surgery. Surg endoscopy. (2022) 36:3994–4003. doi: 10.1007/s00464-021-08720-7
74. Inoue S, Shiina H, Arichi N, Mitsui Y, Hiraoka T, Wake K, et al. Identification of lymphatic pathway involved in the spreading of prostate cancer by fluorescence navigation approach with intraoperatively injected indocyanine green. Can Urological Assoc J = J l’Association Des urologues du Canada. (2011) 5:254–9. doi: 10.5489/cuaj.10159
75. Yuen K, Miura T, Sakai I, Kiyosue A, Yamashita M. Intraoperative Fluorescence Imaging for Detection of Sentinel Lymph Nodes and Lymphatic Vessels during Open Prostatectomy using Indocyanine Green. J urology. (2015) 194:371–7. doi: 10.1016/j.juro.2015.01.008
76. Inoue S, Shiina H, Mitsui Y, Yasumoto H, Matsubara A, Igawa M. Identification of lymphatic pathway involved in the spread of bladder cancer: Evidence obtained from fluorescence navigation with intraoperatively injected indocyanine green. Can Urological Assoc J = J l’Association Des urologues du Canada. (2013) 7:E322–8. doi: 10.5489/cuaj.1251
77. Hautmann RE, de Petriconi RC, Pfeiffer C, Volkmer BG. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur urology. (2012) 61:1039–47. doi: 10.1016/j.eururo.2012.02.028
78. Matsumoto A, Nakagawa T, Kanatani A, Ikeda M, Kawai T, Miyakawa J, et al. Preoperative chronic kidney disease is predictive of oncological outcome of radical cystectomy for bladder cancer. World J urology. (2018) 36:249–56. doi: 10.1007/s00345-017-2141-2
79. Ku JH, Kang M, Kim HS, Jeong CW, Kwak C, Kim HH. Lymph node density as a prognostic variable in node-positive bladder cancer: a meta-analysis. BMC cancer. (2015) 15:447. doi: 10.1186/s12885-015-1448-x
80. Bruins HM, Veskimae E, Hernandez V, Imamura M, Neuberger MM, Dahm P, et al. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: a systematic review. Eur urology. (2014) 66:1065–77. doi: 10.1016/j.eururo.2014.05.031
81. Bi L, Huang H, Fan X, Li K, Xu K, Jiang C, et al. Extended vs non-extended pelvic lymph node dissection and their influence on recurrence-free survival in patients undergoing radical cystectomy for bladder cancer: a systematic review and meta-analysis of comparative studies. BJU Int. (2014) 113:E39–48. doi: 10.1111/bju.2014.113.issue-5b
82. Evangelista L, Guttilla A, Zattoni F, Muzzio PC, Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur urology. (2013) 63:1040–8. doi: 10.1016/j.eururo.2012.09.039
83. Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J urology. (2003) 169:943–5. doi: 10.1097/01.ju.0000032474.22093.06
84. Kassouf W, Agarwal PK, Herr HW, Munsell MF, Spiess PE, Brown GA, et al. Lymph node density is superior to TNM nodal status in predicting disease-specific survival after radical cystectomy for bladder cancer: analysis of pooled data from MDACC and MSKCC. J Clin Oncol. (2008) 26:121–6. doi: 10.1200/JCO.2007.12.9247
85. Zehnder P, Moltzahn F, Mitra AP, Cai J, Miranda G, Skinner EC, et al. Radical cystectomy with super-extended lymphadenectomy: impact of separate vs en bloc lymph node submission on analysis and outcomes. BJU Int. (2016) 117:253–9. doi: 10.1111/bju.2016.117.issue-2
86. Davies JD, Simons CM, Ruhotina N, Barocas DA, Clark PE, Morgan TM. Anatomic basis for lymph node counts as measure of lymph node dissection extent: a cadaveric study. Urology. (2013) 81:358–63. doi: 10.1016/j.urology.2012.10.025
87. Chen JJ, Zhu ZS, Zhu YY, Shi HQ. Applied anatomy of pelvic lymph nodes and its clinical significance for prostate cancer: a single-center cadaveric study. BMC cancer. (2020) 20:330. doi: 10.1186/s12885-020-06833-1
88. Gschwend JE, Heck MM, Lehmann J, Rübben H, Albers P, Wolff JM, et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur urology. (2019) 75:604–11. doi: 10.1016/j.eururo.2018.09.047
89. Morgan TM, Kaffenberger SD, Cookson MS. Surgical and chemotherapeutic management of regional lymph nodes in bladder cancer. J urology. (2012) 188:1081–8. doi: 10.1016/j.juro.2012.06.008
90. Gakis G, Efstathiou J, Lerner SP, Cookson MS, Keegan KA, Guru KA, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur urology. (2013) 63:45–57. doi: 10.1016/j.eururo.2012.08.009
91. Briganti A, Blute ML, Eastham JH, Graefen M, Heidenreich A, Karnes JR, et al. Pelvic lymph node dissection in prostate cancer. Eur urology. (2009) 55:1251–65. doi: 10.1016/j.eururo.2009.03.012
92. Van den Bergh L, Lerut E, Haustermans K, Deroose CM, Oyen R, Isebaert S, et al. Final analysis of a prospective trial on functional imaging for nodal staging in patients with prostate cancer at high risk for lymph node involvement. Urologic Oncol. (2015) 33:109.e23–31. doi: 10.1016/j.urolonc.2014.11.008
93. Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N, et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. (2014) 32:3939–47. doi: 10.1200/JCO.2013.54.7893
94. Joniau S, Van den Bergh L, Lerut E, Deroose CM, Haustermans K, Oyen R, et al. Mapping of pelvic lymph node metastases in prostate cancer. Eur urology. (2013) 63:450–8. doi: 10.1016/j.eururo.2012.06.057
95. Leissner J, Ghoneim MA, Abol-Enein H, Thüroff JW, Franzaring L, Fisch M, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J urology. (2004) 171:139–44. doi: 10.1097/01.ju.0000102302.26806.fb
96. Nakagawa T. Lymph node dissection for bladder cancer: Current standards and the latest evidence. Int J Urol. (2021) 28:7–15. doi: 10.1111/iju.14398
97. Fossati N, Willemse PM, Van den Broeck T, van den Bergh RCN, Yuan CY, Briers E, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: A systematic review. Eur urology. (2017) 72:84–109. doi: 10.1016/j.eururo.2016.12.003
98. Jeschke S, Nambirajan T, Leeb K, Ziegerhofer J, Sega W, Janetschek G. Detection of early lymph node metastases in prostate cancer by laparoscopic radioisotope guided sentinel lymph node dissection. J urology. (2005) 173:1943–6. doi: 10.1097/01.ju.0000158159.16314.eb
99. Weckermann D, Dorn R, Trefz M, Wagner T, Wawroschek F, Harzmann R. Sentinel lymph node dissection for prostate cancer: experience with more than 1,000 patients. J urology. (2007) 177:916–20. doi: 10.1016/j.juro.2006.10.074
100. Lestingi JFP, Guglielmetti GB, Trinh QD, Coelho RF, Pontes J Jr., Bastos DA, et al. Extended versus limited pelvic lymph node dissection during radical prostatectomy for intermediate- and high-risk prostate cancer: early oncological outcomes from a randomized phase 3 trial. Eur urology. (2021) 79:595–604. doi: 10.1016/j.eururo.2020.11.040
101. Preisser F, van den Bergh RCN, Gandaglia G, Ost P, Surcel CI, Sooriakumaran P, et al. Effect of extended pelvic lymph node dissection on oncologic outcomes in patients with D’Amico intermediate and high risk prostate cancer treated with radical prostatectomy: A multi-institutional study. J urology. (2020) 203:338–43. doi: 10.1097/JU.0000000000000504
102. Chalouhy C, Gurram S, Ghavamian R. Current controversies on the role of lymphadenectomy for prostate cancer. Urologic Oncol. (2019) 37:219–26. doi: 10.1016/j.urolonc.2018.11.020
103. Ploussard G, Briganti A, de la Taille A, Haese A, Heidenreich A, Menon M, et al. Pelvic lymph node dissection during robot-assisted radical prostatectomy: efficacy, limitations, and complications-a systematic review of the literature. Eur urology. (2014) 65:7–16. doi: 10.1016/j.eururo.2013.03.057
104. Briganti A, Chun FK, Salonia A, Suardi N, Gallina A, Da Pozzo LF, et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur urology. (2006) 50:1006–13. doi: 10.1016/j.eururo.2006.08.015
105. Cacciamani GE, Maas M, Nassiri N, Ortega D, Gill K, Dell’Oglio P, et al. Impact of pelvic lymph node dissection and its extent on perioperative morbidity in patients undergoing radical prostatectomy for prostate cancer: A comprehensive systematic review and meta-analysis. Eur Urol Oncol. (2021) 4:134–49. doi: 10.1016/j.euo.2021.02.001
106. Martini A, Wever L, Soeterik TFW, Rakauskas A, Fankhauser CD, Grogg JB, et al. Unilateral pelvic lymph node dissection in prostate cancer patients diagnosed in the era of magnetic resonance imaging-targeted biopsy: A study that challenges the dogma. J urology. (2023) 210:117–27. doi: 10.1097/JU.0000000000003442
107. Abdollah F, Gandaglia G, Suardi N, Capitanio U, Salonia A, Nini A, et al. More extensive pelvic lymph node dissection improves survival in patients with node-positive prostate cancer. Eur urology. (2015) 67:212–9. doi: 10.1016/j.eururo.2014.05.011
108. Keegan KA, Cookson MS. Complications of pelvic lymph node dissection for prostate cancer. Curr Urol Rep. (2011) 12:203–8. doi: 10.1007/s11934-011-0179-z
109. Wit EMK, Acar C, Grivas N, Yuan C, Horenblas S, Liedberg F, et al. Sentinel node procedure in prostate cancer: A systematic review to assess diagnostic accuracy. Eur urology. (2017) 71:596–605. doi: 10.1016/j.eururo.2016.09.007
110. Heck MM, Retz M, Bandur M, Souchay M, Vitzthum E, Weirich G, et al. Topography of lymph node metastases in prostate cancer patients undergoing radical prostatectomy and extended lymphadenectomy: results of a combined molecular and histopathologic mapping study. Eur urology. (2014) 66:222–9. doi: 10.1016/j.eururo.2013.02.007
111. Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. (1977) 39:456–66. doi: 10.1002/1097-0142(197702)39:2<456::AID-CNCR2820390214>3.0.CO;2-I
112. Grivas N, Wit EMK, Kuusk T, KleinJan GH, Donswijk ML, van Leeuwen FWB, et al. The impact of adding sentinel node biopsy to extended pelvic lymph node dissection on biochemical recurrence in prostate cancer patients treated with robot-assisted radical prostatectomy. J Nucl medicine: Off publication Soc Nucl Med. (2018) 59:204–9. doi: 10.2967/jnumed.117.195644
113. Aoun F, Albisinni S, Zanaty M, Hassan T, Janetschek G, van Velthoven R. Indocyanine green fluorescence-guided sentinel lymph node identification in urologic cancers: a systematic review and meta-analysis. Minerva urologica e nefrologica = Ital J Urol nephrology. (2018) 70:361–9. doi: 10.23736/S0393-2249.17.02932-0
114. Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur urology. (2012) 61:480–7. doi: 10.1016/j.eururo.2011.10.044
115. Claps F, Ramírez-Backhaus M, Mir Maresma MC, Gómez-Ferrer Á, Mascarós JM, Marenco J, et al. Indocyanine green guidance improves the efficiency of extended pelvic lymph node dissection during laparoscopic radical prostatectomy. Int J Urol. (2021) 28:566–72. doi: 10.1111/iju.14513
116. Jeschke S, Lusuardi L, Myatt A, Hruby S, Pirich C, Janetschek G. Visualization of the lymph node pathway in real time by laparoscopic radioisotope- and fluorescence-guided sentinel lymph node dissection in prostate cancer staging. Urology. (2012) 80:1080–6. doi: 10.1016/j.urology.2012.05.050
117. Hruby S, Englberger C, Lusuardi L, Schätz T, Kunit T, Abdel-Aal AM, et al. Fluorescence guided targeted pelvic lymph node dissection for intermediate and high risk prostate cancer. J urology. (2015) 194:357–63. doi: 10.1016/j.juro.2015.03.127
118. Manny TB, Patel M, Hemal AK. Fluorescence-enhanced robotic radical prostatectomy using real-time lymphangiography and tissue marking with percutaneous injection of unconjugated indocyanine green: the initial clinical experience in 50 patients. Eur urology. (2014) 65:1162–8. doi: 10.1016/j.eururo.2013.11.017
119. de Korne CM, Wit EM, de Jong J, Valdés Olmos RA, Buckle T, van Leeuwen FWB, et al. Anatomical localization of radiocolloid tracer deposition affects outcome of sentinel node procedures in prostate cancer. Eur J Nucl Med Mol imaging. (2019) 46:2558–68. doi: 10.1007/s00259-019-04443-z
120. Sinha A, West A, Hayes J, Teoh J, Decaestecker K, Vasdev N. Methods of sentinel lymph node detection and management in urinary bladder cancer-A narrative review. Curr Oncol (Toronto Ont). (2022) 29:1335–48. doi: 10.3390/curroncol29030114
121. Leadbetter WF, Cooper JF. Regional gland dissection for carcinoma of the bladder; a technique for one-stage cystectomy, gland dissection, and bilateral uretero-enterostomy. J urology. (1950) 63:242–60. doi: 10.1016/S0022-5347(17)68763-9
122. Polom W, Markuszewski M, Cytawa W, Czapiewski P, Lass P, Matuszewski M. Fluorescent versus radioguided lymph node mapping in bladder cancer. Clin genitourinary cancer. (2017) 15:e405–e9. doi: 10.1016/j.clgc.2016.11.007
123. Sherif A, de la Torre M, Malmström PU, Thörn M. Lymphatic mapping and detection of sentinel nodes in patients with bladder cancer. J urology. (2001) 166:812–5. doi: 10.1016/S0022-5347(05)65842-9
124. Liedberg F, Chebil G, Davidsson T, Gudjonsson S, Månsson W. Intraoperative sentinel node detection improves nodal staging in invasive bladder cancer. J urology. (2006) 175:84–8;discussion 8-9. doi: 10.1016/S0022-5347(05)00066-2
125. Knapp DW, Adams LG, Degrand AM, Niles JD, Ramos-Vara JA, Weil AB, et al. Sentinel lymph node mapping of invasive urinary bladder cancer in animal models using invisible light. Eur urology. (2007) 52:1700–8. doi: 10.1016/j.eururo.2007.07.007
126. Schaafsma BE, Verbeek FP, Elzevier HW, Tummers QR, van der Vorst JR, Frangioni JV, et al. Optimization of sentinel lymph node mapping in bladder cancer using near-infrared fluorescence imaging. J Surg Oncol. (2014) 110:845–50. doi: 10.1002/jso.v110.7
127. Manny TB, Hemal AK. Fluorescence-enhanced robotic radical cystectomy using unconjugated indocyanine green for pelvic lymphangiography, tumor marking, and mesenteric angiography: the initial clinical experience. Urology. (2014) 83:824–9. doi: 10.1016/j.urology.2013.11.042
128. Rosenblatt R, Johansson M, Alamdari F, Sidiki A, Holmström B, Hansson J, et al. Sentinel node detection in muscle-invasive urothelial bladder cancer is feasible after neoadjuvant chemotherapy in all pT stages, a prospective multicenter report. World J urology. (2017) 35:921–7. doi: 10.1007/s00345-016-1952-x
129. Alvaeus J, Rosenblatt R, Johansson M, Alamdari F, Jakubczyk T, Holmström B, et al. Fewer tumor draining sentinel nodes in patients with progressing muscle invasive bladder cancer, after neoadjuvant chemotherapy and radical cystectomy. World J urology. (2020) 38:2207–13. doi: 10.1007/s00345-019-03025-w
130. Thoeny HC, Barbieri S, Froehlich JM, Turkbey B, Choyke PL. Functional and targeted lymph node imaging in prostate cancer: current status and future challenges. Radiology. (2017) 285:728–43. doi: 10.1148/radiol.2017161517
131. Purkayastha A, Sharma N, Vashisth R, Kishore B. Transitional cell carcinoma of urinary bladder manifesting as extensive retroperitoneal and axillary lymph node metastasis: an extremely rare case scenario detected by (18)F-fluorodeoxyglucose positron emission tomography scan. Indian J Nucl medicine: IJNM: Off J Soc Nucl Medicine India. (2017) 32:351–4. doi: 10.4103/ijnm.IJNM_52_17
132. Shankar PR, Barkmeier D, Hadjiiski L, Cohan RH. A pictorial review of bladder cancer nodal metastases. Trans andrology urology. (2018) 7:804–13. doi: 10.21037/tau.2018.08.25
133. Tian R, Ke C, Rao L, Lau J, Chen X. Multimodal stratified imaging of nanovaccines in lymph nodes for improving cancer immunotherapy. Advanced Drug delivery Rev. (2020) 161-162:145–60. doi: 10.1016/j.addr.2020.08.009
134. Hu H. Recent advances of bioresponsive nano-sized contrast agents for ultra-high-field magnetic resonance imaging. Front Chem. (2020) 8:203. doi: 10.3389/fchem.2020.00203
135. Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. New Engl J Med. (2003) 348:2491–9. doi: 10.1056/NEJMoa022749
136. Modo M, Hoehn M, Bulte JW. Cellular MR imaging. Mol imaging. (2005) 4:143–64. doi: 10.1162/15353500200505145
137. Scheenen TWJ, Zamecnik P. The role of magnetic resonance imaging in (Future) cancer staging: note the nodes. Invest radiology. (2021) 56:42–9. doi: 10.1097/RLI.0000000000000741
138. Mehralivand S, van der Poel H, Winter A, Choyke PL, Pinto PA, Turkbey B. Sentinel lymph node imaging in urologic oncology. Trans andrology urology. (2018) 7:887–902. doi: 10.21037/tau.2018.08.23
139. Winter A, Woenkhaus J, Wawroschek F. A novel method for intraoperative sentinel lymph node detection in prostate cancer patients using superparamagnetic iron oxide nanoparticles and a handheld magnetometer: the initial clinical experience. Ann Surg Oncol. (2014) 21:4390–6. doi: 10.1245/s10434-014-4024-8
140. Winter A, Engels S, Goos P, Süykers MC, Gudenkauf S, Henke RP, et al. Accuracy of magnetometer-guided sentinel lymphadenectomy after intraprostatic injection of superparamagnetic iron oxide nanoparticles in prostate cancer: the sentiMag pro II study. Cancers. (2019) 12. doi: 10.3390/cancers12010032
141. Geißen W, Engels S, Aust P, Schiffmann J, Gerullis H, Wawroschek F, et al. Diagnostic accuracy of magnetometer-guided sentinel lymphadenectomy after intraprostatic injection of superparamagnetic iron oxide nanoparticles in intermediate- and high-risk prostate cancer using the magnetic activity of sentinel nodes. Front Pharmacol. (2019) 10:1123. doi: 10.3389/fphar.2019.01123
142. Ploussard G, Rouvière O, Rouprêt M, van den Bergh R, Renard-Penna R. The current role of MRI for guiding active surveillance in prostate cancer. Nat Rev Urology. (2022) 19:357–65. doi: 10.1038/s41585-022-00587-0
143. Muehllehner G, Karp JS. Positron emission tomography. Phys Med Biol. (2006) 51:R117–37. doi: 10.1088/0031-9155/51/13/R08
144. Hutton BF. The origins of SPECT and SPECT/CT. Eur J Nucl Med Mol imaging. (2014) 41 Suppl 1:S3–16. doi: 10.1007/s00259-013-2606-5
145. Abikhzer G, Keidar Z. SPECT/CT and tumor imaging. Eur J Nucl Med Mol imaging. (2014) 41 Suppl 1:S67–80. doi: 10.1007/s00259-013-2534-4
146. Zalewski K, Benke M, Mirocha B, Radziszewski J, Chechlinska M, Kowalewska M. Technetium-99m-based radiopharmaceuticals in sentinel lymph node biopsy: gynecologic oncology perspective. Curr Pharm design. (2018) 24:1652–75. doi: 10.2174/1381612824666180515122150
147. Hameed S, Chen H, Irfan M, Bajwa SZ, Khan WS, Baig SM, et al. Fluorescence guided sentinel lymph node mapping: from current molecular probes to future multimodal nanoprobes. Bioconjugate Chem. (2019) 30:13–28. doi: 10.1021/acs.bioconjchem.8b00812
148. Sampson CB. Adverse reactions and drug interactions with radiopharmaceuticals. Drug safety. (1993) 8:280–94. doi: 10.2165/00002018-199308040-00003
149. Klingenberg S, Jochumsen MR, Ulhøi BP, Fredsøe J, Sørensen KD, Borre M, et al. (68)Ga-PSMA PET/CT for primary lymph node and distant metastasis NM staging of high-risk prostate cancer. J Nucl medicine: Off publication Soc Nucl Med. (2021) 62:214–20. doi: 10.2967/jnumed.120.245605
150. Sprute K, Kramer V, Koerber SA, Meneses M, Fernandez R, Soza-Ried C, et al. Diagnostic accuracy of (18)F-PSMA-1007 PET/CT imaging for lymph node staging of prostate carcinoma in primary and biochemical recurrence. J Nucl Med. (2021) 62:208–13. doi: 10.2967/jnumed.120.246363
151. Stabile A, Pellegrino A, Mazzone E, Cannoletta D, de Angelis M, Barletta F, et al. Can negative prostate-specific membrane antigen positron emission tomography/computed tomography avoid the need for pelvic lymph node dissection in newly diagnosed prostate cancer patients? A systematic review and meta-analysis with backup histology as reference standard. Eur Urol Oncol. (2022) 5:1–17. doi: 10.1016/j.euo.2021.08.001
152. Girard A, Rouanne M, Taconet S, Radulescu C, Neuzillet Y, Girma A, et al. Integrated analysis of (18)F-FDG PET/CT improves preoperative lymph node staging for patients with invasive bladder cancer. Eur radiology. (2019) 29:4286–93. doi: 10.1007/s00330-018-5959-0
153. Meads C, Sutton AJ, Rosenthal AN, Małysiak S, Kowalska M, Zapalska A, et al. Sentinel lymph node biopsy in vulval cancer: systematic review and meta-analysis. Br J cancer. (2014) 110:2837–46. doi: 10.1038/bjc.2014.205
154. van Leeuwen FWB, Schottelius M, Brouwer OR, Vidal-Sicart S, AChilefu S, Klode J, et al. Trending: radioactive and fluorescent bimodal/hybrid tracers as multiplexing solutions for surgical guidance. J Nucl medicine: Off publication Soc Nucl Med. (2020) 61:13–9. doi: 10.2967/jnumed.119.228684
155. van der Poel HG, Buckle T, Brouwer OR, Valdés Olmos RA, van Leeuwen FW. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur urology. (2011) 60:826–33. doi: 10.1016/j.eururo.2011.03.024
156. Wit EMK, KleinJan GH, Berrens AC, van Vliet R, van Leeuwen PJ, Buckle T, et al. A hybrid radioactive and fluorescence approach is more than the sum of its parts; outcome of a phase II randomized sentinel node trial in prostate cancer patients. Eur J Nucl Med Mol imaging. (2023) 50:2861–71. doi: 10.1007/s00259-023-06191-7
157. Hinsenveld FJ, Wit EMK, van Leeuwen PJ, Brouwer OR, Donswijk ML, Tillier CN, et al. Prostate-specific membrane antigen PET/CT combined with sentinel node biopsy for primary lymph node staging in prostate cancer. J Nucl medicine: Off publication Soc Nucl Med. (2020) 61:540–5. doi: 10.2967/jnumed.119.232199
158. Rietbergen DDD, van Gennep EJ, KleinJan GH, Donswijk M, Valdés Olmos RA, van Rhijn BW, et al. Evaluation of the hybrid tracer indocyanine green- 99m tc-nanocolloid for sentinel node biopsy in bladder cancer-A prospective pilot study. Clin Nucl Med. (2022) 47:774–80. doi: 10.1097/RLU.0000000000004301
159. Xi L, Jiang H. Image-guided surgery using multimodality strategy and molecular probes. Wiley Interdiscip Rev Nanomedicine nanobiotechnology. (2016) 8:46–60. doi: 10.1002/wnan.2016.8.issue-1
160. Bai J, Wang JT, Rubio N, Protti A, Heidari H, Elgogary R, et al. Triple-modal imaging of magnetically-targeted nanocapsules in solid tumors in vivo. Theranostics. (2016) 6:342–56. doi: 10.7150/thno.11918
161. Zhang X, Ding B, Qu C, Li H, Sun Y, Gai Y, et al. A thiopyrylium salt for PET/NIR-II tumor imaging and image-guided surgery. Mol Oncol. (2020) 14:1089–100. doi: 10.1002/1878-0261.12674
162. Jia B, Zhang X, Wang B, Chen M, Lv F, Wang S, et al. Dual-modal probe based on polythiophene derivative for pre- and intraoperative mapping of lymph nodes by SPECT/optical imaging. ACS Appl materials interfaces. (2018) 10:6646–51. doi: 10.1021/acsami.8b01032
163. Shi H, Yan R, Wu L, Sun Y, Liu S, Zhou Z, et al. Tumor-targeting CuS nanoparticles for multimodal imaging and guided photothermal therapy of lymph node metastasis. Acta biomaterialia. (2018) 72:256–65. doi: 10.1016/j.actbio.2018.03.035
164. Shi H, Sun Y, Yan R, Liu S, Zhu L, Liu S, et al. Magnetic semiconductor gd-doping cuS nanoparticles as activatable nanoprobes for bimodal imaging and targeted photothermal therapy of gastric tumors. Nano letters. (2019) 19:937–47. doi: 10.1021/acs.nanolett.8b04179
165. Patras L, Shaashua L, Matei I, Lyden D. Immune determinants of the pre-metastatic niche. Cancer Cell. (2023) 41:546–72. doi: 10.1016/j.ccell.2023.02.018
166. Mohan V, Das A, Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol. (2020) 62:192–200. doi: 10.1016/j.semcancer.2019.09.004
167. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. (2013) 13:97–110. doi: 10.1038/nrc3447
168. Huang X, Zhang F, Lee S, Swierczewska M, Kiesewetter DO, Lang L, et al. Long-term multimodal imaging of tumor draining sentinel lymph nodes using mesoporous silica-based nanoprobes. Biomaterials. (2012) 33:4370–8. doi: 10.1016/j.biomaterials.2012.02.060
169. Muteganya R, Goldman S, Aoun F, Roumeguère T, Albisinni S. Current imaging techniques for lymph node staging in prostate cancer: A review. Front surgery. (2018) 5:74. doi: 10.3389/fsurg.2018.00074
170. Xie D, Gu D, Lei M, Cai C, Zhong W, Qi D, et al. The application of indocyanine green in guiding prostate cancer treatment. Asian J urology. (2023) 10:1–8. doi: 10.1016/j.ajur.2021.07.004
171. Małkiewicz B, Kiełb P, Kobylański M, Karwacki J, Poterek A, Krajewski W, et al. Sentinel lymph node techniques in urologic oncology: current knowledge and application. Cancers. (2023) 15. doi: 10.3390/cancers15092495
172. Chahal B, Aydin A, Amin MSA, Khan A, Khan MS, Ahmed K, et al. The learning curves of major laparoscopic and robotic procedures in urology: a systematic review. Int J Surg (London England). (2023) 109:2037–57. doi: 10.1097/JS9.0000000000000345
173. Tummers WS, Warram JM, van den Berg NS, Miller SE, Swijnenburg RJ, Vahrmeijer AL, et al. Recommendations for reporting on emerging optical imaging agents to promote clinical approval. Theranostics. (2018) 8:5336–47. doi: 10.7150/thno.27384
174. Wit EMK, van Beurden F, Kleinjan GH, Grivas N, de Korne CM, Buckle T, et al. The impact of drainage pathways on the detection of nodal metastases in prostate cancer: a phase II randomized comparison of intratumoral vs intraprostatic tracer injection for sentinel node detection. Eur J Nucl Med Mol imaging. (2022) 49:1743–53. doi: 10.1007/s00259-021-05580-0
Keywords: fluorescence imaging, fluorescent contrast agent, pelvic lymph node dissection, lymph node metastasis, multimodal imaging
Citation: Xu M, Li P, Wei J, Yan P, Zhang Y, Guo X, Liu C and Yang X (2024) Progress of fluorescence imaging in lymph node dissection surgery for prostate and bladder cancer. Front. Oncol. 14:1395284. doi: 10.3389/fonc.2024.1395284
Received: 05 March 2024; Accepted: 13 September 2024;
Published: 04 October 2024.
Edited by:
Mukund Seshadri, University at Buffalo, United StatesReviewed by:
Giuseppe Fallara, San Raffaele Hospital (IRCCS), ItalyPierre Bourgeois, hospital Erasme, Belgium
Copyright © 2024 Xu, Li, Wei, Yan, Zhang, Guo, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Liu, bGl1Y2hhb18wODMxQDE2My5jb20=; Xiaofeng Yang, eXhmeWxxQDE2My5jb20=
 Mingquan Xu
Mingquan Xu Panpan Li
Panpan Li Jinzheng Wei
Jinzheng Wei Pengyu Yan
Pengyu Yan Yunmeng Zhang
Yunmeng Zhang Xinyu Guo
Xinyu Guo Chao Liu1,2*
Chao Liu1,2* Xiaofeng Yang
Xiaofeng Yang