
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 22 May 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1394168
Secondary prostate cancer typically occurs from direct seeding of a renal or bladder tumor. Metastasis via hematogenous spread is exceedingly rare and is typically identified incidentally at autopsy. This report describes a 72-year-old male with lung adenocarcinoma initially staged as Stage IA2 who developed oligometastatic disease of the prostate. He was initially treated with radiation therapy and was found to have a hypermetabolic focus in the prostate gland during surveillance PET/CT imaging 6 months following treatment. Subsequent biopsy revealed metastatic lung adenocarcinoma in 6/6 core samples, leading to diagnosis of oligometastatic disease of the prostate. To our knowledge, this is the first report of isolated oligometastatic disease to the prostate from a primary lung adenocarcinoma.
Prostatic metastasis is uncommon, occurring in less than one percent of all prostatic surgical specimens (1). Leukemia and lymphoma are the most common neoplasms to metastasize to the prostate (1). Solid tumor metastases typically occurs by direct extension from nearby organs and is most commonly seen in patients with widely disseminated metastatic disease (2). Hematogenous spread is extremely rare, and only a few cases have been documented, including metastasis from primary lung, gastrointestinal, skin and endocrine organs (1). Due to their propensity to be identified at later stages, and typically in widely disseminated disease, these malignancies commonly confer poor prognoses and are typically identified incidentally during autopsy (2). In this report, we describe an unusual case of isolated oligometastatic disease of the prostate from a primary lung adenocarcinoma (Figure 1).
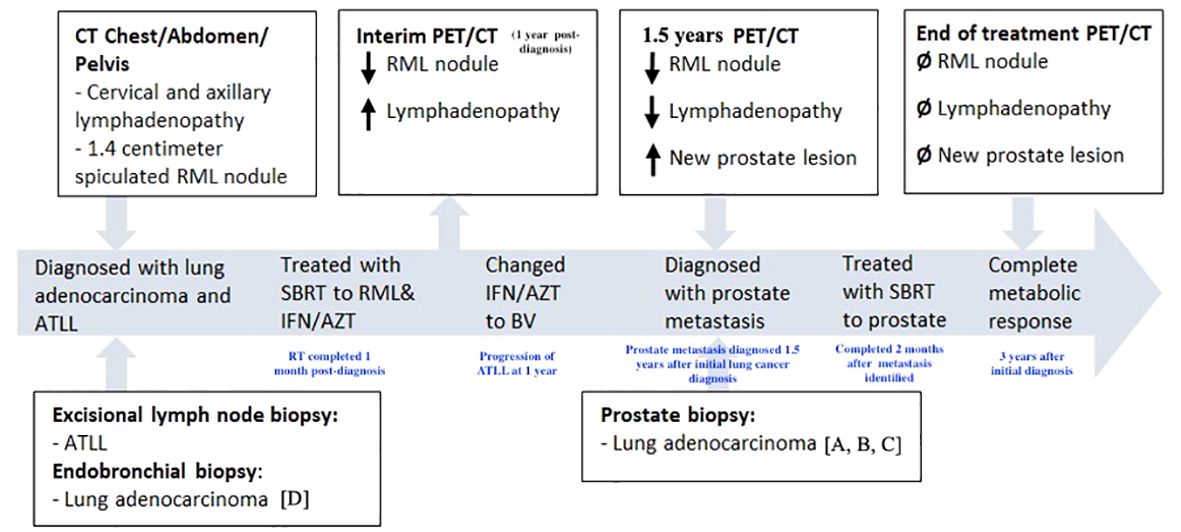
Figure 1 Timeline of treatment course. CT, computed tomography. PET, positron emission tomography. RML, right middle lobe. ATLL, adult T-cell leukemia/lymphoma. SBRT, stereotactic body radiation therapy. IFN/AZT, interferon/zidovudine. BV, brentuximab vedotin. Letters presented in brackets (ex: [A]) refer to parts of pathology shown in Figure 3.
A 72-year-old male with a 20-pack-year smoking history, presented with a painless right neck mass and axillary lymphadenopathy. Imaging of the neck showed multiple slightly enlarged right cervical nodes and right axillary nodes, as well as a 1.4 cm right middle lobe (RML) spiculated nodule (Figure 2A). PET/CT demonstrated absence of increased signal activity in other organs, including the prostate gland (Figure 2B). Excisional biopsy of a right neck lymph node revealed mature CD4+, CD7-, CD8-, CD25+, CD30+ T- cell lymphoma. He tested positive for human-T-lymphocytic virus type 1 (HTLV-1) and was diagnosed with Adult T Cell Leukemia/Lymphoma (ATLL). Staging positron emission tomography/computed tomography (PET)/CT did not reveal additional sites of disease. There was no bone marrow involvement. The patient underwent endobronchial ultrasound-guided biopsy of the spiculated RML pulmonary nodule, which revealed lung adenocarcinoma. There was no nodal involvement and it was classified as a Stage IA2 (cT1bN0M0) tumor. Molecular testing revealed EGFR-negative status and PD-L1 TPS < 1%. It was treated with 54 Gy of stereotactic body radiation therapy (SBRT). The patient’s ATLL was treated with Interferon and Zidovudine. Repeat PET/CT showed interval decrease of RML nodule but progression of ATLL prompting change of treatment to brentuximab vedotin (BV). Repeat PET/CT 6 months later showed resolution of lymphadenopathy and previously seen RML nodule but identified an ill-defined right lower lobe (RLL) opacity attributed to post-radiation changes.
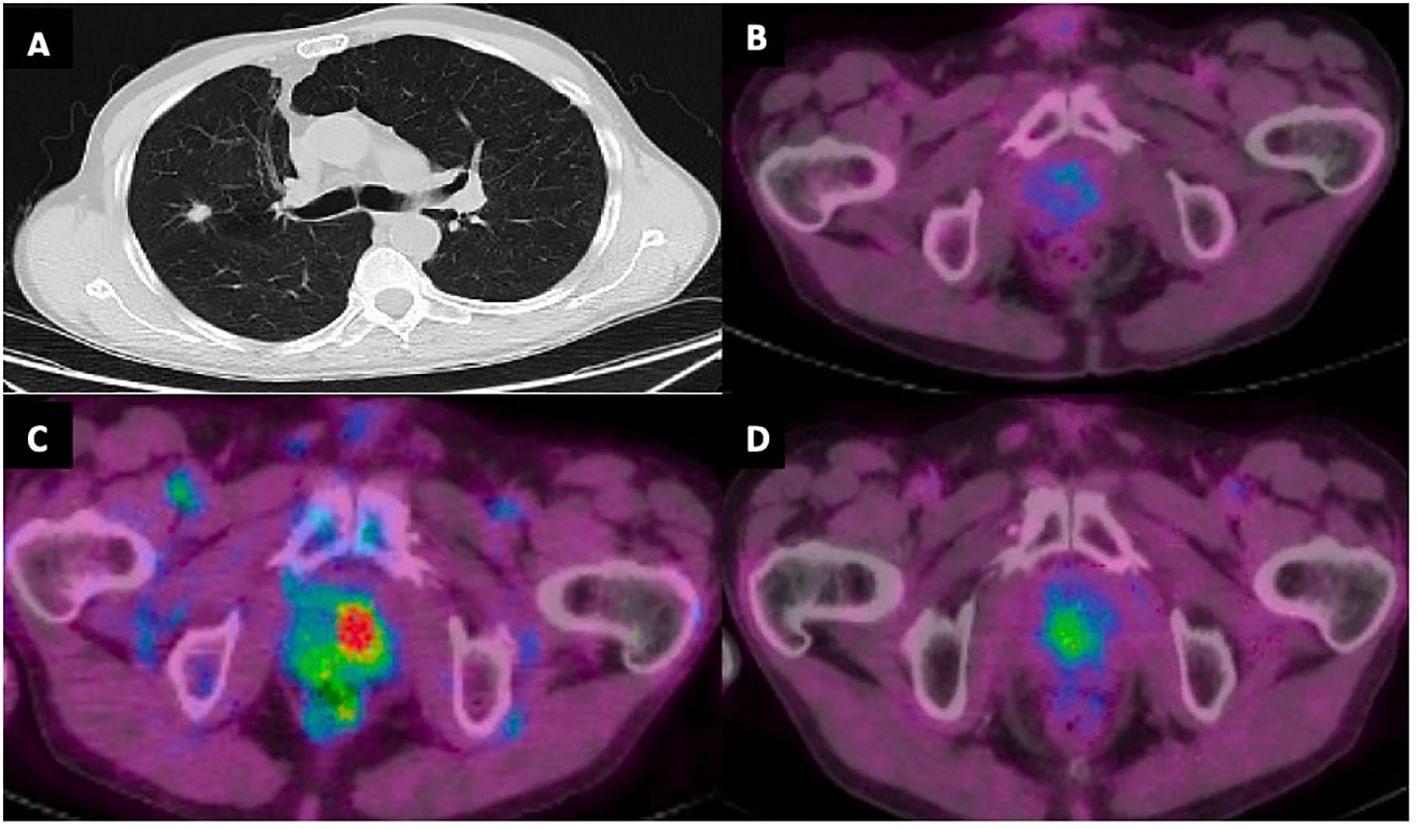
Figure 2 (A) CT chest with 1.4 cm right middle lobe spiculated nodule at time of initial diagnosis. (B) PET/CT demonstrating absence of increased FDG activity in the prostate gland at time of diagnosis. (C) Increased FDG activity in the prostate gland with maximum standardized uptake value (SUV) 8.4 concerning for prostatic metastasis. (D) Decreased FDG activity in prostate gland following SBRT with SUV max 3.6.
Subsequent PET/CT 1.5 years following initial diagnosis revealed interval enlargement of the RLL opacity consistent with post-radiation changes and a new hypermetabolic focus in the left prostate concerning for malignant process at both locations (Figure 2C). Prostate Specific Antigen (PSA) testing was negative. A prostate biopsy identified infiltration of prostatic gland by a poorly differentiated adenocarcinoma in 6/6 core samples. Tumor cells were positive for TTF1, CK7, AE1/3 and negative for NKX3, suggestive of metastatic lung adenocarcinoma (Figure 3). Given oligometastatic, low burden disease he was treated with SBRT at both sites. He received 45 Gy and 24 Gy at the RLL and prostate, respectively. He completed 16 months of BV with continuous complete metabolic response of his lymphoma on post-treatment PET/CT (Figure 2D). At the time of the writing of this report the patient has been in remission for one year.
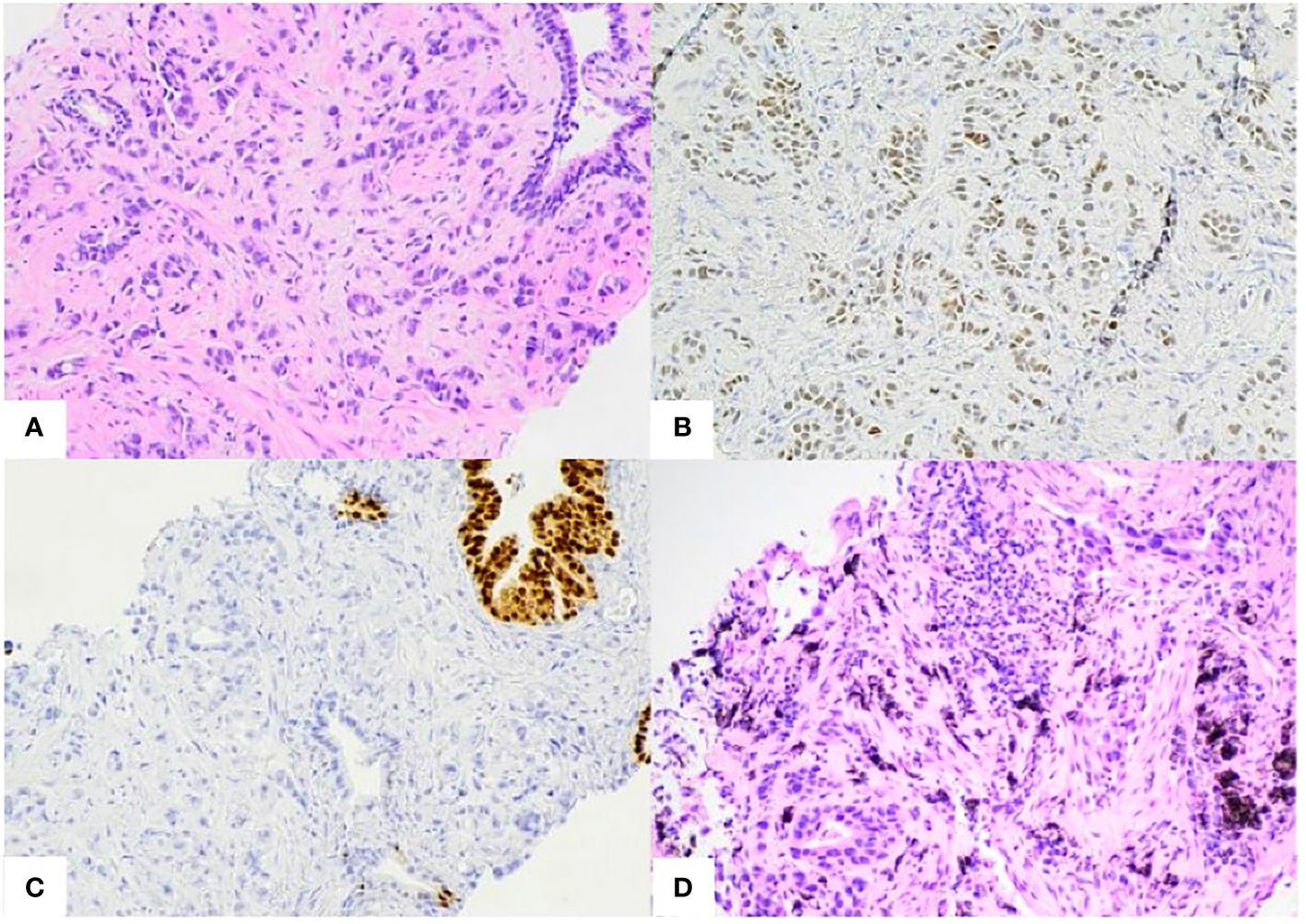
Figure 3 (A) Prostate biopsy. Poorly differentiated pulmonary adenocarcinoma infiltrating between benign prostate glands. (B, C). Prostate biopsy immunohistochemistry stains. Positive for TTF1 [B], CK7, AE1/3 (not shown) and negative for NKX3.1[C] and Napsin-A (not shown). (D) Right middle lobe biopsy. Invasive pulmonary adenocarcinoma. Positive for TTF-1, Napsin-1, and negative for p40 (not shown).
Over half of lung adenocarcinoma cases are metastatic at diagnosis, commonly involving the liver, adrenal glands, bone, and brain (3). Lung cancer metastasis to prostate is uncommon, with most cases found incidentally during autopsy (1). A study reporting 1,474 autopsy cases of prostate tumors identified only 18 (1.2%) cases with metastatic prostatic lesions;5 (0.34%) cases were derived from primary lung malignancies (4). In a series of approximately 30,000 patients with metastatic cancer, a diagnosis of metastasis to the prostate was made in 3 (0.01%) of cases (4). Another study at the Royal London Hospital examined post-mortem samples from 11,945 prostatic resections and biopsies, obtained from 1907 to 2002. Of these, 51 secondary prostatic tumors were identified, including eight that originated from primary lung cancers. All of these patients represented had widely disseminated disease and not isolated metastasis to the prostate (1). A post-mortem pathological examination of 10,791 cancer patients at the Roswell Park Comprehensive Cancer Center in New York also identified 185 cases of secondary neoplasms of the prostate. In 181 patients tumor was present in at least five or more organ systems. Only one of the four remaining cases involved a primary lung malignancy and this patient may have had disease in multiple sites as well (5). These cases of prostate metastasis were identified at autopsy, not during the patient’s active clinical management, and they occurred almost exclusively in the context of widely disseminated disease. This contrasts with our patient, who was diagnosed during ongoing clinical treatment and exhibited only isolated oligometastasis to the prostate, without evidence of disease at additional sites.
A PubMed search for cases of prostatic tumors composed of a primary lung cancer revealed 6 cases (Table 1) (2, 3, 6–9). Disseminated disease was present in five cases; one report did not specify. In the majority of cases the PSA was not significantly elevated, and patients had lower urinary tract symptoms such as hematuria, pelvic pain, and the sequelae of urethral obstruction (1). These symptoms are associated with benign prostatic hyperplasia, primary prostate tumors, and secondary prostate tumors. Because the symptoms of primary and secondary neoplasms of the prostate overlap it is impossible to distinguish them cinically (1).
Differentiating between primary and secondary disease of the prostate by close histological examination is critical, as hormonal treatments and surgical excision may not be indicated for the latter. Primary prostate cancers have highly variable microscopic features (10). Secondary neoplasms of the prostate typically have well-circumscribed appearance on pathology, tumor invasion to the non-neoplastic glands, and absence of intraepithelial neoplasia in adjacent glands (4). To further delineate tissue of origin, immunohistochemistry for tissue-specific markers is informative. Secondary prostatic tumors are likely negative for PSA and Prostatic Acid Phosphatase (PAP), as these enzyme secretions are intrinsic and specific to the prostate gland (4). Secondary lung adenocarcinomas typically overexpresses CK7, TTF-1, and AE1/3. TTF-1, in particular, is highly sensitive and specific for primary lung adenocarcinoma and can be reliably used to differentiate between metastatic lung disease and primary prostatic tumors (3). Prostate adenocarcinoma typically stains positive for NKX3.1 and Napsin-A, although the latter is also frequently positive in lung adenocarcinoma as well. And while it is not yet standard of care recent research suggests that RNA expression of prostate cancer antigen 3 (PSA3) is specific to primary prostate tumors, and may represent a valuable tool as a cancer-specific biomarker in distinguishing primary from secondary disease (9, 11–13).
The prostate is a rare but possible site of metastasis of primary lung adenocarcinoma. To our knowledge, this is the first case of biopsy-proven lung adenocarcinoma with isolated oligometastatic disease to the prostate emphasizing the importance of paying close attention to and pursuing workup of all hypermetabolic lesions identified on PET/CT. Prompt identification of tissue origin allows for timely and appropriately tailored therapeutic intervention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JK: Data curation, Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. KF: Conceptualization, Writing – original draft, Writing – review & editing. FS: Data curation, Writing – original draft, Writing – review & editing. JC: Data curation, Writing – review & editing. IM: Conceptualization, Data curation, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
These authors would like to thank the patient represented in this case and their family for their willingness to let us share their experiences in this case report, thus contributing to the sharing of medical knowledge.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bates AW, Baithun SI. Secondary solid neoplasms of the prostate: a clinico-pathological series of 51 cases. Virchows Archiv: Int J Pathol. (2002) 440:392–6. doi: 10.1007/s004280100505
2. Yoo JH, Lee JH, Kim EK, Hong YK, Lee Y, Jeong HC. Prostatic metastasis of large cell neuroendocrine carcinoma of the lung. Respirol (Carlton Vic). (2009) 14:772–5. doi: 10.1111/j.1440-1843.2009.01545.x
3. González Yañez I, Pérez López ME, Rodríguez López J.Á., Arias Santos MD, García Gómez J, García Mata J. Metástasis única en próstata de carcinoma microcítico pulmonar. Archivos Españoles Urología (Ed Impresa). (2009) 62: 141–4. doi: 10.4321/s0004-06142009000200010
4. Johnson DE, Chalbaud R, Ayala AG. Secondary tumors of the prostate. J Urol. (1974) 112:507–8. doi: 10.1016/s0022-5347(17)59776-1
5. Zein TA, Huben RP, Lane WW, Pontes JE, Englander LS. Secondary tumors of the prostate. J Urol. (1985) 133:615–6. doi: 10.1016/s0022-5347(17)49111-7
6. Smedley HM, Brown C, Turner A. Ectopic ACTH-producing lung cancer presenting with prostatic metastasis. Postgrad Med J. (1983) 59:371–2. doi: 10.1136/pgmj.59.692.371
7. Ohmori K, Nishimura K, Nishizaka Y. A case of small cell lung cancer metastasizing to prostatic cancer. Urol Internationalis. (1998) 60:251–3. doi: 10.1159/000030267
8. Barba Abad J, Robles García JE, Tolosa Eizaguirre E, Panizo Santos A, Zudaire Bergera JJ. Lung cancer metastasizing to the prostate: case report and literature review. Actas Urologicas Espanolas. (2010) 34:918–20. doi: 10.1016/S2173-5786(10)70232-8
9. Gilmour N, Mampuya WA, Jumeau R, Dattner N, Ozsahin M. Lung adenocarcinoma metastasis to the prostate: A case report. Pract Radiat Oncol. (2019) 9:pp.77–80. doi: 10.1016/j.prro.2018.11.010
10. Humphrey PA. Histopathology of prostate cancer. Cold Spring Harbor Perspect Med. (2017) 7:a030411. doi: 10.1101/cshperspect.a030411
11. Qiu M-T, Hu J-W, Yin R, Xu L. Long noncoding RNA: an emerging paradigm of cancer research. Tumor Biol. (2013) 34:pp.613–620. doi: 10.1007/s13277-013-0658-6
12. Ueno T, Linder S, Elmberger G. Aspartic proteinase napsin is a useful marker for diagnosis of primary lung adenocarcinoma. Br J Cancer. (2003) 88:1229–33. doi: 10.1038/sj.bjc.6600879
13. Turner BM, Cagle PT, Sainz IM, Fukuoka J, Shen SS, Jagirdar J, et al. A new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med. (2012) 136:163–71. doi: 10.5858/arpa.2011-0320-OA
Keywords: prostate cancer, lung adenocarcinoma, tumor to tumor metastasis, secondary prostate cancer, lung adenocarcinoma with oligometastatic disease
Citation: Kamel JM, Arjani S, Fedorov K, Sapna F, Cheng J and Mantzaris I (2024) Case report: Isolated oligometastatic disease of the prostate from a primary lung adenocarcinoma. Front. Oncol. 14:1394168. doi: 10.3389/fonc.2024.1394168
Received: 01 March 2024; Accepted: 29 April 2024;
Published: 22 May 2024.
Edited by:
Francesca Sanguedolce, University of Foggia, ItalyReviewed by:
Alessandro Caputo, University of Salerno, ItalyCopyright © 2024 Kamel, Arjani, Fedorov, Sapna, Cheng and Mantzaris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ioannis Mantzaris, aW1hbnR6YXJAbW9udGVmaW9yZS5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.