
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 03 May 2024
Sec. Gynecological Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1391546
Objectives: The objective of this network meta-analysis is to systematically compare the efficacy of diverse progestin-based combination regimens in treating patients diagnosed with endometrial cancer or atypical endometrial hyperplasia. The primary goal is to discern the optimal combination treatment regimen through a comprehensive examination of their respective effectiveness.
Methods: We systematically searched four prominent databases: PubMed, Web of Science, Embase, and Cochrane Central Register of Controlled Trials, for randomized controlled trials addressing the efficacy of progestins or progestin combinations in the treatment of patients with endometrial cancer or atypical endometrial hyperplasia. The search spanned from the inception of these databases to December 2023. Key outcome indicators encompassed survival indices, criteria for assessing efficacy, as well as pregnancy and relapse rate. This study was registered in PROSPERO (CRD42024496311).
Results: From the 1,558 articles initially retrieved, we included 27 studies involving a total of 5,323 subjects in our analysis. The results of the network meta-analysis revealed that the mTOR inhibitor+megestrol acetate (MA)+tamoxifen regimen secured the top rank in maintaining stable disease (SD) (SUCRA=73.4%) and extending progression-free survival (PFS) (SUCRA=72.4%). Additionally, the progestin combined with tamoxifen regimen claimed the leading position in enhancing the partial response (PR) (SUCRA=75.2%) and prolonging overall survival (OS) (SUCRA=80%). The LNG-IUS-based dual progestin regimen emerged as the frontrunner in improving the complete response (CR) (SUCRA=98.7%), objective response rate (ORR) (SUCRA=99.1%), pregnancy rate (SUCRA=83.7%), and mitigating progression (SUCRA=8.0%) and relapse rate (SUCRA=47.4%). In terms of safety, The LNG-IUS-based dual progestin regimen had the lowest likelihood of adverse events (SUCRA=4.2%), while the mTOR inhibitor regimen (SUCRA=89.2%) and mTOR inbitor+MA+tamoxifen regimen (SUCRA=88.4%) had the highest likelihood of adverse events.
Conclusions: Patients diagnosed with endometrial cancer or atypical endometrial hyperplasia exhibited the most favorable prognosis when undergoing progestin combination therapy that included tamoxifen, mTOR inhibitor, or LNG-IUS. Notably, among these options, the LNG-IUS-based dual progestin regimen emerged as particularly promising for potential application.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42024496311.
Endometrial cancer (EC), arising from the epithelial lining of the uterus, stands as the most prevalent malignant tumor in the female reproductive tract and ranks as the sixth most common cancer in women globally. The disease exhibits an escalating morbidity and mortality rate worldwide. In 2020 alone, there were 417,000 new cases of endometrial cancer, resulting in 97,000 related deaths. The cumulative risk of developing this cancer by the age of 75 is 1%, with a corresponding risk of death at 0.2%. Despite its widespread occurrence, endometrial cancer is generally associated with a favorable prognosis, primarily because, in the majority of cases, the disease is confined to the uterus. The overall 5-year survival rates for endometrial cancer differ by stage: stage I disease boasts an 80% survival rate, stage II at 60%, stage III at 30%, and stage IV at 5%. The incidence of endometrial cancer varies significantly across regions, with a tenfold difference observed globally. The highest incidence rates are documented in Northern America, Europe, Micronesia/Polynesia, and Australia/New Zealand, while the lowest rates are found in most African regions and South Central Asia. In terms of mortality rates, regional disparities are relatively modest. Eastern Europe, Micronesia/Polynesia, the Caribbean, and Northern America report the highest mortality rates (1–4), highlighting the need for increased awareness and targeted interventions in these regions.
Endometrioid neoplastic lesions of the endometrium follow a histological continuum, spanning from endometrial hyperplasia without atypia (EH) to endometrial hyperplasia with atypia (AEH) and well-differentiated endometrial cancer (EC) (5). Broadly categorized into type I and type II, endometrial cancer exhibits distinct characteristics. Type I cancers are primarily associated with obesity-related sequelae, marked by excessive proliferation of endometrial cells. Patients with type I cancer often suffer from hyperestrogenism, hyperlipidemia, diabetes, and anovulatory uterine bleeding-all linked to metabolic syndrome. These type I cancers, mainly highly to moderately differentiated endometrioid tumors, typically originate from atypical endometrial hyperplasia, with at least 90% expressing medium to high levels of estrogen receptors. Conversely, type II cancers are unrelated to metabolic or endocrine disorders and do not stem from endometrial hyperplasia, commonly appearing in non-obese women. This category includes more aggressive histological variants such as clear cell carcinoma, serous carcinoma, or uterine carcinosarcoma (6, 7). Several risk factors contribute to the likelihood of endometrial cancer, with age, race, metabolic syndrome, unopposed estrogen exposure, and genetic predispositions being significant. Unopposed estrogen replacement notably increases the risk of EC by up to 20-fold, with a higher risk associated with prolonged exposure. However, the combined use of progestin significantly reduces this risk (8). Given the dependence of endometrial proliferation on estrogen, well-differentiated tumors are inclined to retain their estrogen and progestin receptors. Additionally, progestin assumes an anti-estrogen role in the endometrium (9).
The standard treatment for endometrial cancer involves surgery, typically with total abdominal hysterectomy and bilateral salpingectomy. In cases with high-risk factors, lymph node dissection followed by individualized adjuvant therapy becomes necessary. Adjuvant treatment strategies for endometrial cancer encompass external-beam pelvic radiotherapy, vaginal brachytherapy, chemotherapy, and the combination of chemotherapy and radiotherapy. Approximately 20% of endometrial cancers occur in premenopausal women, posing a significant concern for maintaining fertility. Surgical treatment, while standard, carries substantial risks for certain patients, particularly those who are morbidly obese or experiencing serious complications (9, 10). Progestin therapy stands as the primary fertility-preserving treatment for young women with atypical endometrial hyperplasia (AEH) and well-differentiated endometrial cancer (EC) (11). Enhancing the efficacy of progestin therapy has become a focal point in addressing the needs of this patient population. For women with AEH who do not intend to bear children, hysterectomy with adnexal removal is strongly recommended, given the approximately 30% risk of developing invasive carcinoma. Conversely, conservative treatment is an option for women who wish to preserve fertility or are at higher surgical risk. These individuals should receive relatively high-dosed progestin therapy, and the use of a progestin-containing intrauterine device is also a viable approach (12). As progestin therapy becomes more prevalent in clinical applications, adverse reactions, including progesterone resistance, thrombosis, and weight gain, are frequently observed, impacting therapeutic efficacy and patient compliance (13). Additionally, a potential consequence of progestin therapy is the reduction in progestin receptor concentration, leading to a shorter duration of response (14). Clinical trials have indicated that progestin combination therapy can mitigate the side effects of long-term progestin use while maintaining its therapeutic benefits. However, there is no consensus on the outcome of progestin combination therapy. For instance, the levonorgestrel-releasing intrauterine system (LNG-IUS) is a device placed in the uterus that increases the concentration of progestin in localized tissues. Fang et al. demonstrated that LNG-IUS combined with oral progestin therapy was more effective in improving menstrual status and endometrial thickness compared to the control group, reducing adverse effects (15). In a 3-year randomized controlled trial, Xu et al. found no difference in treatment efficacy between megestrol acetate (MA) alone, LNG-IUS alone, and MA combined with LNG-IUS (16). Despite these studies, there is still a lack of evidence-based consensus on the efficacy of progestin combination therapy, necessitating further exploration.
Network meta-analyses serve as a valuable tool by amalgamating evidence from both direct and indirect comparisons of different trials, constructing a comprehensive network of treatments. This approach enables researchers to assess the impact of multiple interventions simultaneously for the same condition. Commonly reported outcomes in network meta-analyses include mean ranks or the surface under the cumulative ranking curve (SUCRA), which provides a numerical summary of the distribution to establish hierarchies of treatment comparisons (17, 18). In this study, we employed a network meta-analysis to compare the effectiveness of various progestin combination regimens for patients with endometrial cancer (EC) or atypical endometrial hyperplasia (AEH). The aim is to identify the optimal combination treatment regimen, offering a theoretical reference for the clinical management of AEH or EC patients.
The investigators systematically searched PubMed, Web of Science, Embase, and Cochrane Central Register of Controlled Trials for studies published from inception to December 2023. The search strategy was meticulously crafted in accordance with the PICOS tool: (P) Population: patients with endometrial cancer (EC) or atypical endometrial hyperplasia (AEH), (I) Intervention: progestin or progestin combined therapy, (C) Comparator: other conventional treatments (including surgical or irradiation therapy) or progestin, (O) Outcomes: survival index, evaluation criteria for efficacy, pregnancy rate, relapse rate and adverse events, (S) Study type: Randomized Controlled Trials (RCTs). A comprehensive list of the search terms is provided in Table 1 (using PubMed as an example). Concurrently, Chinese databases such as CNKI were searched, but no literature meeting the inclusion criteria was identified.
The study inclusion criteria encompassed the following key aspects (1): The experimental group involved diverse progestin or progestin combined therapies as interventions for patients (2). The control group received routine treatment or progestin (3). Patients diagnosed with primary endometrial cancer (EC) or atypical endometrial hyperplasia (AEH) were clearly defined and confirmed (4). The study included analyzable data and relevant outcome indices (5). The study design adhered to the Randomized Controlled Trial (RCT) framework.
Studies were excluded based on the following criteria (1): Incomplete or non-extractable data (2). Inconsistencies in diagnostic criteria (3). Studies derived from animal or cell experiments, case reports, scientific experiment plans, protocols, conference abstracts, or correspondence.
The literature management process involved importing articles into EndNote 20, followed by a thorough screening and exclusion procedure conducted by two investigators. Initially, the titles of the literature were scrutinized for duplication and the exclusion of non-randomized controlled trial studies, animal or cell experiments, case reports, scientific experiment plans, protocols, conference abstracts, or correspondence. Subsequently, the abstracts of the literature were reviewed by the same investigators to further narrow down the selection. The remaining literature was then meticulously examined in full by the two investigators, who independently identified articles for inclusion, recording the reasons for exclusion. Throughout this elimination process, the two researchers worked independently, and any disparities were resolved through discussion and comparison. If an agreement was reached between the two investigators regarding the inclusion of a particular piece of literature, it was ultimately included. In cases of disagreement, a third investigator made the final decision. This rigorous approach aimed to ensure the meticulous selection of literature for the study.
Data extraction was meticulously conducted using Excel 2010 software. The extracted information encompassed critical details, including author, year of publication, type of disease, sample size, experimental group intervention, control group intervention, mean age, median follow-up, and outcome indicators of the literature. To ensure a standardized and systematic approach, a nine-item, pre-selected data extraction form was employed for recording data, contributing to the comprehensive inclusion of relevant information from the selected studies. This methodical process aimed to uphold consistency and accuracy in the extraction of pertinent data for subsequent analysis. The data reported in the studies we included were obtained based on diagnostic follow-up (hysteroscopic evaluation and curettage biopsy) of the results explored. The outcome indicators were defined as follows (1):. OS was defined as the time from the start of randomization group to death, regardless of cause (2). PFS was defined as the time from the start of randomization group to tumor progression or death from any cause (3). CR was defined as no evidence of hyperplasia or cancerous lesion (4). PR was defined as the improvement in pathological findings (5). ORR was defined as the sum of CR and PR (6). SD was defined as the persistence of disease at initial diagnosis (7). PD was defined as the presence of grade II-III EC, myometrial infiltration or extrauterine lesions in patients with well-differentiated EC and any endometrial malignancy in patients with AEH (8). We included pregnant patients who were in complete response after progestin treatment, with or without assisted reproductive technology (ART) (9). Relapse was defined as the presence of EC or AEH after complete response on progestin therapy (10). Adverse events were defined as any recorded adverse events (AEs) and serious adverse events (SAEs).
Two investigators independently assessed the risk of bias (ROB) using the Cochrane Handbook version 5.1.0 tool. The assessment tool comprises seven domains: (a) randomized sequence generation, (b) concealment of allocation, (c) blinding of participants and personnel, (d) blinding of outcome assessment, (e) incomplete outcome data addressed, (f) freedom from selective reporting bias, and (g) other sources of bias. The risk of bias in each domain is categorized into three levels based on the number of components for which high ROB might exist: high risk (five or more), some concerns (three or four), and low risk (two or less). In cases of disagreement, resolution was achieved through the involvement of a third investigator. Ultimately, a risk of bias graph was generated to visually represent the assessment outcomes. This systematic approach aimed to ensure a rigorous evaluation of potential biases in the included studies.
In the intervention studies, all variables were treated as dichotomous and expressed as odds ratios (OR), accompanied by the calculation of the corresponding 95% confidence intervals (CI). Acknowledging the heterogeneity among the studies, this meta-analysis opted for a random-effects model over a fixed-effects model. The analysis was performed using State software (version 15.1) (19). For the Network Meta-Analysis (NMA), Markov chain Monte Carlo simulation chains were employed within a Bayesian-based framework. The nodal method was utilized to quantify consistency between direct and indirect comparisons. The verification of consistency relied on assessing whether the calculated p-value exceeded 0.05 (20).
Network diagrams generated by State software serve to illustrate and elucidate various treatment interventions. Within these diagrams, each node symbolizes a distinct intervention or control condition, while the connecting lines depict direct comparisons between interventions. The size of each node and the thickness of the lines are proportional to the number of patients and relevant studies, respectively (21).
The Surface Under the Cumulative Ranking Curve (SUCRA) metric was employed in this meta-analysis to assess and rank the effectiveness of each treatment. Additionally, it facilitated the identification of the most effective interventions. More precisely, the SUCRA metric comprehensively considers all possible rankings and uncertainties (22). SUCRA values, ranging from 0 to 1, indicate the likelihood of a specific intervention achieving favorable outcomes. A higher SUCRA value suggests a greater probability of the intervention being optimal for the given outcome. A SUCRA value of 1 implies certainty that an intervention is the best, while a value of 0 denotes certainty that an intervention is the least effective. However, differences in SUCRA values do not inherently imply variations in intervention efficacy. A funnel plot was utilized for visual assessment of publication bias, aiding in the identification of potential biases arising from smaller studies (23, 24).
Utilizing specified search strings, a total of 1553 documents were identified in the database. Additionally, five documents were manually retrieved. Following the removal of 998 duplicates, the remaining 560 documents underwent screening based on titles and abstracts, resulting in the exclusion of 238 documents. The remaining 322 papers were thoroughly read and assessed for eligibility, leading to the exclusion of 295 papers due to various reasons, including non-randomized controlled trials (n=147), incomplete data (n=45), other literature types such as conference proceedings (n=56), study protocol (n=20) and interventions not meeting inclusion criteria (n=27). Ultimately, the study included 27 documents (Figure 1).
A total of 27 randomized controlled trials, encompassing 5,323 patients diagnosed with endometrial cancer or atypical endometrial hyperplasia, were included in this study. These trials spanned the period from 1978 to 2023, with sample sizes ranging from 6 to 1084. The participants’ average age varied between 28.3 and 68.1 years. Among the selected trials, nine were conducted in China (11, 13, 15, 16, 25–29), four in the United States (30–33), three in the United Kingdom (34–36), two in Norway (37, 38), two in Italy (39, 40), and one each in Poland (41), Australia (42), Russia (43), Germany (44), Belgium (45), Iran (46), and the former Soviet Union (47). Of these, 11 trials investigated the effects of progestin-based combination therapy versus progestin alone or other treatments (11, 13, 15, 16, 25, 27, 28, 31, 32, 42, 43), another 11 trials explored the efficacy of progestin alone versus non-progestin treatments (26, 30, 33–35, 37, 40, 41, 44, 45, 47), and 5 trials compared the efficacy of different progestin treatments used alone (29, 36, 38, 39, 46). Most studies incorporated two or more outcome indicators, while a few focused solely on a single survival metric, Overall Survival (OS) (33, 34, 37, 41, 43, 47). Table 2 provides a summary of the basic characteristics of the included studies.
Among the randomized controlled trials included in the analysis, 10 studies exhibited a low overall risk of bias, while 15 studies raised some concerns, and only 2 studies presented a high overall risk of bias. The majority of studies (25 out of 27, or 92.3%) seemed to carry a low to moderate risk of bias. 6 studies did not specify how random sequences were generated, and 15 did not provide details about allocation concealment. Given the objective nature of the outcome indicators, blinding of outcome assessment was deemed low risk in all studies. Additionally, 26 studies elucidated the treatment of incomplete data, and 14 studies demonstrated selective reporting. Notably, only eight studies achieved blinding of both participants and assessors to treatment allocation. This limitation arose from the diverse interventions in these studies, involving progestin as well as surgical procedures and radiotherapy, making simultaneous blinding challenging. The included studies did not exhibit evidence of other types of bias (Supplementary Figure 1).
We assessed p-values for consistency and inconsistency in all direct and indirect comparisons related to survival indicators. The findings revealed that all p-values exceeded 0.05, signifying acceptable consistency among the studies (Supplementary Tables 1, 2). The network maps depicting the included interventions are presented in Supplementary Figures 2A, B.
A comprehensive analysis of 10 randomized controlled trials encompassing 13 interventions was conducted to evaluate their impact on Overall Survival (OS). The outcomes from the network meta-analysis, focusing on OS, revealed that none of the interventions demonstrated a clear superiority in terms of OS (Table 3). Hydroxyprogesterone caproate (HC) emerged as the most effective intervention for enhancing overall survival, boasting a SUCRA score of 81.4%. Additionally, among all combined treatment regimens, the Hydroxyprogesterone caproate+tamoxifen combination secured the top rank in the probability of improving overall survival, with a SUCRA score of 80% (Figure 2A).
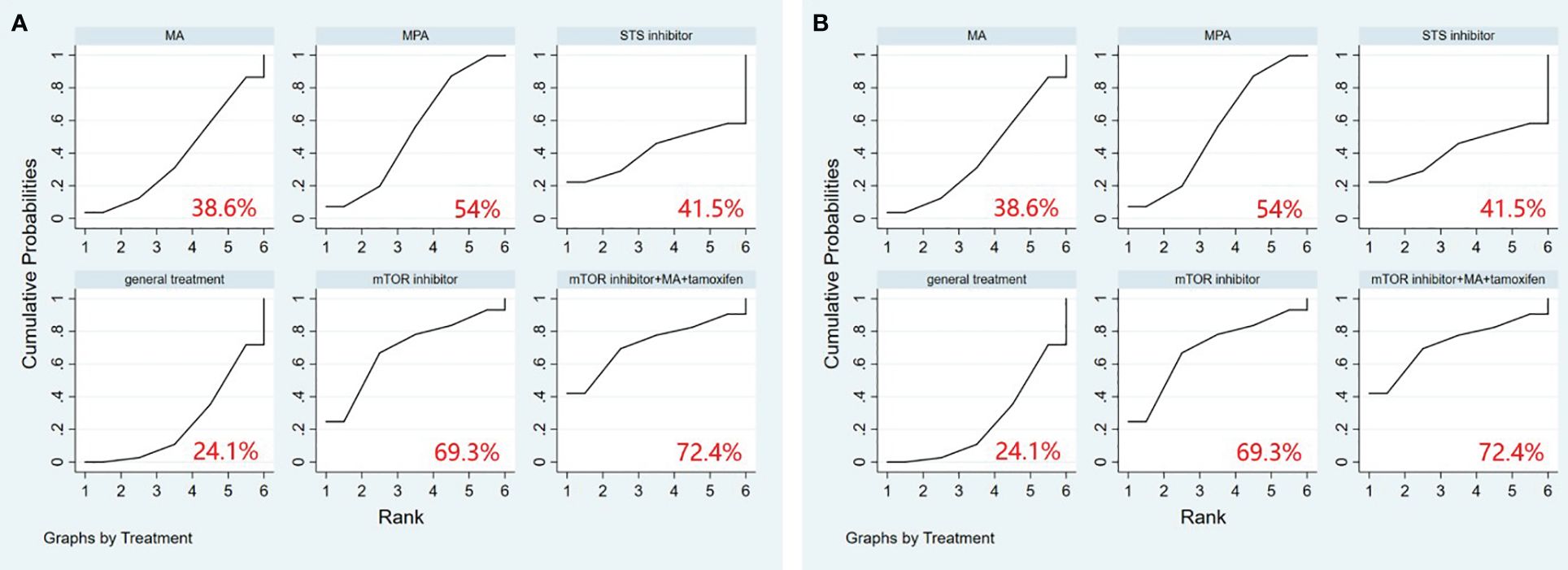
Figure 2 (A) SUCRA plot for overall survival (OS), (B) SUCRA plot for progression-free survival (PFS). HC, hydroxyprogesterone caproate, MA, megestrol acetate, MPA, medroxyprogesterone acetate, STS, steroid sulphatase, mTOR, mammalian target of rapamycin.
A comprehensive analysis was conducted on 5 randomized controlled trials involving 6 interventions to assess their impact on Progression-Free Survival (PFS). Among these interventions, Medroxyprogesterone acetate (MPA) [OR=1.44, 95% CI= (1.05, 1.98)] demonstrated superiority over general treatment in comparison to the control group (Table 4). According to the Surface Under the Cumulative Ranking Curve (SUCRA), the combination of mTOR inhibitor, megestrol acetate (MA), and tamoxifen exhibited the most significant influence on PFS, achieving a SUCRA score of 72.4% (Figure 2B).
All p-values comparing the involved studies were assessed for both consistency and inconsistency. In the non-consistency test, all p-values exceeded 0.05. Similarly, in the consistency test, the majority of p-values were greater than 0.05. In summary, these results suggest that the consistency across studies was acceptable (Supplementary Tables 3–7). The network maps illustrating the included interventions are presented in Supplementary Figures 3A–E.
A total of 19 randomized controlled trials involving 17 interventions were analyzed for their impact on Complete Response (CR). The findings indicated that the LNG-IUS+MPA group [OR=14.75, 95% CI=(4.58, 47.52)], LNG-IUS+general treatment group [OR=4.20, 95% CI=(1.25, 14.13)], MA+metformin group [OR=3.75, 95% CI=(1.03, 13.68)], LNG-IUS group [OR=3.23, 95% CI=(1.64, 6.37)], and MPA+metformin group [OR=1.93, 95% CI=(1.01, 3.71)] were more effective than the MPA group in increasing the number of CR post-treatment. Additionally, when compared with the tamoxifen group, the LNG-IUS+MPA group [OR=19.18, 95% CI=(3.73, 98.69)], LNG-IUS+general treatment group [OR=5.46, 95% CI=(1.03, 28.99)], and LNG-IUS group [OR=4.20, 95% CI=(1.11, 15.92)] outperformed the MPA group. Ultimately, compared to the MPA cycle group, the LNG-IUS+MPA group [OR=220.92, 95% CI=(11.00, 4437.09)], LNG-IUS+general treatment group [OR=62.88, 95% CI=(3.08, 1285.05)], MA+metformin group [OR=56.08, 95% CI=(2.63, 1196.31)], LNG-IUS group [OR=48.37, 95% CI=(2.81, 831.86)], LNG-IUS+metformin group [OR=41.92, 95% CI=(2.10, 835.53)], LNG-IUS+MA group [OR=38.92, 95% CI=(1.96, 771.98)], MA group [OR=34.54, 95% CI=(1.76, 678.22)], MPA+metformin group [OR=28.92, 95% CI=(1.44, 578.99)], general treatment group [OR=23.48, 95% CI=(1.10, 500.67)], and MPA continue group [OR=8.41, 95% CI=(2.08, 34.06)] were all superior to the MPA group (Table 5). The LNG-IUS+MPA group ranked first in the SUCRA probability rankings (SUCRA=98.7%) (Figure 3A).

Figure 3 (A) SUCRA plot for complete response (CR), (B) SUCRA plot for partial response (PR), (C) SUCRA plot for objective response rate (ORR), (D) SUCRA plot for stable disease (SD), (E) SUCRA plot for progressive disease (PD). MA, megestrol acetate, MPA, medroxyprogesterone acetate, LNG-IUS, levonorgestrel-releasing intrauterine system, STS, steroid sulphatase, mTOR, mammalian target of rapamycin.
A total of 13 randomized controlled trials involving 12 interventions were analyzed for their impact on Partial Response (PR). The outcomes of the network meta-analysis, with Partial Response (PR) as the outcome, revealed that the MA group [OR=4.07, 95% CI=(1.02, 16.31)] demonstrated superiority in increasing the number of individuals in PR compared to the STS inhibitor group (Table 6). Megestrol acetate (MA) emerged as the most effective intervention to enhance overall survival among all interventions (SUCRA=75.6%). Within combined treatment regimens, the MA+tamoxifen group secured the top position in the probability ranking for improving overall survival (SUCRA=75.2%) (Figure 3B).
In total, 19 randomized controlled trials involving 17 interventions were analyzed for their effects on Objective Response Rate (ORR). The results revealed that, in comparison to the tamoxifen group, the LNG-IUS+MPA group [OR=27.17, 95%CI=(5.41, 136.41)], MA+metformin [OR=5.21, 95%CI=(1.12, 24.26)], LNG-IUS+general treatment group [OR=6.44, 95%CI=(1.48, 28.08)], LNG-IUS group [OR=4.95, 95%CI=(1.69, 14.52)], LNG-IUS+metformin group [OR=4.29, 95%CI=(1.04, 17.77)], and MPA+metformin group [OR=4.10, 95%CI=(1.40, 11.97)] demonstrated superior efficacy. Similarly, compared to the Steroid Sulphatase Inhibitor (STS) group, the LNG-IUS+MA group [OR=5.95, 95%CI=(1.27, 27.96)], LNG-IUS+MPA group [OR=40.55, 95%CI=(5.37, 306.32)], MA group [OR=5.28, 95%CI=(1.34, 20.73)], MA+metformin group [OR=7.77, 95%CI=(1.67, 36.19)], LNG-IUS+treatment group [OR=9.61, 95%CI=(1.42, 65.00)], and LNG-IUS group [OR=7.39, 95%CI=(1.46, 37.56)] exhibited superiority. Furthermore, in comparison to the MPA cycle group, the LNG-IUS+MA group [OR=38.92, 95%CI=(1.96, 771.97)], LNG-IUS+MPA group [OR=265.28, 95%CI=(12.09, 5822.25)], MA group [OR=34.54, 95%CI=(1.76, 678.21)], MA+metformin group [OR=50.86, 95%CI=(2.39, 1083.85)], LNG-IUS+general treatment group [OR=62.88, 95%CI=(3.08, 1285.05)], LNG-IUS group [OR=48.37, 95%CI=(2.81, 831.86)], LNG-IUS+metformin group [OR=41.92, 95%CI=(2.10, 835.53)], MPA+metformin group [OR=39.99, 95%CI=(1.99, 804.54)], MA+tamoxifen group [OR=32.51, 95%CI=(1.24, 850.80)], and MPA continue group [OR=8.41, 95%CI=(2.08, 34.06)] surpassed the tamoxifen group (Table 7). Lastly, compared to the LNG-IUS+MPA group, the LNG-IUS+MA group [OR=0.15, 95%CI=(0.03,0.66)] did not exhibit a significant advantage in improving ORR. According to the SUCRA, the LNG-IUS+MPA group ranked first (SUCRA=99.1%) in the probability ranking of different interventions for improving ORR (Figure 3C).
In total, 13 randomized controlled trials involving 12 interventions were analyzed for their effects on Stable Disease (SD). The results of the network meta-analysis with SD as the outcome revealed that the mTOR inhibitor group [OR=20.62, 95%CI=(1.15, 369.19)] outperformed the LNG-IUS+MPA group in increasing the number of SD after treatment (Table 8). According to the SUCRA, the mTOR inhibitor was identified as the most effective intervention for improving overall survival among all interventions (SUCRA=75.2%). Additionally, among all combined treatment regimens, the mTOR inhibitor+MA+tamoxifen group achieved the top rank in the probability ranking of improving overall survival (SUCRA=73.4%) (Figure 3D).
In total, 13 randomized controlled trials involving 12 interventions were analyzed for their effects on Progressive Disease (PD). The results of the network meta-analysis indicated that there were more instances of disease progression in the mTOR inhibitor+MA+tamoxifen group [OR=22.37, 95%CI=(1.75, 285.42)] compared to the MPA+metformin group. Moreover, in comparison to the mTOR inhibitor group, both the mTOR inhibitor+MA+tamoxifen group [OR=24.50, 95%CI=(2.78, 216.27)] and the MPA group [OR=2.64, 95%CI=(1.11, 6.29)] showed an increased likelihood of disease progression after treatment. Furthermore, when compared to the LNG-IUS+MPA group, the mTOR inhibitor+MA+tamoxifen group [OR=288.57, 95%CI=(2.77, 30039.79)] exhibited a higher risk of disease progression after treatment (Table 9). In the SUCRA analysis, the LNG-IUS+MPA group ranked last (SUCRA=8.0%) in the probability ranking of different interventions leading to disease progression (Figure 3E).
All p-values for comparisons between the studies were examined for both consistency and inconsistency, and the majority of p-values exceeded 0.05, signifying an acceptable level of consistency across studies (Supplementary Tables 8–10). The network maps illustrating the included interventions are presented in Supplementary Figures 4A–C.
A total of 9 randomized controlled trials and 7 interventions were included in the analysis of their effects on the pregnancy rate. The results of the network meta-analysis, with pregnancy rate as the outcome, indicated that both LNG-IUS+MA group [OR=9.05, 95% CI=(1.92, 42.62)] and MA group [OR=3.40, 95% CI=(1.17, 9.91)] were more effective than the general treatment group in enhancing the pregnancy rate (Table 10). In the SUCRA analysis, LNG-IUS+MA group ranked first (SUCRA=83.7%) in the probability ranking of different interventions for improving the pregnancy rate (Figure 4A).
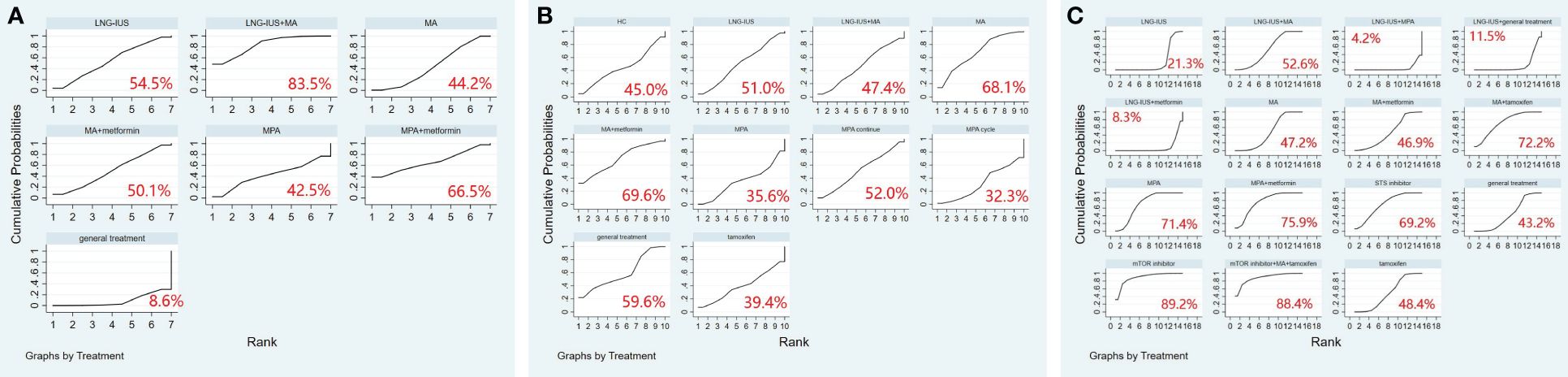
Figure 4 (A) SUCRA plot for pregnancy rate, (B) SUCRA plot for relapse rate, (C) SUCRA plot for adverse events. MA, megestrol acetate; MPA, medroxyprogesterone acetate; LNG-IUS, levonorgestrel-releasing intrauterine system; STS, steroid sulphatase; mTOR, mammalian target of rapamycin; HC, hydroxyprogesterone caproate.
A total of 10 randomized controlled trials and 10 interventions underwent analysis to assess their impact on relapse rate. The results of the network meta-analysis, considering relapse rate as the outcome, revealed that the general treatment group [OR=1.38, 95% CI=(1.02, 1.85)] caused more relapses compared to the MPA group (Table 11). In the SUCRA analysis, LNG-IUS+MA group ranked last (SUCRA=47.4%) in the probability ranking of different interventions for increasing the relapse rate among all combined treatment regimens (Figure 4B).
We included 15 randomized controlled trials and 15 interventions to analyze their safety. The results of the network meta-analysis showed that more adverse events occurred in mTOR inhibitor group [OR=4.38, 95% CI=(1.34, 7.42)], mTOR+ MA+tamoxifen group [OR=4.41, 95% CI=(1.10, 7.73)], and MPA group [OR=2.94, 95% CI=(1.03, 4.85)] compared to LNG-IUS+MA group. Similarly, more adverse events occurred in mTOR inhibitor group [OR=4.59, 95% CI=(1.62, 7.57)], mTOR+ MA+tamoxifen group [OR=4.63, 95% CI=(1.38, 7.88)], MPA+metformin group [OR=3.40, 95% CI=(1.27, 5.53)], MA+tamoxifen group [OR=3.22, 95% CI=(1.16, 5.27)], MPA group [OR=3.16, 95% CI=(1.36, 4.96)], and STS inhibitor group [OR=3.07, 95% CI=(1.09, 5.06)] compared to LNG-IUS+ metformin group (Supplementary Table 11). In the SUCRA analysis, Among all treatment regimens, LNG-IUS+MPA group ranked last (SUCRA=4.2%) in the probability ranking of the incidence of adverse events due to the different interventions and correspondingly, mTOR inhibitor group and mTOR inbitor+MA+tamoxifen group were ranked first (SUCRA=89.2%) and second (SUCRA=88.4%) in the probability ranking, respectively (Figure 4C).
We generated distinct funnel plots for each outcome indicator to identify potential publication bias. Upon careful examination of the funnel plots, no significant publication bias was observed. Detailed information is presented in Supplementary Figure 5.
In this investigation, we conducted a comprehensive comparison of the effectiveness of various progestin-based regimens for treating patients with endometrial cancer or endometrial atypia. A total of 5,323 patients diagnosed with endometrial cancer or atypical hyperplasia, sourced from 27 studies, were included, encompassing 19 different interventions. Among these interventions, 9 comprised progestin-based combination therapy programs. Our findings indicated that the Hydroxyprogesterone caproate (HC)+tamoxifen regimen demonstrated superior efficacy in extending overall survival (OS), while the mTOR inhibitor+megestrol acetate (MA)+tamoxifen regimen exhibited the best performance in prolonging progression-free survival (PFS). Regarding efficacy indicators, the LNG-IUS+medroxyprogesterone acetate (MPA) regimen emerged as the top choice for increasing the number of complete responses (CR). The MA+tamoxifen regimen proved to be the optimal treatment for enhancing the number of partial responses (PR). For the objective response rate (ORR), the LNG-IUS+MPA regimen stood out as the most effective combination therapy. In terms of stable disease (SD), the mTOR inhibitor+MA+tamoxifen regimen demonstrated superior efficacy. In the context of disease progression, the LNG-IUS+MPA regimen exhibited the lowest likelihood of causing disease progression. Additionally, the LNG-IUS+MA regimen emerged as the preferred combination therapy for improving pregnancy rates, and it was also the least likely to result in disease relapse. However, in terms of safety, mTOR inhibitor and mTOR inhibitor+MA+tamoxifen had the highest likelihood of adverse events, while LNG-IUS+MPA was the safest combination regimen. Overall, combination therapy based on mTOR inhibitors had advantages in improving certain indicators, but it also faced the risk of adverse events. The combination therapy based on tamoxifen had a lower risk of adverse events compared to mTOR inhibitor combination therapy, but the indicators it improves were limited. The LNG-IUS-based dual progestin regimen not only had an advantage in the number of indicators improved but was also the safest approach. Therefore, among all the combination regimens, the LNG-IUS-based dual progestin regimen may represent the most suitable approach for treating endometrial cancer or atypical endometrial hyperplasia.
In our investigation, tamoxifen featured prominently in optimal combination regimens associated with prolonged survival index, and it was also a key component in the optimal combination regimen employing PR and SD as outcome indicators. Existing studies have highlighted that estrogen replacement therapy without progestin supplementation leads to a substantial increase (4- to 14-fold) in the relative risk of endometrial cancer (48). Tamoxifen, classified as a selective estrogen receptor modulator (SERM), functions by obstructing estrogen binding to estrogen receptors in endometrial cancer (44, 49). Consequently, it holds potential beneficial effects in managing endometrial cancer or atypical endometrial hyperplasia. Hald et al. investigated tamoxifen’s efficacy in advanced endometrial cancer, reporting assessable disease remission (partial and complete) in 8 of 33 patients (50). Quinn et al. observed a 20% remission rate in 10 out of 49 patients with advanced/recurrent endometrial cancer treated with tamoxifen (51). However, the relationship between tamoxifen and an elevated risk of endometrial cancer is a subject of extensive discourse. Leeuwen et al. conducted a case-control study in the Netherlands, revealing a trend toward a significantly increased risk of endometrial cancer with longer duration of tamoxifen use and an escalating cumulative dose (52). In conclusion, the role of tamoxifen in endometrial cancer, along with its specific mechanisms, warrants further exploration.
High-frequency aberrations on the PI3K/Akt/mTOR pathway have been identified in endometrial cancer (31). Elevated activity of the PI3K/Akt/mTOR pathway is linked to resistance to progesterone treatment in endometrial cancer cell lines and mouse models, and inhibition of this pathway can reverse such resistance (53). Consequently, mTOR inhibitors demonstrate promise as targeted agents for endometrial cancer treatment. In our investigation, mTOR inhibitors emerged as crucial components in the optimal combination regimen for achieving positive outcomes in terms of progression-free survival (PFS) and stable disease (SD). First-generation mTOR inhibitors currently undergoing clinical trials for advanced or recurrent endometrial cancer include everolimus (RA001), temsirolimus (CCI779), and ridaforolimus (AP2357) (54). A phase II clinical trial of ridaforolimus (AP2357) in patients with advanced and recurrent endometrial cancer revealed a clinical benefit response (CBR) in 9 out of 27 patients with evaluable responses (55). Tsoref et al. conducted a phase II trial assessing the tolerability and activity of oral ridaforolimus (AP2357) in patients with metastatic or recurrent endometrial cancer, reporting that among the 31 evaluable patients, 3 achieved partial response (PR), and an additional 18 achieved stable disease (SD) (56). The application of mTOR inhibitors restrains the proliferation and progression of cancer cells, and when combined with progestins, they may exert synergistic effects.
Among the various optimal combination regimens, the LNG-IUS-based dual progestin regimen emerged prominently, underscoring the crucial role of LNG-IUS in treating endometrial cancer or atypical endometrial hyperplasia. LNG-IUS serves as a long-acting contraceptive option for women and is employed in the management of abnormal and heavy bleeding (heavy periods). Its mechanism involves the inhibition of endometrial proliferation, leading to endometrial atrophy through metaplasia and suppression of endometrial glands (57). This effect may stem from LNG-IUS inhibiting the synthesis of endometrial estrogen receptors, rendering the endometrium insensitive to circulating estrogen. Furthermore, the therapeutic efficacy relies significantly on local progestogen action within the uterine cavity, potentially making it preferable to other progestins (58). LNG-IUS demonstrates heightened effectiveness in reversing endometrial hyperplasia. For example, Varma et al. conducted a prospective study involving 105 women diagnosed with endometrial hyperplasia treated with LNG-IUS, revealing a remarkable 90% regression within 2 years, with 92% resolution of atypical endometrial hyperplasia (59). In another study, Hashim et al. conducted a randomized controlled trial involving 120 perimenopausal women with atypical endometrial hyperplasia, noting a significantly higher regression rate in the LNG-IUS group compared to the oral progestogen group, achieving regression within 1 year (60). Additionally, LNG-IUS plays a pivotal role in endometrial cancer treatment. Westin et al. conducted a phase II trial of LNG-IUS in 57 patients diagnosed with complicated atypical hyperplasia and early endometrial cancer, reporting an overall 83% 12-month remission rate (RR), with a 66.7% remission rate for grade 1 endometrial-looking endometrial cancer (61). However, it’s worth noting that LNG-IUS may face challenges in delivering a uniform progesterone dose to the entire endometrial cavity, potentially creating a ‘myometrial sanctuary’ for tumors (62).
In terms of progestin therapy for EC or AEH, relevant guidelines are also described, for example, for patients with grade 1, stage IA endometrial cancer who wish to preserve fertility, both the National Comprehensive Cancer Network (NCCN) and European guidelines recommend continuous progestin-based therapy, which includes MA, MPA, or LNG-IUS. In addition, for patients with disseminated metastases, who are unsuitable for local therapy, or who have further recurrence, both the NCCN and the European guidelines recommend hormonal therapy, with the European Society of Gynaecological Oncology (ESGO) guidelines recommending the use of MA and MPA (63). Treatment guidelines recommend that patients with atypical endometrial hyperplasia should be treated with hysterectomy. However there is still controversy over the use of progestins (64). Although some guidelines do not make a clear statement about progestin-based combination therapy regimens, this is the innovation we wanted to explore. The results of a meta-analysis showed that oral progestins still resulted in high CR rates and relapse rates within the accepted range for patients with AEH or EC who relapsed after receiving conservative treatment (65). Through this study, we want to find an efficacious and safe progestin-based combination therapy regimen, to raise awareness of progestin therapy for endometrial cancer, and to lay the foundation for subsequent research.
Notably, alterations occurring in the endometrium can link reproductive and oncological diseases. It has been shown that polycystic ovary syndrome (PCOS), a heterogeneous endocrine disease, causes dysregulation of endometrial sex hormone receptor and co-receptor expression, endometrial insulin resistance, impaired glucose transport and utilization, chronic inflammation, immune dysfunction, uterine vascular alterations, and even abnormal gene expression and cellular abnormalities of the endometrium, which make the endometrium dysfunctional (66). A national case-control study from Australia showed a four-fold increased risk of endometrial cancer in patients with PCOS compared to those without PCOS, and in addition, hirsutism and extremely irregular menstruation among the symptoms of PCOS were significantly associated with the risk of endometrial cancer (67). Results from another cohort study from Denmark showed a similar fourfold increase in endometrial risk (68). This is consistent with the results of a number of meta-analyses of which we are aware. For example a meta-analysis that included 10 studies showed that women with PCOS had a significantly increased risk of endometrial cancer compared to controls (69), with a further increase in risk when postmenopausal people were excluded from the meta-analysis. Similarly, adenomyosis is a benign gynaecological disease, whereas EC can originate from malignant transformation of ectopic endometrium within the adenomyosis lesion, leading to endometrial carcinoma arising in adenomyosis (EC-AIA). This disease is difficult to diagnose preoperatively, but differs from EC in postoperative histological features and prognosis (70). We therefore envisage that the combination of hysteroscopic surgery and progestins will yield beneficial results in the treatment of endometrial carcinoma and its rarer types.
Previous risk stratification of EC has been based on pathological features, and studies of The Cancer Genome Atlas (TCGA) may have changed our view. In TCGA, EC can be classified into four prognostically relevant groups based on mutation load and somatic copy number variation, namely POLE-mutant, mismatch repair (MMR)-deficient, p53-abnormal, and “no specific molecular profile” (NSMP). Each group differs in pathological characteristics, prognosis, and response to different therapies, making the TGCA classification of great advantage for risk stratification and management of EC (71).
This study has certain advantages. Firstly, our study investigates the potential of progestin-based combination therapy regimens in treating endometrial cancer or atypical endometrial hyperplasia and finds the combination regimen with the best efficacy and the least number of adverse events, offering significant clinical implications. Secondly, the rigorous application of the network meta-analysis method ensures a high level of medical evidence, delivering crucial information for clinicians and policymakers. We also assessed the risk of bias for each of the included studies, and none found the presence of serious bias. Finally, the inclusion of randomized controlled trials and a rigorous data extraction process ensure the quality of the study. However, our study has some limitations. While we incorporated 27 studies and analyzed data from 5323 patients, the persuasiveness of our findings could be further strengthened with a more extensive literature review. Additionally, we acknowledge a lack of in-depth consideration of heterogeneity among the studies. Factors like patient age, weight, progesterone dosage, and administration route were not thoroughly addressed and may introduce confounding variables. For the EC and AEH patients involved in this study, we did not analyze them separately. Finally, although we chose multiple indicators to assess, the number of studies included in each indicator was not the same, which may have had some impact on the results.
In summary, our study indicates that while tamoxifen and mTOR inhibitors show efficacy in combined progestin-based regimens, a dual progestin-based approach utilizing LNG-IUS has better efficacy and safety and holds greater promise for treating endometrial cancer or endometrial atypical hyperplasia. This insight could be valuable for clinicians in selecting the most suitable progestin treatment for patients with endometrial cancer or atypical endometrial hyperplasia.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
JC: Conceptualization, Methodology, Writing – original draft. YZ: Data curation, Writing – review & editing. LS: Writing – review & editing. T-JW: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1391546/full#supplementary-material
1. Makker V, MacKay H, Ray-Coquard I, Levine DA, Westin SN, Aoki D, et al. Endometrial cancer. Nat Rev Dis Primers. (2021) 7:88. doi: 10.1038/s41572-021-00324-8
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Van Nyen T, Moiola CP, Colas E, Annibali D, Amant F. Modeling endometrial cancer: past, present, and future. Int J Mol Sci. (2018) 19:2348. doi: 10.3390/ijms19082348
4. Purdie DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. (2001) 15:341–54. doi: 10.1053/beog.2000.0180
5. Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ 2nd, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. (2006) 106:812–19. doi: 10.1002/cncr.21650
6. Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. (2019) 69:258–79. doi: 10.3322/caac.21561
7. Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. (2012) 379:1352–60. doi: 10.1016/s0140-6736(12)60442-5
8. Passarello K, Kurian S, Villanueva V. Endometrial cancer: an overview of pathophysiology, management, and care. Semin Oncol Nurs. (2019) 35:157–65. doi: 10.1016/j.soncn.2019.02.002
9. Cade TJ, Quinn MA, Rome RM, Neesham D. Progestogen treatment options for early endometrial cancer. Bjog. (2010) 117:879–84. doi: 10.1111/j.1471-0528.2010.02552.x
10. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. (2016) 387:1094–108. doi: 10.1016/s0140-6736(15)00130-0
11. Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng YL, Shan WW, et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial. Bjog. (2020) 127:848–57. doi: 10.1111/1471-0528.16108
12. Denschlag D, Ulrich U, Emons G. The diagnosis and treatment of endometrial cancer: progress and controversies. Dtsch Arztebl Int. (2010) 108:571–7. doi: 10.3238/arztebl.2011.0571
13. Yuan F, Hu Y, Han X, Li Q. Metformin in combination with progesterone improves the pregnancy rate for patients with early endometrial cancer. Contrast Media Mol Imaging. (2022) 2022:1961016. doi: 10.1155/2022/1961016
14. Fiorica JV, Brunetto VL, Hanjani P, Lentz SS, Mannel R, Andersen W. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. (2004) 92:10–4. doi: 10.1016/j.ygyno.2003.11.008
15. Fang F, Xu H, Wu L, Hu L, Liu Y, Li Y, et al. LNG-IUS combined with progesterone ameliorates endometrial thickness and pregnancy outcomes of patients with early-stage endometrial cancer or atypical hyperplasia. Am J Transl Res. (2021) 13:5412–9.
16. Xu Z, Yang B, Shan W, Liao J, Shao W, Wu P, et al. Comparison of the effect of levonorgestrel-intrauterine system with or without oral megestrol acetate on fertility-preserving treatment in patients with atypical endometrial hyperplasia: A prospective, open-label, randomized controlled phase II study. Gynecol Oncol. (2023) 174:133–41. doi: 10.1016/j.ygyno.2023.05.001
17. Buti J, Glenny AM, Worthington HV, Nieri M, Baccini M. Network meta-analysis of randomised controlled trials: direct and indirect treatment comparisons. Eur J Oral Implantol. (2011) 4:55–62.
18. Faltinsen EG, Storebø OJ, Jakobsen JC, Boesen K, Lange T, Gluud C. Network meta-analysis: the highest level of medical evidence? BMJ Evid Based Med. (2018) 23:56–9. doi: 10.1136/bmjebm-2017-110887
19. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
20. Lin C, Chu CM, Su SL. Epistasis test in meta-analysis: A multi-parameter markov chain monte carlo model for consistency of evidence. PloS One. (2016) 11:e0152891. doi: 10.1371/journal.pone.0152891
21. Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int. (2014) 34:1489–96. doi: 10.1007/s00296-014-2994-2
22. Wang Z, Carter RE. Ranking of the most effective treatments for cardiovascular disease using SUCRA: Is it as sweet as it appears? Eur J Prev Cardiol. (2018) 25:842–3. doi: 10.1177/2047487318767199
23. Xu Y, Deng G, Zhang J, Zhu J, Liu Z, Xu F, et al. Efficacy of four anti-vascular endothelial growth factor agents and laser treatment for retinopathy of prematurity: A network meta-analysis. Biomol BioMed. (2023). doi: 10.17305/bb.2023.9829
24. Chen L, Xue Q, Yan C, Tang B, Wang L, Zhang B, et al. Comparative safety of different recommended doses of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis of randomized clinical trials. Front Endocrinol. (2023) 14:1256548. doi: 10.3389/fendo.2023.1256548
25. Shan W, Wang C, Zhang Z, Gu C, Ning C, Luo X, et al. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. J Gynecol Oncol. (2014) 25:214–20. doi: 10.3802/jgo.2014.25.3.214
26. Kuang J, Sun S, Xu H, Ni R, Ke F. Curative effects of hysteroscopic resection combined with progesterone on early-stage endometrial cancer and its prognosis. J buon. (2021) 26:1320–6.
27. Xu Z, Yang B, Guan J, Shan W, Liao J, Shao W, et al. Comparison of the effect of oral megestrol acetate with or without levonorgestrel-intrauterine system on fertility-preserving treatment in patients with early-stage endometrial cancer: a prospective, open-label, randomized controlled phase II trial (ClinicalTrials.gov NCT03241914). J Gynecol Oncol. (2023) 34:e32. doi: 10.3802/jgo.2023.34.e32
28. Kong WY, Liu ZA, Zhang N, Wu X, Zhao XB, Yan L. A prospective cohort study of metformin as an adjuvant therapy for infertile women with endometrial complex hyperplasia/complex atypical hyperplasia and their subsequent assisted reproductive technology outcomes. Front Endocrinol. (2022) 13:849794. doi: 10.3389/fendo.2022.849794
29. Mao Y, Wan X, Chen Y, Lv W, Xie X. Outcomes of conservative therapy for young women with early endometrial adenocarcinoma. Fertil Steril. (2010) 93:283–85. doi: 10.1016/j.fertnstert.2009.07.999
30. Oza AM, Pignata S, Poveda A, McCormack M, Clamp A, Schwartz B, et al. Randomized phase II trial of ridaforolimus in advanced endometrial carcinoma. J Clin Oncol. (2015) 33:3576–82. doi: 10.1200/jco.2014.58.8871
31. Fleming GF, Filiaci VL, Marzullo B, Zaino RJ, Davidson SA, Pearl M, et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: a gynecologic oncology group study. Gynecol Oncol. (2014) 132:585–92. doi: 10.1016/j.ygyno.2014.01.015
32. Pandya KJ, Yeap BY, Weiner LM, Krook JE, Erban JK, Schinella RA, et al. Megestrol and tamoxifen in patients with advanced endometrial cancer: an Eastern Cooperative Oncology Group Study (E4882). Am J Clin Oncol. (2001) 24:43–6. doi: 10.1097/00000421-200102000-00007
33. Malkasian GD Jr., Burĕs J. Adjuvant progesterone therapy for stage I endometrial carcinoma. Int J Gynaecol Obstet. (1978) 16:48–9. doi: 10.1002/j.1879-3479.1978.tb00391.x
34. Haylock BJ, Murrell DS, Bourne H, Acworth P. Stage I endometrial carcinoma: the role of neoadjuvant progesterone therapy. Clin Oncol. (1993) 5:102–6. doi: 10.1016/S0936-6555(05)80857-X
35. Groups C-N-UECS. Adjuvant medroxyprogesterone acetate in high-risk endometrial cancer. Int J Gynecol Cancer. (1998) 8:387–91. doi: 10.1046/j.1525-1438.1998.09865.x
36. Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK. LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study. Hum Reprod. (2013) 28:2966–71. doi: 10.1093/humrep/det320
37. Vergote I, Kjørstad K, Abeler V, Kolstad P. A randomized trial of adjuvant progestagen in early endometrial cancer. Cancer. (1989) 64:1011–6. doi: 10.1002/1097-0142(19890901)64:5<1011::aid-cncr2820640507>3.0.co;2-7
38. Ørbo A, Arnes M, Vereide AB, Straume B. Relapse risk of endometrial hyperplasia after treatment with the levonorgestrel-impregnated intrauterine system or oral progestogens. Bjog. (2016) 123:1512–9. doi: 10.1111/1471-0528.13763
39. Falcone F, Laurelli G, Losito S, Di Napoli M, Granata V, Greggi S. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J Gynecol Oncol. (2017) 28:e2. doi: 10.3802/jgo.2017.28.e2
40. Rendina GM, Donadio C, Fabri M, Mazzoni P, Nazzicone P. Tamoxifen and medroxyprogesterone therapy for advanced endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. (1984) 17:285–91. doi: 10.1016/0028-2243(84)90071-6
41. Urbański K, Karolewski K, Kojs Z, Klimek M, Dyba T. Adjuvant progestagen therapy improves survival in patients with endometrial cancer after hysterectomy. Results of one-institutional prospective clinical trial. Eur J Gynaecol Oncol. (1993) 14 Suppl:98–104.
42. Janda M, Robledo KP, Gebski V, Armes JE, Alizart M, Cummings M, et al. Complete pathological response following levonorgestrel intrauterine device in clinically stage 1 endometrial adenocarcinoma: Results of a randomized clinical trial. Gynecol Oncol. (2021) 161:143–51. doi: 10.1016/j.ygyno.2021.01.029
43. Vishnevsky AS, Bokhman Ya V, Loutfi G. Favourable influence of adjuvant hormone therapy by oxyprogesterone caproate (OPC) and by its combination with tamoxifen on 5-year survival rate of surgical and combined treatment of primary endometrial carcinoma patients. Eur J Gynaecol Oncol. (1993) 14:150–3.
44. von Minckwitz G, Loibl S, Brunnert K, Kreienberg R, Melchert F, Mösch R, et al. Adjuvant endocrine treatment with medroxyprogesterone acetate or tamoxifen in stage I and II endometrial cancer–a multicentre, open, controlled, prospectively randomised trial. Eur J Cancer. (2002) 38:2265–71. doi: 10.1016/s0959-8049(02)00378-7
45. Pautier P, Vergote I, Joly F, Melichar B, Kutarska E, Hall G, et al. A phase 2, randomized, open-label study of irosustat versus megestrol acetate in advanced endometrial cancer. Int J Gynecol Cancer. (2017) 27:258–66. doi: 10.1097/igc.0000000000000862
46. Behnamfar F, Ghahiri A, Tavakoli M. Levonorgestrel-releasing intrauterine system (Mirena) in compare to medroxyprogesterone acetate as a therapy for endometrial hyperplasia. J Res Med Sci. (2014) 19:686–90.
47. Bokhman JV, Chepick OF, Volkova AT, Vishnevsky AS. Adjuvant hormone therapy of primary endometrial carcinoma with oxyprogesterone caproate. Gynecol Oncol. (1981) 11:371–8. doi: 10.1016/0090-8258(81)90051-2
48. Cohen CJ, Rahaman J. Endometrial cancer. Management of high risk and recurrence including the tamoxifen controversy. Cancer. (1995) 76:2044–52. doi: 10.1002/1097-0142(19951115)76:10+<2044::aid-cncr2820761323>3.0.co;2-n
49. DeMichele A, Troxel AB, Berlin JA, Weber AL, Bunin GR, Turzo E, et al. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol. (2008) 26:4151–9. doi: 10.1200/jco.2007.14.0921
50. Hald I, Salimtschik M, Mouridsen HT. Tamoxifen treatment of advanced endometrial carcinoma. A phase II study. Eur J Gynaecol Oncol. (1983) 4:83–7.
51. Quinn MA, Campbell JJ. Tamoxifen therapy in advanced/recurrent endometrial carcinoma. Gynecol Oncol. (1989) 32:1–3. doi: 10.1016/0090-8258(89)90839-1
52. van Leeuwen FE, Benraadt J, Coebergh JW, Kiemeney LA, Gimbrère CH, Otter R, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. (1994) 343:448–52. doi: 10.1016/s0140-6736(94)92692-1
53. Gu C, Zhang Z, Yu Y, Liu Y, Zhao F, Yin L, et al. Inhibiting the PI3K/Akt pathway reversed progestin resistance in endometrial cancer. Cancer Sci. (2011) 102:557–64. doi: 10.1111/j.1349-7006.2010.01829.x
54. Husseinzadeh N, Husseinzadeh HD. mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: a critical review. Gynecol Oncol. (2014) 133:375–81. doi: 10.1016/j.ygyno.2014.02.017
55. Colombo N, McMeekin S, Schwartz P, Kostka J, Sessa C, Gehrig P, et al. A phase II trial of the mTOR inhibitor AP23573 as a single agent in advanced endometrial cancer. J Clin Oncol. (2007) 25:5516–6. doi: 10.1200/jco.2007.25.18_suppl.5516
56. Tsoref D, Welch S, Lau S, Biagi J, Tonkin K, Martin LA, et al. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol Oncol. (2014) 135:184–9. doi: 10.1016/j.ygyno.2014.06.033
57. Dore M, Filoche S, Danielson K, Henry C. Efficacy of the LNG-IUS for treatment of endometrial hyperplasia and early stage endometrial cancer: Can biomarkers predict response? Gynecol Oncol Rep. (2021) 36:100732. doi: 10.1016/j.gore.2021.100732
58. Giannopoulos T, Butler-Manuel S, Tailor A. Levonorgestrel-releasing intrauterine system (LNG-IUS) as a therapy for endometrial carcinoma. Gynecol Oncol. (2004) 95:762–4. doi: 10.1016/j.ygyno.2004.09.010
59. Varma R, Soneja H, Bhatia K, Ganesan R, Rollason T, Clark TJ, et al. The effectiveness of a levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of endometrial hyperplasia–a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. (2008) 139:169–75. doi: 10.1016/j.ejogrb.2008.02.022
60. Abu Hashim H, Zayed A, Ghayaty E, El Rakhawy M. LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: a randomized controlled trial. J Gynecol Oncol. (2013) 24:128–34. doi: 10.3802/jgo.2013.24.2.128
61. Westin SN, Fellman B, Sun CC, Broaddus RR, Woodall ML, Pal N, et al. Prospective phase II trial of levonorgestrel intrauterine device: nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am J Obstet Gynecol. (2021) 224:191. doi: 10.1016/j.ajog.2020.08.032
62. Dhar KK, NeedhiRajan T, Koslowski M, Woolas RP. Is levonorgestrel intrauterine system effective for treatment of early endometrial cancer? Report of four cases and review of the literature. Gynecol Oncol. (2005) 97:924–7. doi: 10.1016/j.ygyno.2004.10.031
63. Restaino S, Paglietti C, Arcieri M, Biasioli A, Martina MD, Mariuzzi L, et al. Management of patients diagnosed with endometrial cancer: Comparison of guidelines. Cancers. (2023) 15:1091. doi: 10.3390/cancers15041091
64. Reed SD, Voigt LF, Newton KM, Garcia RH, Allison HK, Epplein M, et al. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. (2009) 113:655–62. doi: 10.1097/AOG.0b013e318198a10a
65. Raffone A, Raimondo D, Rovero G, Travaglino A, Lopez G, Di Maio CM, et al. Conservative re-treatment of women with atypical endometrial hyperplasia and early endometrial carcinoma: We can hope, at least. Int J Gynaecol Obstet. (2024) 165:542–51. doi: 10.1002/ijgo.15146
66. Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum Reprod Update. (2021) 27:584–618. doi: 10.1093/humupd/dmaa051
67. Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM, Australian Ovarian Cancer Study Group and Australian National Endometrial Cancer Study Group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control. (2010) 21:2303–8. doi: 10.1007/s10552-010-9658-7
68. Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. (2015) 136:99–103. doi: 10.1016/j.ygyno.2014.11.012
69. Johnson JE, Daley D, Tarta C, Stanciu PI. Risk of endometrial cancer in patients with polycystic ovarian syndrome: A meta−analysis. Oncol Lett. (2023) 25:168. doi: 10.3892/ol.2023.13754
70. Raffone A, Raimondo D, Maletta M, Travaglino A, Renzulli F, Neola D, et al. Endometrial cancer arising in adenomyosis (EC-AIA): A systematic review. Cancers (Basel). (2023) 15:1142. doi: 10.3390/cancers15041142
Keywords: endometrial cancer, progestin, atypical endometrial hyperplasia, combination therapy, systematic review, meta-analysis
Citation: Cui J, Zhao Y-C, She L-Z and Wang T-J (2024) Comparative effects of progestin-based combination therapy for endometrial cancer or atypical endometrial hyperplasia: a systematic review and network meta-analysis. Front. Oncol. 14:1391546. doi: 10.3389/fonc.2024.1391546
Received: 26 February 2024; Accepted: 22 April 2024;
Published: 03 May 2024.
Edited by:
Stefano Palomba, Sapienza University of Rome, ItalyReviewed by:
Aris Besharat, Sapienza University of Rome, ItalyCopyright © 2024 Cui, Zhao, She and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tie-Jun Wang, dGp3YW5nQGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.