- 1Department of Oncology, Hubei Cancer Hospital, TongJi Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Intensive Care Unit, Chengdu Shuangliu Hospital of Traditional Chinese Medicine, Chengdu, China
Non-small cell lung cancer (NSCLC) is one of the most common malignancies in the world. EGFR tyrosine inhibitors are the preferred first-line treatment for patients with epidermal growth factor-cell receptor mutant (EGFR mutant) advanced NSCLC. Unfortunately, drug resistance inevitably occurs leading to disease progression. Activation of the ALK and BRAF bypass signaling pathways is a rare cause of acquired drug resistance for EGFR-TKIs.We report two NSCLC-patients with EGFR- mutations, in exon 19, and exon 18, correspondingly, who were treated with EGFR-TKIs. The first case shows acquired BRAF-mutation, and the second case demonstrates acquired ALK-fusion. The overall survival of patients was significantly prolonged by drug-match therapies. As it is well-known that ALK-fusion and BRAF-mutations are described forms of acquired resistance. These two case reports contribute to the previous reports that ALK-fusion and BRAF-mutation are potential underlying mechanisms of EGFR-TKI resistance.
Introduction
Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 80% -85% of the cases (1). In recent years, significant progress has been made in the molecular genetics of lung cancer, and the treatment of NSCLC has entered the era of targeted therapy (2). The most common driver gene mutation in NSCLC is the epidermal growth factor receptor (EGFR), which is found in 45% of Asian patients with adenocarcinoma histology and in 20% of Caucasian patients (3). The most common gene mutations include EGFR 19 del and EGFR 21 L858R. In patients with advanced NSCLC harboring sensitizing EGFR mutations, EGFR-tyrosine kinase inhibitor (EGFR-TKI) treatment was significantly better effective than conventional chemotherapy. Globally, neither treatment guidelines nor clinical practice considers EGFR-TKIs as the first-line treatment option for EGFR-mutated metastatic NSCLC patients (4).
However, the vast majority of patients inevitably develop acquired drug resistance, which seriously affects the survival prognosis of patients (5). The resistance mechanisms can be generally classified into two categories: on-target and off-target resistance. We report two cases of rare resistance mechanisms involving ALK and BRAF pathway that occurred under EGFR-TKI treatment. To some extent, our cases contribute to previously reported new treatment approaches.
Case 1
A 60-year-old male smoker presented with complaints of cough and fatigue. A chest CT scan revealed multiple nodules in both lungs, with the largest measuring approximately 1.1x0.8cm, suggesting the presence of lung cancer with multiple pulmonary metastases. Additionally, multiple liver metastases were observed, with the largest measuring about 2.1x1.3cm. The patient was diagnosed with stage cT1bN3M1c IVB based on biopsy from the tumor in the right lung. Real-Time PCR indicated the presence of EGFR 19 E746_A750del(1) deletion mutation. As the first-line treatment, the patient received furmonertinib (80 mg once daily) for 27 months. A follow-up chest CT scan revealed an enlargement of the right lung nodule to approximately 2.6x2.1cm. Subsequently, the patient underwent 4 cycles of chemotherapy with pemetrexed combined with cisplatin. One month later, the chest CT scan showed the development of massive pleural effusion on the left side and enlarged liver lesions, measuring about 5.5x4.6cm. PCR genetic testing of tumor liver tissue revealed a mutation in V600E in exon 15 of the BRAF gene. The patient was then treated with dabrafenib (150 mg twice daily) and trametinib (2 mg once daily), and a follow-up CT scan after eight weeks showed improvement in the left pleural effusion and liver metastasis (Figure 1). So far, the patient responses well to targeted drugs and remains healthy with no signs of recurrence.
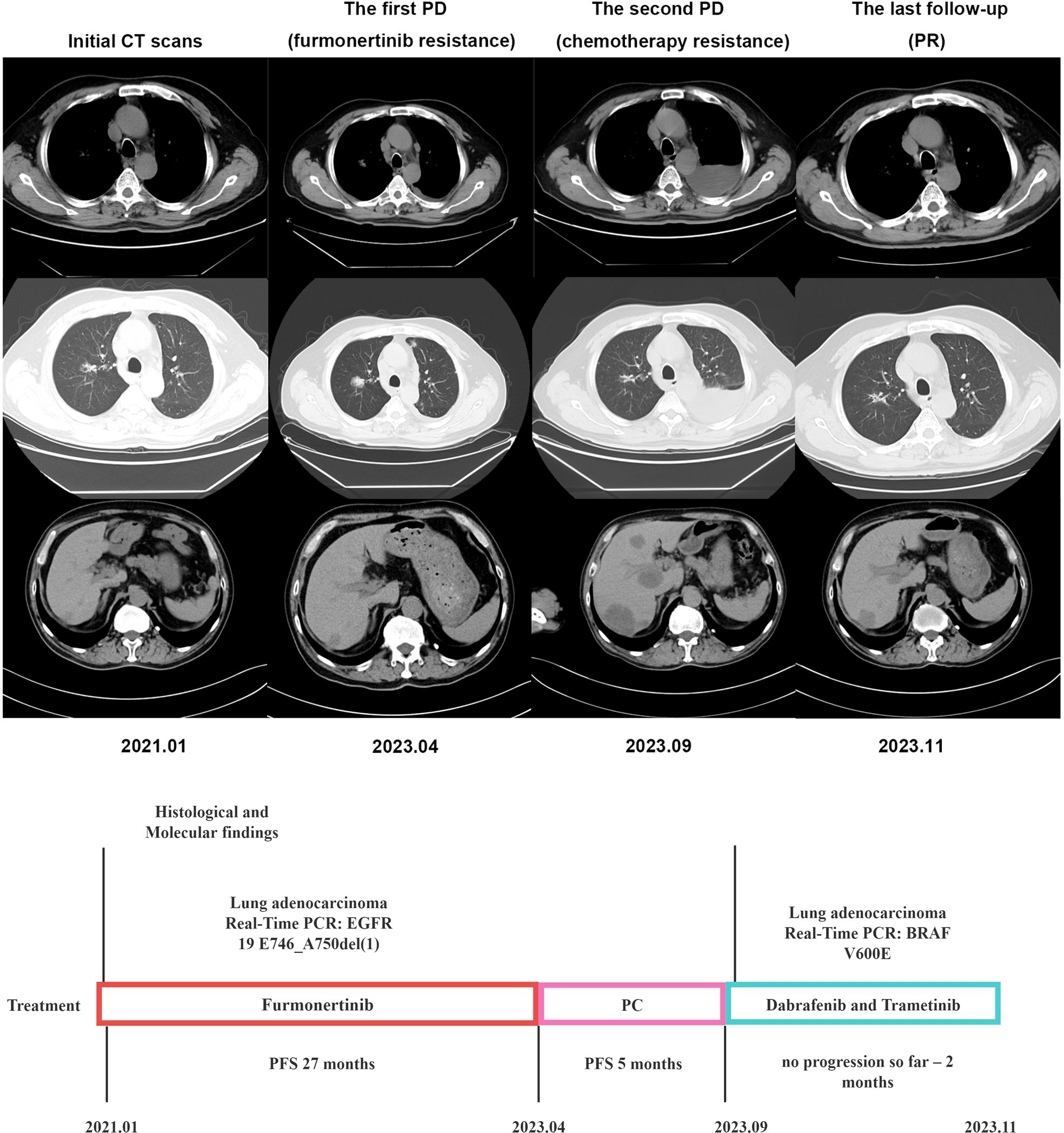
Figure 1. Timeline illustrating the changes in therapeutic regimen in correlation with molecular and radiological findings. PCR, digital polymerase chain reaction; PFS, progress free survival; PC, pemetrexed combined with cisplatin; PD, progression of disease; PR, partial remission.
Case 2
A 77-year-old female non-smoking patient presented with a cough. Imaging examination revealed the presence of nodules (1.7x1.5 cm in size) in the left lower lobe, along with multiple brain metastases. Genetic testing revealed a mutation in EGFR18-G719X. The patient was initially treated with gefitinib (250 mg once daily) with good tolerance and treatment for 20 months. NGS analysis of peripheral blood showed no mutations. Subsequently, the treatment was changed to icotinib, but a re-evaluation after 4 months demonstrated progression of the lung and brain metastases. Then the patient was treated with 6 cycles of Pemetrexed and Bevacizumab. The following MRI of the brain revealed enlarging lesions with extensive adjacent edema. Rebiopsy from progression lung tumor investigated by illumina NGS showed the presence of ALK-fusion, leading to the initiation of treatment with Alectinib. After eight weeks, reexamination showed a reduction in the size of lung lesions and disappearance of brain lesions (Figure 2). So far, the patient remains stable with no signs of recurrence.
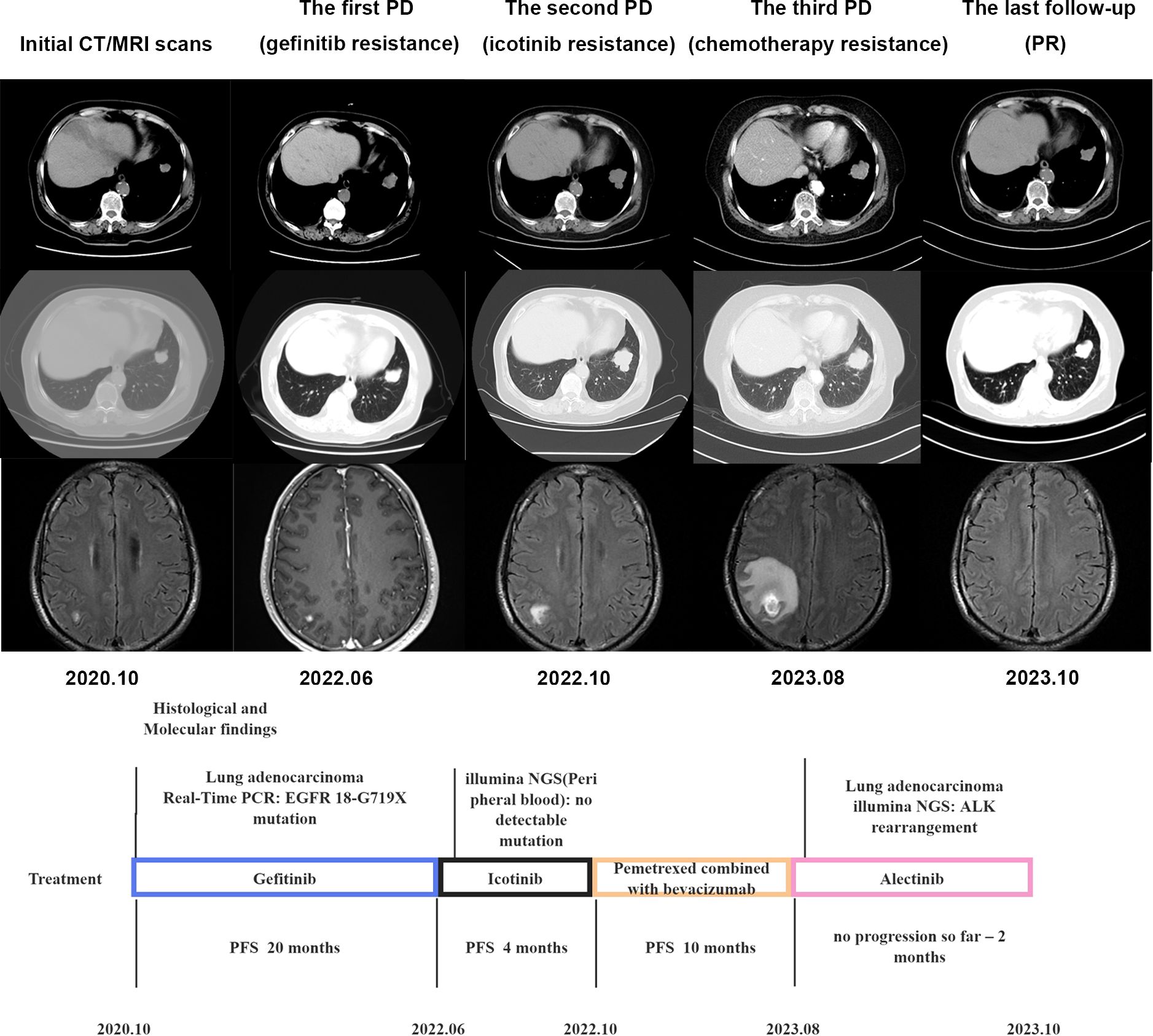
Figure 2. Timeline illustrating the changes in therapeutic regimen in correlation with molecular and radiological findings. PCR, digital polymerase chain reaction; PFS, progress free survival; NGS, next-generation sequencing; PD, progression of disease; PR, partial remission.
Discussion
We present two cases of advanced lung adenocarcinoma with two EGFR -mutations at initial diagnosis. In both cases progression on EGFR-TKIs was observed, leading to the identification of acquired resistance mechanisms in form of ALK(EML4 exon20) fusion and BRAF mutation. Interestingly, both patients achieved long-term survival with the use of ALK and BRAF inhibitors. These findings offer valuable insights for selecting therapeutic drugs in patients with acquired ALK fusion and BRAF mutation.
Mechanisms of EGFR-TKI resistance include EGFR mutations such as C797S, T790M, G796D, G724S, and L718Q, with T790M being the most common (50-60%). Additionally, resistance can arise from MET amplification (20%), HER2 amplification (13%), activation of bypass pathways through mutation or amplification of tyrosine kinase receptor genes like c-MET, FGFR, and HER2, mutations in downstream signaling genes like KRAS, BRAF, PIK3CA, and RAS/RAF/MEK/ERK, and transformation into small cell lung cancer (10%) (6). ALK fusion is also involved in mediating EGFR-TKI resistance and may occur as intrinsic or acquired resistance mechanism (7). Primary fusion occurs in patients with dual alteration of EGFR mutation and ALK fusion genes, with a prevalence ranging from 0.9% to 6% in NSCLC (8). For patients with double alteration, the effectiveness of EGFR-TKI or ALK-TKI may depend on the mutation level of the involved gene (9, 10). Secondary fusion refers to ALK fusion may occur as a rare acquired resistance mechanism under treatment with EGFR-TKI (11). However, cases of secondary ALK fusion following acquired resistance are rarely reported, and the optimal choice of targeted therapy remains inconclusive. In 2016, a case study reported the first patient with EGFR exon 19 deletion and ALK wild-type NSCLC. The study demonstrated the coexistence of primary EGFR mutation and acquired EML4-ALK gene fusion in plasma under EGFR-TKI treatment. The patient was treated with the EGFR inhibitor Osimertinib along with crizotinib, which resulted in sustained efficacy in the liver metastatic lesions (Extrahepatic lesions were stable) (12). In a study involving 3505 patients treated with EGFR-TKI, new ALK-fusions were detected in 7 patients. These fusion partners included EML4 (n=4), STRN (n=1), TGF (n=1), and PLEKHA7 (n=1). The patient with acquired STRN-ALK fusion did not experience the effect of Crizotinib. Additionally, in the patient with acquired PLEKHA7-ALK fusion, partial remission in six months was observed under the treatment with Alectinib and Osimertinib (13). Based on the hitherto published reports and the current case, combining of EGFR- and ALK-TKI may be an effective treatment in EGFR-mutated NSCLC with ALK-fusion acquired on the EGFR-TKI therapy. It is important to note that further research with a larger sample size is necessary to validate these results.
As one of the rare driver genes in NSCLC, BRAF mutations play a crucial role in the development and occurrence of tumors. Among these mutations, BRAF V600 is the most commonly observed. With significant advancements in precision therapy, targeted therapy has emerged as a primary treatment approach for BRAF mutations. Dual-target combination therapy, in particular, has gained widespread recognition for its clinical value. This therapy involves the use of dabrafenib, a BRAF inhibitor, and trametinib, a MAPK-inhibitor. The combination of these two drugs allows for precise targeting of dual pathways and complete inhibition of the upstream and downstream pathways of MAPK (14). The results of the BRF113928 trial demonstrated an overall response rate (ORR) of 63.9% and a 5-year overall survival (OS) rate of 22% in first-line treatment with dabrafenib + Trametinib (D+T). Among the 36 subjects treated with D + T as after line, the ORR reached 68.4%, and the duration of remission (DOR) was 9.8 (95% CI 6.9-18.3) months. Furthermore, the trial confirmed the safety of this treatment regimen (15). The Chinese Lung Cancer Registration Clinical Study also provided evidence of the favorable antitumor activity and safety of dabrafenib + trametinib in Chinese NSCLC patients. Consequently, in 2023, dabrafenib and trametinib were recommended as grade I treatments in the Clinical Diagnosis and Treatment Guidelines of the Chinese Society of Clinical Oncology (CSCO).
BRAF mutation is also considered one of the mechanisms of EGFR-TKI resistance. However, the occurrence of BRAF mutation in EGFR-TKI resistance is rare, with an incidence of only about 1%. On the other hand, the rate of BRAF mutation after osimertinib resistance is higher, ranging from 3% to 10% (16). There was a study (17) showing that a combination treatment of dabrafenib, trametinib, and osimertinib resulted in clinical remission with an overall response rate (ORR) of 80% in five EGFR+ patients who had received osimertinib as a primary treatment. This suggests that the combination treatment may have a better therapeutic effect for patients with BRAF mutation after EGFR resistance. However, it is important to note that this study had a small sample size and there were significant differences in patient baseline data. Therefore, further studies with larger sample sizes are needed to validate these findings.
Conclusion
While TKIs are widely used in NSCLC treatment, the occurrence of drug resistance is inevitable. The resistance mechanisms of 3rd generation EGFR-TKIs are complex and lack specific treatment. ALK-fusion and BRAF-mutations, although rare, cannot be ignored as they may emerge as mechanisms of EGFR-TKIs acquired resistance in a small number of NSCLC-patients. To develop more accurate personalized treatment plans, it is crucial to continuously explore potential drug resistance mechanisms and for finding therapeutic options under EGFR-TKIs resistance. This will further optimize the application of targeted therapy in NSCLC and provide precise guidance for individualized treatment of patients.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Writing – original draft, Writing – review & editing. QZ: Data curation, Formal analysis, Writing – original draft. BY: Writing – original draft. YH: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guo CL, Mei JD, Jia YL, Gan FY, Tang YD, Liu CW, et al. Impact of thymosin α1 as an immunomodulatory therapy on long-term survival of non-small cell lung cancer patients after R0 resection: a propensity score-matched analysis. Chin Med J (Engl). (2021) 134:2700–9. doi: 10.1097/CM9.0000000000001819
2. Deng B, Chen X, Xu L, Zheng L, Zhu X, Shi J, et al. Chordin-like 1 is a novel prognostic biomarker and correlative with immune cell infiltration in lung adenocarcinoma. Aging (Albany NY). (2022) 14:389–409. doi: 10.18632/aging.203814
3. Yang L, He YT, Dong S, Wei XW, Chen ZH, Zhang B, et al. Single-cell transcriptome analysis revealed a suppressive tumor immune microenvironment in EGFR mutant lung adenocarcinoma. J Immunother Cancer. (2022) 10:e003534. doi: 10.1136/jitc-2021-003534
4. Huang WC, Yadav VK, Cheng WH, Wang CH, Hsieh MS, Huang TY, et al. The MEK/ERK/miR-21 signaling is critical in osimertinib resistance in EGFR-mutant non-small cell lung cancer cells. Cancers (Basel). (2021) 13:6005. doi: 10.3390/cancers13236005
5. Bahcall M, Awad MM, Sholl LM, et al. Amplification of wild-type KRAS imparts resistance to crizotinib in MET exon 14 mutant non-small cell lung cancer. Clin Cancer Res. (2018) 24:5963–76. doi: 10.1158/1078-0432.CCR-18-0876
6. Xu H, Chen R, Shen Q, Yang D, Peng H, Tong J, et al. Overexpression of Circular RNA circ_0013587 Reverses Erlotinib Resistance in Pancreatic Cancer Cells Through Regulating the miR-1227/E-Cadherin Pathway. Front Oncol. (2021) 11:754146. doi: 10.3389/fonc.2021.754146
7. Lo Russo G, Imbimbo M, Corrao G, Proto C, Signorelli D, Vitali M, et al. Concomitant EML4-ALK rearrangement and EGFR mutation in non-small cell lung cancer patients: a literature review of 100 cases. Oncotarget. (2017) 8:59889–900. doi: 10.18632/oncotarget.17431
8. Li M, Hou X, Zhou C, Feng W, Jiang G, Long H, et al. Prevalence and clinical impact of concomitant mutations in anaplastic lymphoma kinase rearrangement advanced non-small-cell lung cancer (Guangdong association of thoracic oncology study 1055). Front Oncol. (2020) 10:1216. doi: 10.3389/fonc.2020.01216
9. Yang JJ, Zhang XC, Su J, Xu CR, Zhou Q, Tian HX, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res. (2014) 20:1383–92. doi: 10.1158/1078-0432.CCR-13-0699
10. Lou NN, Zhang XC, Chen HJ, Zhou Q, Yan LX, Xie Z, et al. Clinical outcomes of advanced non-small-cell lung cancer patients with EGFR mutation, ALK rearrangement and EGFR/ALK co-alterations. Oncotarget. (2016) 7:65185–95. doi: 10.18632/oncotarget.11218
11. Zhang L, Li Y, Zhang S, Gao C, Nie K, Ji Y, et al. Primary resistance to crizotinib treatment in a non-small cell lung cancer patient with an EML4-ALK rearrangement: a case report. Cancer Biol Med. (2018) 15:178–81. doi: 10.20892/j.issn.2095-3941.2018.0003
12. Liang W, He Q, Chen Y, Chuai S, Yin W, Wang W, et al. Metastatic EML4-ALK fusion detected by circulating DNA genotyping in an EGFR-mutated NSCLC patient and successful management by adding ALK inhibitors: a case report. BMC Cancer. (2016) 16:62. doi: 10.1186/s12885-016-2088-5
13. Schrock AB, Zhu VW, Hsieh WS, Madison R, Creelan B, Silberberg J, et al. Receptor tyrosine kinase fusions and BRAF kinase fusions are rare but actionable resistance mechanisms to EGFR tyrosine kinase inhibitors. J Thorac Oncol. (2018) 13:1312–23. doi: 10.1016/j.jtho.2018.05.027
14. Xu H, Zhou S, Xia H, Yu H, Tang Q, Bi F, et al. MEK nuclear localization promotes YAP stability via sequestering β-TrCP in KRAS mutant cancer cells. Cell Death Differ. (2019) 26:2400–15. doi: 10.1038/s41418-019-0309-6
15. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. (2017) 18:1307–16. doi: 10.1016/S1470-2045(17)30679-4
16. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. (2019) 121:725–37. doi: 10.1038/s41416-019-0573-8
Keywords: EGFR-TKI resistance mechanisms, acquired ALK-fusion, acquired BRAF-mutation, NSCLC, case report
Citation: Zeng Y, Zeng Q, Yang B and Hu Y (2024) Therapeutic strategies to overcome ALK-fusion and BRAF-mutation as acquired resistance mechanism in EGFR-mutated non-small cell lung cancer: two case reports. Front. Oncol. 14:1390523. doi: 10.3389/fonc.2024.1390523
Received: 23 February 2024; Accepted: 14 October 2024;
Published: 01 November 2024.
Edited by:
Shiyou Wei, Sichuan University, ChinaReviewed by:
Songxiao Xu, University of Chinese Academy of Sciences, ChinaLuis Mas, Auna Oncosalud, Peru
Copyright © 2024 Zeng, Zeng, Yang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Hu, SFloeTMyMUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Yuan Zeng
Yuan Zeng Qiang Zeng2†
Qiang Zeng2†