
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 19 June 2024
Sec. Cancer Epidemiology and Prevention
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1390052
This article is part of the Research Topic Community Series in Reducing Adverse Effects of Cancer Immunotherapy: Volume II View all 18 articles
 Yilkal Abebaw Wassie1*
Yilkal Abebaw Wassie1* Alebachew Ferede Zegeye1
Alebachew Ferede Zegeye1 Deresse Abebe Gebrehana2
Deresse Abebe Gebrehana2 Sintayehu Simie Tsega1
Sintayehu Simie Tsega1 Getasew Kibralew3
Getasew Kibralew3 Setegn Fentahun3
Setegn Fentahun3 Abebaw Setegn4
Abebaw Setegn4 Girum Nakie3
Girum Nakie3Introduction: Currently, the problem of cancer has been increasing around the world, predominantly in middle- and low-income countries. Anemia, a major and often overwhelming health burden for cancer patients, significantly distorts their quality of life. It is well-established that the length of treatment increases the frequency of anemia, with hematological malignancies experiencing nearly double the rate compared to solid tumors. Despite this established knowledge, data on the prevalence of anemia among cancer patients in Ethiopia remains scarce, according to the investigators.
Objective: This study aimed to assess the prevalence of baseline anemia and associated factors among adult cancer patients at Northwest Amhara Comprehensive Specialized Hospitals, oncology treatment units, Northwest Ethiopia, in 2021.
Methods: This study employed an institutional-based cross-sectional design and was conducted in Northwest Amhara Comprehensive Specialized Hospitals. A systematic random sampling technique was used to select 315 participants. The data were collected using interviewer-administered questionnaires and chart reviews of existing medical records using a structured and pretested questionnaire format. The data were entered into Epi. Data version 4.6 and analyzed using Stata version 14.0. Bivariable and multivariable logistic regression were carried out to identify factors associated with anemia. Adjusted odds ratios with a 95% confidence interval and variables with a p-value of < 0.05 were considered significantly associated with anemia.
Results: The prevalence of baseline anemia among adult patients with cancer was found to be 34.84%. Being a woman (AOR = 1.97; 95% CI: 1.00–3.87), being underweight (AOR = 1.96; 95% CI: 1.09–3.52), and having stage III cancer (AOR = 2.35; 95% CI: 1.12–3.01) were significantly associated with anemia.
Conclusion: The prevalence of baseline anemia among adult cancer patients was significant. Women, cancer patients with an underweight body mass index, and those diagnosed with clinical-stage III cancer were more likely to have baseline anemia. For health policymakers and healthcare providers, it is better to give special attention to female patients, patients who are underweight, and patients with advanced-stage cancer to reduce the risk of developing the outcome. This would allow for timely intervention to manage anemia and potentially improve treatment tolerance and quality of life for cancer patients.
Approximately one-sixth of all fatalities globally are caused by cancer, making it the most prevalent public health issue. In 2020 alone, nearly 9.6 million deaths were reported worldwide, which may be attributed to something (1). Cancer patients are particularly vulnerable to anemia, which is a major and debilitating health condition that can significantly impair their quality of life. A pathological decrease or reduction in hemoglobin (Hb) concentration, hematocrit, or the number of red blood cells per liter under the reference value for a healthy individual of similar age, gender, and race in a congruent environmental setting brought on by various pathophysiological mechanisms is what is meant to be understood (2–4).
Anemia is a global public health concern that affects both advanced and low-income nations, having a significant impact on social and economic advancement as well as human health. Around one-fourth of the world’s population, or 1.93 billion people, suffers from anemia globally. The majority of anemia-related impairments (89%) are seen in low-income nations (5). The magnitude is highest in Central and Western Sub-Saharan Africa, South Asia, Southeast Asia, and East Asia. It occurs at all stages of the life cycle but is more prevalent in patients with chronic disease, specifically cancer (6). Cancer is one of the most common conditions associated with anemia, a chronic disease, while anemia is a common complication of cancer (7). Most cancer patients, especially those who are receiving therapy, suffer anemia while their condition is still being treated. However, it most likely happens for unknown causes/means most of the time, following myelosuppressive chemotherapy or radiotherapy (8, 9).
Different articles announced that the magnitude of anemia varies ranging from 30% to 90% of cancer patients for the time of the course of their diseases (10). Numerous factors are recognized to be significant with anemia, as well as sociodemographic factors such as age, gender, ethnicity, locality, and marital status; nutritional factors, which are determined by overweight, obesity, and an imbalanced diet; lifestyle factors such as physical activity; and psychological factors such as depression and anxiety (7, 11).
Chemotherapy is one of the most essential causes of anemia in cancer patients, and the association between the dose and duration of chemotherapy with anemia is well known. The length of treatment increases the frequency of anemia (12). Before the administration of chemotherapy, every patient has an electrolyte assessment, including the measurement of hemoglobin. The frequency of anemia in hematological malignancies is nearly double that of solid tumors (13). This anemia can severly impact a patient’s mental, physical, and social development in both the short and long term; it can lead to problems with the immune system, hinder motor and cognitive growth, cause poor performance in school, and reduce work productivity throughout the patient’s life, thereby reducing earning capacities and adversely affecting national economic development (14).
Despite the challenges, chemotherapy may still be justified in patients with anemia by considering several factors, including cancer type and stage (15). The potential benefits of chemotherapy for a particular cancer and its stage outweigh the risks associated with anemia (16). Severity of anemia: the degree of anemia can influence the decision (17). Milder anemia might be manageable with supportive measures. Patient’s overall health: a patient’s overall health and ability to tolerate treatment are crucial considerations (18).
Generally, anemia can also distort many features of quality of life (QOL) and result in functional deficits (3, 7, 8). However, there are little data known about anemia among patients with cancer in African countries, including Ethiopia. Therefore, the purpose of this study was to determine the magnitude of baseline anemia and its associated factors among patients living with cancer.
The purpose of this study is to assess the prevalence of baseline anemia and associated factors among adult cancer patients at Northwest Amhara Comprehensive Specialized Hospitals, oncology treatment units, Northwest Ethiopia, in 2021.
This study, conducted in 2021 at Northwest Amhara Comprehensive Specialized Hospitals, oncology treatment units, Northwest Ethiopia, aimed to determine the prevalence of baseline anemia among adult cancer patients and to identify factors associated with baseline anemia in these patients.
An institutional-based cross-sectional study was adopted for this research, which was conducted from 15 March to 15 May 2021.
The study was conducted at Northwest Amhara Comprehensive Specialized Hospitals. There are a total of eight comprehensive specialized hospitals in the Amhara Region, of which five—Debre Markos, Felege Hiwot, Tibebe Gion, Debre Tabor, and University of Gondar—are found in the Northwest of Amhara. Each comprehensive specialized hospital serves 3.5–5 million people (19). Of the five referral hospitals, two [University of Gondar Comprehensive Specialized Referral Hospital (UoGCSH) and Felege Hiwot Comprehensive Specialized Hospital (FHRH)] have oncology treatment units. Those two referral hospitals are located in the Amhara Regional State, Northwest Ethiopia, 738 km and 565 km away from the capital city of Ethiopia, Addis Ababa, respectively. There are a total of 980 cancer patients in the two referral hospitals. The Oncology Treatment Unit of UoGCSH was established in 2014 G.C. and currently has 500 cancer patients and 17 beds for the management of cancer patients. Whereas the oncology treatment unit of FHCSH was established in 2016 G.C. and has 480 cancer patients, it currently has 18 beds for inpatient treatment of cancer patients. A 1-month average number of cancer patients who had follow-up treatments in UoGCSH and FHCSH were 250 and 240, respectively.
All adult cancer patients who were ≥ 18 years old and patients who attended Northwest Amhara Comprehensive Specialized Hospitals and oncology treatment units during the data collection period were included in the study. Those adult cancer patients with transferred-in record information were excluded because these charts may lack baseline information.
The sample size was calculated using the single population proportion formula, considering the following: a 95% confidence interval (CI), a 23% proportion of anemia from the previous study conducted in Addis Ababa (20), and a 5% margin of error. The final sample size was 315, considering a 10% nonrespondent rate. A systematic random sampling method was employed to select study participants in the study area by using the order of the cases. The sample size was proportionally allocated for each selected hospital based on their number of cancer patients and based on the report obtained from the oncology treatment units. The first participant to be included in the study was selected using the lottery method (K = N/n, where K is the interval, N is the total population, and n is the sample size), and then every three participants were interviewed.
This study classified participants as anemic or nonanemic based on the WHO criteria: hemoglobin concentration < 13 g/dL for men and < 12 g/dL for women (21).
Physical ability was assessed using the ECOG performance status grade (22). This reflects a patient’s general well-being and his/her ability to perform activities of daily life.
In this study, the calculation of body mass index (BMI) involves dividing the weight of an individual in kilograms by their height in square meters (23).
The data were collected by using interviewer-administered and chart-reviewed structured pretested questionnaires that are adapted from a questionnaire developed by a previous study, which contains four sections. The first section contains eight questions regarding sociodemographic characteristics of the study participants; the second section contains seven questions related to the clinical characteristics of a patient; the third section includes the nutritional status of a patient; and the fourth section is related to the behavioral factors of a patient with cancer. The data were collected by four BSc nurses who have working experience in the oncology treatment unit. Adult cancer patients were identified during their follow-up visits to the oncology unit. Patients who met the inclusion criteria were then interviewed after obtaining their consent to participate in the study.
After data collection, the collected data were cleaned and checked for completeness. Data were entered using Epi Data version 4.6 after being coded and analyzed using Stata version 14.0. Descriptive statistics were used in the analysis of medians and frequencies, and percentages were computed for all variables. Data were presented in tables. The association between dependent and independent variables was assessed using a binary logistic regression analysis model to estimate the strength of association using odds ratios (OR). All variables associated with baseline anemia with a p-value less of than 0.25 in the bivariable analysis were further analyzed using multivariable analyses to control potential confounding factors. Variables with a p-value of less than 0.05 were declared to be associated with baseline anemia. Multicollinearity and model goodness-of-fit tests were checked by using variance inflation factors (VIF = 1.05–4.17) and Hosmer and Lemeshow goodness-of-fit test (p = 0.91), respectively.
A pretest was done on 5% of the total sample size to make sure whether the questionnaire was appropriate and to ensure its validity in the study population before the actual data collection time. After the pretest, training was given to all data collectors and supervisors on the purpose of the study, how to get informed consent, and the technique of selecting the study participants from each oncology treatment unit. Supervision was conducted by the supervisors and the principal investigator. All questionnaires were translated into local languages (Amharic) before data collection. Consistency was checked by a backtranslation by another expert fluent both in English and in local languages. At the end of each data collection day, the supervisors were checked for the completeness or fulfillment of the questionnaires and the quality of the recorded information.
Ethical clearance was obtained from the School of Nursing research ethical review committee on behalf of the University of Gondar institutional review board. Written permission letters were obtained from hospital managers. Participants were informed about the purpose of the study, and written informed consent was obtained from them. Confidentiality was maintained by omitting direct personal identifiers on the questionnaire, using code numbers, storing data locked with a password, and not misusing or disclosing their information. Participants were also informed that participation was voluntary and they had the right to withdraw from the study at any stage if they were not comfortable with the investigation. The issues of privacy and confidentiality were strictly maintained.
A total of 315 study participants were enrolled in the study, with a response rate of 310 (98.41%). The mean age of the study participants was 45.81, with a standard deviation of ± 10.98 years. Nearly one-third (99; 31.94%) of them were between the ages of 40 and 49 years. A bit less than three-fourths (218; 70.32%) were women, and the majority (284; 91.61%) were followers of Orthodox Christianity. Most of them (292; 94.19%) were Amhara by ethnicity, and 209 (67.42%) were rural dwellers (Table 1).
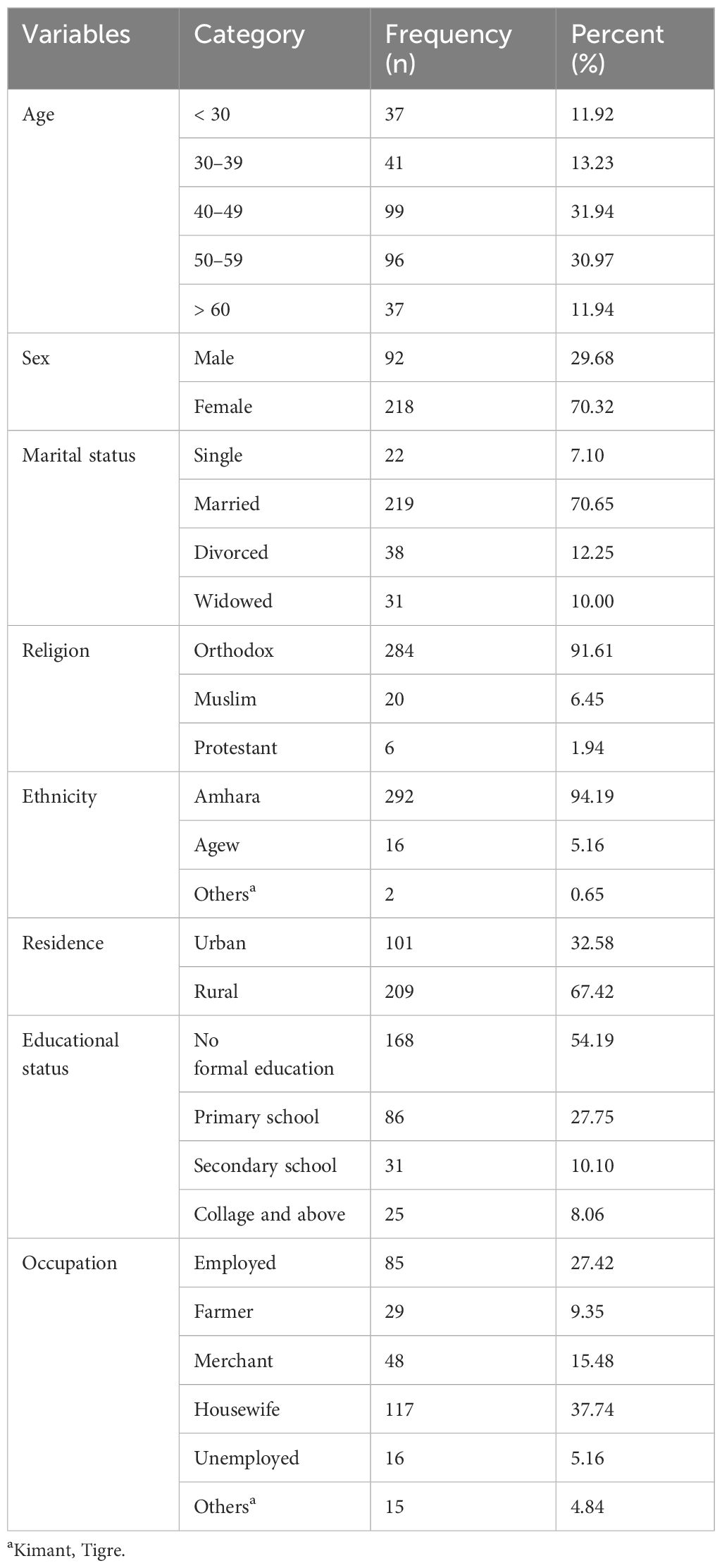
Table 1 Sociodemographic characteristics of adult patients with cancer in Northwest Amhara Comprehensive Specialized Hospitals, oncology treatment units, 2021 (n = 310).
Of the total (310) cancer patients who participated in the study, nearly one-fourth (74; 23.87%) had gastrointestinal cancer; three-fourths (224; 42.26%) presented at a late stage of cancer (stages III and IV); and nearly half (143; 46.13%) had comorbidities, of which hypertension accounted for 18.71% (Table 2).

Table 2 Clinical-related characteristics of adult cancer patients in Northwest Amhara Comprehensive Specialized Hospitals, oncology treatment units, 2021.
The prevalence of baseline anemia among patients with cancer in Northwest Amhara Comprehensive Specialized Hospitals, oncology treatment units was found to be 34.84% (95% CI: 29.71–40.34). Of which, women made up 28.38% of the group, with men comprising the remaining 6.46%.
A bivariate analysis was carried out to identify factors associated with baseline anemia among adult cancer patients, and gender, age, occupation, BMI, and stage of cancer were significantly associated with anemia. Finally, multivariable logistic regressions were conducted and gender, body mass index, and stage of cancer were significantly associated with anemia among adult cancer patients. Women are nearly two times more likely to develop anemia as compared to men (AOR = 1.97; 95% CI: 1.00–3.87), underweight patients are nearly two times more likely to develop baseline anemia as compared to their counterpart (AOR = 1.96; 95% CI: 1.09–3.52), and patients with stage III cancer are nearly two and a half times more likely to develop anemia as compared to their counterparts (AOR = 2.35; 95% CI: 1.12–3.01) (Table 3).
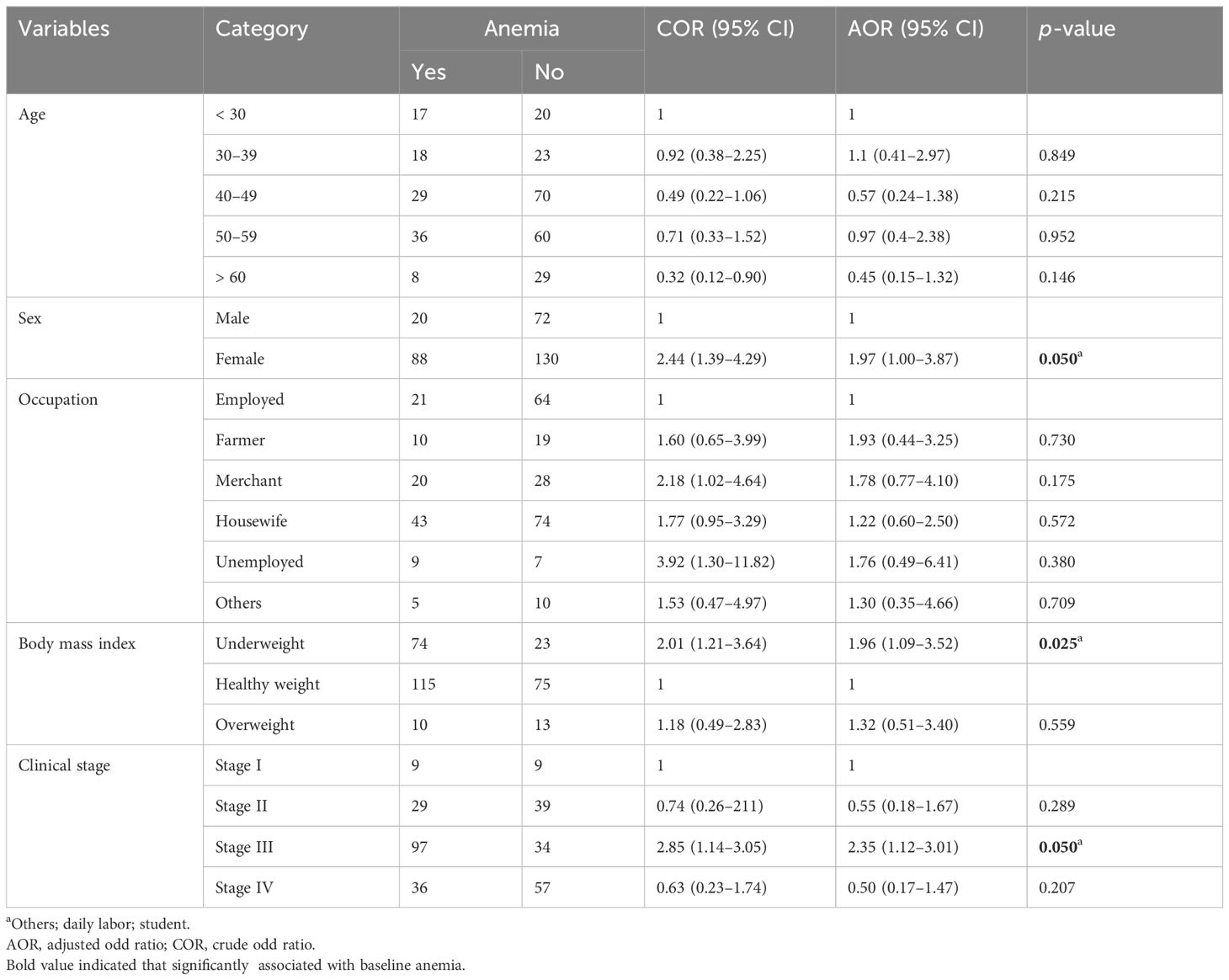
Table 3 Bivariable and multivariable logistic regression of factors associated with baseline anemia among adult cancer patients, 2021 (n = 310).
This study revealed the prevalence of baseline anemia and its associated factors among adult cancer patients in Northwest Amhara Referral Hospitals and oncology treatment units. The overall prevalence of baseline anemia among study participants was found to be 34.84% (95% CI: 29.71–40.34). The prevalence of baseline anemia found in this study was in agreement with a study conducted in Europe (39.3%) (24) and Australia (35%) (25). The prevalence of baseline anemia found in this study was higher than the findings of a study conducted in Ethiopia: Addis Ababa (23%) (20). The possible reason for this difference may be due to the difference in sampling method and study time because the prevalence of cancer is increasing every day in the world. Similarly, the result was also higher than the studies done in China (18.98%) (26) and South Korea (14.2%) (27). The discrepancy between our findings and those of these studies might be due to procedural differences, especially sensitivity differences between the different screening apparatuses. It may be due to the fact that classifications of anemia differ somewhat from several references; the World Health Organization explains anemia as an Hb level < 10.9 g/dL (15), whereas the European Organization for Research and Treatment of Cancer (EORTC) and the National Cancer Institute (NCI) (16) describe anemia as a Hb level < 12.0 g/dL (28).
On the contrary, the prevalence of baseline anemia in this study was lower than in studies conducted in India, New York, Thailand, Belgium, Spain, Saudi Arabia, and Israel: 54.7%, 41%, 54.4%, 55.7%, 48.1%, 44.1%, and 53%, respectively (11, 28–33). The difference might be due to differences in the study design. This inconsistency could also be due to differences in the socioeconomic, cultural, and lifestyle differences between those countries. The possible reason may be due to healthcare delivery policy dissimilarities and the country’s right of way to cancer management and prevention in the case of developed countries.
Regarding the associated factors of baseline anemia among adult patients with cancer, women were nearly two times more likely to develop anemia as compared to men. This finding was supported by studies conducted in Belgium (28). This might be due to the physiological effects of women, such as they are more vulnerable to anemia at a reproductive age due to menstrual blood loss and childbearing iron loss. In addition to this, the reason behind this may also be that, due to the present study, most of the respondents were women.
The current study found that patients with advanced stage of cancer were nearly two and a half times more likely to develop anemia as compared to their counterparts. This result was similar to a study conducted in the USA (10), Australia (29), and Norway (34). This might be due to complex communications between tumor cells and the immune system. Overpowering certain inflammatory cytokines results in reduced existence of red blood cells, a clampdown of erythroid progenitor cells, diminished iron utilization, and insufficient erythropoietin production (35, 36). Furthermore, a direct result of the malignancy is that it attacks normal tissues, causing blood loss and marrow infiltration, which prevents the production of red cells, or inflammation, leading to functional iron deficiency.
According to this study, cancer patients with an underweight body mass index were nearly two times more likely to develop baseline anemia as compared to their counterparts. This finding was in line with other studies conducted in Mikki et al. (37). The possible reason might be that, typically, the causes of anemia and underweight (malnutrition) are similar and aggravated by poverty and food insecurity. Food insecurity affects the dietary patterns of cancer patients by compromising the quantity and quality of nutritional consumption, which contributes to the development of anemia (38). This shows that malnutrition has a substantial role in the health of people, and maintaining a healthy weight is essential to improving health in general. Moreover, this may be due to the different dietary intakes, where poor nutritional practices that lead to insufficiencies of iron, folate, or vitamin B12 are also reasons that might contribute to anemia in underweight patients.
This research raises awareness about a common problem—anemia—among cancer patients even before treatment begins. This can help with early diagnosis and intervention. Next, it is used to identify factors associated with anemia, which can help healthcare providers predict which patients are more at risk. Early intervention can improve their prognosis and quality of life. Finally, these studies focusing on certain cancers, like cervical cancer, provide valuable data on that specific patient group.
This study appears to have limitations. Firstly, it only included individuals accompanying cancer patients, not the cancer patients themselves. Secondly, it did not assess the level or type of anemia, which are crucial factors for understanding the condition. If the study relies on medical records, the data might not have been collected for the study and could be inaccurate or incomplete. Most variables were self-reported and therefore may be affected by social desirability bias or defensive reactions.
This study highlights the prevalence of baseline anemia among adult cancer patients. Identifying factors associated with it, like being female, cancer patients with an underweight body mass index, and cancer patients with stage III, could help predict at-risk patients. Early detection of anemia through screening programs for high-risk patients (identified by this study’s factors) could be beneficial. This would allow for timely intervention to manage anemia and potentially improve treatment tolerance and quality of life for cancer patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by School of Nursing research ethical review commute on the behalf of the University of Gondar institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
YW: Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration. AZ: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. DG: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. ST: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. GK: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. SF: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. AS: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. GN: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The investigators would like to extend their special thanks to data collectors, supervisors, and study participants for their great contributions to the success of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMI, body mass index; EORTC, European Organization for Research and Treatment of Cancer; WHO, World Health Organization; ECOG, Eastern Cooperative Oncology Group performance; FHCSH, Felege Hiwot Comprehensive Specialized Hospital; GUCSH, University of Gondar Comprehensive Specialized Hospital; QOL, quality of life; SSA, Sub-Saharan Africa, National Cancer Institute.
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin D, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International journal of cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
2. Gebreweld A, Ali N, Ali R, Fisha T. Prevalence of anemia and its associated factors among children under five years of age attending Guguftu Health Center, South Wollo, Northeast Ethiopia. PloS One. (2019) 14:e0218961. doi: 10.1371/journal.pone.0218961
3. Balducci LJCC. Anemia, cancer, and aging. Sage journal section: Cancer Control (2003) 10(6):478–86. doi: 10.1177/107327480301000606
4. Corona LP, Duarte Y, Lebrão ML. Prevalence of anemia and associated factors in older adults: evidence from the SABE Study. SciELO Brasil (2014) 48:723–431. doi: 10.1590/S0034-8910.2014048005039
5. Kassebaum NJ, America GAC. The global burden of anemia. Elsevier (2016) 30(2):247–308. doi: 10.1016/j.hoc.2015.11.002
6. De Benoist B, Cogswell M, Egli I, McLean E. Worldwide prevalence of anemia 1993–2005. WHO global database of anemia, English (2008).
7. Rodgers GM, Becker PS, Blinder M, Cella D, Chanan-Khan A, Cleeland C, et al. Cancer-and chemotherapy-induced anemia. Journal of the National Comprehensive Cancer Network (2012) 10(5):628–53. doi: 10.6004/jnccn.2012.0064
8. Demetri G. Anaemia and its functional consequences in cancer patients: current challenges in management and prospects for improving therapy. British Journal of Cancer (2001) 84(1):31–7. doi: 10.1054/bjoc.2001.1750
9. Dicato M, Plawny L, Diederich M. Anemia in cancer. Elsevier section: Annals of Oncology (2010) 21:vii167–vii72. doi: 10.1093/annonc/mdq284
10. Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. The American journal of medicine, 2004 - Elsevier (2004) 116(7):11–26. doi: 10.1016/j.amjmed.2003.12.008
11. Steegmann JL, Torres JS, Colomer R, Vaz A, López J, Jalón I, et al. Prevalence and management of anemia in patients with non-myeloid cancer undergoing systemic therapy: a Spanish survey. Clinical and Translational Oncology (2013) 15(6):477–83. doi: 10.1007/s12094-012-0953-5
12. Birgegård G, Gascón P, Ludwig H. Evaluation of anemia in patients with multiple myeloma and lymphoma: findings of the European CANCER ANAEMIA SURVEY. Eur J hematology. (2006) 77:378–86. doi: 10.1111/j.1600-0609.2006.00739.x
13. Coiffier B, Guastalla J-P, Pujade-Lauraine Ef, Bastit P, Group AS. Predicting cancer-associated anemia in patients receiving non-platinum chemotherapy: results of a retrospective survey. Eur J Cancer. (2001) 37:1617–23. doi: 10.1016/S0959-8049(01)00169-1
14. Barrett-Lee PJ, Ludwig H, Birgegård G, Bokemeyer C, Gascón P, Kosmidis PA, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology (2006) 70(1):34–48. doi: 10.1159/000091675
15. Madeddu C, Gramignano G, Astara G, Demontis R, Sanna E, Atzeni V, et al. Pathogenesis and treatment options of cancer-related anemia: perspective for a targeted mechanism-based approach. Front Physiol. (2018) 9:1294. doi: 10.3389/fphys.2018.01294
16. Madeddu C, Neri M, Sanna E, Oppi S, Macciò A. Experimental drugs for chemotherapy-and cancer-related anemia. J Exp Pharmacol. (2021) 225:593–611.
17. Wiciński M, Liczner G, Cadelski K, Kołnierzak T, Nowaczewska M, Malinowski B. Anemia of chronic diseases: wider diagnostics—better treatment? Nutrients. (2020) 12:1784. doi: 10.3390/nu12061784
18. Barni S, Gascòn P, Petrelli F, García-Erce JA, Pedrazzoli P, Rosti G, et al. Position paper on management of iron deficiency in adult cancer patients. Expert Rev Hematology. (2017) 10:685–95. doi: 10.1080/17474086.2017.1343140
19. Alebachew A, Waddington C. Improving health system efficiency: Ethiopia: human resources for health reforms. World Health Organization, Africa (2015).
20. Kifle E, Hussein M, Alemu J, Tigeneh W. Prevalence of anemia and associated factors among newly diagnosed patients with solid Malignancy at Tikur Anbessa Specialized Hospital, radiotherapy center, Addis Ababa, Ethiopia. Advances in Hematology Journal (2019) 2019:. doi: 10.1155/2019/8279789
21. Murphy J. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. In: Vitamin and Mineral Nutrition Information System. World Health Organization, Geneva (2011). 2002.
22. Azam F, Latif MF, Farooq A, Tirmazy SH, AlShahrani S, Bashir S, et al. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Karger, Oncology (2020) 12(3):728–36. doi: 10.1159/000503095
23. Lim JU, Lee JH, Kim JS, Hwang YI, Kim T-H, Lim SY, et al. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. International Journal of Chronic Obstructive Pulmonary Disease (2017), 2465–75. doi: 10.2147/COPD
24. Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anemia in cancer patients. European Journal of Cancer (2004) 40(15):2293–306. doi: 10.1016/j.ejca.2004.06.019
25. Seshadri T, Prince HM, Bell DR, Coughlin PB, James PP, Richardson GE, et al. The Australian Cancer Anaemia Survey: a snapshot of anemia in adult patients with cancer. Medical journal of Australia (2005) 182(9):453–7. doi: 10.5694/j.1326-5377.2005.tb06784.x
26. Men JG, Tang J, Ding J, Chen X, Su J-M, Liu J-Y. Prevalence and characteristics of anemia in patients with solid cancers at diagnosis in southwest China. Asian Pacific Journal of Cancer Prevention (2011) 12:2825–8.
27. Park S, Jung CW, Kim K, Kim SJ, Kim WS, Jang JH. Iron deficient erythropoiesis might play a key role in the development of anemia in cancer patients. Oncotarget. (2015) 6(40):42803. doi: 10.18632/oncotarget.v6i40
28. Verbeke N, Beguin Y, Wildiers H, Canon J, Bries G, Bosly A, et al. High prevalence of anemia and limited use of therapy in cancer patients: a Belgian survey (Anaemia Day 2008). Supportive care in cancer (2012) 20(1):23–8. doi: 10.1007/s00520-010-1045-0
29. Birgegård G, Aapro MS, Bokemeyer C, Dicato M, Drings P, Hornedo J, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Karger Oncology (2005) 68(Suppl. 1):3–11. doi: 10.1159/000083128
30. Bahl A, Sharma D, Basu J, Rath G, Julka P. Pre-treatment anemia evaluation in Cancer patients attending Radiotherapy Clinic: Results from a single Indian Center. Indian journal of medical sciences (2008) 62(10):417–20. doi: 10.4103/0019-5359.44022
31. Ahlberg K, Ekman T, Gaston-Johansson F. Fatigue, psychological distress, coping resources, and functional status during radiotherapy for uterine cancer. Oncol Nurs Forum. (2005). doi: 10.1188/05.ONF.633-640
32. Almehmadi M, Salih M, Elmissbah TE, Alsharif A, Alsiwiehri N, Alzahrani K, et al. Prevalence of anemia among Saudi patients with solid cancers at diagnosis in King Faisal Hospital, Taif Province, Kingdom of Saudi Arabia. PLoS One (2021) 16(1):. doi: 10.1371/journal.pone.0246202
33. Mahasittiwat P, Pataranutraporn P, Ieumwananonthachai N, Dankulchai P, Chansilpa Y, Sangruchi S, et al. Prevalence, incidence, and management of anemia in cancer patients treated in the radiation oncology division, Siriraj Hospital. Siriraj Medical Journal (2008) 60(5):235–9.
34. Edna T-H, Karlsen V, Jullumstro E, Lydersen S. Prevalence of anemia at diagnosis of colorectal cancer: assessment of associated risk factors. Hepato-gastroenterology (2012) 59(115):713–6.
35. Weiss G, Goodnough LT. Anemia of chronic disease. New England Journal of Medicine (2005) 352(10):1011–23. doi: 10.1056/NEJMra041809
36. Adamson JW. The anemia of inflammation/malignancy: mechanisms and management. ASH Education Program Book (2008) 2008(1):159–65. doi: 10.1182/asheducation-2008.1.159
37. Mikki N, Abdul Rahim H, Stigum H, Holmboe Ottesen G. Anemia prevalence and associated sociodemographic and dietary factors among Palestinian adolescents in the West Bank. EMHJ-Eastern Mediterranean Health Journal (2011) 17:208–17. doi: 10.26719/2011.17.3.208
Keywords: anemia, prevalence, cancer, Gondar, Ethiopia
Citation: Wassie YA, Zegeye AF, Gebrehana DA, Tsega SS, Kibralew G, Fentahun S, Setegn A and Nakie G (2024) Baseline anemia and its associated factors among adult cancer patients at Northwest Amhara Regional State Referral Hospitals, Northwest Ethiopia, 2021. Front. Oncol. 14:1390052. doi: 10.3389/fonc.2024.1390052
Received: 23 February 2024; Accepted: 03 June 2024;
Published: 19 June 2024.
Edited by:
Daniele Maria-Ferreira, Instituto de Pesquisa Pelé Pequeno Príncipe, BrazilReviewed by:
Sezaneh Haghpanah, Shiraz University of Medical Sciences, IranCopyright © 2024 Wassie, Zegeye, Gebrehana, Tsega, Kibralew, Fentahun, Setegn and Nakie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yilkal Abebaw Wassie, bGlrbmF3YWJlQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.