
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 17 June 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1389975
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive system. They usually occur in the gastrointestinal tract. However, we discovered a rare phenomenon in which small cell carcinoma infiltrated the GIST of a patient. The patient came to the hospital and presented with chest tightness and shortness of breath for 2 months and a dry cough for half a month. As the ancillary tests were refined, it was discovered that he also had a lesion in the pelvic cavity. After pathological examination of the core needle biopsy (CNB) samples from the pelvic cavity lesion, the patient was diagnosed with GIST with small cell carcinoma infiltration. The patient is currently receiving a chemotherapy regimen of etoposide combined with cisplatin.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive system (1). The histological appearance of GISTs is mainly characterized by spindle-shaped cells (2). Morphologically, epithelioid cells, palisaded–vacuolated cells, and sclerotic patterns may also be present (2). GISTs generally express positive indicators such as CD117 and DOG1 according to immunohistochemistry (IHC) staining (3).
Small cell carcinoma is a type of neuroendocrine carcinoma. When small cell carcinoma occurs in the lung, it can also be known as small cell lung cancer (SCLC). SCLC accounts for approximately 15% of all lung cancers (4). In SCLC, finely granular nuclear chromatin, absent nucleoli, and common fusiform shapes are histologically found, and the nuclear/cytoplasmic ratio is generally high (5). In terms of IHC staining, SCLC is usually positive for thyroid transcription factor-1 (TTF-1), cytokeratin (CK), and synaptophysin (Syn) (6, 7).
SCLC commonly metastasizes to the brain, bones, liver, and adrenal glands (8), but the metastasis of SCLC to other solid tumors is not commonly reported. GISTs have been reported to accompany SCLC (9). However, small cell carcinoma infiltrating GISTs has not been found.
A 69-year-old male patient with a 10-year history of arterial hypertension presented to the Medical Oncology Outpatient with chest tightness and shortness of breath for 2 months and a dry cough for half a month. A chest CT revealed an infection in the upper lobe of the left lung and a lesion in the left hilar region. After antibiotic treatment, the patient underwent a repeat chest CT scan, which revealed no significant changes in the mediastinum or left hilar mass. On admission, he underwent a chest contrast-enhanced CT, which revealed a lesion in the left hilar region measuring approximately 43 × 63 mm and encroaching on the left pulmonary artery, suggesting malignancy. The patient then underwent a CT-guided percutaneous core needle biopsy for the left hilar lesion. The patient’s left lung biopsy tissue was sent for pathological examination. The contrast-enhanced CT of the abdomen revealed a hepatic cyst. Pelvic cavity CT revealed a pelvic cavity soft tissue lesion, approximately 61 × 63 mm in size.
In response to the discovery of the pelvic cavity lesion, the patient underwent additional contrast-enhanced MRI, which revealed a posterior bladder lesion (Figures 1A, B). It presented a well-defined roundish mass, mostly with slightly low and uneven signals, suggesting possible malignancy. Subsequently, the patient underwent a CT-guided percutaneous core needle biopsy for the pelvic cavity lesion. CT revealed a mostly well-defined roundish mass. As expected, hypodense lesions were observed in a small portion of the ill-defined part of the mass (Figure 1C). Clinicians then chose a core needle biopsy site that allows obtaining lesions of different densities at the same time (Figure 1D). The biopsy tissue was sent for pathological examination.
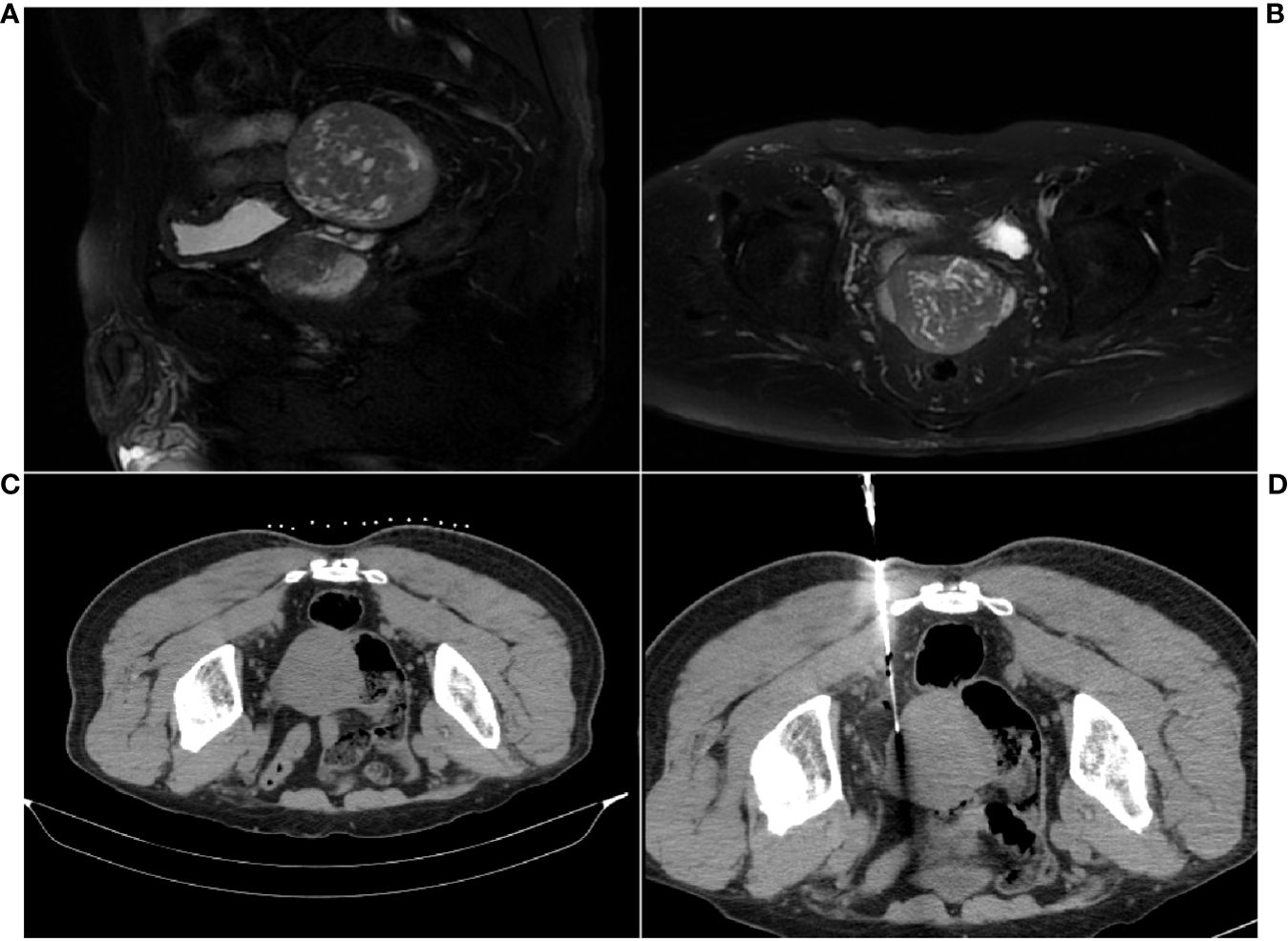
Figure 1 MRI and CT scan images of the pelvic cavity lesion. (A) Sagittal MR image. (B) Axial MR image. (C) Well-defined roundish mass. (D) CT scan during the core needle biopsy for sample collection.
The left hilar biopsy tissue was susceptible to stretching and deformation, and analysis of the morphological features revealed nests, beams, and chrysanthemum-shaped clusters of arranged cells. The tumor cells were small, with little cytoplasm, indistinct cell boundaries, fine chromatin, and a lack of nucleoli (Figure 2A). IHC staining revealed that the tumor cells were positive for CK, TTF-1, CD56, and Syn, and the Ki-67 index was approximately 90%. Ultimately, the IHC staining results and morphological features were combined to pathologically diagnose poorly differentiated neuroendocrine carcinoma (small cell carcinoma) (Figures 2B–F).
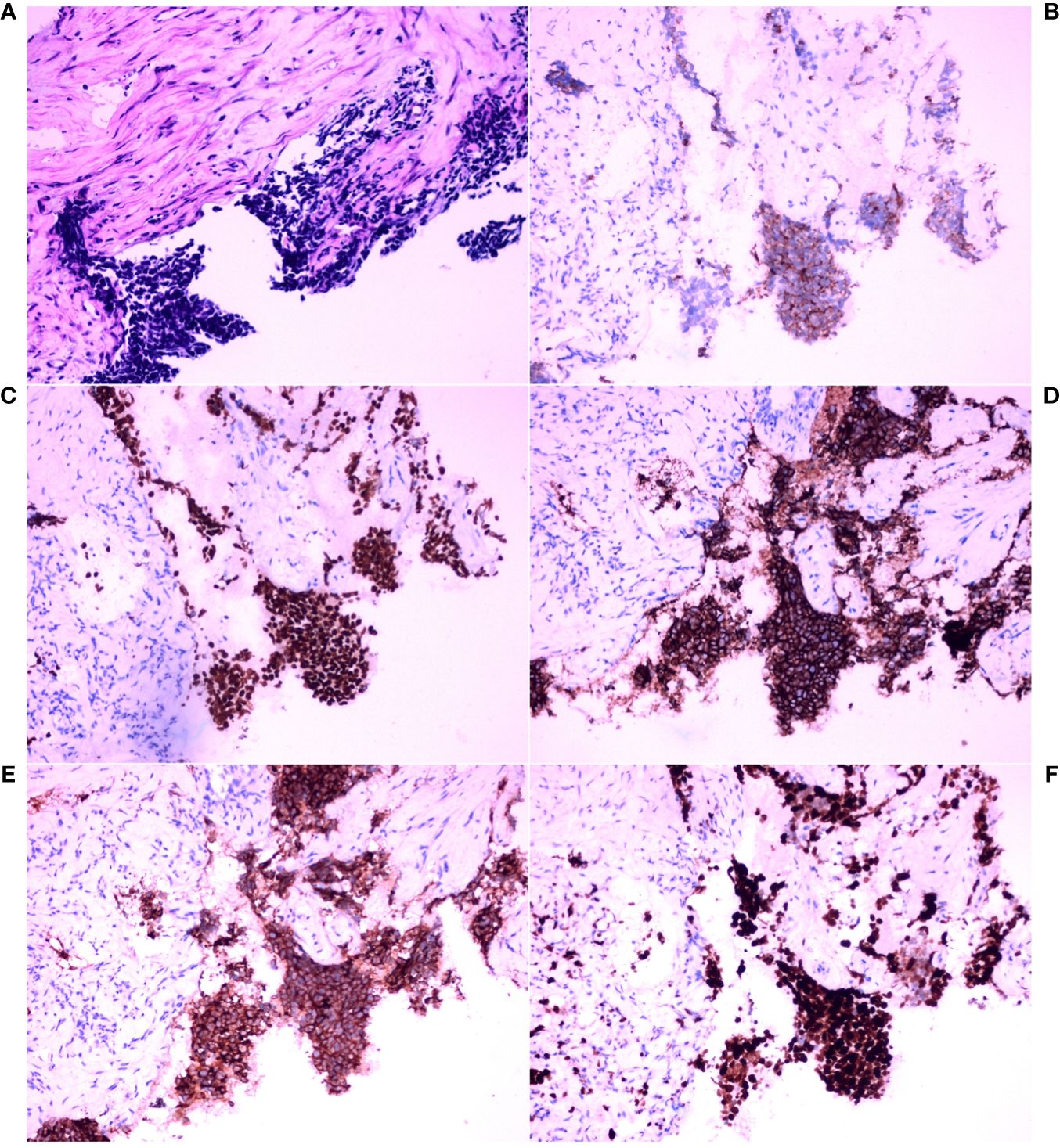
Figure 2 Histopathological analysis and immunohistochemical examination of the core needle biopsy tissue in the left hilar tumor. (A) Hematoxylin and eosin (HE) staining (×200). (B) Immunohistochemical (IHC) staining for cytokeratin (CK) (×200). (C) IHC staining for thyroid transcription factor-1 (TTF-1) (×200). (D) IHC staining for CD56 (×200). (E) IHC staining for synaptophysin (Syn) (×200). (F) IHC staining for Ki-67 (×200).
Thereafter, morphological analysis of the biopsy tissue from the pelvic cavity lesion revealed that some areas presented a chrysanthemum cluster-like structure, consisting of spindle cells and epithelioid cells, with visible paranuclear vacuoles (Figure 3A). These histological features were consistent with the diagnosis of GIST. Immunohistochemical staining of the spindle and epithelioid cell tumor region revealed that the tumor was positive for CD117 and DOG1 (Figures 3B, C), but negative for smooth muscle actin (SMA), CD34, S-100, CD10, HMB45, and desmin. Genetic testing confirmed the diagnosis of GIST, and a gain-of-function mutation was identified in exon 13 c.1924A>G (p.K642E) of the c-kit gene by Sanger sequencing (Figure 4).
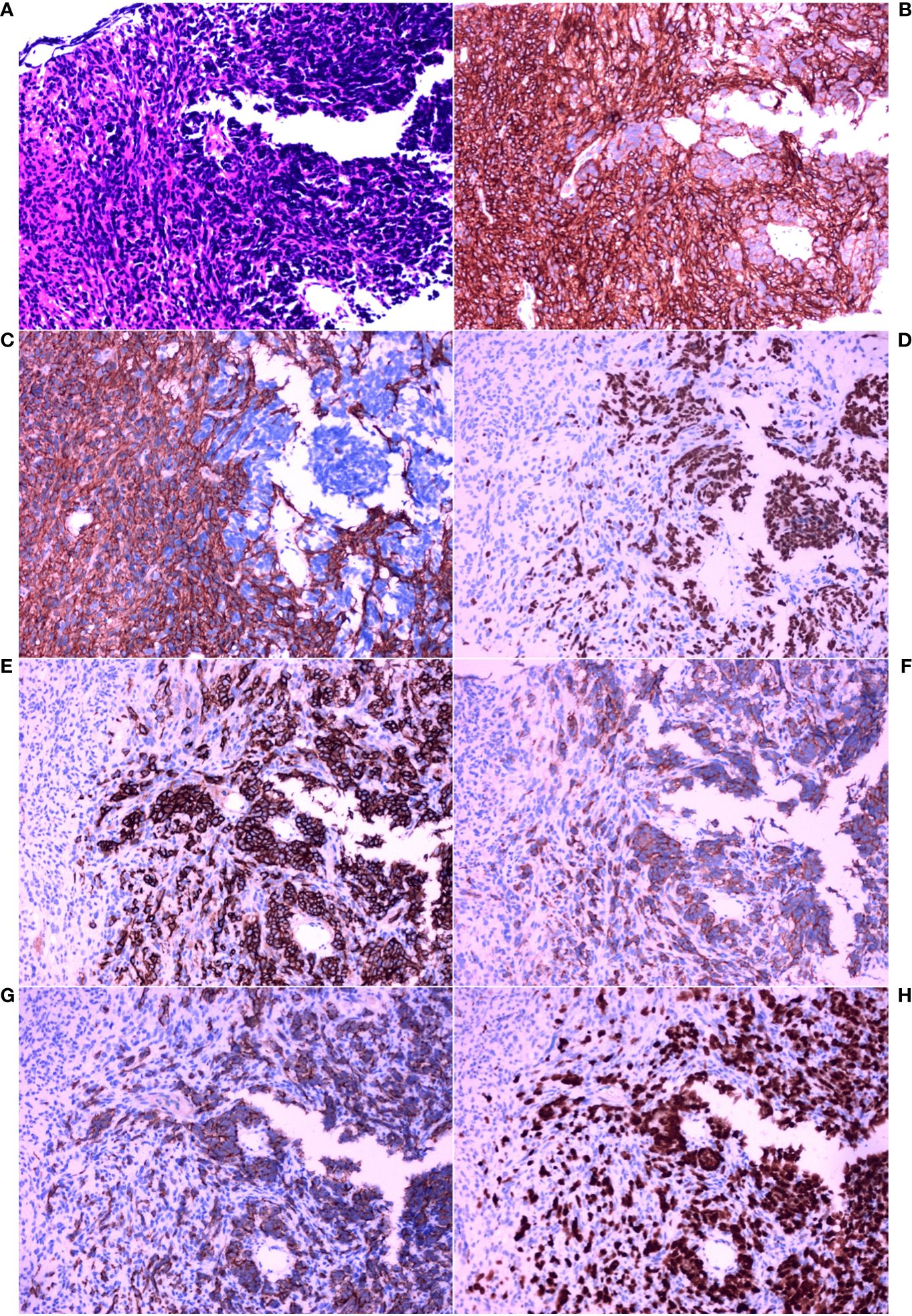
Figure 3 Histopathological analysis and immunohistochemical examination of the pelvic cavity lesion sample obtained by core needle biopsy. (A) HE staining (×200). (B) IHC staining for CD117 (×200). (C) IHC staining for DOG1 (×200). (D) IHC staining for thyroid transcription factor-1 (TTF-1) (×200). (E) IHC staining for CD56 (×200). (F) IHC staining for synaptophysin (Syn) (×200). (G) IHC staining for cytokeratin (CK) (×200). (H) IHC staining for Ki-67 (×200).
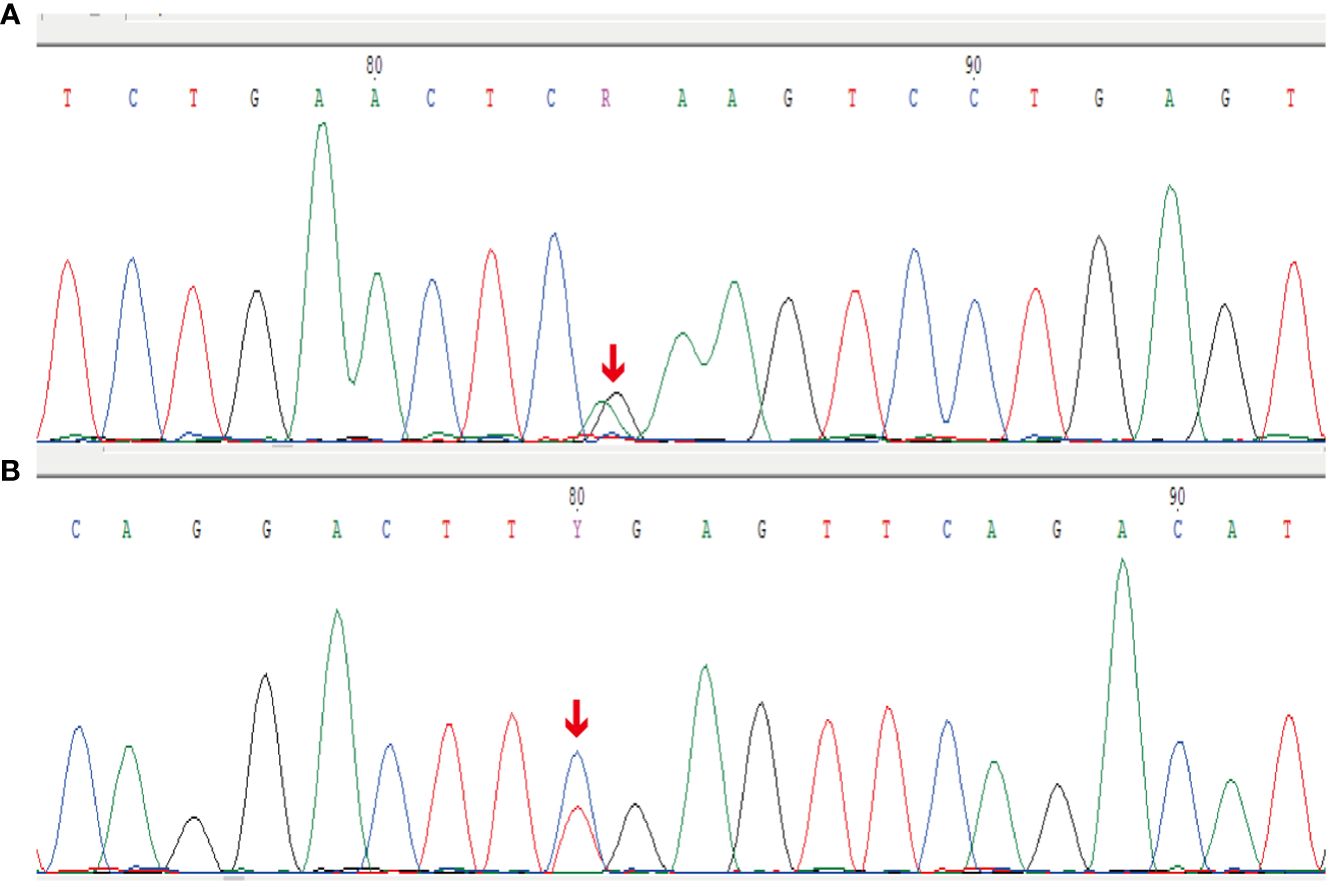
Figure 4 Sanger sequencing results for the c-kit gene. (A) Forward reads from exon 13 c.1924A>G (p.K642E) gain-of-function mutation of the c-kit gene. (B) Reverse reads from exon 13 c.1924A>G (p.K642E) gain-of-function mutation of the c-kit gene.
However, between these spindle-shaped and epithelioid cells, there were some basal cells arranged in a fenestrated pattern. These infiltrating cells were small, round or short, shuttle-shaped, with a naked nucleus, deep nuclear staining, and inconspicuous nucleoli. These features were similar to those of small cell carcinoma.
Therefore, additional targeted IHC staining of the biopsy tissue from the pelvic cavity lesion was carried out. Infiltration of small cell carcinoma in the GIST was found (Figure 3A). The infiltrating fraction expressed TTF-1, CD56, Syn, and CK and had a proliferation index of approximately 90%, as measured using Ki67 IHC staining (Figures 3D–H). Considering these findings and the morphological features, it was concluded that the lesion in the pelvic cavity was GIST with small cell carcinoma infiltration. Fortunately, there was no evidence that the SCLC had metastasized to any site other than the pelvic cavity.
The patient first underwent treatment with etoposide combined with cisplatin for 21 days per cycle. After three cycles of chemotherapy, the patient’s GIST had ruptured and bled, necessitating surgery. Postoperative histopathology revealed necrosis in the area infiltrated by small cell carcinoma. The patient is still undergoing chemotherapy for small cell carcinoma of the lungs. Postoperative histopathology of the GIST revealed that the current chemotherapy regimens are effective.
SCLC often metastasizes to the bone and the brain in advanced stages (10). Metastasis to other sites is rare. Only a few studies have reported GISTs with neuroendocrine carcinoma components. Phan et al. (9) reported on a patient with pancreatic GIST and limited-stage SCLC. However, this patient’s GIST and SCLC occurred in two different lesion tissues. Our patient suffered from advanced-stage SCLC. The pathological features of our patient corroborate the aggressiveness of small cell carcinoma: it may invade less malignant tumors. A patient with simultaneous poorly differentiated neuroendocrine carcinoma and GIST of the stomach was identified by Ding et al. (11). These authors explained this phenomenon by the fact that GISTs and neuroendocrine tumors (NETs) have some commonalities in their immunoreactivity profiles, which could indicate a common oncogenic mechanism for neuroendocrine cancer and GISTs (11). Their cases had a histological pattern typical of GIST. Moreover, the expression of IHC in the neuroendocrine carcinoma was similar to that of our patient. The difference was that, in their cases, the neuroendocrine carcinoma did not invade the GIST. Our case likely presented metastasis from the primary tumor in the left lung, which led to the GIST being infiltrated by small cell carcinoma.
The literature shows that GIST and neuroendocrine carcinoma have some common immunophenotypes. CD117 and DOG1 positivity is commonly used clinically to aid in the diagnosis of GIST (12). However, Akintola-Ogunremi et al. (13) demonstrated that CD117 is expressed in some neuroendocrine carcinomas and has potential therapeutic implications. Marando et al. (14) reported a statistically significant correlation between DOG1 expression and neuroendocrine tumors of the gastrointestinal tract. This evidence may suggest that it is not a coincidence that our case of GIST was infiltrated by small cell neuroendocrine carcinoma. GISTs could also coexist with a single well-differentiated, sporadic, small NET (15).
The metastasis and infiltration of SCLC into the GIST in our patient may not have been coincidental. Thus, the potential underlying mechanism was explored based on previous studies. An in vivo experiment on SCLC showed that tumors are generally composed of phenotypically distinct cells characterized by neuroendocrine or mesenchymal markers, and these cells have a common origin (16). The biological behavior of tumor cells is affected by the crosstalk between mesenchymal and neuroendocrine cells. Mesenchymal cells can confer metastatic capacity on neuroendocrine cells (16). GISTs are tumors derived from mesenchymal cells. This might represent a potential connection. Genetically, GIST and SCLC share some commonly mutated genes, such as FGFR1 and KIT (17, 18). The diseases also share abnormal expression of some molecules in common pathways such as PI3K/AKT and MAPK (19–21).
Moreover, SCLC is highly vascularized, and angiogenesis greatly contributes to its metastatic process (22). Small cell carcinomas could metastasize via the bloodstream with a tumor-homing effect and are more likely to grow in the setting of gastrointestinal mesenchymal tumors. Epithelial-to-mesenchymal transition (EMT) could also play an important role in the metastasis of small cell carcinoma. A study of drug responses in small cell carcinoma revealed that it partly exhibits features of EMT (23). In this process, small cell carcinomas may express chemokines that target the GIST. The molecular and cellular mechanisms of small cell carcinoma homing and EMT in specific environments are poorly understood.
Exploring these mechanisms could lead to the design of better treatments for patients with poorly differentiated small cell carcinoma. However, our case is an isolated one. Our findings do not rule out the possibility that these tumors occurred coincidentally. More experiments are needed to ascertain the link between the two diseases. The patient had been treated with chemotherapy for SCLC for several months prior to undergoing resection of the GIST. This may have led to necrosis in the area infiltrated by the small cell carcinoma in the GIST, causing the GIST to rupture. Hence, the small cell carcinoma infiltrating area became a range of necrosis in the resection specimens. It is difficult to explore the mechanisms in resection specimens.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Dalian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. LS: Supervision, Validation, Visualization, Writing – review & editing. LL: Conceptualization, Resources, Validation, Writing – review & editing. SL: Conceptualization, Resources, Validation, Writing – review & editing. QW: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author would like to thank QW for her guidance and suggestions for revision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. (2006) 23:70–83. doi: 10.1053/j.semdp.2006.09.001
2. WHO. Classification of tumours editorial board. In: Soft tissue and bone tumours, 5th Edition, vol. 3. International Agency for Research on Cancer, Lyon (2020). Available at: https://publications.iarc.fr/588.
3. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. (1998) 279:577–80. doi: 10.1126/science.279.5350.577
4. Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, et al. Small cell lung cancer. J Natl Compr Canc Netw. (2013) 11:78–98. doi: 10.6004/jnccn.2013.0011
5. Travis WD. Pathology and diagnosis of neuroendocrine tumors. Thorac Surg Clin. (2014) 24:257–66. doi: 10.1016/j.thorsurg.2014.04.001
6. Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. (2020) 15:1823–35. doi: 10.1016/j.jtho.2020.09.009
7. Guinee DG, Fishback NF, Koss MN, Abbondanzo SL, Travis WD. The spectrum of immunohistochemical staining of small-cell lung carcinoma in specimens from transbronchial and open-lung biopsies. Am J Clin Pathol. (1994) 102:406–14. doi: 10.1093/ajcp/102.4.406
8. Ko J, Winslow MM, Sage J. Mechanisms of small cell lung cancer metastasis. EMBO Mol Med. (2021) 13:e13122. doi: 10.15252/emmm.202013122
9. Phan M, Jones S, Jenkins J, Pant S, Khawandanah M. Pancreatic GIST in a patient with limited stage small cell lung cancer: a case report and review of published cases. Case Rep Oncol Med. (2016) 2016:9604982. doi: 10.1155/2016/9604982
10. Megyesfalvi Z, Tallosy B, Pipek O, Fillinger J, Lang C, Klikovits T, et al. The landscape of small cell lung cancer metastases: organ specificity and timing. Thorac Cancer. (2021) 12:914–23. doi: 10.1111/1759–7714.13854
11. Ding J, Sun P, Cai XY, Fei SH, Wu J, Qi YK, et al. Synchronous poorly-differentiated neuroendocrine carcinoma and gastrointestinal stromal tumor of the stomach: a case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA. Int J Clin Exp Pathol. (2014) 7:9076–80.
12. Kiśluk J, Zińczuk J, Kemona A, Guzińska-Ustymowicz K, Żurawska J, Kędra B. Expression of CD117, DOG-1, and IGF-1R in gastrointestinal stromal tumours - an analysis of 70 cases from 2004 to 2010. Prz Gastroenterol. (2016) 11:115–22. doi: 10.5114/pg.2015.52587
13. Akintola-Ogunremi O, Pfeifer JD, Tan BR, Yan Y, Zhu X, Hart J, et al. Analysis of protein expression and gene mutation of c-kit in colorectal neuroendocrine carcinomas. Am J Surg Pathol. (2003) 27:1551–8. doi: 10.1097/00000478–200312000–00008
14. Marando A, Di Blasi E, Tucci F, Aquilano MC, Bonoldi E. DOG1 expression in neuroendocrine neoplasms: potential applications and diagnostic pitfalls. Pathol Res Pract. (2023) 248:154623. doi: 10.1016/j.prp.2023.154623
15. Samaras VD, Foukas PG, Triantafyllou K, Leontara V, Tsapralis D, Tsompanidi EM, et al. Synchronous well differentiated neuroendocrine tumour and gastrointestinal stromal tumour of the stomach: a case report. BMC Gastroenterol. (2011) 11:27. doi: 10.1186/1471–230X-11–27
16. Calbo J, van Montfort E, Proost N, van Drunen E, Beverloo HB, Meuwissen R, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell. (2011) 19:244–56. doi: 10.1016/j.ccr.2010.12.021
17. Napolitano A, Ostler AE, Jones RL, Huang PH. Fibroblast Growth Factor Receptor (FGFR) signaling in GIST and soft tissue sarcomas. Cells. (2021) 10:1533. doi: 10.3390/cells10061533
18. Raso MG, Bota-Rabassedas N, Wistuba II. Pathology and classification of SCLC. Cancers. (2021) 13:820. doi: 10.3390/cancers13040820
19. Miettinen M, Felisiak-Golabek A, Wang Z, Inaguma S, Lasota J. GIST manifesting as a retroperitoneal tumor: clinicopathologic immunohistochemical, and molecular genetic study of 112 cases. Am J Surg Pathol. (2017) 41:577–85. doi: 10.1097/PAS.0000000000000807
20. Kern JA, Kim J, Foster DG, Mishra R, Gardner EE, Poirier JT, et al. Role of mTOR as an essential kinase in SCLC. J Thorac Oncol. (2020) 15:1522–34. doi: 10.1016/j.jtho.2020.05.026
21. Gupta A, Ma S, Che K, Pobbati AV, Rubin BP. Inhibition of PI3K and MAPK pathways along with KIT inhibitors as a strategy to overcome drug resistance in gastrointestinal stromal tumors. PloS One. (2021) 16:e0252689. doi: 10.1371/journal.pone.0252689
22. Lu H, Jiang Z. Advances in antiangiogenic treatment of small-cell lung cancer. Onco Targets Ther. (2017) 10:353–9. doi: 10.2147/OTT.S119714
Keywords: gastrointestinal stromal tumors, small cell carcinoma, small cell lung cancer (SCLC), neoplasm metastasis, case report
Citation: Zhou Y, Song L, Lyu L, Li S and Wang Q (2024) Gastrointestinal stromal tumor with small cell carcinoma infiltration: a case report. Front. Oncol. 14:1389975. doi: 10.3389/fonc.2024.1389975
Received: 22 February 2024; Accepted: 24 May 2024;
Published: 17 June 2024.
Edited by:
Lizza E. L. Hendriks, Maastricht University Medical Centre, NetherlandsReviewed by:
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyCopyright © 2024 Zhou, Song, Lyu, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qimin Wang, ZGx3YW5ncW1AMTYzLmNvbQ==; Li Lyu, Mjk0MTI0MzUwQHFxLmNvbQ==; Shengjie Li, MjQwNzc5NzIyQHFxLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.