- 1Dipartimento di Scienze di Laboratorio ed Ematologiche, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy
- 2Sezione di Ematologia, Dipartimento di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Rome, Italy
Introduction: Indications for HSCT are increasing worldwide, paralleled by a growing demand for donors of therapeutic cells.
Methods: Herein, we report our real-world experience of adult HPC donor assessment during a 5-year study period (2018–2023): we have retrospectively revised data of 455 potential related stem cell donors, consecutively evaluated at our center. Donor medical history was assessed by a questionnaire and an interview with a trained physician experienced in donation procedures to evaluate donor fitness and medical history. Pre-existing health disorders were fully investigated. Behavioral risk factors for communicable infectious diseases were also routinely explored.
Results and discussion: Overall, 351 donors were finally assessed as eligible for HPC donation, and 233 underwent stem cell collection, 158 through apheresis from mobilized peripheral blood, and 75 through bone marrow harvest. Among them, 27 donors were selected despite the presence of pre-existing health conditions, which would be potential exclusion criteria for unrelated donors: 16 suffered from well-controlled cardiovascular diseases (CVD) and 11 from allergic diathesis. Most of the selected donors with pre-existing disorders were candidates for apheresis HPC collection (21, 77.8%), while only six (22.2%) underwent BM harvest. We then analyzed the data relative to the corresponding 233 allogeneic HSCT to explore if the presence of pre-existing diseases in the donors could show any association with transplant characteristics. Transplants from CVD and allergy donors showed no significant disparities in comparison with those from healthy donors. A significant difference emerged regarding the disease severity, with a higher proportion of patients with high/very high disease risk index (DRI) among those receiving grafts from CVD donors (68.7% in transplants from CVD donors versus 36.0% in transplants from healthy donors, p=0.005). Multivariate analysis confirmed that high/very high DRI patients had an increased probability of receiving donations from CVD donors (OR, 4.89; 95%CI, 1.15–20.86; p=0.031). Among donors with well-controlled pre-existing conditions, no adverse events were recorded during stem cell collection or at follow-up. Our results suggest that in patients at high risk for relapse requiring a prompt allogeneic transplant, a familiar donor might be accepted for HPC apheresis donation on less strict criteria than unrelated donors, without risk for both donor and patient.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an established treatment for a wide range of acquired or congenital disorders (1–4). At present, available stem cell sources include bone marrow (BM), mobilized peripheral blood (PB), and cord blood. Indications for HSCT are increasing worldwide, paralleled by a growing demand for donors of therapeutic cells. The practice of haploidentical HSCT, together with the advances in transplant technique, supportive care, and conditioning regimens, allows the treatment of older patients with elderly familial donors or donors with comorbidities, imposing different challenges to hematologists (5, 6). Pretransplant hemopoietic progenitor cells (HPC) donor assessment and testing are critical processes that affect the quality and safety of donation. Many issues on donor safety have been addressed in recent years, but most data are collected from unrelated donors, while consistent information from related donors is sparse (7). For unrelated donors, detailed recommendations for the health assessment have been published (8, 9), allowing HPC donations only if they are in good health, without any medical conditions. The donors must have a performance status that permits safe apheretic HPC collection or be able to tolerate anesthesia during BM harvest, with adequate cardiac, pulmonary, hepatic, and renal function. Eligibility criteria for related donors are less rigorous and may vary between centers. In 2015, the Worldwide Network for Blood and Marrow Transplantation (WBMT) Standing Committee developed a consensus document with recommendations for donor workup and final clearance of family donors who were not eligible as unrelated donors because of their age or pre-existing diseases (10). The document has been recently updated (11). Despite that specific diagnosis and/or disease severity directly imply the donor exclusion, the presence of other well-controlled conditions may be overcome and does not prevent per se the donation.
Herein, we report our real-world experience of HPC donor assessment during a 5-year study period (January 2018–October 2023); we have retrospectively revised anamnestic data of potential related stem cell donors, consecutively evaluated at our center. The study aims to explore if the presence of one or more pre-existing health disorders in donors deemed eligible according to the recent WBMT recommendations (11) may have an impact on transplant outcomes.
Methods
Study population
We conducted a retrospective observational study, including potential related donors consecutively evaluated at the Transfusion Medicine Department of Fondazione Policlinico A. Gemelli IRCCS of Rome (Italy) and allo-HSCT consecutively performed from selected related donors at the Transplant Unit of the same hospital between 1 January 2018 and 31 October 2023. Transplants with BM grafts (HPC Marrow, HPC-M) and PB apheresis collections (HPC Apheresis, HPC-A) were included, while transplants with cord blood units were excluded from the study.
The study followed the tenets of the Declaration of Helsinki and received approval from the Ethics Committee of Fondazione Policlinico Universitario A. Gemelli IRCCS (Prot. 0030921/20).
Donor assessment
All donors were qualified according to JACIE standards (12) and Italian National regulation for transfusion activities (13, 14).
Donor medical history was assessed by a questionnaire and an interview with a trained physician experienced in donation procedures, to evaluate donor fitness, medical history, and willingness to donate before HLA-typing. Pre-existing health disorders were explored to exclude inherited or genetic coexisting diseases and behavioral risk factors for communicable infectious diseases. Donors characteristics that resulted in deferral from HPC donation were grouped into 11 categories according to WBMT standards (11): 1) infectious disease, 2) autoimmune disorders (AID), 3) low weight (defined as a weight lower than 50 kg), 4) abuse of alcohol or drugs, 5) CVD), 6) promiscuous sexual activity or cohabitation with hepatitis carriers, 7) allergic diathesis, including drug allergies, 8) lack of proper venous access, 9) history of cancer, 10) recent history of major surgery, and 11) other conditions. CVD specifically comprise arterial hypertension, hypotension, coronary heart disease, disturbance of heart rate and rhythm, congestive heart failure, cardiomyopathy, valvular heart disease, pericarditis, myocarditis, atherosclerotic peripheral vascular disease, aortic aneurism, and cardiac surgery for congenital heart disease. According to the updated WBMT standards (11), donors affected by CVD are eligible for HPC apheresis collection if the American Society of Anesthesiology Physical Status (ASA-PS) classification (15) are ≤2, according to the European Society of Anaesthesiology (ESA) (16) and American College of Cardiology/American Heart Association guidelines (17). Among the autoimmune disorders, diseases with systemic multiorgan involvement are investigated such as ankylosing spondylitis, polymyositis, arteritis, dermatomyositis, polymyalgia rheumatic, arteritis, antiphospholipid antibody syndrome, multiple sclerosis/optic neuritis, systemic lupus erythematosus, scleroderma, Sjögren’s syndrome, vasculitis syndromes, and Behçet’s (11). Among single-organ autoimmune diseases, Hashimoto thyroiditis, Graves’ disease, pernicious anemia, psoriasis, alopecia areata, and vitiligo are included (11).
Laboratory tests were performed on HPC donors deemed potentially eligible according to their medical history and after collecting a medical interview. These tests included complete peripheral blood count, serum creatinine, electrolyte and liver function studies, coagulation test, thrombophilia screening, microbiological screening for communicable infections (serologic studies for cytomegalovirus, herpes viruses, syphilis, anti-HIV antibodies HIV RNA, hepatitis B and C viruses, including nucleic acid amplification testing) blood typing and red cell antibody screening, human leukocyte antigen (HLA) typing, chest X-ray, electrocardiography, and abdominal ultrasound.
All donors eligible for BM harvest underwent a preventive anesthesiological assessment, while for HPC-A donors, an evaluation of peripheral vascular access was requested.
Mobilization protocol and apheresis procedures
Allogeneic peripheral blood stem cell (PBSC) donation was performed with subcutaneous (sc) administration of granulocyte colony-stimulating factor (G-CSF for days +1 to +4), followed by leukapheresis on day +5. G-CSF was administered at a standard dose of G-CSF 12 μg/kg sc daily. Prophylaxis with paracetamol was administered to prevent potential side effects of G-CSF. All collection procedures were performed at the Apheresis Unit using the COBE Spectra or Spectra Optia continuous flow cell separators (Terumo BCT, Shinagawa, Tokyo) with the mononuclear cell collection program, and a ratio anticoagulant: blood of 1:12. Anticoagulant always consisted of sodium citrate solution (Fresenius Kabi, Bad Homburg, Germany). In all patients, 2.5–3 total blood volumes (TBV, defined as the processing blood volume divided by the patient’s blood volume) were processed. The apheresis procedures aimed to achieve a CD34+ cell dose of 4.0x106 per kg of the recipient’s body weight. If the required number of CD34+ cells/kg was not accomplished, G-CSF administration was continued, and a second collection procedure was performed on day +6. According to institutional procedures, in donors with circulating CD34+ cells <20/µL on day +5 or estimated collection harvest <1×106/kg of the recipient’s body weight, sc plerixafor at the dose of 240 μg/kg/day was planned in the consecutive night, from 4 to 6 h before leukapheresis. Red blood cell depletion in major and bidirectional AB0 mismatched transplants, and plasma removal in minor AB0 mismatched transplants, or transplants with transfused donors or female donors with previous pregnancies, were performed as previously reported (18). From March 2020 onward, all HPC products were cryopreserved. This procedure was introduced due to the COVID-19 pandemic and is still in place (19).
Bone marrow collection
BM harvest and processing were managed as previously reported (18, 20–22). The perioperative autologous donation practice was discontinued in April 2020, replaced by iron (ferric carboxymaltose 500 mg intravenous), and B12 vitamin supplementation (1 mg sc) 1 week before BM collection (22). BM was harvested from posterior iliac crests in the operating room under general anesthesia, with the goal to collect 20–22 ml/kg of the donor weight. After harvesting, donors were admitted to the Hematology Department and observed for the following 24–48 h or more, as necessary.
Donor follow-up
All donors were followed for 1 year after the donation. Hematological and biochemical tests were routinely performed at 1 week, 6 months, and 12 months after donation.
Donor, patient, and graft data
Donor variables included gender, age, weight, HLA match (HLA-identical, haploidentical, and 7/8 mismatch), and ABO match. Laboratory data included WBC and, for apheresis donors, CD34+ cell count in peripheral blood on the day of collection. Apheresis data included blood volume processed, content of total nucleated cells (TNC), and content of CD34+ cells in the apheresis product. Patient variables included basic demographics; diagnosis; date of transplant; disease status (complete remission or not), disease risk index (DRI) (23); hematopoietic cell transplantation comorbidity index (HCT-CI) (24); dates of neutrophil, platelet, and erythrocyte engraftment; the incidence of acute and chronic graft versus host disease (aGVHD and cGVHD, respectively) (25–27); relapse; and status at the last follow-up. Graft variables included source (BM or PBSC), TNC content, CD34+ cell content, and CD3+ cell content. Cell contents were expressed as cell dose (i.e., the number of cells per kilogram of the recipient’s body weight) and were obtained as previously reported (28).
Study outcomes and definitions
We investigated the association of pre-donation health disorders with recipient features and transplant characteristics and outcomes, including patient’s age, HCT-CI, and DRI; conditioning regimens; neutrophil, platelet, and erythrocyte engraftment; all grade aGVHD and cGVHD; relapse; and recipient status at the last follow-up (alive or death). Neutrophil and platelet engraftments were defined as the achievement of an absolute neutrophil count (ANC) ≥ 0.5 × 109/L and a PLT count ≥ 20 × 109/L unsupported by transfusion, respectively; erythrocyte engraftment was defined as a reticulocyte count ≥ 2%. HCT-CI was defined according to Sorror et al. (24) DRI was defined according to Armand et al. (23) Diagnosis and grading of aGVHD and cGVHD were made according to standard criteria (25–27). Regimens were classified as myeloablative conditioning (MAC) or non-myeloablative (NMA) conditioning, including reduced-intensity conditioning (RIC) (18). In particular, MAC regimens consisted of fludarabine and total body irradiation (FLU-TBI; fludarabine 120 mg/m2, followed by 9–12 Gy TBI) or thiotepa, busulfan, fludarabine (TBF; thiotepa 5 mg/kg on day −6 and −5 total; intravenous busulfan 3.2 mg/kg on day −4, −3, and −2; fludarabine 50 mg/m2 on day −4, −3, and −2). Reduced and NMA regimens consisted of busulfan or fludarabine/TBI 2 Gy-based regimens.
Statistical analysis
Continuous variables were expressed as median with relative interquartile range (IQR) and categorical variables as n (%). Univariate analysis of continuous variables was performed by the Mann–Whitney U test or the Wilcoxon rank test, as appropriate. For categorical variables, Fisher’s exact test or the χ2 test was used, as appropriate. The association between donor status (fully eligible or eligible despite pre-existing diseases) and patients or transplant characteristics was assessed by multivariate logistic regression analysis incorporating the donor status as the dependent variable and recipients and transplant variables as covariates. The results were expressed as odds ratio (OR) with the relative 95% CI. All tests were two-sided, and a p-value <0.05 was considered statistically significant. Missing data were always <5% and were not considered. Analyses were performed using the IBM SPSS Statistics 25.0 and NCSS 10 v 10.0.19.
Results
Donor eligibility evaluation
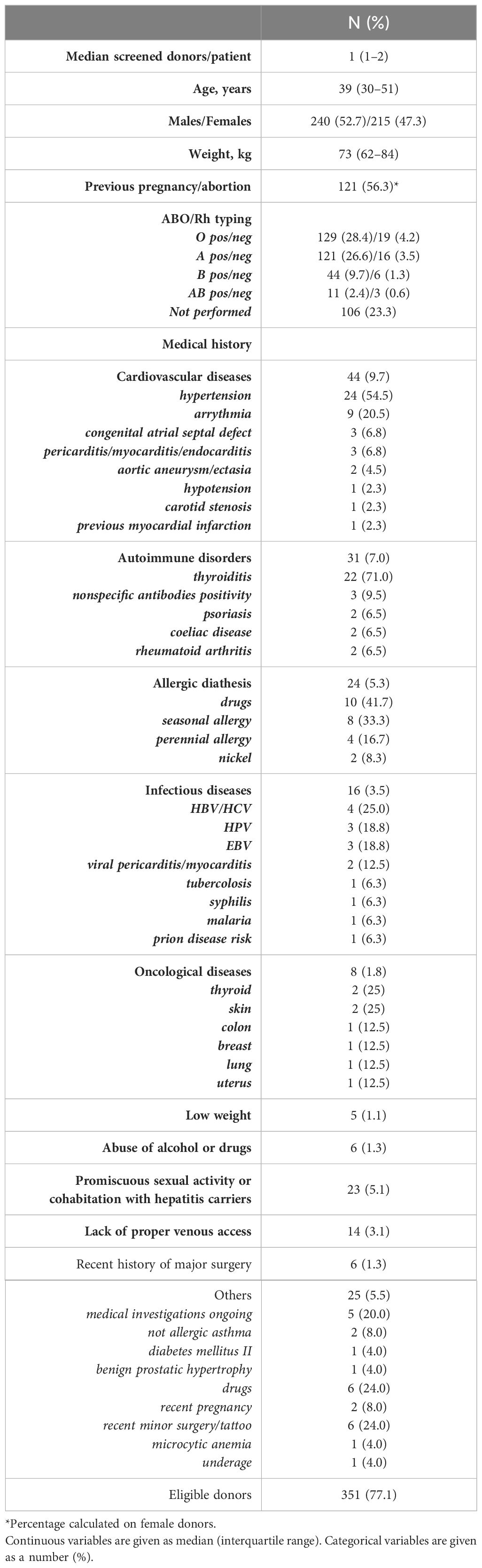
Overall, during the study period, we examined 455 potential HSC donors (ratio M/F 240/215), accounting for a median number of 1 donor for the patient (range, 1–7). The median age at first donation screening was 39 years (IQR, 30–51). Table 1 summarizes the main characteristics of all evaluated donors.
At donor assessment, one or more pre-existing health disorders were identified (Table 1). Among 455 donors, 16 (3.6%) were excluded for previous hepatitis B and C infection, 15 (4.7%) for promiscuous sexual activities or cohabitation with hepatitis carriers, eight (1.8%) for oncological diseases, six (1.3%) for a recent history of major surgery, and six (1.3%) for a previous history of alcohol or drug abuse. In addition, 14 donors (3.1%) were deferred from donation because of lack of peripheral venous access and five (1.1%) for a low body weight (< 50 kg). Overall, 31 donors (7% of the total), mainly female (26 F/5 M, 84% vs. 16%), were affected by one or more AID. Finally, 25 potential donors (5.5%) had several miscellaneous conditions that precluded HPC donation (Table 1).
Following our policy, we allowed HPC donation in donors with CVD if classified as ASA <3, in diabetes mellitus type 2 with no organ damage, or with allergic diathesis including drug allergies to a known pharmacological agent and nickel allergy. Accordingly, among 44 identified donors (9.7%) with CVD, 28 (63.6%) were definitively deferred from HPC donations, while 16 (36.3%) who were in well-controlled clinical conditions were deemed eligible for donation. Moreover, among 24 donors (5.3%) with allergic diathesis, 11 with allergies due to known pharmacological agents (9) or nickel allergy (2) were deemed eligible for donating.
Finally, after medical evaluation, 351 out of 455 donors were finally considered eligible for HPC donation and 233 underwent stem cell collection: 158 underwent apheresis and 75 bone marrow harvest. In total, among 233 selected donors, 27 had pre-existing well-controlled diseases, which would have prevented donation in the setting of unrelated HPC donors. Table 2 details the main features of this group of related donors. Pre-existing CVDs were equally distributed in both female and male donors, while allergic diathesis was more frequent in women. CVD donors were more frequently aged >60 years, and they more frequently were HLA-identical siblings. Conversely, drug allergies were mostly reported in the haploidentical setting. Most part of selected donors with pre-existing disorders were candidates to apheresis HPC collection (21, 77.8%), while only six (22.2%) underwent BM harvest, and they were considered suitable to be exposed to general anesthesia (Table 2).
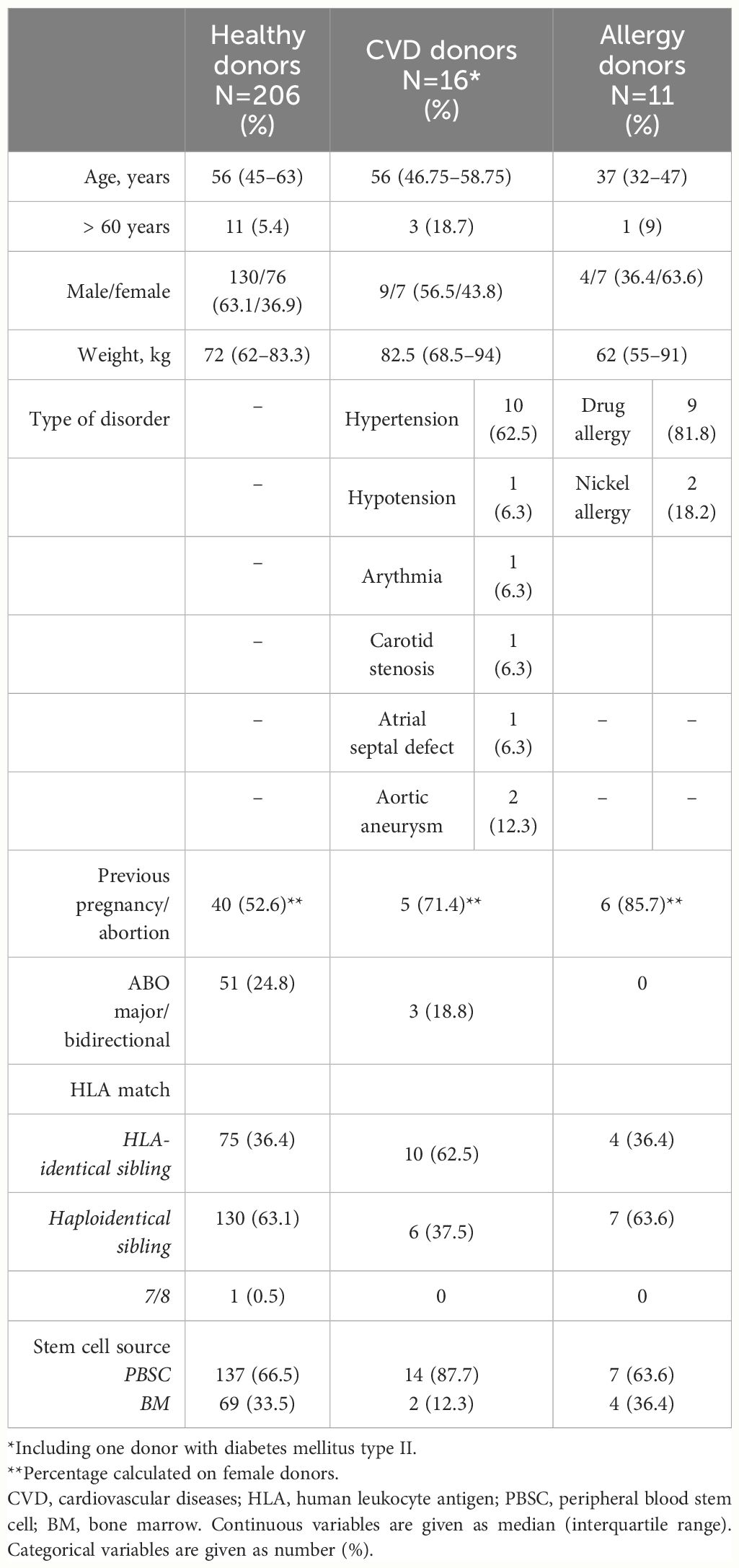
Table 2 Characteristics of 27 related donors with pre-existing well-controlled clinical conditions and 206 healthy ones.
Graft collection
No adverse events were observed during mobilization or collection. Among 158 HPC-A donors, 141 (89.2%) underwent one single collection procedure, while in 17 cases (10.8%), a second collection procedure was performed to achieve the required CD34+ cell dose (Table 3). Moreover, two HPC-A donors received plerixafor the night between day+5 and day +6 after starting of G-CSF. Five donors (3.2%) received a low prophylactic dose of enoxaparin the evening before stem cell apheresis because of hereditary thrombophilia without a personal medical history of venous thromboembolism (two heterozygous factor V Leiden, two protein S deficiency, and one antithrombin III deficiency). Neither thrombotic nor hemorrhagic complications were subsequently observed. Regarding donors with pre-existing well-controlled medical conditions, only one apheresis donor with CVD experienced a second-day collection, while none received plerixafor or anticoagulant prophylaxis (Table 3). Similarly, no adverse events were recorded among BM donors.
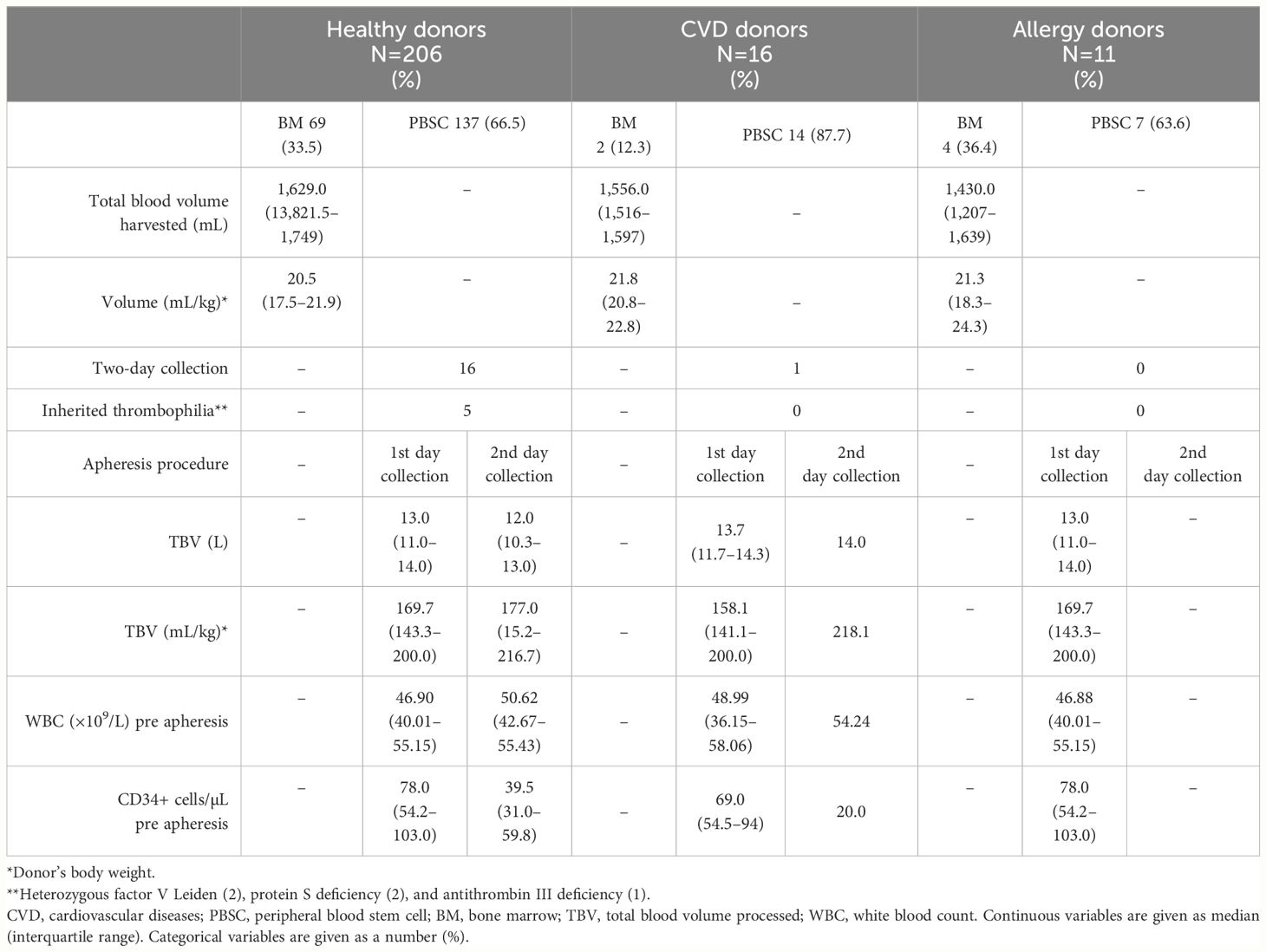
Table 3 Characteristics of stem cell collections grouped according to the medical history of HPC-related donors.
Donor follow-up
At follow-up, one healthy donor reported a hospital admission for cholecystectomy due to gallstones; the surgery occurred 3 months after the stem cell collection. No further adverse events were recorded among healthy donors or donors with pre-existing diseases.
Recipients and transplants
Overall, 233 allo-HSCT were performed in 227 patients (male/female, 140/87). Six patients received a second HSCT due to graft failure in four cases and relapse in additional two cases. Diagnoses were acute myeloid leukemia and myelodysplastic syndromes (118 transplants), acute lymphoid leukemia (29 transplants), primary or post-myeloproliferative neoplasm myelofibrosis (45 transplants), Hodgkin’s and non-Hodgkin’s lymphomas, chronic lymphocytic leukemia, and multiple myeloma (34 transplants), and severe aplastic anemia (seven transplants).
Table 4 illustrates the characteristics of recipients and transplants grouped according to the donor status (healthy donors, CVD donors, and allergy donors).
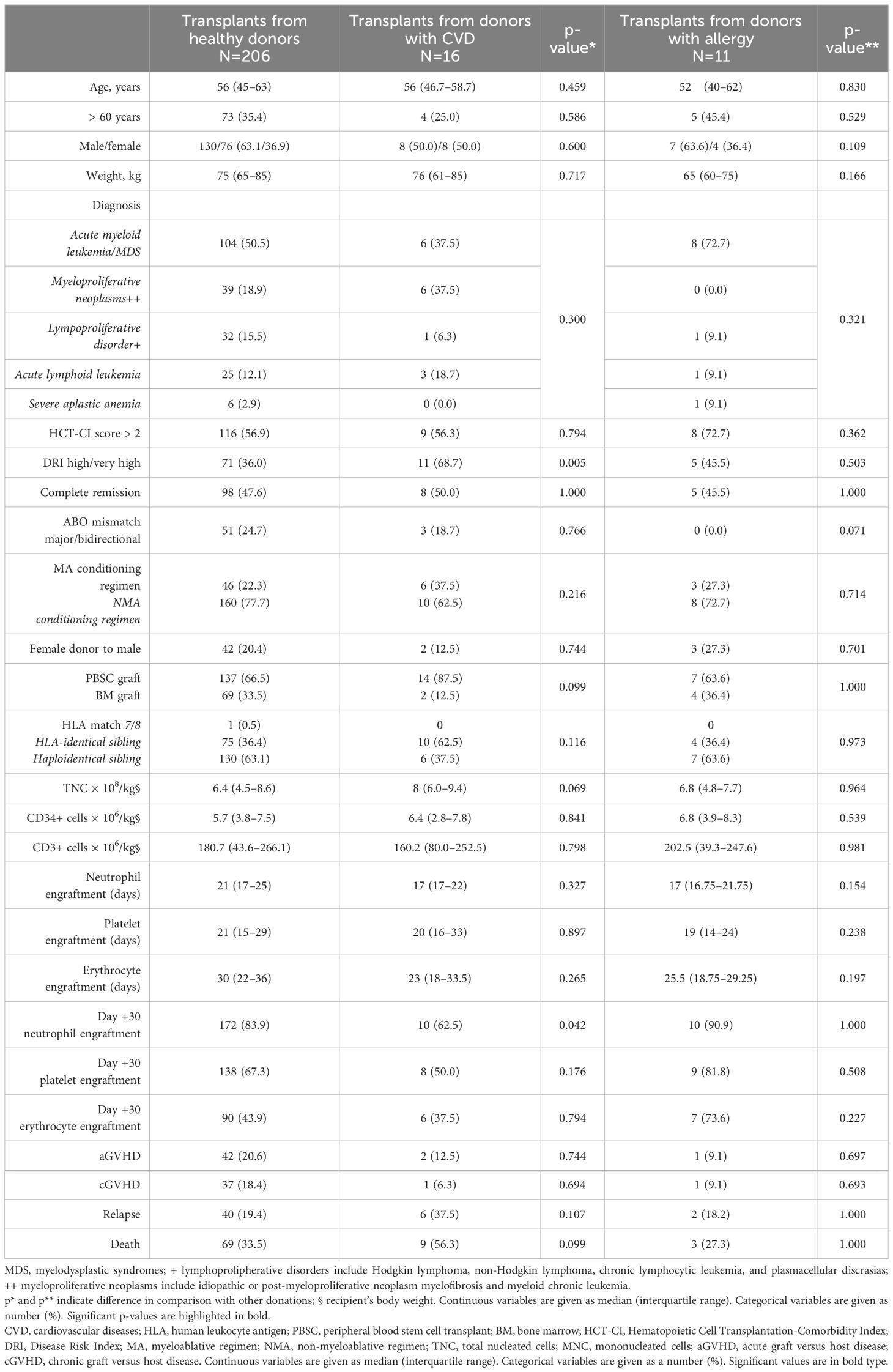
Table 4 Characteristics of 233 allogeneic stem cell transplantations, and results of univariate analysis comparing transplants from healthy donors with those from HPC-related donors with pre-existing medical conditions.
Regarding transplants from CVD donors, no significant disparities were observed for baseline characteristics (patients’ age, diagnosis, conditioning type, donor type, graft type, and cell content). A significant difference emerged regarding the disease severity, with a higher proportion of patients with DRI > 2 among those receiving grafts from CVD donors (68.7% in transplants from CVD donors in comparison with 36.0% in transplants from healthy donors, p=0.005). There was no difference in the transplant cell doses, including TNC, CD34+ cells, and CD3+ cells (Table 4). Regarding transplants from allergy donors, no differences were found in comparison with transplants from healthy donors.
Outcomes
Regarding engraftment, the median time to obtain neutrophils, platelet, and erythrocyte engraftment was similar in all patients, independently from the donor type (Table 4). However, a trend for a lower proportion of recipients with day-30 neutrophil engraftment was observed among transplants from CVD donors (62.5% in transplants from CVD donors in comparison with 83.9% in transplants from healthy donors, p=0.042) (Table 4). Moreover, similar proportions of patients in the three transplant groups developed aGVHD, cGVHD, or relapsed or died (Table 4).
Considering that the significant findings emerged only relative to transplants from CVD donors, we further evaluated in a multivariate regression logistic model the association between the CVD donor status and several recipient or transplant variables. We considered the CVD donor status as the dependent variable and included among covariates stem cell source, the CD34+ cell, and TNC graft content; HCT-CI>2; high/very high DRI; day-30 neutrophil; platelet and erythrocyte engraftment; and incidence of acute and chronic GVHD. We found that high/very high DRI patients had an increased probability of receiving donations from CVD donors (OR, 4.89; 95% CI, 1.15–20.86; p=0.031; Table 5).
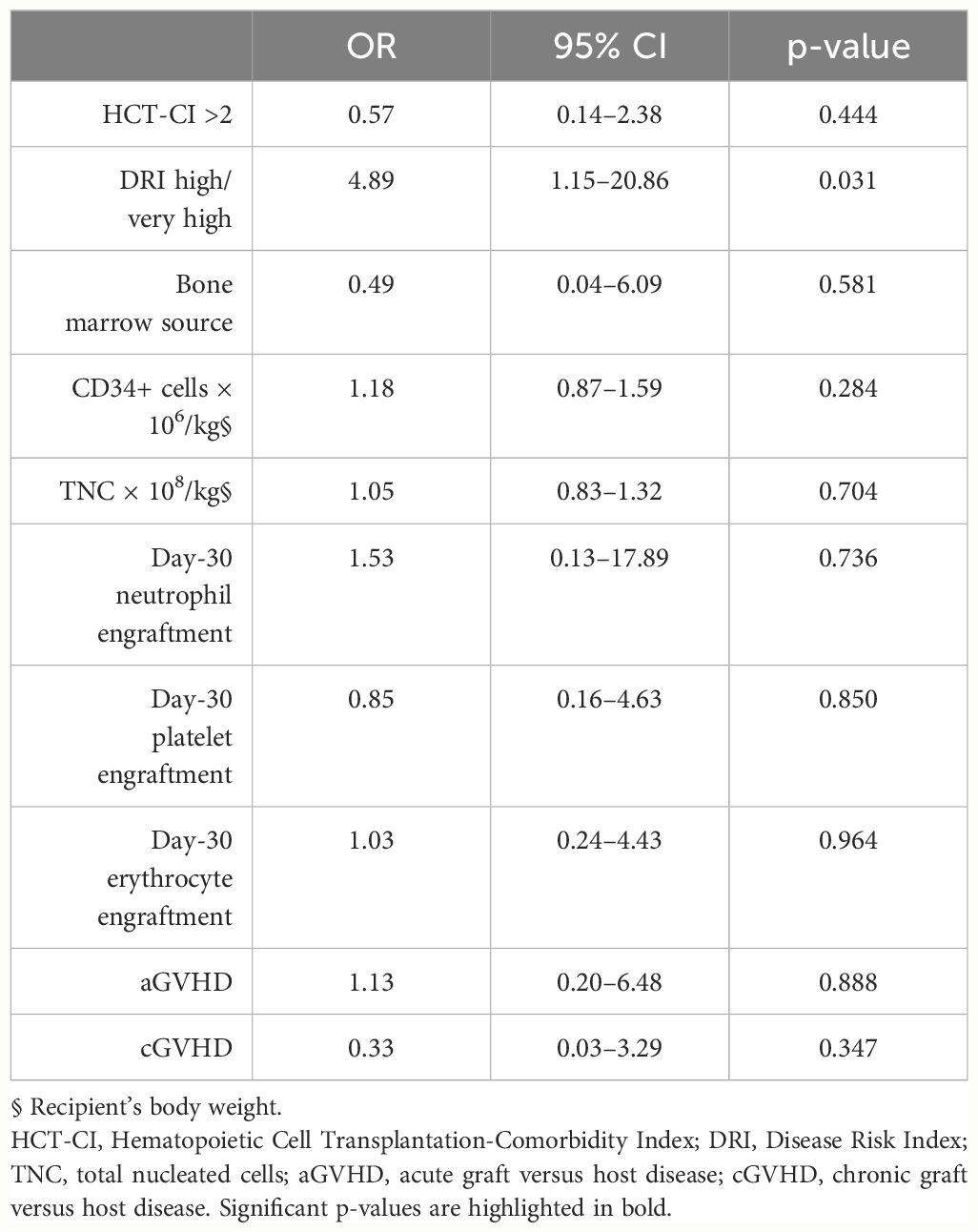
Table 5 Multivariate analysis of the association of pre-existing well-controlled cardiovascular disorders in HPC donors’ medical history on patient conditions, graft cellular doses, and transplant outcomes.
Discussion
This study reported our monocentric experience in the health assessment of HPC-related donors and investigated for the first time the impact of pre-existing medical conditions in donors considered eligible on transplant outcomes. Our data showed that HPC collection can be safely performed in related donors suffering from some pre-existing diseases such as well-controlled cardiovascular disorders. In our setting, this situation occurred most for the transplant of patients at the highest risk for relapse, in whom allo-HSCT could not be postponed. Moreover, the presence of medical conditions that would have deferred donation in the unrelated setting, did not affect engraftment or was associated with an increased rate of acute or chronic GVHD.
The safety and welfare of the donor are recognized as major concerns for the transplantation community. Historically, HLA-matched sibling donors, available for 30% of patients, have been considered the best choice for both practical and biological reasons (29–31). Transplantation techniques have evolved over the past two decades. The practice of haploidentical HSCT, together with the advances in transplant technique, supportive care, and reduced-intensity conditioning regimens, allows the treatment of older patients with elderly familial donors or donors with comorbidities, imposing different challenges to hematologists. As a result of such substantial changes in the donation process, the best accessible graft for many patients may be from an older donor such as HLA identical-sibling or haploidentical relative, highlighting the importance of understanding if any characteristic of donors may negatively influence recipient outcomes. There are an increasing number of studies that have evaluated the risk associated with HPC collection in related donors (32–34), but no report has been published evaluating the impact of benign medical conditions of donors on transplant outcome.
Donor assessment and the final decision on donor clearance are under the responsibility of the collection center’s physicians. The evaluation of related and unrelated donors followed the same procedure based on the currently valid quality standards and recommendations [WMDA (35, 36), FACT-JACIE (37)]. Compared with the strict recommendations on the suitability of unrelated donors, criteria for related donors allow for more discretion. In 2015, the donor outcome committee of WBMT proposed consensus recommendations of suitability criteria for pediatric and adult-related donors, which have been recently updated (10, 11). The WBMT standards allow related donors with pre-existing health conditions that would have been deferred as unrelated donors might still undergo donation if these medical conditions are not expected to lead to a significant reduction in donor safety.
We excluded all potential donors with medical conditions or lifestyles that would have represented a serious risk for both the donor and the recipient. In our experience, 23% of related HPC donors would not fit the eligibility criteria of an unrelated donor registry because of pre-existing conditions, and medical history of autoimmune diseases, primarily thyroid affections, represented the main reason for non-eligibility. CVDs were the second cause of medical contraindications to HPC donation, mainly severe acute heart issues, uncontrolled hypertension, and tachyarrhythmias. Donors with ongoing malignancies or a history of a malignant condition other than minor skin cancers such as basal cell carcinomas were excluded from further consideration. As for eligible HPC donors, 12% would have been deferred according to WBMT standards (11), mainly because of hypertension. Of note, four of them were over 60 years of age. Similar results are reported from a Dutch study evaluating short- and long-term adverse reactions in 268 related donors who underwent PBSC mobilization (32). The 15% of donors would have been deferred based on NMDP criteria for unrelated donors due to age (older than 60 years), BMI (at least >40 kg/m2), and hypertension (higher than 160/95 mmHg); other medical contraindications to HPC collection included clotting issues, diabetes, or severe heart issues. Of note, the authors detailed the follow-up of donors that would not have been eligible due to NMDP criteria, not reporting an increase in cardiovascular events, autoimmune diseases, or malignancy post-PBSC collection (32). Likewise, in the subsequent follow-up of related donors, we did not report the onset of additional medical conditions in the group of HPC donors with pre-existing benign medical conditions.
WBMT standards (11) recommend that in case of an active cardiac disorder, detailed evaluation by a cardiologist is required, and cardiac risk stratification should be performed by a specialist. Among CVD reported in our study population study, the most frequent pre-existing medical conditions were hypertension. All related donors were classified as ASA-PS <3. In addition, if a donor were considered suitable, we planned an in-depth cardiological evaluation with final approval for apheresis and extracorporeal circulation during the leukapheresis procedure, and an additionally fully anesthesiologic assessment in HPC-M donors, with continuous monitoring of the donor’s vital parameters during and immediately after the collection procedure. Among CVD donors, stem cell collection was performed mainly through apheresis, while only two donors were subjected to BM harvesting; in none of them, we recorded severe adverse events. At the same time, donors suffering from allergic diathesis did not experience side effects during HPC collection, nor patients who received grafts from donors with drug allergies develop an allergy after HSCT.
The WBMT board agreed that HPC donation is typically to be disregarded in donors with systemic multiorgan involvement related to AID; on the other hand, among donors affected by single-organ autoimmune diseases such as Hashimoto thyroiditis, Graves’ disease, pernicious anemia, psoriasis, alopecia areata, or vitiligo, the experts recommended that donation must be deferred only for candidate donors receiving systemic treatment (11). In our institution, all donors with AID were deferred from HPC donations, and a medical history of autoimmune diseases represented the main reason for non-eligibility. The impact on the recipient’s immune reconstitution is not yet known. However, it is widely acknowledged that the pathogenesis of autoimmune disorders is multifactorial, with genetic and environmental factors combining to determine disease onset and evolution. For many autoimmune disorders, adoptive transfer of diseases from the donor to the recipient during allogeneic HSCT has been documented. They include thyroid diseases, type 1 diabetes, immune thrombocytopenia, vitiligo, and psoriasis (38–42).
Large registry-based studies have shown that younger donor age is the most important secondary donor characteristic after HLA matching and has been associated with improved overall and disease-free survival, with a 3% improvement in 2-year survival when a donor 10 years younger is selected (43–45). We did not find a significant correlation between the age of related donors and transplant outcome; the median donor age of our population was 40 years old (range, 16–75), which is usually used as a cutoff for younger versus older donors. Notably, 15 donors were older than 60 years, and three of them also suffered from CVD, in agreement with the increasing prevalence of conditions such as atherosclerotic cardiovascular disorders with aging. Aging is marked by the acquisition of somatic mutations in hematopoietic stem cells due to cumulative genomic DNA damage: this condition is commonly referred to as clonal hematopoiesis of indeterminate potential (CHIP) (46). The proportion of CHIP carriers increases exponentially with age even if mutations within genes including DNMT3A or TET2 can be detected for up to 95% of healthy individuals, with a mean age of 50 years old (47). CHIP is associated with a 0.5%–1% risk per year of leukemia (47). Remarkably, it confers a twofold increase in cardiovascular risk independent of traditional risk factors (46). In fact, CHIP is associated with a pro-inflammatory state that has been linked to coronary artery disease, myocardial infarction, and venous thromboembolic disease (48, 49). In the context of HSCT, the potential transfer of CHIP from donor to patient may bring up concerning implications. An early report first described the transfer of CHIP-mutated hematopoietic stem cells to recipients through HSCT (50). Indeed, these data imply precaution in selecting aging relatives suffering from CVD for donation and suggest that the CHIP screening could be worthwhile in these cases.
Our study exhibits some limitations. First, the short length of the follow-up of the study population requires caution in the interpretation of data. Second, the retrospective design does not allow to reach definite evidence. Finally, recipients were affected by different types of diseases, and the effect of various immunosuppressive regimens was not considered on the GVHD outcome.
Nevertheless, our results provide insights into the related donor selection and suggest that relatives may be accepted for safe HPC donation based on less strict criteria than unrelated donors, offering a lifesaving opportunity for patients whose allogeneic transplants cannot be postponed. Further multicenter studies on larger donor populations are worthy to compare donor selection policies and come to definite conclusions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Largo Agostino Gemelli, 8, 00168, Rome, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Author contributions
CGV: Writing – original draft, Writing – review & editing. SC: Writing – original draft. FF: Writing – original draft. EM: Writing – original draft. CP: Writing – original draft. PC: Writing – review & editing. SS: Writing – review & editing. LT: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Cámara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. (2021) 56:1651–64. doi: 10.1038/s41409-021-01227-8
2. Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. (2010) 303:1617–24. doi: 10.1001/jama.2010.491
3. Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. (2016) 51:786–92. doi: 10.1038/bmt.2016.20
4. van Walraven SM, Nicoloso-de Faveri G, Axdorph-Nygell UA, Douglas KW, Jones DA, Lee SJ, et al. Family donor care management: principles and recommendations. Bone Marrow Transplant. (2010) 45:1269–73. doi: 10.1038/bmt.2009.354
5. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. (2017) 52:811–7. doi: 10.1038/bmt.2017.34
6. Gagelmann N, Bacigalupo A, Rambaldi A, Hoelzer D, Halter J, Sanz J, et al. Haploidentical stem cell transplantation with posttransplant cyclophosphamide therapy vs other donor transplantations in adults with hematologic cancers: A systematic review and meta-analysis. JAMA Oncol. (2019) 5:1739–48. doi: 10.1001/jamaoncol.2019.3541
7. Sacchi N, Costeas P, Hartwell L, Hurley CK, Raffoux C, Rosenmayr A, et al. Haematopoietic stem cell donor registries: World Marrow Donor Association recommendations for evaluation of donor health. Bone Marrow Transplant. (2008) 42:9–14. doi: 10.1038/bmt.2008.76
8. Halter JP, van Walraven SM, Worel N, Bengtsson M, Hägglund H, Nicoloso de Faveri G, et al. Allogeneic hematopoietic stem cell donation-standardized assessment of donor outcome data: a consensus statement from the Worldwide Network for Blood and Marrow Transplantation (WBMT). Bone Marrow Transplant. (2013) 48:220–5. doi: 10.1038/bmt.2012.119
9. Lown RN, Philippe J, Navarro W, van Walraven SM, Philips-Johnson L, Fechter M, et al. Unrelated adult stem cell donor medical suitability: recommendations from the World Marrow Donor Association Clinical Working Group Committee. Bone Marrow Transplant. (2014) 49:880–6. doi: 10.1038/bmt.2014.67
10. Worel N, Buser A, Greinix HT, Hägglund H, Navarro W, Pulsipher MA, et al. Suitability criteria for adult related donors: A consensus statement from the worldwide network for blood and marrow transplantation standing committee on donor issues. Biol Blood Marrow Transplant. (2015) 21:2052–60. doi: 10.1016/j.bbmt.2015.08.009
11. Worel N, Aljurf M, Anthias C, Buser AS, Cody M, Fechter M, et al. Suitability of haematopoietic cell donors: updated consensus recommendations from the WBMT standing committee on donor issues. Lancet Haematol. (2022) 9:e605–14. doi: 10.1016/S2352-3026(22)00184-3
12. JOINT ACCREDITATION COMMITEE-ISCT and EBMT (JACIE). JACIE/FACT International Standards for Hematopoietic Cellular Therapy Product Collection, Processing and Administration. Eight Edition Version 8.2. Springer. (2021)
13. MINISTERO DELLA SALUTE. DECRETO 2 novembre 2015. Disposizioni relative ai requisiti di qualita' e sicurezza del sangue e degli emocomponenti. (15A09709) (GU Serie Generale n.300 del 28-12-2015 - Suppl. Ordinario n. 69). GAZZETTA UFFICIALE DELLA REPUBBLICA ITALIANA. (2015).
14. RSWG-IBMDR (Italian Bone Marrow Donor Registry). Italian Bone Marrow Donor Registry Standard di funzionamento del Programma nazionale italiano di donazione di Cellule Staminali Emopoietiche da non familiare. Versione XXIV. (2021). Available at: https://ibmdr.galliera.it/accoglienza-area-professionisti-e-manuale-operativo/manuale-operativo/standard-ibmdr-versione-2021/@@download/file (accessed 22th May 2024).
15. Horvath B, Kloesel B, Todd MM, Cole DJ, Prielipp RC. The evolution, current value, and future of the American society of anesthesiologists physical status classification system. Anesthesiology. (2021) 135:904–19. doi: 10.1097/ALN.0000000000003947
16. Kristensen SD, Knuuti J. New ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur Heart J. (2014) 35:2344–5. doi: 10.1093/eurheartj/ehu285
17. Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. (2014) 130:2215–45. doi: 10.1161/CIR.0000000000000105
18. Valentini CG, Metafuni E, Gallo L, Giammarco S, Orlando N, Bianchi M, et al. ABO mismatch in allogeneic hematopoietic stem cell transplant: effect on short- and long-term outcomes. Transplant Direct. (2021) 7:e724. doi: 10.1097/TXD.0000000000001179
19. Valentini CG, Chiusolo P, Bianchi M, Metafuni E, Orlando N, Giammarco S, et al. Coronavirus disease 2019 pandemic and allogeneic hematopoietic stem cell transplantation: a single center reappraisal. Cytotherapy. (2021) 23:635–40. doi: 10.1016/j.jcyt.2020.12.001
20. Teofili L, Bianchi M, Valentini CG, Bartolo M, Orlando N, Sica S. Validation plan of bone marrow collection, processing and distribution using the failure mode and effect analysis methodology: a technical report. Cytotherapy. (2022) 24:356–64. doi: 10.1016/j.jcyt.2021.10.005
21. Teofili L, Valentini CG, Bianchi M, Pellegrino C, Bellesi S, Chiusolo P, et al. Preoperative autologous blood donation in adult bone marrow donors: reappraisal of a single-centre experience. Vox Sang. (2019) 114:762–8. doi: 10.1111/vox.12834
22. Valentini CG, Innocenti I, Pellegrino C, Draisci G, Teofili L, ATREMA group. Autologous transfusion requirements in bone marrow harvest: results of the ATREMA study. Blood Transfus. (2024) 22:90–2. doi: 10.2450/BloodTransfus.669
23. Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. (2014) 123:3664–71. doi: 10.1182/blood-2014-01-552984
24. Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. (2005) 106:2912–9. doi: 10.1182/blood-2005-05-2004
25. Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. (2018) 53:1401–15. doi: 10.1038/s41409-018-0204-7
26. Malard F, Holler E, Sandmaier BM, Huang H, Mohty M. Acute graft-versus-host disease. Nat Rev Dis Primers. (2023) 9:27. doi: 10.1038/s41572-023-00438-1
27. Hamilton BK. Updates in chronic graft-versus-host disease. Hematol Am Soc Hematol Educ Program. (2021) 2021:648–54. doi: 10.1182/hematology.2021000301
28. Teofili L, Chiusolo P, Valentini CG, Metafuni E, Bellesi S, Orlando N, et al. Bone marrow haploidentical transplant with post-transplantation cyclophosphamide: does graft cell content have an impact on main clinical outcomes? Cytotherapy. (2020) 22:158–65. doi: 10.1016/j.jcyt.2020.01.007
29. Anthias C, Shaw BE, Kiefer DM, Liesveld JL, Yared J, Kamble RT, et al. Significant improvements in the practice patterns of adult related donor care in US transplantation centers. Biol Blood Marrow Transplant. (2016) 22:520–7. doi: 10.1016/j.bbmt.2015.11.008
30. Shouval R, Fein JA, Labopin M, Kröger N, Duarte RF, Bader P, et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. (2019) 6:e573–84. doi: 10.1016/S2352-3026(19)30158-9
31. Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. (2014) 371:339–48. doi: 10.1056/NEJMsa1311707
32. Wiersum-Osselton JC, van Walraven SM, Bank I, Lenselink AM, Fibbe WE, van der Bom JG, et al. Clinical outcomes after peripheral blood stem cell donation by related donors: a Dutch single-center cohort study. Transfusion. (2013) 53:96–103. doi: 10.1111/j.1537-2995.2012.03676.x
33. Pulsipher MA, Logan BR, Chitphakdithai P, Kiefer DM, Riches ML, Rizzo JD, et al. Effect of aging and predonation comorbidities on the related peripheral blood stem cell donor experience: report from the related donor safety study. Biol Blood Marrow Transplant. (2019) 25:699–711. doi: 10.1016/j.bbmt.2018.11.004
34. Pulsipher MA, Logan BR, Kiefer DM, Chitphakdithai P, Riches ML, Rizzo JD, et al. Related peripheral blood stem cell donors experience more severe symptoms and less complete recovery at one year compared to unrelated donors. Haematologica. (2019) 104:844–54. doi: 10.3324/haematol.2018.200121
35. World Marrow Donor Association World marrow donor association international standards for unrelated hematopoietic stem cell donor registries (2021). Available online at: https://wmda.info/wp-content/uploads/2021/01/WMDA-2020-Standards_AM1_Jan2021-1.pdf (Accessed 20th February 2024).
36. World Marrow Donor Association WMDA international standards unrelated hematopoietic stem cell donor registries (2021). Available online at: https://wmda.info/wp-content/uploads/2021/01/Amendment-1-to-2020-Std_AM1_Jan2021.pdf (Accessed 20th February 2024).
37. Foundation for the Accreditation of Cellular Therapy. International standards for hematopoietic cellular therapy product collection, processing, and administration accreditation manual (2021). Available online at: https://www.ebmt.org/jacie-accreditation (Accessed 20th February 2024).
38. Campbell-Fontaine A, Coad JE, Kovach R, Ericson SG. Adoptive transfer of vitiligo after allogeneic peripheral blood stem cell transplant. Bone Marrow Transplant. (2005) 36:745–6. doi: 10.1038/sj.bmt.1705137
39. Olivares JL, Ramos FJ, Olivé T, Fillat C, Bueno M. Autoimmune thyroiditis after bone marrow transplantation in a boy with Wiskott-Aldrich syndrome. J Pediatr Hematol Oncol. (2002) 24:772–6. doi: 10.1097/00043426-200212000-00020
40. Lampeter EF, McCann SR, Kolb H. Transfer of diabetes type 1 by bone-marrow transplantation. Lancet. (1998) 351:568–9. doi: 10.1016/S0140-6736(05)78555-X
41. Snowden JA, Heaton DC. Development of psoriasis after syngeneic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br J Dermatol. (1997) 137:130–2.
42. Thomson JA, Wilson RM, Franklin IM. Transmission of thyrotoxicosis of autoimmune type by sibling allogeneic bone marrow transplant. Eur J Endocrinol. (1995) 133:564–6. doi: 10.1530/eje.0.1330564
43. Ayuk F, Zabelina T, Wortmann F, Alchalby H, Wolschke C, Lellek H, et al. Donor choice according to age for allo-SCT for AML in complete remission. Bone Marrow Transplant. (2013) 48:1028–32. doi: 10.1038/bmt.2013.14
44. Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. (2001) 98:2043–51. doi: 10.1182/blood.v98.7.2043
45. Shaw BE, Logan BR, Spellman SR, Marsh SGE, Robinson J, Pidala J, et al. Development of an unrelated donor selection score predictive of survival after HCT: donor age matters most. Biol Blood Marrow Transplant. (2018) 24:1049–56. doi: 10.1016/j.bbmt.2018.02.006
46. Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J Mol Cell Cardiol. (2021) 161:98–105. doi: 10.1016/j.yjmcc.2021.07.004
47. Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. (2016) 7:12484. doi: 10.1038/ncomms12484
48. Senguttuvan NB, Subramanian V, Venkatesan V, Muralidharan TR, Sankaranarayanan K. Clonal hematopoiesis of indeterminate potential (CHIP) and cardiovascular diseases-an updated systematic review. J Genet Eng Biotechnol. (2021) 19:105. doi: 10.1186/s43141-021-00205-3
49. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. (2014) 371:2488–98. doi: 10.1056/NEJMoa1408617
Keywords: related donor, donor assessment, eligibility criteria, hematopoietic stem cell transplantation, engraftment
Citation: Valentini CG, Ceglie S, Fatone F, Metafuni E, Pellegrino C, Chiusolo P, Sica S and Teofili L (2024) Hematopoietic stem cell transplantation: an Italian monocentric experience on the health assessment and eligibility of adult-related donors. Front. Oncol. 14:1389068. doi: 10.3389/fonc.2024.1389068
Received: 20 February 2024; Accepted: 14 May 2024;
Published: 30 May 2024.
Edited by:
Salvatore Leotta, Independent Researcher, Catania, ItalyReviewed by:
Elisa Sala, Ulm University Medical Center, GermanyW. Scott Goebel, Indiana University Bloomington, United States
Copyright © 2024 Valentini, Ceglie, Fatone, Metafuni, Pellegrino, Chiusolo, Sica and Teofili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caterina Giovanna Valentini, Y2F0ZXJpbmFnaW92YW5uYS52YWxlbnRpbmlAcG9saWNsaW5pY29nZW1lbGxpLml0
 Caterina Giovanna Valentini
Caterina Giovanna Valentini Sara Ceglie1,2
Sara Ceglie1,2 Elisabetta Metafuni
Elisabetta Metafuni Claudio Pellegrino
Claudio Pellegrino Patrizia Chiusolo
Patrizia Chiusolo Simona Sica
Simona Sica Luciana Teofili
Luciana Teofili