- 1Department of Respiratory Medicine, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Child Health Advocacy Institute, China Hospital Development Institute, Shanghai Jiao Tong University, Shanghai, China
- 3Department of Respiratory Medicine, Sanya Women and Children’s Hospital Affiliated to Hainan Medical College, Hainan Branch of Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Sanya, Hainan, China
- 4Department of Respiratory Medicine, Linyi Maternal and Child Healthcare Hospital, Linyi Branch of Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Linyi, Shandong, China
- 5Shanghai Children’s Medical Center Pediatric Medical Complex (Pudong), Shanghai, China
- 6Pediatric AI Clinical Application and Research Center, Shanghai Children’s Medical Center, Shanghai, China
- 7Shanghai Engineering Research Center of Intelligence Pediatrics (SERCIP), Shanghai, China
Background: With the widespread use of computed tomography (CT), the detection rate of pulmonary nodules in children has gradually increased. Due to the lack of epidemiological evidence and clinical guideline on pulmonary nodule treatment in children, we aimed to provide a reference for the clinical diagnosis and management of pediatirc pulmonary nodules.
Methods: This retrospective study collected consecutive cases from April 2012 to July 2021 in the Shanghai Children’s Medical Center. The sample included children with pulmonary nodules on chest CT scans and met the inclusion criteria. All patients were categorized into tumor and non-tumor groups by pre-CT clinical diagnosis. Nodule characteristics between groups were analyzed. To establish a clinical assessment model for the benign versus malignant pulmonary nodules, patients who have been followed-up for three months were detected and a decision tree model for nodule malignancy prediction was constructed and validated.
Results: The sample comprised 1341 patients with an average age of 7.2 ± 4.6 years. More than half of them (51.7%) were diagnosed with malignancies before CT scan. 48.3% were diagnosed with non-tumor diseases or healthy. Compared to non-tumor group, children with tumor were more likely to have multiple nodules in both lungs, with larger size and often be accompanied by osteolytic or mass lesions. Based on the decision tree model, patients’ history of malignancies, nodules diameter size≥5mm, and specific nodule distribution (multiple in both lungs, multiple in the right lung or solitary in the upper or middle right lobe) were important potential predictors for malignity. In the validation set, sensitivity, specificity and AUC were 0.855, 0.833 and 0.828 (95%CI: 0.712-0.909), respectively.
Conclusion: This study conducted a clinical assessment model to differentiate benignity and malignancy of pediatric pulmonary nodules. We suggested that a nodule’s diameter, distribution and patient’s history of malignancies are predictable factors in benign or malignant determination.
1 Introduction
Pulmonary nodules are defined as round, oval or irregular lesions, surrounded by lung parenchyma, with a diameter of ≤3cm (1), and can be ground glass, solid, or contain both solid and ground glass components (2). Pulmonary nodules are more common in adults because primary lung cancer is one of the most common cancers in this population. The etiology of pediatric pulmonary nodules differs from that in adults, and often lack specificity. Common causes in pediatric pulmonary nodules include infectious lung diseases, pulmonary metastases, connective tissue diseases and other less common etiologies (such as chronic granulomatous disease, pulmonary nodulosis and multiple pulmonary arteriovenous fistulas) (3–5).
In recent years, the widespread use of chest computed tomography (CT) has made an increase in the detection of pediatric pulmonary nodules. The prevalence of incidental lung nodules in healthy children is not low. Alves et al. (6) evaluated preoperative thoracic CT scans of 99 children who were scheduled for surgery for pectus carinatum or excavatum and found the detection rate of nodule was 75%. In 2017, Samim and colleagues found a detection rate of pulmonary nodules on CT scan was 38.0% in pediatric patients after trauma (7). Similarly, researchers (8) retrospectively reviewed 259 trauma chest CT scans in pediatric patients and demonstrated a total of 86 patients (33%) with incidental pulmonary nodules. A large proportion of the studies revealed that pulmonary nodules are also not uncommonly identified in the pediatric population with extra thoracic malignancies. Silva and colleagues (9) reviewed 488 patients with a noncentral nervous system solid tumor or lymphoma and identified 111 patients (23%) with pulmonary nodules on the presentation CT scan. Recently, a study (10) reported more than one-fifth had pulmonary nodules in 316 pediatric patients with rhabdomyosarcoma. Another study discovered that 57.0% of the nodules detected by CT were malignant in children with osteosarcoma (11). These existing studies evaluated features of pulmonary nodules in children, but there is still no consensus on reliable features to determine a benign incidental pulmonary nodule versus metastatic disease. Therefore, the pulmonary nodule in pediatric population is not uncommonly encountered and becomes a challenging situation.
There were few accepted criteria for pediatric pulmonary nodule evaluation. In 2014, an American study found that 40% of pediatric pulmonologists referred to the Fleischner guidelines (12). This guideline aims at patients ≥35 years old, indicating a tendency towards overtreatment of pediatric pulmonary nodules (13). The development of standard guidelines for pediatric pulmonary nodules is an urgent demand (14). Although lung biopsy is the gold standard for nodule diagnosis, it is not the first choice due to its invasiveness. Chest CT is the first line guide for determining benignity or malignancy (6, 8, 15, 16). Our study is designed to provide a reference for clinical diagnosis, treatment, and management of pulmonary nodules for children.
2 Materials and methods
2.1 Study design and data collection
We retrospectively collected consecutive cases from April 2012 to July 2021 in the Shanghai Children’s Medical Center. The inclusion criteria were as follows: (1) age <18 years; (2) first detection of pulmonary nodules on CT; (3) pulmonary nodules with a diameter ≤3cm, round or irregular in shape and presenting as high-density shadows. The exclusion criteria were: (1) pulmonary mass with a diameter >3cm on CT; (2) missing clinical data or radiological data was unavailable. Patients’ demographic information (age, sex), clinical record (clinical department, respiratory symptoms, Pre-CT clinical diagnosis, past medical history and follow-up information etc.) and chest CT data (nodule diameter, density, distribution, accompanied radiological features, and radiological diagnosis) were collected by the electronic medical record system. All subjects were categorized into tumor and non-tumor groups by pre-CT diagnosis. Patients having infections, non-tumor hematological diseases, autoimmune diseases, immunodeficiencies and other diagnosis like trauma were set into non-tumor group.
To establish a clinical assessment model, children with pulmonary nodule follow-up for ≥3 months were subdivided into malignant and benign nodule subgroups. Data collected in subgroups met the following criteria: (1) radiological diagnosis was made by two experienced radiologists’ analysis of changes in the imaging characteristics of the nodules during follow-up (including size, density, distribution, etc.); (2) clinical judgment of the nodule was made by clinician based on the therapeutic effect (including tumor chemotherapy, anti-infection, anti-inflammatory treatment, etc.). The collected samples were then randomly divided into a training set and a validation set, and the features of the training set data were analyzed to build a decision tree algorithm using R language.
The study was approved by the Institutional Review Board of the Shanghai Children’s Medical Center, Shanghai Jiao Tong University (SCMCIRB-K2022085-2).
2.2 Statistical analysis
SPSS 26.0 software was utilized for statistical analysis of demographic information, clinical record and chest CT data. The t-test, Mann-Whitney U test and χ2 test were used to analyze data between groups. A P-value <0.05 was considered statistically significant.
To fit the potential nonlinear relationships in the data and provide an intuitive explanation of the decision paths, we used a decision tree model to predict the malignancy of nodules. It was constructed using the rpart () and prp () functions from the rpart package in R version 4.2.2. The training dataset was used to grow the decision tree model. Five-fold cross-validation and grid search were employed on the training set to tune the hyperparameters. The model achieved optimal performance when the nodes of supersets and subsets were set to 10 and 5, respectively, and the maximum growth depth was set to 3 layers. The cutoff value was determined from the maximum value of the Youden index, and the corresponding sensitivity and specificity were calculated.
3 Results
3.1 Clinical characteristics and CT features between tumor and non-tumor groups
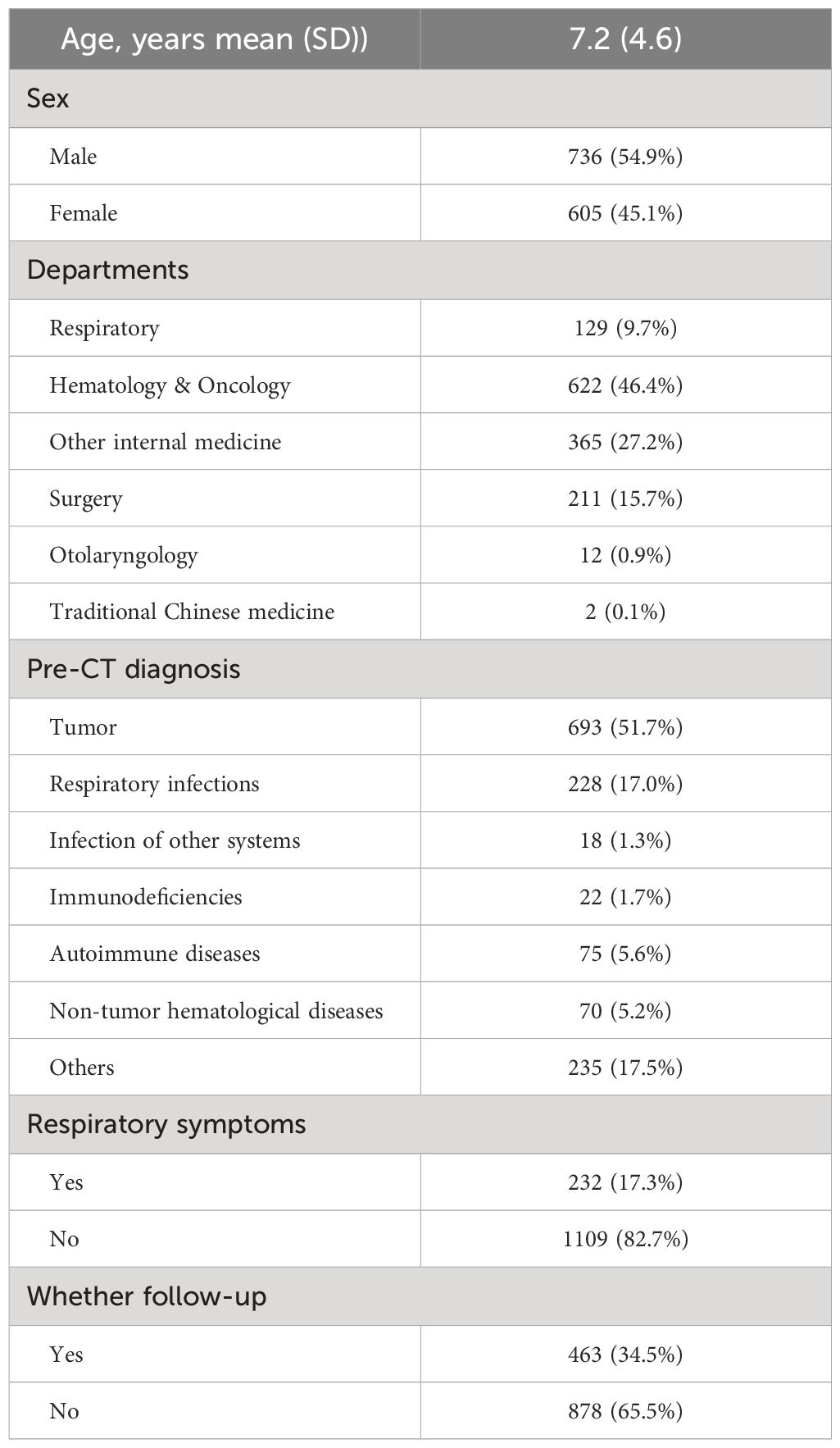
A total of 1341 children were detected with pulmonary nodules for the first time on chest CT scan, with 736 males (54.9%) and 605 females (45.1%). The average age was 7.2 ± 4.6 years. In Table 1, more than half of patients (n=693, 51.7%) were diagnosed with malignant tumors before the CT examination.
Children with tumor had significantly larger pulmonary nodules and were more likely to have multiple nodules in both lungs, often be accompanied by osteolytic or mass lesions on imaging. In non-tumor group, nodules were frequently located in the lower right lobe and were accompanied by patchy and striated shadows on imaging, children in this group showed more respiratory symptoms. Gender and nodule density showed no difference between groups (Table 2).
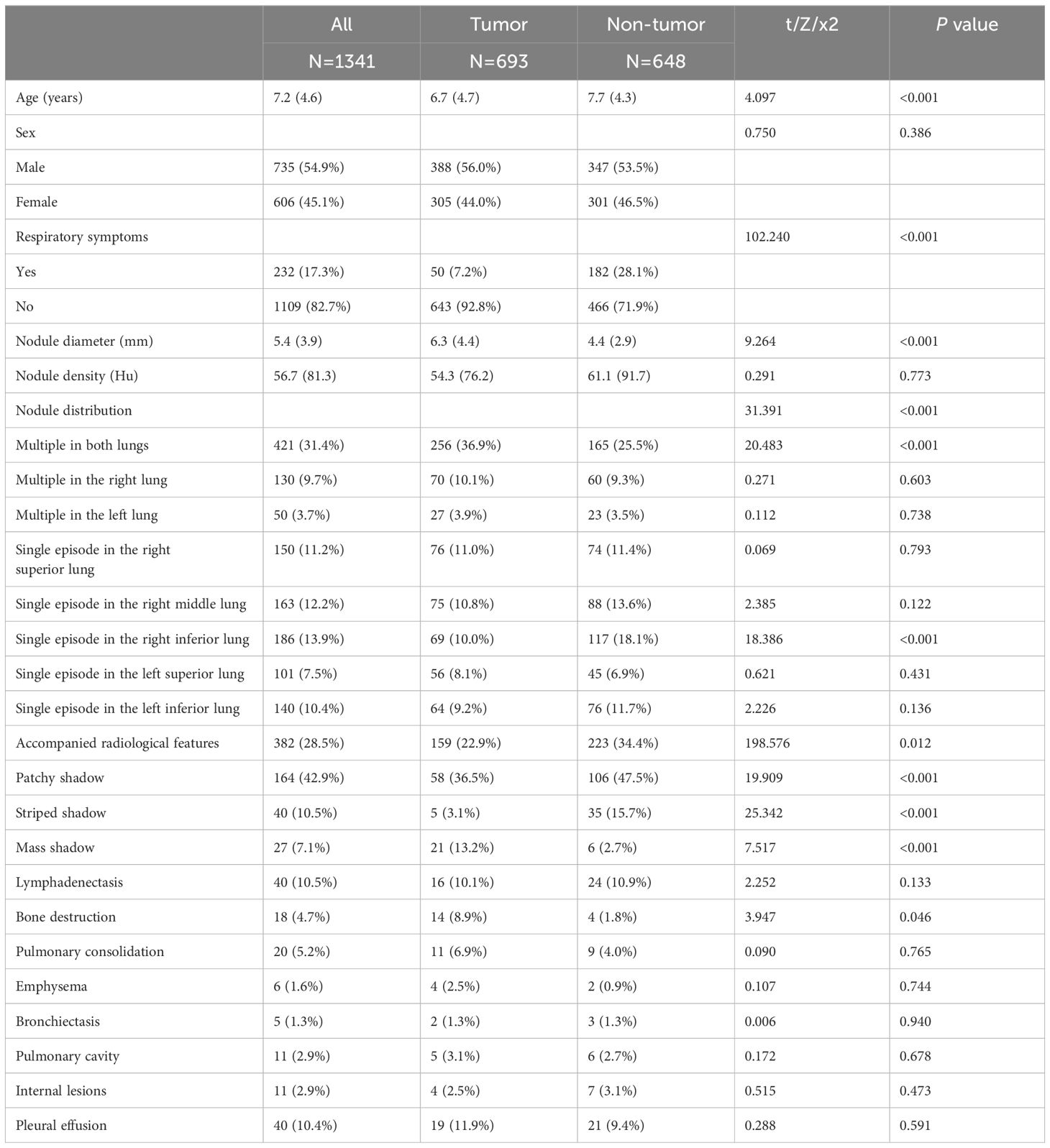
Table 2 Comparison of general data and pulmonary nodule characteristics between children in the tumor and non-tumor groups.
3.2 General conditions and CT features between benign and malignant nodule subgroups
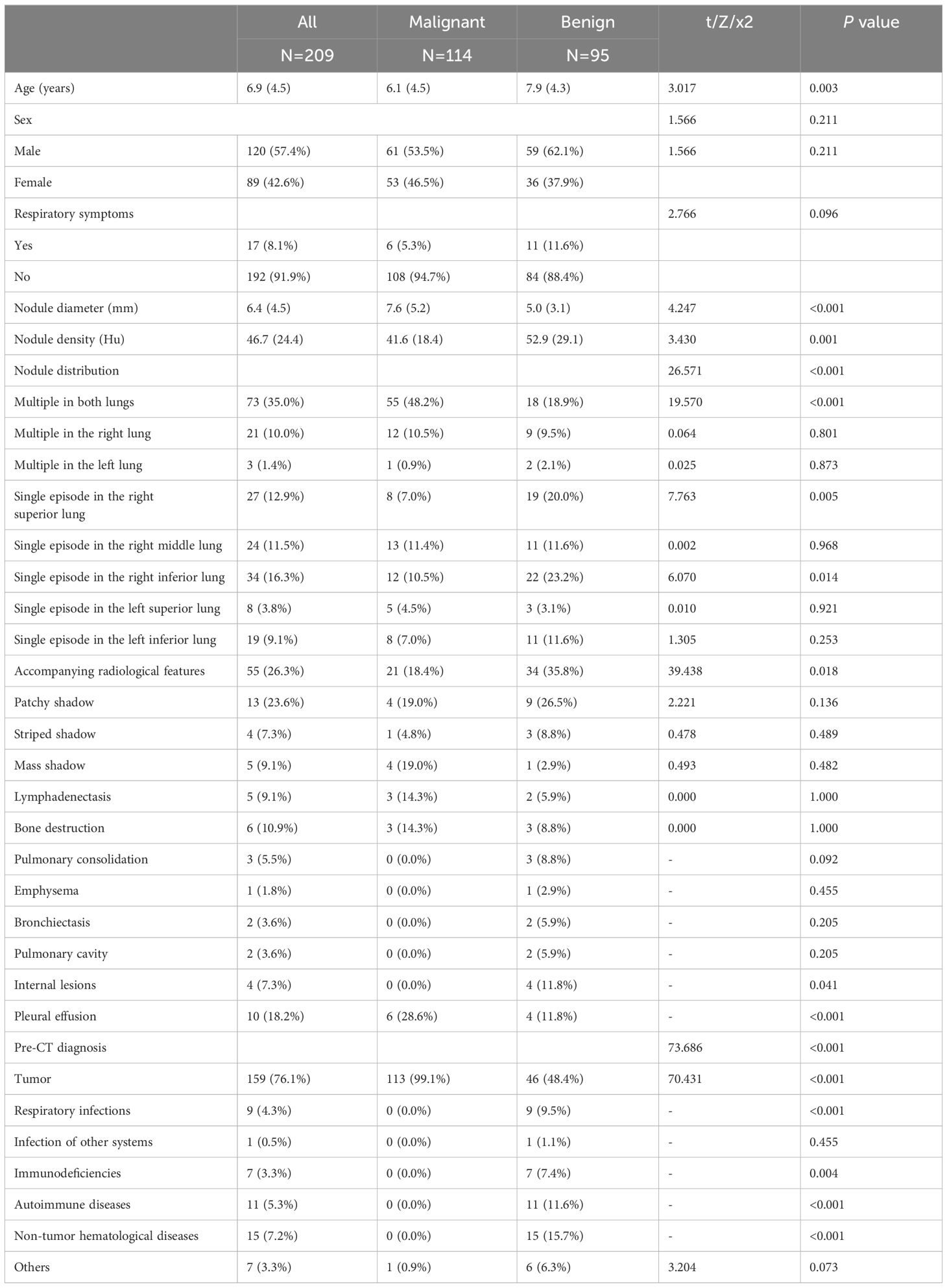
Based on the judgment of the radiologists and clinicians, a total of 209 cases with diagnosed nodules were detected. In the benign subgroup, 53.7% (n=51) were caused by infections, the rest were caused by thickened pleura, interstitial changes in both lungs, small contusions, hemangiomas, vascular malformations, etc. In the malignant nodule group, most of the nodules were metastases. 91% of the children showed a history of tumor. In terms of CT features, the nodule diameter (P<0.001), density (P=0.001), distribution (P<0.001) and accompanying radiological features (P=0.018) were markedly different between groups. Age (P=0.003) and pre-CT diagnosis (P<0.001) were statistically significant between the subgroups. No statistical difference showed in gender and clinical symptoms between subgroups (Table 3).
3.3 Decision tree model for determining the benignity or malignity of pediatric pulmonary nodules
Potential factors including age, gender, clinical symptoms, primary clinical diagnosis, nodule diameter, density, distribution location and accompanying radiological features were put into the decision tree model. After pruning strategies to achieve the best sensitivity and specificity, a clinical decision tree model was developed based on the primary clinical diagnosis, nodule diameter, and distribution pattern. This model has 3 specific nodes and 4 terminal nodes. Starting from the root node, it accurately distinguishes between the subgroups based on the threshold values of each classification or indicator. As the result showed in Figure 1, when the primary clinical diagnosis was not tumor, the nodule was considered benign; when the primary clinical diagnosis was tumor, further assessment of the nodule diameter was required. If the diameter was ≥5mm, the nodule was considered malignant, if it was <5mm, further assessment of the nodule distribution was necessary. Nodules multiple in both lungs, multiple only in the right lung, or solitary in the upper or middle right lobe are considered malignant, otherwise they were considered benign. Following the decision thresholds of the model, judgments are made step by step from the root node to determine the benignity versus malignity of pulmonary nodules.
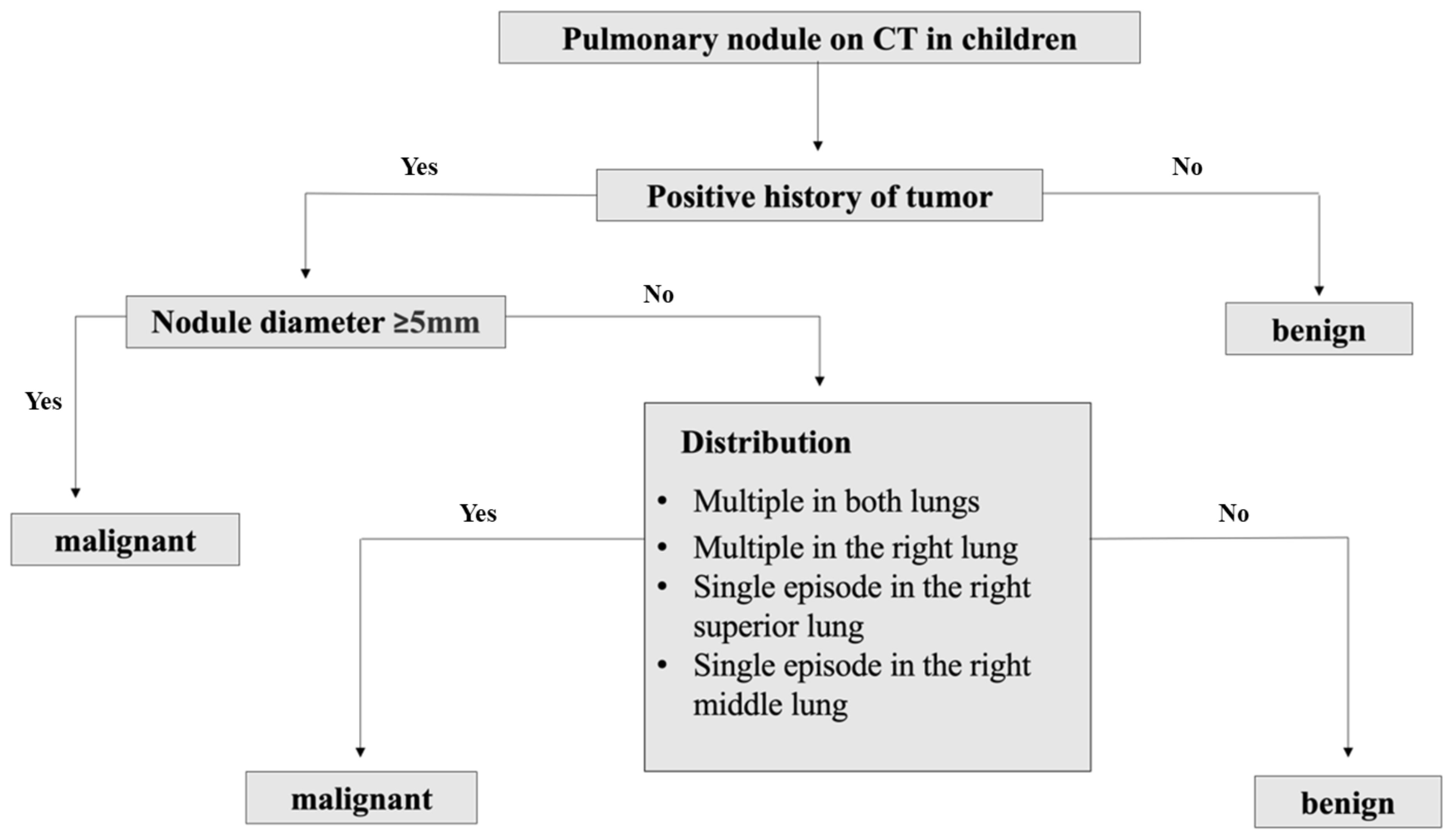
Figure 1 Clinical decision tree model of benign and malignant pulmonary nodules prediction in children.
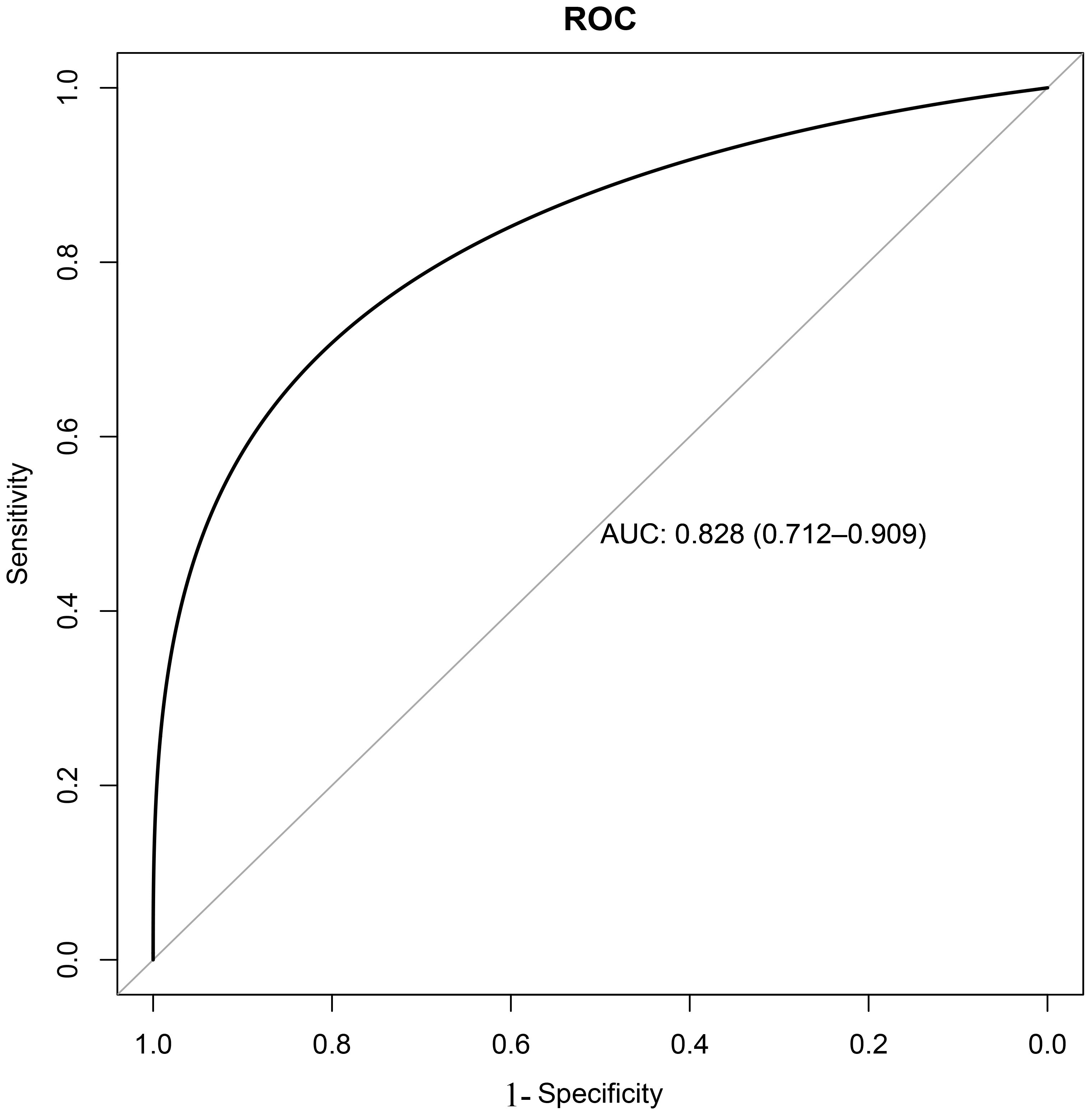
Based on the training set, the results of the clinical decision tree for pediatric pulmonary nodules were compared with the original clinical diagnosis of the nodule nature. The optimal cutoff value was 34.00%. The sensitivity, specificity, accuracy and AUC in the validation set were 0.855, 0.833, 0.810 and 0.828 (95%CI: 0.712-0.909) respectively. The diagnostic model showed significantly high sensitivity (Figure 2).
4 Discussion
This study retrospectively analyzed the clinical and radiological characteristics of pulmonary nodules in 1341 children. The detection rate of pediatric pulmonary nodules was low. Children with the history of malignancies, nodule diameter ≥5mm, and specific nodule distribution (multiple in both lungs, multiple in the right lung, solitary in the upper or middle right lobe) were important predictable factors for malignity. Clinical and radiological dynamic follow-up was important for those patients.
The etiological spectrum of pulmonary nodules in children is different from that in adults. Clarifying the causes of pulmonary nodules is a challenge in their diagnosis and treatment. Previous studies have shown that many causes were associated with pediatric pulmonary nodules, such as infectious diseases, hematological disorders, autoimmune diseases, granulomatous diseases, obliterative bronchiolitis, pulmonary nodulosis, Crohn’s disease (17–19). In our finding, the primary clinical diagnoses before examination were malignant tumors, including hepatoblastoma, neuroblastoma, nephroblastoma, osteosarcoma, leukemia, lymphoma, which was consistent with previous study (3). We found The most common non-tumor cause was infection (including respiratory infections and other systemic infectious diseases). We also revealed that most pulmonary nodules in children are incidental. Only 17.3% of all children showed respiratory symptoms such as coughing, wheezing, shortness of breath or chest pain before CT scanning. In addition, children with a history of malignant tumors were younger, showed larger nodule diameters, and presented with multiple nodules often accompanied by osteolytic lesions and mass effect, which is consistent with previous findings (7, 8, 20).
The differentiation and etiological diagnosis of benign versus malignant pulmonary nodules is crucial in clinical management. Guidelines for adult pulmonary nodules offer detailed assessment and clinical management plans for physicians. Generally, nodules<6 mm are considered as low probability of malignancy (18), and nodules>5mm are recommended for clinical follow-up, those with solid nodules should undergo annual follow-up (21). Lung biopsy is the golden standard method for defining the nature of nodules and widely used in adult (22). One study (23) found that lung biopsy results led to changes in the management of 63% of children, particularly affecting the use of steroids. But biopsy is not frequently performed in children due to its invasiveness and parents’ worries. Therefore, the clinical features and radiological presentations of children are important clue to nodule assessment. In 2015, American Society for Pediatric Radiology’s Imaging Committee published the first guide for pediatric pulmonary nodules offering individualized management suggestions based on the CT characteristics and clinical history features (24, 25), but it lacks clear follow-up guidelines and criteria for judging benignity or malignancy. Several studies show that a history of malignant tumors, nodule diameter size, distribution, and margin can help in judging the nature of the nodule (8, 9, 15, 17, 26). Similarly, our decision tree model suggested that a nodule diameter≥5mm, a history of malignancies and nodule distribution are important predictors of malignancy. Multiple nodules also have predictable effect on malignancy (27). In our findings, not only multiple lesions (in the right lung or both lungs), solitary lesions in the upper or middle right lobe were also suggestive of malignancy.
Our study has some limitations. The selection bias exists due to the single-center study. Multicenter studies are highly recommended in the future with larger sample size to reduce bias. In addition, we failed to collect lung biopsy report of nodules in these cases, this reduced the reliability of the model. Biopsy is invasive with potential complications. Pathological evidence is hard to obtain when patient shows no indication for biopsy or surgery. We believe that further studies or meta-analysis are necessary to confirm our preliminary findings in the future.
In conclusions, our study demonstrated a history of malignancies, nodule diameter ≥5cm in size and distribution were potential predictors for malignant nodules. We suggested that children with potential malignant tumors have a higher probability of malignancy in pulmonary nodules. These patients require frequent follow-up and an early pulmonary nodule diagnosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the Shanghai Children’s Medical Center, Shanghai Jiao Tong University (SCMCIRB-K2022085-2). The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
MD: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. SY: Data curation, Formal Analysis, Methodology, Writing – review & editing. HJ: Data curation, Supervision, Writing – original draft. YW: Data curation, Supervision, Writing – original draft. HM: Data curation, Supervision, Writing – original draft. SS: Data curation, Software, Supervision, Writing – original draft. JL: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Writing – review & editing. JC: Conceptualization, Methodology, Software, Supervision, Validation, Writing – review & editing. YY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study has received funding by Health and Family Planning Scientific Research Project of Pudong New Area Health Committee (PW2021E-06), Fundamental Research Funds for the Central Universities (YG2021QN109), Shanghai Municipal Health Commission Healthcare Industry Clinical Research Special Project (20224Y0180); Shanghai Municipal Science and Technology Commission ‘Yangfan Plan’ (23YF1425100); Pudong New Area Science and Technology Development Fund Livelihood Research Special Fund Healthcare Project (PKJ2023-Y50).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou Q, Fan Y, Wang Y, Qiao Y, Wang G, Huang Y, et al. [China national guideline of classification, diagnosis and treatment for lung nodules (2016 version)]. Zhongguo Fei Ai Za Zhi. (2016) 19:793–8. doi: 10.3779/j.issn.1009-3419.2016.12.12
2. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. (2008) 246:697–722. doi: 10.1148/radiol.2462070712
3. Li J, You J, Peng L, Hu J, Gao Y, Shu C. Clinical characteristics and etiological analysis of lung nodules in 98 children. J Clin Pediatr. (2017) 35:585–8. doi: 10.369/j.issn.1000-3606.201.08.006
4. Rosenfield NS, Keller MS, Markowitz RI, Touloukian R, Seashore J. CT differentiation of benign and Malignant lung nodules in children. J Pediatr Surg. (1992) 27:459–61. doi: 10.1016/0022-3468(92)90336-6
5. Barber A, Passarelli P, Dworsky ZD, Gatcliffe C, Ryu J, Lesser DJ, et al. Clinical implications of pulmonary nodules detected in children. Pediatr Pulmonol. (2021) 56(1):203–10. doi: 10.1002/ppul.25146
6. Alves GR, Marchiori E, Irion KL, Guimarães MD, da Cunha CF, de Souza VV, et al. Mediastinal lymph nodes and pulmonary nodules in children: MDCT findings in a cohort of healthy subjects. AJR Am J Roentgenol. (2015) 204:35–7. doi: 10.2214/AJR.14.12773
7. Samim A, Littooij AS, van den Heuvel-Eibrink MM, Wessels FJ, Nievelstein RAJ, de Jong PA. Frequency and characteristics of pulmonary nodules in children at computed tomography. Pediatr Radiol. (2017) 47:1751–8. doi: 10.1007/s00247-017-3946-2
8. Renne J, Linderkamp C, Wacker F, Berthold LD, Weidemann J. Prevalence and configuration of pulmonary nodules on multi-row CT in children without Malignant diseases. Eur Radiol. (2015) 25:2651–6. doi: 10.1007/s00330-015-3675-6
9. Silva CT, Amaral JG, Moineddin R, Doda W, Babyn PS. CT characteristics of lung nodules present at diagnosis of extrapulmonary Malignancy in children. AJR Am J Roentgenol. (2010) 194:772–8. doi: 10.2214/AJR.09.2490
10. Vaarwerk B, Bisogno G, McHugh K, Brisse HJ, Morosi C, Corradini N, et al. Indeterminate pulmonary nodules at diagnosis in rhabdomyosarcoma: are they clinically significant? A report from the european pediatric soft tissue sarcoma study group. J Clin Oncol. (2019) 37:723–30. doi: 10.1200/JCO.18.01535
11. Cho YJ, Kim WS, Choi YH, Ha JY, Lee S, Park SJ, et al. Computerized texture analysis of pulmonary nodules in pediatric patients with osteosarcoma: Differentiation of pulmonary metastases from non-metastatic nodules. PloS One. (2019) 14:e0211969. doi: 10.1371/journal.pone.0211969
12. MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. (2005) 237:395–400. doi: 10.1148/radiol.2372041887
13. Assefa D, Atlas AB. Management of pulmonary nodules in pediatric patients. A survey of practicing pediatric pulmonologist in the USA. Am J Respir Crit Care Med. (2014) 189:A1801.
14. Thacker PG. The incidental pulmonary nodule: An impetus for guidelines. Pediatr Radiol. (2015) 45:777. doi: 10.1007/s00247-013-2762-6
15. McCarville MB, Lederman HM, Santana VM, Daw NC, Shochat SJ, Li CS, et al. Distinguishing benign from Malignant pulmonary nodules with helical chest CT in children with Malignant solid tumors. Radiology. (2006) 239:514–20. doi: 10.1148/radiol.2392050631
16. Alpert JB, Naidich DP. Imaging of incidental findings on thoracic computed tomography. Radiol Clin North Am. (2011) 49:267–89. doi: 10.1016/j.rcl.2010.10.005
17. Breen M, Zurakowski D, Lee EY. Clinical significance of pulmonary nodules detected on abdominal CT in pediatric patients. Pediatr Radiol. (2015) 45:1753–60. doi: 10.1007/s00247-015-3407-8
18. Anderson IJ, Davis AM. Incidental pulmonary nodules detected on CT images. JAMA. (2018) 320:2260–1. doi: 10.1001/jama.2018.16336
19. Brader P, Abramson SJ, Price AP, Ishill NM, Emily ZC, Moskowitz CS, et al. Do characteristics of pulmonary nodules on computed tomography in children with known osteosarcoma help distinguish whether the nodules are Malignant or benign? J Pediatr Surg. (2011) 46:729–35. doi: 10.1016/j.jpedsurg.2010.11.027
20. Assefa D, Atlas AB. Natural history of incidental pulmonary nodules in children. Pediatr Pulmonol. (2015) 50:456–9. doi: 10.1002/ppul.23141
21. Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. (2015) 70 Suppl 2:ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168
22. Huang MD, Weng HH, Hsu SL, Hsu LS, Lin WM, Chen CW, et al. Accuracy and complications of CT-guided pulmonary core biopsy in small nodules: a single-center experience. Cancer Imaging. (2019) 19:51. doi: 10.1186/s40644-019-0240-6
23. Hafezi N, Heimberger MA, Lewellen KA, Maatman T, Montgomery GS, Markel TA. Lung biopsy in children’s interstitial and diffuse lung disease: Does it alter management? Pediatr Pulmonol. (2020) 55:1050–60. doi: 10.1002/ppul.24683
24. Westra SJ, Brody AS, Mahani MG, Guillerman RP, Hegde SV, Iyer RS, et al. The incidental pulmonary nodule in a child. Part 1: recommendations from the SPR Thoracic Imaging Committee regarding characterization, significance and follow-up. Pediatr Radiol. (2015) 45:628–33. doi: 10.1007/s00247-014-3267-7
25. Westra SJ, Thacker PG, Podberesky DJ, Lee EY, Iyer RS, Hegde SV, et al. The incidental pulmonary nodule in a child. Part 2: Commentary and suggestions for clinical management, risk communication and prevention. Pediatr Radiol. (2015) 45:634–9. doi: 10.1007/s00247-014-3269-5
26. Liang TI, Lee EY. Pediatric pulmonary nodules: imaging guidelines and recommendations. Radiol Clin North Am. (2022) 60:55–67. doi: 10.1016/j.rcl.2021.08.004
Keywords: pulmonary nodule, children, benign and malignant differentiation, diagnostic model, computed tomography
Citation: Dilimulati M, Yuan S, Jiang H, Wang Y, Ma H, Shen S, Lin J, Chen J and Yin Y (2024) Imaging features and clinical evaluation of pulmonary nodules in children. Front. Oncol. 14:1385600. doi: 10.3389/fonc.2024.1385600
Received: 13 February 2024; Accepted: 19 July 2024;
Published: 08 August 2024.
Edited by:
Lisa States, Children’s Hospital of Philadelphia, United StatesReviewed by:
Duilio Divisi, University of L’Aquila, ItalyJie Tian, Sichuan University, China
Mehmet Ali Bedirhan, Yedikule Teaching Hospital, Türkiye
Copyright © 2024 Dilimulati, Yuan, Jiang, Wang, Ma, Shen, Lin, Chen and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Yin, eWlueW9uZzk5OTlAMTYzLmNvbQ==; Jilei Lin, amlsZWlfbGluQDE2My5jb20=; Jiande Chen, MjUwNDY0NDQ3QHFxLmNvbQ==
†These authors share first authorship
 Muheremu Dilimulati
Muheremu Dilimulati Shuhua Yuan1†
Shuhua Yuan1† Hejun Jiang
Hejun Jiang Yahua Wang
Yahua Wang Jilei Lin
Jilei Lin Jiande Chen
Jiande Chen Yong Yin
Yong Yin