- 1Department of Medical Oncology, Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
- 2Department of Medical Oncology, Tata Medical Center, Kolkata, West Bengal, India
- 3Department of Surgical Oncology, Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
- 4Department of Pediatric Surgery, All India Institute of Medical Sciences, New Delhi, India
- 5Department of Orthopedics, All India Institute of Medical Sciences, New Delhi, India
Background: The medical management of DT comprises tyrosine kinase inhibitors (TKIs), hormonal agents, anti-inflammatory drugs with the recently approved gamma secretase inhibitor nirogacestat being the current standard of care. Real-world data on evolving treatment landscapes of DT remains scarce.
Methods: This is a retrospective study of patients with DT registered between 1995 and 2020 at All India Institute of Medical Sciences, New Delhi and Tata Medical Center, Kolkata. Baseline characteristics were analyzed in form of median values and interquartile range. Categorical and continuous variables were compared by chi square and independent samples T- tests respectively. Anxiety, depression and QoL were prospectively measured among 30 patients using Hospital Anxiety and Depression (HADS) and Functional Assessment of Cancer Therapy-General (FACT-G) scales respectively between 2022 to 2023.
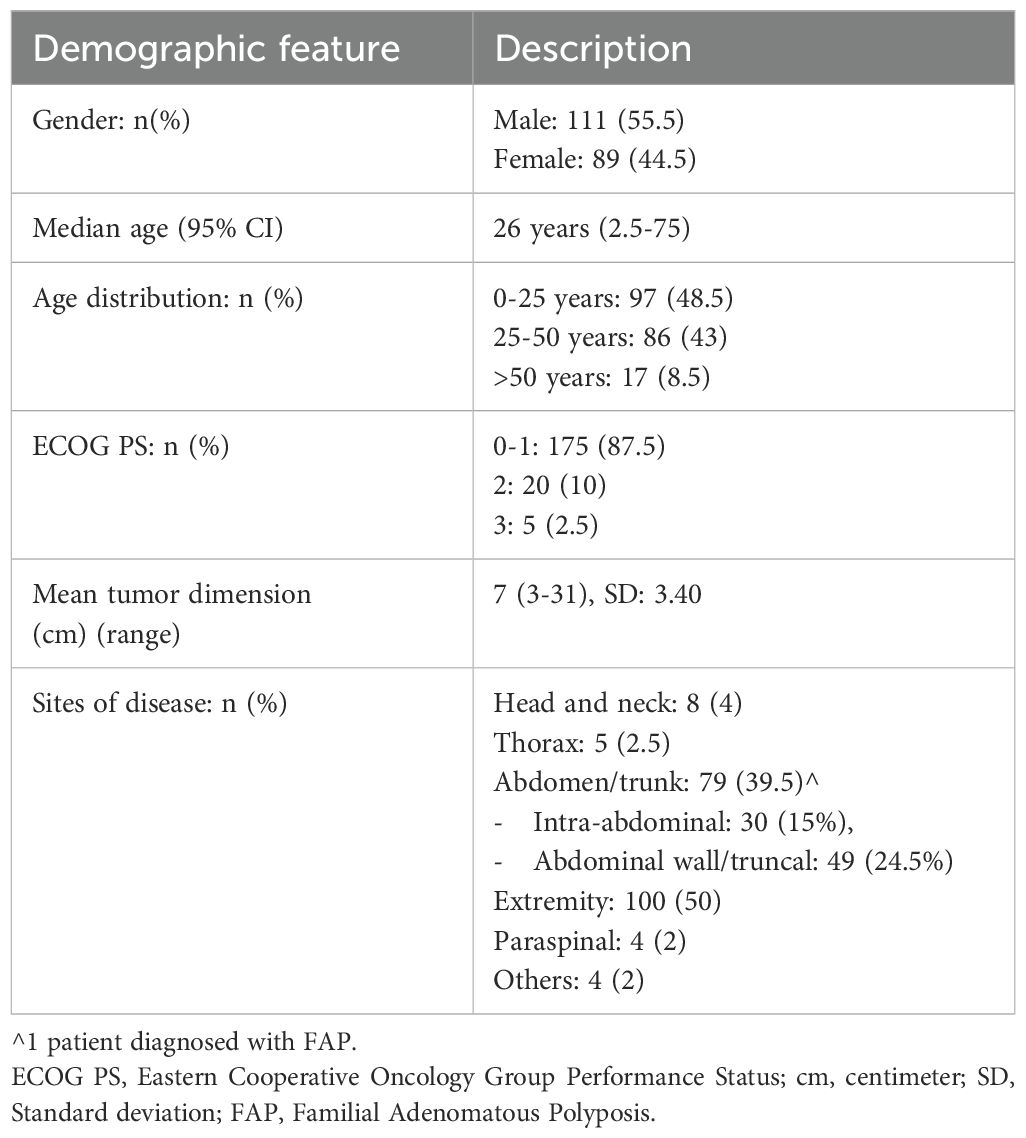
Results: 200 patients were included with a male-predominant (n=111, 55.5%) population and median age 26.5 (2.5-75) years. Extremity (n=100, 50%) and abdomen (n=65, 32.5%) were commonest primary sites and median of 2 (1–4) lines of treatment were received. First-line included surgery (n=116, 58%), systemic therapy (n=67, 33.5%), radiotherapy (10, n=5%) and active surveillance (n=7, 3.5%). First-line systemic agents included tamoxifen (n=55, 27.5%), imatinib (n=7, 3.5%), sorafenib (n=1, 0.5%) and chemotherapy (n=4, 2%). 2019 onward, 3% and 63% underwent active surveillance and surgery respectively. Best radiological response obtained with tamoxifen was stable disease (SD) (n=76, 59%) and partial response (PR) (n=31, 24.2%). Best radiological response obtained with sorafenib was PR (n=17, 60.7%) and SD (n=9, 32.1%). Thirty patients underwent HADS and FACT-G scale assessment. Mean HADS-Anxiety subscale score was 3.6 (+/-3.9 SD) and HADS-Depression sub-scale score was 2.6 (+/-3.5 SD) with clinically significant anxiety and depression in 2 (6.7%) patients each. The overall mean FACT-G score was 87.5 (+/-12.6 SD) and lower mean physical well-being (p=0.006) and emotional well-being (0.017) scores were significantly associated with higher HADS-anxiety (>/=8) scores.
Conclusions: Assessment of anxiety, depression and QoL are paramount to gauge the psychological impact of DT. This study gives an overview of clinical and management profile of patients with DT in India, with limitations of selection bias, heterogeneous population and small sample size for QoL assessment.
Background
Desmoid tumor (DT) is a locally aggressive and recurrent connective tissue annual incidence of 5-6 cases per million population (1). This is a rare disease that affects younger patients with a median age of onset at 30-40 years and arises at sites such as extremity, abdomen and abdominal wall (1, 2). The treatment trends of DT have undergone a paradigm change in recent years with chemotherapy, surgical resection and radiotherapy reserved for life or function-threatening scenarios (3). The treatment options for medical management of DT include the gamma secretase inhibitor nirogacestat and tyrosine kinase inhibitors (TKIs) (4, 5). Nirogacestat is the only drug approved by the Food and Drug Administration for the management of DT and is the standard of care (6).
Other therapeutic options are hormonal agents and nonsteroidal anti-inflammatory agents (NSAID) and oral metronomic therapy (OMT). The data for use of OMT while available in refractory solid tumors, is scarce in DT (7–9). The choice of treatment depends on the clinical presentation, location of the tumor, availability of the therapeutic option and adverse event profiles (10–12).
The unique feature of DT is that even though it is a benign disease with low mortality, the morbidity of the disease remains significant (13). Abdominal DT, especially in Familial Adenomatous Polyposis (FAP) produces significant morbidity and mortality with symptoms such as bowel obstruction and ulceration (14). Patients with DT experience significant stress and anxiety owing to the chronic nature of the disease, lack of awareness among medical practitioners regarding and the lack of support groups for DT (15). This necessitates the assessment of their HRQoL as an endpoint in addition to radiological response rates. QoL measures reflect the perspective of patients regarding their symptom burden, impact on their functioning and side effects of treatment. Few studies have explored the HRQoL of patients with DT and have revealed low global health QoL (16).
Data on the evolving treatment landscapes and QoL measures among patients with DT is scarce. Moreover, real-world data on tamoxifen, NSAIDs and OMT are deficient in published literature. We thus performed this study across two tertiary-care centers in India to evaluate the changing trends of treatment, outcomes with various modalities and QoL measured assessment among patients with DT.
Methods
We conducted a retrospective analysis of consecutive patients with histologically confirmed DT treated at the All India Institute of Medical Sciences (AIIMS), New Delhi and Tata Memorial Center, Kolkata managed between 1995 to 2020. Ethical clearance was obtained from the Institutional Review Boards of respective institutions (IEC-748/02.09.2022, RP-27/2022 and EC/WV/TMC/06/24). Diagnosis of DT made at outside centers was confirmed by the institute pathologist. Patient details including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and treatment received were included.
Treatment modalities were classified into surgery, definitive radiotherapy, TKI (including imatinib and sorafenib), tamoxifen (with or without NSAID), chemotherapy (including methotrexate-vinblastine and vincristine doxorubicin cyclophosphamide) and oral metronomic therapy. Oral metronomic therapy comprised of thalidomide 100 milligram (mg) once daily, celecoxib 200 mg twice daily, etoposide 50 mg alternate day to five days/week alternating with cyclophosphamide 50 mg alternate day to 5 days/week for three weeks, six weekly (17). Assessment by Response Evaluation Criteria in Solid Tumors (RECIST) performed, and the radiological responses given by our expert radiologists (18).
A randomly selected subset of the study population aged greater than 18 years at the time of interview was included in the study conducted for assessment of anxiety, depression and QoL at AIIMS. Participants with any cognitive or psychiatric impairment, any communication disability, lack of understanding of English or Hindi language, or unwillingness to provide informed consent were excluded from the study. Institutional review board clearance was obtained prior to commencement of the study. The prospective assessment was conducted between 2022-2023 irrespective of phase of therapy with the help of a study nurse not involved in the treatment of the participant. The interview was conducted in the hospital when the patient reported for their visit. Clinical, epidemiological and treatment details of the participants were noted. FACT-G (Functional Assessment of Cancer Therapy-General) and HADS (Hospital Anxiety and Depression Scale) questionnaires were administered in English or Hindi languages.
The FACT-G is a 27-item questionnaire consisting of the subdomains of physical well being (PWB) with 7 items (score 0-28), social/family well-being (SWB) with 7 items (score 0-28), emotional well-being (EWB) with 7 items and functional well-being (FWB) with 6 items. The answers are given as per Likert scale of 0 (“not at all”) to 4 (“very much”) leading to a possible total score of 0-108. For the responses to be acceptable, more than 80% answers overall and more than 50% in each subdomain had to be given. The scoring of each item was done as per the FACT-G scoring manual (19) and a higher score implied a higher QoL.
The HADS questionnaire is a 14-item questionnaire with 7 questions each targeting anxiety and depression. The answers are to be scored from 0 to 3 and a total score of 11-21 is considered clinically abnormal, 8-10 is borderline and 0-7 is normal (20).
Statistical analysis
The participants’ baseline clinical characteristics were presented in the form of median (inter-quartile range), mean (standard deviation) and/or frequencies. Categorical variables were compared using the chi-square test and continuous variables following normal distribution were compared using the independent samples T-test. Overall survival (OS) was defined as the duration between date of diagnosis and date of death due to any cause. Kaplan-Meier curve was constructed for depiction of overall survival analyses while Cox proportional hazards model was used to assess the association of clinical variables and survival. Associations between mean QoL and anxiety/depression scores with clinico-radiological factors were analyzed by the Chi-square test. A p-value of <0.05 was considered to be statistically significant. Statistical analysis was carried out using the SPSS software version 26.0.
Results
Demographic profile
Two hundred consecutive patients with DT registered between April 1995 and July 2020 were included in the study from both the institutes with baseline characteristics shown in Table 1.
There was male predominance (n=111, 55.5%) and median age was 26 years (range 2.5-75) at the time of diagnosis of DT. Median ECOG PS upon presentation was 1 (range 0-3). The common primary sites of disease consisted of extremity (n=100, 50%), abdomen (n=65, 32.5%), thorax/chest wall (n=5, 2.5%) and with a median symptom duration of 12 months (range 1-140) prior to presentation. Clinical and management details based on anatomic site of disease are provided in Supplementary Table S6.
Treatment modalities with time trends
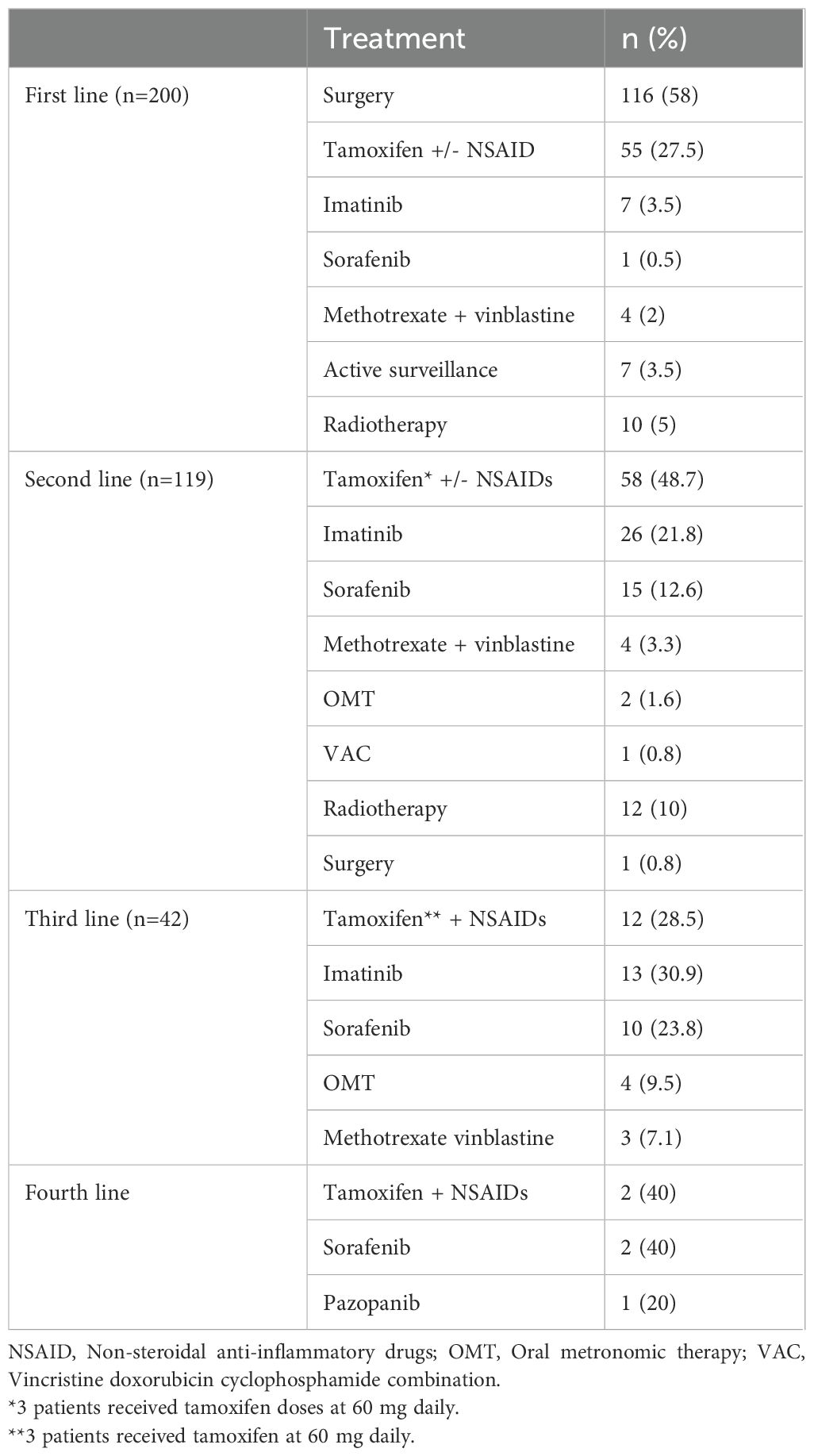
Patients received a median of two lines (range 1-4) of treatment including surgical resection, systemic therapy, radiotherapy and active surveillance. Systemic therapies (n=220) consisted of tamoxifen (n=127, 57.7%), TKI (n=75, 34%), cytotoxic chemotherapy (n=12, 5.4%) and OMT (17) (n=6, 2.7%) across lines. The first-line treatment (n=200) consisted of surgical resection (n=116, 58%), systemic therapy (n=67, 33.5%), definitive radiotherapy (10, n=5%) and active surveillance (n=7, 3.5%). The types of systemic treatment given in the first-line included tamoxifen (with and without NSAID) (n=55, 27.5%), imatinib (n=7, 3.5%), sorafenib (n=1, 0.5%) and chemotherapy (n=4, 2%). The details of all lines of treatment are provided in Table 2.
In the first line, the proportion of patients undergoing surgical resection across the years was 39% (1995–2002), 52% (2003-2010), 66% (2011-2018) and 63% (2019 onwards). Among patients who progressed after upfront surgery (n=76), 64 (84.3%) underwent medical management and 12 (15.7%) received radiotherapy in the second line. Active surveillance formed a part of first-line therapy in 6% patients in 1995-2002, 2% in 2003-2010, 3% patients each in 2011-2018 and 2019 onwards respectively. 1 (14.2%) patient progressed after active surveillance and was given second-line medical therapy (Figure 1). The use of frontline tamoxifen was undertaken in 55% (1995-2002), 24% (2003-2010), 23% (2011-2018) and 17% (2019 onwards) patients. TKIs in the first line setting were used in 4% (2011-2018) and 13% (2019 onwards) patients (Figure 2A). In the second line, tamoxifen was used in 85% (1995-2002), 70% (2003-2010), 42% (2011-2018) and 10% (2019 onward) patients. The use of TKI in second line was distributed as 0% (1995-2002), 9% (2003-2010), 38% (2011-2018) and 81% (2019 onwards) (Figure 2B).
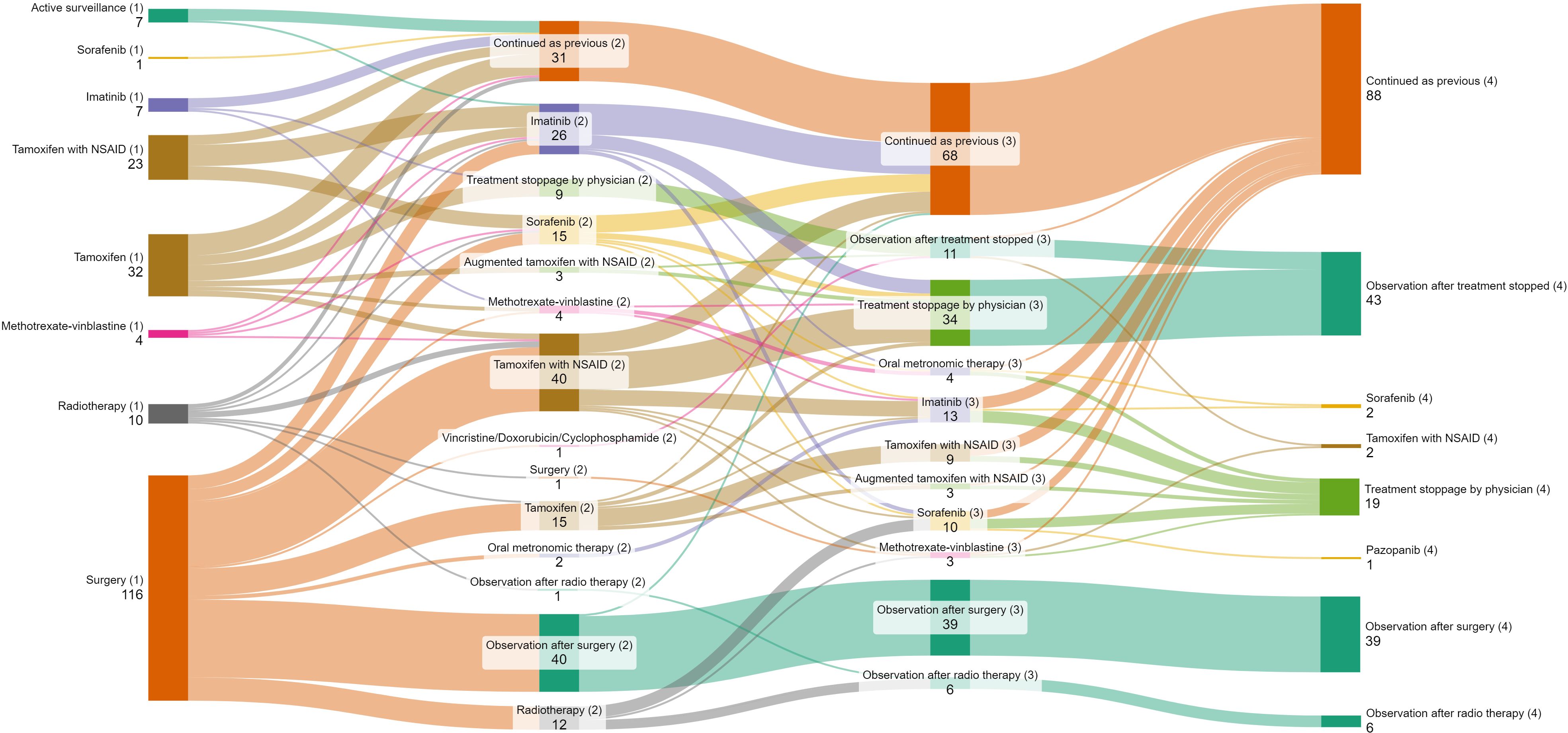
Figure 1. Sankey diagram depicting lines of treatment of patients with desmoid tumor. Bracketed numbers represent each line of therapy (1) First line (2) Second line (3) Third line (4) Fourth line; Non-bracketed numbers represent the number of patients receiving each type of therapy.
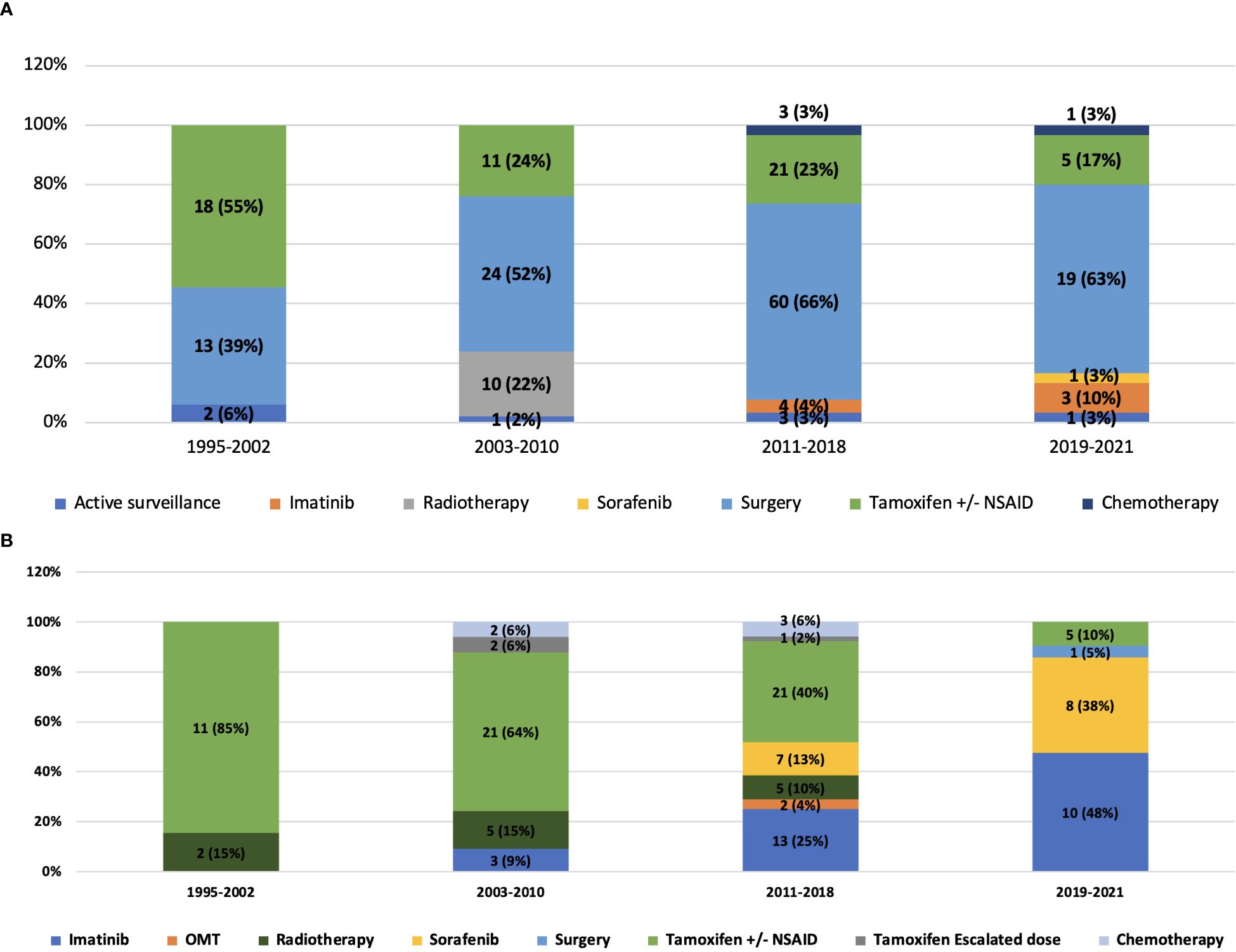
Figure 2. (A) Distribution of treatment received in the first line by the study population and its evolution across years of follow-up. (B) Distribution of treatment received in the second line by the study population and its evolution across years of follow-up.
Treatment outcomes
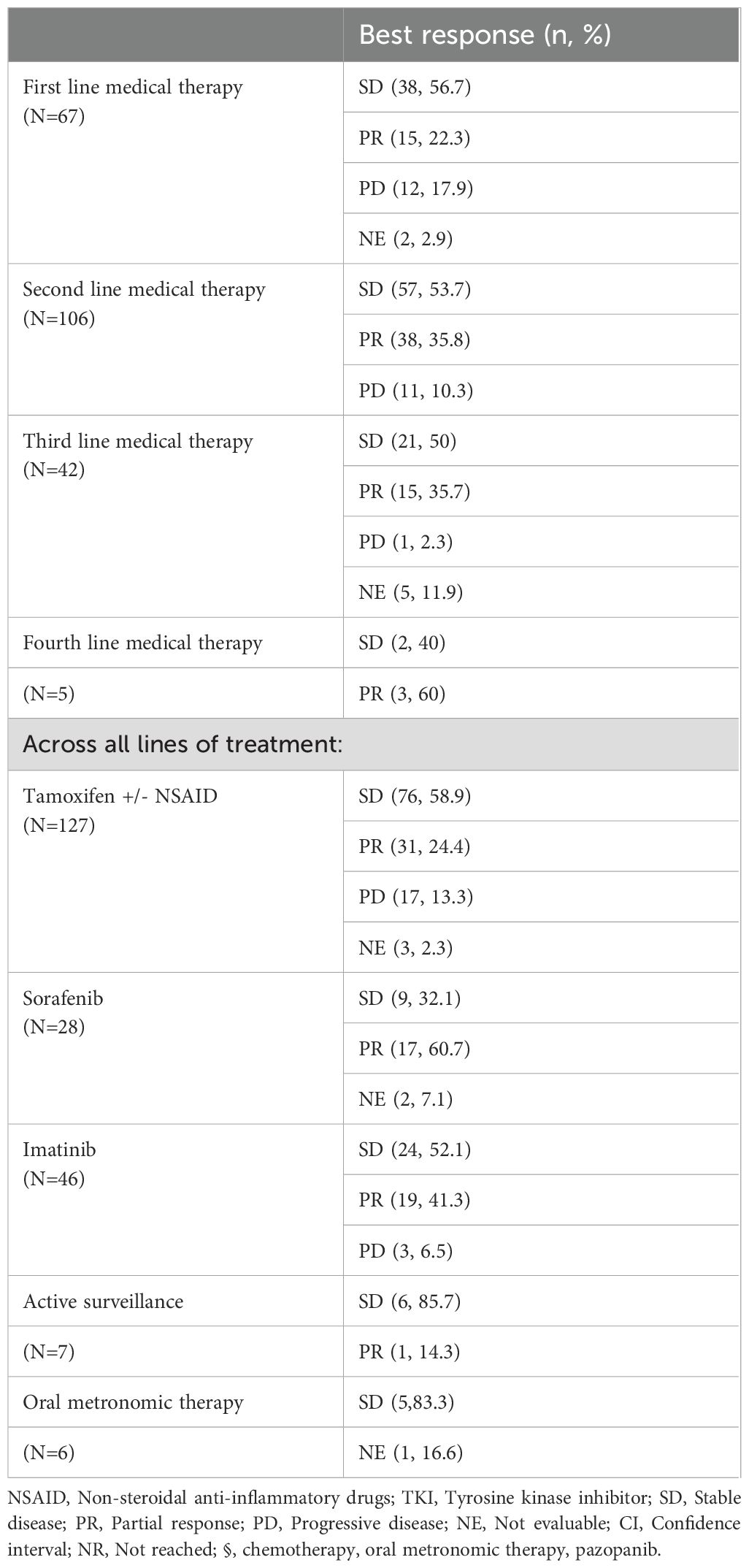
The best radiological response according to RECIST v1.1 to systemic treatments in the first line (n=67) was stable disease (38, 80.8%), partial response (n=15, 22.3%) and progressive disease (n=12, 17.9%) (Table 3). The best response with systemic therapies in the second line (n=106) was stable disease (n=57, 53.7%), partial response (n=38, 35.8%) and progressive disease (n=11, 10.3%).
Across all treatment lines, the predominant documented best response with tamoxifen was disease stabilization (n=76, 59%) followed by partial response (n=31, 24.2%). Among TKIs across lines, the best response obtained with sorafenib was partial response (n=17, 60.7%) and stable disease (n=9, 32.1%). The best response with imatinib was stable disease (n=23, 51.1%) and partial response (n=19, 41.3%).
Among the 145 patients who were given medical therapy across all line, treatment was discontinued in 61 (42%) patients following a median duration of 73 months (95% confidence interval: 3-300), and followed with observation till the data cut-off on 1st August 2023. No patients who were observed after treatment discontinuation have had disease progression. At a median follow-up of 89 months (95% confidence interval: 70.2-107.7), there were no deaths in our study population.
Assessment of anxiety and depression
Thirty patients with DT were interviewed using the HADS questionnaire. The interviewees exhibited a female predominance (19, 63.3%) and with a median time of 5 years (range: 1-9 years) from diagnosis. The median age of the patients was 21 years (18–43) at the time of diagnosis of DT. The median tumor dimension was 10 centimeters (4–31) with tumor size of greater than 10 centimeters present in 16 (53.3%) patients.
All the patients responded to more than 90% of the questions of the questionnaires. The mean HADS-Anxiety sub-scale score was 3.6 (+/- 3.9 SD) and HADS-Depression sub-scale score was 2.6 (+/- 3.5 SD) (Supplementary Table S1). The mean scores of HADS-depression and anxiety sub-scales were significantly different on univariate analysis among patients according to time from diagnosis (less than versus greater than 5 years) and number of lines of therapy (less than versus greater than 2). Factors not associated with the HADS-Depression scores were gender, age, performance status, tumor dimension (less than 10 centimeters versus greater than 10 centimeters), primary site (extremity and non-extremity), and phase of therapy (observation versus active treatment) (Supplementary Table S2). Multivariate analysis did not yield any statistically significant factors associated with HADS-Anxiety and Depression scales (Supplementary Table S3). As per the predefined scoring criteria, clinically significant and borderline anxiety was prevalent in 2 (6.7%) and 3 (10%) patients respectively. Clinically significant and borderline depression were present in 2 (6.7%) patients each.
Quality of life assessment
Thirty patients were interviewed using the FACT-G questionnaire. The median number of unanswered questions in the FACT-G questionnaire was 1 (0-3) and 28 (93.3%) patients responded to more than 90% of the questionnaire. The least answered question (n=19, 63.3% unanswered) in the questionnaire was GS7 of the social well-being subdomain about satisfaction of the participants with their sex life. The mean PWB score was 22.9 (+/- 5.8 SD), SWB score was 20.6 (+/- 2.1 SD), EWB score was 21.2 (+/- 3.9 SD) and FWB was 22.4 (+/- 5.3 SD) with the overall mean FACT-G score of 87.5 (+/- 12.6 SD).
In the Physical well-being sub-scale, the maximum responses of “quite a bit/very much” were given to the questions regarding “I have nausea” (93.3%), “I am forced to spend time in bed” (90%) and “I feel ill” (82.8%). The assessment of the Social/Family well-being yielded that patients obtained “quite a bit/very much” support from family (100%), partner (96.7%) and friends (83.3%). Sexual dissatisfaction was reported in 3 out of 11 respondents (27.3%) answering “not at all/a little bit” sexually satisfied. In the Emotional well-being parameter, patients answered “quite a bit/very much” in terms of nervousness (96.7%), worry about dying (96.7%), sadness (86.7%), losing hope of fighting against their illness (86.7%) and worry about worsening of their condition (83.3%). In the Functional well-being scale, participants answered “quite a bit/very much” to questions on enjoying the things they do for fun (93.3%), being content with their quality of life (93.3%), sleeping well (89.7%), the ability to work (63.3%), and finding their work fulfilling (51.7%).
On univariate analysis, time from diagnosis less than 5 years had significantly positive association with mean FACT-G scores compared to time from diagnosis greater than 5 years (EWB and FWB). Less than 2 lines of therapy had significant positive association compared to greater than 2 lines (PWB and EWB) while observation phase of treatment had significant positive association with mean FACT-G scores compared to active treatment (SWB) (Supplementary Table S4). Multivariate analysis did not yield any factors with statistically significant association with FACT-G scores (Supplementary Table S5). Lower mean PWB (p=0.006) and EWB (0.017) scores were significantly associated with higher HADS-anxiety (>/=8) score but not significant at the threshold of clinically significant anxiety (>/=11). No association was found between the FACT-G mean subdomain scores with HADS-depression scale.
Discussion
In this study, we describe the clinical profile and treatment outcomes of patients with DT who were managed at two referral centers of India. This cohort of patients comprised both pediatric and adult patients, with a median age that falls in the age distribution described in previous studies (1). The predominance of extremity and abdominal primary sites in our patients is also in accordance with published literature (2). The gender distribution of our study population, however, was discordant as compared to that published in Western literature (2). While DT is not a malignant condition, this observation could mirror the gender disparities associated with presentation of patients with cancer to healthcare facilities in India (21). We observed that most patients had a good performance status even though they presented to our centers after a symptom duration as high as 12 years. This can be explained by both the chronicity of the disease as well as significant delays in diagnosis due to the rarity of the disease (22). Patients with DT usually experience diagnostic lags due to non-specific symptoms, slow tempo of disease, lack of awareness among physicians as well as pathologic misdiagnoses (23).
Our study covers a population treated over almost 3 decades, allowing an assessment of the evolution of management of DT over the years. Studies have now established that an initial surgical approach does not provide benefit in terms of event-free survival and long-term disease control compared to conservative measures (24). This paradigm shift has now led to surgery having a very limited role in the management of DT as per current guidelines (10). Yet the fact that referrals from surgical disciples remain frequent in our study highlights the need for further iteration that DT is essentially a medically manageable disease. The role of radiotherapy also has been deemed limited, both in the adjuvant and definitive settings (25). The risk of radiation-induced sarcomas in these young patients is an additional factor that restricts the role of radiotherapy to scenarios where other options are exhausted. Active surveillance of asymptomatic patients irrespective of the size and location of tumor is an accepted strategy in current times (26). Due to rarity of disease and lack of awareness among practitioners in peripheral centers of developing countries, patients with DT are initially managed by general surgeons and subjected to surgical resections and then referred to specialized centers. Thus, we find that 60% of our patients had undergone upfront surgery, and that active surveillance was carried out in less than 5% patients only. Though this is a small subset, it is still notable that around 86% patients did not experience disease progression while on surveillance. Thus, active surveillance for DT should be considered as an option for eligible patients after careful assessment by connective tissue tumor experts.
Among systemic therapy options, randomized phase 3 data is available for nirogacestat (5) and sorafenib only (26). While clinical benefit in treatment of DT has been demonstrated by TKIs such as sorafenib and pazopanib, real-world data remains sparse. Variable results have also been observed with the use of low-dose conventional chemotherapy in small studies (27). In the developing world, the paradigm of medical therapies is shifting from anti-hormonal therapies to TKIs with the improved accessibility, affordability and physician awareness of newer drugs such as sorafenib.
This is reflected among our patients also, with the use of tamoxifen/NSAID combination reducing across the years both in first and second lines. Indeed, TKIs were prescribed more frequently after 2010 and comprised more than 80% of second-line systemic treatments. The high rates of disease stabilization with tamoxifen and imatinib among our patients are in concordance with previous studies (28, 29). Sorafenib exhibited overall response rates higher than described in a previous randomized controlled trial (30), but this observation has limited interpretation because only a small number of patients received the drug in our population. While the use of oral chemotherapy has been sparsely documented among DT (7, 31), we used OMT among a few pre-treated patients in this study. With disease stabilization achieved in more than 80% of them, OMT could be explored as a treatment option especially in resource-limited settings. The disease control rate (DCR) obtained with various treatment agents was similar across all groups of medical therapies such as tamoxifen (83.3%), imatinib (93.4%), sorafenib (92.8%) and OMT (83.3%). Hence, the sequencing of therapies could be based on the side effect profile and local availability of the agents.
Assessment of anxiety and depression in our patients yielded a significant association between longer times to diagnosis and number of lines of therapies received. This finding reiterates previous reports of the burden that diagnostic lags and morbidity associated with DT cause (9). The prevalence of clinically significant depression in 6.7% of the interviewed patients compared to another study from our center that found 7.8% patients with DT who fulfilled criteria of major depressive disorder (16). We found a significant impact on the QoL of the patients especially in the aspects of physical, and emotional sub-scales. A majority of patients experienced negative emotions such as nervousness, worry about death and sadness along with physical symptoms of nausea and ill-health. We notably found that anxiety, depression and QoL scores were not significantly different in patients who were on active treatment or observation at the time of interview. Similar findings have also been described in previous analyses; wherein active surveillance did not jeopardize QoL scores among patients with DT compared to those who received active therapy (32). We could also demonstrate that worse emotional and functional scores correlated with greater anxiety, however, the patient numbers are too low to establish significance. The high prevalence of nausea in the patients is an interesting observation in the physical well-being sub-scale. With the caveat of a small sample size, our findings could potentially depict higher degree of adverse events than experienced by the Western population (29). The fear of death was found in close to 97% of the patients, possibly denoting the lower health literacy among Indian patients as demonstrated in previous studies (33). Our findings denote the important concerns in patients with DT in terms of anxiety, depression and QoL deterioration. There is also a need of proper counseling regarding the prognosis and outcomes of this disease to improve their understanding about the disease. The lacunae are expected to be addressed by the recently developed DT-specific GODDESS questionnaire (34).
The limitations of our study include selection bias, single time-point measurement of QoL, anxiety and depression and heterogeneous study population with adult and pediatric patients. Radiological assessment was not carried out at pre-defined intervals and was done on the treating oncologists’ discretion. The small sample size assessed for anxiety, depression and QoL limits the interpretation of the observations, and a larger subset would be desirable for better assessment. Toxicity profile of various treatment agents could not be adequately recorded due to the retrospective nature of the study. However, this is the largest study of its kind to give an overview of the clinical and therapeutic profile of patients with DT. Long follow-up and description of treatment paradigms especially from the developing world are other strengths of our study.
Conclusions
This study gives a detailed profile of patients with DT managed at two referral centers in India. The evolving treatment patterns with increasing use of TKIs in recent years compared to hormonal agents was demonstrated. There is a limited role of conventional chemotherapy, surgery and radiotherapy in the current era, and active surveillance is an acceptable strategy in selected patients. Though in this real-world study, all types of medical treatments produced similar DCR, the choice of therapy depends on multiple disease and patient-related factors. Systemic treatment options of choice in the current era include sorafenib and nirogacestat, depending on availability. In developing countries, other medical treatments could serve as alternatives as our study demonstrates. The morbidity in patients with DT can be assessed by QoL and scales for anxiety and depression, which can serve as an endpoint for response to treatment. Being a rare disease, a robust referral system should be established to provide patients with DT specialized expertise care for their treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institute Ethics Committee, All India Institute of Medical Sciences, New Delhi. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
GT: Data curation, Writing – original draft, Writing – review & editing. AS: Conceptualization, Investigation, Writing – review & editing, Writing – original draft. BB: Conceptualization, Writing – original draft, Writing – review & editing. SS: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. SR: Conceptualization, Resources, Writing – original draft, Writing – review & editing. SD: Conceptualization, Resources, Writing – original draft, Writing – review & editing. SA: Resources, Writing – original draft, Writing – review & editing. SK: Conceptualization, Resources, Writing – original draft, Writing – review & editing. SB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. DP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1382856/full#supplementary-material
References
1. Kasper B, Ströbel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. (2011) 16:682–93. doi: 10.1634/theoncologist.2010-0281
2. Moorthy GD, Amini B, Nikolaidis P, Assing M, Vikram R. Current update on desmoid fibromatosis. J Comput Assist Tomogr. (2019) 43:29–38. doi: 10.1097/RCT.0000000000000790
3. Riedel RF, Agulnik M. Evolving strategies for management of desmoid tumor. Cancer. (2022) 128:3027–40. doi: 10.1002/cncr.v128.16
4. Kasper B, Raut CP, Gronchi A. Desmoid tumors: To treat or not to treat, That is the question. Cancer. (2020) 126:5213–21. doi: 10.1002/cncr.v126.24
5. Gounder M, Ratan R, Alcindor T, Schöffski P, van der Graaf WT, Wilky BA, et al. Nirogacestat, a γ-secretase inhibitor for desmoid tumors. New Engl J Med. (2023) 388:898–912. doi: 10.1056/NEJMoa2210140
6. Research C for DE and FDA approves nirogacestat for desmoid tumors. FDA (2023). Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nirogacestat-desmoid-tumors.
7. Bahl A, Bakhshi S. Metronomic chemotherapy in progressive pediatric Malignancies: old drugs in new package. Indian J Pediatr. (2012) 79:1617–22. doi: 10.1007/s12098-012-0759-z
8. Pramanik R, Agarwala S, Gupta YK, Thulkar S, Vishnubhatla S, Batra A, et al. Metronomic chemotherapy vs best supportive care in progressive pediatric solid Malignant tumors: A randomized clinical trial. JAMA Oncol. (2017) 3:1222–7. doi: 10.1001/jamaoncol.2017.0324
9. Pramanik R, Bakhshi S. Metronomic therapy in pediatric oncology: A snapshot. Pediatr Blood Cancer. (2019) 66:e27811. doi: 10.1002/pbc.27811
10. Alman B, Attia S, Baumgarten C, Benson C, Blay JY, Bonvalot S, et al. The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer. (2020) 127:96–107. doi: 10.1016/j.ejca.2019.11.013
11. Spolverato G, Capelli G, Kasper B, Gounder M. Management of desmoid tumors. Surg Oncol Clinics North America. (2022) 31:447–58. doi: 10.1016/j.soc.2022.03.008
12. Kasper B, Baldini EH, Bonvalot S, Desmoid Tumor Working Grp, Callegaro D, Cardona K, et al. Current management of desmoid tumors: A review. JAMA Oncol. (2024) 10:1121–8. doi: 10.1001/jamaoncol.2024.1805
13. Penel N, Chibon F, Salas S. Adult desmoid tumors: biology, management and ongoing trials. Curr Opin Oncol. (2017) 29:268–74. doi: 10.1097/CCO.0000000000000374
14. Desurmont T, Lefèvre JH, Shields C, Colas C, Tiret E, Parc Y. Desmoid tumour in familial adenomatous polyposis patients: responses to treatments. Fam Cancer. (2015) 14:31–9. doi: 10.1007/s10689-014-9760-1
15. Ingley KM, Klein R, Theobalds N, Burtenshaw S, Abdul Razak AR, Chen B, et al. High prevalence of persistent emotional distress in desmoid tumor. Psychooncology. (2020) 29:311–20. doi: 10.1002/pon.v29.2
16. Garg V, Rastogi S, Kalra K, Bhoriwal S, Barwad A, Dhamija E, et al. Health-related quality of life (HRQoL), anxiety, and depression in patients with desmoid type fibromatosis. Support Care Cancer. (2022) 30:10089–98. doi: 10.1007/s00520-022-07445-0
17. Sharma A, Kataria B, Biswas B, Bakhshi S, Pushpam D. Oral metronomic chemotherapy is a cost effective alternative to pazopanib in advanced soft tissue sarcoma. J Oncol Pharm Pract. (2022) 28:560–8. doi: 10.1177/10781552211000113
18. Eisenhauer EA, Therasse P, Bogaerts J, LH S, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
19. Interpretation. Available from: https://www.facit.org/interpretation (accessed October 11, 2024).
20. Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. (2003) 1:29. doi: 10.1186/1477-7525-1-29
21. Bhatia KP, Ganguly S, Sasi A, Kumar V, Deo S, Agarwala S, et al. Sex disparity in childhood cancer in India: a multi-centre, individual patient data analysis. Lancet Oncol. 24(1):54–63. doi: 10.1016/S1470-2045(22)00688-X
22. Husson O, Younger E, Dunlop A, Dean L, Strauss DC, Benson C, et al. Desmoid fibromatosis through the patients’ eyes: time to change the focus and organisation of care? Support Care Cancer. (2019) 27(3):965–80. doi: 10.1007/s00520-018-4386-8
23. Penel N, Coindre JM, Bonvalot S, Italiano A, Neuville A, Le Cesne A, et al. Management of desmoid tumours: A nationwide survey of labelled reference centre networks in France. Eur J Cancer. (2016) 58:90–6. doi: 10.1016/j.ejca.2016.02.008
24. Fernandez MM, Bell T, Tumminello B, Khan S, Zhou S, Oton AB. Disease and economic burden of surgery in desmoid tumors: a review. Expert Rev Pharmacoecon Outcomes Res. (2023) 23:607–18. doi: 10.1080/14737167.2023.2203915
25. Figueredo C, Schiano T. A review of the clinical presentation, outcomes, and treatments of patients having desmoid tumors. Gastro Hep Adv. (2023) 2:588–600. doi: 10.1016/j.gastha.2023.01.010
26. Timbergen MJM, Schut ARW, Grünhagen DJ, Sleijfer S, Verhoef C. Active surveillance in desmoid-type fibromatosis: A systematic literature review. Eur J Cancer. (2020) 137:18–29. doi: 10.1016/j.ejca.2020.06.022
27. Skapek SX, Ferguson WS, Granowetter L, Devidas M, Perez-Atayde AR, Dehner LP, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol. (2007) 25:501–6. doi: 10.1200/JCO.2006.08.2966
28. Skapek SX, Anderson JR, Hill DA, Henry D, Spunt SL, Meyer W, et al. Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children’s Oncology Group (COG) phase II study. Pediatr Blood Cancer. (2013) 60:1108–12. doi: 10.1002/pbc.24457
29. Chugh R, Wathen JK, Patel SR, Maki RG, Meyers PA, Schuetze SM, et al. Efficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res. (2010) 16:4884–91. doi: 10.1158/1078-0432.CCR-10-1177
30. Gounder MM, Mahoney MR, Van Tine BA, Ravi V, Attia S, Deshpande HA, et al. Sorafenib for advanced and refractory desmoid tumors. New Engl J Med. (2018) 379:2417–28. doi: 10.1056/NEJMoa1805052
31. Mir O, Rahal C, Rimareix F, Adam J, Terrier P, Domont J, et al. Efficacy of oral vinorelbine in advanced/progressive desmoid tumours: An updated retrospective study in 50 patients. J Clin Oncol. (2016) 34:11050–0. doi: 10.1200/JCO.2016.34.15_suppl.11050
32. Duhil de Bénazé G, Vigan M, Corradini N, Minard-Colin V, Marie-Cardine A, Verite C, et al. Functional analysis of young patients with desmoid-type fibromatosis: Initial surveillance does not jeopardize long term quality of life. Eur J Surg Oncol. (2020) 46:1294–300. doi: 10.1016/j.ejso.2020.02.028
33. Harding R, Salins N, Sharan K, Ekstrand ML. Health literacy in communication, decision-making and outcomes among cancer patients, their families and clinicians in India: A multicentre cross-sectional qualitative study. Psychooncology. (2022) 31:532–40. doi: 10.1002/pon.v31.3
Keywords: Desmoid tumor, tyrosine kinase inhibitors, oral metronomic therapy, quality of life, rare diseases
Citation: Tansir G, Sharma A, Biswas B, Sah SN, Roy S, Deo SVS, Agarwala S, Khan SA, Bakhshi S and Pushpam D (2024) A real-world study on the clinicopathological profile, treatment outcomes and health-related quality of life, anxiety and depression among patients with desmoid tumor at two tertiary care centers in India. Front. Oncol. 14:1382856. doi: 10.3389/fonc.2024.1382856
Received: 06 February 2024; Accepted: 01 October 2024;
Published: 21 October 2024.
Edited by:
Dorit Di Gioia, LMU Munich University Hospital, GermanyReviewed by:
Naonori Kawakubo, Kyushu University, JapanBruce Brockstein, NorthShore University HealthSystem, United States
Copyright © 2024 Tansir, Sharma, Biswas, Sah, Roy, Deo, Agarwala, Khan, Bakhshi and Pushpam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deepam Pushpam, ZGVlcGFtcHVzaHBhbUBnbWFpbC5jb20=
 Ghazal Tansir
Ghazal Tansir Aparna Sharma
Aparna Sharma Bivas Biswas2
Bivas Biswas2 Shah Alam Khan
Shah Alam Khan Sameer Bakhshi
Sameer Bakhshi Deepam Pushpam
Deepam Pushpam