
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 May 2024
Sec. Cancer Epidemiology and Prevention
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1382197
This article is part of the Research Topic Advancements in Multimodal Data Analysis for Lung Tumor Diagnosis View all 12 articles
 Huijuan Mu1,2†
Huijuan Mu1,2† Xing Yang1,3†
Xing Yang1,3† Yanxia Li2
Yanxia Li2 Bingzheng Zhou3,4
Bingzheng Zhou3,4 Li Liu1,2
Li Liu1,2 Minmin Zhang1,3
Minmin Zhang1,3 Qihao Wang1,3
Qihao Wang1,3 Qian Chen1,3
Qian Chen1,3 Lingjun Yan1,3
Lingjun Yan1,3 Wei Sun1,3
Wei Sun1,3 Guowei Pan1,3*
Guowei Pan1,3*Background: No studies in China have assessed the guideline-concordance level of the first-course of non-small cell lung cancer (NSCLC) diagnosis and treatment and its relationship with survival. This study comprehensively assesses the current status of guideline-concordant diagnosis (GCD) and guideline-concordant treatment (GCT) of NSCLC in China and explores its impact on survival.
Methods: First course diagnosis and treatment data for NSCLC patients in Liaoning, China in 2017 and 2018 (n=1828) were used and classified by whether they underwent GCD and GCT according to Chinese Society of Clinical Oncology (CSCO) guidelines. Pearson’s chi-squared test was used to determine unadjusted associations between categorical variables of interest. Logistic models were constructed to identify variables associated with GCD and GCT. Kaplan–Meier analysis and log-rank tests were used to estimate and compare 3-year survival rates. Multivariate Cox proportional risk models were constructed to assess the risk of cancer mortality associated with guideline-concordant diagnosis and treatment.
Results: Of the 1828 patients we studied, 48.1% underwent GCD, and 70.1% underwent GCT. The proportions of patients who underwent both GCD and GCT, GCD alone, GCT alone and neither GCD nor GCT were 36.7%, 11.4%, 33.5% and 18.4%, respectively. Patients in advanced stage and non-oncology hospitals were significantly less likely to undergo GCD and GCT. Compared with those who underwent neither GCD nor GCT, patients who underwent both GCD and GCT, GCD alone and GCT alone had 35.2%, 26.7% and 35.7% higher 3-year survival rates; the adjusted lung cancer mortality risk significantly decreased by 29% (adjusted hazard ratio[aHR], 0.71; 95% CI, 0.53–0.95), 29% (aHR, 0.71; 95% CI, 0.50–1.00) and 32% (aHR, 0.68; 95% CI, 0.51–0.90).
Conclusion: The 3-year risk of death is expected to be reduced by 29% if patients with NSCLC undergo both GCD and GCT. There is a need to establish an oncology diagnosis and treatment data management platform in China to monitor, evaluate, and promote the use of clinical practice guidelines in healthcare settings.
Lung cancer is one of the most common malignant tumors. In 2020, there were approximately 2.207 million new lung cancer patients worldwide, ranking second in new malignant tumors, and 1.796 million new lung cancer deaths, ranking first among all malignant tumors (1). At the same time, the survival rate of lung cancer worldwide is low, with a 5-year survival rate of less than 30% (2). In addition to the biological factors that affect the prognosis of lung cancer (such as age, sex, disease stage, etc.) (3–5), the diagnosis and treatment of lung cancer is also critical (6–9).
During recent decades, authoritative clinical practice guidelines for the diagnosis and treatment of lung cancer, including those from the American College of Chest Physicians (ACCP) (10), American Society for Clinical Oncology (ASCO) (11), National Cancer Institute (NCI) (12), European Society for Medical Oncology (ESMO) (13), and other academic societies, have been adopted globally for lung cancer diagnosis and treatment. The clinical practice guidelines compile existing evidence and expert consensus and can be viewed as the established basis for guideline-concordant care (GCC) for lung cancer (14). Studies of clinical practice patterns in the United States have documented differences in lung cancer management in terms of age, race, education, comorbidities, insurance and type of hospital (15–22). In particular, some studies based on Surveillance, Epidemiology and End Results (SEER) and the National Cancer Data-base (NCDB) have demonstrated significant improvements in survival outcomes for patients with lung cancer undergoing GCC (23, 24).
Nadpara et al. reported that 44.7% of old lung cancer patients in the SEER-Medicare database (2002–2007) underwent GCC, and the three-year median survival time was significantly longer for patients undergoing GCC (747 days) than non-GCC patients (260 days) (23). A study specifically investigating the association between GCC and overall survival in patients with locally advanced non-small cell lung cancer (NSCLC) found that 23% of patients underwent GCC, that socioeconomic factors, including lack of insurance and geographic location, were associated with non-GCC, that patient- and disease-specific factors, including advanced adenocarcinoma histology and gender, were also associated with non-GCC, and that non-GCC patients had higher mortality rates than GCC patients (hazard ratio [HR], 1.42) (24). However, it is unclear whether these results are broadly generalizable given the limited comparability of existing studies, which typically examine only specific subgroups of clinical cases, define guideline-concordant lung cancer care based primarily on treatment undergone, and fail to capture the appropriateness of the lung cancer diagnostic process prior to undergoing treatment (23–25).
In China, the number of new cases of lung cancer in 2018 was 781,000 (85% of which were NSCLC), the highest in the world, but the level of survival is in the low, with a 3-year survival rate of only 19.8% (26, 27). Although many oncology diagnostic and treatment guidelines and related documents have been published in China in recent years, and the “Three-year Action Plan for Cancer Prevention and Treatment in China (2015–2017)” issued in 2016 emphasized the standardization of oncology diagnosis and treatment practices (28), there is no authoritative database similar to SEER containing information on the first course of diagnosis and treatment of tumors and survival, the level of guideline-concordant diagnosis and treatment of NSCLC has not been fully evaluated, and the associated health outcomes are unclear. Liaoning Province, located in northeastern China, is an important industrial area with a high incidence of lung cancer (29).
Therefore, in this study, we used data from patients with NSCLC in Liaoning Province with a similar structure to SEER, with the following study objectives:
1. To assess the level of guideline concordance, distribution characteristics and non-concordance issues for the first course of diagnosis and treatment;
2. To assess the impact of guideline-concordant diagnosis (GCD) and guideline-concordant treatment (GCT) on survival, including GCD alone, GCT alone and both GCD and GCT.
This study was conducted using multi-stage cluster sampling to select 2756 patients with lung cancer from 20 hospitals in Liaoning Province who were diagnosed and treated with their first course between January 1, 2017 and December 31, 2018. We define the first course of diagnosis and treatment as the first diagnosis and cancer-oriented treatment administered within four months of diagnosis (30). Patients with non-small cell carcinoma by pathological type were selected; patients with other organ insufficiency, patients with other malignant tumors, and patients who gave up treatment were excluded. Based on the inclusion and exclusion criteria, we excluded 161 cases of SCLC, 123 cases with other combined tumors, 95 cases that did not receive any treatment, and 549 cases that were not the first course of diagnosis and treatment, resulting in the final selection of 1,828 patients with NSCLC (Figure 1).
First course diagnosis and treatment monitoring data: to ensure comparability with the general population in the United States, this study took SEER dataset monitoring and evaluation system as the main framework, combined with the latest and authoritative diagnosis and treatment guidelines for lung cancer in China, in which the monitoring and evaluation plan for lung cancer is formulated. This project invited experts in the diagnosis and treatment of lung cancer from surgery, internal medicine, pathology and other specialties to set up a lung cancer expert group to determine and evaluate the guideline consistency questionnaire for the first course of diagnosis and treatment. The questionnaire included 22 diagnostic indicators, 16 surgical indicators, 38 radiotherapy and chemotherapy indicators, 19 targeted therapy indicators and other indicators. It was completed by physicians and continuously validated for data quality and accuracy through internal monitoring, data quality reviews, and on-site surveys.
Survival outcome follow-up surveys: passive follow-up (Cancer Death Registration System of Liaoning Provincial Center for Disease Control and Prevention) and active follow-up (telephone follow-up) were combined to investigate the three-year survival outcomes of patients with lung cancer; the last follow-up time was December 31, 2021. Of the 1828 patients included in the analysis, 149 patients were lost due to moving out of the area, for a loss rate of 8.15% in this study.
According to evidence-based medicine and in combination with the country’s national conditions, the Chinese Society of Clinical Oncology (CSCO) first issued the “Guidelines for the diagnosis and treatment of primary lung cancer” in 2016 (31). This study refers to the “Guidelines for the diagnosis and treatment of primary lung cancer” of the Chinese Society of Clinical Oncology (2016 edition) and the updated content of the 2017 edition, combined with expert opinions to define guideline-concordant diagnosis and treatment (Table 1).
GCD: defined as correct clinical TNM staging and pathotyping prior to treatment, and molecular pathology testing in advanced stages. Clinical staging is completed according to the 8th edition of the AJCC TNM staging system, T (primary tumor staging: T0, T1s, T1a-T4), N (regional lymph node staging: N0-N2), M (distant metastasis staging: Mx-M1c), and then divided into I A, I B, II A, II B, III A, III B, III C, IV A, IV B (32). To clarify the small-cell lung carcinoma (SCLC) and NSCLC, and to clarify the squamous and adenocarcinoma in NSCLC. After histologic diagnosis, sufficient tissue should be preserved for molecular testing, and treatment should be guided according to molecular typing.
GCT: after accurate staging by a panel of experts based on tumor size, lymph node metastasis and information on distant metastasis, the first course of treatment that patients should undergo at each stage was determined according to CSCO guidelines:
Stage I: Although recent studies have pointed out that sublobar resection is not inferior to lobectomy in terms of disease-free survival (33), the current clinical guidelines in China still recommend anatomic lobectomy as the main minimal treatment for stage I; Stereotactic Body Radiotherapy (SBRT) is an effective, low-division, non-invasive ablative treatment that delivers high doses of radiation only to specific targets and has a high rate of tumor control, well tolerated by normal tissues, and is the basic treatment strategy for patients who are not suitable for surgery (34, 35). Therefore, both lobectomy and SBRT are considered to be GCT for stage I NSCLC. SBRT is defined as chest radiation therapy with a total radiation dose of 45 grays or more delivered in fractions of 5 or less.
Stage II: the standard of care for stage II NSCLC is surgical resection combined with chemotherapy, if the patient is unable to tolerate surgery, the minimum recommendation is radiotherapy, possibly with adjuvant chemotherapy.
Stage III: stage III NSCLC is a very heterogeneous group of diseases and the minimum recommended treatment depends on operability, if surgery is possible, the minimum recommendation is surgery combined with chemotherapy; for the majority of patients who cannot undergo surgery, the minimum recommendation is a combination of radiotherapy and chemotherapy.
Stage IV: for driver gene-positive advanced NSCLC, the recommended treatment is targeted therapy, and for patients whose genotype cannot be specified for various reasons the minimum recommended treatment is conventional chemotherapy.
We used clinical stage at diagnosis for creating clinical subgroups, because pathological stage can only be known after the outcome of interest (initial treatment) has occurred. We assessed the proportion of GCD and GCT for each clinical subgroup, and then intersected diagnosis and treatment to assess the proportion of both GCD and GCT, GCD alone, GCT alone and neither GCD or GCT. Pearson’s chi-squared test was used to determine unadjusted associations between categorical variables of interest. The logistic regression model was used to predict GCD and GCT status, and propensity scores for sex, age, pathological type, stage, area, hospital type, etc. were calculated. Kaplan–Meier analysis and log-rank tests were used to estimate and compare 3-year survival rates. Hazard ratios (HRs) were calculated using Cox proportional hazard modeling. All analyses were performed using SPSS 25.0; the threshold for statistical significance for all tests was 0.05.
Table 2 shows the distribution of clinical and sociodemographic characteristics of these patients. There are slightly more women than men (55.0% vs. 45.0%), and there are roughly equal numbers of patients younger than 60 and older than 60. The pathological type of most tumors was lung adenocarcinoma (LUAD) (77.9%), followed by lung squamous cell carcinoma (LUSC) (12.8%); large cell carcinoma, atypical carcinoma and adenosquamous carcinoma were combined with others (9.3%), and more than half of the patients were in stage I (63.7%). Most patients were from urban areas (62.0%), only 18.5% of patients presented at specialist oncology hospitals.
In the study population, less than half of the patients (48.1%) underwent GCD, but most patients (70.1%) underwent GCT. Pathological type, stage, city type, and hospital type were associated with undergoing GCD (p < 0.001); sex, pathology type, stage and hospital type were associated with undergoing GCT (p < 0.001). In addition, the proportions of patients who underwent both GCD and GCT, GCD alone, GCT alone, and neither GCD nor GCT were 36.7%, 11.4%, 33.5%, and 18.4%, respectively, and there were statistically significant differences for sex, pathology type, stage and hospital type. However, there was no difference in the distribution of patients who lost follow-up in the four groups (Table 3).
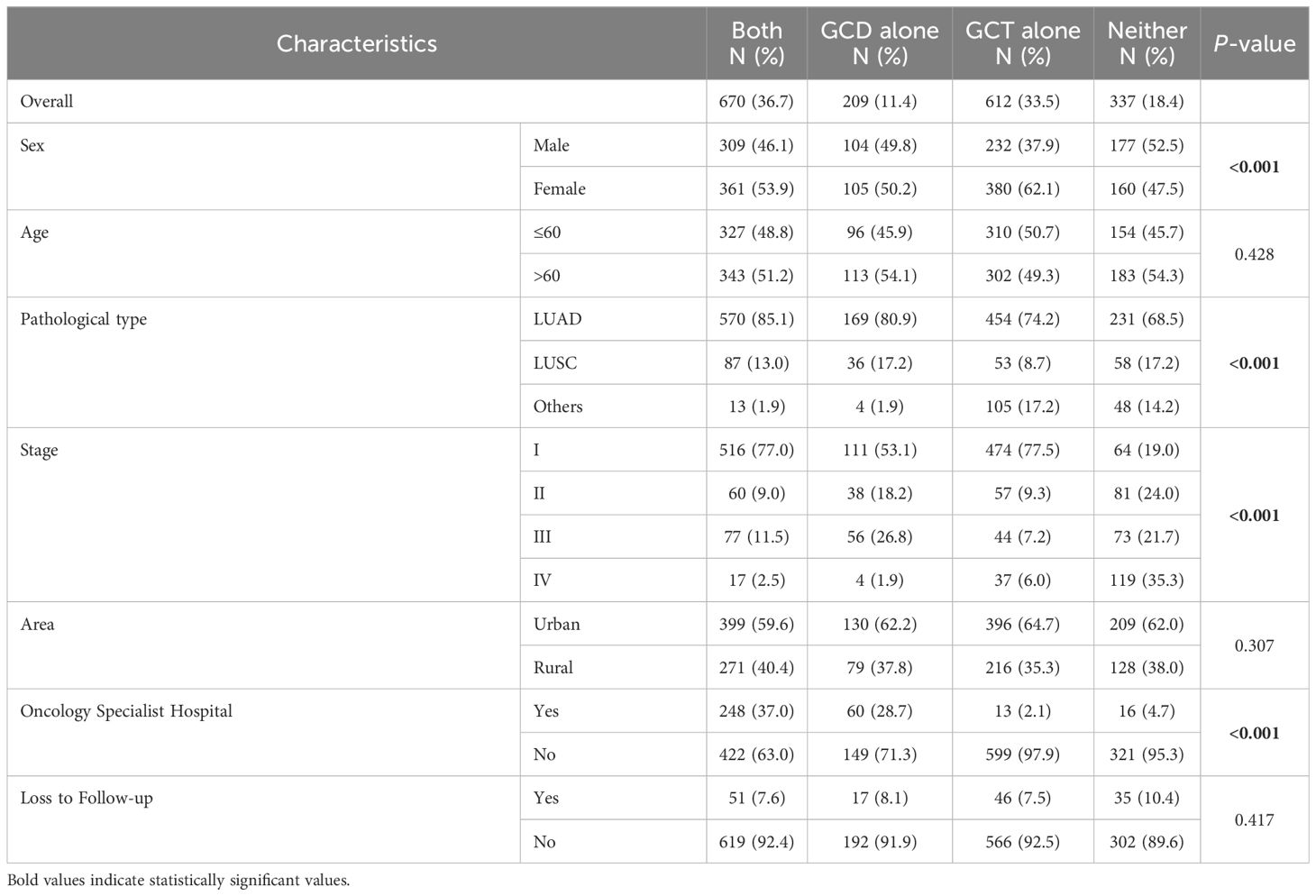
Table 3 Descriptive characteristics of lung cancer cases by both GCD and GCT, GCD alone, GCT alone, or neither.
The results of the unordered multicategorical logistic regression analysis showed that the factors influencing the diagnosis and treatment patterns were pathology type, stage, and hospital type. Taking “Neither” as a control, LUAD was associated with “Both” (adjusted odds ratio [aOR], 14.54; 95% CI, 6.79–31.12) and “GCD alone” (aOR, 16.29; 95% CI, 5.30–50.08), similarly, LUSC was associated with “Both” (aOR, 12.91; 95% CI, 5.55–30.02) and “GCD alone” (aOR, 13.58; 95% CI, 4.13–44.74). Stages I was associated with “Both” (aOR, 39.13; 95% CI, 10.35–68.42), “GCD alone” (aOR, 34.17; 95% CI, 18.72–97.05) and “GCT alone” (aOR, 22.86; 95% CI, 14.46–36.15), Stages II was associated with “Both” (aOR, 6.75; 95% CI, 3.27–13.92), “GCD alone” (aOR, 17.91; 95% CI, 5.88–54.58) and “GCT alone” (aOR, 2.24; 95% CI, 1.36–3.72), Stages III was associated with “Both” (aOR, 10.04; 95% CI, 4.90–20.58), “GCD alone” (aOR, 30.60; 95% CI, 10.14–92.32) and “GCT alone” (aOR, 1.99; 95% CI, 1.16–3.39). In addition, oncology specialist hospital was associated with “Both” (aOR, 26.95; 95% CI, 14.20–51.57) and “GCD alone” (aOR, 15.84; 95% CI, 8.00–31.35) (Table 4).
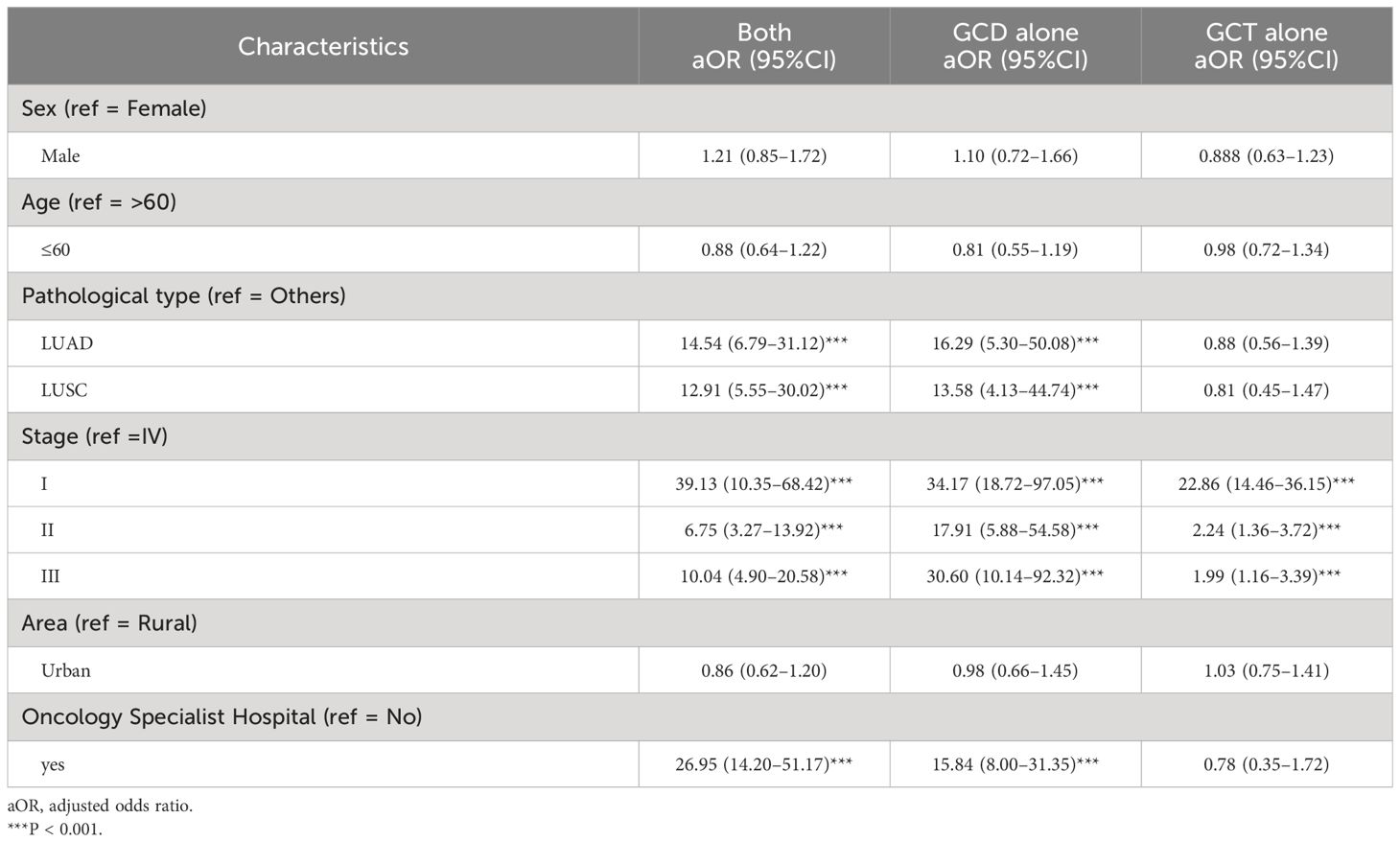
Table 4 Multifactorial logistic regression of factors influencing acceptance of both GCD and GCT, GCD alone, and GCT alone (compare with Neither).
As shown in Table 5, inappropriate staging was the most common non-GCD in stages I–III, accounting for 44.1%, 56.4% and 40.4%, while in stage IV, lack of molecular pathological testing was the most common non-GCD (79.0%). Sublobectomy was the most common non-GCT in stage I, accounting for 96.6%, and the most common non-GCT in stage II and III was surgery only, accounting for 95.8% and 60.5%; however, in advanced NSCLC, non-targeted therapy with unknown driver genes was the most common non-GCT, accounting for 69.1%.
Survival was greatest in patients who underwent GCT alone (3-year overall survival, 79.2%; 95% CI, 76.0%–82.5%), followed by patients who underwent both GCD and GCT (3-year overall survival, 78.8%; 95% CI, 75.7%–81.9%), and patients who underwent GCD alone (3-year overall survival, 70.3%; 95% CI, 64.1%–76.6%), and worst in those who underwent neither (3-year overall survival, 43.6%; 95% CI, 38.3%–48.9%) (Table 6, Figure 2). Three-year survival analysis for each clinical stage showed that for stage I patients, survival was significantly higher for those who underwent both GCD and GCT than for the other patterns of care(3-year survival, 87.4%; 95% CI, 84.5%–90.3%), but this difference in survival was not significant in other stages of NSCLC patients (Table 6, Figure 3).
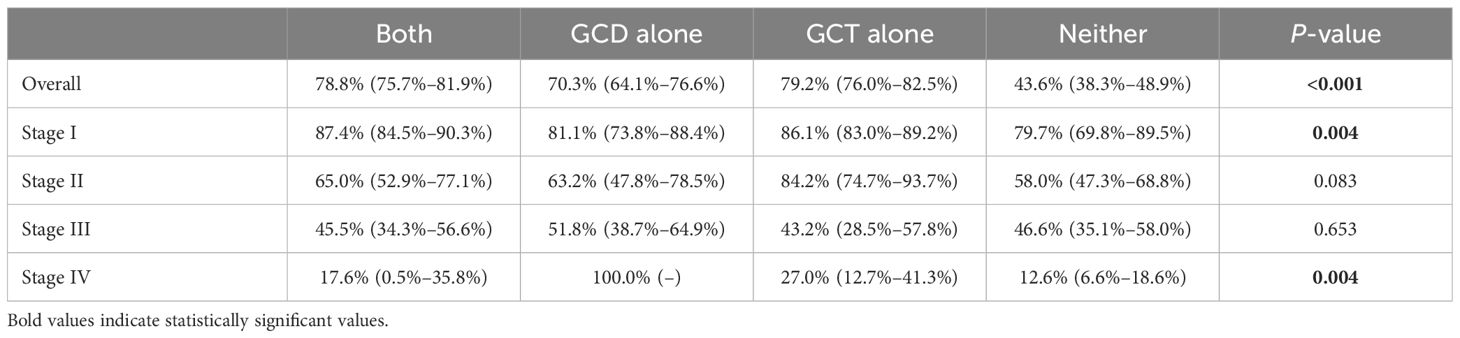
Table 6 3-year survival rate of patients underwent both GCD and GCT, GCD alone, GCT alone, or Neither.
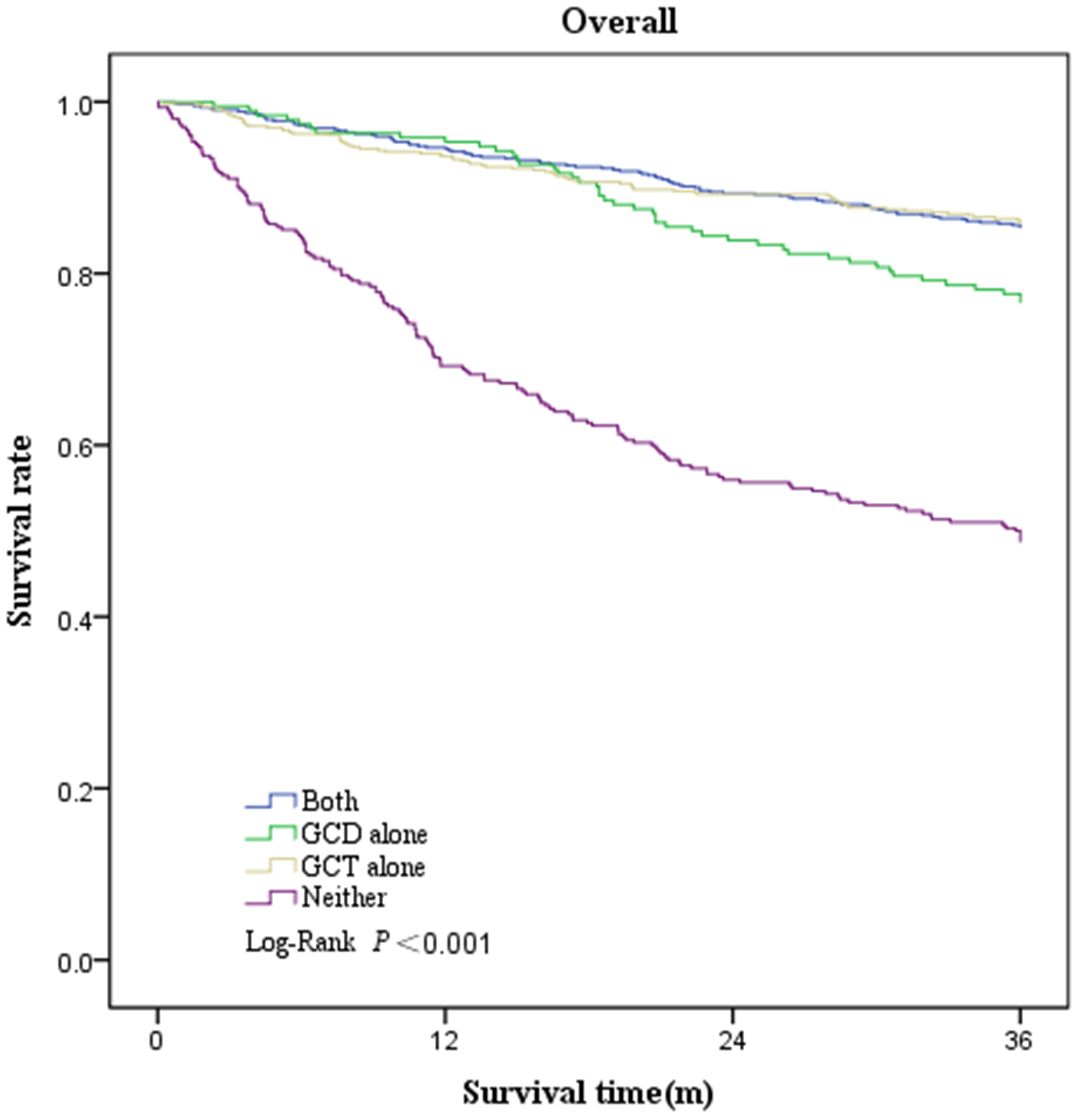
Figure 2 Kaplan–Meier plot showing overall survival rate among patients underwent both GCD and GCT, GCD alone, GCT alone, or Neither.
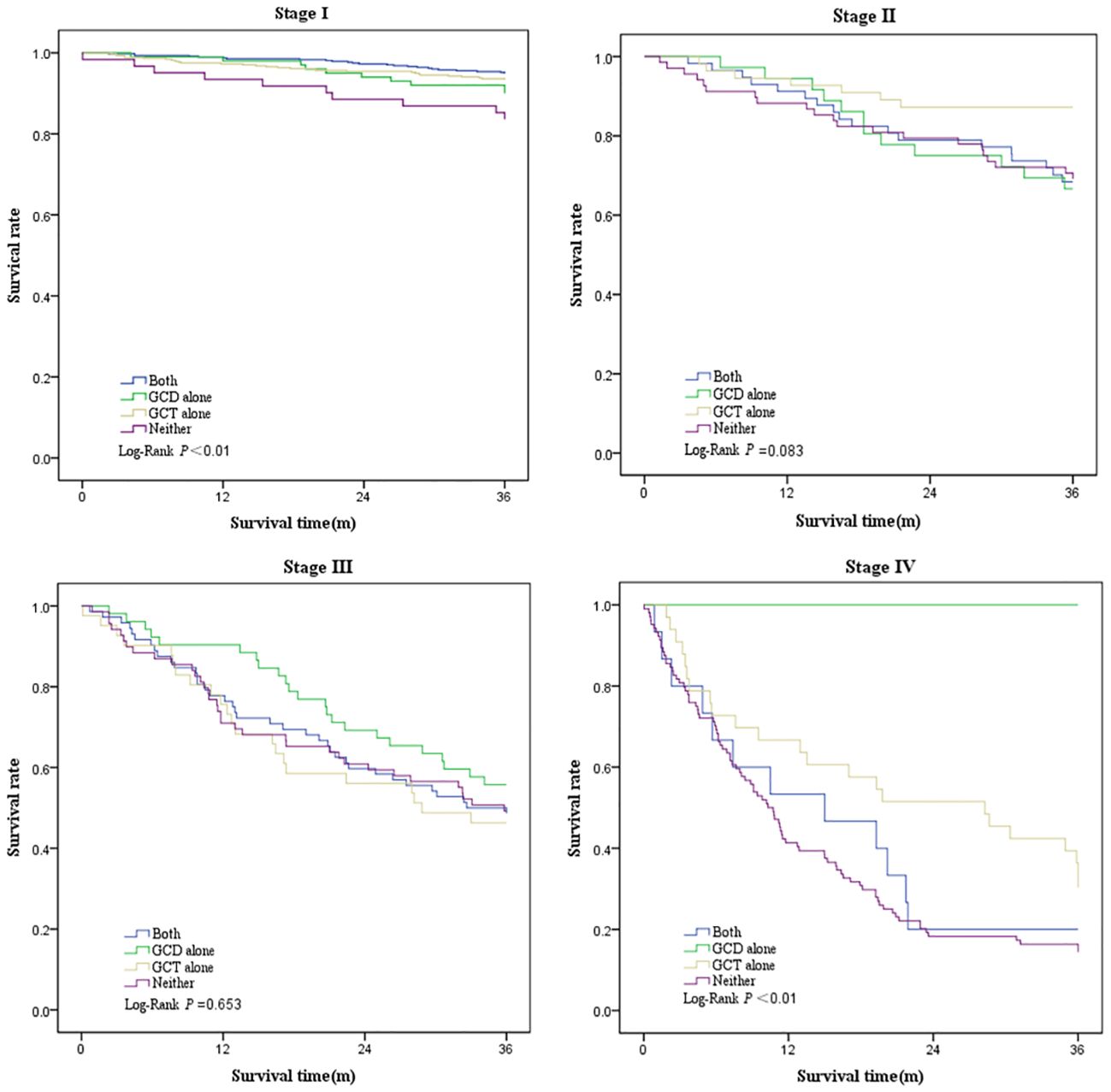
Figure 3 Kaplan–Meier plot showing survival rate by clinical stage among patients underwent both GCD and GCT, GCD alone, GCT alone, or Neither.
Of the overall patients, after adjusting for age, sex, pathological type, and stage, patients undergoing both GCD and GCT showed a 29% reduction in the risk of death compared with those undergoing neither (aHR, 0.71; 95% CI, 0.53–0.95), patients who underwent GCD alone showed a lower hazard of death compared with those who underwent neither (aHR, 0.71; 95% CI, 0.50–1.00), and those who received only GCT showed significantly better survival than those receiving neither (aHR, 0.68; 95% CI, 0.51–0.90).The results of the subgroup analysis showed that the risk of death was significantly lower in patients in stage I who underwent both GCD and GCT or GCT alone than in those who underwent neither (both vs. neither: aHR, 0.28 [95% CI, 0.13–0.59]; GCT alone vs. neither: aHR, 0.37 [95% CI, 0.18–0.75]). In stages II and IV, the risk of death was significantly lower in patients who underwent GCT (both vs. neither: aHR, 0.39 [95% CI, 0.17–0.92]; both vs. neither: aHR, 0.60 [95% CI, 0.37–0.96]) (Table 7).
There are major differences between China and Europe and the United States in terms of health insurance, new drugs and individual FDA-approved therapies, as well as varying levels of economic and medical resources across China (36–38). CSCO guidelines not only take into account the imbalance of development in various regions of China, but also make relevant adjustments to the guidelines in many aspects such as the accessibility of drugs and treatment measures and the value of tumor treatment, and therefore can be said to be the most suitable guidelines for China’s national conditions (31). To the best of our knowledge, this study is the first in China to assess the guideline concordance in lung cancer diagnosis and treatment using first course lung cancer diagnosis and treatment data with a similar structure to SEER data and in combination with Chinese clinical guidelines, the results are more comprehensive and credible than previous case report studies in China.
Diagnosis is a prerequisite for treatment, but there is now no international common standard for assessing guideline concordant diagnosis. In this study, three evaluation components were identified according to the guidelines: clinical TNM staging, pathological staging, and molecular pathology examination in advanced stages. Accurate clinical TNM staging and pathology reporting are important for clinical treatment options and disease outcomes (39, 40), but from our results, more than half of patients did not undergo a GCD, the majority of these were attributed to inappropriate staging, accounting for 41.0% of all patients, a decrease from the 63.1% reported in the 1992–1999 case review in Shanghai, China (41), reflecting the importance of quality of care and tumor staging by medical institutions, and signaling that the implementation of guidelines should continue to increase and be targeted.
Foreign studies have not yet focused on the completeness of pre-treatment clinical TNM staging and pathology type, but some studies have analyzed the appropriateness of biomarker testing among patients with advanced stage cancer; only 20.9% of patients with advanced disease in this study underwent biomarker testing, much lower than the 68.7% in the United States during the same period. This may be due to the high cost of such tests and the fact that they are not covered by medical insurance in China, resulting in their rejection by most patients (42). It is worth noting that the consistency of the diagnosis and guidelines experienced by patients in the oncology specialist hospital was very high, which may be because specialist hospitals are more focused on the professional and characteristic diagnosis and treatment of tumors, the discipline is developing rapidly, and the technology and standardization of diagnosis and treatment are higher (43, 44).
Other studies in the United States have reported that approximately 44.7%–76% of patients underwent GCT; the degree of GCT acceptance varies by cancer type and stage, and this determines the choice of appropriate treatment (23, 45–47). Our study is concordant with previous findings that the likelihood of undergoing GCT decreases with disease progression in NSCLC cases, was higher in stage I–II NSCLC than in stage III–IV NSCLC, this may be related to more direct patient selection and increased expectations of treatment outcomes. The majority of patients who did not undergo GCT presented with advanced disease, with quality of life (QOL) rather than survival likely to be an important consideration, and a previous study reported that patient preference for treatment was the most common reason for not seeking expert advice (48).
Sublobectomy was the most common non-GCT in early stage patients, accounting for 96.6% of stage I non-GCT and 13.1% of stage I surgery patients, an improvement over a previous study in Shanghai (49). However, in recent years there have also been studies showing that sublobar resection is noninferior to lobectomy in terms of disease-free survival (risk ratio for disease recurrence or death, 1.01; 90% [CI], 0.83–1.24), and although these results have not yet been reflected in the guidelines, this trend has been observed and may be influencing the choice of resection (50). In addition, non-GCT for advanced lung cancer is primarily non-targeted, due in large part to the lack of definitive pathologic molecular testing.
In our study, the proportions of patients who underwent both GCD and GCT, GCD alone, GCT alone and neither GCD nor GCT were 36.7%, 11.4%, 33.5% and 18.4%, respectively. A study by Meadows-Taylor et al. showed that these rates for NSCLC in Tennessee from 2014 to 2019 were 61.2%, 12.7%, 17.2% and 8.9%, respectively (51). This indicates that the overall procedures in the first course of diagnosis and treatment of lung cancer in China are poorly adherent to the guidelines and require enhanced supervision and management.
Our follow-up results suggest that either GCD or GCT reduces the risk of death by more than a quarter, concordant with previous studies (24, 52). However, subgroup analysis showed that the effect of GCD on survival was not significant, probably because we focused on GCD as the completeness of the pre-treatment diagnostic report, a procedure that is less likely to affect patient prognosis directly and more likely to affect patient survival indirectly by influencing treatment choice. For advanced NSCLC, highly effective targeted therapies have been approved in many places for patients with EGFR-activating mutations, ALK fusions and ROS1 fusions (53, 54). The development of novel targeted therapies for patients with advanced NSCLC with specific genetic alterations (driver mutations) has greatly improved response rates and survival compared to previously available treatments (6, 55). However, the accessibility of targeted therapies for lung cancer depends on the accurate identification of patient biomarkers through molecular testing (56). Very few patients with advanced NSCLC in this study underwent biomarker testing, with negative impact on their survival. For stage I NSCLC, those who received both GCD and GCT showed the lowest risk of death, suggesting that GCD and GCT were complementary in their association with improved survival in stage I. Therefore, providing both GCD and GCT to patients with stage I NSCLC may have higher benefits for them, especially with the development of lung cancer screening programs in recent years, increasing numbers of cases are being detected at an early stage, with 63.7% of patients in this study being in stage I (57). Although the results of this study show that GCT has a greater survival benefit compared to GCD in almost all stages of NSCLC, as mentioned earlier about the positive impact of molecular pathology testing on survival, proper staging and pathology testing prior to treatment is also important for the survival of NSCLC patients (51).
Therefore, it is critical to provide NSCLC patients with the most appropriate diagnostic procedures and deploy optimal treatment within a reasonable limit of the specific clinical situation, patient preferences and available resources. In the future, more rigorous, prospectively designed and executed studies at the intersection of these two critical components of lung cancer care are needed to validate the impact of GCD and GCT on patients’ survival.
There are some limitations to this study. First, owing to the lack of large databases such as SEER and NCDB in China, our cohort used data from only one province, Liaoning, with a small sample size. As such, there is some uncertainty about comparability when comparing with previous results based on large databases, but we believe that the exploration of the application of clinical practice guidelines in China is complementary to this area of research. The patients’ performance status and comorbidities were used as part of the rationale for treatment, which may be related to a patient’s decision to avoid aggressive treatment, but this information is not available in our data source. In addition, we excluded patients who did not undergo any treatment, although “no treatment” may be considered appropriate treatment given the heterogeneity of patients, therefore, we may have overestimated the proportion of patients undergoing GCT. Future research could collect more comprehensive information, including individual patient income, education, functional status, treatment preferences, and factors related to the doctor, such as the degree of expertise, treatment choice, etc. Besides that, the cases in this study are from 2017-2018, and the use of immune checkpoint inhibitors related to lung cancer is not as widespread; however, with their increasing use as first-line therapies in lung cancer in recent years, the results of this study may change, relevant and more recent studies are still needed for the future.
This study is the first in China to assess concordance with guidelines for the first course of lung cancer diagnosis and treatment. We found a poor level of guideline concordance for NSCLC diagnosis and treatment in China, which was lower than that in the United States during the same period. Patients with early-stage NSCLC, as well as those from oncology specialist hospitals, are more likely to undergo GCD and GCT. Undergoing GCD and GCT improves the survival of patients with NSCLC. Therefore, there is a need to establish an oncology diagnosis and treatment data management platform in China to monitor, evaluate and promote the use of clinical practice guidelines in healthcare institutions in order to maximize the survival rate of lung cancer patients in China.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Liaoning Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HM: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. XY: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. YL: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. BZ: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. LL: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. MZ: Data curation, Formal analysis, Investigation, Writing – original draft. QW: Data curation, Formal analysis, Investigation, Writing – original draft. QC: Data curation, Formal analysis, Investigation, Writing – original draft. LY: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. WS: Conceptualization, Methodology, Project administration, Writing – review & editing. GP: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Liao Ning Revitalization Talents Program [grant number: XLYC1802131], the Key Laboratory Program of Liaoning Province [grant number: 2018225113] and Science and Technology Innovation Team of China Medical University [grant number: CXTD2022006].
We thank all participants for their cooperation in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
aHR, adjusted hazard ratio; aOR, adjusted odds ratio; CSCO, Chinese Society of Clinical Oncology; GCC, guideline-concordant care; GCD, guideline-concordant diagnosis; GCT, guideline-concordant treatment; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; SEER, Surveillance, Epidemiology and End Results.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
3. Yang Z, Li X, Bai J, Li D, Ma Z, Deng C, et al. Prognostic factors for survival of stage IB non-small cell lung cancer patients: A 10-year follow-up retrospective study. Ann Surg Oncol. (2023) 30:7481–91. doi: 10.1245/s10434-023-14016-y
4. Kinoshita FL, Ito Y, Morishima T, Miyashiro I, Nakayama T. Sex differences in lung cancer survival: long-term trends using population-based cancer registry data in Osaka, Japan. Jpn J Clin Oncol. (2017) 47:863–9. doi: 10.1093/jjco/hyx094
5. Kristiansen C, Schytte T, Hansen KH, Holtved E, Hansen O. Trends in lung cancer in elderly in Denmark, 1980-2012. Acta Oncol. (2016) 55 Suppl 1:46–51. doi: 10.3109/0284186X.2015.1114676
6. Chi SA, Yu H, Choi YL, Park S, Sun JM, Lee SH, et al. Trends in survival rates of non-small cell lung cancer with use of molecular testing and targeted therapy in Korea, 2010-2020. JAMA network Open. (2023) 6:e232002. doi: 10.1001/jamanetworkopen.2023.2002
7. Vlăsceanu S, Mahler B, Marghescu A, Bădărău IA, Moldovan H, Gheorghiţă D, et al. The nine-year survival of patients operated for non-small-cell lung carcinoma in a tertiary centre: the impact of the tumour stage and other patient-related parameters. Medicina (Kaunas Lithuania). (2024) 60:415–30. doi: 10.3390/medicina60030415
8. Lee JD, Zheng R, Okusanya OT, Evans NR 3rd, Grenda TR. Association between surgical quality and long-term survival in lung cancer. Lung Cancer (Amsterdam Netherlands). (2024) 190:107511. doi: 10.1016/j.lungcan.2024.107511
9. Remon J, Hendriks LEL, Besse B. Paving the way for long-term survival in non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. (2021) 39:2321–3. doi: 10.1200/JCO.21.00760
10. Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, et al. Treatment of small-cell lung cancer: american society of clinical oncology endorsement of the american college of chest physicians guideline. J Clin Oncol Off J Am Soc Clin Oncol. (2015) 33:4106–11. doi: 10.1200/JCO.2015.63.7918
11. Absenger G, Terzic J, Bezan A. ASCO update: lung cancer. Memo. (2017) 10:224–7. doi: 10.1007/s12254-017-0373-x
12. Parascandola M, Pearlman PC, Eldridge L, Gopal S. The development of global cancer research at the United States national cancer institute. J Natl Cancer Inst. (2022) 114:1228–37. doi: 10.1093/jnci/djac104
13. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv1–iv21. doi: 10.1093/annonc/mdx222
14. Benson AB 3rd, Brown E. Role of NCCN in integrating cancer clinical practice guidelines into the healthcare debate. Am Health Drug Benefits. (2008) 1(1):28–33.
15. Harrison S, Judd J, Chin S, Ragin C. Disparities in lung cancer treatment. Curr Oncol Rep. (2022) 24:241–8. doi: 10.1007/s11912-022-01193-4
16. Møller H, Coupland VH, Tataru D, Peake MD, Mellemgaard A, Round T, et al. Geographical variations in the use of cancer treatments are associated with survival of lung cancer patients. Thorax. (2018) 73:530–7. doi: 10.1136/thoraxjnl-2017-210710
17. Bick KJ, Ding L, Ebner PJ, Kim AW, Atay SM, Wightman SC, et al. Regional variation in treatment for highest-risk patients with non-small cell lung cancer. Ann Thorac surgery. (2022) 113:1282–90. doi: 10.1016/j.athoracsur.2021.04.067
18. Dwyer LL, Vadagam P, Vanderpoel J, Cohen C, Lewing B, Tkacz J. Disparities in lung cancer: A targeted literature review examining lung cancer screening, diagnosis, treatment, and survival outcomes in the United States. J racial ethnic Health disparities. (2024) 11:1489–500. doi: 10.1007/s40615-023-01625-2
19. Salloum RG, Smith TJ, Jensen GA, Lafata JE. Factors associated with adherence to chemotherapy guidelines in patients with non-small cell lung cancer. Lung Cancer. (2012) 75:255–60. doi: 10.1016/j.lungcan.2011.07.005
20. Landrum MB, Keating NL, Lamont EB, Bozeman SR, McNeil BJ. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer. (2012) 118:3345–55. doi: 10.1002/cncr.26628
21. Gould MK, Munoz-Plaza CE, Hahn EE, Lee JS, Parry C, Shen E. Comorbidity profiles and their effect on treatment selection and survival among patients with lung cancer. Ann Am Thorac Society. (2017) 14:1571–80. doi: 10.1513/AnnalsATS.201701-030OC
22. Walter J, Tufman A, Holle R, Schwarzkopf L. Differences in therapy and survival between lung cancer patients treated in hospitals with high and low patient case volume. Health Policy (Amsterdam Netherlands). (2020) 124:1217–25. doi: 10.1016/j.healthpol.2020.07.012
23. Nadpara PA, Madhavan SS, Tworek C, Sambamoorthi U, Hendryx M, Almubarak M. Guideline-concordant lung cancer care and associated health outcomes among elderly patients in the United States. J geriatric Oncol. (2015) 6:101–10. doi: 10.1016/j.jgo.2015.01.001
24. Ahmed HZ, Liu Y, O'Connell K, Ahmed MZ, Cassidy RJ, Gillespie TW, et al. Guideline-concordant care improves overall survival for locally advanced non-small-cell lung carcinoma patients: A national cancer database analysis. Clin Lung cancer. (2017) 18:706–18. doi: 10.1016/j.cllc.2017.04.009
25. Bott MJ, Patel AP, Verma V, Crabtree TD, Morgensztern D, Robinson CG, et al. Patterns of care in hilar node-positive (N1) non-small cell lung cancer: A missed treatment opportunity? J Thorac Cardiovasc Surg. (2016) 151:1549–58.e2. doi: 10.1016/j.jtcvs.2016.01.058
26. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
27. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Global Health. (2018) 6:e555–e67. doi: 10.1016/S2214-109X(18)30127-X
28. National Health Commission of the People's Republic of China. China’s cancer prevention and treatment three-year action plan (2015-2017) (2016). Available online at: http://en.nhc.gov.cn/2016-01/08/c_68740.htm.
29. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
30. Kang MJ, Lim J, Han SS, Park HM, Park SJ, Won YJ, et al. First course of treatment and prognosis of exocrine pancreatic cancer in korea from 2006 to 2017. Cancer Res Treat. (2022) 54:208–17. doi: 10.4143/crt.2021.421
31. Zhou Q, Wu YL. Developing CSCO lung cancer practice guidelines stratified by resource availability and treatment value. J Glob Oncol. (2017) 3:285–8. doi: 10.1200/JGO.2016.006734
32. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
33. Romero D. Sublobar resection is non-inferior to lobectomy in very early stage NSCLC. Nat Rev Clin Oncol. (2023) 20:209. doi: 10.1038/s41571-023-00743-4
34. Schneider BJ, Daly ME, Kennedy EB, Antonoff MB, Broderick S, Feldman J, et al. Stereotactic body radiotherapy for early-stage non-small-cell lung cancer: american society of clinical oncology endorsement of the american society for radiation oncology evidence-based guideline. J Clin Oncol Off J Am Soc Clin Oncol. (2018) 36:710–9. doi: 10.1200/JCO.2017.74.9671
35. Udelsman BV, Canavan ME, Zhan PL, Ely S, Park HS, Boffa DJ, et al. Overall survival in low-comorbidity patients with stage I non-small cell lung cancer who chose stereotactic body radiotherapy compared to surgery. J Thorac Cardiovasc surgery. (2024) 167:822–33.e7. doi: 10.1016/j.jtcvs.2023.07.021
36. Wang Y, Li Y, Qin S, Kong Y, Yu X, Guo K, et al. The disequilibrium in the distribution of the primary health workforce among eight economic regions and between rural and urban areas in China. Int J Equity Health. (2020) 19:28. doi: 10.1186/s12939-020-1139-3
37. Ao Y, Feng Q, Zhou Z, Chen Y, Wang T. Resource allocation equity in the China's rural three-tier healthcare system. Int J Environ Res Public Health. (2022) 19:6589–605. doi: 10.3390/ijerph19116589
38. Yao S, Wang R, Qian K, Zhang Y. Real world study for the concordance between IBM Watson for Oncology and clinical practice in advanced non-small cell lung cancer patients at a lung cancer center in China. Thorac Cancer. (2020) 11:1265–70. doi: 10.1111/1759-7714.13391
39. Kutob L, Schneider F. Lung cancer staging. Surg Pathol clinics. (2020) 13:57–71. doi: 10.1016/j.path.2019.10.003
40. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network JNCCN. (2017) 15:504–35. doi: 10.6004/jnccn.2017.0050
41. Li D, Zheng Y, Chen H, Xu Y, Lu W. Evaluation of implement of the diagnosis and treatment criterion for common cancer in China in shanghai. China Oncol. (2001) 4:8–11. doi: 10.3969/j.issn.1004-0242.2001.04.003
42. John A, Yang B, Shah R. Clinical impact of adherence to NCCN guidelines for biomarker testing and first-line treatment in advanced non-small cell lung cancer (aNSCLC) using real-world electronic health record data. Adv Ther. (2021) 38:1552–66. doi: 10.1007/s12325-020-01617-2
43. Chiu AS, Resio B, Hoag JR, Monsalve AF, Blasberg JD, Brown L, et al. Why travel for complex cancer surgery? Americans react to 'Brand-sharing' Between specialty cancer hospitals and their affiliates. Ann Surg Oncol. (2019) 26:732–8. doi: 10.1245/s10434-018-6868-9
44. Liu M, Hu L, Xu Y, Wang Y, Liu Y. Patient healthcare experiences of cancer hospitals in China: A multilevel modeling analysis based on a national survey. Front Public Health. (2023) 11:1059878. doi: 10.3389/fpubh.2023.1059878
45. Wah W, Stirling RG, Ahern S, Earnest A. Association between receipt of guideline-concordant lung cancer treatment and individual- and area-level factors: A spatio-temporal analysis. Cancer epidemiology Biomarkers Prev Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol. (2020) 29:2669–79. doi: 10.1158/1055-9965.EPI-20-0709
46. Nadpara PA, Madhavan SS, Tworek C. Disparities in lung cancer care and outcomes among elderly in a medically underserved state population-A cancer registry-linked database study. Population Health management. (2016) 19:109–19. doi: 10.1089/pop.2015.0027
47. Blom EF, Ten Haaf K, Arenberg DA, de Koning HJ. Disparities in receiving guideline-concordant treatment for lung cancer in the United States. Ann Am Thorac Society. (2020) 17:186–94. doi: 10.1513/AnnalsATS.201901-094OC
48. Oliveri S, Lanzoni L, Veldwijk J, de Wit GA, Petrocchi S, Janssens R, et al. Balancing benefits and risks in lung cancer therapies: patient preferences for lung cancer treatment alternatives. Front Psychol. (2023) 14:1062830. doi: 10.3389/fpsyg.2023.1062830
49. Zhao X, Qian L, Luo Q, Huang J. Segmentectomy as a safe and equally effective surgical option under complete video-assisted thoracic surgery for patients of stage I non-small cell lung cancer. J Cardiothoracic Surgery. (2013) 8:116. doi: 10.1186/1749-8090-8-116
50. Altorki N, Wang X, Kozono D, Watt C, Landrenau R, Wigle D, et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. New Engl J Med. (2023) 388:489–98. doi: 10.1056/NEJMoa2212083
51. Meadows-Taylor MB, Faris NR, Smeltzer MP, Ray MA, Fehnel C, Akinbobola O, et al. The relative survival impact of guideline-concordant clinical staging and stage-appropriate treatment of potentially curable non-small cell lung cancer. Chest. (2022) 162:242–55. doi: 10.1016/j.chest.2022.01.046
52. Stokes SM, Massarweh NN, Stringham JR, Varghese TK Jr. Clinical-pathologic correlation and guideline concordance in resectable non-small cell lung cancer. Ann Thorac surgery. (2019) 108:837–44. doi: 10.1016/j.athoracsur.2019.03.062
53. Herrera-Juárez M, Serrano-Gómez C, Bote-de-Cabo H, Paz-Ares L. Targeted therapy for lung cancer: Beyond EGFR and ALK. Cancer. (2023) 129:1803–20. doi: 10.1002/cncr.34757
54. Xiao Y, Liu P, Wei J, Zhang X, Guo J, Lin Y. Recent progress in targeted therapy for non-small cell lung cancer. Front Pharmacol. (2023) 14:1125547. doi: 10.3389/fphar.2023.1125547
55. Król K, Mazur A, Stachyra-Strawa P, Grzybowska-Szatkowska L. Non-small cell lung cancer treatment with molecularly targeted therapy and concurrent radiotherapy-A review. Int J Mol Sci. (2023) 24:5858–80. doi: 10.3390/ijms24065858
56. Smeltzer MP, Wynes MW, Lantuejoul S, Soo R, Ramalingam SS, Varella-Garcia M, et al. The international association for the study of lung cancer global survey on molecular testing in lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2020) 15:1434–48. doi: 10.1016/j.jtho.2020.05.002
Keywords: lung cancer, first course, diagnosis and treatment, guideline-concordant, survival
Citation: Mu H, Yang X, Li Y, Zhou B, Liu L, Zhang M, Wang Q, Chen Q, Yan L, Sun W and Pan G (2024) Three-year follow-up study reveals improved survival rate in NSCLC patients underwent guideline-concordant diagnosis and treatment. Front. Oncol. 14:1382197. doi: 10.3389/fonc.2024.1382197
Received: 19 February 2024; Accepted: 15 May 2024;
Published: 28 May 2024.
Edited by:
Liang Zhao, Dalian University of Technology, ChinaReviewed by:
Catherine Rufatto Sears, Indiana University Bloomington, United StatesCopyright © 2024 Mu, Yang, Li, Zhou, Liu, Zhang, Wang, Chen, Yan, Sun and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guowei Pan, Z3dwYW5AY211LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.