
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 02 May 2024
Sec. Cancer Metabolism
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1381894
This article is part of the Research TopicMetabolic Crosstalk between Cancer Cells and Immune Cells in the Tumor Microenvironment: Cellular and Molecular Insights, and their Therapeutic ImplicationsView all 12 articles
Arachidonic acid (AA) is a crucial polyunsaturated fatty acid in the human body, metabolized through the pathways of COX, LOX, and cytochrome P450 oxidase to generate various metabolites. Recent studies have indicated that AA and its metabolites play significant regulatory roles in the onset and progression of ovarian cancer. This article examines the recent research advancements on the correlation between AA metabolites and ovarian cancer, both domestically and internationally, suggesting their potential use as biological markers for early diagnosis, targeted therapy, and prognosis monitoring.
Ovarian cancer(OC) is a common gynecological malignant tumor with a hidden onset, high degree of malignancy, and high mortality rate (1). Globally, there are nearly 240,000 new cases annually, with the ovarian cancer mortality rate expected to significantly increase by 2040 (2). However, the lack of specific markers for ovarian cancer means that most patients are diagnosed in the late stages of the disease (51% in stage III or 29% in stage IV), missing the optimal timing for surgery, resulting in a 5-year survival rate of only 26-42% (3). Therefore, there is an urgent need to develop new molecular markers for the prevention and treatment of ovarian cancer. Extensive research indicates a close relationship between abnormal lipid metabolism and tumor development, increasing the possibility of detecting subtle metabolic changes in early tumor formation, making it a promising candidate as an ideal biomarker for ovarian cancer diagnosis (4–6). Among them, arachidonic acid is considered a key lipid metabolism product, playing a significant role in cell proliferation, apoptosis, invasion, and metastasis. Therefore, research on arachidonic acid and other lipid metabolites can not only help us better understand the pathogenesis of ovarian cancer but also offer new insights and methods for the treatment and prevention of this disease.
Arachidonic acid (AA) is an essential omega-6 polyunsaturated fatty acid in the human body (7). The endogenous generation of arachidonic acid (AA) is derived from phospholipids in the cell membrane, which is catalyzed by the superfamily of phospholipase A2 (PLA2). This process is induced by various cellular activation signals, including stimulation of tumor necrosis factor receptor (TNFR) and toll-like receptor 4 (TLR4) in the course of inflammation or infection. Among the members of the PLA2 enzyme superfamily, three contribute to eicosanoid production and are involved in distinct functions within eicosanoid metabolism (8–10). The cytosolic calcium-dependent PLA2 alpha (cPLA2α) primarily facilitates the production of free fatty acids (FFAs) and generation of AA, which plays a crucial role in cellular signaling (11). The cytosolic calcium-independent PLA2 alpha (iPLA2α) contributes to cellular homeostasis through synthesis of specialized pro-resolving mediators (SPMs) and reacylation of free AA, while secretory PLA2 (sPLA2), operating in a paracrine manner, controls the release of free AA and induces local inflammatory responses. In addition to PLA2 enzymes, other enzymes known as phospholipase C (PLC) and phospholipase D (PLD), generate AA via intermediate products such as diacylglycerol(DAG) (12–14). Free AA is converted in three ways: ① Cyclooxygenase (COX) enzymes catalyze the metabolic conversion of arachidonic acid to prostanoids including prostaglandins (PGs), prostacyclin, and thromboxane(TXs). ② Lipoxygenase (LOX) pathways catalyze the conversion of arachidonic acid to leukotrienes and lipoxins. ③ Cytochrome P450 enzyme (CYP) pathway (15) (Figure 1). Previous studies have demonstratedthat AA and its metabolites promote the occurrence and development of tumors by regulating the process of cell carcinogenesis, progression, and differentiation such as cell proliferation, chemotaxis, mitosis, migration, and apoptosis (16–18). Therefore, AA metabolism is considered to be one of the active metabolisms in tumor metabolism.
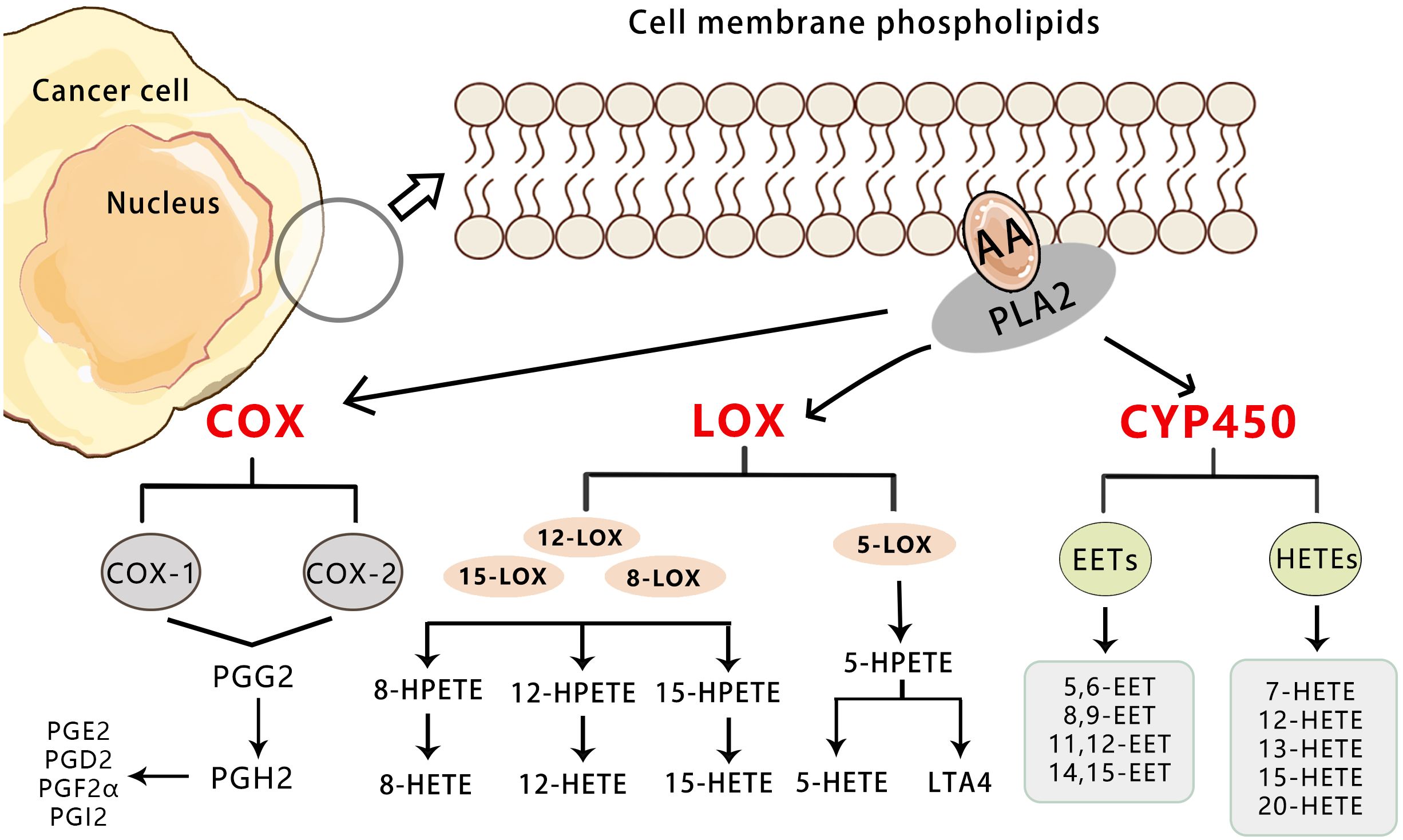
Figure 1 Overview of the three pathways for AA metabolism in cancer. Upon cell stimulation, phospholipase A2 induces FFAs, primarily AA derived from the lipid bilayer. The released AA is metabolized into bioactive lipid signaling molecules by three different enzymatic pathways. COX-1/2 metabolize AA to a series of prostaglandins (PGD2, PGE2, PGF2, PGH2, and PGI2).5-LO metabolizes AA to 5-HETE, LTB4, and cysteine leukotrienes, and 12-LO metabolizes AA to 12-HETE. Both subtypes of 15-LO metabolize AA to 15-HETE and, to a lesser extent, 12-HETE. Cytochrome P450 metabolizes AA to 19-/20-HETEand so on.
The COX enzyme is the initial catalyst in the arachidonic acid pathway for prostaglandin (PG) and thromboxane (Tx) synthesis, existing in three isomeric forms: COX-1, COX-2, and COX-3 (19). Both COX-1 and COX-2 enzymes facilitate the conversion of cell membrane phospholipids to arachidonic acid via phospholipase A2, followed by its conversion to PGH2 through PGG2 (20)(Figure 2).COX-1 is a constitutive enzyme essential for normal physiological functions and widely distributed across various tissues to safeguard cellular integrity (21). There is no significant difference in the expression level of COX-1 between tumor tissues and normal tissues (22).
COX-2 is an inducible enzyme that performs a crucial function in the pathophysiological process of various cancers, including pancreatic, breast, prostate, lung, liver, cervical, bowel, and skin cancer (23–28). It is either not expressed or expressed at low levels in most normal tissues. The expression of COX-2 is associated with inflammation, cell survival, proliferation, angiogenesis, invasion, and metastasis. In response to growth factors and endotoxins, COX-2 is briefly but strongly expressed. The overexpression of COX-2 significantly enhances the production of PGE2, leading to increased cell aggressiveness (29). PGE2 contributes to the development and progression of many cancers by activating the membrane receptors EP (including EP1, EP2, EP3, and EP4 receptors) and the nuclear receptor PPARδ of target cells (30). Other studies have indicated that high expression of COX-2 can hinder the apoptosis of tumor cells, which is crucial in the early stages of tumor formation (31). The overexpression of COX-2 can result in increased synthesis of PG, which plays a significant biological role in tumor growth and proliferation. PGE2 can impede apoptosis induced by selective COX-2 inhibitors by upregulating the anti-apoptotic protein bc-l2 (32). Kajita et al. discovered that the level of specific enzymes in the PG synthesis pathway, such as TXA2 synthetase, increased in papillary thyroid carcinoma. Additionally, the expression of COX-2 protein in papillary thyroid carcinoma was higher than in normal thyroid tissue and varied greatly, indicating that COX-2 could promote the growth of the thyroid papillary gland (33). These findings suggest that the increased expression of these enzymes may contribute to the pathogenesis of tumors.
What’s more, angiogenesis is the physiological basis of solid cancer growth and metastasis (34). The high expression of COX-2 and its metabolite PGE2 promotes angiogenesis by up-regulating angiogenic factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) (35). COX-2 also promotes the metastasis and invasion of ovarian cancer by inducing matrix metalloproteinases (MMPs) in the extracellular matrix and the decomposition of collagen matrix, which may be involved in the activation of the PI3K/AKT signaling pathway (36–38). Inhibition of COX-2 with its specific inhibitor NS-398 can increase the expression of E-cadherin and inhibit the expression of slug, vimentin, MMP2, and MMP9, thereby suppressing the invasion and metastasis of ovarian cancer cells under estrogen treatment (39–41). Moreover, overexpression of COX-2 in ovarian cancer cells can directly up-regulate Bcl-2 expression through the increased synthesis of PGs. In addition, COX-2 can inhibit the proliferation of T and B lymphocytes through its product PGE2, and also inhibit the synthesis of cytokines to reduce the cytotoxicity of natural killer cells (42). Therefore, the role of COX-2 and PGE2 in promoting tumor proliferation and invasion, reducing tumor cell apoptosis, and promoting tumor angiogenesis has been confirmed, and it proved that they play an important role in carcinogenesis, and have the function of promoting tumor by inhibiting the body’s immunity (43).
LOX is the initial enzyme in the leukotriene (LT) pathway of arachidonic acid. Isoenzymes of LOX consist of 5-LOX, 12-LOX, and two isomers of 15-LOX (15-LOX-1, 15-LOX-2). However, 5-LOX, 12-LOX, and 15-LOX-1 have pro-tumor effects, while 15-LOX-2 appears to have anticancer effects (44).
LOX catalyzes the oxidation of arachidonic acid to 5-HPETE, which is subsequently metabolized to 5-HETE (5-hydroxyeicosatetraenoic acid) and LTB4 (leukotriene B4) (45). As a member of the arachidonic acid oxygenase family, 5-LOX is composed of 674 amino acids and is a monomeric enzyme containing iron ions. 5-LOX can be transcriptionally regulated by transcription factor Er, Sp1, nuclear factor-κB (NF-κB), and GATA (46). 5-LOX is activated by 5-LOX activating protein (FLAP) to catalyze arachidonic acid, which is released from the phospholipid bilayer by phospholipase A2 (47). Arachidonic acid is transformed into 5-HPETE, which can be metabolized by glutathione peroxidase into 5-HETE (48). The activity of 5-LOX leads to the formation of unstable LTA4, which can be converted to LTB4, LTC4,LTD4 and LTE4 (49). More significantly, the average endogenous level of LOX metabolites such as 12-HETE (12-hydroxyeicosatetraenoic acid) in primary prostate cancer were found to be significantly higher than that in non-neoplastic prostate tissue. It is suggested that 12-HETE is crucial in the progression of prostate cancer and the LOX pathway may be a target for the treatment and prevention of prostate cancer (50). 12-HETE has also been shown to play an important role in cancer adhesion, invasion, and metastasis. It stimulates NF-κB activation and NF-κB-dependent ICAM-1 expression through RhoA and PKCα signaling pathways, and the Rho/Rac family of GTases also play a role in cell adhesion and migration. 12-HETE can also promote the secretion of protease and enhance the motor capacity of tumor cells (51)(Figure 3).
Various environmental and chemical carcinogens activate pro-tumor mediators during carcinogenesis, including 5-LOX, whose metabolite 5S-HERE acts as a substrate for COX-2 to form bicyclic and is further transformed into two pro-angiogenic mediators (52). However, the specific role of these products in cancer development needs better understanding, given the pro-angiogenic role of these enzymes. As a result, 5-LOX is considered a new target for cancer prevention and treatment, while LETA4 hydrolase is considered a tumor promoter whose inhibitors can reduce tumor growth and development (53). Although most of the metabolites synthesized by the COX and LOX pathways can promote tumorigenesis. Additionally, CYP450 monooxygenase-derived metabolites can also regulate the occurrence and growth of cancer, playing both a pro-tumor and anti-tumor role (54).
Cytochrome P450 (CYP) belongs to the group of hemoglobin enzymes. It is a super-family gene encoding isoenzymes with a structure and function related to CYP. The combination of reduced CYP and carbon monoxide (CO) has a special light absorption peak at 450 nm (55). The expression of CYP surface oxidase is significantly high in cancer tissues, while almost no expression is found in adjacent normal tissues. This confirms that CYP surface oxidase and its metabolites can regulate the occurrence and growth of cancer, playing both a pro-tumor and anti-tumor role. It has been shown that reducing the expression and activity of CYP450 has anti-tumor effects, making it a promising anti-cancer treatment method (56) (Figure 4).
Arachidonic acid produces epoxyeicosatrienoic acid (EETs) and hydroxyeicosatetraenoic acid (HETEs) through the catalysis of cytochrome P450 surface oxidase. EETs are further categorized into four types: 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET (57, 58). Recent studies have focused on the role and mechanism of EETs in important biological processes of tumors, including proliferation, apoptosis, and metastasis (59). These studies have revealed that EETs can significantly enhance the proliferation of tumor cells and protect them from TNF-α-induced apoptosis by up-regulating anti-apoptotic proteins Bcl-2 and Bcl-XL, while down-regulating pro-apoptotic proteins Bax and Bak, thereby reducing the activity of caspase-3 protein (60).
In light of the characteristics of the CYP gene superfamily, research on the correlation between CYP and gynecologic tumors primarily focuses on specific CYP polymorphisms involved in sterol hormone metabolism. The polymorphisms of CYPlAl, CYPlBl, CYPl7, and CYPl9 have garnered increasing attention in relation to gynecologic tumors (61, 62). CYPlAl is an enzyme involved in the metabolism of polycyclic aromatic hydrocarbons (PAHs), which are significant metabolically active chemical carcinogens, and is expressed in various sterol hormone-reactive tissues such as the ovary, mammary gland, and prostate. Studies have shown that the CYPlAl Ile/Val and Val/Val genotypes are significantly more common in patients with epithelial ovarian tumors, indicating that women with these genotypes are at higher risk for ovarian cancer (63). CYP and its lipid metabolites play a role in the development of ovarian tumors, and their expression may be a crucial marker for tumor development (64).
AA catalyzes the synthesis of prostaglandins (PGs) and thromboxanes (TXs) through cyclooxygenase (COX). There are two subtypes of COX: COX-1 and COX-2. COX-1 is widely distributed in tissues, with no significant difference in expression levels between tumor tissues and normal tissues. On the other hand, COX-2 is closely associated with the development of inflammation and tumors. Overexpression of COX-2 significantly promotes the biosynthesis of prostaglandin E2 (PGE2). PGE2 stimulates the occurrence and progression of various cancers by activating membrane receptors EP and nuclear receptor PPARδ in target cells.
Recently, Mauricio A. Cuello discussed the impact of COX-2 on the immune spectrum of high-grade serous ovarian cancer (HGSOC). Elevated levels of COX-2 may hinder NK cell activity, promote the expression of cytotoxic T lymphocyte-associated protein-4 (CTLA-4), affecting the efficacy of anti-CTLA-4 immunotherapy in HGSOC patients. Targeting COX-2 before anti-CTLA-4 immunotherapy could be a promising strategy to enhance the effectiveness of immunotherapy in ovarian cancer patients (42). Additionally, Ning Ding’s research found that the overexpression of COX-2 in SKOV3 cells (human ovarian cancer cells) is associated with the acquisition of stem cell properties, inflammatory microenvironment, enhanced tumor sphere formation, increased cell proliferation, and enhanced metastatic potential, all contributing to ovarian cancer progression and metastasis (65). Based on current research, the roles of COX- and LOX-derived class of diterpenes in cancer have been fairly well studied. COX-1, COX-2, mPGES-1, EP1, and EP2 are mainly expressed in epithelial cells of human epithelial ovarian cancer (66). The expression of COX-2 in ovarian cancer cells is regulated by various cytokines, such as EGF, vitamin D, IL-1β, which can stimulate the proliferation, migration, and angiogenesis of ovarian cancer cells. It mainly enhances the proliferation and migration of human ovarian cancer CAOV-3 cells by activating the phosphatidylinositol 3 kinase/protein kinase B (PI3-k/Akt) pathway (67). Moreover, by analyzing epithelial ovarian cancer tissues and cell lines, COX-2 also regulates cell growth and apoptosis through the PI3K/AKT signaling pathway in ovarian cancer tissues (68).
In the Lipoxygenase (LOX) pathway, AA is catalyzed by the LOX enzyme, undergoing an oxidation reaction at specific sites to form hydroxyl-containing products, namely Hydroxyeicosatetraenoic acid (HETEs, including 5-, 8-, 12-HETE, etc.). Additionally, there are lipoxins (LXs), leukotrienes (LTs), and other products, which will not be the focus here.
Research in ovarian cancer has found an increased expression level of 5-LOX in the immune stroma of tissues, suggesting a specific impact on the tumor microenvironment during tumor initiation and progression (47). Under hypoxic conditions, the transcription levels of 5-LOX and ALOX5AP in ovarian cancer cell lines also increase, significantly correlating with poor overall survival, progression-free survival, lymphatic infiltration after initial treatment, rapid relapse, and other adverse clinical pathological features. Current studies have discovered that various LOX pathway metabolites, such as HETEs, play a role in inhibiting tumor cell apoptosis, stimulating angiogenesis, enhancing cell proliferation, and promoting metastasis. The presence of 12-HETE has been identified in high-grade serous ovarian cancer and normal ovarian epithelial tissues. The 12-LOX - 12-HETE system is present in two epithelial ovarian cancer cell lines, OVCAR-3 and SK-OV-3, with high levels of 12-LOX mRNA and protein (69, 70). Exposure to arachidonic acid increases the production of 12-HETE in both epithelial ovarian cancer cell lines. Numerous studies have confirmed its ability to promote the proliferation, adhesion, migration, and angiogenesis of various cancer cells such as breast, prostate, and colon cancer. These findings collectively indicate that metabolites produced by the LOX pathway can play a role in the development of ovarian cancer through different isoenzymes (71).
Most studies on gynecologic malignant tumors have focused on the arachidonic acid oxygenase pathway, particularly examining the roles of PGE2 and PGF2a along with their respective receptors. While some research has touched upon the P450 cytochrome epoxygenase pathway in ovarian cancer, there is a lack of in-depth mechanistic studies in this area (72). Research indicates that CYP2C8 and CYP3A5 are prominently expressed in the majority of ovarian tumors, showing higher IHC staining intensity of CYP3A5 and other CYP enzymes in primary ovarian cancer tissues. In contrast, CYP3A4 is expressed at significantly lower levels in ovarian cancer (73, 74).
Arachidonic acid is converted into four eicosanoids (EETs) by cytochrome P450 oxygenases (CYP oxygenases). Human EETs are synthesized by the CYP2 family, including the CYP2C and CYP2J families. EETs are intermediate products downstream of the VEGF signaling pathway, regulating vascular tone and maintaining vascular homeostasis. They have been shown to promote endothelial cell proliferation, enhance endothelial cell migration through pathways such as eNOS, MEK/MAPK, and PI3K, and boost VEGF-mediated angiogenesis. Angiogenesis plays a crucial role in tumor development, enabling tumors to access more nutrients and oxygen for continued growth, spread, and metastasis by providing pathways for tumor cells to travel through the bloodstream to distant sites.
AA is a highly concentrated polyunsaturated fatty acid present in the microenvironment of ovarian cancer, with multiple research findings indicating its association with adverse clinical outcomes (75). Enzymes and metabolites related to the AA metabolic pathway regulate various pathophysiological processes in the cellular system, establishing the tumor microenvironment (TME) (76). The progression, metastasis, and spread of cancer, as well as the evasion of tumor cells from immune surveillance, crucially depend on the signaling network of the tumor microenvironment. In ovarian cancer, ascites is a significant component of the peritoneal TME, containing numerous tumor spheroids and immune cells, particularly tumor-associated macrophages (TAMs), T cells, and NK cells (77). Studies have reported a correlation between ovarian cancer survival and the abundance of immunosuppressive CD163-CD206-high tumor-associated macrophages (TAMs) and high levels of arachidonic acid (AA) in the tumor microenvironment. A research also indicates that the high expression of CD163 and CD206/MRC1 in TAMs is closely related to the inhibition of cytokine-triggered signals, reflecting impaired interferon and IL-6 transcriptional responses in monocyte-derived macrophages by AA (78). How does arachidonic acid affect the cytokine-triggered signals in macrophages? It influences the signal transduction of cytokine-triggered signals in macrophages by inhibiting the transcriptional responses to interferon and IL-6. This inhibition of pro-inflammatory signals is caused by dysfunction of homologous receptors, manifested as inhibition of JAK1, JAK2, STAT1, and STAT3 phosphorylation, as well as the relocation of interferon receptor IFNAR1, STAT1, and other immune regulatory proteins in lipid rafts. Exposure to AA leads to significant accumulation of free AA in lipid rafts, which appears to be a crucial mechanism, as inhibiting its binding to phospholipids does not affect AA-mediated interference with STAT1 phosphorylation. Therefore, the association between arachidonic acid and TAMs in the ovarian cancer microenvironment involves high levels of arachidonic acid impairing the signal transduction and transcriptional responses of TAMs, leading to an immunosuppressive environment. Additionally, macrophages play a key role in tumor growth, metastasis, immune suppression, and chemoresistance, contributing significantly to the progression and treatment of drug resistance in ovarian cancer (79).
An article entitled “Targeting LTA4H facilitates the reshaping of the immune microenvironment mediated by CCL5 and sensitizes ovarian cancer to Cisplatin” discusses the establishment of a prognosis model for ovarian cancer based on pufa-related genes, the role of the downstream LTA4H gene in the progression and drug resistance of ovarian cancer, and potential treatment strategies for ovarian cancer (80). The article highlights the significant role of LTA4H in influencing tumor characteristics and the immune microenvironment in the context of ovarian cancer. Positive correlation between LTA4H and poor prognosis in ovarian cancer has been observed, with the lack of LTA4H enhancing sensitivity to Cisplatin. Knockdown of LTA4H has been shown to inhibit the proliferation of ovarian cancer cells, while high expression of LTA4H leads to a decrease in infiltrating CD8+ T cells, which are crucial for anti-tumor immune responses. Furthermore, LTA4H is associated with abnormal metabolism in the arachidonic acid (AA) pathway, resulting in a reduction of certain chemokines such as CCL5. The decrease in chemokines may lead to changes in the composition of immune cells in the tumor microenvironment. LTA4H has been identified as a potential therapeutic target. Targeting LTA4H, whether through genetic manipulation or chemical inhibition, can yield favorable therapeutic effects for ovarian cancer. However, targeted therapies and drug researches related to arachidonic acid metabolism in ovarian cancer are still in the exploratory stage and have not yet been widely applied in clinical settings. Due to the complexity of the biochemical properties of AA and its metabolites, inhibitors of AA have been continuously discovered to have effects in tumors. Additionally, COX-2, HETEs, and key enzymes in their metabolic pathways may become potential targets for early cancer detection and treatment.
COX-2, LOX-5, and HETEs, along with the key enzymes in their metabolic pathways, could be potential targets for the early detection and treatment of cancer. The standard initial treatment for ovarian cancer is a combination of platinum and taxane (81, 82). However, due to the complexity of the biochemical properties of AA and its metabolites, the role of its inhibitors in tumors is continually being uncovered.
The COX inhibitor SC-560 was initially used as a pharmacological tool to study the role of COX-1-derived prostaglandins in inflammation and pain. It was later found that at specific doses of COX-1 inhibition, SC-560 exhibited mild to moderate inhibitory effects on tumor growth. Its anti-tumor activity has been demonstrated in various ovarian and colorectal cancer in vitro models and other types of tumor tissue. SC-560 also enhanced the sensitivity of paclitaxel-resistant ovarian cancer cell lines with MDR1/p-glycoprotein upregulation to paclitaxel. It belongs to a group of small molecules that may target specific genes in ovarian cancer stem cells (OVCSC), suggesting that SC-560 could be a promising lead compound for ovarian cancer (83).
Reduced expression of COX-2 and PGE is positively associated with decreased severity and occurrence of ovarian cancer. Currently, COX-2 has been recognized as a new cancer chemoprevention and therapeutic target. Selective COX-2 inhibitors like celecoxib, when used in combination with anti-cancer drugs, can overcome multidrug resistance in various cancers. They can also reduce cell growth, increase cleaved caspase-3 activity, and induce cell cycle G1 phase arrest in a dose-dependent manner in ovarian cancer cells (84).
Additionally, recent studies shown that the CYP4A/F-20-HETE pathway has a positive feedback regulatory effect (85). The AA pathway inhibits tumor cell migration and invasion by inhibiting 20-HETE synthesis. This is achieved by using the selective 20-HETE inhibitor N-hydroxy-N’-(4-butyl-2-methylphenyl)formamidine (HET0016), which significantly reduces the levels of vascular endothelial growth factor responsible for tumor cell communication with the microenvironment (86, 87), whether used alone or in combination. It is worth noting that ovarian cancer shows upregulation of CYP4A/F family enzymes involved in 20-HETE production and widespread use of HET0016 as a therapeutic approach against excessive proliferation (88).
Studies have also indicated that berberine, a natural compound with low cytotoxicity in normal cells, effectively inhibits the regeneration of post-chemotherapy ovarian cancer cells, particularly SKOV3 cells induced by VP16 (82, 89). Berberine can lower AA levels while increasing PGE2 levels, thus reversing the caspase-3-iPLA2-AA-COX-2-PGE2 pathway induced by chemotherapy drugs in SKOV-3 cells (90). This confirms that berberine can prevent recurrence of ovarian cancer, suggesting that combining chemotherapy drugs with berberine may prove to be an effective approach for preventing its recurrence (89).
To comprehensively evaluate the function and signaling pathways of AA metabolism related genes in OC, we obtained transcriptome expression profile data from TCGA-OV cohort and normal ovarian tissues, and after analysis, a total of 8885 differentially expressed genes (DEGs) were identified (Figure 5A).Then, veen analysis was utilized to identify the above DEGs and AA metabolism related genes,resulting in a total of 34 overlapping DEGs (Figure 5B). To further determine the function of 34 candidate genes with related signaling pathways in OC, enrichment analysis was performed. Gene Ontology (GO)-biological process (BP) analysis showed that candidate genes were primarily involved in carboxylic acid metabolic process and monocarboxylic acid biosynthetic process, while GO-cellular component (CC) analysis showed that candidate genes were mainly located in endomembrane system and organelle membrane, with molecular functions (MF) mainly including aromatase activity, metal ion binding, and cation binding (Figure 5C).
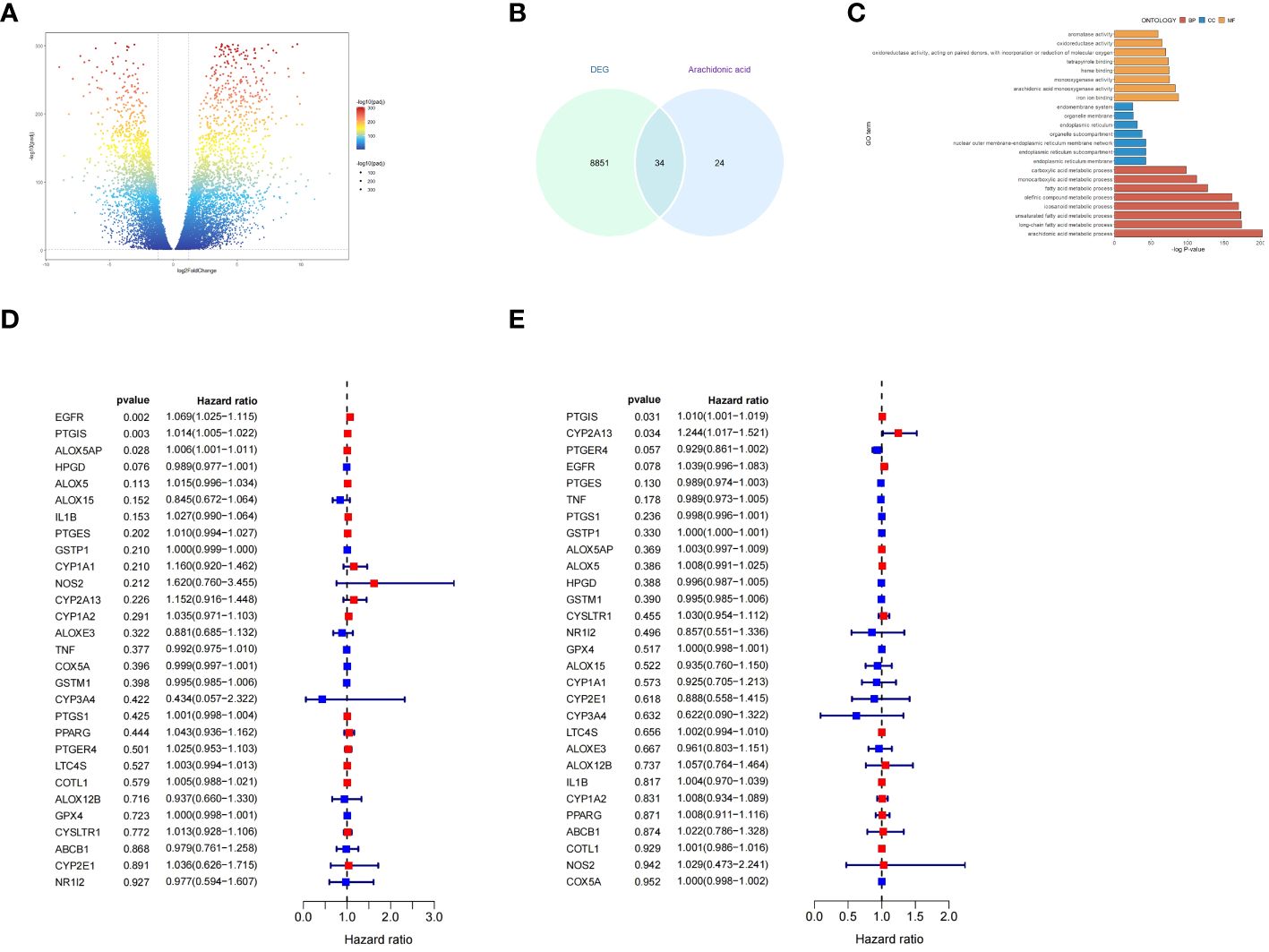
Figure 5 Functional and signaling pathway enrichment analysis of AA metabolism related genes in OC. (A) Volcano plot of DEGs between ovarian cancer and non-tumor tissues based on the TCGA (OV for ovarian cancer, accessed on 20240329) and GTEx (Ovary, accessed on 20240329) database. (B) Venn diagram showing the number of DEGs and AA metabolism related genes; the intersection part is the total number of genes in the two datasets. (C) Results of GO analysis of overlapping DEGs. (D, E) Forest plot demonstrating the univariate Cox model results of 34 OS/PFS-related key genes of AA metabolism.
To discover the potential prognostic significance of key genes of AA metabolism, we performed a univariate Cox hazard regression analysis. The expressions of 3 genes were found to be significantly associated with OC patient with overall survival(OS) and 2 genes associated with OC patient with progression-free survival (PFS) (Figures 5D, E).
With the continuous development of research methods and tools, it has been gradually discovered that AA and its metabolites play important roles in the proliferation, metastasis, apoptosis, angiogenesis, and inflammatory responses of various tumor cells such as ovarian cancer, as well as in treatment and prognosis. High levels of COX, LOX, CYP, and key enzymes in related metabolic pathways may serve as potential targets for early detection and treatment of ovarian cancer. These pathways have been utilized to assess the progression of ovarian cancer, which is crucial for clinical diagnosis of the disease, especially early diagnosis. As an important metabolite of AA metabolism, an increased level of 15(S)-HETE has been identified in various cancers, including non-small cell lung cancer and breast cancer, which suggests that 15(S)-HETE could be served as a potential biomarker for cancer diagnosis (44).245 epithelial ovarian cancer samples were explored by tissue microarray and revealed a higher expression of 12-LOX. Furthermore, it was found that free fatty acid metabolism via LOX pathway leads to an elevated level of 8-HETE in women at risk for developing ovarian cancer. Therefore, measuring levels of 8-HETE could be proposed as an important indicator for assessing the risk of ovarian cancer (91).
This review emphasizes the critical role of AA and its metabolites in the occurrence, development, and metastasis of ovarian cancer. However, due to the complex biochemical nature of AA and its metabolites, their role in ovarian cancer remains challenging. Further research into the relationship between AA and tumor development is essential, requiring a more comprehensive and systematic exploration of the association between inhibitors and combination therapy efficacy. It is anticipated that with the discovery of more biomarkers, AA will usher in a new era of gene-targeted cancer therapy.
QX: Visualization, Writing – original draft. WG: Conceptualization, Writing – review & editing. JY: Formal Analysis, Writing – review & editing. ZX: Project administration, Writing – review & editing. ZJ: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Yangfan Plan of Shanghai Science and Technology Commission (No. 22YF1404700 for ZJ), the Research Start Up Fund of Huashan Hospital (No.2021QD043 for ZJ),the Medical Health Science and Technology Project of Zhejiang Province (No.2022KY627 and 2024KY793 for WG).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1381894/full#supplementary-material
1. Zhang R, Siu MKY, Ngan HYS, Chan KKL. Molecular biomarkers for the early detection of ovarian cancer. Int J Mol Sci. (2022) 23(19):12041. doi: 10.3390/ijms231912041
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. (2018) 68:284–96. doi: 10.3322/caac.21456
4. Beeghly-Fadiel A, Wilson AJ, Keene S, El Ramahi M, Xu S, Marnett LJ, et al. Differential cyclooxygenase expression levels and survival associations in type I and type II ovarian tumors. J Ovarian Res. (2018) 11(1):17. doi: 10.1186/s13048-018-0389-9
5. Yu W, Lei Q, Yang L, Qin G, Liu S, Wang D, et al. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J Hematol Oncol. (2021) 14:187. doi: 10.1186/s13045-021-01200-4
6. Yin X, Xu R, Song J, Ruze R, Chen Y, Wang C, et al. Lipid metabolism in pancreatic cancer: emerging roles and potential targets. Cancer Commun (Lond). (2022) 42:1234–56. doi: 10.1002/cac2.12360
7. Zhang Y, Liu Y, Sun J, Zhang W, Guo Z, Ma Q. Arachidonic acid metabolism in health and disease. MedComm (2020). (2023) 4:e363. doi: 10.1002/mco2.363
8. Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci. (2004) 25:331–6. doi: 10.1016/S0165-6147(04)00115-4
9. McFadyen MC, Cruickshank ME, Miller ID, McLeod HL, Melvin WT, Haites NE, et al. Cytochrome P450 CYP1B1 over-expression in primary and metastatic ovarian cancer. Br J Cancer. (2001) 85:242–6. doi: 10.1054/bjoc.2001.1907
10. Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med. (2017) 23:631–7. doi: 10.1038/nm.4297
11. Moran JH, Weise R, Schnellmann RG, Freeman JP, Grant DF. Cytotoxicity of linoleic acid diols to renal proximal tubular cells. Toxicol Appl Pharmacol. (1997) 146:53–9. doi: 10.1006/taap.1997.8197
12. Medina-Gomez G, Gray S, Vidal-Puig A. Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1). Public Health Nutr. (2007) 10:1132–7. doi: 10.1017/S1368980007000614
13. Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond). (2011) 8:36. doi: 10.1186/1743-7075-8-36
14. Yarla NS, Bishayee A, Sethi G, Reddanna P, Kalle AM, Dhananjaya BL, et al. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol. (2016) 40-41:48–81. doi: 10.1016/j.semcancer.2016.02.001
15. Bosma KJ, Kaiser CE, Kimple ME, Gannon M. Effects of arachidonic acid and its metabolites on functional beta-cell mass. Metabolites. (2022) 12(4):342. doi: 10.3390/metabo12040342
16. Wang B, Wu L, Chen J, Dong L, Chen C, Wen Z, et al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther. (2021) 6:94. doi: 10.1038/s41392-020-00443-w
17. Cui L, Zhao Y, Pan Y, Zheng X, Shao D, Jia Y, et al. Chemotherapy induces ovarian cancer cell repopulation through the caspase 3-mediated arachidonic acid metabolic pathway. Onco Targets Ther. (2017) 10:5817–26. doi: 10.2147/OTT
18. Ma Y, Zheng L, Wang Y, Gao Y, Xu Y. Arachidonic acid in follicular fluid of PCOS induces oxidative stress in a human ovarian granulosa tumor cell line (KGN) and upregulates GDF15 expression as a response. Front Endocrinol (Lausanne). (2022) 13:865748. doi: 10.3389/fendo.2022.865748
19. Yoshimura. Arachidonic acid pathway: A molecular target in human testicular cancer (Review). Mol Med Rep. (2009) 2(4):527–31. doi: 10.3892/mmr
20. Wang T, Fu X, Chen Q, Patra JK, Wang D, Wang Z, et al. Arachidonic acid metabolism and kidney inflammation. Int J Mol Sci. (2019) 20(15):3683. doi: 10.3390/ijms20153683
21. Pannunzio A, Coluccia M. Cyclooxygenase-1 (COX-1) and COX-1 inhibitors in cancer: A review of oncology and medicinal chemistry literature. Pharm (Basel). (2018) 11(4):101. doi: 10.3390/ph11040101
22. Uddin MJ, Wilson AJ, Crews BC, Malerba P, Uddin MI, Kingsley PJ, et al. Discovery of furanone-based radiopharmaceuticals for diagnostic targeting of COX-1 in ovarian cancer. ACS Omega. (2019) 4:9251–61. doi: 10.1021/acsomega.9b01093
23. Renaldi K, Simadibrata M, Rahadiani N, Handjari DR, William A, Sinuraya F, et al. Prognostic value of COX-2, NF-κB, and sp1 tissue expressions in pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Turk J Gastroenterol. (2021) 32:956–70. doi: 10.5152/tjg
24. Balamurugan K, Poria DK, Sehareen SW, Krishnamurthy S, Tang W, McKennett L, et al. Stabilization of E-cadherin adhesions by COX-2/GSK3β signaling is a targetable pathway in metastatic breast cancer. JCI Insight. (2023) 8(6):e156057. doi: 10.1172/jci.insight.156057
25. Musalam A, Andarawi M, Osman M, Al-Shriam M, Elrefaie A, Mahfouz AA, et al. Alterations of COX-2, HER-2/neu and E-Cadherin protein expression in the prostatic adenocarcinoma: preliminary findings. Am J Transl Res. (2019) 11:1653–67.
26. Desind SZ, Iacona JR, Yu CY, Mitrofanova A, Lutz CS. PACER lncRNA regulates COX-2 expression in lung cancer cells. Oncotarget. (2022) 13:291–306. doi: 10.18632/oncotarget.v13
27. Alves AF, Baldissera VD, Chiela ECF, Cerski CTS, Fontes PRO, Fernandes MDC, et al. Altered expression of COX-2 and TNF-α in patients with hepatocellular carcinoma. Rev Esp Enferm Dig. (2019) 111:364–70. doi: 10.17235/reed.2019.5898/2018
28. Tudor DV, Bâldea I, Lupu M, Kacso T, Kutasi E, Hopârtean A, et al. COX-2 as a potential biomarker and therapeutic target in melanoma. Cancer Biol Med. (2020) 17:20–31. doi: 10.20892/j.issn.2095-3941.2019.0339
29. Xin C, Chu L, Zhang L, Geng D, Wang Y, Sun D, et al. Expression of cytosolic phospholipase A2 (cPLA2)-arachidonic acid (AA)-cyclooxygenase-2 (COX-2) pathway factors in lung cancer patients and its implication in lung cancer early detection and prognosis. Med Sci Monitor. (2019) 25:5543–51. doi: 10.12659/MSM.915314
30. Ye Y, Wang X, Jeschke U, von Schönfeldt V. COX-2-PGE(2)-EPs in gynecological cancers. Arch Gynecol Obstet. (2020) 301:1365–75. doi: 10.1007/s00404-020-05559-6
31. Lai ZZ, Yang HL, Ha SY, Chang KK, Mei J, Zhou WJ, et al. Cyclooxygenase-2 in endometriosis. Int J Biol Sci. (2019) 15:2783–97. doi: 10.7150/ijbs.35128
32. Sato N, Yako Y, Maruyama T, Ishikawa S, Kuromiya K, Tokuoka SM, et al. The COX-2/PGE(2) pathway suppresses apical elimination of RasV12-transformed cells from epithelia. Commun Biol. (2020) 3:132. doi: 10.1038/s42003-020-0847-y
33. Kajita S, Ruebel KH, Casey MB, Nakamura N, Lloyd RV. Role of COX-2, thromboxane A2 synthase, and prostaglandin I2 synthase in papillary thyroid carcinoma growth. Modern Pathol. (2005) 18:221–7. doi: 10.1038/modpathol.3800285
34. Hyde CA, Missailidis S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int Immunopharmacol. (2009) 9:701–15. doi: 10.1016/j.intimp.2009.02.003
35. Xu L, Croix BS. Improving VEGF-targeted therapies through inhibition of COX-2/PGE2 signaling. Mol Cell Oncol. (2014) 1:e969154. doi: 10.4161/23723548.2014.969154
36. Cheng HW, Chen YF, Wong JM, Weng CW, Chen HY, Yu SL, et al. Cancer cells increase endothelial cell tube formation and survival by activating the PI3K/Akt signalling pathway. J Exp Clin Cancer Res. (2017) 36:27. doi: 10.1186/s13046-017-0495-3
37. Ghoneum A, Said N. PI3K-AKT-mTOR and NFκB pathways in ovarian cancer: implications for targeted therapeutics. Cancers (Basel). (2019) 11(7):949. doi: 10.3390/cancers11070949
38. Liu L, Wu N, Wang Y, Zhang X, Xia B, Tang J, et al. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K / AKT oncogenic signaling. J Exp Clin Cancer Res. (2019) 38:106. doi: 10.1186/s13046-019-1061-y
39. Joki T, Heese O, Nikas DC, Bello L, Zhang J, Kraeft SK, et al. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res. (2000) 60:4926–31.
40. Zhang X, Qu P, Zhao H, Zhao T, Cao N. COX−2 promotes epithelial−mesenchymal transition and migration in osteosarcoma MG−63 cells via PI3K/AKT/NF−κB signaling. Mol Med Rep. (2019) 20:3811–9. doi: 10.3892/mmr
41. Seo JM, Park S, Kim JH. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J Biol Chem. (2012) 287:13840–9. doi: 10.1074/jbc.M111.317131
42. Gómez-Valenzuela F, Wichmann I, Suárez F, Kato S, Ossandón E, Hermoso M, et al. Cyclooxygenase-2 Blockade Is Crucial to Restore Natural Killer Cell Activity before Anti-CTLA-4 Therapy against High-Grade Serous Ovarian Cancer. Cancers (Basel). (2023) 16(1):80. doi: 10.3390/cancers16010080
43. Frejborg E, Salo T, Salem A. Role of cyclooxygenase-2 in head and neck tumorigenesis. Int J Mol Sci. (2020) 21(23):9246. doi: 10.3390/ijms21239246
44. Hada M, Edin ML, Hartge P, Lih FB, Wentzensen N, Zeldin DC, et al. Prediagnostic serum levels of fatty acid metabolites and risk of ovarian cancer in the prostate, lung, colorectal, and ovarian (PLCO) cancer screening trial. Cancer Epidemiol Biomarkers Prev. (2019) 28:189–97. doi: 10.1158/1055-9965.EPI-18-0392
45. Korbecki J, Rębacz-Maron E, Kupnicka P, Chlubek D, Baranowska-Bosiacka I. Synthesis and significance of arachidonic acid, a substrate for cyclooxygenases, lipoxygenases, and cytochrome P450 pathways in the tumorigenesis of glioblastoma multiforme, including a pan-cancer comparative analysis. Cancers (Basel). (2023) 15(3):946. doi: 10.3390/cancers15030946
46. Xia C, Sadeghi L, Strååt K, Merrien M, Wright AP, Sander B, et al. Intrinsic 5-lipoxygenase activity regulates migration and adherence of mantle cell lymphoma cells. Prostaglandins Other Lipid Mediat. (2021) 156:106575. doi: 10.1016/j.prostaglandins.2021.106575
47. Smith PG, Roque D, Ching MM, Fulton A, Rao G, Reader JC. The role of eicosanoids in gynecological Malignancies. Front Pharmacol. (2020) 11:1233. doi: 10.3389/fphar.2020.01233
48. Moore GY, Pidgeon GP. Cross-talk between cancer cells and the tumour microenvironment: the role of the 5-lipoxygenase pathway. Int J Mol Sci. (2017) 18(2):236. doi: 10.3390/ijms18020236
49. Poczobutt JM, Nguyen TT, Hanson D, Li H, Sippel TR, Weiser-Evans MCM, et al. Deletion of 5-lipoxygenase in the tumor microenvironment promotes lung cancer progression and metastasis through regulating T cell recruitment. J Immunol. (2016) 196:891–901. doi: 10.4049/jimmunol.1501648
50. Yang P, Cartwright CA, Li J, Wen S, Prokhorova IN, Shureiqi I, et al. Arachidonic acid metabolism in human prostate cancer. Int J Oncol. (2012) 41:1495–503. doi: 10.3892/ijo.2012.1588
51. Liu Q, Tan W, Che J, Yuan D, Zhang L, Sun Y, et al. 12-HETE facilitates cell survival by activating the integrin-linked kinase/NF-κB pathway in ovarian cancer. Cancer Manage Res. (2018) 10:5825–38. doi: 10.2147/CMAR.S180334
52. Wasilewicz MP, Kołodziej B, Bojułko T, Kaczmarczyk M, Sulzyc-Bielicka V, Bielicki D, et al. Overexpression of 5-lipoxygenase in sporadic colonic adenomas and a possible new aspect of colon carcinogenesis. Int J Colorectal Dis. (2010) 25:1079–85. doi: 10.1007/s00384-010-0980-z
53. Bishayee K, Khuda-Bukhsh AR. 5-lipoxygenase antagonist therapy: a new approach towards targeted cancer chemotherapy. Acta Biochim Biophys Sin (Shanghai). (2013) 45:709–19. doi: 10.1093/abbs/gmt064
54. Alzahrani AM, Rajendran P. The multifarious link between cytochrome P450s and cancer. Oxid Med Cell Longev. (2020) 2020:3028387. doi: 10.1155/2020/3028387
55. Elfaki I, Mir R, Almutairi FM, Duhier FMA. Cytochrome P450: polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac J Cancer Prev. (2018) 19:2057–70. doi: 10.22034/APJCP.2018.19.8.2057
56. Shi Z, He Z, Wang DW. CYP450 epoxygenase metabolites, epoxyeicosatrienoic acids, as novel anti-inflammatory mediators. Molecules. (2022) 27(12):3873. doi: 10.3390/molecules27123873
57. Reddy KK, Vidya Rajan VK, Gupta A, Aparoy P, Reddanna P. Exploration of binding site pattern in arachidonic acid metabolizing enzymes, Cyclooxygenases and Lipoxygenases. BMC Res Notes. (2015) 8:152. doi: 10.1186/s13104-015-1101-4
58. Kim HS, Moon SJ, Lee SE, Hwang GW, Yoo HJ, Song JW. The arachidonic acid metabolite 11,12-epoxyeicosatrienoic acid alleviates pulmonary fibrosis. Exp Mol Med. (2021) 53:864–74. doi: 10.1038/s12276-021-00618-7
59. Zhang Z, Hu D, Zhou M, Liu H, Wu J, Huang S, et al. 14,15-Epoxyeicosatrienoic acid induces the proliferation and anti- apoptosis of human carcinoma cell. Daru. (2011) 19:462–8.
60. Rand AA, Barnych B, Morisseau C, Cajka T, Lee KSS, Panigrahy D, et al. Cyclooxygenase-derived proangiogenic metabolites of epoxyeicosatrienoic acids. Proc Natl Acad Sci. (2017) 114:4370–5. doi: 10.1073/pnas.1616893114
61. Wang F, Chen J, Wang L, Ma Y, Mayinuer N. CYP1A1 genetic polymorphisms and uterine leiomyoma risk: a meta-analysis. Int J Clin Exp Med. (2015) 8:3590–4.
62. Bozina N, Bradamante V, Lovrić M. Genetic polymorphism of metabolic enzymes P450 (CYP) as a susceptibility factor for drug response, toxicity, and cancer risk. Arh Hig Rada Toksikol. (2009) 60:217–42. doi: 10.2478/10004-1254-60-2009-1885
63. Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer. (2009) 9:187. doi: 10.1186/1471-2407-9-187
64. Al-Saraireh YM, Alshammari F, Abu-Azzam OH, Al-Dalain SM, Al-Sarayra YM, Haddad M, et al. Targeting cytochrome P450 enzymes in ovarian cancers: new approaches to tumor-selective intervention. Biomedicines. (2023) 11(11):2898. doi: 10.20944/preprints202309.0152.v1
65. Ding Y, Zhuang S, Li Y, Yu X, Lu M, Ding N. Hypoxia-induced HIF1α dependent COX2 promotes ovarian cancer progress. J Bioenergetics Biomembranes. (2021) 53:441–8. doi: 10.1007/s10863-021-09900-9
66. Sun H, Zhang X, Sun D, Jia X, Xu L, Qiao Y, et al. COX-2 expression in ovarian cancer: an updated meta-analysis. Oncotarget. (2017) 8:88152–62. doi: 10.18632/oncotarget.v8i50
67. Song JH, Lee CJ, An HJ, Yoo SM, Kang HC, Lee JY, et al. Magnolin targeting of ERK1/2 inhibits cell proliferation and colony growth by induction of cellular senescence in ovarian cancer cells. Mol Carcinog. (2019) 58:88–101. doi: 10.1002/mc.22909
68. Song G, Cai QF, Mao YB, Ming YL, Bao SD, Ouyang GL. Osteopontin promotes ovarian cancer progression and cell survival and increases HIF-1alpha expression through the PI3-K/Akt pathway. Cancer Sci. (2008) 99:1901–7. doi: 10.1111/j.1349-7006.2008.00911.x
69. Liu Q, Tan W, Che J, Yuan D, Zhang L, Sun Y, et al. 12-HETE facilitates cell survival by activating the integrin-linked kinase/NF-κB pathway in ovarian cancer. Cancer Manag Res. (2018) 10:5825–38. doi: 10.2147/CMAR
70. Zheng Z, Li Y, Jin G, Huang T, Zou M, Duan S. The biological role of arachidonic acid 12-lipoxygenase (ALOX12) in various human diseases. BioMed Pharmacother. (2020) 129:110354. doi: 10.1016/j.biopha.2020.110354
71. Guo AM, Liu X, Al-Wahab Z, Maddippati KR, Ali-Fehmi R, Scicli AG, et al. Role of 12-lipoxygenase in regulation of ovarian cancer cell proliferation and survival. Cancer Chemother Pharmacol. (2011) 68:1273–83. doi: 10.1007/s00280-011-1595-y
72. Chistyakov DV, Kovalenko LV, Donnikov MY, Sergeeva MG. Blood oxylipin profiles as markers of oncological diseases. Biochem (Moscow). (2023) 88:621–9. doi: 10.1134/S000629792305005X
73. Gréen H, Khan MS, Jakobsen-Falk I, Åvall-Lundqvist E, Peterson C. Impact of CYP3A5*3 and CYP2C8-HapC on paclitaxel/carboplatin-induced myelosuppression in patients with ovarian cancer. J Pharm Sci. (2011) 100:4205–9. doi: 10.1002/jps.22680
74. van Eijk M, Boosman RJ, Schinkel AH, Huitema ADR, Beijnen JH. Cytochrome P450 3A4, 3A5, and 2C8 expression in breast, prostate, lung, endometrial, and ovarian tumors: relevance for resistance to taxanes. Cancer Chemother Pharmacol. (2019) 84:487–99. doi: 10.1007/s00280-019-03905-3
75. Schoutrop E, Moyano-Galceran L, Lheureux S, Mattsson J, Lehti K, Dahlstrand H, et al. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment. Semin Cancer Biol. (2022) 86:207–23. doi: 10.1016/j.semcancer.2022.03.027
76. Dietze R, Hammoud MK, Gomez-Serrano M, Unger A, Bieringer T, Finkernagel F, et al. Phosphoproteomics identify arachidonic-acid-regulated signal transduction pathways modulating macrophage functions with implications for ovarian cancer. Theranostics. (2021) 11:1377–95. doi: 10.7150/thno.52442
77. Bamias A, Koutsoukou V, Terpos E, Tsiatas ML, Liakos C, Tsitsilonis O, et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNFalpha in ascites from advanced ovarian cancer: Association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol Oncol. (2008) 108:421–7. doi: 10.1016/j.ygyno.2007.10.018
78. Hammoud MK, Dietze R, Pesek J, Finkernagel F, Unger A, Bieringer T, et al. Arachidonic acid, a clinically adverse mediator in the ovarian cancer microenvironment, impairs JAK-STAT signaling in macrophages by perturbing lipid raft structures. Mol Oncol. (2022) 16:3146–66. doi: 10.1002/1878-0261.13221
79. Nowak M, Klink M. The role of tumor-associated macrophages in the progression and chemoresistance of ovarian cancer. Cells. (2020) 9(5):1299. doi: 10.3390/cells9051299
80. Guo Z, Huang J, Huo X, Huang C, Yu X, Sun Y, et al. Targeting LTA4H facilitates the reshaping of the immune microenvironment mediated by CCL5 and sensitizes ovarian cancer to Cisplatin. Sci China Life Sci. (2024). doi: 10.1007/s11427-023-2444-5
81. Sørensen BH, Thorsteinsdottir UA, Lambert IH. Acquired cisplatin resistance in human ovarian A2780 cancer cells correlates with shift in taurine homeostasis and ability to volume regulate. Am J Physiology-Cell Physiol. (2014) 307:C1071–80. doi: 10.1152/ajpcell.00274.2014
82. Zhang Q, Yan G, Lei J, Chen Y, Wang T, Gong J, et al. The SP1-12LOX axis promotes chemoresistance and metastasis of ovarian cancer. Mol Med. (2020) 26:39. doi: 10.1186/s10020-020-00174-2
83. Li W, Wan L, Zhai LY, Wang J. Effects of SC-560 in combination with cisplatin or taxol on angiogenesis in human ovarian cancer xenografts. Int J Mol Sci. (2014) 15:19265–80. doi: 10.3390/ijms151019265
84. Spyra S, Meisner A, Schaefer M, Hill K. COX-2-selective inhibitors celecoxib and deracoxib modulate transient receptor potential vanilloid 3 channels. Br J Pharmacol. (2017) 174:2696–705. doi: 10.1111/bph.13893
85. Capdevila JH, Wang W, Falck JR. Arachidonic acid monooxygenase: Genetic and biochemical approaches to physiological/pathophysiological relevance. Prostaglandins Other Lipid Mediat. (2015) 120:40–9. doi: 10.1016/j.prostaglandins.2015.05.004
86. Chen L, Ackerman R, Saleh M, Gotlinger KH, Kessler M, Mendelowitz LG, et al. 20-HETE regulates the angiogenic functions of human endothelial progenitor cells and contributes to angiogenesis in vivo. J Pharmacol Exp Ther. (2014) 348:442–51. doi: 10.1124/jpet.113.210120
87. Pascale JV, Wolf A, Kadish Y, Diegisser D, Kulaprathazhe MM, Yemane D, et al. 20-Hydroxyeicosatetraenoic acid (20-HETE): Bioactions, receptors, vascular function, cardiometabolic disease and beyond. Adv Pharmacol. (2023) 97:229–55. doi: 10.1016/bs.apha.2023.01.002
88. Borin T, Angara K, Rashid M, Achyut B, Arbab A. Arachidonic acid metabolite as a novel therapeutic target in breast cancer metastasis. Int J Mol Sci. (2017) 18(12):2661. doi: 10.3390/ijms18122661
89. Zhao Y, Cui L, Pan Y, Shao D, Zheng X, Zhang F, et al. Berberine inhibits the chemotherapy-induced repopulation by suppressing the arachidonic acid metabolic pathway and phosphorylation of FAK in ovarian cancer. Cell Prolif. (2017) 50(6):e12393. doi: 10.1111/cpr.12393
90. Liu L, Fan J, Ai G, Liu J, Luo N, Li C, et al. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol Res. (2019) 52:37. doi: 10.1186/s40659-019-0243-6
Keywords: arachidonic acid, ovarian cancer, metabolic pathway, biological marker, targeted therapy
Citation: Xia Q, Gao W, Yang J, Xing Z and Ji Z (2024) The deregulation of arachidonic acid metabolism in ovarian cancer. Front. Oncol. 14:1381894. doi: 10.3389/fonc.2024.1381894
Received: 04 February 2024; Accepted: 19 April 2024;
Published: 02 May 2024.
Edited by:
Shivendra Vikram Singh, St. Jude Children’s Research Hospital, United StatesReviewed by:
Shafiq Khan, Clark Atlanta University, United StatesCopyright © 2024 Xia, Gao, Yang, Xing and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaodong Ji, bnRqemRfMTk5MEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.