
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 15 April 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1378993
This article is part of the Research TopicGenomic Discoveries and Pharmaceutical Development in Urologic TumorsView all 17 articles
Objective: This study aimed to evaluate the relative efficacy and safety of first-line treatment options for metastatic castration-resistant prostate cancer (mCRPC).
Methods: We systematically searched electronic databases, including PubMed and Web of Science, for studies published from their inception to April 3rd, 2023. Inclusion criteria were: 1) Completed Phase III or IV randomized controlled trials (RCTs) registered on ClinicalTrials.gov; 2) Patients with a confirmed diagnosis of mCRPC who had not previously received chemotherapy or novel endocrine therapies. We conducted a network meta-analysis using R software (version 3.4.0). Network graphs and risk of bias graphs were generated using Stata 14.0 and RevMan 5.4, respectively. The primary outcome was overall survival (OS), and the secondary outcome was the incidence of severe adverse events (SAEs).
Results: Seven RCTs encompassing 6,641 patients were included. The network meta-analysis revealed that both docetaxel+prednisone (DP) and cabazitaxel+prednisone (CP) significantly improved OS compared to abiraterone. Compared to placebo, DP showed comparable results to both cabazitaxel 20 mg/m^2+prednisone (C20P) and cabazitaxel 25 mg/m^2+prednisone (C25P) in terms of OS. For SAEs, both DP and C20P were superior to C25P, with no statistical difference between C20P and DP. The probability ranking plots indicated that C25P ranked highest for OS, while DP ranked highest for SAEs.
Conclusions: Based on our network meta-analysis, we recommend cabazitaxel 20 mg/m^2+prednisone (C20P) as the primary choice for first-line management of mCRPC, followed by DP. Enzalutamide and abiraterone are suggested as subsequent options. Radium-223 may be considered for patients presenting with bone metastases.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023443943.
Prostate cancer (PC), the most prevalent malignancy in the male genitourinary system, has recently emerged as the second most common cancer globally (1). The world age-standardized incidence rate is 37.5 per 100,000, with higher prevalence in regions with a high Human Development Index, such as Europe and North America. Many PC patients undergo Androgen Deprivation Therapy (ADT) post-laparoscopic or robotic surgery, showing promising efficacy in the initial and intermediate stages. However, due to various mechanisms such as androgen receptor amplification, mutation, PI3K pathway, or NF-κB pathway aberrations, tumors often develop resistance to ADT, progressing to mCRPC within 18-24 months, frequently accompanied by distant metastases (2). This phase is marked by a dismal prognosis and escalated treatment costs (3). Current therapeutic approaches include second-generation antiandrogens (e.g., abiraterone, enzalutamide, apalutamide), chemotherapy (docetaxel, cabazitaxel), and radionuclide therapy (Radium-223, 177Lu-PSMA) (4, 5). Abiraterone, a CYP17 inhibitor, diminishes androgen levels by inhibiting a crucial enzyme in androgen synthesis. Enzalutamide and apalutamide, as androgen receptor antagonists, prevent androgen from binding to its receptor. Clinical trials have demonstrated the effectiveness of abiraterone and enzalutamide in extending progression-free survival (PFS) and overall survival (OS) in mCRPC patients (6, 7).
Despite this, the absence of direct comparative trials for first-line treatments leaves a gap in knowledge regarding the optimal balance of efficacy and safety. This study aims to fill this void by comparing the effectiveness and safety of first-line mCRPC treatments as reported in randomized clinical trials (RCTs), thereby guiding clinical decision-making.
The inclusion criteria for trials were as follows: 1) Phase III or IV randomized controlled trials (RCTs); 2) Participants diagnosed with metastatic castration-resistant prostate cancer (mCRPC); 3) No history of cytotoxic therapy or androgen receptor inhibitor therapy; 4) Interventions including abiraterone acetate, enzalutamide, apalutamide, docetaxel, cabazitaxel, or Radium-223; 5) Outcomes measuring overall survival (OS) and severe adverse events (SAEs). Exclusion criteria comprised: 1) Studies with incomplete data; 2) Non-English language publications; 3) Trials terminated prematurely for various reasons.
Following initial study selection, a preliminary network graph was produced. In cases where key studies were missing, additional relevant studies were identified and included after thorough discussion, ensuring the completeness of the network graph. The study protocol was registered with PROSPERO (Registration number: CRD42023443943). Our approach to study selection and inclusion aligned with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (8).
We conducted a comprehensive search of electronic databases, including PubMed and Web of Science, for studies published from their inception through April 3rd, 2023. The search included: 1) Completed Phase III or IV randomized controlled trials (RCTs) registered with ClinicalTrials.gov; 2) Patient cohorts with a confirmed diagnosis of metastatic castration-resistant prostate cancer (mCRPC) who had not previously received chemotherapy or novel endocrine therapies. The literature search employed the following terms, used as title/abstract keywords or MeSH terms: ‘castration-resistant prostate cancer’, ‘abiraterone’, ‘enzalutamide’, ‘docetaxel’, ‘Radium-223’, ‘cabazitaxel’.
Data extraction was independently conducted by two reviewers (ZD and WH), following a thorough assessment of all potential abstracts and titles for eligibility. In instances of disagreement or insufficient information, a third reviewer (MY) was consulted to examine the full text for eligibility. Extracted information included patient characteristics (median age, treatment descriptions, and doses) and sites of metastatic disease.
The analysis focused on median overall survival (OS) as the efficacy criterion, while toxicity criteria included the incidence of Grade 3-5 toxicities as per the National Cancer Institute Common Toxicity Criteria, along with the incidence of serious adverse events.
Methodological quality of the included studies was assessed independently by two investigators, utilizing the Cochrane Handbook for Systematic Reviews of Interventions. Each trial was evaluated on the following criteria and assigned a risk of bias rating as low, medium, or high: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting, and presence of any other biases. A trial was deemed to have an overall low risk of bias if all domains were rated as low risk, and high risk if any domain was assessed as high risk. Discrepancies in assessment were resolved through discussion between the two investigators, or by consulting a third investigator for an adjudicated decision.
The network meta-analysis was performed using a Bayesian framework model, employing R software (version 4.3.0) with the gemtc package (9). For the outcomes, overall survival (OS) was estimated using pooled hazard ratios (HRs) with 95% confidence intervals (CIs), and severe adverse events (SAEs) were analyzed using odds ratios (ORs) with 95% CIs. Both fixed-effects and random-effects models were fitted, with the latter accounting for heterogeneity between studies. The results presented in this study are based on the fixed-effects model.
Initial database screening yielded 4,273 references from PubMed and 3,751 from Web of Science (Figure 1). This was narrowed down to 835 potentially relevant trials after initial screening. Upon detailed examination, 7 studies fulfilling the inclusion criteria were selected for analysis. A network graph depicting treatment comparisons is illustrated in Figure 2.
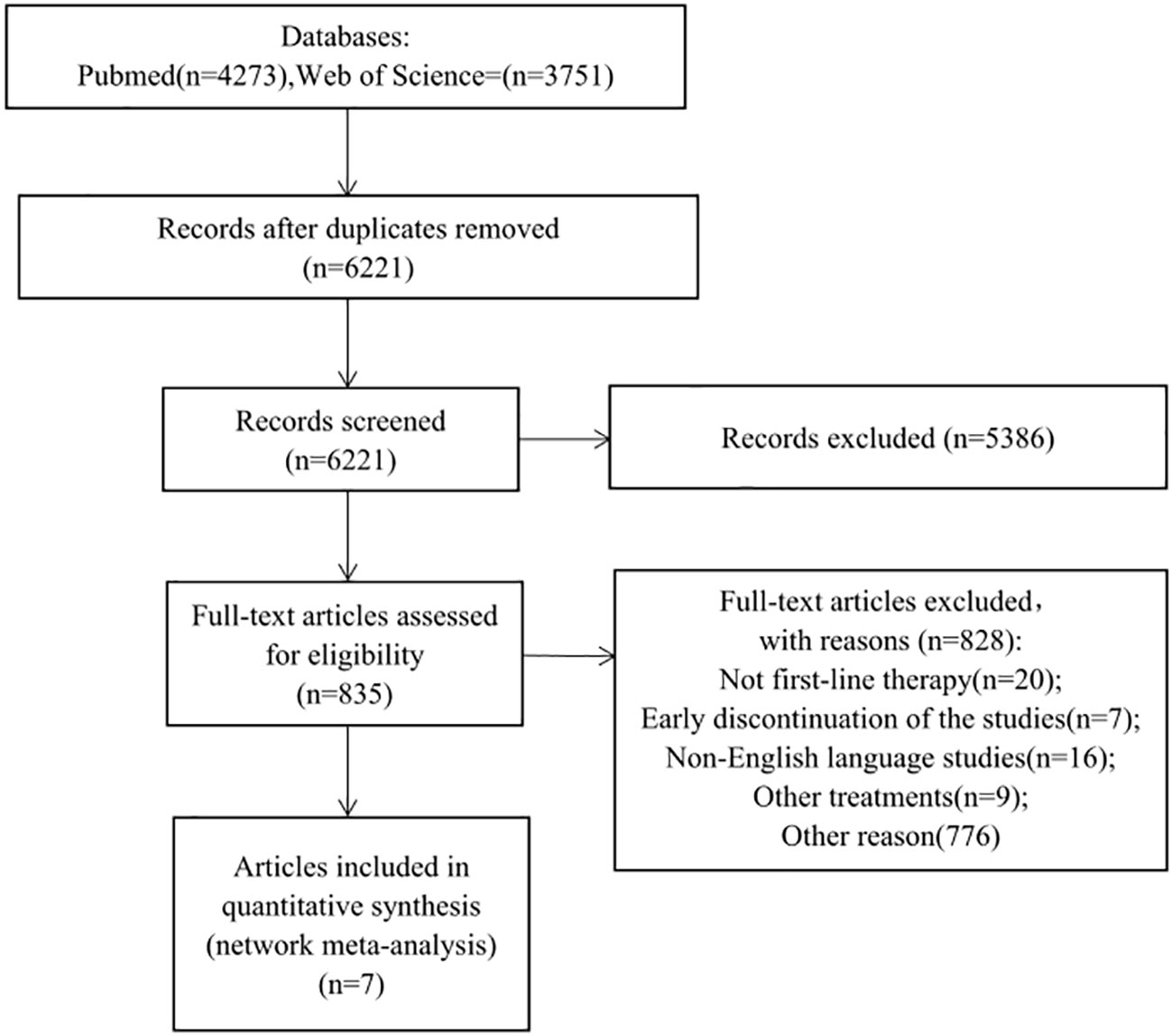
Figure 1 Study fow chart. This network meta-analysis incorporated 6 phase III and 1 phase IV randomized controlled trials (RCTs), enrolling a total of 6,411 patients with metastatic castration-resistant prostate cancer (mCRPC). Eight treatment modalities were analyzed: placebo/prednisone, abiraterone acetate + prednisone, enzalutamide, cabazitaxel 20/25mg/m^2, docetaxel, Radium-223 + abiraterone acetate + prednisone, and apalutamide + abiraterone acetate + prednisolone.
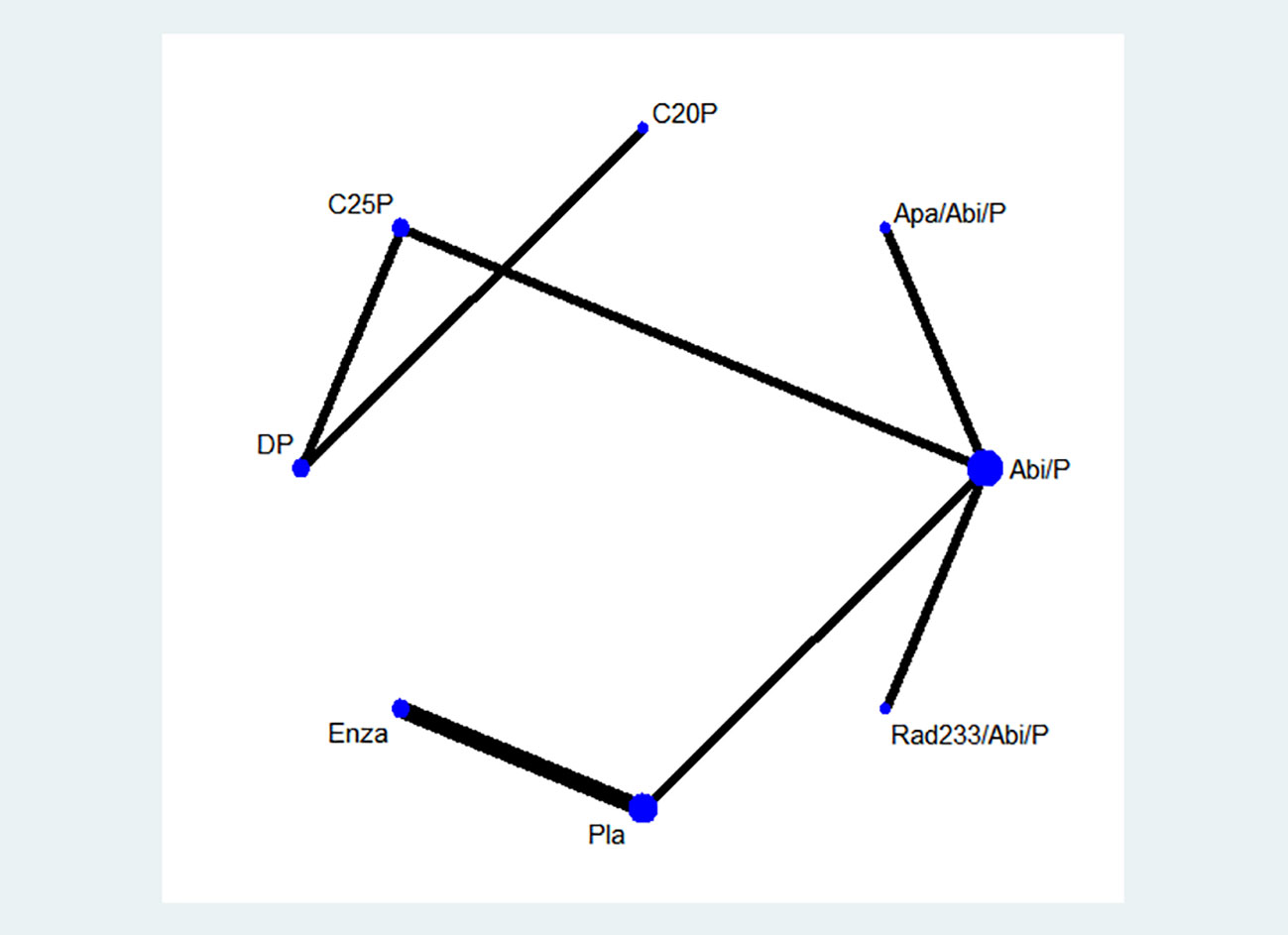
Figure 2 A network graph depicting treatment comparisons is illustrated.DP-docetaxel+prednisone. C25P-cabazitaxel 25 mg/m^2+prednisone, C20P-cabazitaxel 20 mg/m^2+prednisone, Apa/abi/p-apalutamide + abiraterone acetate + prednisolone, Abi/p-abiraterone acetate + prednisone, Rad233/abi/p-Radium-223 + abiraterone acetate + prednisone, Pla-placebo, Enza-enzalutamide.
The most frequently studied treatment was abiraterone acetate + prednisone (4 trials). To complete the network graph, a phase IV second-line treatment RCT comparing cabazitaxel 25mg/m^2 and abiraterone acetate was included after discussion.
The analysis included seven multicenter RCTs, predominantly phase III first-line treatments, with the exception of one phase IV second-line treatment RCT included for network completeness (10–16). These trials spanned 2015 to 2020, involving a total of 6,411 participants. Median sample size per treatment arm was 396 (range, 126-872) patients; median age was 70.6 years (range, 68-71.6 years); median overall survival (OS) was 30.15 months (range, 11-39.1 months). Eligibility criteria primarily required newly diagnosed prostate adenocarcinoma with radiographic evidence of metastasis and adequate performance status, excluding or restricting prior chemotherapy and hormone therapy in the metastatic setting. To ensure network completeness, the CARD trial was additionally included. Baseline characteristics of the 7 studies are detailed in Table 1.
The Cochrane Collaboration tool was employed for quality assessment of the included trials. Bias risk was evaluated across six domains mentioned in the selection criteria. Five of the seven studies demonstrated adequate randomization. The remaining studies lacked specific details on sequence generation methods. Allocation concealment was reported in five trials, with two trials employing open-label designs. Attrition and reporting biases were assessed and managed effectively in the included studies. A summary of the risk-of-bias assessment for each trial is presented in Figure 3.
Network meta-analyses incorporated all eight treatments, evaluating both effectiveness (OS) and safety (SAEs) outcomes (Figure 4).
Treatments showing significant OS improvement over placebo included: docetaxel (HR, 0.53; 95% CI, 0.36-0.79), abiraterone acetate (HR, 0.81; 95% CI, 0.70-0.94), enzalutamide (HR, 0.70; 95% CI, 0.61-0.82), cabazitaxel 20 mg/m^2 (HR, 0.53; 95% CI, 0.35-0.84), and cabazitaxel 25 mg/m^2 (HR, 0.52; 95% CI, 0.36-0.75). Docetaxel also demonstrated superior OS improvement compared to abiraterone and Radium-223 + abiraterone, and was comparable with enzalutamide, cabazitaxel, and apalutamide (Figure 4). Treatment ranking probabilities indicated cabazitaxel 25 mg/m^2 as the most likely best treatment for OS (45% probability).
Regarding SAEs, treatments ranked from safest to least safe were: docetaxel, cabazitaxel 20mg/m^2, cabazitaxel 25mg/m^2, abiraterone, Radium-223 combined with abiraterone, enzalutamide, and apalutamide combined with abiraterone. There were no significant differences between docetaxel, cabazitaxel 20/25 mg/m^2, and placebo in terms of SAEs.
The heterogeneity of our findings (I2) was less than 30%, which indicated that our findings were homogeneous. Therefore, we did not conduct subgroup analysis to identify the source of heterogeneity. Comprehensive results can be found in Supplementary Figure 3.
Out of 7 RCTs, 4 of them were categorized as low risk of bias. Due to the lack of sufficient blinding methods, 3 RCTs were revealed to have high risk of bias. Based on grading the evidence in Table 2, 3 low risk of bias articles were included and produced high certainty of evidence. Based on the grading analysis, it is revealed that all 3 studies not only have low risk of bias but are also not serious in terms of inconsistency, indirectness, and imprecision. All these criteria increase the certainty level and can guide clinicians and policymakers for future events or discussions. Comprehensive results can be found in Table 2.
This network meta-analysis systematically evaluated first-line treatments for metastatic castration-resistant prostate cancer (mCRPC) as delineated in existing Phase III and IV randomized controlled trials (RCTs). A notable majority of these treatments had not previously been directly compared in face-to-face trials. Our comprehensive analysis revealed that chemotherapy regimens, specifically docetaxel and cabazitaxel, demonstrated superior efficacy and safety compared to second-generation anti-hormonal therapies, including abiraterone, enzalutamide, and apalutamide, in the first-line management of mCRPC.
The findings of this network meta-analysis provide new insights into the first-line treatment of metastatic castration-resistant prostate cancer (mCRPC). Current guidelines from the American Urological Association (AUA) recommend abiraterone and enzalutamide as grade A treatments, and docetaxel as a grade B treatment for mCRPC (17). Similarly, the Apccc expert consensus endorses abiraterone and enzalutamide as primary treatments (18). These recommendations contrast with our results, prompting an exploration of potential reasons for these discrepancies. Several factors may contribute to this variation:
AR-V7 Presence: AR-V7, a variant of the androgen receptor (AR) lacking the ligand-binding domain, is frequently observed in mCRPC patients, with about a 30% mutation rate. Antonarakis (19) demonstrated a significant correlation between AR-Vs in circulating tumor cells and clinical outcomes in CRPC patients receiving new AR-targeted therapies. Studies indicate that AR-V7 positivity is associated with resistance and poor efficacy in patients treated with enzalutamide and abiraterone (20). Conversely, AR-V7 status does not significantly affect responses to paclitaxel-based therapies like docetaxel or cabazitaxel (21).
PTEN Deficiency: The tumor suppressor gene PTEN, frequently lost or mutated in cancers, regulates the PI3K−AKT−mTOR signaling pathway. In mCRPC, PTEN gene deletion occurs in 40-60% of cases (22–24). Studies have shown that PTEN deficiency negatively impacts the effectiveness of abiraterone, but does not affect the antitumor activity of docetaxel (25, 26).
DDR Gene Mutations: The impact of DDR (DNA damage response) gene mutations on second-generation hormone therapy and paclitaxel-based therapy remains unclear. Some studies suggest that DDR gene mutations attenuate the efficacy of second-generation hormone therapies, but their impact on the efficacy of cabazitaxel is less certain (27, 28).
Tumor Neuroendocrine Differentiation (NED): NED in mCRPC is a significant factor in treatment response. Hormone therapy is generally less effective in patients with NED. A study found greater OS benefit with a Docetaxel+Prednisone (DP) - Abiraterone Acetate (AA) treatment sequence in patients with elevated NED, compared to an AA-DP sequence (29).In light of these findings, our analysis suggests that the choice of first-line treatment for mCRPC should consider molecular and genetic tumor characteristics to optimize patient outcomes.
The objective of this network meta-analysis was to elucidate the efficacy of cabazitaxel 20 mg/m^2 (C20P) and cabazitaxel 25 mg/m^2 (C25P) over docetaxel+prednisone (DP) in chemotherapy- or hormone therapy-naive patients with metastatic castration-resistant prostate cancer (mCRPC), focusing on overall survival (OS). Our recommendation favors C20P, as it shows comparable OS to C25P but exhibits superior safety in terms of severe adverse events (SAEs), suggesting enhanced tolerability at the lower dose.
The choice of optimal treatment for mCRPC remains a subject of debate. Recently, some scholars have also performed network meta-analysis for first-line treatment of mCRPC (30). Unlike our analysis, this analysis included all period RCTs to form a network and was therefore more exploratory. Our analysis includes Phase III and IV RCTs, and focuses on the high level of evidence to guide clinical application. This analysis included 29 RCTs, involving 12,706 patients and investigating 16 interventions. According to the OS results of this analysis, in addition to docetaxel and cabazitaxel-based chemotherapy regimens, chemotherapy combined with targeted therapy (capivasertib or cabozantinib) and chemotherapy combined with PD-1 (ipilimumab) showed significant effects. Cabozantinib is a tyrosine kinase inhibitor that targets multiple genes, including MET, VEGFR1/2/3, ROS1, RET, AXL, NTRK, and KIT, and is currently used to treat renal cancer (31). Capivasertib is an AKT inhibitor with potential efficacy in patients with PIK3CA, AKT1, and or PTEN mutations. These therapeutic strategies of targeted therapy combined with chemotherapy are providing a new direction for mCRPC.
Different levels of genetic mutations are often found in malignant tumors, which can cause poor response to castration therapy or chemotherapy. Therefore, the use of monotherapy in cancer therapy has limitations, and monotherapy specifically inhibits a therapeutic target and triggers compensatory mechanisms or other signal transduction bypasses, which require the assistance of other drugs to improve efficacy. Researchers are increasingly interested in using combinations of low-dose anti-cancer agents with different modes of action rather than administering single agents at high doses. Combinations of anticancer drugs with different mechanisms of action may show synergistic effects in inhibiting the growth of prostate cancer cells and inducing apoptosis. In response to the aberrant activation of PI3K and NF-κB pathways in the late stage of docetaxel chemotherapy in mCRPC, drugs that can inhibit the transduction of this signaling pathway are required.
In order to achieve precise treatment of mCRPC, genetic testing of patients with mCRPC is required. For patients who have undergone surgical treatment, tumor specimens can be sampled and tested. For inoperable patients, prostate biopsy may be used for testing. Both approaches can be applied to most scenarios. In addition, circulating tumor cells (CTC) can be used to detect novel mutations. CTCs are tumor cells disseminated from primary and/or metastatic tumor sites that circulate in the vasculature with potential for distant seeding. Studies have shown that CTC detection has been performed in mCRPC patients by a useful platform to detect the presence or absence of AR-V7 mutations (32). A multicentric study replicated these findings using an open-source Automated CTC Classification Enumeration and PhenoTyping software for the prognostication of mCRPC patients (33). In addition to CTC, mCRPC can also be genetically classified by detecting circulating nucleic acids, extracellular vesicles. After that targeted or immune treatment regimens can be used for different types mutations to achieve precise treatment of mCRPC.
According to our analysis, cabazitaxel or docetaxel is preferable over abiraterone or enzalutamide for initial chemotherapy or hormone therapy in mCRPC patients who have not undergone genetic testing. Moreover, there is considerable potential for advancement in prostate cancer treatment. The efficacy of many therapeutics is closely linked to tumor genetic mutations, indicating a need for further research in this area.
Our study is not without limitations. Firstly, due to exclusion criteria, we were unable to include emerging treatments such as targeted therapies (olaparib, ipatasertib), vaccine therapies (sipuleucel-T), and radiation therapy (177Lu-PSMA-617). Secondly, the inclusion of a second-line treatment RCT for mCRPC (CARD) was necessary to complete the network graph, which may have introduced bias. It is hoped that future analyses will incorporate more Phase III RCTs focused on first-line mCRPC treatments. Thirdly, the field of prostate cancer treatment is yet to fully embrace precision therapy, and many studies lack genetic data. Therefore, a subgroup analysis of genetic factors in the included patients was not feasible.
We recommend cabazitaxel 20 mg/m^2 as the primary option for first-line treatment of mCRPC. Genetic testing for mCRPC patients is also advised to tailor treatment choices based on mutation profiles. Given the limitations of our network meta-analysis, the need for more comprehensive, high-quality studies for further evaluation is evident.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DZ: Writing – original draft, Investigation, Data curation, Conceptualization. HW: Writing – original draft, Investigation, Data curation, Conceptualization. ZZ: Writing – original draft, Data curation, Conceptualization. WG: Writing – original draft, Data curation, Conceptualization. YM: Writing – review & editing, Supervision, Project administration, Investigation, Funding acquisition, Writing – original draft, Data curation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Project of Zhejiang Provincial Scientific Research Fund for Traditional Chinese Medicine (China). (2022ZB154).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1378993/full#supplementary-material
1. Sweeney C, Bracarda S, Sternberg CN, Chi KN, Olmos D, Sandhu S, et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. (2021) 398:131–42. doi: 10.1016/s0140-6736(21)00580-8
2. Chen J, Wu Z, Ding W, Xiao C, Zhang Y, Gao S, et al. SREBP1 siRNA enhance the docetaxel effect based on a bone-cancer dual-targeting biomimetic nanosystem against bone metastatic castration-resistant prostate cancer. Theranostics. (2020) 10:1619–32. doi: 10.7150/thno.40489
3. Chandrasekar T, Yang JC, Gao AC, Evans CP. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl Androl Urol. (2015) 4:365–80. doi: 10.3978/j.issn.2223-4683.2015.05.02
4. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. (2020) 20:74–88. doi: 10.1038/s41568-019-0216-7
5. Desai K, McManus JM, Sharifi N. Hormonal therapy for prostate cancer. Endocr Rev. (2021) 42:354–73. doi: 10.1210/endrev/bnab002
6. Mori K, Mostafaei H, Sari Motlagh R, Pradere B, Quhal F, Laukhtina E, et al. Systemic therapies for metastatic hormone-sensitive prostate cancer: network meta-analysis. BJU Int. (2022) 129:423–33. doi: 10.1111/bju.15507
7. Hernandez I, Cohen M. Linking cell-surface GRP78 to cancer: From basic research to clinical value of GRP78 antibodies. Cancer Lett. (2022) 524:1–14. doi: 10.1016/j.canlet.2021.10.004
8. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
9. van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. (2016) 7(1):80–93. doi: 10.1002/jrsm.1167
10. Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. (2015) 16:152–60. doi: 10.1016/S1470-2045(14)71205-7
11. Loriot Y, Miller K, Sternberg CN, Fizazi K, De Bono JS, Chowdhury S, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. (2015) 16:509–21. doi: 10.1016/S1470-2045(15)70113-0
12. Oudard S, Fizazi K, Sengelov L, Daugaard G, Saad F, Hansen S, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: A randomized phase III trial-FIRSTANA. J Clin Oncol. (2017) 35:3189–97. doi: 10.1200/JCO.2016.72.1068
13. Smith M, Parker C, Saad F, Miller K, Tombal B, Ng QS, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2019) 20:408–19. doi: 10.1016/S1470-2045(18)30860-X
14. Fizazi K, Kramer G, Eymard JC, Sternberg CN, de Bono J, Castellano D, et al. Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): an analysis of a randomised, multicentre, open-label, phase 4 study. Lancet Oncol. (2020) 21:1513–25. doi: 10.1016/S1470-2045(20)30449-6
15. Saad F, Efstathiou E, Attard G, Flaig TW, Franke F, Goodman OB Jr, et al. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. (2021) 22:1541–59. doi: 10.1016/S1470-2045(21)00402-2
16. Pu YS, Ahn H, Han W, Huang SP, Wu HC, Ma L, et al. Enzalutamide in chemotherapy-naive metastatic castration-resistant prostate cancer: an asian multiregional, randomized study. Adv Ther. (2022) 39:2641–56. doi: 10.1007/s12325-022-02140-2
17. Lowrance W, Dreicer R, Jarrard DF, Scarpato KR, Kim SK, Kirkby E, et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023). J Urol. (2023) 209:1082–90. doi: 10.1097/JU.0000000000003452
18. Gillessen S, Bossi A, Davis ID, de Bono J, Fizazi K, James ND, et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer. (2023) 185:178–215. doi: 10.1016/j.ejca.2023.02.018
19. Antonarakis ES, Armstrong AJ, Dehm SM, Luo J. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis. (2016) 19:231–41. doi: 10.1038/pcan.2016.17
20. Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. (2017) 35:2149–56. doi: 10.1200/jco.2016.70.1961
21. Isebia KT, Mostert B, Belderbos BPS, Buck SAJ, Helmijr JCA, Kraan J, et al. CABA-V7: a prospective biomarker selected trial of cabazitaxel treatment in AR-V7 positive prostate cancer patients. Eur J Cancer. (2022) 177:33–44. doi: 10.1016/j.ejca.2022.09.032
22. Christine A, Park MK, Song SJ, Song MS. The equilibrium of tumor suppression: DUBs as active regulators of PTEN. Exp Mol Med. (2022) 54:1814–21. doi: 10.1038/s12276-022-00887-w
23. Herberts C, Murtha AJ, Fu S, Wang G, Schonlau E, Xue H, et al. Activating AKT1 and PIK3CA mutations in metastatic castration-resistant prostate cancer. Eur Urol. (2020) 78:834–44. doi: 10.1016/j.eururo.2020.04.058
24. Chakraborty G, Nandakumar S, Hirani R, Nguyen B, Stopsack KH, Kreitzer C, et al. The impact of PIK3R1 mutations and insulin-PI3K-glycolytic pathway regulation in prostate cancer. Clin Cancer Res. (2022) 28:3603–17. doi: 10.1158/1078-0432.CCR-21-4272
25. Ferraldeschi R, Nava Rodrigues D, Riisnaes R, Miranda S, Figueiredo I, Rescigno P, et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol. (2015) 67:795–802. doi: 10.1016/j.eururo.2014.10.027
26. Rescigno P, Lorente D, Dolling D, Ferraldeschi R, Rodrigues DN, Riisnaes R, et al. Docetaxel treatment in PTEN- and ERG-aberrant metastatic prostate cancers. Eur Urol Oncol. (2018) 1:71–7. doi: 10.1016/j.euo.2018.02.006
27. Annala M, Struss WJ, Warner EW, Beja K, Vandekerkhove G, Wong A, et al. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair-deficient prostate cancer. Eur Urol. (2017) 72:34–42. doi: 10.1016/j.eururo.2017.02.023
28. Castro E, Romero-Laorden N, Del Pozo A, Lozano R, Medina A, Puente J, et al. PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol. (2019) 37:490–503. doi: 10.1200/jco.18.00358
29. Fan L, Yang Y, Chi C, Ma X, Wang R, Gong Y, et al. Neuroendocrine differentiation markers guide treatment sequence selection in metastatic castration-resistant prostate cancer. Prostate. (2019) 79:567–73. doi: 10.1002/pros.23762
30. Liu Y, Deng X, Wen Z, Huang J, Wang C, Chen C, et al. Comparing efficacy of first-line treatment of metastatic castration resistant prostate cancer: a network meta-analysis of randomized controlled trials. Front Pharmacol. (2023) 14:1290990. doi: 10.3389/fphar.2023.1290990
31. Krawczyk K, Śladowska K, Holko P, Kawalec P. Comparative safety of tyrosine kinase inhibitors in the treatment of metastatic renal cell carcinoma: a systematic review and network meta-analysis. Front Pharmacol. (2023) 14:1223929. doi: 10.3389/fphar.2023.1223929
32. Scher H, Armstrong A, Schonhoft J, Gill A, Zhao JL, Barnett E, et al. Development and validation of circulating tumour cell enumeration (Epic Sciences) as a prognostic biomarker in men with metastatic castration-resistant prostate cancer. Eur J Cancer. (2021) 150:83–94. doi: 10.1016/j.ejca.2021.02.042
Keywords: castration resistant prostate cancer, first-line treatment, chemotherapy, antihormone therapy, network meta-analysis
Citation: Zhang D, Weng H, Zhu Z, Gong W and Ma Y (2024) Evaluating first-line therapeutic strategies for metastatic castration-resistant prostate cancer: a comprehensive network meta-analysis and systematic review. Front. Oncol. 14:1378993. doi: 10.3389/fonc.2024.1378993
Received: 30 January 2024; Accepted: 02 April 2024;
Published: 15 April 2024.
Edited by:
Lei Yin, Shanghai Jiao Tong University, ChinaReviewed by:
Jagpreet Singh Nanda, Cedars Sinai Medical Center, United StatesCopyright © 2024 Zhang, Weng, Zhu, Gong and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinfeng Ma, eWluZmVuZ19tYUAxNjMuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.