Corrigendum: Second malignant neoplasms within 5 years from first primary diagnosis in pediatric oncology patients in Canada: a population-based retrospective cohort study
- 1Lifespan Chronic Disease and Conditions Division, Public Health Agency of Canada, Ottawa, ON, Canada
- 2Department of Pediatrics, Division of Hematology-Oncology, Izzak Walton Killam (IWK) Health Centre, Halifax, NS, Canada
- 3Faculty of Medicine, Dalhousie University, Halifax, NS, Canada
- 4Surveillance Systems and Data Management Division, Public Health Agency of Canada, Ottawa, ON, Canada
- 5Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
- 6Centre for Health Sciences Research, University of Queensland, Brisbane, QLD, Australia
- 7Division of Pediatric Hematology-Oncology, CHU de Québec-Centre Mère-Enfant Soleil, Quebec City, QC, Canada
- 8Research Centre of the CHU de Québec, Axe Reproduction, Santé de la Mère et de l’Enfant, Quebec City, QC, Canada
- 9C17 Research Network, C17 Council, Edmonton, AB, Canada
- 10Department of Pediatrics and Child Health, University of Manitoba, Winnipeg, MB, Canada
- 11Department of Pediatrics, Division of Pediatric Hematology-Oncology, Charles-Bruneau Cancer Center, Centre Hospitalier Universitaire (CHU) Sainte-Justine, Montréal, QC, Canada
- 12Immune Diseases and Cancers Axis, CHU Sainte-Justine Research Center, Montréal, QC, Canada
- 13Department of Pediatric Hematology-Oncology, CancerCare Manitoba, Winnipeg, MB, Canada
- 14Division of Hematology Oncology, The Hospital for Sick Children, Toronto, ON, Canada
- 15Department of Anesthesia and Intensive Care, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 16Department of Pharmacy, Izzak Walton Killam (IWK) Health, Halifax, NS, Canada
- 17Faculty of Health Professions, Dalhousie University, Halifax, NS, Canada
- 18Child Health Evaluative Sciences, Research Institute, The Hospital for Sick Children, Toronto, ON, Canada
Introduction: From the advancement of treatment of pediatric cancer diagnosis, the five-year survival rate has increased significantly. However, the adverse consequence of improved survival rate is the second malignant neoplasm. Although previous studies provided information on the incidence and risk of SMN in long term survivors of childhood cancer, there is still scarce information known for short term (< 5 years) prognosis. This study aims to assess the incidence, characteristics, management, and outcome of children who develop SMN malignancies within 5 years of diagnosis of their initial cancer.
Method: This is a retrospective cohort study of early Second Malignant Neoplasms (SMN) in pediatric oncology patients. The Cancer in Young People – Canada (CYP-C) national pediatric cancer registry was used and reviewed pediatric patients diagnosed with their first cancer from 2000-2015.
Results: A total of 20,272 pediatric patients with a diagnosis of a first malignancy were analyzed. Of them, 0.7% were diagnosed with a SMN within the first 5 years following their first cancer diagnosis. Development of a SMN impacted survival, shown by an inferior survival rate in the SMN cohort (79.1%) after three years compared to that of the non-SMN cohort (89.7%). Several possible risk factors have been identified in the study including the use of epipodophyllotoxins, exposure to radiation, and hematopoietic stem cell 169 transplant.
Discussion: This is the first national study assessing the incidence, 170 characteristics, risk factors and outcome of early SMN in Canadian children 171 from age 0-15 from 2000-2015.
1 Introduction
The five-year survival of children following a cancer diagnosis currently exceeds 84% (1). However, survivors of childhood cancer still experience a diverse spectrum of short-term and long-term complications (1). Second malignant neoplasms (SMN) are one such adverse outcome and pediatric cancer survivors carry a higher risk of developing SMN compared to the general population (2, 3). Fortunately, there has been a significant improvement in the long-term mortality outcomes for five-year cancer survivors, with a decrease in 15-year overall mortality rates from 10.7% to 5.8% in the current era (4). As the five-year cancer survival rate increased, it became evident that the long-term survivors of childhood cancer were at increased risk for severe treatment-related late effects (4). Unfortunately, this late effect may be associated with reduced survival rate of those who develop a second malignant neoplasms as reported by the inferior five-year relative survival rate in this population compared to those who did not develop SMN (3). It is noted that Armstrong et al. described reductions in late SMN specific deaths over their study period (4). Furthermore, the inferior survival rate of those who developed SMN was associated with the reduction in therapeutic radiation dose (5).
These current epidemiological data have helped with the development of guideline recommendations for surveillance and strategies to reduce therapeutic exposure, both of which have played a vital role in improving the short-term and long-term outcomes among five-year survivors of their first cancer (6–11). A study that followed patients for up to 26.4 years demonstrated that about 40% of SMNs are diagnosed in the first 5 years after a first primary malignancy (12). However, acknowledging that the SMN incidence does not plateau over time, studies describing this cohort of early-onset SMN are limited (12). Currently, extensive data and literature exist for specific follow-up after initial childhood cancer. For example, breast cancer screening among survivors at risk begin at 8 years after radiation or at age 25, whichever occurs last (13) and colorectal cancer screening begins 5 years after radiation or at age 30, whichever occurs last (14). Additionally, other potential subsequent neoplasms, such as t-AML after exposure to epipodophyllotoxins, alkylating agents and anthracyclines are meant to be screened annually for 10 years post-exposure (14). These guidelines are based on extensive literature review and updated every few years. However, additional strategies for surveillance of early onset SMN are required to identify those with early presentation of SMNs in the first 5 years following diagnosis of their first primary malignancy (3, 15) because poorer outcomes are seen in patients with early onset SMNs (2, 3, 15). Nearly half of the non-relapse causes of mortality among the five-year first primary cancer survivors can be the result of SMNs (16, 17).
The primary aim of this national population-based surveillance study was to assess the cumulative incidence, clinical characteristics, and outcomes of pediatric patients with a diagnosis of SMN within 5 years of the first primary cancer diagnosis. The secondary aim was to analyze potential risk factors for SMN within the first five years using a proportional sub-distribution hazards regression model.
2 Methodology
2.1 Data sources
The primary data source used for this retrospective cohort study is the Cancer in Young People in Canada (CYP-C), a national pediatric cancer surveillance database. Since January 1, 2001, the CYP-C has captured demographic and clinical information on cancers diagnosed and treated at one of the 17 pediatric oncology centers across Canada prior to the age of 15 years, and 18 years since January 1, 2015. Patient data were abstracted by Clinical Research Associates at each pediatric oncology center and submitted to the CYP-C database.
All eligible patients aged 0-18 years who were diagnosed with a primary malignancy from 2001-2019 in Canada were included in the study to have the largest sample size possible. The last date of follow-up was February 28, 2021, and data for this study were extracted on March 3, 2021. The CYP-C privacy rules entail that cases under five are suppressed and not published to protect participant identity.
2.2 Cohort and cancer diagnosis
The types of cancer were defined according to the International Classification of Childhood Cancer, Third Edition (ICCC-3) criteria, which is based on the World Health Organization’s ICD-O-3 cancer site and morphology coding definitions from 2008 (18). All neoplasms with any ICD-O-3 Behavior code were included in the analysis including in-situ neoplasms. Patient sociodemographic data captured in the database included sex, age at diagnosis, ethnicity, and national neighborhood income quintile. Neighborhood income quintiles were assigned using Statistics Canada’s Postal Conversion File Plus (PCCF+) and rural/urban status were assigned using the child’s residential postal codes captured at the time of diagnosis (19, 20). We denoted if someone lived in a rural setting based on the presence of “0” as the second character on the child’s Forward Sortation Area (FSA). According to Statistics Canada, the presence of a zero in the second position of the FSA code identifies the rural postal codes (20). To define the income quintiles, census data were used to rank dissemination area-level average household income into quintiles ranging from least (Quintile 1) to most affluent (Quintile 5), adjusting for household size, cost of living, and regional differences. Further details can be found in previous Statistics Canada documentation (16). Genetic predisposition was defined as any predisposing condition (e.g., ataxia-telangiectasia, DICER1 syndrome, etc.), comorbidity which modifies therapy, genetic condition, and various other conditions such as neurofibromatosis and Li-Fraumeni Syndrome. This genetic predisposition information was collected for all provinces with the exception of Ontario.
A SMN was defined as a cancer diagnosed after 60 days but within the first 5 years of the initial cancer diagnosis, that was histologically or morphologically distinct from the first primary cancer. Subsequent malignancies were determined to be histologically or morphological distinct from the primary cancer using morphology and topography codes in the CYP-C dataset. Since comprehensive examination at the first diagnosis can artificially inflate the risk of SMN in the first 60 days following a diagnosis, cancer diagnoses within 60 days of the first diagnosis were considered part of the initial diagnosis. This 60-day window has been used in other studies to decrease the risk of differential surveillance (16, 21, 22) into one of the seven main diagnostic types of childhood cancer (Table 1) to mitigate small numbers of events. Only the first SMN was used to indicate a SMN as there were very few cases with multiple SMNs.
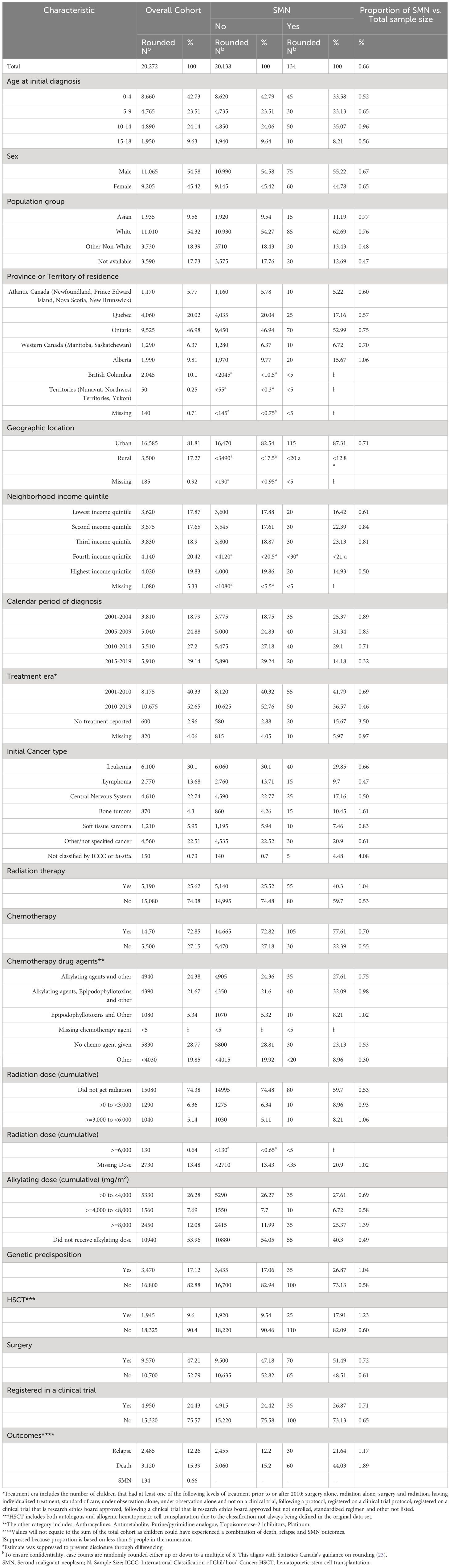
Table 1 Characteristics of patients first diagnosed with cancer at age 0 to 18 years, between 2001 and 2019 in Canada, by SMN status during five-year follow up.
A very-early onset SMN was defined as a SMN that occurred more than 60 days but within 2 years of the initial cancer diagnosis, and an early SMN defined as one that occurred between 2-5 years from the first primary cancer diagnosis.
2.3 Treatment information
All treatment related information including surgery, radiation, hematopoietic stem cell transplant (HSCT), and chemotherapy was obtained from the CYP-C. The treatment era presented in Table 1 distinguishes patients who had received any treatment prior to 2010 from those who received treatment in 2010 or later. This categorization accounts for treatment advancements that occurred in childhood cancer after 2010, which have helped reduce the incidence of SMNs (24, 25). All treatment variables were modeled as dichotomous except for type of chemotherapy, cumulative dose of alkylating agent, and cumulative dose of radiation.
There were six main groupings for the chemotherapeutic agents included in the study, as seen in Table 1. The alkylating agents’ cumulative dose was captured for those who had an alkylating agent as part of their treatment. Cumulative doses (mg/m2) of alkylating agents were categorized as: 1) did not receive alkylating agents; 2) greater than zero to less than 4,000; 3) greater than or equal to 4,000 to less than 8,000; 4) and greater than or equal to 8,000 (26).
The cumulative radiation doses in centigray (cGy) were categorized as: 1) did not receive radiation therapy; 2) greater than 0 to less than 3,000; 3) greater than or equal to 3,000 but less than 6,000; 4) greater than or equal to 6,000 cGy; and 5) missing cumulative radiation dose.
2.4 Statistical analyses
All statistical analyses were performed using SAS Enterprise Guide 7.1 (27). Proportions for first primary cancers and SMNs were based on cancers registered in the CYP-C, similarly, grouped by demographic and treatment factors.
To assess the overall risk of death among our SMN cohort, regardless of competing risks, a cox proportional hazards curve was used to calculate the probability of survival over time among the CYP-C patients. We used the cumulative incidence function to estimate the cumulative probability of being diagnosed with a SMN with (28) 95% confidence intervals estimated according to Hosmer, Lemeshow, and May method (29). A proportional sub-distribution hazards regression model was developed to examine the factors associated with the development of a SMN in the presence of the competing event of death (30, 31). For the competing risk model, participants in the CYP-C contributed person-time from the date of initial diagnosis until the earliest of either when they were diagnosed with a second cancer, died, reached the end of their five-year follow-up or February 28, 2021 for those who did not have a full 5 years of follow-up yet. The sub-distribution hazard function treats the event of interest mutually exclusive from the competing risks and the hazard of the event of interest is adjusted for the cumulative incidence of the competing events. This was done using the %PSHREG SAS macro (31). We developed three models to examine cancer occurrences at different time periods from the first cancer diagnosis. The first model examined all SMNs within the first 5 years, the second model examined very-early SMNs diagnosis, and the last model included the early SMNs. To determine which variables to account for, we decided on several variables a priori, and then used a stepwise model building approach at the 0.05 level to decide on the variables that would be used as confounders in the model. We initially considered sex, age at diagnosis, calendar period of diagnosis, treatment era, population group, first primary cancer type, urban/rural status, neighborhood income quintile, province or territory of residence and various treatment options and dosages. If the removal of a variable considerably changed the estimates for the remaining variables by more than 10%, then that variable was retained. Despite this, we kept chemotherapy agent and HSCT given that previous studies have shown these variables as risk factors for SMN development (12, 32–34). Values were reported as hazard ratios (HR) with 95% confidence intervals.
3 Results
A total of 20,272 pediatric patients with a diagnosis of a first malignancy were analyzed during the study period from 2001 to 2019 (Table 1). From these, 134 (0.7%) were diagnosed with a SMN within the first 5 years following their first cancer diagnosis.
3.1 Characteristics of the first primary cancer cohort
Relative to the cohort without a SMN, those treated in earlier study time periods were more likely to develop SMNs. The period from 2015-2019 had the lowest number of the SMN cases diagnosed; however, this may be due to early censoring of the study data as we do not have the 5-year follow-up for those diagnosed in 2019. The majority of the patients with first malignancy and SMN came from an urban setting. There were no major differences among the SMN and non-SMN groups with respect to income backgrounds. We found the proportion of children and youth with cancer who developed a SMN in Alberta was higher (1.1%) compared to the other provinces; however, the reason remains unclear and could be an artifact of responsive reporting of SMNs in Alberta (Table 1).
Among children with SMN, the most common therapies used to treat the first malignancy were again chemotherapy (77.6%), surgery (51.5%) and radiation (40.3%). Among those who had chemotherapy in the SMN cohort, 59.7% had been exposed to alkylating agents, and 40.3% to epipodophyllotoxins. An underlying genetic cancer predisposition was identified in 17.1% of pediatric patients with a first primary cancer diagnosis. Also, 26.9% of patients who developed a SMN were diagnosed with a cancer predisposition syndrome (Table 1). Since there was no genetic cancer predisposition information for Ontario, this is likely an underestimation of data.
3.2 Characteristics of the SMN cohort
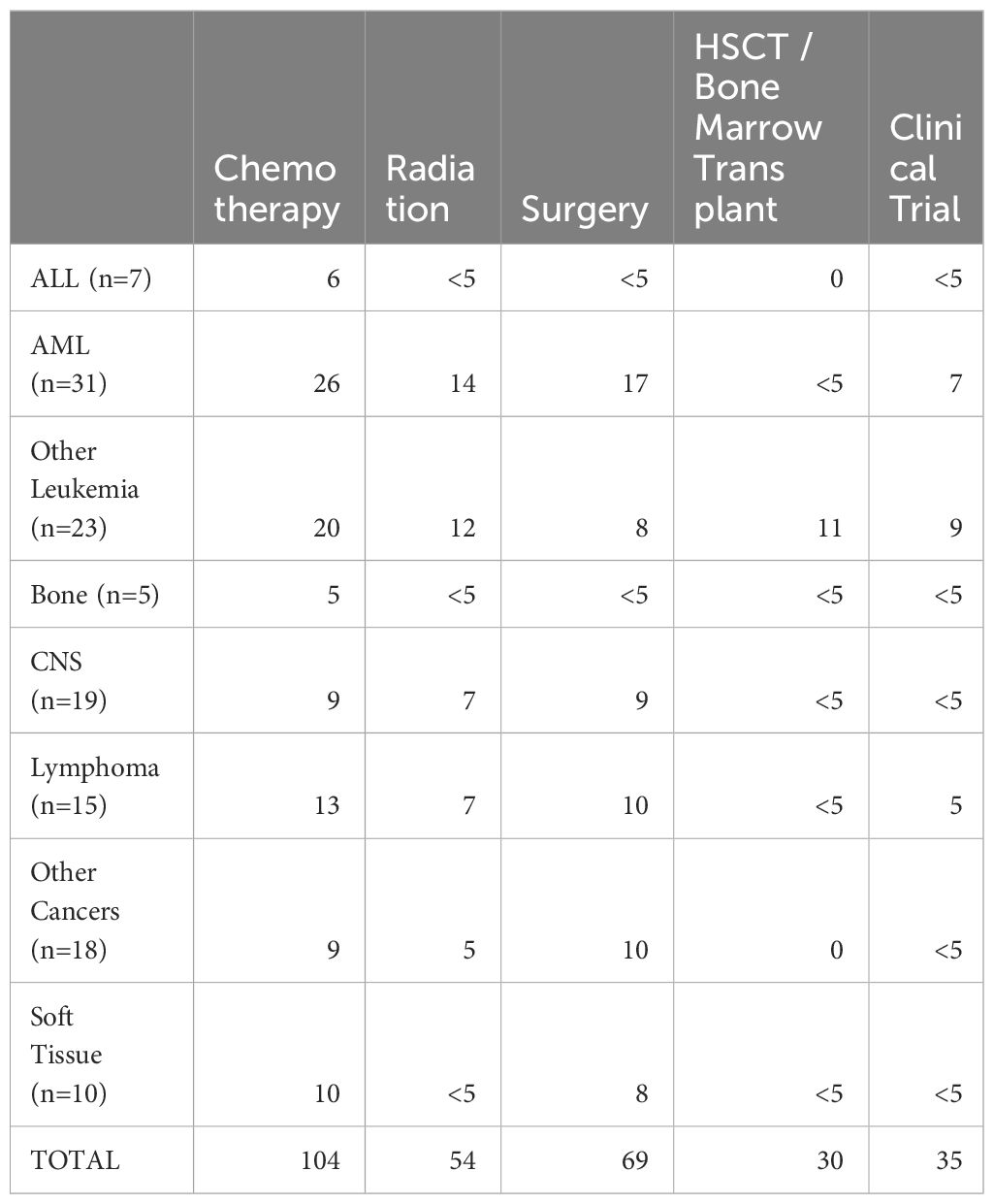
Among the 134 patients diagnosed with SMN, 73 (54.5%) children developed a very-early onset SMN within 2 years of their first cancer diagnosis and 61 (46.5%) had an early onset SMN diagnosed between 2-5 years after their first cancer diagnosis. Leukemia (45.5%) was the most common SMN diagnosis within the first 5 years of their first cancer diagnosis (Table 2) CNS malignancy was the second most common SMN (14.2%) (Figure 1). 10.5% of those who developed a SMN had a prior diagnosis of a bone tumor, mostly Ewing sarcoma. This means that 1.6% of those whose first cancer was a bone tumor developed a SMN (Table 1). Leukemia was the most common second malignancy in both the very-early onset and early onset SMN cohorts (Figure 1) and acute myeloid leukemia was the most common type of leukemia constituting 85.0% of leukemia diagnoses in the SMN cohort [data not shown]. Of the 40 cases of leukemia who developed an SMN, 21 developed a secondary leukemia, 11 as very-early SMNs and 10 as early SMNs. Chemotherapy was the most common therapeutic modality used in the management of SMN (Appendix 1). Within the very-early SMN cohort, chemotherapy was used in 69.9% of patients and within the early SMN cohort it was used in 86.9% [data not shown].
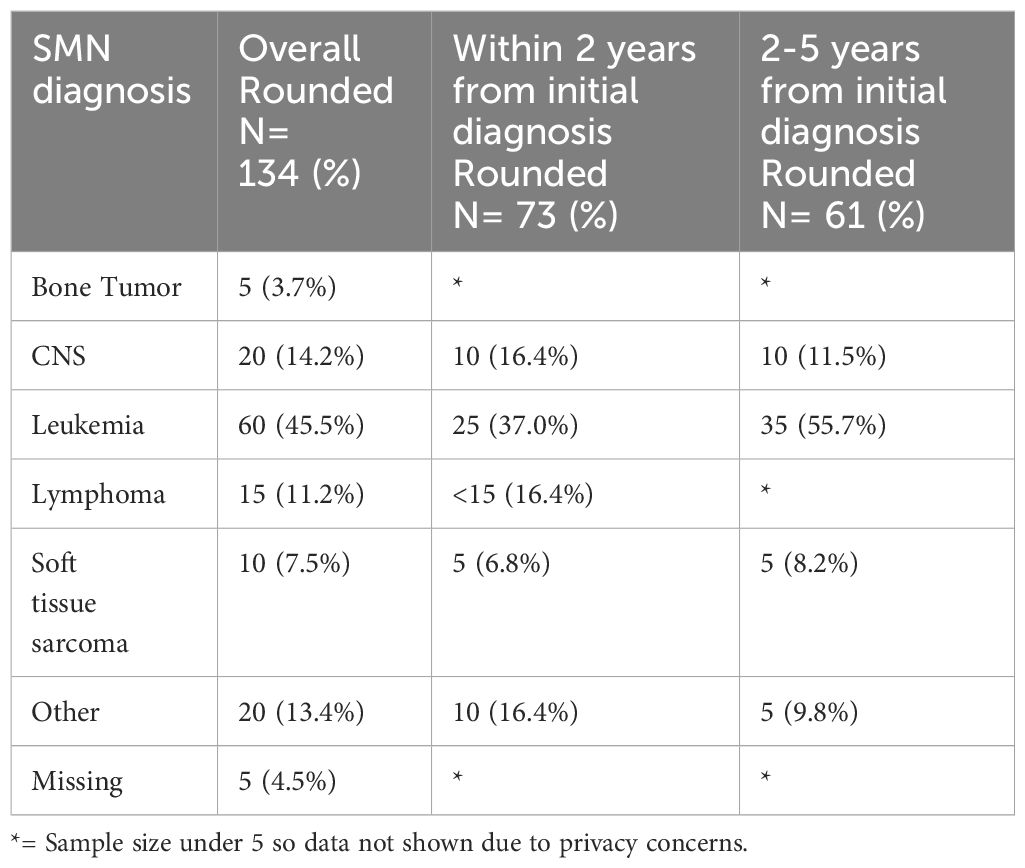
Table 2 Types of SMN in overall cohort (5 years from diagnosis of first primary malignancy), 0-2 years and 2-5 years from diagnosis of first primary malignancy.
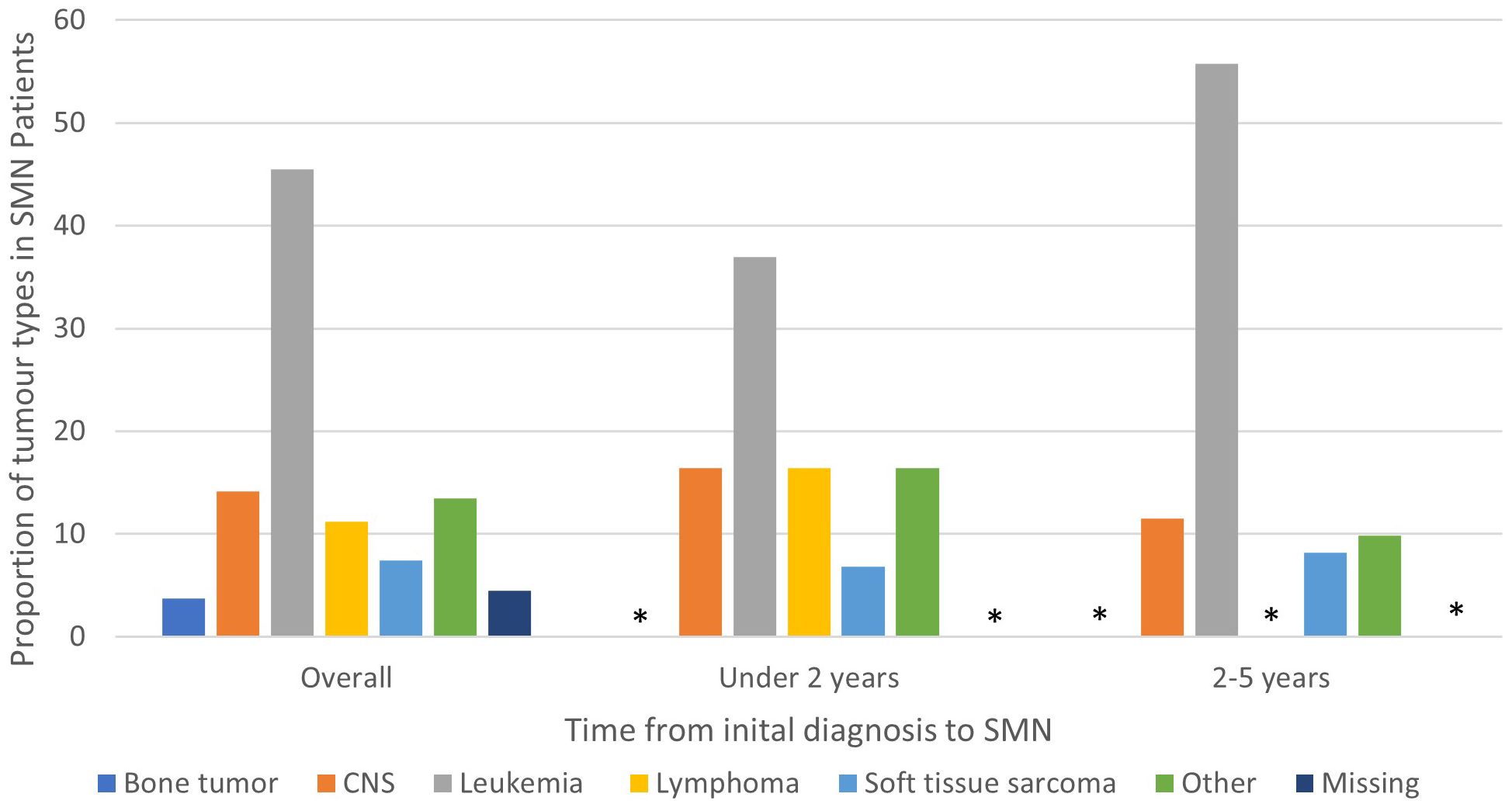
Figure 1 Types of SMN in overall cohort (5 years from diagnosis of first primary malignancy), 0-2 years and 2-5 years from diagnosis of first primary malignancy. Star means number not shown since less than 5.
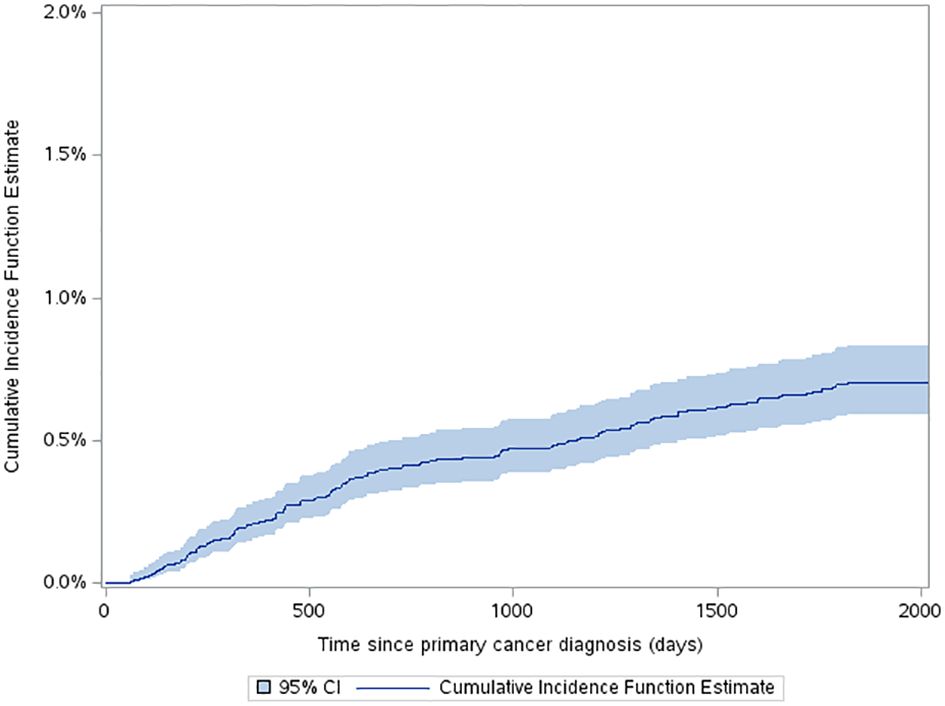
The estimated cumulative incidence function (CIF) for a very-early SMN was 0.42% (95% CI: 0.34% to 0.52%) and this increased to 0.7% (95% CI: 0.59% to 0.83%) by 5 years after diagnosis (Figure 2). The three-year probability of survival among patients with a SMN was 79.1% (95% CI: 71.2% to 85.1%) compared to 89.3% (95% CI: 88.8% to 89.7%) in the non-SMN cohort. The five-year probability of survival was 59.0% (95% CI: 50.1% to 66.7%) in the SMN cohort vs. 86.5% (95% CI: 86.0% to 86.9%) among the non-SMN cohort (Figure 3).
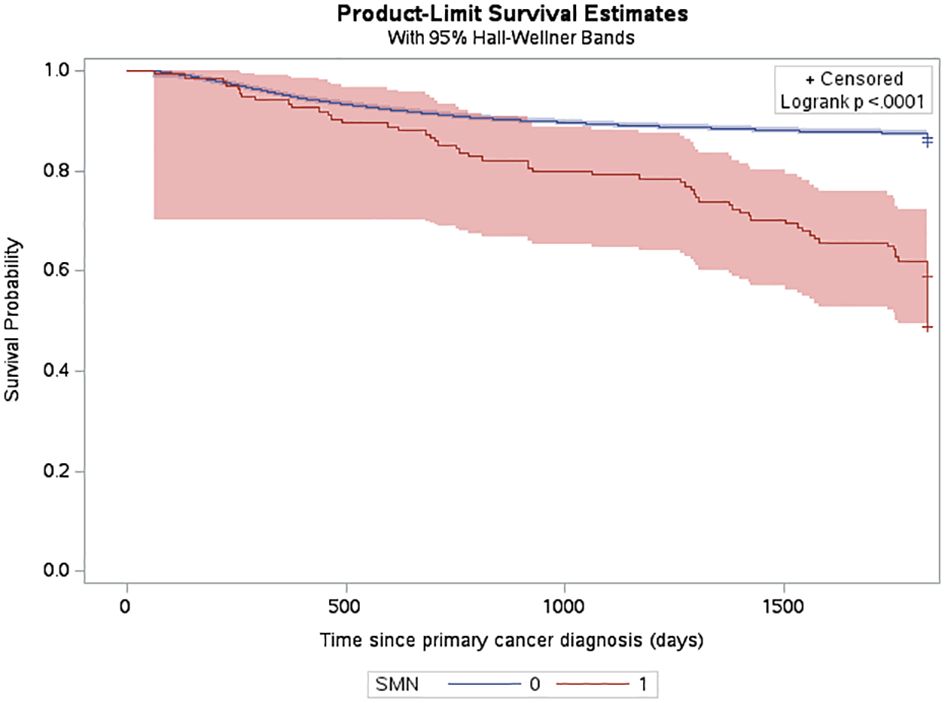
Figure 3 Probability of death among CYP-C patients from 2001-2019 shown by SMN status, censored 5 years after diagnosis or on their death status.
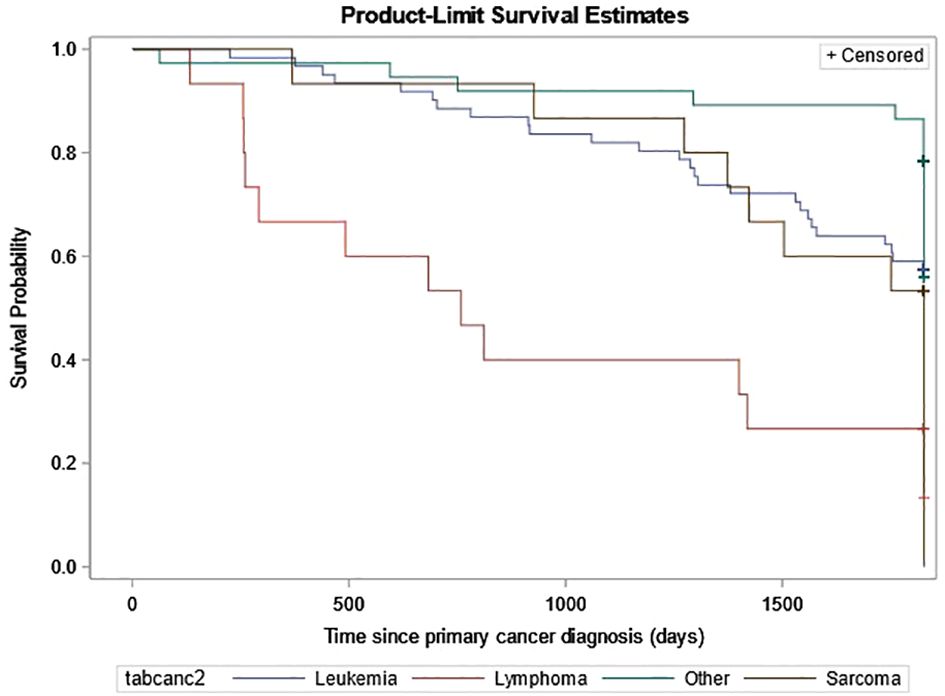
In the full cohort of all first primary cancers, a relapse within 5 years of the first primary cancer was documented in 12.3% of the non-SMN cohort compared to 21.6% of the SMN cohort. Of the 61 patients with leukemia as their SMN diagnosis, 26 patients died. Of those 26 deaths, 20 of them occurred within one year of the SMN diagnosis and the remaining six patients between one to five years. The probability of survival was the lowest for patients diagnosed with lymphoma as their SMN (Figure 4).
Appendix 1 shows management of patients with SMN.
3.3 Risk factors
The proportional hazard regression model derived possible risk factors for developing a SMN based on variables identified in Table 1, accounting for the competing risk of death. All the hazard ratios were adjusted for HSCT, chemotherapy agent categories in Table 1, cancer types, cumulative radiation and alkylating doses, surgery, year of diagnosis, ethnicity, neighborhood income quintile and age at diagnosis. A very few therapeutic exposures reached statistical significance as risk factors. In patients who were 0-4 years old at their initial diagnosis they have a 10% lower hazard [0.1 (95% CI: 0.0-0.8) of developing an early SMN compared to those who were 15-18 years old. In patients who had other chemotherapy treatments in their first diagnosis, they have a 30% lower hazard [0.3 (95% CI: 0.1-0.8) of developing a very-early SMN compared to not having had a chemotherapy treatment. Although not statistically significant, exposure to epipodophyllotoxins [1.7 (95% CI: 0.8 to3.5)], radiation therapy of 3000-6000 cGy [1.6 (95% CI: 0.8 to 3.2)], and/or HSCT [1.6 (95% CI: 0.9 to 2.8)] were clinical risk factors associated with a higher hazard of SMN development compared to their reference groups (Figure 5). Alkylating agents and epipodophyllotoxins still constitute major components of many treatment protocols. We saw a trend for very-early onset SMN among patients with exposure to epipodophyllotoxins, this trend was also true in the overall cohort for onset of a SMN (Figures 5, 6). Contrary to this, we saw the use of alkylating agents had a trend of lowering (Figures 5, 6) or no effect (Figure 7) on the onset of a SMN. However, the cumulative alkylating dose response relationship was inconsistent with some showing using 4,000-8,000 mg/m2 as lowering SMN risk more than using 0-4,000 mg/m2 (Figure 5). For those who had both radiation and an alkylating agent for their treatment of primary cancer, 1.2% had developed SMN, which is slightly higher than the 1% we see in general population for the development of SMN.
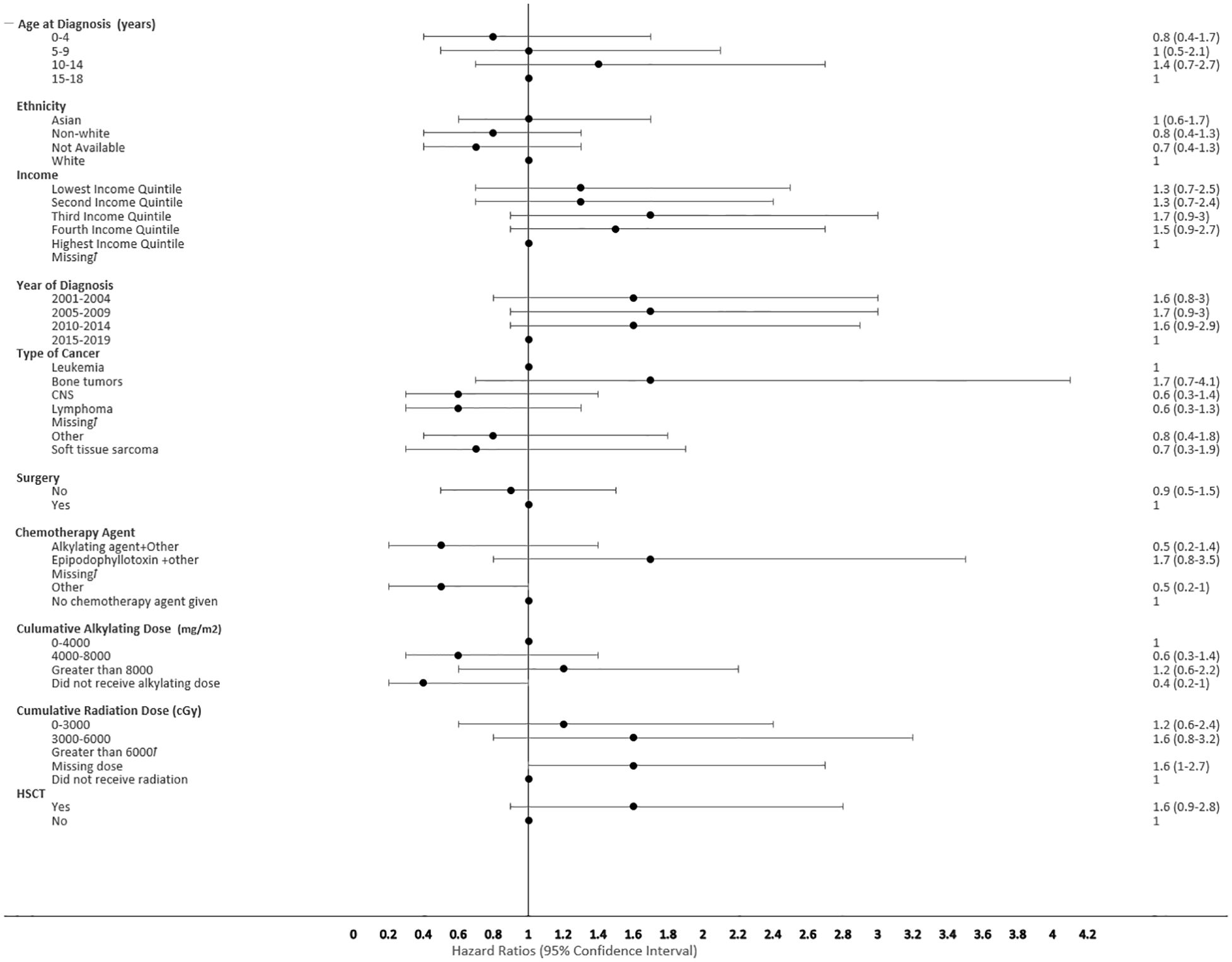
Figure 5 Forest plot depicting the variables and adjusted hazard ratio for SMN. ƚ indicates this hazard ratio has been suppressed because less than 5 patients have the characteristic.
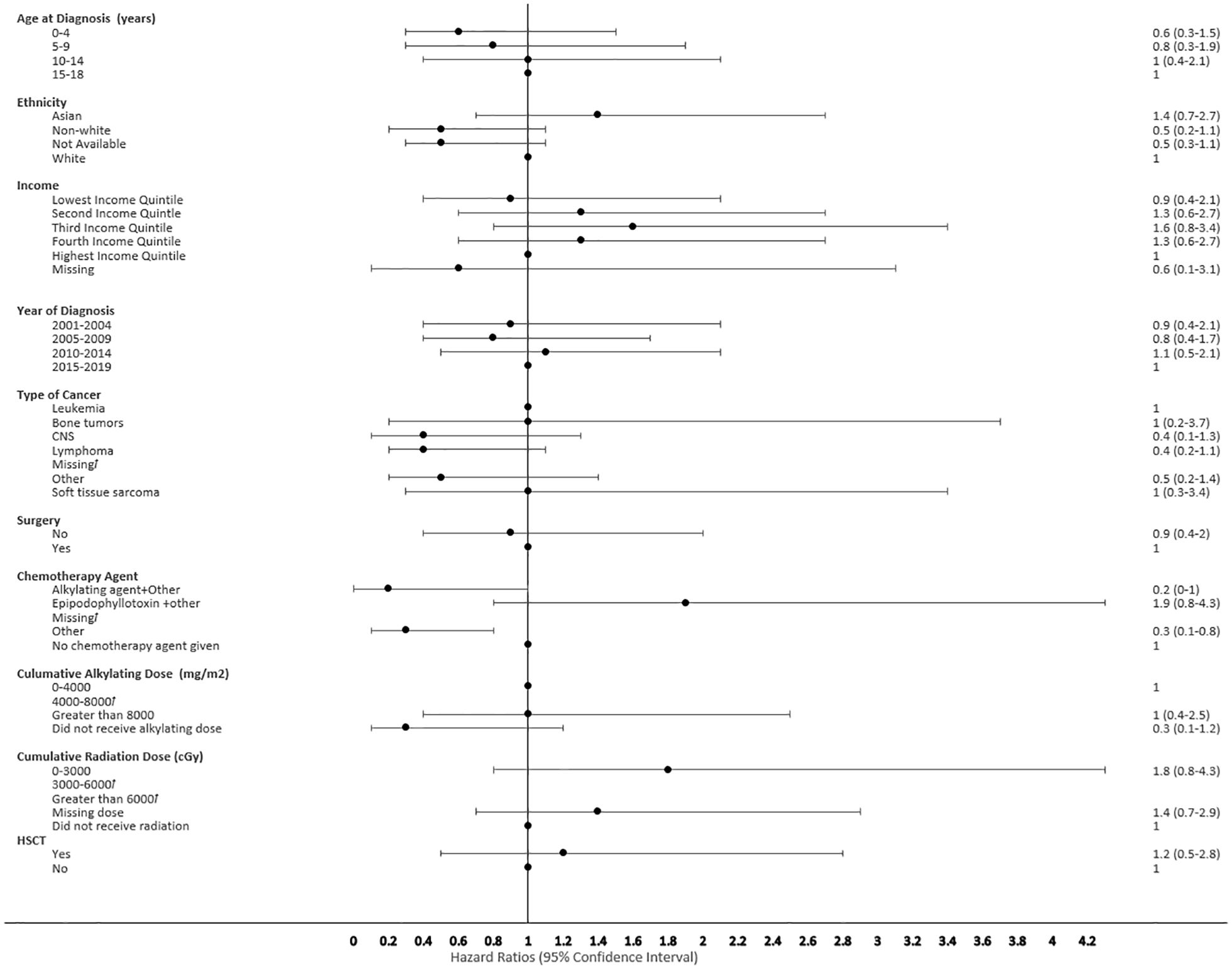
Figure 6 Forest plot depicting the variables and adjusted hazard ratio for SMN developed within 0-2 years. ƚ indicates this hazard ratio has been suppressed because less than 5 patients have the characteristic.
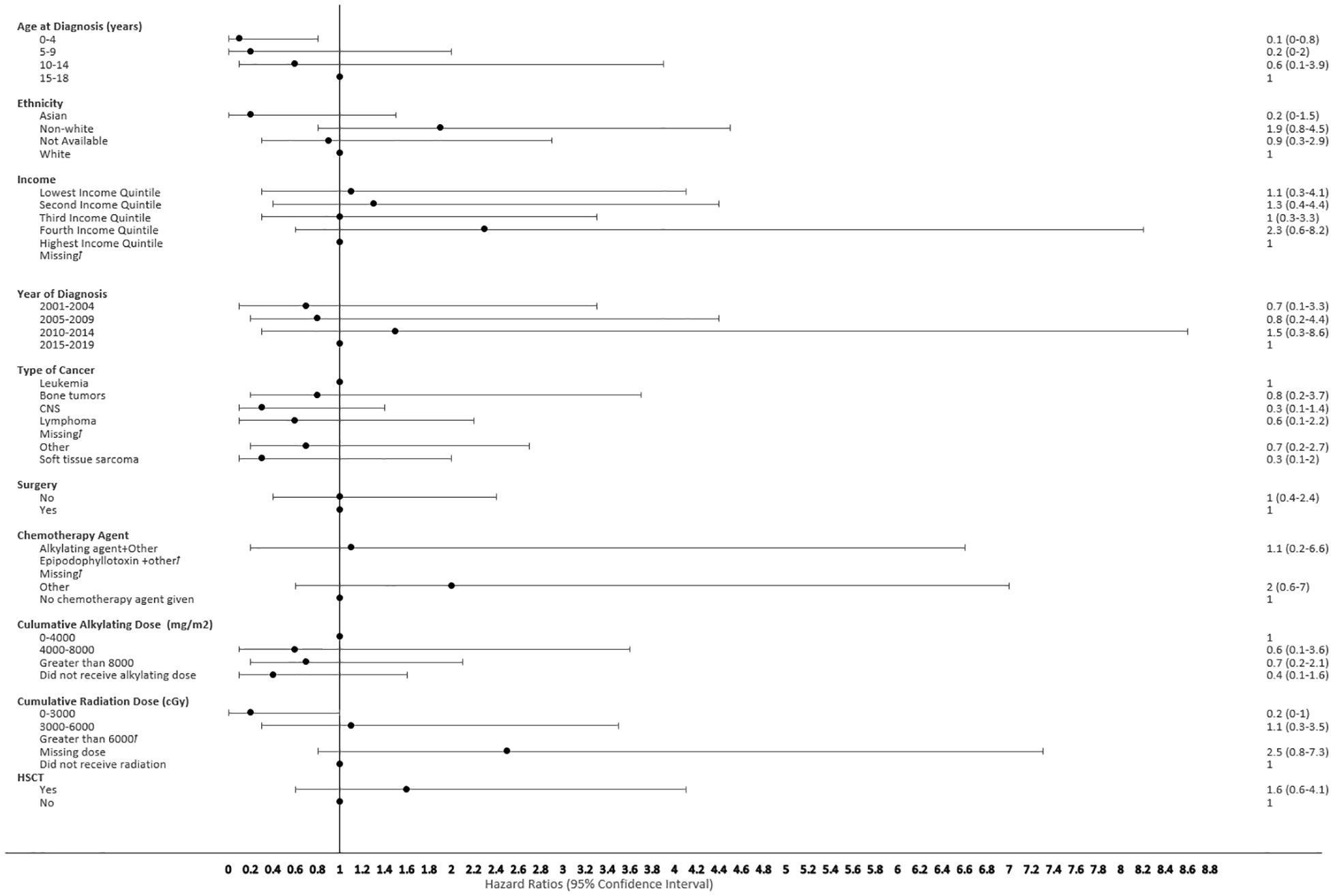
Figure 7 Forest plot depicting the variables and adjusted hazard ratio for SMN developed within 2-5 years. ƚ indicates this hazard ratio has been suppressed because less than 5 patients have the characteristic.
4 Discussion
4.1 Interpretation of findings
The five-year cumulative probability of SMN in our cohort is comparatively lower than the previous Canadian study published by Pole et al. (22) The cumulative incidence derived from our risk model is likely more conservative due to the following two reasons. Firstly, we used multiple competing risk factors and mutually exclusive outcome variables in our model and secondly, our cohort was more recent; therefore, treated using more risk stratified therapeutic approach and consequently exposed to fewer risk factors like high dose radiation or alkylating agents. A recent pediatric cancer survivor population study by Ju et al. showed a similar estimated five-year cumulative incidence of SMN at 0.7% (2). The incidence of SMN in the 15-18 years old age group is much lower in our study, likely due to their more recent diagnosis (i.e., since January 1, 2015) in an era of risk-stratified therapeutic approaches, less complete follow up, and under-representation of adolescent and young adult (AYA) population within the CYP-C dataset. As previously mentioned, there is a lack of continuity and completeness of data available for this subset of the adolescent population. It is crucial to link pediatric cancer databases with the adult registries to avoid early censure and loss of follow up data among the AYA population.
Risk factors associated with second neoplasms have been well described among the late onset SMN, which is defined as those occurring more than 5 years after the first primary cancer, with exposure to radiation therapy being the major factor. The risk of developing a SMN post-radiation therapy has been described to increase with advancing age of the survivors, but its impact on very-early onset SMN is less well described (35, 36). We also did not see a clear association of early SMNs with radiation, given that a smaller proportion of children received upfront radiation therapy for their first cancer diagnosis. Chemotherapy agents still constitute first line therapy for many pediatric cancers (Table 1). HSCT is a well-described risk factor for SMN, especially among recipients of myeloablative conditioning regimens during transplant, and this risk was seen in our cohort (34, 37). Many of the HSCT protocols used total body radiation and/or high dose alkylating agents in myeloablative conditioning regimens, which reiterates the risk of SMN secondary to high dose chemotherapy and radiation therapy (Figure 5). Given the multi-modal treatment approach in pediatric cancer, there is a complex and cumulative impact of treatment in the development of a SMN. This potentially could be due to the size of SMN cohort, methods of capturing data and duration of follow up available.
By using a sub-distributional hazard model, the effects of an individual risk co-variate on SMN were analyzed in the context of the competing risk of death. However, the retrospective nature of the study limited us to only propose an association of SMN with individual risk factors such as exposure to epipodophyllotoxins, high dose alkylating agents, radiation, and HSCT toward the development of a SMN. The hazard ratios might be lower compared to the other studies because the model tries to analyze the impact of an individual risk factor amidst competing factors and is not quantifying the synergic interactions possible between multiple risk factors. Though we did not see any collinearity among the factors in our analysis, we are aware that these covariates have a synergistic relationship clinically. Building future models based on mediation analysis can be used to derive an individual’s risk for SMN from independent co-variates, which can potentially be helpful with personalized surveillance recommendations.
4.2 Strengths and limitations
This is one of the largest population-surveillance databases based SMN studies in pediatrics that utilized trained scientific personnel input study data, and thereby minimizing the potential risk of selection and recall bias. Extensive data quality improvement steps (e.g., mapping, merging, creating derived variables, etc.) were taken for these data, and the CYP-C diligently attempted to minimize such errors by comprehensive training of data collectors and conducting regular data audits.
Another strength was our modeling approach, which utilized the Fine Grey method where you create a sub-distribution hazard model based on the cumulative incidence function accounting for covariates and the presence of competing risks. Competing risk analysis allows you to better assess censoring in a population where a patient is at risk of more than one mutually exclusive events occurring, such as in a population of pediatric oncology patients.
Despite the strengths of our study, we did not have the ability to look at other important SMN risk factors, such as cumulative dose of epipodophyllotoxins, which have been shown to be associated with therapy-related leukemias. Also, the limited sample size of our study, while large for SMN studies but not large for the general population, created model instability and we were unable to stratify the chemotherapy agents (e.g., anthracyclines) more granularly and they were grouped into another category. Additionally, the CYP-C started to gather data on children aged 15-18 after 2015; however, this data is not comprehensive or complete due to the inconsistency in capturing this patient population by the pediatric cancer centers vs. the adult cancer centers. Therefore, data for this age group should be interpreted with caution. The high frequency of SMN among bone tumors could be associated with cyclophosphamide (38). Additionally, although 17.1% of pediatric first primary cancer patients and 26.9% of SMN patients had an underlying genetic cancer predisposition, this may be an underestimation as not all patients had genetic testing. Another limitation of the study is the low yield of CYP-C data in differentiating histologically or morphologically distinct SMN from the primary cancer. Future studies with sufficient treatment data should investigate the association of anthracyclines in SMN risk more closely. Finally, although many efforts were made to increase the accuracy of findings, the study did not consider the interactions between the risk factors. Therefore, future research should include those interactions to enhance the understanding of the overall risk. Currently, there are no large-scale Canadian studies on the early SMN among survivors of childhood cancer. Our study adds valuable data and information from the Canadian perspective to the existing literature on this topic. Furthermore, this data will assist both short term and long term follow up clinics in Canada to maintain heightened surveillance for SMNs in high-risk categories of patients.
4.3 Clinical takeaways
Though the cumulative incidence of SMN has decreased, the five-year survival outcome in our early onset SMN cohort has not significantly improved compared to previously published data (39). Acute leukemia constituted more than 50% of our study cohort, with acute myeloid leukemia being the most common type of SMN in the first 2 years. Although previous literature indicated thyroid cancer as one of the most common SMNs (5), we still consider leukemia as the most common SMN as this was considered with or without receiving radiation therapy for the first cancer. The historical outcomes for early onset SMN with hematological malignancies have been poor (40, 41). Given the inconsistent nature of pediatric cancer centers’ long-term monitoring and surveillance of mortality rates and the quality of life of pediatric cancer survivors across the nation, we, subsequently, also have insufficient data on the adolescent population. Our data suggests the need to incorporate awareness and surveillance for SMN within 5 years of diagnosis in follow-up clinics across Canada. The linking of pediatric cancer databases with the adult registries will allow a better systematic approach to surveillance and follow-up among the adolescent and young adults population.
Data availability statement
The datasets presented in this article are not readily available because individual participant data cannot be shared due to Cancer in Young People in Canada policies. Requests to access the datasets should be directed to the C17 Council website, available at https://www.c17.ca/index.php?cID=70 (login required).
Ethics statement
The studies involving humans were approved by IWK Health Research Ethics Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
CR: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. KL: Writing – original draft, Writing – review & editing. DS: Formal analysis, Writing – original draft, Writing – review & editing. NS: Data curation, Writing – review & editing. JK: Data curation, Writing – review & editing. LX: Writing – review & editing. ML: Writing – review & editing. DZ: Writing – review & editing. JP: Writing – review & editing. M-CP-M: Writing – review & editing. RB: Writing – review & editing. SI: Writing – review & editing. T-HT: Writing – review & editing. SO: Writing – review & editing. SR: Writing – review & editing. TM: Writing – review & editing. LS: Writing – review & editing. KK: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We acknowledge Sulaf Elkhalifa for her help with data curation and analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content and views expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
References
1. Public Health Agency of Canada. Cancer in young people in Canada data tool. 2020 edition (2023). Public Health Infobase. Available at: https://health-infobase.Canada.ca/data-tools/cypc/ (Accessed February 6, 2024).
2. Ju HY, Moon EK, Lim J, Park BK, Shin HY, Won YJ, et al. Second Malignant neoplasms after childhood cancer: A nationwide population-based study in Korea. PloS One. (2018) 13:e0207243. doi: 10.1371/journal.pone.0207243
3. Keegan THM, Bleyer A, Rosenberg AS, Li Q, Goldfarb M. Second primary Malignant neoplasms and survival in adolescent and young adult cancer survivors. JAMA Oncol. (2017) 3:1554–7. doi: 10.1001/jamaoncol.2017.0465
4. Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. (2016) 374:833–42. doi: 10.1056/NEJMoa1510795
5. Turcotte LM, Liu Q, Yasui Y, Arnold MA, Hammond S, Howel RM, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA. (2017) 317:814. doi: 10.1001/jama.2017.0693
6. Armstrong GT, Liu Q, Yasui Y, Neglia J, Leisenring W, Robinson L, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. (2009) 27:2328–38. doi: 10.1200/JCO.2008.21.1425
7. Kremer LCM, Mulder RL, Oeffinger KC, Bhatia S, Landier W, Levitt G, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. (2013) 60:543–9. doi: 10.1002/pbc.24445
8. Fidler MM, Reulen RC, Winter DL, Kelly J, Jenkinson HC, Skinner R, et al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ. (2016) 354:i4351. doi: 10.1136/bmj.i4351
9. Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. (2017) 390:2569–82. doi: 10.1016/S0140-6736(17)31610-0
10. Miller KD, Pandey M, Jain R, Mehta R. Cancer survivorship and models of survivorship care: A review. Am J Clin Oncol. (2015) 38:627–33. doi: 10.1097/COC.0000000000000153
11. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. (2006) 355:1572–82. doi: 10.1056/NEJMsa060185
12. Pole JD, Gu LY, Kirsh V, Greenberg ML, Nathan PC. Subsequent Malignant neoplasms in a population-based cohort of pediatric cancer patients: A focus on the first 5 years. Cancer Epidemiol Biomarkers Prev. (2015) 24:1585–92. doi: 10.1158/1055-9965.EPI-15-0360
13. Mulder RL, Hudson MM, Bhatia S, Landier W, Levitt G, Constine LS, et al. Updated breast cancer surveillance recommendations for female survivors of childhood, adolescent, and young adult cancer from the international guideline harmonization group. J Clin Oncol. (2020) 38:4194–207. doi: 10.1200/JCO.20.00562
14. Children’s Oncology Group. The children’s oncology group long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers (COG LTFU guidelines). Seattle, WA, US: Children's Oncology Group. (2023).
15. Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. (2010) 102:1083–95. doi: 10.1093/jnci/djq238
16. Inskip PD, Curtis RE. New Malignancies following childhood cancer in the United States, 1973-2002. Int J Cancer. (2007) 121:2233–40. doi: 10.1002/ijc.22827
17. Yavvari S, Makena Y, Sukhavasi S, Makena MR. Large population analysis of secondary cancers in pediatric leukemia survivors. Children (Basel). (2019) 6(12):130. doi: 10.3390/children6120130
18. Surveillance E and ERPNCI. ICCC recode ICD-O-3/WHO 2008 table - SEER recodes. Surveillance, epidemiology, and end results program (2008). National Cancer Institute. Available at: https://seer.cancer.gov/iccc/iccc-who2008.html (Accessed February 6, 2024).
19. Canadian Institute for Health Information. Health indicators 2013: definitions, data sources and rationale, may 2013 (2013). Available at: https://publications.gc.ca/collections/collection_2013/icis-cihi/H115-67-2013-eng.pdf (Accessed February 6, 2024).
20. Statistics Canada. Postal code conversion file (PCCF), reference guide. Statistics Canada (2011). Available at: https://www150.statcan.gc.ca/n1/en/catalogue/92-154-G (Accessed February 6, 2024).
21. Zakaria D, Shaw A, Xie L. Risk of a second cancer in Canadians diagnosed with a first cancer in childhood or adolescence. EClinicalMedicine. (2019) 16:107–20. doi: 10.1016/j.eclinm.2019.10.002
22. Zong X, Pole JD, Grundy PE, Mahmud SM, Parker L, Hung RJ. Second Malignant neoplasms after childhood non-central nervous system embryonal tumours in North America: A population-based study. Eur J Cancer. (2017) 84:173–83. doi: 10.1016/j.ejca.2017.06.035
23. Statistics Canada. Statistics Canada’s guidance on rounding. Ottawa, Canada: Statistics Canada (2006).
24. Grisham J. A decade of progress in cancer care, and what’s next (2020). Memorial Sloan Kettering Cancer Center. Available at: https://www.mskcc.org/news/decade-progress-cancer-care-and-what-s-next (Accessed February 6, 2024).
25. Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. (2018) 9:1300. doi: 10.3389/fphar.2018.01300
26. Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. (2014) 61:53–67. doi: 10.1002/pbc.24679
28. Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. Br J Clin Pharmacol. (1995) 41:432.
29. Hosmer DW, Lemeshow S, May S. Applied survival analysis. John Wiley & Sons, Inc (2008). doi: 10.1002/9780470258019
30. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. (2016) 133:601–9. doi: 10.1161/CIRCULATIONAHA.115.017719
31. Kohl M, Plischke M, Leffondré K, Heinze G. PSHREG: A SAS macro for proportional and nonproportional subdistribution hazards regression. Comput Methods Prog Biomed. (2015) 118:218–33. doi: 10.1016/j.cmpb.2014.11.009
32. Pui CH, Behm FG, Raimondi SC, Dodge RK, George SL, Rivera GK, et al. Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. New Engl J Med. (1989) 321:136–42. doi: 10.1056/NEJM198907203210302
33. Pui CH, Ribeiro RC, Hancock ML, Rivera GK, Evans WE, Raimondi SC, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. New Engl J Med. (1991) 325:1682–7. doi: 10.1056/NEJM199112123252402
34. Keslova P, Formankova R, Riha P, Sramkova L, Snajderova M, Malinova B, et al. Total body irradiation is a crucial risk factor for developing secondary carcinomas after allogeneic hematopoietic stem cell transplantation in childhood. Neoplasma. (2020) 67:1164–9. doi: 10.4149/neo_2020_200214N131
35. Turcotte LM, Liu Q, Yasui Y, Henderson TO, Gibson TM, Leisenring W, et al. Chemotherapy and risk of subsequent Malignant neoplasms in the childhood cancer survivor study cohort. J Clin Oncol. (2019) 37:3310–9. doi: 10.1200/JCO.19.00129
36. Cooper JS, Pajak TF, Rubin P, Tupchong L, Brady LW, Leibel SA, et al. Second Malignancies in patients who have head and neck cancer: incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys. (1989) 17:449–56. doi: 10.1016/0360-3016(89)90094-1
37. Hoeben BAW, Wong JYC, Fog LS, Losert C, Filippi AR, Bentzen SM, et al. Total body irradiation in haematopoietic stem cell transplantation for paediatric acute lymphoblastic leukaemia: review of the literature and future directions. Front Pediatr. (2021) 9:774348. doi: 10.3389/fped.2021.774348
38. Teepen JC, van Leeuwen FE, Tissing WJ, Van Dulmen-den Broeder E, Van den Heuvel-Eibrink MM, Van der Pal HJ, et al. Long-term risk of subsequent Malignant neoplasms after treatment of childhood cancer in the DCOG LATER study cohort: role of chemotherapy. J Clin Oncol. (2017) 35:2288–98. doi: 10.1200/JCO.2016.71.6902
39. National Cancer Institute SE and ERP. Second cancers - landmark studies. National cancer institute surveillance, epidemiology, and end results program (2003). Available online at: https://seer.cancer.gov/archive/studies/endresults/study28.html (Accessed February 6, 2024).
40. National Cancer Institute SE and ERP. About the SEER registries. National cancer institute, surveillance, epidemiology, and end results program. Available at: https://seer.cancer.gov/registries/index.html (Accessed February 6, 2024).
41. Byrne J, Schmidtmann I, Rashid H, Hagberg O, Bagnasco F, Bardi E, et al. Impact of era of diagnosis on cause-specific late mortality among 77 423 five-year European survivors of childhood and adolescent cancer: The PanCareSurFup consortium. Int J Cancer. (2022) 150:406–19. doi: 10.1002/ijc.33817
Appendix 1 Management of SMN cancer for the whole SMN cohort.
Keywords: oncology, pediatrics, risk factors, early second malignant neoplasm (early SMN), Canada, surveillance
Citation: Ricci C, Subburaj D, Lim K, Shukla N, Kaur J, Xie L, Laverty M, Zakaria D, Pole J, Pelland-Marcotte M-C, Barber R, Israels SJ, Tran T-H, Oberoi S, Renzi S, MacDonald T, Sung L and Kulkarni K (2024) Second malignant neoplasms within 5 years from first primary diagnosis in pediatric oncology patients in Canada: a population-based retrospective cohort study. Front. Oncol. 14:1376652. doi: 10.3389/fonc.2024.1376652
Received: 25 January 2024; Accepted: 11 March 2024;
Published: 28 March 2024.
Edited by:
Paraskevi Panagopoulou, Aristotle University of Thessaloniki, GreeceCopyright © 2024 Ricci, Subburaj, Lim, Shukla, Kaur, Xie, Laverty, Zakaria, Pole, Pelland-Marcotte, Barber, Israels, Tran, Oberoi, Renzi, MacDonald, Sung and Kulkarni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate Lim, a2F0ZS5saW1AZGFsLmNh; Ketan Kulkarni, S2V0YW4uS3Vsa2FybmlAaXdrLm5zaGVhbHRoLmNh
†These authors share first authorship
 Christina Ricci
Christina Ricci Divya Subburaj2†
Divya Subburaj2† Kate Lim
Kate Lim Jason Pole
Jason Pole Marie-Claude Pelland-Marcotte
Marie-Claude Pelland-Marcotte Sara J. Israels
Sara J. Israels Samuele Renzi
Samuele Renzi Tamara MacDonald
Tamara MacDonald Ketan Kulkarni
Ketan Kulkarni