
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 08 April 2024
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1376498
Objectives: This study aimed to examine Ki-67’s correlation with clinicopathological characteristics of head and neck squamous cell carcinoma (HNSCC), evaluate its prognostic significance, and develop a Ki-67 integrated prognostic model.
Methods: The retrospective study included 764 HNSCC patients hospitalized from 2012 to 2022. Data were sourced from medical records and immunohistochemical analysis of surgical specimens.
Results: Ki-67 expression was significantly associated with sex, pathological grade, clinical stage, and metastasis, but not with age or recurrence. Higher Ki-67 levels were linked to poorer prognosis, as indicated by Kaplan-Meier survival analysis. Utilizing a Cox proportional hazards model, four prognostic factors were identified: age, recurrence, metastasis, and Ki-67 expression. These factors were used to construct a prognostic model and a nomogram. The model’s predictive accuracy was confirmed by a high concordance index and a reliable calibration curve.
Conclusion: Ki-67 expression in HNSCC patients correlates with several clinicopathological features and serves as a negative prognostic marker. A prognostic model incorporating Ki-67 was successfully developed, offering a new tool for patient prognosis assessment in HNSCC.
Head and neck cancer, comprising malignancies in the oral cavity, pharynx, larynx, thyroid gland, salivary glands, and other head and neck regions, is a prevalent malignancy worldwide. In 2020, global incidence rates reported approximately 377,713 new cases of oral cancer, 98,412 of oropharyngeal cancer, 84,254 of hypopharyngeal cancer, and 184,615 of laryngeal cancer (1). Squamous cell carcinoma (SCC), the predominant form of head and neck cancer, originates from the malignant transformation of the squamous epithelium in these regions and constitutes about 90% of all such cancers (2). Common primary sites include the oral cavity, pharynx, and larynx (3). Head and neck squamous cell carcinoma (HNSCC) is notably characterized by invasive growth and a propensity for lymph node metastasis, posing significant diagnostic and treatment challenges globally.
In this context, substantial research has been dedicated to identifying biomarkers for HNSCC (4–6), aiming to enhance disease management, particularly in diagnosis and prognosis. However, limitations in biomarker verification, insufficient quantity or quality of marker molecules, and detection challenges persist. Moreover, methodological flaws in some studies, such as inadequate sample sizes, further complicate these challenges (7–9). Currently, there is no universally accepted definitive prognostic or predictive biomarker for HNSCC.
Despite the heterogeneity observed in tumors, a common characteristic is their uncontrolled proliferative capacity (10). Ki-67, a prominent proliferation marker in human tumor cells, is crucial during interphase and mitosis, with its cellular distribution varying significantly throughout the cell cycle (11). It has been identified as an independent marker for predicting the prognosis of various malignancies, including those of the lung, breast, kidney, and prostate (12–15). In HNSCC, substantial evidence supports Ki-67’s prognostic value (16–19). Nonetheless, the validity of this evidence is often questioned due to small sample sizes, and conflicting views exist regarding Ki-67’s correlation with HNSCC prognosis (20, 21). Furthermore, the nomogram model based on Ki-67 characteristic expression for head and neck squamous carcinoma is currently not a focal point of research.
To address these discrepancies, this study endeavors to evaluate the prognostic value of Ki-67 and construct a prognostic model incorporating Ki-67 in HNSCC by analyzing a large patient cohort, thus offering more substantial and reliable evidence.
This investigation encompassed a retrospective cohort of 764 patients diagnosed with four principal subtypes of head and neck squamous cell carcinoma (HNSCC): oral squamous cell carcinoma (OSCC), oropharyngeal squamous cell carcinoma (OPSCC), hypopharyngeal squamous cell carcinoma (HPSCC), and laryngeal squamous cell carcinoma (LSCC). These patients were admitted to our institution between 2012 and 2022. A thorough analysis of their medical records yielded vital clinicopathological data, including age, sex, primary tumor site, pathological grade, and clinical TNM stage, adjudicated per the 8th edition of the Union for International Cancer Control’s Cancer Staging Manual. Additionally, information on recurrence, metastasis, survival status, and duration of survival as of December 31, 2022, was meticulously collected.
To augment this data, immunohistochemical staining was performed on formalin-fixed, paraffin-embedded surgical specimens, primarily focusing on the expression of Ki-67. This study received full approval from our Institutional Review Board (Ethics approval number: WCHSIRB-D-2023-437) and was rigorously conducted in alignment with the ethical guidelines set forth in the Declaration of Helsinki.
For immunohistochemical staining, 2μm thick sections were prepared from paraffin-embedded tissue blocks. The initial stage involved dewaxing and rehydrating these sections through sequential immersion in xylene, followed by ethanol solutions at concentrations of 100%, 85%, and 75%. Subsequently, they were rinsed in distilled water and immersed in citrate buffer. Antigen retrieval was facilitated by heating the sections in a microwave, comprising a 10-minute cycle at high power, an 8-minute interval, and a further 10-minute cycle at medium power. This process is crucial for exposing antigens to enhance antibody binding efficiency.
To quench endogenous peroxidase activity, the sections were treated with a 3% hydrogen peroxide solution for 10 minutes at ambient temperature. This was followed by the application of normal goat serum (Product code: AR1009, Boster Biological Technology, diluted 1:9) for 20 minutes to reduce non-specific binding. The sections were then incubated with the primary antibody (Product code: GB121141-100, Wuhan Servicebio Technology, diluted 1:100) overnight at 4°C. The following day, a secondary antibody (Product code: SP9002, Beijing Zhong Shan -Golden Bridge Biological Technology) was applied for 30 minutes at 37°C. Subsequent development with a diaminobenzidine (DAB) solution imparted a brownish-yellow coloration to the cell nuclei. Finally, the sections were washed, counterstained with hematoxylin for three minutes, rinsed, and mounted using gum.
The DAB staining methodology is employed to discern Ki-67-positive cells, characterized by the distinctive brownish-yellow coloration of their nuclei. A cell is classified as Ki-67 positive when its nucleus exhibits this specific hue, irrespective of the intensity of the staining. Regions manifesting the highest expression of Ki-67 are subjected to detailed examination under high magnification. This process involves quantifying the total number of Ki-67 positive cells and comparing it with the aggregate count of tumor cells present in the sample. The resulting ratio is subsequently converted into a percentage, representing the level of Ki-67 expression.
In this study, the expression of Ki-67 was evaluated through various categorical variables such as age, sex, pathological grade, clinical TNM stage, recurrence, and metastasis. Owing to the non-Gaussian distribution of Ki-67 expression within these groups, nonparametric statistical methods were employed for analysis. The Wilcoxon rank sum test facilitated the comparison of Ki-67 expression between two distinct groups, while the Kruskal-Wallis H test was used to evaluate differences among three or more groups. Specific group comparisons were conducted using Dunn’s multiple comparison test for specific group assessments.
To categorize patients into high and low Ki-67 expression cohorts, the optimal cut-off value derived from the receiver operating characteristic (ROC) curve and the median value of Ki-67 expression were utilized. Kaplan-Meier survival curves, coupled with log-rank tests, were applied to discern the disparities in overall survival between these groups. A Cox proportional hazards model was formulated, excluding pathological grade and clinical TNM stage as mediating variables. This model incorporated five key variables: Ki-67 expression, age, sex, recurrence, and metastasis. Nomograms were constructed from significant predictors identified in the Cox regression analysis to prognosticate patient outcomes. The model’s accuracy was quantified using the concordance index (C-index) and further validated through Bootstrap resampling techniques.
Considering the heterogeneity inherent in head and neck tumors, separate Cox models were developed for different anatomical regions.
For statistical analysis and graphic visualization, R software version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism version 10.0.2 (GraphPad Software, San Diego, California, USA) were employed. A threshold of p < 0.05 was set to determine statistical significance.
This study encompassed a cohort of 764 patients. Among these, 372 individuals (48.7%) were aged 60 or younger, while 392 (51.3%) were older than 60. The distribution by sex was notably skewed, with 596 patients (78.0%) being male and 168 (22.0%) females. Pathologically, the majority, 529 patients (69.2%), were diagnosed with moderately differentiated squamous cell carcinoma (MDSCC). This was followed by 109 (14.3%) with well-differentiated squamous cell carcinoma (WDSCC) and 126 (16.5%) with poorly differentiated squamous cell carcinoma (PDSCC). In terms of clinical staging, 433 patients (56.7%) presented with advanced stage disease (Stage III-IV), while 331 (43.3%) were at an early stage (Stage I-II). A significant majority, 683 patients (89.4%), exhibited no recurrence, in stark contrast to the 81 patients (10.6%) who experienced a recurrence. Neck or distant metastases occurred in 480 (62.8%) patients, while 284 (37.2%) did not have any. As for Ki-67 expression, the median value was 50%, with an interquartile range of 40% and an average of 48.787 ± 22.165%. Regarding tumor location, 443 cases (58.0%) of HNSCC occurred in the oral cavity, 26 (3.4%) in the oropharynx, 76 (9.9%) in the hypopharynx, and 219 (28.7%) in the larynx (Table 1).
In this study, no statistically significant variance was observed in the mean Ki-67 expression when comparing patients aged 60 or younger with those older than 60, as well as between patients with local recurrence and those without. However, a notable difference in Ki-67 mean expression was evident between sexes, with males exhibiting higher levels than females. Additionally, a clear trend was observed in Ki-67 expression relative to pathological grade; as the grade worsened, Ki-67 expression notably increased. This suggests that tumor cells with poorer differentiation are associated with elevated Ki-67 expression.
Moreover, a significant variation in Ki-67 expression was identified between different clinical TNM stages. Patients in the early stages exhibited lower Ki-67 expression compared to those in advanced stages. This difference was statistically significant, highlighting the potential of Ki-67 as a marker for disease progression.
Furthermore, there was a demonstrable correlation between Ki-67 expression and the occurrence of metastasis. Ki-67 levels were significantly higher in patients who had lymph node or distant metastasis compared to those without such metastatic spread (Table 2) (Figure 1).
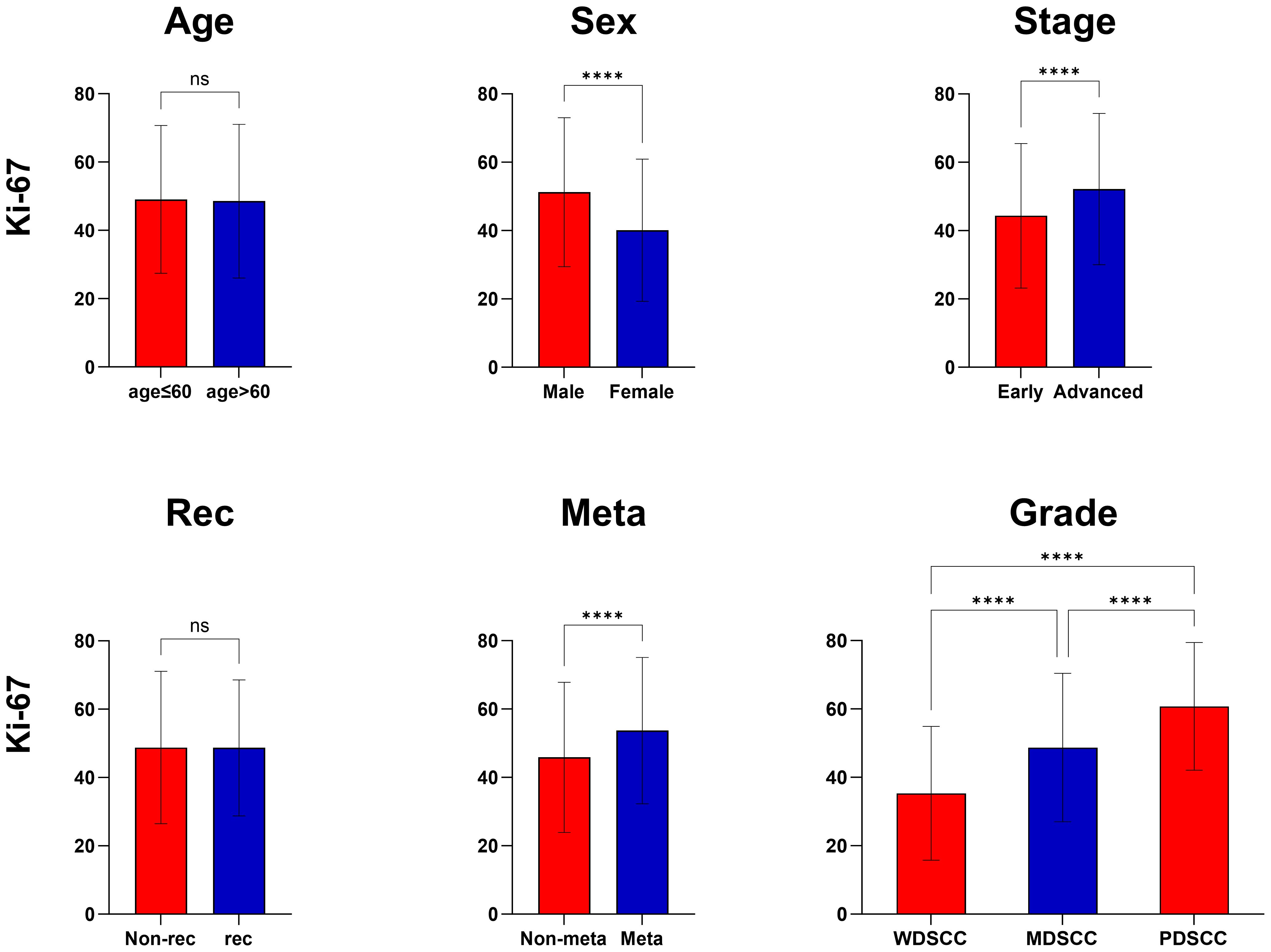
Figure 1 Correlation of Ki-67 expression with clinicopathological features. ****, P<0.0001, ns, no significance.
This study employed two distinct cut-off values to optimize the grouping of Ki-67 expression, thereby enabling a more precise evaluation of its expression levels. The first method involved dividing the sample into two groups using the median value of 50% as the cut-off. An alternative approach determined the optimal cut-off value via the receiver operating characteristic (ROC) curve, which was identified as 57.5%. This cut-off value demonstrated a specificity of 0.589, a sensitivity of 0.579, and an area under the curve (AUC) of 0.605. Kaplan-Meier survival curves and log-rank test results indicated that the prognosis for patients in the high Ki-67 expression group was significantly poorer compared to those in the low expression group (P<0.05). Notably, the cut-off value derived from the ROC curve proved more effective in stratifying Ki-67 expression than the median-based approach (Figure 2).
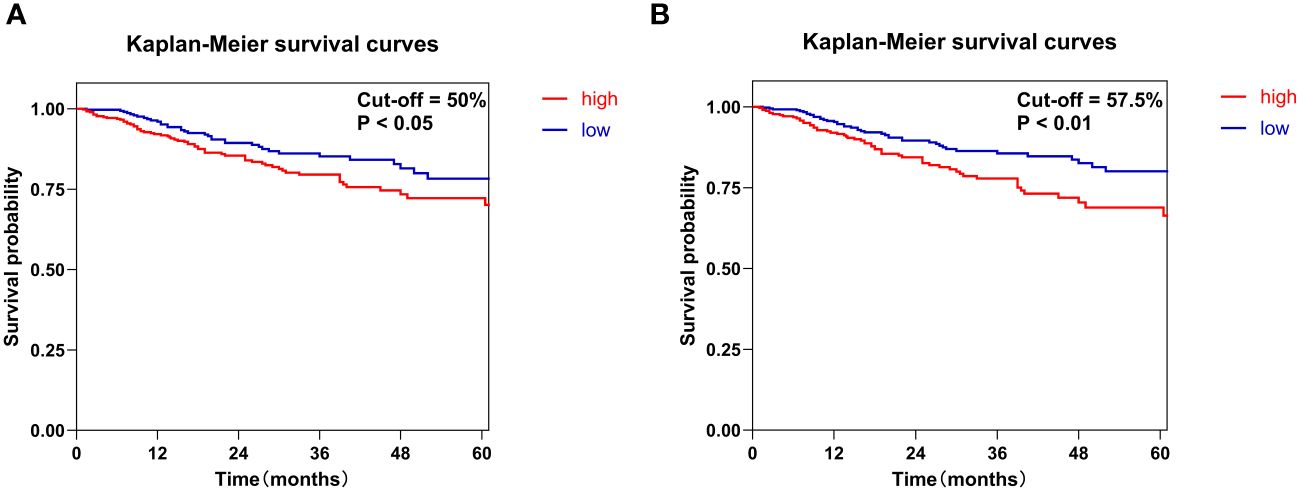
Figure 2 Kaplan-Meier survival curves were constructed to evaluate the overall survival of patients with HNSCC based on Ki-67 expression levels. Specifically, Ki-67 expression was classified into high and low groups using two different methods. (A) The median value of Ki-67 expression of 50% served as the cut-off. (B) The cut-off was set at the optimal threshold of 57.5%, which was determined from the ROC curve.
Multivariate Cox regression analysis of HNSCC identified age, recurrence, metastasis, and Ki-67 expression as independent prognostic risk factors (all hazard ratios >1, all P<0.05) (Table 3). Patients with high Ki-67 expression, advanced age, presence of metastasis, and recurrence had a poorer prognosis than those with lower Ki-67 levels, younger age, absence of metastasis, and no recurrence. Sex did not show a significant impact on prognosis (P>0.05).
Furthermore, separate analyses of Cox proportional hazards models for OSCC, OPSCC, HPSCC, and LSCC revealed that Ki-67 expression was an independent prognostic factor solely in OSCC. In contrast, its prognostic relevance was not significant in OPSCC, HPSCC, and LSCC. (Due to the limited sample size, the HRs for Sex and Recurrence in HPSCC were calculated to be 0.000, lacking a 95% confidence interval (CI). Similarly, in LSCC, the HRs for sex were also found to be 0.000 without a 95% CI. Consequently, we opted to exclude these variables from the separate Cox regression models) (Table 3).
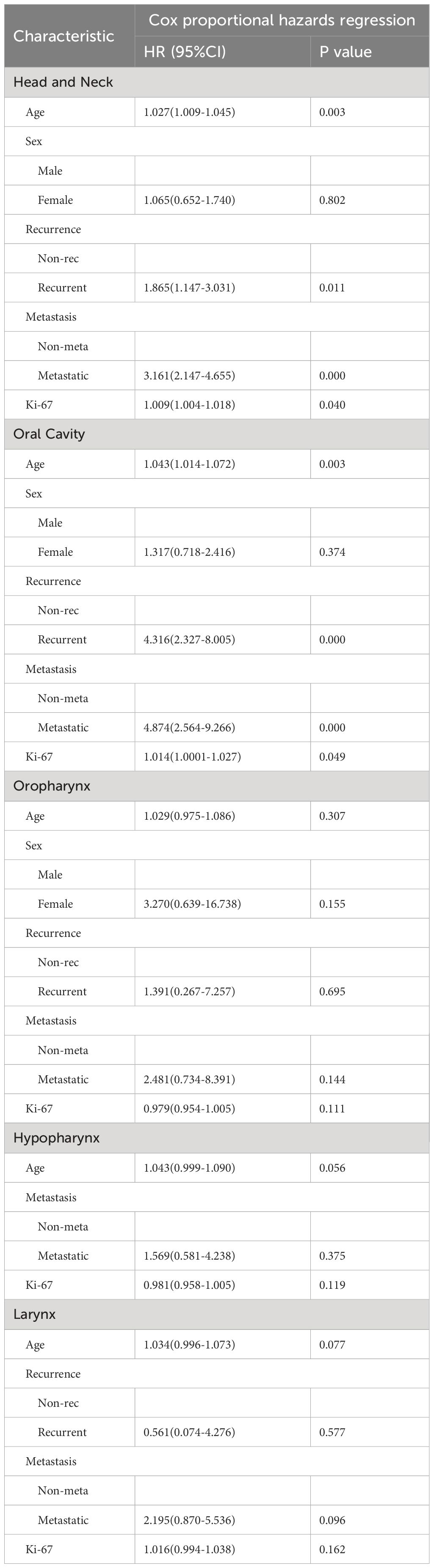
Table 3 Multivariate analysis of overall survival by Cox proportional hazards model in the whole head and neck region and in sub-sites.
Using significant predictors from the Cox model, a nomogram was constructed (Figure 3), enabling the prediction of 1-, 3-, and 5-year survival rates based on the total points accumulated from a patient’s age, Ki-67 expression, recurrence status, and metastasis presence. For example, a 60-year-old patient with cervical lymph node metastasis, no recurrence, and a Ki-67 expression of 70% would score a total of 130 points, correlating to predicted survival rates of 87.5% at 1 year, 68% at 3 years, and 55% at 5 years. The nomogram’s C-index was 0.721, indicating good predictive accuracy for overall survival, with the calibration curve showing close alignment between predicted and observed 3-year survival rates (Figure 4).
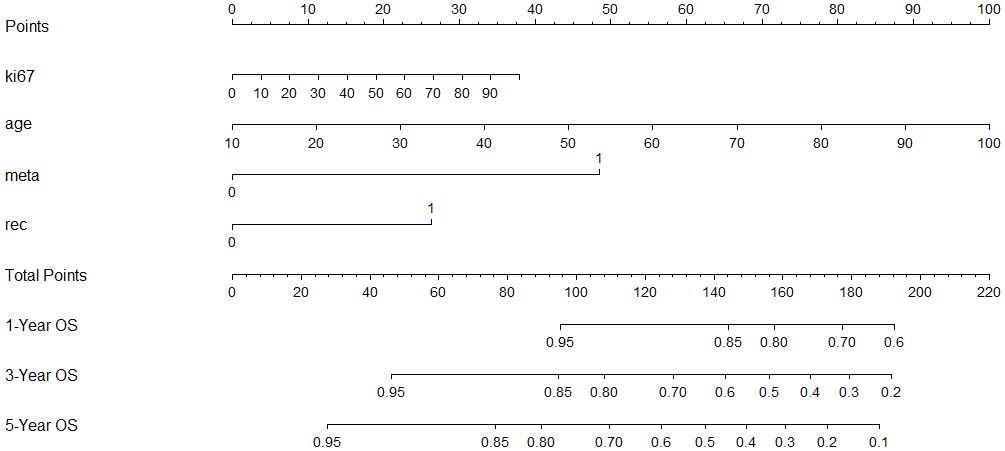
Figure 3 A constructed nomogram for prognostic prediction of a patient with HNSCC. The total points were calculated by summing up the points related to the patient’s age, Ki-67 expression, recurrence, and metastasis. The patients’ 1-, 3-, and 5-year predicted survival rates could be obtained using the total points.
The site-specific Cox proportional hazards model revealed an adverse effect of Ki-67 expression on the prognosis of OSCC. In addition, the overall cohort contained a large proportion of OSCC patients. Therefore, based on the nomogram for head and neck squamous carcinoma, a separate nomogram for OSCC was constructed (Figure 5).
The identification of prognostic biomarkers and the development of prognostic models for head and neck squamous cell carcinoma (HNSCC) are pivotal for predicting patient outcomes and guiding clinical decision-making. This facilitates the adoption of personalized clinical strategies (22). Ki-67, a nuclear protein initially discovered in proliferating Hodgkin’s lymphoma cells by Gerdes et al. (23), is exclusively expressed during mitosis and absent in quiescent cells. Its expression, regulated by cell cycle-dependent transcription and protein degradation (24), marks it as a critical cell proliferation marker. Despite its established prognostic efficacy in various tumors, insights into Ki-67’s role in HNSCC remain varied, influenced by factors like sample size, detection techniques, and cut-off values for grouping (25–27).
In our study, Kaplan-Meier curves and log-rank tests identified Ki-67 as a prognostic risk factor for HNSCC. Cox multivariate analysis, controlling for confounders, confirmed Ki-67’s role as an independent prognostic factor alongside age, recurrence, and metastasis. Our study’s prognostic model, incorporating Ki-67, age, recurrence, and metastasis, revealed that recurrence and metastasis had a more substantial impact on prognosis than Ki-67 expression and age, as evidenced by their higher hazard ratio values. The model’s C-index was 0.721, indicating excellent predictive efficacy (28). The calibration curve corroborated the nomogram’s accuracy in reflecting actual patient survival.
Furthermore, within the Cox proportional hazards model, while the HR for Ki-67 expression exceeded 1, it was lower than that of other variables. This can be attributed to the Ki-67 expression being analyzed as a continuous variable with values ranging from 0 to 100. The HR for Ki-67 expression in the overall cohort was 1.009, representing that if the expression of Ki-67 increased by 1, the risk of patient death increased by 1.009. Upon further analysis, by stratifying Ki-67 expression into two groups based on a predefined cut-off value for the Cox model, we observed a substantially increased HR for Ki-67 expression. Nevertheless, we advocate for the utilization of Ki-67 expression as a continuous variable. This approach not only preserves the integrity of the data but also ensures that the Nomogram remains uninfluenced by arbitrary cut-off values for Ki-67 expression, thereby enhancing its utility.
Unlike prior studies that often relied solely on clinical, gene transcription, or gene expression data for prognostic modeling, our research uniquely integrated clinical data with immunohistochemical staining. Immunohistochemical staining, a standard procedure in tumor pathology, is both cost-effective and time-efficient. It proves more manageable in clinical settings than gene expression analysis. By simply assessing Ki-67 levels alongside key clinicopathological features, one can predict a patient’s prognosis using the nomogram. This predictive insight equips clinicians with solid evidence, facilitating informed decision-making about subsequent treatment steps. For instance, a poor predicated prognosis may prompt the consideration of active measures, including postoperative radiotherapy and chemotherapy. This has a positive effect on the overall prognosis of the patient. Moreover, the prognostic model’s presentation through a nomogram offers clarity, ease of understanding, and practical applicability.
Our research also explored Ki-67 expression’s correlation with clinicopathological features in HNSCC patients. Elevated Ki-67 expression in advanced-stage and poorly differentiated tumors highlighted its link to cell proliferation (29). Notably, Ki-67 expression was significantly higher in males than females, possibly reflecting variations in sex hormones or lifestyle factors like tobacco and alcohol use (30, 31). HPV infection status also appeared to influence proliferative activity (32). A significant difference in Ki-67 expression across different sites necessitated subgroup analysis, revealing Ki-67 as a prognostic biomarker only in OSCC. Because of this, we additionally constructed a separate nomogram for OSCC, demonstrating a prognostic model for containing Ki-67 expression. The prognostic significance of Ki-67 expression in OPSCC, HPSCC, and LSCC was not established, highlighting the need for further research, particularly due to sample size constraints.
Previous studies on Ki-67 expression in HNSCC have employed various cut-off values, including ROC curve-derived values (33), medians (13), quartiles (34), and data distribution-based values (17). Our study utilized both the median and ROC-derived optimal cut-off values, finding the latter to offer more significant prognostic variance. In calculating the cut-off value via the ROC curve, we analyzed a substantial cohort to establish this threshold. In future research, it is imperative to undertake prospective studies to affirm the prognostic significance of Ki-67 expression and to address pivotal concerns, including the determination of cut-off values. Various methodologies for quantifying Ki-67 expression, such as positive cell and area ratios, underscore the need for standardized assessment procedures. The lack of standardization, as highlighted in breast cancer studies (35, 36), calls for the establishment of consistent protocols for Ki-67 assessment in HNSCC.
In summary, this research encompassed a comprehensive analysis of 764 patients hospitalized from 2012 to 2022. It meticulously investigated the correlation between Ki-67 expression and a spectrum of clinicopathological characteristics in HNSCC. Our survival analysis unequivocally established Ki-67 as a negative prognostic biomarker for HNSCC. We developed a nomogram as a prognostic prediction model, integrating Ki-67 expression, which promises to contribute significantly to future clinical practice. However, it is crucial to acknowledge the inherent heterogeneity of head and neck tumors, underscoring the need for further validation of our findings in specific sites such as the oropharynx, hypopharynx, and larynx. Future research directions should focus on refining methodologies, including Ki-67 counting techniques, antigen retrieval processes, and determining the optimal cut-off value for Ki-67 expression. The ultimate goal is to establish a standardized and universally applicable procedure for assessing Ki-67 in HNSCC, thereby enhancing the accuracy and reliability of prognostic predictions in clinical settings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by West China Hospital of Stomatology Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
TW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. LX: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. ZL: Data curation, Formal analysis, Investigation, Writing – review & editing. ZH: Data curation, Investigation, Writing – review & editing. NH: Data curation, Investigation, Writing – review & editing. YL: Methodology, Supervision, Validation, Writing – review & editing. BY: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Fujian Province, China (Grant No. 2021J011361).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. (2019) 16:669–83. doi: 10.1038/s41571-019-0227-z
3. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. (2020) 6:92. doi: 10.1038/s41572-020-00224-3
4. Bellairs JA, Hasina R, Agrawal N. Tumor DNA: an emerging biomarker in head and neck cancer. Cancer Metastasis Rev. (2017) 36:515–23. doi: 10.1007/s10555-017-9685-x
5. Gavrielatou N, Doumas S, Economopoulou P, Foukas PG, Psyrri A. Biomarkers for immunotherapy response in head and neck cancer. Cancer Treat Rev. (2020) 84:101977. doi: 10.1016/j.ctrv.2020.101977
6. Schaaij-Visser TBM, Brakenhoff RH, Leemans CR, Heck AJR, Slijper M. Protein biomarker discovery for head and neck cancer. J Proteomics. (2010) 73:1790–803. doi: 10.1016/j.jprot.2010.01.013
7. Guglas K, Bogaczyńska M, Kolenda T, Ryś M, Teresiak A, Bliźniak R, et al. lncRNA in HNSCC: challenges and potential. Contemp Oncol (Pozn). (2017) 21:259–66. doi: 10.5114/wo.2017.72382
8. Hussein AA, Forouzanfar T, Bloemena E, de Visscher J, Brakenhoff RH, Leemans CR, et al. A review of the most promising biomarkers for early diagnosis and prognosis prediction of tongue squamous cell carcinoma. Br J Cancer. (2018) 119:724–36. doi: 10.1038/s41416-018-0233-4
9. Rivera C, Oliveira AK, Costa RAP, De Rossi T, Paes Leme AF. Prognostic biomarkers in oral squamous cell carcinoma: A systematic review. Oral Oncol. (2017) 72:38–47. doi: 10.1016/j.oraloncology.2017.07.003
10. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. (2001) 411:342–8. doi: 10.1038/35077213
11. Sun X, Kaufman PD. Ki-67: more than a proliferation marker. Chromosoma. (2018) 127:175–86. doi: 10.1007/s00412-018-0659-8
12. Davey MG, Hynes SO, Kerin MJ, Miller N, Lowery AJ. Ki-67 as a prognostic biomarker in invasive breast cancer. Cancers (Basel). (2021) 13:4455. doi: 10.3390/cancers13174455
13. Kammerer-Jacquet SF, Ahmad A, Møller H, Sandu H, Scardino P, Soosay G, et al. Ki-67 is an independent predictor of prostate cancer death in routine needle biopsy samples: proving utility for routine assessments. Mod Pathol. (2019) 32:1303–9. doi: 10.1038/s41379-019-0268-y
14. Li Z, Li F, Pan C, He Z, Pan X, Zhu Q, et al. Tumor cell proliferation (Ki-67) expression and its prognostic significance in histological subtypes of lung adenocarcinoma. Lung Cancer. (2021) 154:69–75. doi: 10.1016/j.lungcan.2021.02.009
15. Menon SS, Guruvayoorappan C, Sakthivel KM, Rasmi RR. Ki-67 protein as a tumour proliferation marker. Clinica Chimica Acta. (2019) 491:39–45. doi: 10.1016/j.cca.2019.01.011
16. Dumitru CS, Ceausu AR, Comsa S, Raica M. Loss of E-cadherin expression correlates with Ki-67 in head and neck squamous cell carcinoma. In Vivo. (2022) 36:1150–4. doi: 10.21873/invivo.12814
17. Gadbail AR, Sarode SC, Chaudhary MS, Gondivkar SM, Tekade SA, Yuwanati M, et al. Ki67 labelling index predicts clinical outcome and survival in oral squamous cell carcinoma. J Appl Oral Sci. (2021) 29:e20200751. doi: 10.1590/1678-7757-2020-0751
18. Gioacchini FM, Alicandri-Ciufelli M, Magliulo G, Rubini C, Presutti L, Re M. The clinical relevance of Ki-67 expression in laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. (2015) 272:1569–76. doi: 10.1007/s00405-014-3117-0
19. Silva SD, Agostini M, Nishimoto IN, Coletta RD, Alves FA, Lopes MA, et al. Expression of fatty acid synthase, ErbB2 and Ki-67 in head and neck squamous cell carcinoma. A clinicopathological study. Oral Oncol. (2004) 40:688–96. doi: 10.1016/j.oraloncology.2004.01.004
20. Maebayashi T, Ishibashi N, Aizawa T, Sakaguchi M, Saito T, Kawamori J, et al. Roles of Ki-67 and p16 as biomarkers for unknown primary head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. (2019) 276:1221–9. doi: 10.1007/s00405-019-05338-3
21. Woźniak M, Nahajowski M, Hnitecka S, Rutkowska M, Marek G, Agrawal A, et al. A comparative study of osteopontin expression, ki67 index and prognosis in squamous cell carcinoma and cysts of the oral cavity. Transl Cancer Res. (2020) 9:795–808. doi: 10.21037/tcr
22. Joyner MJ, Paneth N. Promises, promises, and precision medicine. J Clin Invest. (2019) 129:946–8. doi: 10.1172/JCI126119
23. Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. (1983) 31:13–20. doi: 10.1002/ijc.2910310104
24. Andrés-Sánchez N, Fisher D, Krasinska L. Physiological functions and roles in cancer of the proliferation marker Ki-67. J Cell Sci. (2022) 135:jcs258932. doi: 10.1242/jcs.258932
25. Almangush A, Heikkinen I, Mäkitie AA, Coletta RD, Läärä E, Leivo I, et al. Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer. (2017) 117:856–66. doi: 10.1038/bjc.2017.244
26. Kara A, Turan G, Guven M, Guven EM, Elden H. Ki-67, p-53, E-cadherin, and β-catenin expression of advanced glotto-subglottic and supraglottic larynx carcinomas. Nigerian J Clin Pract. (2022) 25:1424. doi: 10.4103/njcp.njcp_1693_21
27. Xu QQ, Li QJ, Xu Z, Lan LL, Hou Z, Liu J, et al. Prognostic value of the immunohistochemical score based on four markers in head and neck squamous cell carcinoma. Front Immunol. (2023) 14:1076890. doi: 10.3389/fimmu.2023.1076890
28. Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun (Lond). (2020) 40:301–12. doi: 10.1002/cac2.12067
29. Batelja-Vuletic L, Tomasovic-Loncaric C, Ceppi M, Bruzzone M, Fucic A, Krstanac K, et al. Comparison of androgen receptor, VEGF, HIF-1, ki67 and MMP9 expression between non-metastatic and metastatic stages in stromal and tumor cells of oral squamous cell carcinoma. Life (Basel). (2021) 11:336. doi: 10.3390/life11040336
30. Nainani P, Paliwal A, Nagpal N, Agrawal M. Sex hormones in gender-specific risk for head and neck cancer: A review. J Int Soc Prev Community Dent. (2014) 4:S1–4. doi: 10.4103/2231-0762.144557
31. Padma R, Sundaresan S, Kalaivani A, Thilagavathi R. Assessment of histopathological grade and ki-67 expression in tobacco and non-tobacco habitual buccal mucosa cancer. Indian J Otolaryngol Head Neck Surg. (2019) 71:410–6. doi: 10.1007/s12070-018-1328-1
32. Ragin CCR, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. (2007) 86:104–14. doi: 10.1177/154405910708600202
33. Govindaraj PK, Kallarakkal TG, Mohd Zain R, Tilakaratne WM, Lew HL. Expression of ki-67, cornulin and ISG15 in non-involved mucosal surgical margins as predictive markers for relapse in oral squamous cell carcinoma (OSCC). PloS One. (2021) 16:e0261575. doi: 10.1371/journal.pone.0261575
34. Liu Q, Ran D, Wang L, Feng J, Deng W, Mei D, et al. Association between ki67 expression and therapeutic outcome in colon cancer. Oncol Lett. (2023) 25:272. doi: 10.3892/ol
35. Gown AM. The biomarker Ki-67: promise, potential, and problems in breast cancer. Appl Immunohistochemistry Mol Morphology. (2023) 31:478. doi: 10.1097/PAI.0000000000001087
Keywords: Ki-67, head and neck squamous cell carcinoma, prognosis, survival, nomogram, prognostic prediction model
Citation: Wang T, Xue L, Li Z, Hong Z, Hu N, Li Y and Yan B (2024) A novel nomogram model based on Ki-67 characteristic expression to predict prognosis in head and neck squamous cell carcinoma. Front. Oncol. 14:1376498. doi: 10.3389/fonc.2024.1376498
Received: 25 January 2024; Accepted: 25 March 2024;
Published: 08 April 2024.
Edited by:
Gunnar Wichmann, University Hospital Leipzig, GermanyReviewed by:
Ming Zheng, Academy of Military Medical Sciences, ChinaCopyright © 2024 Wang, Xue, Li, Hong, Hu, Li and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Yan, eWFuYmluZ193ZXN0QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.