
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 21 May 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1375737
This article is part of the Research TopicCytogenetics, Genomics and Epigenomics in Myelodysplastic Neoplasm and Acute Myeloid Leukemia: From Biology to TreatmentView all 6 articles
Background: Acute promyelocytic leukemia (APL) is rarely caused by the PLZF::RARα fusion gene. While APL patients with PLZF::RARα fusion commonly exhibit diverse hematologic symptoms, the presentation of myeloid sarcoma (MS) as an initial manifestation is infrequent.
Case presentation: A 61-year-old patient was referred to our hospital with 6-month history of low back pain and difficulty walking. Before this admission, spine magnetic resonance imaging (MRI) conducted at another hospital revealed multiple abnormal signals in the left iliac bone and vertebral bodies spanning the thoracic (T11-T12), lumbar (L1-L4), and sacral (S1/S3) regions. This led to a provisional diagnosis of bone tumors with an unknown cause. On admission, complete blood count (CBC) test and peripheral blood smear revealed a slightly increased counts of monocytes. Immunohistochemical staining of both spinal and bone marrow (BM) biopsy revealed positive expression for CD117, myeloperoxidase (MPO), and lysozyme. BM aspirate showed a significant elevation in the percentage of promyelocytes (21%), which were morphologically characterized by round nuclei and hypergranular cytoplasm. Multiparameter flow cytometry of BM aspirate revealed that blasts were positive for CD13, CD33, CD117, and MPO. Through the integrated application of chromosome analysis, fluorescence in situ hybridization (FISH), reverse transcriptase polymerase chain reaction (RT-PCR), and Sanger sequencing, it was determined that the patient possessed a normal karyotype and a rare cryptic PLZF::RARα fusion gene, confirming the diagnosis of APL.
Conclusion: In the present study, we report the clinical features and outcome of a rare APL patient characterized by a cryptic PLZF::RARα fusion and spinal myeloid sarcoma (MS) as the initial presenting symptom. Our study not only offers valuable insights into the heterogeneity of APL clinical manifestations but also emphasizes the crucial need to promptly consider the potential link between APL and MS for ensuring a timely diagnosis and personalized treatments.
Acute promyelocytic leukemia (APL), also known as acute myeloid leukemia (AML) subtype M3 according to the French-American-British (FAB) classification, is primarily characterized by an accumulation of immature promyelocytes in bone marrow (BM) (1). APL patients typically appear as one or more of hematologic symptoms, including fever, bleeding, fatigue, infections, bone pain, and others (1). Besides, in some cases, APL may present with extramedullary involvement that causes myeloid sarcoma (MS) (2–6). Although rare in clinical practice, MS is more commonly associated with relapsed or refractory APL cases, with an estimated incidence of 3%–5% (2). However, in newly diagnosed APL, MS occurs even more rarely, potentially contributing to delays in APL diagnosis (3–6). In addition, MS can occur simultaneously in various extramedullary locations, such as skin, soft tissues, bones, lymph nodes, and other organs (3–6). Therefore, MS may produce miscellaneous non-hematologic symptoms that mimic those of other diseases, making it more challenging to timely distinguish MS. Moreover, the atypical morphological characteristics exhibited by leukemia cells at onset of this disease adds complexity to the diagnostic procedure in cases of APL with MS (4–6). Given the substantial risk of disseminated intravascular coagulation (DIC) in association with APL, a condition that can be severe and life-threatening, it is imperative to prioritize early APL diagnosis and the immediate commencement of APL-specific treatments like all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) (1, 7, 8).
One of the key diagnostic features of APL is chromosomal translocation involving the gene that encodes retinoic acid receptor alpha (RARα) on chromosome 17 (1, 9–11). In particular, an overwhelming majority of APL cases exhibit the typical t(15;17)(q22;21) translocation, which results in the fusion of the promyelocytic leukemia (PML) gene and RARα gene, namely PML::RARα fusion gene (9). In exceptionally infrequent cases (1~2%), APL has been observed with rare variant translocations, including t(11;17)(q23;q21), t(11;17)(q13;q21), t(5;17)(q32;q21), and t(17;17)(q11;q21) (10, 11). These variant translocations involve other partner genes and may impact on the clinicopathologic features of APL. For example, APL patients with the classic PML::RARα fusion gene are highly responsive to ATRA and ATO (1, 9). However, patients with the t(11;17)(q23;q21) translocation, resulting in the fusion of the promyelocytic leukemia zinc finger (PLZF)-encoding gene and RARα gene—a fusion less prevalent than PML::RARα—may display a comparatively less robust response to the same treatments (10–12). It is important to note that some thirty APL cases with MS as initial presentation has been documented in literatures so far, and almost all carried the classic t(15;17)(q22;21) translocation (3–6). The development of MS is still scarcely reported in APL with other rare variant translocations. Here, we report a newly diagnosed APL patient (61-year-old male) with spinal MS as the first presentation. Using integrated genetic testing, we identified a normal karyotype, and notably, a rare cryptic PLZF::RARα fusion gene.
A 61-year-old Chinese man was referred to our hospital with 6-month history of low back pain and difficulty walking, which were particularly severe after physical exertion. The patient reported occasional temporary relief of these symptoms through Chinese medical massage treatments. One month before being admitted, his symptoms had worsened progressively without a clear precipitating factor, and he experienced pain that extended to his left thigh accompanied by a sensation of numbness. Subsequently, detailed bone examinations were performed at local hospital. At that time, spine magnetic resonance imaging (MRI) suggested pathologic fracture of lumbar (L3) spine and showed multiple abnormal signals in left iliac bone and in vertebral bodies of the thoracic (T11-T12), lumbar (L1-L4), and sacral (S1/S3) spine. Meanwhile, fluorine-18-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) imaging reported that FDG uptake was slightly increased in those bone lesions, but not in other areas of the body. The patient was tentatively diagnosed with bone tumors of unknown cause and was transferred to our hospital for further diagnosis and treatment.
On admission, our spine MRI confirmed the previous results (Figure 1A). A complete blood count (CBC) test showed a slightly increased counts of monocytes (0.73×109/L; normal range: 0.1-0.6×109/L), and normal results of red blood cells (4.95×1012/L), hemoglobin (142 g/L), platelets (204×109/L), and white blood cells (7.46×109/L) (Table 1). Examination of the peripheral blood smear consistently suggested an increased proportion of monocytes. Moreover, antibody serology tests revealed positive results for antinuclear (ANA) antibody and anti-beta-2 glycoprotein 1 (B2GP1) antibody. With flow cytometry (Becton Dickinson FastImmune™ Cytokine System), we detected elevated levels of interferon gamma (IFN-γ; 13.03 pg/mL; normal range:≤4.43 pg/mL) and interleukin-17A (5.60 pg/mL; normal range:≤4.74 pg/mL) in whole blood. Notably, coagulation tests revealed a significant increase in D-dimers level (4.12 mg/L; normal range: 0-0.8 mg/L), while other coagulation indexes were within normal range (Table 1). Heart, renal, liver, and nervous system functions were normal.
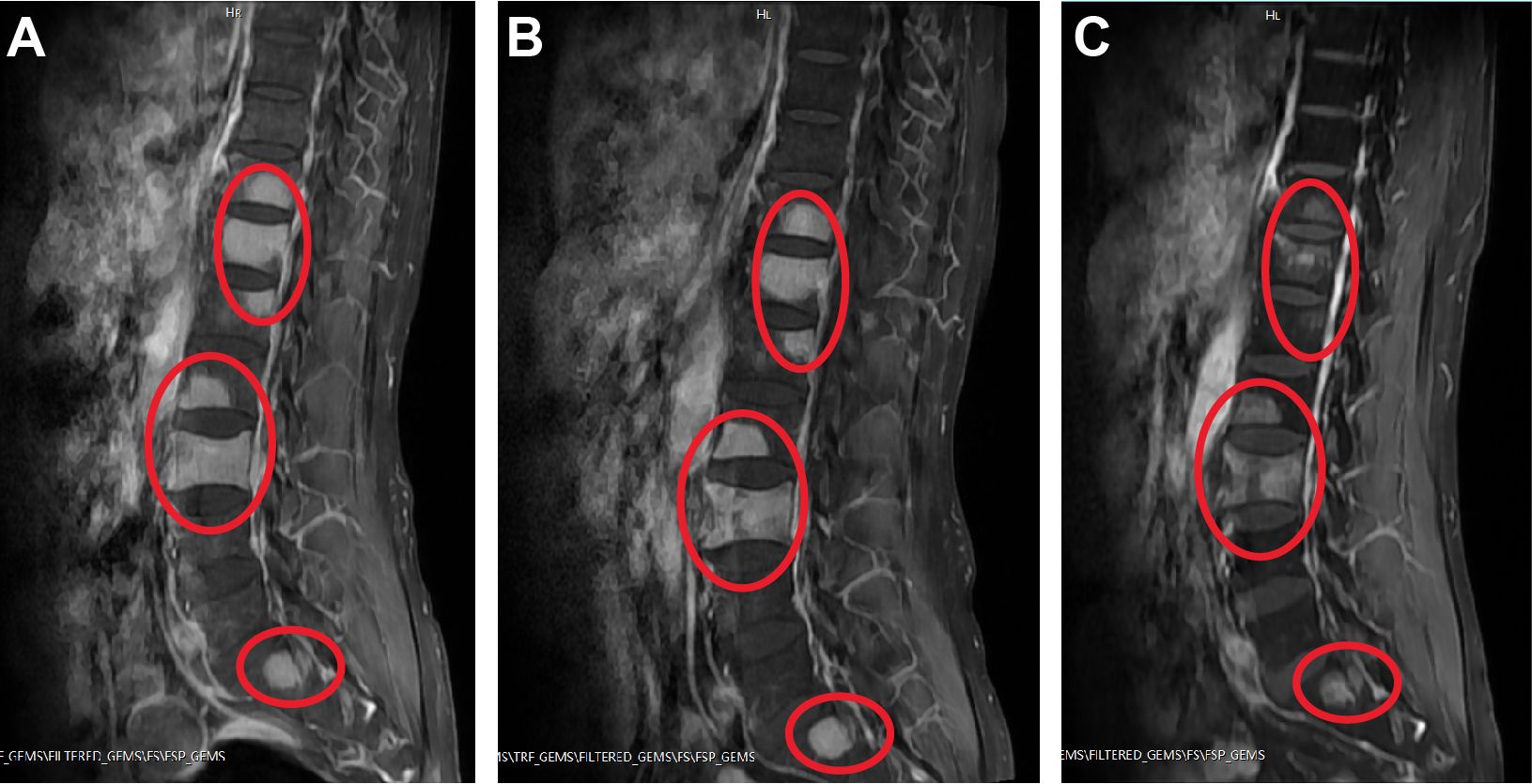
Figure 1 Examples of spinal sagittal T1-weighted MRI of the patient. (A) On admission. (B) After first cycle of therapy. (C) After second cycle of therapy. Abnormal high signals on T1 images are shown circled in red.
In order to assess the histopathological basis of bone lesions, we further performed CT-guided percutaneous needle biopsy of lumbar L3. The spinal biopsy results indicated the presence of immature/blast-like cells with eccentric nuclei within the spaces of the bone trabeculae (Figure 2A). Immunohistochemical staining of the spinal biopsy revealed positive expression for CD117, CD43, myeloperoxidase (MPO), lysozyme, and Ki67 (labelling index about 40%), while it tested negative for CD20, CD34, CD56, CD61, CD79a, CD138, and IgG/M, κ, λ expression. These findings suggested the presence of myeloid neoplasms. Meanwhile, BM biopsy revealed 95% of blast cells and a staining profile characterized by CD117 (+), MPO (+) and lysozyme (part+), which was similar to the results of spinal biopsy. In addition, BM aspirate showed hypercellularity with an elevated myeloid/erythroid (M/E) ratio of 7.52:1. Specifically, there was a significant elevation in the percentage of promyelocytes (21%; normal range: 0.4-3.9%), strongly indicating the likelihood of APL. Erythropoiesis was insufficient, while megakaryopoiesis was normal. Giemsa-stained promyelocytes displayed round nuclei and hypergranular cytoplasm (Figure 2B). However, Auer rods were notably absent. The majority of promyelocytes had positive staining for MPO (Figure 2C). Multiparameter flow cytometry of BM aspirate detected 78% blasts and suggested an immunophenotype that was positive for CD13, CD33, CD117, and MPO, and negative for CD3, CD10, CD11b, CD14, CD15, CD19, CD34, CD71, CD79a, and HLA-DR, corresponding to APL features.
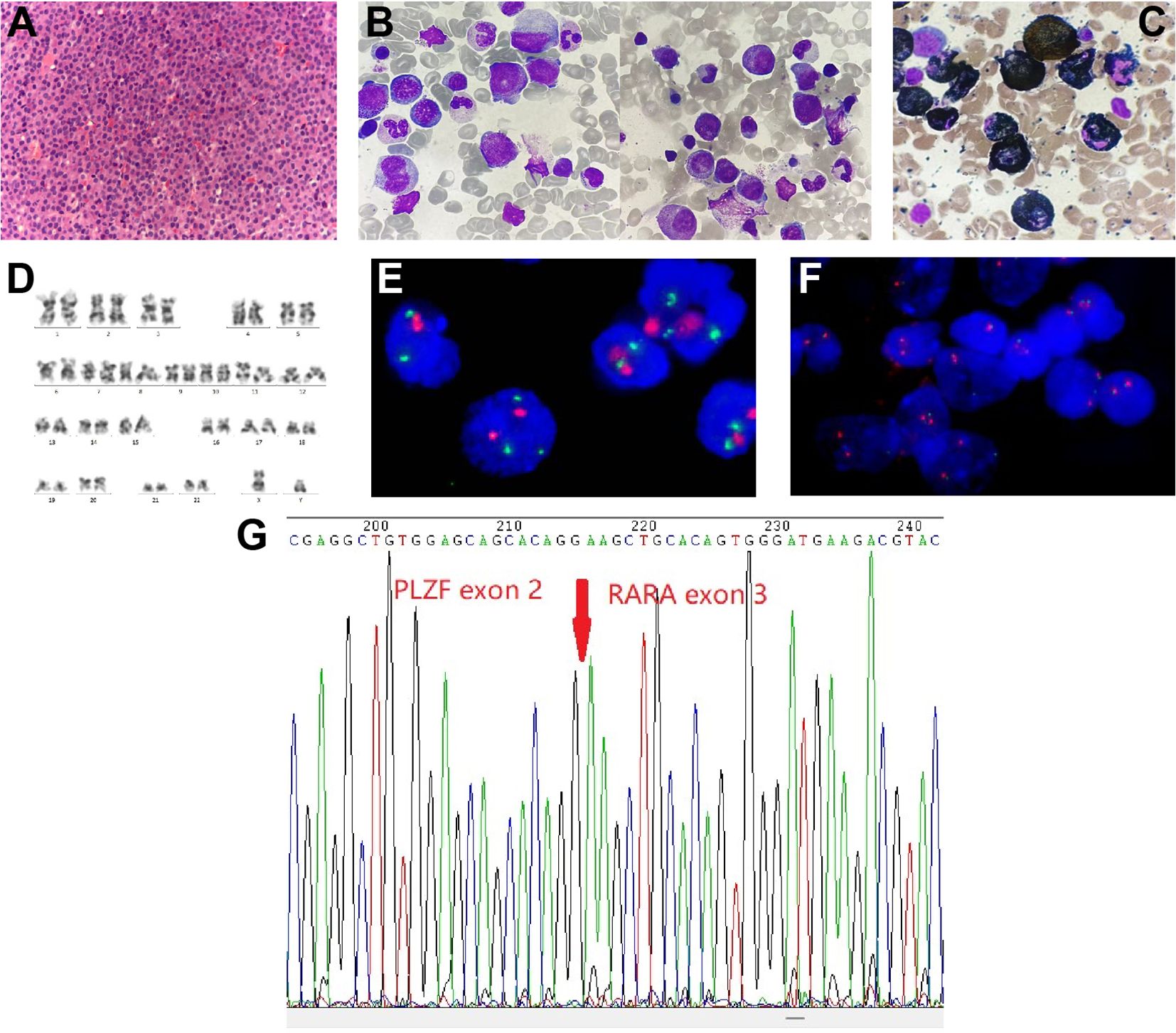
Figure 2 Spine tissue and bone marrow (BM) aspirate examinations and results of karyotype and fluorescence in situ hybridization (FISH) analysis. (A) Histopathology of spinal cord (hematoxylin and eosin staining; magnification, ×400). (B) BM aspirate shows atypical acute promyelocytic leukemia morphology (Wright-Giemsa staining; magnification, ×1,000). (C) BM aspirate showed that promyelocytes were myeloperoxidase (MPO) positivity (MPO staining; magnification, ×1,000). (D) Chromosome analysis of the BM reveals a normal male karyotype. (E, F) Metaphase FISH analysis of the patient. (E) The standard dual-color, dual-fusion probe set probe set for t(15;17) shows the presence of two red signals (PML) and three green signals (RARα), suggesting RARα rearrangement; (F) The RARα break-apart probe detects separated red signals (RARα). (G) Sanger sequencing analysis demonstrates PLZF::RARα fusion transcript.
Notably, cytogenetics G-band analysis of BM cells revealed a normal male karyotype (46, XY) (Figure 2D). Metaphase fluorescence in situ hybridization (FISH) analysis with the PML::RARα dual-color dual-fusion probe kit (FP-005, Wuhan HealthCare Biotechnology Co., Ltd.) on BM aspirate suggested the absence of PML-RARα dual-fusion translocation (Figure 2E). However, three green FISH signals suggested the presence of RARα translocation (Figure 2E). This finding was subsequently validated using the RARα break-apart probe detection kit (FP-043, Wuhan HealthCare Biotechnology Co., Ltd.) (Figure 2F). To further explore the etiology, we performed reverse transcriptase polymerase chain reaction (RT-PCR) (Dian Diagnostics Group Co. Ltd., Hangzhou, China) on BM. It revealed PLZF::RARα fusion by using the reverse primers (NM_000964; RARα 1-R, 5’-AAGCCCTTGCAGCCCTCAC-3’ [external]; RARα 2-R, 5’-CCCATAGTGGTAGCCTGAGGAC-3’ [internal]) located within exon 2 of RARα gene in conjunction with the forward primers (NM_001018011; PLZF 1-F, 5’-CCACAAGGCTGACGCTGTATT-3’ [external]; PLZF 2-F, 5’-GTGGGCATGAAGTCAGAGAGC-3’ [internal]) located within exon 3 of PLZF gene. Sanger sequencing further confirmed the presence of PLZF::RARα exon 3–exon 2 fusion transcript (Figure 2G). Next-generation sequencing (NGS) analysis with the Myeloid Tumor Assay that was consisted of 128 genes panel (Dian Diagnostics Group Co., Hangzhou, China) detected no additional mutations. Taken together, according to FAB classification, a definitive diagnosis of APL was ultimately established.
In the initial induction therapy, the patient was treated with 20 mg/day ATRA (BID) for one week. This was followed by a regimen incorporating subcutaneous azacitidine (120 mg/day, Day 1 to 7) and oral administration of venetoclax with a progressive dose escalation: 100 mg/day (Day 1), 200 mg/day (Day 2), and 400 mg/day (Day 3 to 24) (Table 2). During this period, the patient was also treated with cetirizine for skin itch and rash. Subsequent CBC revealed that his WBC counts reduced to 3.9×109/L, which was still within normal range (Table 1). BM aspirate showed hypercellularity and a decreased M/E ratio of 0.2:1, which was characterized by granulocytic hypoplasia and erythrocytic/megakaryocytic hyperplasia. Importantly, BM aspirate indicated that the percentage of promyelocytes reduced to 0.5%. On BM biopsy, residual leukemia cells were negligible. However, spine MRI showed no significant improvement in MS lesions (Figure 1B). RT-PCR from BM showed the persistence of PLZF::RARα fusion.
As a result, we maintained the patient on oral venetoclax administration at 400 mg/day (Day 1 to 12) and further administered idarubicin intravenously (10 mg/day IV bolus, Day 1 and 2; 20 mg/day IV bolus, Day 3) (Table 2). Meanwhile, the patient developed pancytopenia, and had sustained agranulocytosis for two weeks. To address this, herombopag, recombinant human interleukin-11 (IL-11), and blood transfusion were given (Table 2). Repeated BM aspirate showed reduced cellularity and a decrease in all three blood cell lineages. Notably, the percentage of promyelocytes increased again to 12%, but subsequent flow cytometry immunophenotyping confirmed a normal phenotype of immature granulocytes, which was hypothesized to be a possible manifestation of myeloid hematopoietic recovery. Fortunately, MRI showed that spinal MS lesions were obviously shrunken (Figure 1C). The patient also obtained symptomatic relief of low back pain and difficulty walking. What’s more important, PLZF::RARα fusion transcript became undetectable, indicating the achievement of complete molecular remission (MR). The decision to initiate additional treatment was contingent upon the successful recovery of the patient’s hematopoietic functions.
According to the new International Consensus Classification (ICC) of myeloid neoplasms and acute leukemias, APL with t(11;17)(q23;q21) translocation is now redefined as APL with other RARα rearrangements (13). Since the first report in 1993, only about forty newly diagnosed APL patients with t(11;17)(q23;q21) have been documented in literatures (Table 3). This rare APL impacts individuals across a broad spectrum of ages, ranging from 15 to 81 years old, with an average age of 48.8 years (Table 3). Interestingly, the prevalence of APL with t(11;17)(q23;q21) appears to be higher in males (35/41; 85.4%) compared to females (6/41; 14.6%) (Table 3). The t(11;17)(q23;q21) translocation gives rise to PLZF::RARα fusion gene, also referred to as ZBTB16::RARα. PLZF exhibits the ability to bind to DNA, thereby governing the transcriptional activity of genes pivotal to diverse cellular functions, particularly those involved in the differentiation and maturation of promyelocytic cells (40). However, it’s important to highlight that, in very rare APL cases, the karyotype may appear normal, and the fusion gene may be formed through cryptic or subtle rearrangements that are not readily detected by standard cytogenetic analysis (19, 41). Similar to our patient, Grimwade D et al. previously reported an APL case with a normal karyotype and cryptic formation of the PLZF::RARα fusion gene (19). Meanwhile, studies suggested that cryptic formation was not only limited to PLZF::RARα, but also identified in APL with PML::RARα (41, 42), IRF2BP2::RARα (43), TBL1XR1::RARα (44), and FIP1L1::RARα (45). Such exceptional APL cases underscore the critical importance of employing molecular techniques, such as FISH or RT-PCR, to pinpoint the precise genetic abnormality and confirm the final diagnosis of APL.
Moreover, it’s noteworthy that a majority of APL patients harboring the PLZF::RARα fusion initially manifest with non-specific symptoms that were identical to classical APL, including fever, pancytopenia, fatigue, bone pain, and so forth (Table 3). MS is generally considered a rare extramedullary manifestation of untreated APL, but after induction therapy MS becomes more common (2, 3). As of our current information, our patient was actually the second report of APL with PLZF::RARα fusion and MS as the initial symptom. The previous case was a 56-year-old Korean man characterized by APL and spinal MS (37). Even in classical APL, only around thirty cases with MS have been reported thus far (3–6). In addition, a recent report by Wang, Y., et al. highlighted the presence of skull MS in a 28-month-old girl with APL caused by FIP1L1::RARα fusion (45). The fact that MS has been identified in APL with different variants suggests that MS may not be exclusive to a particular genetic fusion. However, the exact mechanism underlying the development of MS in APL is not fully understood, and it may involve various processes related to the behavior of leukemia cells. In patients with AML or APL, MS can manifest in various sites throughout the body. Bone represents a frequent site of involvement, with MS lesions often observed in spine, skull and long bones (4, 6, 45–47). Additionally, soft tissues including skin, subcutaneous tissue, and lymph nodes are susceptible to MS infiltration (2, 6, 48). In more severe cases, MS can affect visceral organs such as liver, colon, and central nervous system (CNS) (5, 49, 50). The presentation of MS varies widely based on the affected site(s), necessitating a comprehensive diagnostic approach and tailored treatment strategies.
Morphological characteristics of abnormal promyelocytes exhibit variability among APL patients with the PLZF::RARα fusion, occasionally differing significantly from those seen in classic APL (11, 29, 33, 51). In classic APL, distinguishing features of promyelocytes encompass lobulated nuclei, hypergranular cytoplasm, and Auer rods (1, 51). However, a subgroup of APL patients with the PLZF::RARα fusion, similar to our patient, may present with atypical traits, such as round/non-lobulated nuclei, hypogranular or entirely agranular cytoplasm, along with the absence of Auer rods (Table 3). Notably, studies have found that APL cases with the PLZF::RARα fusion may exhibit vacuoles or square crystalline structures within the cytoplasm of promyelocytes (29, 33). Interestingly, we also observed small vacuoles in few abnormal promyelocytes from our patient. Further research is needed to better understand the underlying mechanisms leading to the formation of these atypical intracytoplasmic inclusions and their clinical significance. Hence, in instances with atypical presentations, the use of stains like MPO, Sudan Black B, and immunohistochemical markers such as CD13, CD33, and CD117 can be valuable in reinforcing the diagnosis of APL (1, 13). Nevertheless, it should be noted that APL patients may infrequently show negativity for both MPO and Sudan Black B staining (52, 53), and the immunophenotype may also undergo changes after induction therapy (54).
The immediate initiation of ATRA is now a crucial element in the induction therapy for classic APL, resulting in a notable rise in complete remission (CR) rates and enhanced overall outcomes (8, 9). Currently, there is no established consensus guideline regarding the utilization of ATRA in the treatment of APL with rare variants and MS. Despite demonstrating the ability of leukemia cells carrying the PLZF::RARα fusion to fully differentiate with both ex vivo and in vivo ATRA treatment, the clinical reality is that APL with this rare fusion is commonly considered ATRA-insensitive and is linked to an unfavorable prognosis (10–12, 55). Significantly, it’s also been reported that a small number of APL patients with PLZF::RARα fusion who underwent a combination of ATRA and intensive chemotherapy achieved CR (11, 33). In recent years, the BCL-2 inhibitor venetoclax has exhibited encouraging therapeutic outcomes in AML as well as other hematological malignancies (56). Interestingly, exploratory studies suggested that APL patients who are resistant to conventional chemotherapies may derive benefit from regimens incorporating venetoclax (57). In particular, APL patients harboring exceedingly uncommon RARα::HNRNPC and RARα::THRAP3 fusions have been documented to achieve CR through the administration of venetoclax and hypomethylating agents such as azacytidine or decitabine (58, 59). These findings prompted us to initiate treatment with ATRA, followed by a combination of venetoclax and azacytidine in our patient. The treatment demonstrated a significant efficacy in eradicating leukemic cells from BM aspirate; however, its impact on alleviating his MS and achieving MR was negligible. Fortunately, the substitution of azacitidine with the anthracycline antineoplastic agent idarubicin has ultimately led to the achievement of MR, albeit the occurrence of significant hematological toxicity.
To summarize, we report the clinical features and outcome of a rare APL patient characterized by a cryptic PLZF::RARα fusion and MS as the initial presenting symptom. Our study not only offers valuable insights into the heterogeneity of APL clinical manifestations but also emphasizes the crucial need to promptly consider the potential link between APL and MS for ensuring a timely diagnosis and personalized treatments.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethics committee of Zhongshan Hospital of Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XZ: Data curation, Investigation, Writing – original draft. TW: Formal analysis, Investigation, Writing – review & editing. PC: Formal analysis, Writing – review & editing. YC: Formal analysis, Writing – review & editing. ZW: Project administration, Writing – review & editing. TX: Investigation, Writing – review & editing. PY: Investigation, Writing – review & editing. PL: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Natural Science Foundation of Shanghai (Grant No. 22ZR1411400).
The authors extend their sincere appreciation to the patient and personnel of the Department of Hematology at Zhongshan Hospital, Fudan University, for their valuable support in conducting this study. In addition, we thank Liangjun Zhu from Dian Diagnostics Group Co. Ltd. for technical assistance.
Authors TW and YC were employed by Dian Diagnostics Group Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cingam SR, Koshy NV. Acute Promyelocytic Leukemia. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2023).
2. Vega-Ruiz A, Faderl S, Estrov Z, Pierce S, Cortes J, Kantarjian H, et al. Incidence of extramedullary disease in patients with acute promyelocytic leukemia: a single-institution experience. Int J Hematol. (2009) 89:489–96. doi: 10.1007/s12185-009-0291-8
3. Kasinathan G, Sathar J. Extramedullary disease in acute promyelocytic leukaemia: A rare presentation. SAGE Open Med Case Rep. (2020) 8:2050313X20926076. doi: 10.1177/2050313X20926076
4. Shu X, Wu Q, Guo T, Yin H, Liu J. Acute promyelocytic leukemia presenting with a myeloid sarcoma of the spine: A case report and literature review. Front Oncol. (2022) 12:851406. doi: 10.3389/fonc.2022.851406
5. Wang L, Cai DL, Lin N. Myeloid sarcoma of the colon as initial presentation in acute promyelocytic leukemia: A case report and review of the literature. World J Clin Cases. (2021) 9:6017–25. doi: 10.12998/wjcc.v9.i21.6017
6. Harrer DC, Lüke F, Einspieler I, Menhart K, Hellwig D, Utpatel K, et al. Case report: extramedullary acute promyelocytic leukemia: an unusual case and mini-review of the literature. Front Oncol. (2022) 12:886436. doi: 10.3389/fonc.2022.886436
7. Dombret H, Scrobohaci ML, Ghorra P, Zini JM, Daniel MT, Castaigne S, et al. Coagulation disorders associated with acute promyelocytic leukemia: corrective effect of all-trans retinoic acid treatment. Leukemia. (1993) 7:2–9.
8. Osman AEG, Anderson J, Churpek JE, Christ TN, Curran E, Godley LA, et al. Treatment of acute promyelocytic leukemia in adults. J Oncol Pract. (2018) 14:649–57. doi: 10.1200/JOP.18.00328
9. Zhang X, Sun J, Yu W, Jin J. Current views on the genetic landscape and management of variant acute promyelocytic leukemia. biomark Res. (2021) 9:33. doi: 10.1186/s40364-021-00284-x
10. Sobas M, Talarn-Forcadell MC, Martínez-Cuadrón D, Escoda L, García-Pérez MJ, Mariz J, et al. PLZF-RARα, NPM1-RARα, and other acute promyelocytic leukemia variants: the PETHEMA registry experience and systematic literature review. Cancers (Basel). (2020) 12:1313. doi: 10.3390/cancers12051313
11. Sainty D, Liso V, Cantù-Rajnoldi A, Head D, Mozziconacci MJ, Arnoulet C, et al. A new morphologic classification system for acute promyelocytic leukemia distinguishes cases with underlying PLZF/RARA gene rearrangements. Blood. (2000) 96:1287–96.
12. Chen SJ, Zelent A, Tong JH, Yu HQ, Wang ZY, Derré J, et al. Rearrangements of the retinoic acid receptor alpha and promyelocytic leukemia zinc finger genes resulting from t(11;17)(q23;q21) in a patient with acute promyelocytic leukemia. J Clin Invest. (1993) 91:2260–7. doi: 10.1172/JCI116453
13. Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. (2022) 140:1200–28. doi: 10.1182/blood.2022015850
14. Guidez F, Huang W, Tong JH, Dubois C, Balitrand N, Waxman S, et al. Poor response to all-trans retinoic acid therapy in a t(11;17) PLZF/RAR alpha patient. Leukemia. (1994) 8:312–7.
15. Licht JD, Chomienne C, Goy A, Chen A, Scott AA, Head DR, et al. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17). Blood. (1995) 85:1083–94. doi: 10.1182/blood.V85.4.1083.bloodjournal8541083
16. Culligan DJ, Stevenson D, Chee YL, Grimwade D. Acute promyelocytic leukaemia with t(11;17)(q23;q12-21) and a good initial response to prolonged ATRA and combination chemotherapy. Br J Haematol. (1998) 100:328–30. doi: 10.1046/j.1365-2141.1998.00575.x
17. Hoshi S, Ketsueki R. Acute promyelocytic leukemia with t (11; 17) (q23;q21); a case report. Japan J Clin Hematol. (1999) 40:119–23.
18. Jansen JH, de Ridder MC, Geertsma WM, Erpelinck CA, van Lom K, Smit EM, et al. Complete remission of t(11;17) positive acute promyelocytic leukemia induced by all-trans retinoic acid and granulocyte colony-stimulating factor. Blood. (1999) 94:39–45. doi: 10.1182/blood.V94.1.39.413a26_39_45
19. Grimwade D, Biondi A, Mozziconacci MJ, Hagemeijer A, Berger R, Neat M, et al. Characterization of acute promyelocytic leukemia cases lacking the classic t(15;17): results of the European Working Party. Groupe Français de Cytogénétique Hématologique, Groupe de Français d’Hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOMED 1 European Community-Concerted Action “Molecular Cytogenetic Diagnosis in Haematological Malignancies”. Blood. (2000) 96:1297–308.
20. George B, Poonkuzhali B, Srivastava VM, Chandy M, Srivastava A. Hematological and molecular remission with combination chemotherapy in a patient with PLZF-RARalpha acute promyelocytic leukemia (APML). Ann Hematol. (2005) 84:406–8. doi: 10.1007/s00277-004-0979-z
21. Cassinat B, Guillemot I, Moluçon-Chabrot C, Zassadowski F, Fenaux P, Tournilhac O, et al. Favourable outcome in an APL patient with PLZF/RARalpha fusion gene: quantitative real-time RT-PCR confirms molecular response. Haematologica. (2006) 91:ECR58.
22. Jovanovic JV, Rennie K, Culligan D, Peniket A, Lennard A, Harrison J, et al. Development of real-time quantitative polymerase chain reaction assays to track treatment response in retinoid resistant acute promyelocytic leukemia. Front Oncol. (2011) 1:35. doi: 10.3389/fonc.2011.00035
23. Han SB, Lim J, Kim Y, Kim HJ, Han K. A variant acute promyelocytic leukemia with t(11;17) (q23;q12); ZBTB16-RARA showing typical morphology of classical acute promyelocytic leukemia. Korean J Hematol. (2010) 45:133–5. doi: 10.5045/kjh.2010.45.2.133
24. Rohr SS, Pelloso LA, Borgo A, De Nadai LC, Yamamoto M, Rego EM, et al. Acute promyelocytic leukemia associated with the PLZF-RARA fusion gene: two additional cases with clinical and laboratorial peculiar presentations. Med Oncol. (2012) 29:2345–7. doi: 10.1007/s12032-011-0147-y
25. Liu K, Liu B, Zhou C, Mi Y, Wei S, Zhang G, et al. Combination of arsenic trioxide and chemotherapy in the treatment of PLZF/RAR positive acute promyelocytic leukemia patient: a case report and literature review. J Clin Hematol (China). (2012) 11:719–21. doi: 10.13201/j.issn.1004-2806.2012.06.013
26. Palta A, Dhiman P, Cruz SD. ZBTB16-RARα variant of acute promyelocytic leukemia with tuberculosis: a case report and review of literature. Korean J Hematol. (2012) 47:229–32. doi: 10.5045/kjh.2012.47.3.229
27. Piñán MA, Balerdi A, Iglesias A, Dueñas M, Olazabal I, Puente M, et al. Acute myeloid leukemia with t(11;17)(q23;q21). Ann Hematol Oncol. (2015) 2:1050.
28. Lechevalier N, Dulucq S, Bidet A. A case of acute promyelocytic leukaemia with unusual cytological features and a ZBTB16-RARA fusion gene. Br J Haematol. (2016) 174:502. doi: 10.1111/bjh.14198
29. Dowse RT, Ireland RM. Variant ZBTB16-RARA translocation: morphological changes predict cytogenetic variants of APL. Blood. (2017) 129:2038. doi: 10.1182/blood-2016-10-743856
30. Wen H, Chen S, Wang F, Meng X, Liu L, Sun L. PLZF-RARα positive acute promyelocytic leukemia. Chin J Hematol (China). (2017) 38:805. doi: 10.3760/cma.j.issn.0253-2727.2017.09.016
31. Langabeer SE, Preston L, Kelly J, Goodyer M, Elhassadi E, Hayat A. Molecular profiling: A case of ZBTB16-RARA acute promyelocytic leukemia. Case Rep Hematol. (2017) 2017:7657393. doi: 10.1155/2017/7657393
32. Wang X, Wang J, Zhang L. Characterization of atypical acute promyelocytic leukaemia: Three cases report and literature review. Med (Baltimore). (2019) 98:e15537. doi: 10.1097/MD.0000000000015537
33. Pardo Gambarte L, Franganillo Suárez A, Cornago Navascués J, Soto de Ozaeta C, Blas López C, Atance Pasarisas M, et al. ZBTB16-RARα-positive atypical promyelocytic leukemia: A case report. Med (Kaunas). (2022) 58:520. doi: 10.3390/medicina58040520
34. Liu G, Liu L, Bartolo DD, Li KY, Li X. Acute promyelocytic leukemia with rare genetic aberrations: A report of three cases. Genes (Basel). (2022) 14:46. doi: 10.3390/genes14010046
35. Canali A, Rieu JB. Morphological characterization of acute myeloid leukaemia with t(11;17)(q23;q21)/ZBTB16::RARA fusion. Br J Haematol. (2022) 199:638. doi: 10.1111/bjh.18463
36. Courville EL, Shantzer L, Vitzthum von Eckstaedt HC, Mellot H, Keng M, Sen J, et al. Variant acute promyelocytic leukemia presenting without auer rods highlights the need for correlation with cytogenetic data in leukemia diagnosis. Lab Med. (2022) 53:95–9. doi: 10.1093/labmed/lmab051
37. Cho EJ, Byeon SJ, Hyun J, Kim HS, Jung JY. A ZBTB16-RARα Variant of acute promyelocytic leukemia with concurrent myeloid sarcoma presenting as sudden onset paraplegia. Clin Lab. (2022) 68:10. doi: 10.7754/Clin.Lab.2021.211227
38. Castelijn DAR, Sijm G, Venniker-Punt B, Poddighe PJ, Wondergem MJ. An acute promyelocytic leukemia resistant to all-trans retinoic acid: A case report of the ZBTB16::RARa variant and review of the literature. Case Rep Oncol. (2023) 16:1443–50. doi: 10.1159/000534862
39. Rabade N, Raval G, Chaudhary S, Subramanian PG, Kodgule R, Joshi S, et al. Molecular heterogeneity in acute promyelocytic leukemia - a single center experience from India. Mediterr J Hematol Infect Dis. (2018) 10:e2018002. doi: 10.4084/MJHID.2018.002
40. McConnell MJ, Licht JD. The PLZF gene of t (11;17)-associated APL. Curr Top Microbiol Immunol. (2007) 313:31–48. doi: 10.1007/978-3-540-34594-7_3
41. Grimwade D, Gorman P, Duprez E, Howe K, Langabeer S, Oliver F, et al. Characterization of cryptic rearrangements and variant translocations in acute promyelocytic leukemia. Blood. (1997) 90:4876–85.
42. Zhang Z, Xu Y, Jiang M, Kong F, Chen Z, Liu S, et al. Identification of a new cryptic PML-RARα fusion gene without t(15;17) and biallelic CEBPA mutation in a case of acute promyelocytic leukemia: a case detected only by RT-PCR but not cytogenetics and FISH. Cancer Biol Ther. (2020) 21:309–14. doi: 10.1080/15384047.2019.1702398
43. Jovanovic JV, Chillón MC, Vincent-Fabert C, Dillon R, Voisset E, Gutiérrez NC, et al. The cryptic IRF2BP2-RARA fusion transforms hematopoietic stem/progenitor cells and induces retinoid-sensitive acute promyelocytic leukemia. Leukemia. (2017) 31:747–51. doi: 10.1038/leu.2016.338
44. Osumi T, Watanabe A, Okamura K, Nakabayashi K, Yoshida M, Tsujimoto SI, et al. Acute promyelocytic leukemia with a cryptic insertion of RARA into TBL1XR1. Genes Chromosomes Cancer. (2019) 58:820–3. doi: 10.1002/gcc.22791
45. Wang Y, Rui Y, Shen Y, Li J, Liu P, Lu Q, et al. Myeloid sarcoma type of acute promyelocytic leukemia with a cryptic insertion of RARA into FIP1L1: the clinical utility of NGS and bioinformatic analyses. Front Oncol. (2021) 11:688203. doi: 10.3389/fonc.2021.688203
46. Shah K, Panchal H, Patel A. Spine myeloid sarcoma: A case series with review of literature. South Asian J Cancer. (2021) 10:251–4. doi: 10.1055/s-0041-1742079
47. Sawhney S, Holtzman NG, Davis DL, Kaizer H, Giffi V, Emadi A, et al. Promyelocytic sarcoma of the right humerus: an unusual clinical presentation with unique diagnostic and treatment considerations. Clin Case Rep. (2017) 5:1874–7. doi: 10.1002/ccr3.1212
48. Jadhav T, Baveja P, Sen A. Myeloid sarcoma: an uncommon presentation of myeloid neoplasms; a case series of 4 rare cases reported in a tertiary care institute. Autops Case Rep. (2021) 11:e2021339. doi: 10.4322/acr.2021.339
49. Abu-Zeinah GF, Weisman P, Ganesh K, Katz SS, Dogan A, Abou-Alfa GK, et al. Acute myeloid leukemia masquerading as hepatocellular carcinoma. J Gastrointest Oncol. (2016) 7:E31–5. doi: 10.21037/jgo.2015.12.01
50. Cervantes GM, Cayci Z. Intracranial CNS manifestations of myeloid sarcoma in patients with acute myeloid leukemia: review of the literature and three case reports from the author’s institution. J Clin Med. (2015) 4:1102–12. doi: 10.3390/jcm4051102
51. Castoldi GL, Liso V, Specchia G, Tomasi P. Acute promyelocytic leukemia: morphological aspects. Leukemia. (1994) 8:1441–6.
52. Rastogi P, Sharma S, Sreedharanunni S, Sharma P, Sachdeva MUS, Jain R, et al. Myeloperoxidase deficient acute promyelocytic leukemia: report of two cases. Indian J Hematol Blood Transfus. (2018) 34:372–4. doi: 10.1007/s12288-017-0844-6
53. Cui W, Qing S, Xu Y, Hao Y, Xue Y, He G. Negative stain for myeloid peroxidase and Sudan black B in acute promyelocytic leukemia (APL) cells: report of two patients with APL variant. Haematologica. (2002) 87:ECR16.
54. Yoshii M, Ishida M, Yoshida T, Okuno H, Nakanishi R, Horinouchi A, et al. Clinicopathological features of acute promyelocytic leukemia: an experience in one institute emphasizing the morphological and immunophenotypic changes at the time of relapse. Int J Clin Exp Pathol. (2013) 6:2192–8.
55. Ablain J, de The H. Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood. (2011) 117:5795–802. doi: 10.1182/blood-2011-02-329367
56. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
57. Wang QQ, Wang HF, Zhao JZ, Naranmandura H, Jin J, Zhu HH. Venetoclax for arsenic-resistant acute promyelocytic leukaemia. Br J Haematol. (2022) 197:e58–:e60. doi: 10.1111/bjh.18061
58. Liu M, Zhao X, Pan W, Qian Z, Du M, Wang LM, et al. A novel HNRNPC-RARA fusion in acute promyelocytic leukaemia lacking PML-RARA rearrangement, sensitive to venetoclax-based therapy. Br J Haematol. (2021) 195:e123–8. doi: 10.1111/bjh.17642
Keywords: spine, myeloid sarcoma, acute promyelocytic leukemia, PLZF::RARα fusion, cryptic
Citation: Zhang X, Wang T, Chen P, Chen Y, Wang Z, Xu T, Yu P and Liu P (2024) Spinal myeloid sarcoma presenting as initial symptom in acute promyelocytic leukemia with a rare cryptic PLZF::RARα fusion gene: a case report and literature review. Front. Oncol. 14:1375737. doi: 10.3389/fonc.2024.1375737
Received: 24 January 2024; Accepted: 07 May 2024;
Published: 21 May 2024.
Edited by:
Maria S. Pombo-de-Oliveira, National Cancer Institute (INCA), BrazilReviewed by:
Irma Olarte, Hospital General de México Dr. Eduardo Liceaga, MexicoCopyright © 2024 Zhang, Wang, Chen, Chen, Wang, Xu, Yu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Liu, bGl1LnBlbmdAenMtaG9zcGl0YWwuc2guY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.