
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 May 2024
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1374592
 Gudrun Piringer1,2,3*†
Gudrun Piringer1,2,3*† Florian Ponholzer4†
Florian Ponholzer4† Josef Thaler2,3
Josef Thaler2,3 Thomas Bachleitner-Hofmann5
Thomas Bachleitner-Hofmann5 Holger Rumpold3,6
Holger Rumpold3,6 Alexander de Vries7
Alexander de Vries7 Lukas Weiss8,9
Lukas Weiss8,9 Richard Greil8,9
Richard Greil8,9 Michael Gnant10
Michael Gnant10 Dietmar Öfner4 on behalf of the Austrian Breast & Colorectal Cancer Study Group
Dietmar Öfner4 on behalf of the Austrian Breast & Colorectal Cancer Study GroupPurpose: The aim of this retrospective analysis was to determine if the response to preoperative radio(chemo)therapy is predictive for survival among patients with locally advanced rectal cancer and may act as a potential surrogate endpoint for disease free survival and overall survival.
Results: Eight hundred seventy-eight patients from five centers were analyzed. There were 304 women and 574 men; the median age was 64.7 years. 77.6% and 22.4% of patients received neoadjuvant radiochemotherapy or short-course radiotherapy, resulting in a pathological complete response in 7.3%. T-downstaging and N-downstaging occurred in 50.5% and 37% of patients after neoadjuvant therapy. In patients with T-downstaging, the 10-year DFS and 10-year OS were 64.8% and 66.8% compared to 37.1% and 45.9% in patients without T-downstaging. N-downstaging resulted in 10-year DFS and 10-year OS in 56.2% and 62.5% compared to 47.3% and 52.3% without N-downstaging. Based on routinely evaluated clinical parameters, an absolute risk prediction calculator was generated for 5-year disease-free survival, and 5-year overall survival.
Conclusion: T-downstaging and N-downstaging after neoadjuvant radiochemotherapy or short-course radiotherapy resulted in better DFS and OS compared to patients without response. Based on clinical parameters, 5-year DFS, and 5-year OS can be predicted using a prediction calculator.
Since the publication of the German trial by Sauer et al. (1), neoadjuvant fluoropyrimidine-based radiotherapy (RCT) followed by total mesorectal excision (TME) became a standard treatment for locally advanced rectal cancers (LARC). The administration of adjuvant chemotherapy is a highly debated issue as several trials and meta-analysis found no or only a marginal survival benefit (2–7). Nevertheless, the consensus-based guidelines from the National Comprehensive Cancer Network and the ESMO guidelines consider adjuvant chemotherapy and the decision should be risk-balanced (8, 9). Despite optimized local treatment with less than 10% recurrence rates, neoadjuvant RCT has not improved overall survival (OS), and distant metastases still occur in 25–30% (1, 10–13). The primary goals of neoadjuvant treatment for LARC are improvement of local tumor control, tumor downstaging and enabling sphincter-sparing surgery. Patients achieving a complete pathological response, i.e. ypT0N0 (pCR), after neoadjuvant RCT have better long-term outcomes than patients without pCR, which was shown in a pooled analysis by Maas et al. (14). Further developments of neoadjuvant therapy aim to improve primary tumor response and patient outcome. Identifying short-time surrogate endpoints for predicting disease-free survival (DFS) and OS are helpful for individualizing adjuvant therapy and follow-up. There have been several publications concerning short-term surrogate for DFS and OS after neoadjuvant RCT or RT such as tumor regression grade (TRG) (15–18) and Neoadjuvant Rectal Score (NAR score) (19). The NAR score (19) was developed as a short-term clinical trial surrogate endpoint to take variables associated with treatment effects beyond pCR into consideration yet simple enough to support a diversity of clinical trial designs. The NAR score is calculated based on data supported by the Valentini nomogram (20) for OS, but only using the clinical T-stage and pathologic T- and N-stages. Of the eight variables used in the Valentini nomogram, only pN and pT are potentially influenced by neoadjuvant therapy. After establishing the NAR score calculation, it was validated using the NSABP R04 trial patient dataset (21, 22). NAR scores in the NSAPB R-04 trial dataset were categorized as low (NAR<8), intermediate (NAR 8–16), and high (NAR >16) based on the tertiles of the observed scores. These categories were significantly associated with OS (p<0.0001) with 5-year OS values of 92%, 89%, and 68%, respectively. TGR is also predictive of therapeutic response in rectal cancer after RCT followed by curative resection. However, various TGR systems have been suggested, with subjective categorization, resulting in interobserver variability (15–18). Furthermore, even regional lymph node status after RCT is an important prognostic factor, the TRG systems only evaluate the primary tumor with no consideration of regional lymph node status. Due to the subjective classification there is a low concordance rate even in experienced gastrointestinal pathologics using the same TRG system. The Mandard (15) and Dworak (16) TRG systems are classified according to five-point grades based on residual tumor and fibrosis, whereas the Ryan TGR system (17), with three-point grading, is a type of modified Mandard TRG system. The 2010 American Joint Committee on Cancer (AJCC) TRG system (18) is a modification of the Ryan TRG system based on the volume of residual primary tumor cells.
In the ABCSG R02 trial we previously demonstrated that downstaging of the N-level and particularly the achievement of lymph node negativity after neoadjuvant RCT with capecitabine and oxaliplatin (ABCSG R02-Study) exerts a statistically significant influence on 5-year OS and DFS in this patient population (23), whereas downstaging at the T-level showed no statistically significant influence on OS and only a borderline significance in DFS. The finding obtained from the ABCSG R02-trial that downstaging of the N-level has a more significant impact on DFS and OS than T-downstaging was now evaluated in a large cohort receiving neoadjuvant RCT or short-time RT. Furthermore, a prediction calculator for 5-year DFS and 5-year OS was developed based on the available clinical data.
A total of 993 patients with locally advanced and histologically confirmed adenocarcinoma of the rectum with the indication for neoadjuvant RCT or short-term RT were included in this retrospective analysis from six centers in Austria. Patients were included from 2000–2014, and the postoperative follow-up was recorded until March 2019. Patients received a standardized magnetic resonance imaging (MRI) of the pelvis for local staging of the rectal tumor according to an Austrian MRI-Standard Operating Procedure (SOP) to achieve comparable MRI results. The study was approved by the ethics committee in Upper Austria (EK-No: 1074/2018) and was conducted by the Austrian breast and colorectal cancer study group (ABCSG).
All evaluated patients received neoadjuvant RCT concurrent with fluoropyrimidine +/- oxaliplatin or short-RT, followed by surgical resection. Adjuvant chemotherapy was administered according to regional local standards. Surgical techniques included open and laparoscopic approaches.
During a median follow-up of 69 months, patients were clinically evaluated (history and examination) and were referred to radiological assessment (chest X-ray, abdominal-pelvic CT scan, colonoscopy, and other investigations) as per clinical indication and local standards. DFS was defined as the time between surgery and the first recurrence of rectal cancer (local or distant) or death. OS was defined as the time between surgery and death.
Statistical analysis was performed using IBM SPSS Statistics 26 (IBM Corporation, Armon, NY, USA) and the R software environment (24). In R the package ‘survminer’ was used to plot survival curves (25). The likelihood-ratio chi-squared test was used to identify correlations between categorical variables. Homogeneity of variance was assessed by Levene’s test. Depending on homogeneity of variance, analysis of variance (ANOVA) or Welch-ANOVA was used for comparing continuous variables between groups. For post-hoc testing, Tukey’s test and Games-Howell test were used.
Cox’s proportional hazards regression analysis was used for calculating and establishing an absolute risk prediction model for 5-year DFS and 5-year OS based on Jia et al., who provide a detailed explanation of the used model (26). The formula for the absolute risk prediction model was: R(t)=1−[Su0(t)]exp(∑lr=1 Xrβr− ∑lr=1 ˉXrβr), where R is the risk of the event occurring in the calculated time period t (60 months in this case), l is the number of risk factors, Su0 the base survival probability at mean values, Xr is the value of the corresponding risk factor, βr the corresponding regression coefficient. This prediction model study is a Type 1a study with a direct model evaluation using the same data as for model development (27).
The log-rank test was used to compare Kaplan-Meier curves. The missing indicator method was used for missing categorical data, except for the regression analysis, as this would lead to inefficient regression coefficients (28). Statistical significance was assumed for a p-value<0.05. Downstaging is defined as the migration to lower T- or N-classification following neoadjuvant therapy. The extent of regional nodal involvement includes the mesorectal and internal iliac nodes based on size and defined morphologic criteria.
Nine hundred ninety-three patients were included in this retrospective analysis. One center was excluded due to insufficient follow-up and survival data documentation because most patients had follow-up outside the hospital. After data cleaning 878 patients from five centers were involved in this retrospective analysis with a median age of 64.7 years (min.-max.: 27.5–90.1, range: 62.6), 34.6% (304 patients) were female, and 65.4% (574 patients) were male. The pretreatment stage distribution included cT2, cT3, and cT4 in 3.6%, 86.8%, and 9.6% of patients, and cN0, cN1–2, and nodal status missing in 31.1%, 60.0%, and 8.9%. Due to the primary clinical tumor stage, all patients had an indication for neoadjuvant RCT or short-course RT according to the local standard. Neoadjuvant RCT was done in 77.6%, and short-course RT in 22.4%. Patients in the neoadjuvant RCT had a significantly higher rate of pCR with 8.9% in comparison to 2.1% (p<0.001) in the short-course RT cohort. The pretreatment clinical tumor stage and the performed neoadjuvant therapy modality were not balanced between the five involved centers, as is visualized in Figure 1 and Table 1. Approximately 43.7 days (min.-max.: 0–991, range: 991) after completion of RCT and 16.2 days (min.-max.: 1–161, range: 160) after RT, surgery was performed (p<0.001). Adjuvant chemotherapy was administered to 52.5% of patients (ranging from 39.5% – 81.6% between the five centers). Median follow-up was 78.8 months (min.-max.: 0–256, range: 256). All patients received pathological work-up of the resected specimen, with 7.7% being graded as ypT0, 6.8% as ypT1, 30.5% as ypT2, 49.4% as ypT3 and 4.3% as ypT4. About two-thirds of patients were nodal negative after neoadjuvant therapy, and ypN1 and ypN2 status were found in 21.2% and 11.7%. A pCR (ypT0N0) after neoadjuvant RCT/short-term RT was documented in 7.3% of patients. T-downstaging and N-downstaging occurred in 50.5% and 37% of patients after neoadjuvant therapy. Correspondingly, the UICC tumor stage was down-staged in 47% of patients after neoadjuvant therapy. T-downstaging, N-downstaging, and UICC-downstaging were not significantly better if surgery was done >7 weeks after RCT/RT. After a median follow-up of 55 months, 7.6% of patients (n=67) experienced local recurrence, and 21.2% of patients (n=186) developed distant metastases. The 3-year and 5-year DFS were 73.0 months and 64.3 months, respectively. The 5-year and 10-year OS were 75.8 and 55.8 months, respectively.
Univariate analysis demonstrated a significantly better 10-year DFS in patients with downstaging in T-level (64.8% versus 37.1%; p<0.001), downstaging in N-level (56.2% versus 47.3%, p 0.001), downstaging in UICC stage (62.1% versus 39.1%), p< 0.001) and with lower UICC stages, as is described in Table 2 and as can be seen in Figure 2. 10-year DFS was 67.3% versus 48.6% in ypT0 versus ypT1–4 (p<0.001) and 72.6% versus 48.3% (p<0.001) in patients with a complete pathological response (ypT0N0) versus no complete response. Furthermore, a significantly better 10-year OS was demonstrated in patients with downstaging in T-level (66.8% versus 45.9%, p<0.001), downstaging in N-level (62.5% versus 52.3%, p 0.001), downstaging in UICC stage (65.6% vs. 46.7%, p<0.001). Lower UICC stages, as shown in Table 3 and as can be seen in Figure 3. 10-year OS was 66.8% versus 54.7% in ypT0 versus ypT1–4 (p 0.022) and 72.1% versus 54.4% (p 0.006) in patients with a complete pathological response (ypT0N0) versus no complete response. Four patients were ypT0 but ypN1.
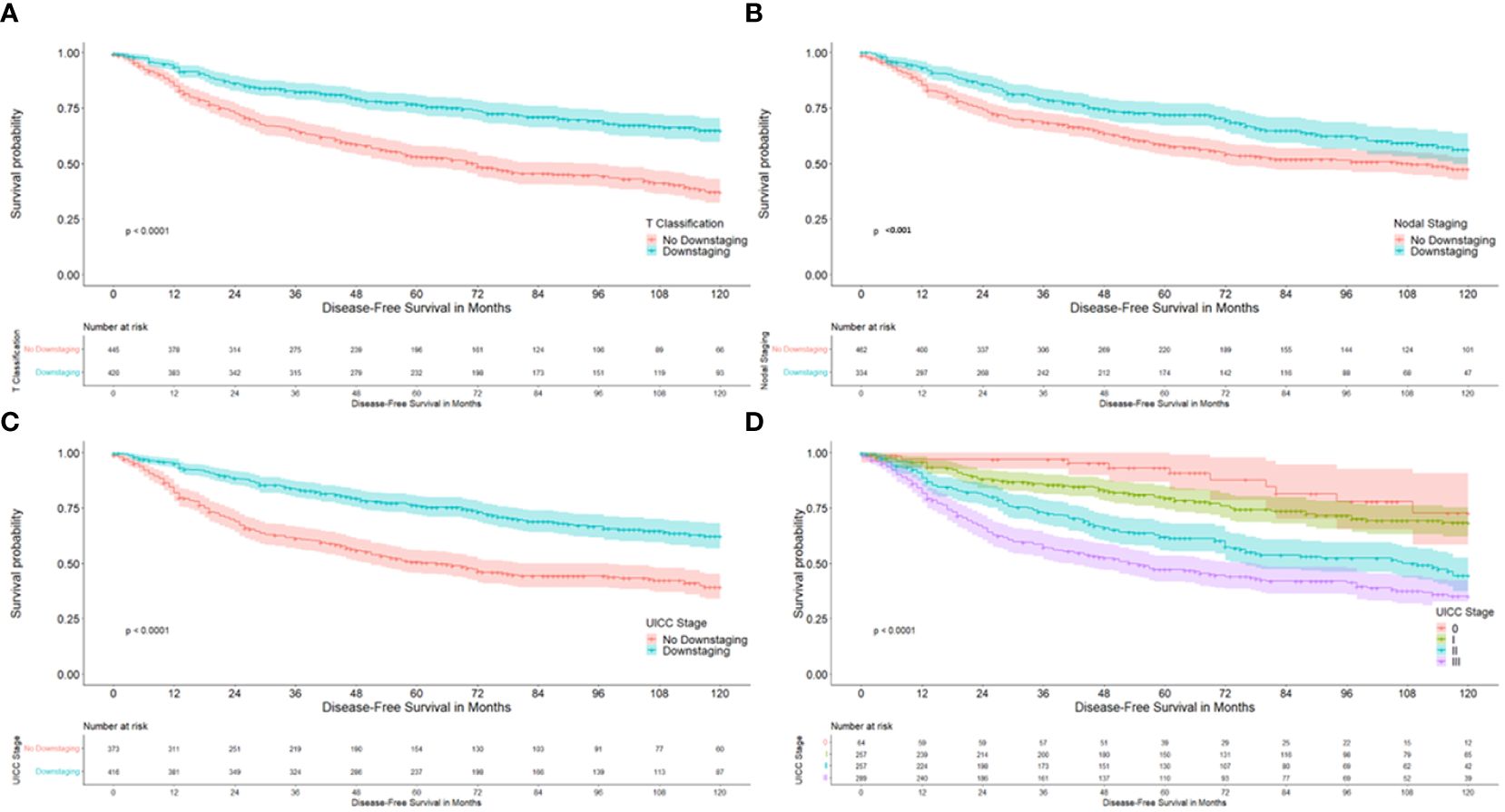
Figure 2 Kaplan-Meier curves with regard to disease free survival. (A) according to downstaging at the T-level; (B) according to downstaging at the nodal status; (C) according to downstaging at UICC stages; (D) according to UICC stage.
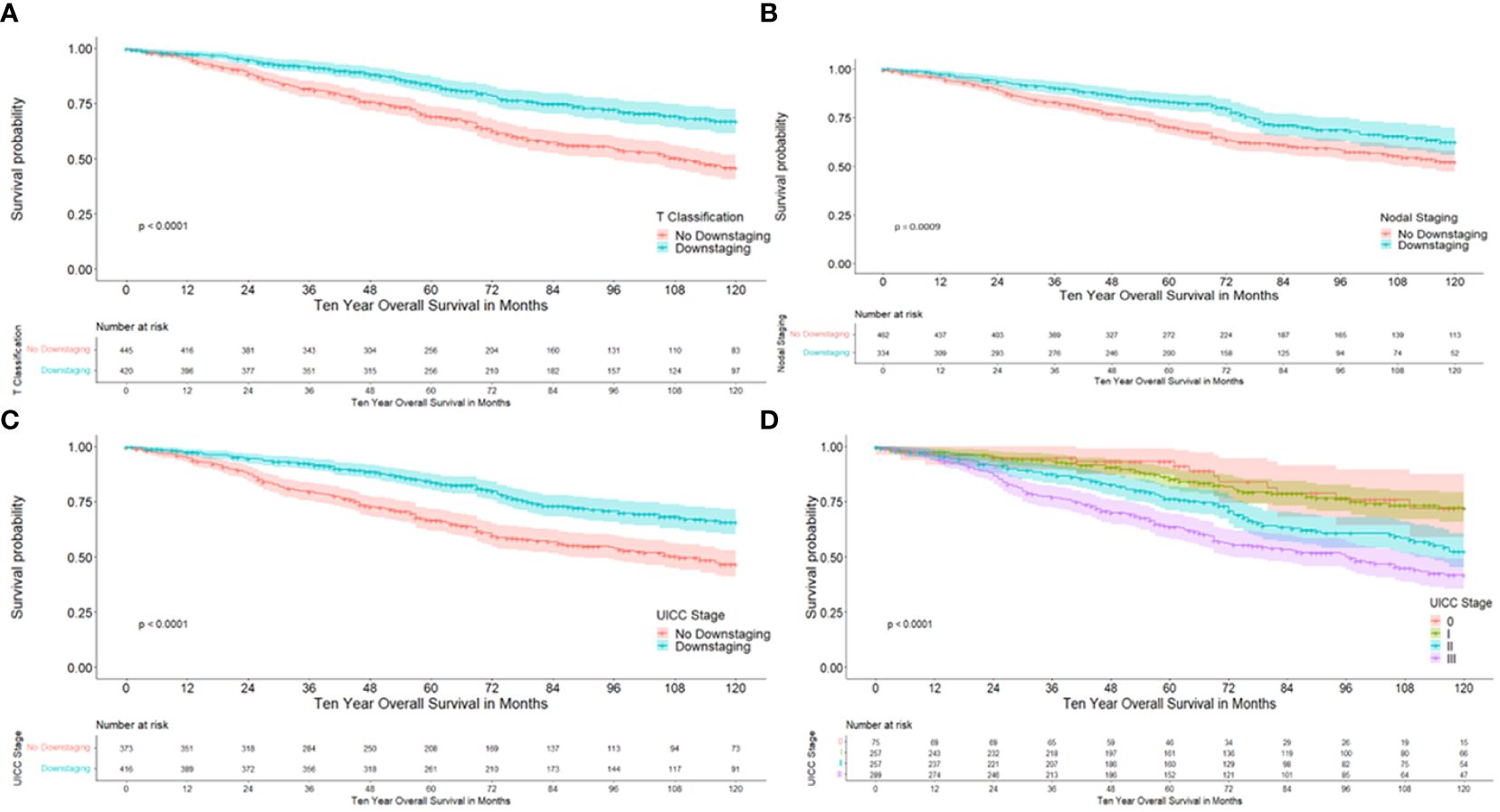
Figure 3 Kaplan-Meier curves with regard to overall survival. (A) according to downstaging at the T-level; (B) according to downstaging at the nodal status; (C) according to downstaging at UICC stages; (D) according to UICC stage.
Evaluation of the NAR scores categorized patients as low (NAR<8) in 14.4%, intermediate (NAR 8–16) in 50.8%, and high (NAR >16) in 33.1%. The NAR score could not be calculated in 15 patients (1.7%) due to missing values.
After performing a univariate Cox regression analysis, significant variables were used for the multivariate analysis to establish a risk prediction score for five-year DFS and five-year OS. Variables used for the multivariate five-year DFS absolute risk prediction model can be seen in Table 4; corresponding covariate means can be found in Supplementary Table 1. Evaluation of the model was performed by using the same data and reached an Area Under the Receiver Operating Characteristic (AUROC) of 0.722 and an overall good model quality with the lower bound of the 95% confidence interval of 0.684. The AUROC curve is visualized in Supplementary Figure 1. Variables used for the multivariate five-year OS absolute risk prediction model can be seen in Table 5; corresponding covariate means can be found in Supplementary Table 2. Evaluation of the model was performed by using the same data and reached an AUROC of 0.716 and an overall good model quality with the lower bound of the 95% confidence interval of 0.673. The AUROC curve is visualized in Supplementary Figure 2.
This retrospective rectal cancer registry was conducted to validate that downstaging in the N-level after neoadjuvant RCT had a more significant impact on DFS and OS than downstaging of the T-level, which was found in the previously published ABCSG-R02 trial (23). The ABCSG-R02 trial aimed to evaluate the efficacy expressed by downstaging at the T-level and safety of preoperative daily capecitabine plus weekly oxaliplatin in combination with RT in treating LARC (29). Furthermore, it was evaluated if tumor downstaging at the T-level and pCR acts as a surrogate for survival. When addressing these endpoints and analyzing their effects on survival rates, our study group was able to show that downstaging of the T-level does not influence OS but does influence DFS with a borderline significance. Assessment of the nodal status of these patients showed that downstaging in the N-level highly influences patient DFS and OS. However, a more significant number of patients was needed to confirm this finding clearly, which has now been performed in this retrospective analysis. The prognostic relevance of lymph node status in patient survival was demonstrated previously, regardless of the applied chemotherapeutic regimen (30–33). Patients with positive lymph nodes after neoadjuvant RCT in LARC had a higher risk for local recurrences and the development of distant metastases than patients with negative lymph nodes. Furthermore, our previous study demonstrates that the downstaging of the N-level acts as a better surrogate for survival than the downstaging of the T-level.
However, this retrospective analysis of 878 patients with LARC who were treated with neoadjuvant RCT or short-term RT followed by TME in five highly experienced centers in Austria could not confirm that only downstaging of the N-level and not of the T-level acts as a surrogate for survival. 10-year DFS and 10-year OS for patients with downstaging of the T-level or the N-level were significant better compared with patients with no downstaging. Clinical lymph node status before any treatment did not impact DFS or OS. The decisive factor for a good outcome is lymph-node negativity after neoadjuvant therapy.
In the univariate analysis, we found that the downstaging of the T-level and the downstaging of the N-level are surrogates for survival. Furthermore, based on multivariant analysis, we developed calculators for absolute risk prediction for 5-year DFS and 5-year OS. These prediction calculators are able to estimate the individual risk for recurrence or death within the first five years after surgery. While a dataset validation is ongoing, the prediction calculators offer an opportunity to incorporate the individual patient follow-up assessment to adjust follow-up in patients at high risk of recurrence. Furthermore, predictors can be used to select patients in clinical trials who would benefit from adjuvant chemotherapy based on their individual risk. Some parameters from the NAR score, such as cT and possible stage migration, were also significant factors in our predictive models. Nevertheless, our analysis showed that further factors like cN status, stage migration, short-course RT, age and gender are of importance for DFS and OS estimation. Compared to the TRG system, we included the lymph node status in our prediction model.
Improvement of OS is the most important goal when treating cancer patients and is the preferred primary clinical endpoint in studies. Its usefulness is limited by several disadvantages, requiring a higher number of patients, longer follow-up, and is associated with higher study costs. Therefore, identifying short-time surrogate endpoints for DFS or OS are useful for individualizing follow-up and adjuvant therapy. Surrogate endpoints in rectal cancer after neoadjuvant therapy are rare due to its complex validation and confirmation in phase III clinical trials. A pCR after neoadjuvant therapy and surgery is commonly associated with better outcomes compared to patients without pCR (14, 34). However, Petrelli et al. showed in a literature-based analysis of 22 randomized trials, that pCR and DFS are not surrogate endpoints for 5-year survival in rectal cancer (35). Nevertheless, we could demonstrate in our retrospective analysis, that patients with a pCR had a significantly better 10-year DFS and 10-year OS compared with patients without pCR. Furthermore, any response to therapy with T-downstaging or N-downstaging resulted in better survival compared to patients without response. This study was performed in a retrospective manner and thus might possess limited accuracy. Collected data on tumor stages did not include substages (e.g. cT3a, cT3b, etc.) and might therefore be a bias. Different time periods between treatment regimens and centers might be a confounding factor for this analysis, especially when comparing the RCT and RT cohorts.
Establishing a cancer registry is a complex process, especially when the data are collected retrospectively in different centers. The advantages of retrospective analysis include a large sample size, the participation of patients who are usually excluded from randomized clinical trials, the evaluation of a broad range of outcomes, and lower costs. Furthermore, the data are available quicker than in a prospective trial. Limitations of retrospective analysis include low internal validity, lack of quality control surrounding data collection, and susceptibility to multiple sources of bias for comparing outcomes. Six centers in Austria participated in our retrospective registry. Data were collected retrospectively utilizing existing data generated during routine clinical practice. Only a few centers had a locally established registry, but with different variables queried. The biggest challenge in our registry was merging the data from the centers across Austria. Strengths of our retrospective analysis are the high quality of local staging in all participating centers using standardized MRI and the large sample size. Nevertheless, of the discussed limitations, we could generate a prediction calculator for 5-year DFS and 5-year OS. The next step is to validate this prediction calculator in another cohort. This validation is still ongoing.
Response to neoadjuvant RCT/RT regarding T-downstaging and N-downstaging in LARC resulted in better DFS and OS compared to patients without response. The most significant benefit was seen in patients with pCR. Furthermore, an easy to handle absolute risk prediction calculator for 5-year DFS and 5-year OS based on routinely collected clinical data was generated. The final dataset validation is ongoing.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethikkommission des Landes Oberösterreich. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
GP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JT: Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing. TB: Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing. HR: Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing. AD: Conceptualization, Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing. LW: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. RG: Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing. MG: Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing. DÖ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funded by the Johannes Kepler Open Access Publishing Fund.
MG reports personal fees/travel support from Amgen, AstraZeneca, Celgene, EliLilly, Invectys, Pfizer, Novartis, Puma, Nanostring, Roche, Medison, and LifeBrain; an immediate family member is employed by Sandoz. LW reports personal fees/travel support from Astellas, AstraZeneca, BMS, Daiichi-Sankyo, Janssen , Merck, MSD, Novocure, Pierre Fabre, Roche, Servier and cosulting or Advisory role from Amgen, Astellas, BMS, GSK, Incyte, Lilly, Merck, MSD, Novocure, Pharmar, Pierre Fabre, Roche.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1374592/full#supplementary-material
Supplementary Figure 1 | AUROC curve of the 5-Year DFS prediction model with an AUROC of 0.722.
Supplementary Figure 2 | AUROC curve of the 5-Year OS prediction model with an AUROC of 0.716.
1. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. (2012) 30:1926–33. doi: 10.1200/JCO.2011.40.1836
2. Sainato A, Cernusco LNV, Valentini V, De Paoli A, Maurici ER, Lupattelli M, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. (2014) 113:223–9. doi: 10.1016/j.radonc.2014.10.006
3. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. (2006) 355:1114–23. doi: 10.1056/NEJMoa060829
4. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomized study. Lancet Oncol. (2014) 15:184–90. doi: 10.1016/S1470-2045(13)70599-0
5. Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, et al. Chronicle: results of a randomized phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomizing postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. (2014) 25:1356–62. doi: 10.1093/annonc/mdu147
6. Breugom AJ, van Gijn W, Muller EW. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo) radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. (2014) 26(4):696–701. doi: 10.1093/annonc/mdu560
7. Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, et al. Adjuvant chemotherapy after preoperative(chemo) radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. (2015) 16:200–7. doi: 10.1016/S1470-2045(14)71199-4
8. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. NCCN clinical practice guidelines in oncology: Rectal Cancer. J Nat. Compr Canc Netw (2022) 20:1139–1167. doi: 10.6004/jnccn.2022.0051
9. Glynne-Jones R, Wyrqicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal Cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:22–40. doi: 10.1093/annonc/mdx224
10. Gerard JP, Conroy T, Bonnetain F, Bouché O, Chapaet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: Results of FFCD 9203. J Clin Oncol. (2006) 24:4620–5. doi: 10.1200/JCO.2006.06.7629
11. Bosset JF, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results - EORTC 22921. J Clin Oncol. (2005) 23:5620–7. doi: 10.1200/JCO.2005.02.113
12. Bosset JF, Collette L, Calais G, Maingon P, Radosevic-Jelic L, Daban A, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. (2006) 355:1114–112. doi: 10.1056/NEJMoa060829
13. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. (2005) 23:5644–50. doi: 10.1200/JCO.2005.08.144
14. Maas M, Nelemans PJ, Valentini V, Das P, Rödel Kuo C. LJ, et al. Long-term outcome in patients with pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. (2010) 11:835–44. doi: 10.1016/S1470-2045(10)70172-8
15. Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: clinicopathologic correlations. Cancer. (1994) 73:2680–6. doi: 10.1002/(ISSN)1097-0142
16. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. (1997) 12:19–23. doi: 10.1007/s003840050072
17. Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. (2005) 47:141–6. doi: 10.1111/j.1365-2559.2005.02176.x
18. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. 3rd. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag (2010).
19. Georg TJ, Allegra CJ and Yothers G. Neoadjuvant rectal (NAR) score: a surrogate endpoint in rectal cancer clinical trials. Curr Colorectal Cancer Res. (2015) 11:275–80. doi: 10.1007/s11888-015-0285-2
20. Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. (2011) 29:3163–72. doi: 10.1200/JCO.2010.33.1595
21. Yothers G, George TJ, Petrelli NJ, O’Connell MJ, Beart RW, Allegra CJ, et al. Neoadjuvant rectal cancer score predicts survival: potential surrogate endpoint for early phase trials. J Clin Oncol. (2014) 32:5s. (suppl; abstr 3533). doi: 10.1200/jco.2014.32.15_suppl.3533
22. O’Connell MJ, Colangelo LH, Beart RW, Petrelli N, Allegra C, Sharif S, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical adjuvant breast and Bowel Project trial R-04. J Clin Oncol. (2014) 32:1927–34. doi: 10.1200/JCO.2013.53.7753
23. Kogler P, DeVries AF, Eisterer W, Thaler J, Sölkner L, Öfner D, et al. Intensified preoperative chemoradiation by adding oxaliplatin in locally advanced, primary operable (cT3NxM0) rectal cancer. Impact on long-term outcome, results of the phase II TAKO 05/ABCSG R02-trial. Strahlenther Onkol. (2018) 194:41–9. doi: 10.1007/s00066-017-1219-5
24. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2021). Available at: https://www.R-project.org.
25. Kassambara A, Kosinski M, Biecek P, Scheipl F. Drawing Survival Curves using’ggplot2’ (2021). Available online at: https://cran.rproject.org/web/packages/survminer/survminer.pdf.
26. Jia X, Baig MM, Mirza F, Hosseini HG. A Cox-based risk prediction model for early detection of cardiovascular disease: Identification of key risk factors for the development of a 10-year CVD risk prediction. Adv Prev Med. (2019). doi: 10.1155/2019/8392348
27. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent Reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. Britisch J Surg. (2015) 102:148–58. doi: 10.1002/bjs.9736
28. Zhuchkova S, Rotmistrov A. How to choose an approach to handling missing categorical data: (un)expected findings from a simulated statistical experiment. Qual Quantity. (2022) 56:1–22. doi: 10.1007/s11135-021-01114-w
29. Öfner D, DeVries A, Schaberl-Moser R, Greil R, Rabl H, Tschmelitsch J, et al. Preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with newly diagnosed, primary operable cT3NxM0, low rectal cancer. Strahlenther Oncol. (2011) 185:488–92. doi: 10.1007/s00066-010-2182-6
30. Loftas P, Arbmann G, Fomichov V, Hallböök O. Nodal involvement in luminal complete response after neoadjuvant treatment for rectal cancer. Eur J Surg Oncol. (2016) 42:801–7. doi: 10.1016/j.ejso.2016.03.013
31. Yeo SG, Kim Dy, Park JW, Hwan J, Kim SY, Chang HE, et al. Tumor volume reduction rate after preoperative chemoradiotherapy as a prognostic factor in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. (2012) 82:193–9. doi: 10.1016/j.ijrobp.2011.03.022
32. Lykke J, Jess P, Roikjaer O, Danish Colorectal Cancer Group. The prognostic value of lymph node ratio in a national cohort of rectal cancer patients. Eur J Surg Oncol. (2016) 42:504–12. doi: 10.1016/j.ejso.2016.01.012
33. Rödel C, Sauer R, Fietkau R. The role of magnetic resonance imaging to select patients for preoperative treatment in rectal cancer. Strahlenther Onkol. (2009) 185:488–92. doi: 10.1007/s00066-009-2043-3
34. Tan Y, Fu D, Li D, Xiangxing K, Jiang K, Chen L, et al. Predictors and risk factors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer: a population-based analysis. Front Oncol. (2019) 9:497. doi: 10.3389/fonc.2019.00497
Keywords: neoadjuvant radiochemotherapy, prediction of survival, T-downstaging, Ndownstaging, locally advanced rectal cancer
Citation: Piringer G, Ponholzer F, Thaler J, Bachleitner-Hofmann T, Rumpold H, de Vries A, Weiss L, Greil R, Gnant M and Öfner D (2024) Prediction of survival after neoadjuvant therapy in locally advanced rectal cancer – a retrospective analysis. Front. Oncol. 14:1374592. doi: 10.3389/fonc.2024.1374592
Received: 22 January 2024; Accepted: 29 April 2024;
Published: 16 May 2024.
Edited by:
Per J. Nilsson, Karolinska Institutet (KI), SwedenCopyright © 2024 Piringer, Ponholzer, Thaler, Bachleitner-Hofmann, Rumpold, de Vries, Weiss, Greil, Gnant and Öfner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gudrun Piringer, gudrun.piringer@kepleruniklinikum.at
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.