
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 21 March 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1373606
This article is part of the Research Topic Radioligand Therapy in Prostate Cancer View all 5 articles
Prostate cancer(PCa), a leading global health concern, profoundly impacts millions of men worldwide. Progressing through two stages, it initially develops within the prostate and subsequently extends to vital organs such as lymph nodes, bones, lungs, and the liver. In the early phases, castration therapy is often employed to mitigate androgen effects. However, when prostate cancer becomes resistant to this treatment, alternative strategies become imperative. As diagnostic and treatment methodologies for prostate cancer continually advance, radioligand therapy (RLT) has emerged as a promising avenue, yielding noteworthy outcomes. The fundamental principle of RLT involves delivering radionuclide drugs to cancerous lesions through specific carriers or technologies. Subsequently, these radionuclide drugs release radioactive energy, facilitating the destruction of cancer cell tissues. At present, the positron emission tomography (PET) targeting PSMA has been widely developed for the use of diagnosis and staging of PCa. Notably, FDA-approved prostate-specific membrane antigen (PSMA) targeting agents, such as 68Ga-PSMA-11 and 177Lu-PSMA-617, represent significant milestones in enhancing diagnostic precision and therapeutic efficacy. This review emphasizes the current research status and outcomes of various radionuclide-labeled PSMA ligands. The objective is to provide valuable insights for the continued advancement of diagnostic and therapeutic approaches in the realm of prostate cancer.
As of 2020, the global landscape of cancer incidence and mortality reveals a staggering burden, with around 19.3 million new cancer cases reported worldwide (excluding non-melanoma skin cancer). Unfortunately, approximately 10 million cancer patients succumbed to the disease (excluding non-melanoma skin cancer). Prostate cancer constituted a significant portion of these cases, with approximately 1.4 million new diagnoses globally, accounting for 7.3% of all cancer cases. Regrettably, there were approximately 375,000 deaths attributed to prostate cancer, making it the second most common cancer and the fifth leading cause of death among men in 2020 (1). The anticipated cancer burden in 2022 underscores the significant impact on public health in both China and the United States. It is projected that China will experience around 4.82 million new cancer cases and approximately 3.21 million cancer-related deaths. In China, prostate cancer specifically contributes over 125,000 new cases and more than 56,000 deaths. Similarly, the United States is expected to see around 2.37 million new cancer cases and approximately 640,000 cancer-related deaths in 2022. For prostate cancer in the United States, the estimates are approximately 126,900 new cases and 34,600 deaths. These figures highlight the substantial health challenges posed by cancer and the need for continued efforts in prevention, early detection, and effective treatment strategies (2). The data underscores a consistent rise in the incidence of prostate cancer, a trend attributed to advancements in medical technology. Improved diagnostic methods, heightened awareness prompting proactive screening, and demographic shifts, including an aging population, contribute to the increasing detection rates. As healthcare technologies continue to progress, early detection becomes more achievable, playing a pivotal role in effectively managing and treating prostate cancer (3, 4). While many prostate cancers exhibit slow growth, their impact on a patient’s health and life can be profound, potentially progressing into castration-resistant prostate cancer, which stands as the primary cause of death in prostate cancer patients. Hence, the early diagnosis and precise evaluation of prostate cancer hold immense significance in mitigating the potential adverse outcomes associated with the disease. Early detection allows for timely intervention and tailored treatment strategies, contributing to better patient outcomes and quality of life.
PET/CT stands as a widely utilized nuclear medicine technique for comprehensive tumor examinations. By integrating Positron Emission Tomography (PET) and Computed Tomography (CT), PET/CT provides valuable insights into the metabolic activity throughout the body. This imaging modality aids in the early detection of tumor lesions, offering crucial information on tumor size, location, local invasion, lymph node metastasis, and distant metastasis. Additionally, PET/CT serves as a valuable tool in distinguishing between focal changes and tumor recurrence following radiotherapy. Its versatility and effectiveness make PET/CT one of the most commonly employed methods for oncology evaluations today. Moreover, its application in targeted cancer therapy further enhances its value in contributing to improved patient care (5, 6).
18F-FDG (fluorodeoxyglucose) stands as a frequently employed PET tracer for assessing tumor metabolic activity and diagnosing tumor lesions. Its potential utility extends across various facets of prostate cancer care, encompassing diagnosis, staging, treatment evaluation, and prognosis, especially in cases of castration-resistant metastatic prostate cancer. The versatility of 18F-FDG PET proves valuable in providing comprehensive insights into prostate cancer, aiding in precise diagnosis, effective staging, informed treatment decisions, and prognostic assessments for patients (7, 8). While 18F-FDG is extensively utilized in oncology, it exhibits lower specificity, occasionally accumulating in inflammation, infections, and normal tissues. Additionally, it may be less sensitive to certain tumor types, such as breast and prostate cancer. In instances where these limitations are notable, alternative PET tracers may prove more suitable for achieving higher specificity and sensitivity in imaging and diagnosis (9–12). PSMA (Prostate Specific Membrane Antigen) is a membrane antigen characterized by high expression in prostate tissue and on the surface of prostate cancer cells. Due to its heightened expression in prostate cancer, PSMA has evolved into a pivotal marker for targeted diagnosis and treatment of prostate cancer. The application of PET-CT imaging with radionuclide-labeled PSMA has demonstrated significant potential in detecting and staging prostate cancer, offering a promising approach for improved visualization and assessment of the disease (13, 14). Currently, PSMA imaging has gained recognition in the latest international guidelines and is poised to become the forefront choice for the diagnosis and treatment of prostate cancer in the future. This acknowledgment underscores the increasing importance of PSMA-based imaging methods in refining the accuracy and precision of prostate cancer diagnostics, guiding targeted therapeutic approaches. As guidelines evolve, the prominence of PSMA imaging is expected to play a central role in the comprehensive management of prostate cancer (15). PSMA-617, PSMA-1007, PSMA-11, PSMA-I&T, and similar compounds are chemical reagents designed to target the prostate-specific membrane antigen (PSMA). They possess the ability to bind to PSMA present on the surface of tumor cells, facilitating the visualization of tumor signals and offering the potential for targeted therapy. These PSMA ligands are preferred due to their advantages, including small molecular weight, robust tissue permeability, rapid blood clearance, and ease of large-scale synthesis. Consequently, they have emerged as the primary choice for molecular imaging probes in prostate cancer, finding widespread applications in the targeted therapy of prostate cancer (13). This review seeks to provide a comprehensive overview of the current research status of commonly used PSMA ligand drugs for radioisotope labeling. The focus is on exploring their properties, efficacy, and toxicity, along with examining the outcomes of their combined application with other treatments. The goal is to offer a consolidated understanding of the current landscape of PSMA ligand drugs, shedding light on their characteristics, therapeutic effectiveness, and potential synergies when employed in conjunction with other therapeutic modalities.
68Ga-PSMA-11 is the small molecule imaging agent known for its favorable biological distribution characteristics. It was initially utilized in clinical settings in 2012, and by the end of December 2020, 68Ga-PSMA-11 had become the earliest PSMA imaging agent to receive approval from the FDA (U.S. Food and Drug Administration).
The complexation of the radionuclide gallium-68 is achieved using the bifunctional non-cyclic chelating agent HBED-CC (N,N’-bis[2-hydroxy-5-(carboxyethyl)]-N,N’-diacetic acid). This chelating agent is hexadentate and adopts an octahedral geometry. In the complex, gallium-68 is coordinated with 2 nitrogen atoms, 2 hydroxyl groups, and 2 carboxyl groups. The 68Ga-HBED-CC group binds to the PSMA analogue Glu-NH-CO-NH-Lys(Ahx). Figure 1 depicts the structure of 68Ga-PSMA-11 (16). 68Ga-PSMA-11 PET/CT has shown notable specificity and sensitivity in the diagnosis and staging of primary prostate cancer, re-staging of patients with recurrent prostate cancer (PCa), and evaluating castration-resistant prostate cancer (CRPC). The use of this imaging technique can contribute to more accurate diagnosis, staging, and assessment of treatment response in patients with prostate cancer.
68Ga-PSMA-11 PET/CT has shown notable specificity and sensitivity in the diagnosis and staging of primary prostate cancer, re-staging of patients with recurrent prostate cancer (PCa), and evaluating castration-resistant prostate cancer (CRPC). The use of this imaging technique can contribute to more accurate diagnosis, staging, and assessment of treatment response in patients with prostate cancer (17).
68Ga-PSMA-11 has been extensively demonstrated to exhibit high sensitivity and specificity in the diagnosis of prostate cancer. It significantly enhances the detection rate compared to other imaging agents such as 18F-FDG PET/CT, 18F-PSMA-1007 PET/CT, and 64CuCl2 PET-CT (18). In particular, 68Ga-PSMA-11 PET/CT displays higher sensitivity for prostate cancer diagnosis (19). Yang J. et al. utilized the 68Ga-PSMA-11 PET Maximum Normalized Threshold (SUVmax) to predict clinically significant prostate cancer (PCa) and PSA levels in the gray area (4-10 ng/ml), which is challenging for PCa diagnosis. In this study, the sensitivity and specificity were reported as 86.21% and 86.54%, respectively. 68Ga-PSMA-11 PET facilitates the screening and early diagnosis of prostate cancer and can help avoid unnecessary biopsy procedures (20).
In a study conducted by Hope et al., 68Ga-PSMA-11 PET imaging scans were employed for preliminary staging in 764 patients with prostate cancer and pelvic lymph node metastasis. The results indicated a positive 68Ga-PSMA-11 PET scan, with reported sensitivity and specificity for pelvic lymph node metastasis of 0.40 and 0.95, respectively (21). Consequently, 68Ga-PSMA-11 PET is considered beneficial for preoperative staging and assisting in lymph node dissection. The study findings suggest that 18F-PSMA-11 PET/MRI can help reduce false negatives for clinically significant prostate cancer (csPCa) when compared to MRI alone. This potential improvement in diagnostic accuracy may lead to a reduction in the number of unnecessary prostate biopsies required to diagnose clinically significant prostate cancer. The combined information from PET and MRI imaging could enhance the detection and localization of prostate cancer, thereby aiding in more targeted and effective clinical decision-making (22).
Indeed, 68Ga-PSMA-11 PET is increasingly recommended by various guidelines for detecting biochemical recurrent prostate cancer. Its high accuracy in detection, along with its capability to assess stage and prognosis, has contributed to its recognition and adoption in clinical practice. This imaging modality has proven valuable in the management of patients with biochemical recurrence by providing detailed information about the location and extent of disease, aiding in treatment planning, and contributing to more informed clinical decision-making (23, 24). This study examining 635 cases involving prostatectomy and/or radiotherapy with 68Ga-PSMA-11 PET scans and histopathological verification, the researchers found that the detection rates varied with different PSA levels. The results were as follows: for PSA levels <0.5ng/mL (n=136), the detection rate was 38%; for PSA levels 0.5 to <1.0ng/mL (n=79), the detection rate was 57%; for PSA levels 1.0 to <2.0ng/mL (n=89), the detection rate was 84%; for PSA levels 2.0 to <5.0ng/mL (n=158), the detection rate was 86%. The detection rate increased to 97% for PSA levels ≥5.0ng/mL (n=173, P<.001). The study concluded that the rate of PSA detection in localized recurrent prostate cancer with 68Ga-PSMA-11 PET was significantly improved, demonstrating the effectiveness of this imaging modality in detecting recurrent disease at various PSA levels (23). The multicenter study involving 138 prostate cancer (PCa) patients with biochemical recurrent (BCR) lesions, classified as progressive, mixed, or nonprogressive, utilized quantitative parameters (SUVmean, SUVmax, SUVpeak, volume) to quantify tumor response at a focal level following 68Ga-PSMA-11 PET scans. The study demonstrated that patients with systemic progression had a significantly higher risk of death compared to those without progression (HR=5.70), with SUVmean identified as having the highest prognostic value. Furthermore, 68Ga-PSMA-11 PET was found to possess significant prognostic value in progressive patients for overall survival (HR=3.67). These findings suggest that 68Ga-PSMA-11 PET can assist in restaging biochemical recurrent prostate cancer and plays a crucial role in assessing prognosis (24).
68Ga-PSMA-11 PET/CT stands out as a valuable tool for evaluating metastatic castration-resistant prostate cancer (mCRPC), enhancing the precision of staging, and aiding in crucial clinical decision-making processes. With its high sensitivity and specificity, this imaging modality provides detailed insights into the localization and extent of metastatic lesions, empowering doctors to make well-informed decisions on treatment strategies and patient management for those facing advanced prostate cancer (25, 26). In contrast to other imaging techniques, 68Ga-PSMA-11 PET proves highly effective in detecting small, distant, and atypical metastases associated with prostate cancer, including instances of intraocular (27) and isolated peritoneal metastases (28).
PSMA-1007 represents a novel PSMA ligand derived from the chemical structure of PSMA-617 (Figure 2). It targets the gluu-urea-lys motif of the PSMA enzyme pocket S1` and concurrently engages the naphthaln-based hydrophobic accessory pocket S1. The primary distinction lies in the addition of two glutamic acids to the site carrying the radioactive label, simulating the carboxyl group of the DOTA chelating agent (29). Prostate-specific membrane antigen (PSMA) is found in the proximal tubule cells of the kidney, resulting in significant uptake of renal tracers in PSMA-PET. This uptake may contain valuable information about renal function. PSMA-1007 has been validated for its excellent binding and internalization properties in vitro. It exhibits high specific uptake in vivo and demonstrates effective differentiation between normal ganglia and lymph node metastasis in prostate cancer. Research indicates that PET/CT diagnosis using 18F-PSMA-1007 offers advantages over traditional imaging modalities such as CT, MRI, ultrasound, and bone imaging (30). Moreover, in contrast to other well-known PSMA ligands, PSMA-1007 primarily undergoes metabolization through the hepatobiliary pathway, diverging from the renal metabolism observed with other ligands. This distinctive metabolic pathway could potentially confer benefits in the differentiation of lymph node metastasis, particularly in patients with recurrent prostate cancer (31, 32). In a retrospective survey involving 73 prostate cancer patients, Rassek P et al. concluded that renal uptake of 18F-PSMA-1007 can serve as an accurate measure for quantifying renal function, utilizing parameters such as SRFPSMA-TOTAL or SRFSUV (33).. In the latest clinical study, which included 60 patients diagnosed with prostate cancer exhibiting low PSA levels, 18F-PSMA-1007 PET/MRI detected 53 lesions in 45 patients, resulting in a detection rate of 75%. The average PSA value in this cohort was 0.31 ng/mL. These findings suggest that 18F-PSMA-1007 proves to be an excellent molecular probe, particularly beneficial for early-stage biochemical recurrence (BCR) patients with exceptionally low PSA levels (34). Given the chemical structure and biological activity, 18F-PSMA-1007, along with 177Lu-PSMA-617, holds promise in matching the diagnostic capabilities. Further research endeavors will ascertain the clinical significance of this ligand and its potential for practical application in clinical settings. Ongoing studies will shed light on the utility and effectiveness of this ligand, shaping its role in clinical diagnosis and potentially opening avenues for enhanced prostate cancer management (29, 35, 36).
18F-DCFPyL, or 2-(3-(1-carboxy-5-[(6-[18F]fluoro-pyridine3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid, is a specific small molecule imaging agent developed based on the Glu-urea-Lys structure (Figure 3). This agent demonstrates high affinity for binding to the extracellular region of Prostate-Specific Membrane Antigen (PSMA) (37). It is also the second FDA-approved PSMA-targeted PET imaging drug (38).
18F-DCFPyL has been validated in multiple prospective clinical trials such as OSPREY and CONDOR, demonstrating its effectiveness in clinical applications, including staging, re-staging, and efficacy evaluation in patients with prostate cancer (PCa). In the Phase III multicenter CONDOR trial, 18F-DCFPyL-PET/CT imaging was conducted on 208 patients with prostate adenocarcinoma who had undergone radical prostatectomy (RP) due to biochemical recurrence and had negative results on standard imaging. The administered dose was 333 MBq, and it was given intravenously 1 to 2 hours before PET/CT imaging. The results revealed that 63.9% of the patients altered their intended treatment plan after 18F-DCFPyL-PET/CT, and the disease detection rate ranged from 59% to 66% (39). Hence, 18F-DCFPyL-PET/CT proves to be an effective tool for disease imaging in patients with recurrent prostate cancer. In the OSPREY trial, 18F-DCFPyL-PET/CT examinations were conducted on 252 male patients with high-risk prostate cancer after radical prostatectomy plus pelvic lymph node dissection. The median specificity and sensitivity were reported as 97.9% (95% CI: 94.5% to 99.4%) and 40.3% (95% CI: 28.1% to 52.5%), respectively (40), The collective evidence supports the utility of 18F-DCFPyL-PET/CT for detecting lesions in patients with prostate cancer (PCa) and evaluating the staging of lymph node or distant metastasis. Zsolt Szabo was the first to employ 18F-DCFPyL in patients with hormone-independent or castration-resistant prostate cancer. Studies have demonstrated that 18F-DCFPyL exhibits physiological uptake in salivary glands, lacrimal glands, kidneys, liver, spleen, and small intestine, with no uptake observed in the brain. Simultaneously, it is excreted through urine, showing notable accumulation in the kidneys and bladder. Dosimetry studies revealed that the effective dose of 18F-DCFPyL was 0.0165 mSv/MBq or 6.1 mGy (0.61 rem) at an injected dose of 370 MBq. There was significant accumulation in prostate cancer foci (SUVmax up to 9100, tumor/blood ratio up to 950). The highest radiation doses were observed in renal viscera (0.0945 mGy/MBq), bladder wall (0.0864 mGy/MBq), submandibular gland (0.0387 mGy/MBq), and liver viscera (0.0380 mGy/MBq) (41).
Despite the relatively short duration of clinical application, 18F-DCFPyL has undergone several evaluations, all of which report high sensitivity, specificity, and positive detection rates. In a prospective study involving 205 patients experiencing biochemical recurrence (BCR) after initial radical prostate cancer surgery or radiation therapy, separate examinations were conducted using 18F-DCFPyL PET/CT and 18F-fluoromyclocholine PET/CT. The positive detection rate of lesions increased with the rise in PSA value. The overall detection rate of 18F-DCFPyL PET/CT was superior to that of 18F-fluoromethylcholine PET/CT (58% vs 40%, p < 0.0001) (42). The 18F-PSMA PET/CT pair can also be utilized to detect uncommon metastatic sites, including the brain, liver, and penis, among others (43). Simultaneously, when compared to other traditional imaging modalities, 18F-DCFPyL PET/MR demonstrates superiority in pre-treatment screening of prostate cancer patients with pretreatment lesion localization. In a study led by Adriano Basso Dias et al., 18F-DCFPyL PET/MRI was employed to screen prostate cancer patients undergoing focal ablation therapy (FT). mpMRI and/or PET/MRI were conducted on 34 patients with low/medium-risk PCa. 18F-DCFPyL PET/MRI excluded focal treatment in nearly 30% of patients with low/medium-risk PCa and exhibited higher sensitivity (97% vs 76%, P = 0.02) but relatively lower specificity (30% vs 85%, P < 0.001). As a result, its elevated sensitivity effectively detects lesions without missing detections, enhancing the diagnostic efficacy of clinically significant (CS) prostate cancer in patients undergoing focal ablation (FT). Nonetheless, its lower specificity may restrict the use of PET/MRI as a screening tool (44). Therefore, in conjunction with histological characteristics and conventional imaging examinations, 18F-PSMA PET/CT can be more effectively applied in patients with prostate cancer. NCT04461509 is a Phase II clinical trial, which is utilizing 18F-PSMA PET/MRI and enhanced prostate imaging with standard mp/MRI to evaluate the effectiveness of focal high-intensity focused ultrasound (HIFU) therapy on prostate cancer targets Currently. 18F-DCF PET/CT imaging facilitates individualized management of prostate cancer by eliminating unnecessary biopsies through disease staging and risk stratification (39, 45).
The 18F labeled PSMA targeting compound provides a significant improvement in image quality and noise compared to the 68Ga-labeled PSMA-targeting compound. This improvement allows for the detection of subtle lesions, and the longer half-life of 110 minutes facilitates delayed imaging (46).
Although many treatments have emerged for metastatic castration-resistant prostate cancer (mCRPC) over the past decades, recent clinical trials have shown a survival benefit of 177Lu-PSMA-617 in mCRPC following chemotherapy (47). Currently, a relatively novel treatment method for metastatic castration-resistant prostate cancer (mCRPC) involves targeted radioactive oligonucleotide therapy. In this approach, radioactive isotopes are paired with monoclonal antibodies targeting cancer-specific antigens, such as prostate-specific membrane antigen (PSMA). This method is not only simple but also minimizes the impact on normal tissues.
PSMA-617, a novel DOTA-conjugated PSMA inhibitor containing naphthalene, displays elevated uptake in both tumors and kidneys within the LNcaP tumor model, as observed through small animal PET imaging. It efficiently internalizes into LNcaP cells and exhibits swift renal clearance, making it promising for therapeutic applications. The favorable pharmacokinetics lead to target-to-non-target ratios of 1058 (tumor-to-blood) and 529 (tumor-to-muscle) at 24 hours post-injection in animal models (48). This ligand is amenable to labeling with 68Ga, 111In, 177Lu, and 90Y (Figure 4). Early clinical studies have showcased the high-contrast detection capabilities of 68Ga-PSMA-617 in identifying prostate cancer (PCa) lesions. Moreover, it has been utilized in the treatment of metastatic PCa across various medical centers (49, 50).
There is a substantial body of randomized clinical evidence and practical experience regarding the use of the PSMA radioligand 177Lu-PSMA-617 for therapeutic purposes. Since obtaining regulatory approval in 2013, Germany and several other regions in Europe have accumulated significant experience in the application of 177Lu-PSMA-617 for prostate cancer treatment (51–53). In a randomized, parallel-group, open-label, phase 2 non-inferiority trial, Swayamjeet Satapathy et al. prospectively compared the efficacy and safety of 177Lu-PSMA-617 with docetaxel in patients with metastatic castration-resistant prostate cancer (mCRPC). A total of 40 patients underwent randomization, and the best prostate-specific antigen response rate (PSA-RR) was 60% (9/15) in the 177Lu-PSMA-617 group and 40% (8/20) in the docetaxel group. The difference in PSA-RR between the two groups was 20% (95% confidence interval, CI: -12-47, P=0.25). Furthermore, the 6-month progression-free survival rates for the 177Lu-PSMA-617 group and the docetaxel group were 30% and 20%, respectively (difference 10%, 95% CI: -18-38, P=0.50). The study concludes that 177Lu-PSMA-617 is safe and non-inferior to docetaxel in the treatment of mCRPC and can be considered during early stages of the disease process (54). As of March 2022, the FDA has granted approval for the use of the drug exclusively in the treatment of patients diagnosed with PSMA-positive metastatic castration-resistant prostate cancer. This approval is specifically designated for individuals who have undergone previous treatments, such as androgen receptor inhibition or taxane chemotherapy (55). In a phase III trial conducted by O. Sartor et al., 831 out of 1179 screened patients were enrolled for randomization between June 2018 and mid-October 2019. The findings revealed that 177Lu-PSMA-617 led to a significant extension in progression-free survival compared to standard therapy. Despite a higher incidence of grade 3 or higher adverse events in patients using 177Lu-PSMA-617 compared to those who did not, their overall quality of life was not significantly impacted (56).
The fundamental principle of radioactive isotope therapy involves delivering a high dose of radiation to target tissues while minimizing toxicity to healthy tissues. The prerequisite for treatment is the presence of a PSMA-positive tumor phenotype detected through PET or imaging. The 177Lu-PSMA-617 treatment appears to be a safe approach for castration-resistant prostate cancer. The maximum tolerated dose for a single administration may range between 7.4-11.1 GBq, depending on whether the tumor involves the bone marrow (57). Early inclusion of clinical patients in studies has shown that the distribution of lesions and physiological uptake regions is similar to what is observed in early diagnostic 68Ga-PSMA-11 PET scans. This similarity indicates promising therapeutic potential for 177Lu-PSMA-617 treatment in castration-resistant prostate cancer (58).
PSMA I&T, or DOTAGA-(I - y) fk-fk (Sub - KuE), with DOTAGA [1,4,7,10-tetraazacyclododececane-1(-glutaric acid)-4,7,10-triacetic acid, 1,4,7, 10-tetraazecyclodecadecane-1-(glutamic acid)-4,7,10-triethonic acid] as a chelating agent (59, 60). This small molecule PSMA-targeted inhibitor exhibits rapid pharmacokinetics and high affinity for PSMA (Figure 5). PSMA I&T can be labeled with isotopes such as 68Ga, 111In, 177Lu, and 225Ac for imaging and therapeutic purposes (61).
Compared to 177Lu-PSMA-617, 177Lu-PSMA-I&T demonstrates similar mean tumor dose absorption and favorable safety profiles. Notably, 177Lu-PSMA-I&T exhibits lower uptake in salivary glands, resulting in reduced potential damage to these glands. However, it is important to consider that the kidney uptake rate is relatively high with 177Lu-PSMA-I&T (54, 62, 63). 177Lu-PSMA-I&T is utilized for targeted radionuclide therapy and has shown promising efficacy in patients with metastatic castration-resistant prostate cancer (mCRPC). Several clinical trials are currently underway, including NCT05204927, NCT05867615, and NCT04647526. NCT05204927 is a prospective, multicenter, randomized Phase 3 clinical trial involving 400 metastatic prostate cancer patients randomized to receive either 177Lu-PSMA-I&T or hormone therapy. The trial aims to assess disease progression based on solid tumor response criteria and record PSA levels and symptoms.In a retrospective study conducted by Amir Karimzadeh et al., 301 mCRPC patients treated with 177Lu-PSMA-I&T were evaluated. The standard activity of 177Lu-PSMA-I&T was 7.4 GBq, administered every 4-10 weeks (median, 6 weeks) for a total of 1138 cycles of intravenous injection (median, three cycles per patient). Results indicated that 34% of patients demonstrated at least a 50% PSA response, with a median progression-free PSA survival of 16.0 weeks and an overall survival (OS) of 13.8 months (64). Mehmet Onur Demirkol et al. conducted 177Lu-PSMA-I&T radionuclide therapy (RLT) on 33 patients with metastatic castration-resistant prostate cancer (mCRPC) and 5 patients with metastatic hormone-sensitive prostate cancer (mHSPC). Among the mCRPC patients, 56% exhibited a PSA response of ≥30%. Notably, all mHSPC patients showed a high PSA response ranging from 93.0% to 99.9%. These findings suggest that 177Lu-PSMA-I&T RLT demonstrates significant antitumor activity. However, it is important to note that some patients experienced mild renal impairment or anemia during the course of treatment (65).
Furthermore, 68Ga-PSMA-I&T PET/CT has demonstrated successful utility in detecting primary lesions and staging prostate cancer patients. This approach offers advantages in the stratification and follow-up of patients undergoing treatment with the 177Lu-PSMA-I&T (Integrated Diagnosis and Treatment) radioligand (60, 66). 225Ac-PSMA-I&T has demonstrated enhanced anti-tumor effects in patients with advanced metastatic castration-resistant prostate cancer (mCRPC) (67). A Phase I/II clinical trial named AlphaBet (NCT05383079) is underway, combining 223Ra with 177Lu-PSMA-I&T. This combination aims to improve outcomes for patients with mCRPC and bone metastases (68).
Alpha-targeted therapy (TAT) is a therapeutic approach that targets cancer cell vectors based on drugs labeled with radionuclides that emit alpha particles (69). Alpha-nuclides possess distinctive characteristics, including high linear energy transfer, a limited range, and potent cytotoxicity. The current alpha-radionuclides deemed suitable for targeted therapy encompass 149Tb, 212/213Bi, 212Pb (212Bi), 225Ac, and 226/227Th. These radionuclides show promise in targeted alpha-particle therapy due to their ability to deliver focused and intense radiation, making them valuable candidates for precision cancer treatment (70). Due to its extended half-life (t1/2 = 10 days), distinctive decay properties, ease of coordination, and selective destruction of cancer cells with minimal damage to normal tissue, 225Ac stands out as one of the most favorable choices for Targeted Alpha Therapy (TAT). The unique characteristics of 225Ac make it an ideal candidate for precision cancer treatment, offering the potential to effectively combat cancer while minimizing harm to surrounding healthy tissues (71). Research findings indicate that 225Ac-PSMA-617 has demonstrated efficacy in patients with metastatic castration-resistant prostate cancer (mCRPC). In a groundbreaking study conducted by Clemens Kratochwil et al. in 2016, they pioneered the use of an alpha nuclide-labeled PSMA ligand for human therapy. Two patients in the study received treatment with 225Ac-PSMA-617 Radionuclide Therapy (RLT) at a dosage of 100 kBq/kg of body weight every 2 months. Remarkably, these patients exhibited a significant reduction in prostate-specific antigen (PSA) levels, suggesting the therapeutic potential of 225Ac-PSMA-617 in managing mCRPC (72).
The evaluation of 225Ac-PSMA therapy is currently limited by the scarcity of prospective, randomized trials, with ongoing trials such as NCT04597411 aiming to provide more robust insights. In a recent multi-center retrospective study, 488 patients with metastatic castration-resistant prostate cancer (mCRPC) underwent treatment with 225Ac-PSMA for a total of 1174 cycles (median 2 cycles, IQR 2-4). The patients were followed for a median duration of 9 months (IQR 5-17.5). The study reported a median overall survival of 15.5 months (95% CI 13.4 to 18.3) and a median progression-free survival of 7.9 months (6.8 to 8.9). These findings contribute to the evolving understanding of the efficacy and outcomes associated with 225Ac-PSMA therapy in mCRPC patients (73). Madhav Prasad Yadav et al. conducted a study involving 28 patients with metastatic castration-resistant prostate cancer (mCRPC) who were treated with 225Ac-PSMA-617. The average administered activity was 26.5 ± 12 MBq (range, 9.25-62.9 MBq), with a median of 3 cycles (range, 1-7 cycles). Following the first cycle and at the 8th week, PSA reduction of >50% was observed in 25% and 39% of patients, respectively. The median progression-free survival (PFS) and overall survival (OS) were reported as 12 months (95% CI: 9-13 months) and 17 months (95% CI: 16 months - not reaching the upper limit), respectively. The disease control rates were 82% and 63.6%, contributing valuable insights into the clinical outcomes of 225Ac-PSMA-617 therapy in mCRPC patients (74). Therefore, 255Ac-PSMA-617 RLT not only has good anti-tumor effect, but also has good therapeutic safety.
Moreover, the unique capability of alpha rays to eliminate cells that typically display resistance to beta or gamma rays, as well as chemotherapy drugs, positions 225Ac-PSMA-617 treatment as a compelling alternative. This makes it a viable option for patients facing tumors that have developed resistance to the conventional treatment with 177Lu-PSMA-617. The distinctive attributes of alpha radiation introduce a promising avenue for therapeutic intervention, particularly in addressing cells resistant to established treatment approaches (71). Nalan Alan-Selcuk et al. administered 225Ac-PSMA-617 to 23 mCRPC patients who had previously undergone unsuccessful 177Lu-PSMA-617 treatment (2-9 cycles). The median interval between doses was 13 weeks (range, 8-28 weeks), with an average dose activity of 7.6 MBq (range, 6.2-10.0 MBq) per cycle. Following the first treatment cycle (n=18), 50% of patients (n=9) exhibited disease control based on prostate-specific membrane antigen PET progression criteria. The median progression-free survival was 3.1 months, and the median overall survival was 7.7 months (75).
The most prevalent adverse effect of 225Ac-PSMA-617 was dry mouth (72–74, 76–78), and when administered in conjunction with 177Lu-PSMA-617, it may lead to a substantial rise in salivary toxicity. Delayed nephrotoxicity has also been documented following 2 cycles of 225Ac-PSMA-617 RLT (79).
The article comprehensively examines the role of diverse radiolabeled PSMAs in both diagnosing and treating prostate cancer. It aims to consolidate the clinical evidence supporting various PSMA ligands in the context of prostate cancer. The degree of PSMA uptake serves as a crucial biomarker for prostate cancer, with elevated PSMA levels typically signifying the presence of prostate cancer cells in specific regions. Active cancer cells often exhibit heightened PSMA uptake, making PSMA a valuable indicator for prostate cancer, metastasis, or lymph node involvement. This information plays a pivotal role in guiding treatment decisions for prostate cancer patients. Additionally, for individuals undergoing PSMA radionuclide therapy, alterations in PSMA uptake levels can be utilized to assess the treatment’s effectiveness, where a reduction in uptake may indicate a positive treatment response on the tumor (25). Earlier investigations have established that PSMA (Prostate-Specific Membrane Antigen) is a membrane protein that exhibits high expression in prostate tissue. Normally, PSMA is predominantly present on the luminal surface of prostate epithelial cells. However, in cases of prostate cancer, the expression of PSMA significantly elevates, resulting in cancer cells displaying a heightened affinity for PSMA. This characteristic allows tracers like 68Ga-PSMA to selectively accumulate in vivo within prostate cancer cells that overexpress PSMA, as detected by positron emission tomography (PET-CT). Importantly, there is less accumulation of these tracers in normal tissues (23, 80).
While PSMA serves as a valuable visualization tool, it comes with certain limitations in comparison to the widely used imaging agent 18F-FDG. One notable limitation is the high uptake of PSMA in the liver, kidneys, and salivary glands. This heightened uptake in these normal tissues may potentially impact the diagnostic efficacy of PSMA imaging (31). The strength of PSMA lies in its comprehensive role in both radiological diagnosis and treatment. Building on the foundation of 68Ga-PSMA-11 imaging diagnosis, the administration of 177Lu-PSMA-617 for treatment is a significant advancement. Following treatment and the assessment of recurrent lesions in the early stages, 68Ga-PSMA-11 continues to exhibit promising effects. Leveraging the radioactivity of 177Lu, 177Lu-PSMA-617 serves as a targeted therapeutic agent for prostate cancer. It releases beta rays within the body, facilitating the localized destruction of cancer cells. With its high affinity for PSMA, it selectively targets tumor cells, demonstrating efficacy in the local treatment of refractory prostate cancer and metastatic disease, forming an integral part of various treatment regimens (81). Furthermore, 225Ac-PSMA-617 holds particular significance for patients exhibiting resistance to 177Lu-PSMA-617. The unique properties of 225Ac nuclide, including high energy, short range, potent cytotoxicity, and easy coordination, have elevated it to a recent hotspot in the realm of radioligand therapy (RLT) for patients with metastatic castration-resistant prostate cancer (mCRPC) (82). However, a common adverse reaction post-treatment is dry mouth. Concomitant administration with 177Lu-PSMA-617 may result in a notable escalation in salivary toxicity. Simultaneously, careful attention must be directed towards nephrotoxicity that may arise subsequent to the treatment (78).
As a diagnostic and therapeutic tool, the substantial uptake of PSMA in the salivary glands and urinary tract necessitates modification to enhance its diagnostic and therapeutic efficacy. Addressing this issue is crucial in future PSMA research. Moreover, future studies should explore additional structural variants of PSMA to ameliorate certain challenges associated with existing PSMA ligand structures.
The development and application of PSMA ligands in the field of prostate cancer diagnosis and treatment have witnessed remarkable progress in recent years. From the pioneering 68Ga-PSMA-11 to the therapeutic breakthroughs with 177Lu-PSMA-617 and other emerging compounds, these radiolabeled PSMA-targeting agents have significantly enhanced our ability to detect and manage prostate cancer. The continuous evolution and integration of these agents into clinical practice hold tremendous potential for personalized and effective management of prostate cancer patients in the future. (Figures 6–9).
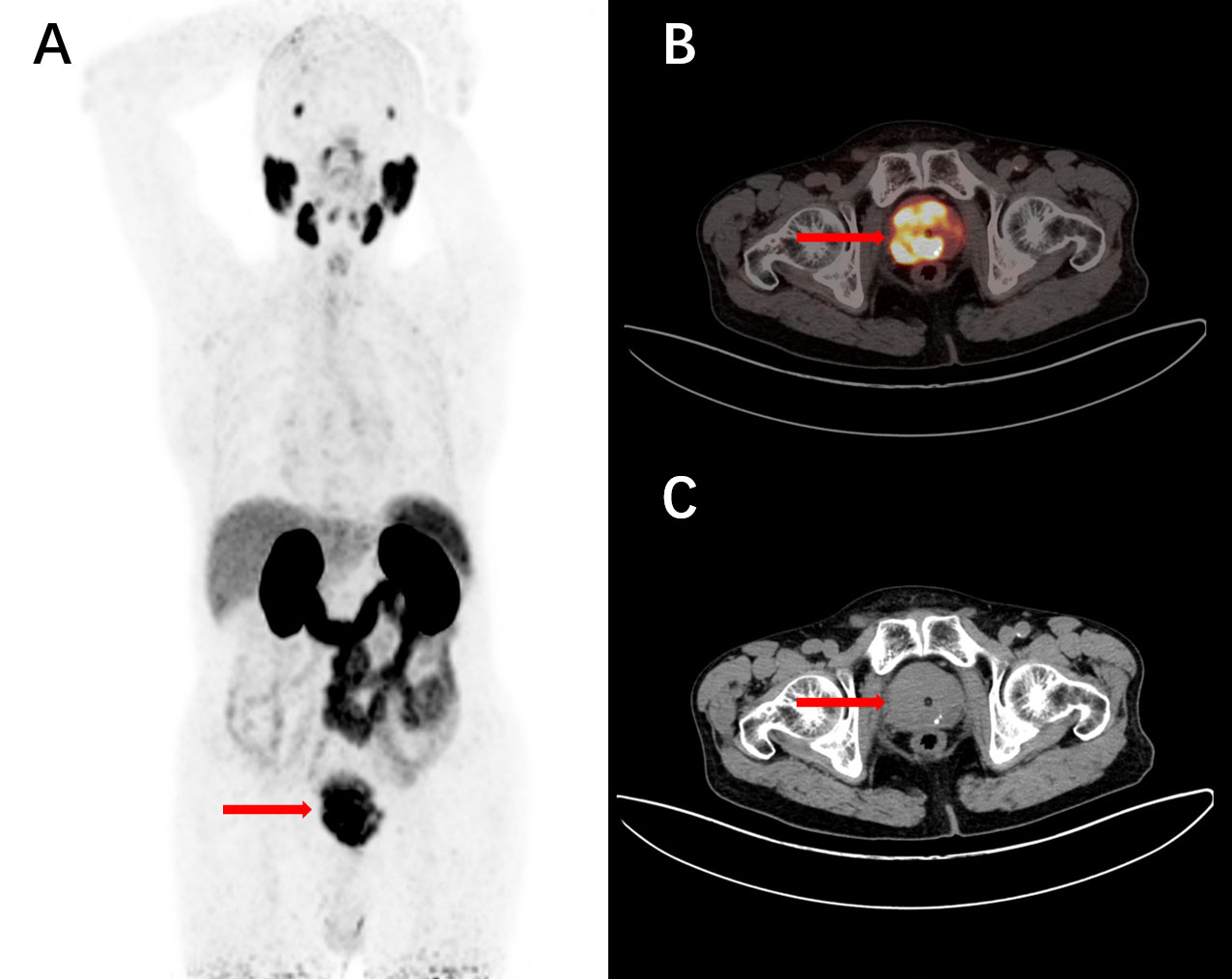
Figure 6 A 77-year-old male, diagnosed with prostate acinar adenocarcinoma through a biopsy, underwent intravenous injection of 68Ga-PSMA-11. 1 hour later, a PET/CT scan was performed, and the maximum intensity projection (MIP) image showed unevenly increased PSMA expression (A). The tomographic images revealed non-uniform internal density, small nodular low-density shadows, and scattered calcifications with increased tracer uptake (B, C). These findings are consistent with the presentation of prostate cancer.
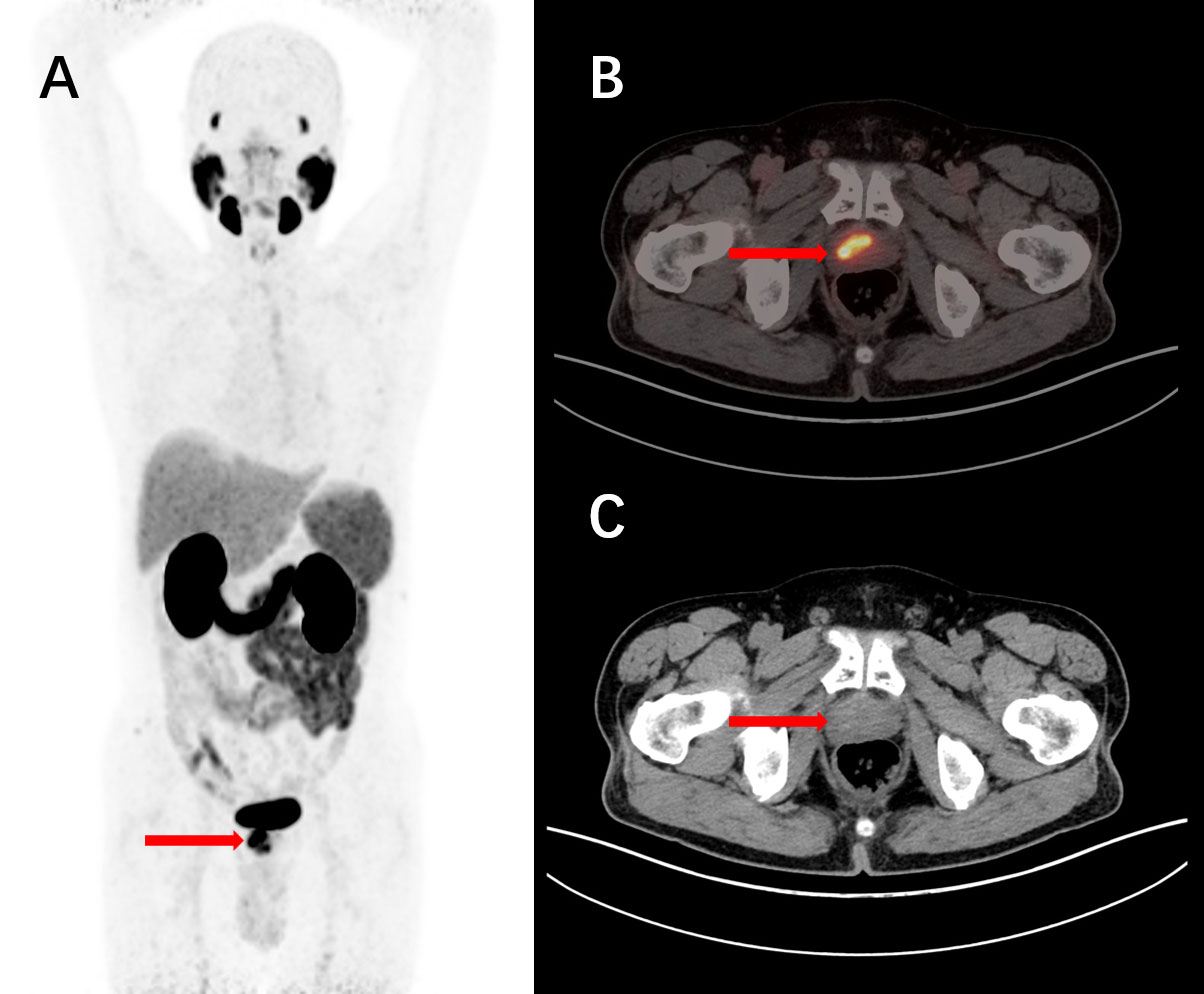
Figure 7 A 76-year-old male, diagnosed with prostate cancer for over 4 years and untreated, underwent intravenous injection of 68Ga-PSMA-11 followed by PET/CT tomographic imaging. The maximum intensity projection (MIP) image showed an elevated focal uptake in the nodular structure of the prostate (A). The tomographic images revealed a nodular increased PSMA expression focus in the right portion of the prostate parenchyma, with a slightly decreased density in the corresponding area. Nodular high-density shadows were observed within the parenchyma (B, C). These findings are consistent with the manifestation of prostate cancer.
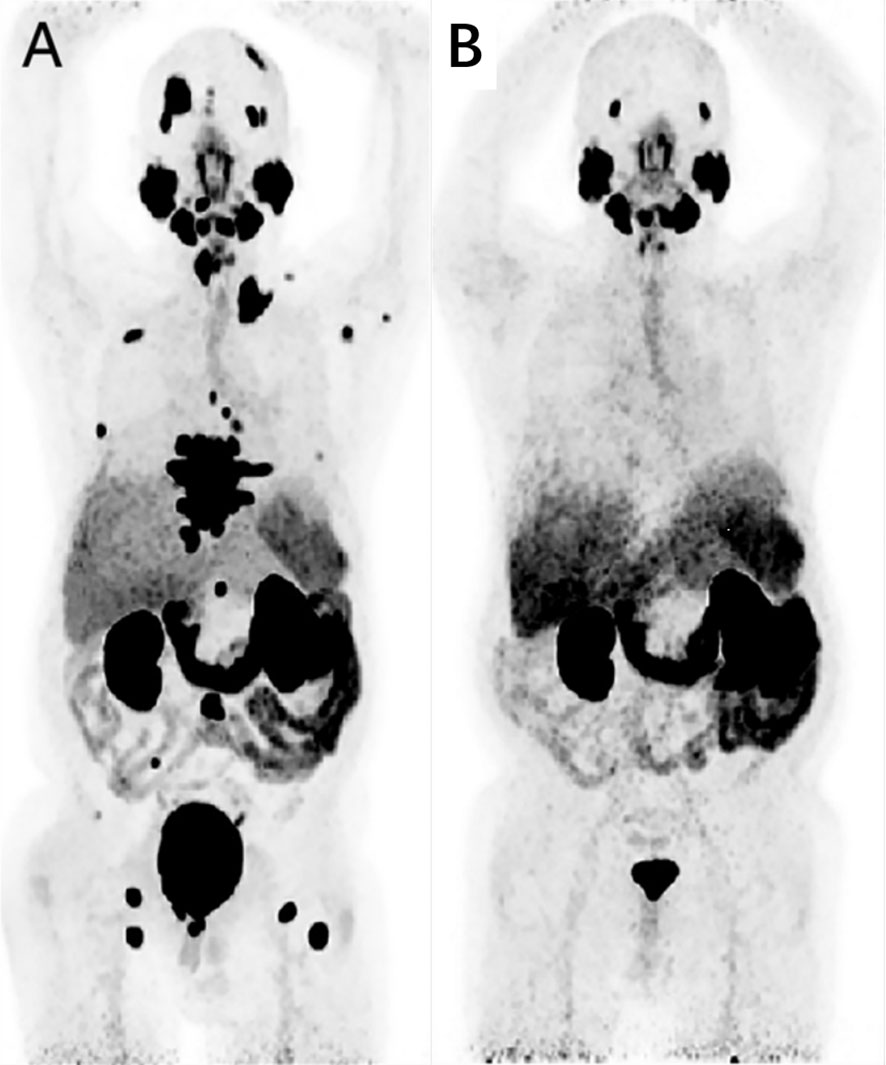
Figure 8 A 65-year-old man, diagnosed with prostate cancer 7 years ago during prostatectomy, experienced biochemical recurrence. A baseline 68Ga-PSMA-11 PET/CT imaging was conducted, and the maximum intensity projection (MIP) image revealed systemic PSMA metastases, particularly in bone metastatic lesions (A). Following 2 cycles of Lu-PSMA-617 treatment, a reassessment of treatment efficacy was performed using 68Ga-PSMA-11 imaging. The MIP image showed no tracer uptake in new lesions, and there was a significant reduction in tracer uptake overall (B). Adapted from Ref (81).
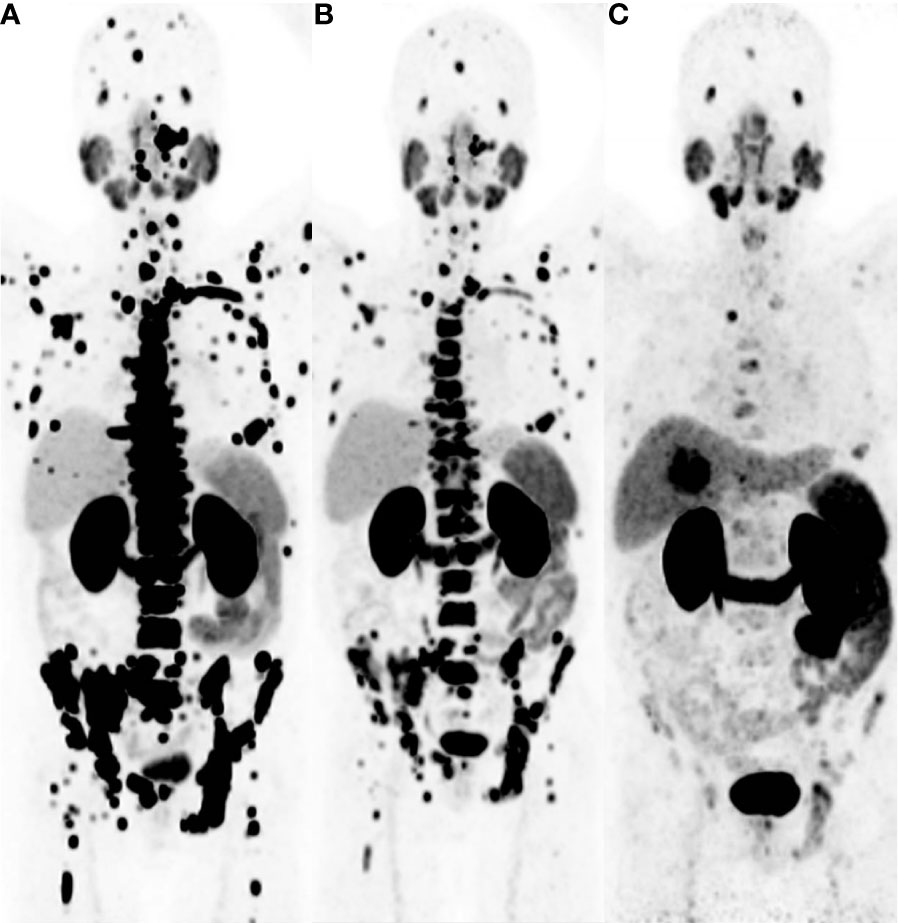
Figure 9 This 63-year-old male patient was diagnosed with prostate cancer 6 years ago and underwent a 68Ga-PSMA PET/CT imaging examination. The maximum intensity projection (MIP) image revealed a significant increase in tracer uptake in the skeletal region (A). The patient received 4 cycles of 225Ac-PSMA-617 therapy, and after 2 treatment cycles, subsequent 68Ga-PSMA PET/CT imaging still showed multiple areas of tracer uptake in the skeletal region, despite a notable reduction in SUVmax (B). A 68Ga-PSMA PET/CT imaging performed 16 weeks after the last treatment showed a substantial decrease in tracer uptake in the skeletal lesions (C). Adapted from Ref (82).
YY: Conceptualization, Writing – review & editing, Writing – original draft. ZH: Writing – review & editing, Visualization. LT: Writing – review & editing, Visualization. ZJ: Writing – review & editing, Visualization. TM: Writing – review & editing, Visualization. CY: Writing – review & editing, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors are grateful to the members of Department of Nuclear Medicine, The Affiliated Hospital, Southwest Medical University and Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province for their guidance, cooperation, and assistance in completing this review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
4. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. (2022) 72:409–36. doi: 10.3322/caac.21731
5. Fonti R, Conson M, Del Vecchio S. PET/CT in radiation oncology. Semin Oncol. (2019) 46:202–9. doi: 10.1053/j.seminoncol.2019.07.001
6. Tan H, Gu Y, Yu H, Hu P, Zhang Y, Mao W, et al. Total-body PET/CT: current applications and future perspectives. AJR Am J Roentgenol. (2020) 215:325–37. doi: 10.2214/AJR.19.22705
7. Jadvar H. Is there use for FDG-PET in prostate cancer? Semin Nucl Med. (2016) 46:502–6. doi: 10.1053/j.semnuclmed.2016.07.004
8. Shen K, Liu B, Zhou X, Ji Y, Chen L, Wang Q, et al. The evolving role of (18)F-FDG PET/CT in diagnosis and prognosis prediction in progressive prostate cancer. Front Oncol. (2021) 11:683793. doi: 10.3389/fonc.2021.683793
9. Lawal I, Sathekge M. F-18 FDG PET/CT imaging of cardiac and vascular inflammation and infection. Br Med Bull. (2016) 120:55–74. doi: 10.1093/bmb/ldw035
10. Pijl JP, Nienhuis PH, Kwee TC, Glaudemans A, Slart R, Gormsen LC. Limitations and pitfalls of FDG-PET/CT in infection and inflammation. Semin Nucl Med. (2021) 51:633–45. doi: 10.1053/j.semnuclmed.2021.06.008
11. Cai J, Xu W, Meng T, Pang Y, Chen H. Visualization of intermetastatic heterogeneity in mixed neuroendocrine carcinoma-acinar adenocarcinoma of the prostate by 68Ga-PSMA, 68Ga-FAPI, and 18F-FDG PET/CT. Clin Nucl Med. (2023) 48:743–5. doi: 10.1097/RLU.0000000000004719
12. Paymani Z, Rohringer T, Vali R, Loidl W, Alemohammad N, Geinitz H, et al. Diagnostic performance of [(18)F]Fluorocholine and [(68)Ga]Ga-PSMA PET/CT in prostate cancer: A comparative study. J Clin Med. (2020) 9(7):2308. doi: 10.3390/jcm9072308
13. Wang F, Li Z, Feng X, Yang D, Lin M. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. (2022) 25:11–26. doi: 10.1038/s41391-021-00394-5
14. Farolfi A, Calderoni L, Mattana F, Mei R, Telo S, Fanti S, et al. Current and emerging clinical applications of PSMA PET diagnostic imaging for prostate cancer. J Nucl Med. (2021) 62:596–604. doi: 10.2967/jnumed.120.257238
15. Schaeffer E, Srinivas S, Antonarakis ES, Armstrong AJ, Bekelman JE, Cheng H, et al. NCCN guidelines insights: prostate cancer, version 1.2021. J Natl Compr Canc Netw. (2021) 19:134–43. doi: 10.6004/jnccn.2021.0008
16. Hennrich U, Eder M. [(68)Ga]Ga-PSMA-11: the first FDA-approved (68)Ga-radiopharmaceutical for PET imaging of prostate cancer. Pharm (Basel). (2021) 14(8):713. doi: 10.3390/ph14080713
17. Sonni I, Eiber M, Fendler WP, Alano RM, Vangala SS, Kishan AU, et al. Impact of (68)Ga-PSMA-11 PET/CT on staging and management of prostate cancer patients in various clinical settings: A prospective single-center study. J Nucl Med. (2020) 61:1153–60. doi: 10.2967/jnumed.119.237602
18. Nazar AK, Kalshetty A, Chakravarty R, Chakraborty S, Basu S. Exploratory analysis of 64CuCl2 PET-CT imaging in carcinoma prostate and its comparison with 68Ga-PSMA-11 and 18F-FDG PET-CT. Nucl Med Commun. (2023) 44:910–23. doi: 10.1097/MNM.0000000000001744
19. Yu W, Zhao M, Deng Y, Liu S, Du G, Yan B, et al. Meta-analysis of (18) F-PSMA-1007 PET/CT, (18) F-FDG PET/CT, and (68)Ga-PSMA PET/CT in diagnostic efficacy of prostate Cancer. Cancer Imaging. (2023) 23:77. doi: 10.1186/s40644-023-00599-y
20. Yang J, Li J, Xiao L, Zhou M, Fang Z, Cai Y, et al. (68)Ga-PSMA PET/CT-based multivariate model for highly accurate and noninvasive diagnosis of clinically significant prostate cancer in the PSA gray zone. Cancer Imaging. (2023) 23:81. doi: 10.1186/s40644-023-00562-x
21. Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, et al. Diagnostic accuracy of 68Ga -PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: A multicenter prospective phase 3 imaging trial. JAMA Oncol. (2021) 7:1635–42. doi: 10.1001/jamaoncol.2021.3771
22. Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): A prospective multicentre study. Eur Urol. (2021) 80:682–9. doi: 10.1016/j.eururo.2021.08.002
23. Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga -PSMA-11 PET accuracy in localizing recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol. (2019) 5:856–63. doi: 10.1001/jamaoncol.2019.0096
24. Kendrick J, Francis RJ, Hassan GM, Rowshanfarzad P, Ong JSL, Barry N, et al. Quantitative [(68)Ga]Ga-PSMA-11 PET biomarkers for the analysis of lesion-level progression in biochemically recurrent prostate cancer: a multicentre study. Sci Rep. (2023) 13:17673. doi: 10.1038/s41598-023-45106-2
25. de Jong AC, Segbers M, Ling SW, Graven LH, Mehra N, Hamberg P, et al. (68)Ga-PSMA PET/CT for response evaluation of (223)Ra treatment in metastatic prostate cancer. J Nucl Med. (2023) 64:1556–62. doi: 10.2967/jnumed.123.265489
26. Denis CS, Cousin F, Laere B, Hustinx R, Sautois BR, Withofs N. Using (68)Ga-PSMA-11 PET/CT for therapy response assessment in patients with metastatic castration-resistant prostate cancer: application of EAU/EANM recommendations in clinical practice. J Nucl Med. (2022) 63:1815–21. doi: 10.2967/jnumed.121.263611
27. Ndum F, Seifert P, Freesmeyer M, Guhne F. Noninvasive verification of a very small intraocular prostate carcinoma metastasis by 68Ga -PSMA-11 PET/CT. Clin Nucl Med. (2023) 48:915–6. doi: 10.1097/RLU.0000000000004790
28. Zhao Q, Dong A, Zuo C. 68Ga -PSMA-11 PET/CT in a case of isolated parietal peritoneal metastasis from prostate adenocarcinoma. Clin Nucl Med. (2023) 48:913–4. doi: 10.1097/RLU.0000000000004805
29. Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. (2017) 44:678–88. doi: 10.1007/s00259-016-3573-4
30. Cardinale J, Schafer M, Benesova M, Bauder-Wust U, Leotta K, Eder M, et al. Preclinical evaluation of (18)F-PSMA-1007, a new prostate-specific membrane antigen ligand for prostate cancer imaging. J Nucl Med. (2017) 58:425–31. doi: 10.2967/jnumed.116.181768
31. Soydal C, Demir B, Sutcu G, Araz M, Kucuk NO. Comparison of (68)Ga-PSMA PET/CT and (18)F-PSMA PET/CT of a patient with prostate cancer recurrence on urinary bladder wall. Mol Imaging Radionucl Ther. (2023) 32:150–2. doi: 10.4274/Mirt
32. Lengana T, Lawal I, Janse Van Rensburg C, Mokoala K, Moshokoa E, Mazibuko S, et al. The Diagnostic Performance of 18F-PSMA-1007 PET/CT in Prostate Cancer Patients with Early Recurrence after Definitive Therapy with a PSA <10 ng/ml. Nuklearmedizin. (2022) 61:120–9. doi: 10.1055/a-1759-1603
33. Rassek P, Schafers M, Rahbar K, Backhaus P. [18F]-PSMA-1007-PET for evaluation of kidney function. Nuklearmedizin. (2023) 62:244–51. doi: 10.1055/a-2127-7880
34. Mojsak M, Szumowski P, Amelian A, Hladunski M, Kubas B, Mysliwiec J, et al. Application of 18F -PSMA-1007 PET/MR imaging in early biochemical recurrence of prostate cancer: results of a prospective study of 60 patients with very low PSA levels ≤ 0.5 ng/mL. Cancers (Basel). (2023) 15(16):4185. doi: 10.3390/cancers15164185
35. Janssen J, Noordzij W, Velleman T, de Jong IJ, Langendijk JA, Verzijlbergen JF, et al. Skeletal (18)F-PSMA-1007 uptake in prostate cancer patients. Ther Adv Med Oncol. (2023) 15:17588359231179311. doi: 10.1177/17588359231179311
36. Hartrampf PE, Huttmann T, Seitz AK, Kubler H, Serfling SE, Schlotelburg W, et al. SUV(mean) on baseline [(18)F]PSMA-1007 PET and clinical parameters are associated with survival in prostate cancer patients scheduled for [(177)Lu]Lu-PSMA I&T. Eur J Nucl Med Mol Imaging. (2023) 50:3465–74. doi: 10.1007/s00259-023-06281-6
37. Chen Y, Pullambhatla M, Foss CA, Byun Y, Nimmagadda S, Senthamizhchelvan S, et al. 2-(3-1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. (2011) 17:7645–53. doi: 10.1158/1078-0432.CCR-11-1357
38. FDA approves a second PSMA targeting agent for PET imaging in men with prostate cancer. BJU Int. (2021) 128:127–30. doi: 10.1111/bju.15538
39. Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic performance of (18)F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase III, multicenter study. Clin Cancer Res. (2021) 27:3674–82. doi: 10.1158/1078-0432.CCR-20-4573
40. Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with (18)F-DCFPyL in prostate cancer patients (OSPREY). J Urol. (2021) 206:52–61. doi: 10.1097/JU.0000000000001698
41. Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. (2015) 17:565–74. doi: 10.1007/s11307-015-0850-8
42. Oprea-Lager DE, Gontier E, Garcia-Canamaque L, Gauthe M, Olivier P, Mitjavila M, et al. [(18)F]DCFPyL PET/CT versus [(18)F]fluoromethylcholine PET/CT in Biochemical Recurrence of Prostate Cancer (PYTHON): a prospective, open label, cross-over, comparative study. Eur J Nucl Med Mol Imaging. (2023) 50:3439–51. doi: 10.1007/s00259-023-06301-5
43. Chen J, Russon A, Mansberg V, Mansberg R. Solitary prostate carcinoma penile metastasis on 18F -DCFPyL PET/CT. Clin Nucl Med. (2024) 49:78–80. doi: 10.1097/RLU.0000000000004926
44. Basso Dias A, Ghai S, Ortega C, Mirshahvalad SA, Perlis N, Berlin A, et al. Impact of 18F -DCFPyL PET/MRI in selecting men with low-/intermediate-risk prostate cancer for focal ablative therapies. Clin Nucl Med. (2023) 48:e462–e7. doi: 10.1097/RLU.0000000000004819
45. Song H, Harrison C, Duan H, Guja K, Hatami N, Franc BL, et al. Prospective evaluation of (18)F-DCFPyL PET/CT in biochemically recurrent prostate cancer in an academic center: A focus on disease localization and changes in management. J Nucl Med. (2020) 61:546–51. doi: 10.2967/jnumed.119.231654
46. Werner RA, Derlin T, Lapa C, Sheikbahaei S, Higuchi T, Giesel FL, et al. (18)F-labeled, PSMA-targeted radiotracers: leveraging the advantages of radiofluorination for prostate cancer molecular imaging. Theranostics. (2020) 10:1–16. doi: 10.7150/thno.37894
47. Sun M, Niaz MO, Nelson A, Skafida M, Niaz MJ. Review of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Cureus. (2020) 12:e8921. doi: 10.7759/cureus.8921
48. Benesova M, Schafer M, Bauder-Wust U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. (2015) 56:914–20. doi: 10.2967/jnumed.114.147413
49. Kratochwil C, Giesel FL, Eder M, Afshar-Oromieh A, Benesova M, Mier W, et al. [(1)(7)(7)Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. (2015) 42:987–8. doi: 10.1007/s00259-014-2978-1
50. Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. (2015) 56:1697–705. doi: 10.2967/jnumed.115.161299
51. Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. (2017) 58:85–90. doi: 10.2967/jnumed.116.183194
52. Hope TA. From compassionate use to phase 3 trial: the impact of Germany's PSMA-617 literature. J Nucl Med. (2020) 61:255S–6S. doi: 10.2967/jnumed.120.252122
53. Sanli Y, Simsek DH, Sanli O, Subramaniam RM, Kendi AT. 177Lu-PSMA therapy in metastatic castration-resistant prostate cancer. Biomedicines. (2021) 9(4):430. doi: 10.3390/biomedicines9040430
54. Satapathy S, Mittal BR, Sood A, Das CK, Mavuduru RS, Goyal S, et al. (177)Lu-PSMA-617 versus docetaxel in chemotherapy-naive metastatic castration-resistant prostate cancer: a randomized, controlled, phase 2 non-inferiority trial. Eur J Nucl Med Mol Imaging. (2022) 49:1754–64. doi: 10.1007/s00259-021-05618-3
55. Hennrich U, Eder M. [177Lu]Lu-PSMA-617 (PluvictoTM): the first FDA-approved radiotherapeutical for treatment of prostate cancer. Pharm (Basel). (2022) 15(10):1292. doi: 10.3390/ph15101292
56. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. (2021) 385:1091–103. doi: 10.1056/NEJMoa2107322
57. Kabasakal L, AbuQbeitah M, Aygun A, Yeyin N, Ocak M, Demirci E, et al. Pre-therapeutic dosimetry of normal organs and tissues of (177)Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. (2015) 42:1976–83. doi: 10.1007/s00259-015-3125-3
58. Das T, Guleria M, Parab A, Kale C, Shah H, Sarma HD, et al. Clinical translation of (177)Lu-labeled PSMA-617: Initial experience in prostate cancer patients. Nucl Med Biol. (2016) 43:296–302. doi: 10.1016/j.nucmedbio.2016.02.002
59. Heck MM, Retz M, D'Alessandria C, Rauscher I, Scheidhauer K, Maurer T, et al. Systemic radioligand therapy with (177)Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J Urol. (2016) 196:382–91. doi: 10.1016/j.juro.2016.02.2969
60. Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu -labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. (2015) 56:1169–76. doi: 10.2967/jnumed.115.158550
61. Neels OC, Kopka K, Liolios C, Afshar-Oromieh A. Radiolabeled PSMA inhibitors. Cancers (Basel). (2021) 13(24):6255. doi: 10.3390/cancers13246255
62. Ruigrok EAM, van Vliet N, Dalm SU, de Blois E, van Gent DC, Haeck J, et al. Extensive preclinical evaluation of lutetium-177-labeled PSMA-specific tracers for prostate cancer radionuclide therapy. Eur J Nucl Med Mol Imaging. (2021) 48:1339–50. doi: 10.1007/s00259-020-05057-6
63. Schuchardt C, Zhang J, Kulkarni HR, Chen X, Muller D, Baum RP. Prostate-specific membrane antigen radioligand therapy using (177)Lu-PSMA I&T and (177)Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: comparison of safety, biodistribution, and dosimetry. J Nucl Med. (2022) 63:1199–207. doi: 10.2967/jnumed.121.262713
64. Karimzadeh A, Heck M, Tauber R, Knorr K, Haller B, D'Alessandria C, et al. (177)Lu-PSMA-I&T for treatment of metastatic castration-resistant prostate cancer: prognostic value of scintigraphic and clinical biomarkers. J Nucl Med. (2023) 64:402–9. doi: 10.2967/jnumed.122.264402
65. Demirkol MO, Esen B, Seymen H, Sen M, Ucar B, Kurtuldu S, et al. Radioligand therapy with 177Lu -PSMA-I&T in patients with metastatic prostate cancer : oncological outcomes and toxicity profile. Clin Nucl Med. (2023) 48:e564–e9.
66. Cytawa W, Seitz AK, Kircher S, Fukushima K, Tran-Gia J, Schirbel A, et al. (68)Ga-PSMA I&T PET/CT for primary staging of prostate cancer. Eur J Nucl Med Mol Imaging. (2020) 47:168–77. doi: 10.1007/s00259-019-04524-z
67. Zacherl MJ, Gildehaus FJ, Mittlmeier L, Boning G, Gosewisch A, Wenter V, et al. First clinical results for PSMA-targeted alpha-therapy using (225)Ac-PSMA-I&T in advanced-mCRPC patients. J Nucl Med. (2021) 62:669–74. doi: 10.2967/jnumed.120.251017
68. Kostos L, Buteau JP, Yeung T, Iulio JD, Xie J, Cardin A, et al. AlphaBet: Combination of Radium-223 and 177Lu -PSMA-I&T in men with metastatic castration-resistant prostate cancer (clinical trial protocol). Front Med (Lausanne). (2022) 9:1059122. doi: 10.3389/fmed.2022.1059122
69. Feuerecker B, Kratochwil C, Ahmadzadehfar H, Morgenstern A, Eiber M, Herrmann K, et al. Clinical translation of targeted alpha-therapy: an evolution or a revolution? J Nucl Med. (2023) 64:685–92. doi: 10.2967/jnumed.122.265353
70. Yang H, Wilson JJ, Orvig C, Li Y, Wilbur DS, Ramogida CF, et al. Harnessing alpha-emitting radionuclides for therapy: radiolabeling method review. J Nucl Med. (2022) 63:5–13. doi: 10.2967/jnumed.121.262687
71. Pretze M, Kunkel F, Runge R, Freudenberg R, Braune A, Hartmann H, et al. Ac-EAZY! Towards GMP-compliant module syntheses of (225)Ac-labeled peptides for clinical application. Pharm (Basel). (2021) 14(7):652. doi: 10.3390/ph14070652
72. Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, et al. 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. (2016) 57:1941–4. doi: 10.2967/jnumed.116.178673
73. Sathekge MM, Lawal IO, Bal C, Bruchertseifer F, Ballal S, Cardaci G, et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): a multicentre, retrospective study. Lancet Oncol. (2024) 25(2):175–83. doi: 10.1016/S1470-2045(23)00638-1
74. Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C. Efficacy and safety of (225)Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics. (2020) 10:9364–77. doi: 10.7150/thno.48107
75. Alan-Selcuk N, Beydagi G, Demirci E, Ocak M, Celik S, Oven BB, et al. Clinical experience with [(225)Ac]Ac-PSMA treatment in patients with [(177)Lu]Lu-PSMA-refractory metastatic castration-resistant prostate cancer. J Nucl Med. (2023) 64:1574–80. doi: 10.2967/jnumed.123.265546
76. Sathekge M, Bruchertseifer F, Vorster M, Lawal IO, Mokoala K, Reed J, et al. (225)Ac-PSMA-617 radioligand therapy of de novo metastatic hormone-sensitive prostate carcinoma (mHSPC): preliminary clinical findings. Eur J Nucl Med Mol Imaging. (2023) 50:2210–8. doi: 10.1007/s00259-023-06165-9
77. Ballal S, Yadav MP, Satapathy S, Raju S, Tripathi M, Damle NA, et al. Long-term survival outcomes of salvage [(225)Ac]Ac-PSMA-617 targeted alpha therapy in patients with PSMA-expressing end-stage metastatic castration-resistant prostate cancer: a real-world study. Eur J Nucl Med Mol Imaging. (2023) 50:3777–89. doi: 10.1007/s00259-023-06340-y
78. Langbein T, Kulkarni HR, Schuchardt C, Mueller D, Volk GF, Baum RP. Salivary gland toxicity of PSMA-targeted radioligand therapy with 177Lu-PSMA and combined 225Ac- and 177Lu-labeled PSMA ligands (TANDEM-PRLT) in advanced prostate cancer: A single-center systematic investigation. Diagnostics (Basel). (2022) 12(8):1926. doi: 10.3390/diagnostics12081926
79. Satapathy S, Sharma A, Sood A, Maheshwari P, Gill HJS. Delayed nephrotoxicity after 225Ac -PSMA-617 radioligand therapy. Clin Nucl Med. (2022) 47:e466–e7. doi: 10.1097/RLU.0000000000004149
80. Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. (2013) 40:486–95. doi: 10.1007/s00259-012-2298-2
81. Qu G, Hua Q, Li H, Zhang Y, Chen Y. 177Lu-PSMA-617 therapy in a case of metastatic castration-resistant prostate cancer. Clin Nucl Med. (2024) 49:152–3. doi: 10.1097/RLU.0000000000005010
Keywords: PSMA, prostate cancer, mCRPC, radiolabeled, RLT, Tat, PET/CT
Citation: Yan Y, Zhuo H, Li T, Zhang J, Tan M and Chen Y (2024) Advancements in PSMA ligand radiolabeling for diagnosis and treatment of prostate cancer: a systematic review. Front. Oncol. 14:1373606. doi: 10.3389/fonc.2024.1373606
Received: 20 January 2024; Accepted: 08 March 2024;
Published: 21 March 2024.
Edited by:
Vikas Prasad, Radiology Mallinckrodt Institute of Radiology Division of Nuclear Medicine Washington University, United StatesReviewed by:
Xiaotian Xia, Huazhong University of Science and Technology, ChinaCopyright © 2024 Yan, Zhuo, Li, Zhang, Tan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Chen, Y2hlbnl1ZTU1MjNAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.