- 1Guangzhou Medical University, Guangzhou, Guangdong, China
- 2State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, Department of Pulmonary and Critical Care Medicine-Section 5, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
- 3Henan University, Kaifeng, Henan, China
Objective: To compare the prognostic differences between non-small cell lung cancer (NSCLC) patients with mild and severe checkpoint inhibitor-associated pneumonitis (CIP), and explore the causes of death and prognostic risk factors in NSCLC patients with severe CIP.
Methods: A retrospective study of a cohort of 116 patients with unresectable stage III or IV NSCLC with any grade CIP from April 2016 to August 2022 were conducted. To analyze the clinical characteristics of patients with different CIP grades, patients were divided into mild CIP group (grade 1-2, n=49) and severe CIP group (grade 3-5, n=67) according to the grade of CIP. To explore the OS-related risk factors in the severe CIP group, the patients were divided into a good prognosis (GP) group (≥ median OS, n=30) and a poor prognosis (PP) group (< median OS, n=37) based on whether their overall survival (OS) were greater than median OS. Baseline clinical and laboratory data were collected for analysis.
Results: The median OS of all NSCLC patients combined with CIP was 11.4 months (95%CI, 8.070–16.100), The median OS for mild CIP and severe CIP was 22.1 months and 4.4 months respectively (HR=3.076, 95%CI, 1.904-4.970, P<0.0001). The results showed that the most common cause of death among severe CIP patients in the PP group was CIP and the most common cause in the GP group was tumor. The univariate regression analysis showed that suspension of antitumor therapy was a risk factor for poor prognosis (OR=3.598, 95%CI, 1.307-9.905, p=0.013). The multivariate logistic regression analysis showed that suspension of anti-tumor therapy (OR=4.24, 95%CI, 1.067-16.915, p=0.040) and elevated KL-6 (OR=1.002, 95%CI, 1.001-1.002, p<0.001) were independent risk factors for poor prognosis.
Conclusion: In conclusion, patients with severe CIP had a poor prognosis, especially those with elevated KL-6, and the main cause of death is immune checkpoint inhibitor-associated pneumonitis complicated with infection. In addition, anti-tumor therapy for severe CIP patients should be resumed in time and should not be delayed for too long.
Introduction
Over the past decade, China has experienced the highest incidence and mortality rates of lung cancer globally, with non-small cell lung cancer (NSCLC) comprising approximately 80% of all diagnosed cases (1, 2). With the advancement of medical research, in addition to conventional chemotherapy, progress in cancer genomics has provided novel avenues for targeted therapy in lung cancer patients harboring driver gene mutations (3–7). However, for patients lacking driver gene mutations and those resistant to chemotherapy and targeted therapies, cancer immunology and immunotherapy offer a fresh perspective in cancer treatment. Use of immune checkpoint inhibitors (ICIs), including antibodies against programmed cell death protein 1 (PD-1) or its ligand PD-L1, has significantly improved overall survival (OS) in NSCLC (8). ICIs mainly mediates the destruction of cancer cells by activating the antitumor function of T cells; however, the deinhibition of T cell function by ICIs can lead to a series of organ-specific inflammatory side effects known as immune-related adverse events (irAEs). These irAEs result from unintended effects of the ICIs-mediated activation of the immune system and can occur in any organ system. Checkpoint inhibitor-associated pneumonitis (CIP) induced by ICIs is considered one of the more serious irAEs (9). Early data from clinical trials and other studies reported CIP in only 3% to 7% of patients, but more recently this phenomenon was reported to occur in nearly 20% of patients with NSCLC who received one or more of these agents outside of clinical trials (10). Therefore, clinicians need to pay more attention to this serious irAE. CIP has a more frequent occurrence and a faster rate of onset in NSCLC compared to other types of cancer (10). Therefore, clinicians need to pay more attention to this serious irAE.
The development of irAEs, including CIP, was considered a good predictive factor for the efficacy of ICIs treatment. Besides, patients who experienced an irAE had significantly longer progression free survival (PFS) and OS compared with those without irAEs (2, 11). However, some other research found that CIP was a serious complication with a poor prognosis in patients with NSCLC undergoing ICIs therapy, and the efficacy of ICIs was significantly worst in patients with severe CIP than in those without severe CIP (12). However, few articles have focused on the prognosis, cause of death, and survival risk factors of severe CIP. Understanding the prognosis of CIP can help us to better manage it.
Herein, the objective of this study is to compare the differences in prognosis between NSCLC patients with mild and severe CIP and to identify the related causes of death and influencing factors of poor prognosis in patients with severe CIP.
Materials and methods
Subjects
A retrospective study of a cohort of 116 patients with unresectable stage III or IV NSCLC with any grade CIP were conducted. All subjects were enrolled from the First Affiliated Hospital of Guangzhou Medical University for analysis from April 2016 to August 2022. To analyze the clinical characteristics of patients with different CIP grades, patients were divided into mild CIP group (grade 1-2, n=49) and severe CIP group (grade 3-5, n=67) according to the grade of CIP. To explore the OS-related risk factors in the severe CIP group, the patients were divided into a good prognosis (GP) group (≥ median OS, n=30) and a poor prognosis (PP) group (< median OS, n=37) based on whether their OS were greater than median OS. This study protocol was formulated in accordance with the requirements of the Declaration of Helsinki of the World Medical Association. This study was approved by the local Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (2005L01528).
Inclusion and exclusion criteria
Inclusion criteria: 1. Patients over 18 years old; 2. Patients with pathologically confirmed advanced (inoperable stage IIIB-IV) primary lung carcinoma with at least one measurable lesion that meets RECIST v1.1 criteria; 3. ICIs treatment was carried out in the clinical practice of the patient. 4. The diagnosis of CIP was established by a panel consisting of two seasoned pulmonologists and a chest radiologist, adhering to the standards outlined by the National Comprehensive Cancer Network, the American Society for Clinical Oncology, and the European Society for Medical Oncology (11–13).
Exclusion criteria: 1. Patients who have experienced tuberculosis, bacterial or fungal infection before ICI treatment (To exclude influence of these diseases on cytokines (14)); 2. Patients with incomplete clinical data. 3. Patients with clinical symptoms or diseases of the heart that are not well controlled, such as: a. NYHA grade 2 or higher heart failure; b. Unstable angina pectoris; c. Myocardial infarction within 1 year; d. Clinically significant supraventricular or ventricular arrhythmias requiring treatment or intervention.
ICIs treatment protocol
All ICIs therapeutic measurements shall apply the standard measurements specified in the National Comprehensive Cancer Network (NCCN) guidelines (15). Patients were given Nivolumab 3 mg/kg, Pembrolizumab 200 mg, Atezolizumab 1200 mg, or other programmed cell death protein-1 (PD-1)/PD-L1 inhibitors according to the requirements of clinical trials. Every 2 or 3 weeks until disease progression or unacceptable ICIs related toxicity was confirmed. All patients were treated with single ICIs.
Data collection
The medical records were reviewed, and the following basic characteristics of patients were collected: age, gender, body mass index (BMI), smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), treatment line, TNM stage, histologic classification, coexisting conditions, etc. The histologic classification of NSCLC was based on the World Health Organization criteria (2015 version) (16). The baseline time point was the time point at which CIP was diagnosed. The coexisting cardiovascular disease included specific diseases of hypertension, coronary artery disease and arrhythmia. The history of prior lung disease encompasses emphysema chronic obstructive pulmonary disease (COPD), obstructive pneumonitis and Interstitial lung disease.
We defined suspension of antitumor therapy as discontinuation of antitumor therapy after CIP occurred for more than 5 treatment cycles.
The laboratory data on the patient were also collected at the time of diagnosis of CIP, including interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor (TNF-α), interferon-gamma receptor (IFN -γ), high sensitivity C reactive protein (hsCRP), lactate dehydrogenase (LDH), albumin (ALB), neutrophil (NEUT), absolute lymphocyte count (ALC), platelet (PLT), neutrophil-to-lymphocyte (NLR), platelet lymphocyte ratio (PLR), D-Dimer and human sialylated carbohydrate antigen 6 (KL-6).
In addition, the specific treatment protocols and follow-up results of patients were also collected. The OS was determined from the date of confirmed CIP to death or last follow-up evaluation.
Evaluation and treatment of CIP
CIP was defined as the emergence of new infiltrates on thoracic imaging, accompanied by or without clinical manifestations such as cough, shortness of breath, or wheezing, all of which were deemed likely to be induced by ICIs. Other potential etiologies were thoroughly excluded from the diagnostic considerations. At the same time, a number of other causes, including lung infection or tumor progression, need to be ruled out by bronchoalveolar perfusion culture, sputum culture, echocardiography, and laboratory tests (routine blood tests, procalcitonin, tumor markers, arterial gas analysis, serous D-dimer, and brain natriuretic peptide, etc.) before diagnosis was made.
Causes of death
The causes of death of patients in this study were divided into the following three categories: 1. Death directly caused by CIP; 2. Death caused by tumors, such as tumor progression, tumor emergencies, or complications during tumor treatment; 3. Other reasons, such as other irAEs or other underlying diseases. The telephone follow-up was performed at bi-monthly intervals from the onset of CIP until loss to follow-up or death through August 2022.
Statistical analysis
The measurement data were expressed by medians and ranges. Associations between continuous variables were by the Wilcoxon-Mann-Whitney test or the Kruskal-Wallis test, when appropriate. Categorical variables will be compared by chi-square test (χ2) or Fisher’s exact test in terms of frequency, and described by frequency and percentage (%). Kaplan–Meier estimates and the log-rank test were used to evaluate the indicator of OS. In addition, the logistic regression models were performed to examine which risk factors were independently associated with OS. For each test, two-sided P values of <0.05 were considered statistically significant. All analyses were conducted using R Studio (4.2.2) and Jamovi (2.3.13). The statistical significance is set to 2 sides with p=0.05.
Results
Baseline clinical characteristics and comparison of OS between mild CIP group and severe CIP group
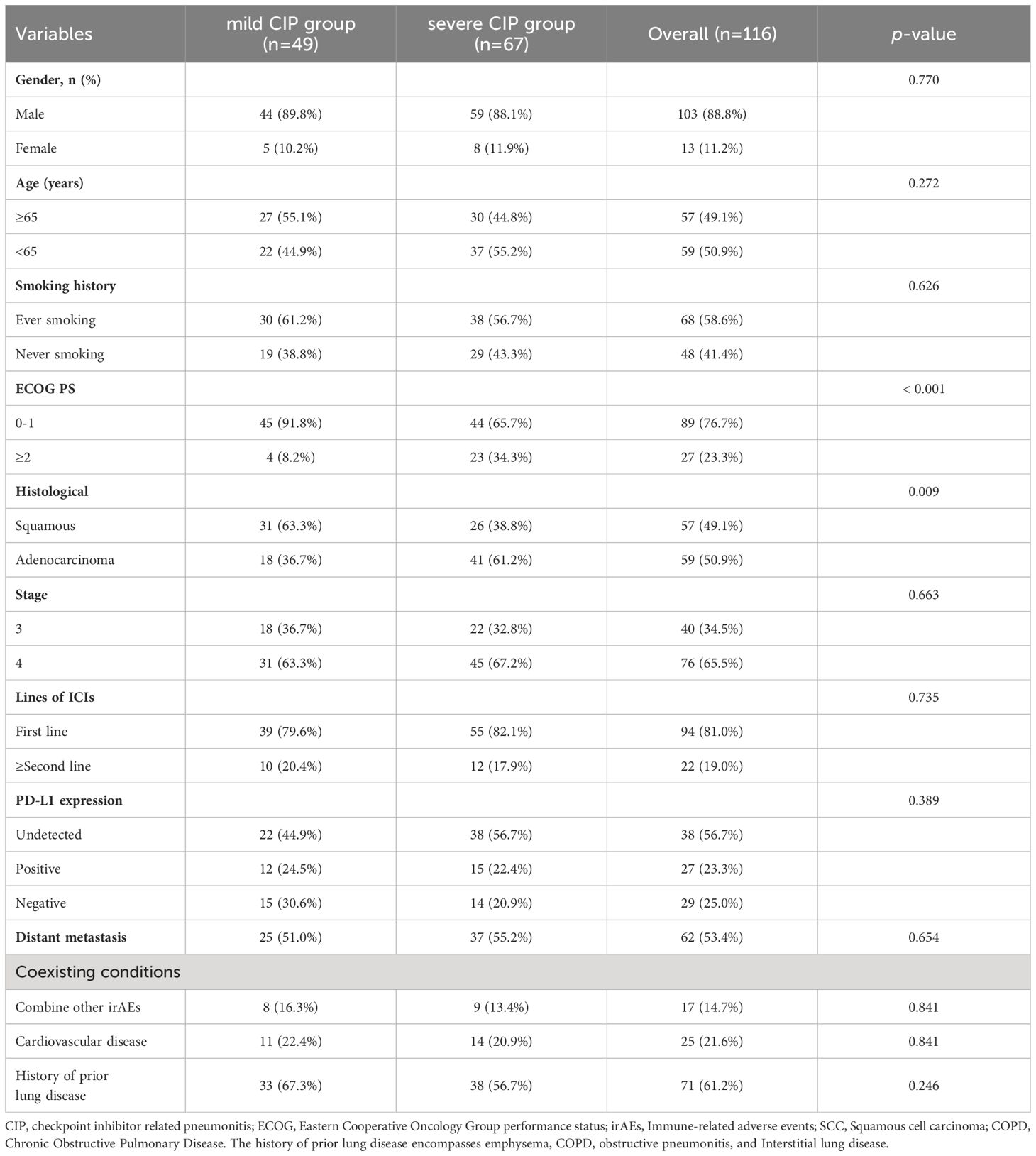
A total of 116 patients were included. The mild CIP group consisted of 49 patients with 44 males and 5 females. The severe CIP group consisted of 67 patients with 59 males and 8 females. In the severe CIP group, the proportion of ECOG score≥2 (p<0.001) and the proportion of NON-SCC (p=0.009) were significantly higher than those in the mild CIP group. Other baseline data were not significantly different between the two groups and were presented in Table 1. The median OS (mOS) of all NSCLC patients combined with CIP was 11.4 months (95%CI,8.070–16.100). The median OS for mild CIP and severe CIP was 22.1 months and 4.4 months respectively (HR=3.076, 95%CI, 1.904-4.970, p<0.0001). The data of OS was shown in Figure 1. Patients in mild CIP group had significantly longer OS than those in severe CIP group (p<0.0001).
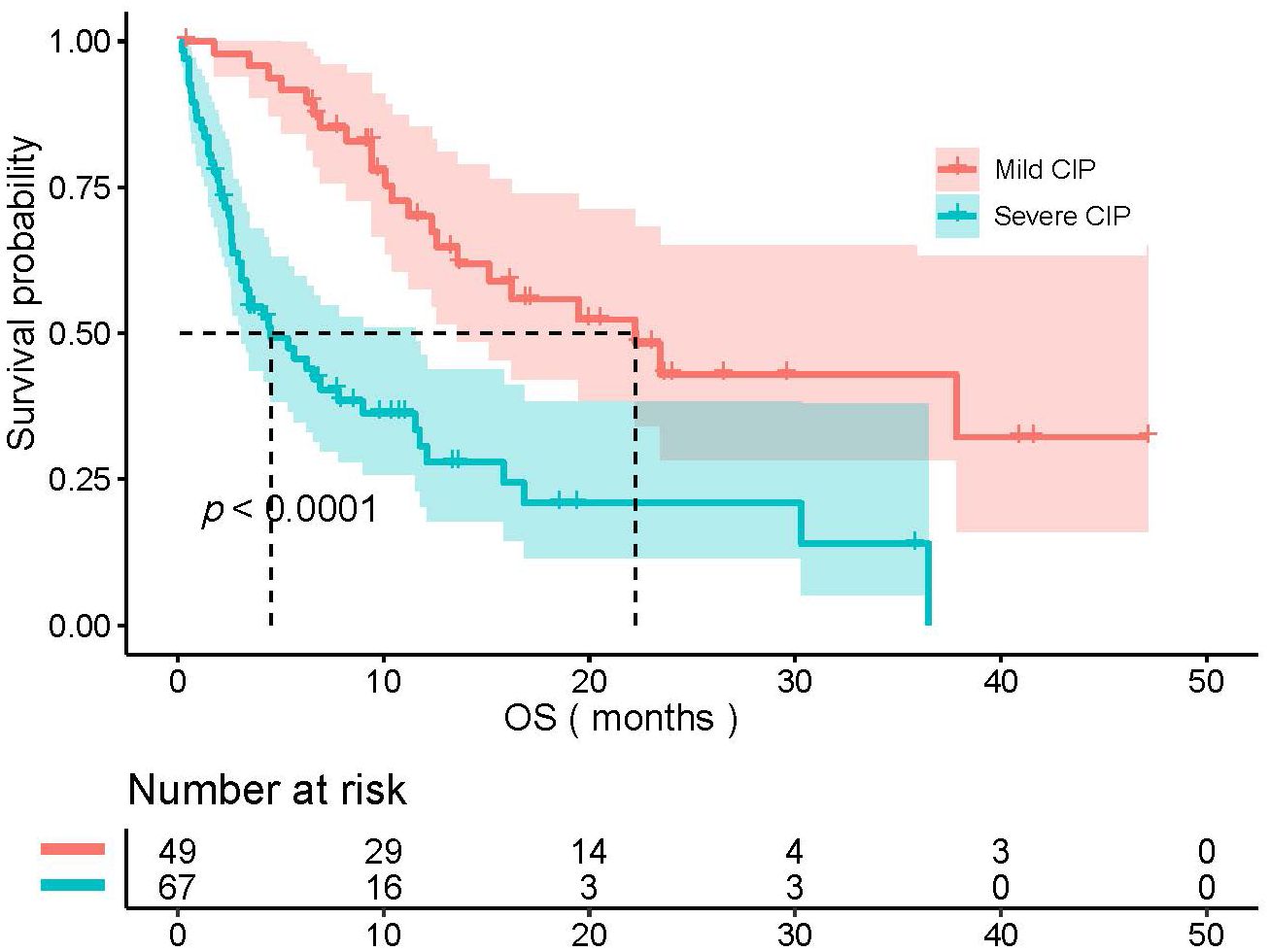
Figure 1 The comparison of OS between mild CIP group and severe CIP group. OS, overall survival; CIP, checkpoint inhibitor pneumonitis.
Clinical characteristics of patients in severe CIP group
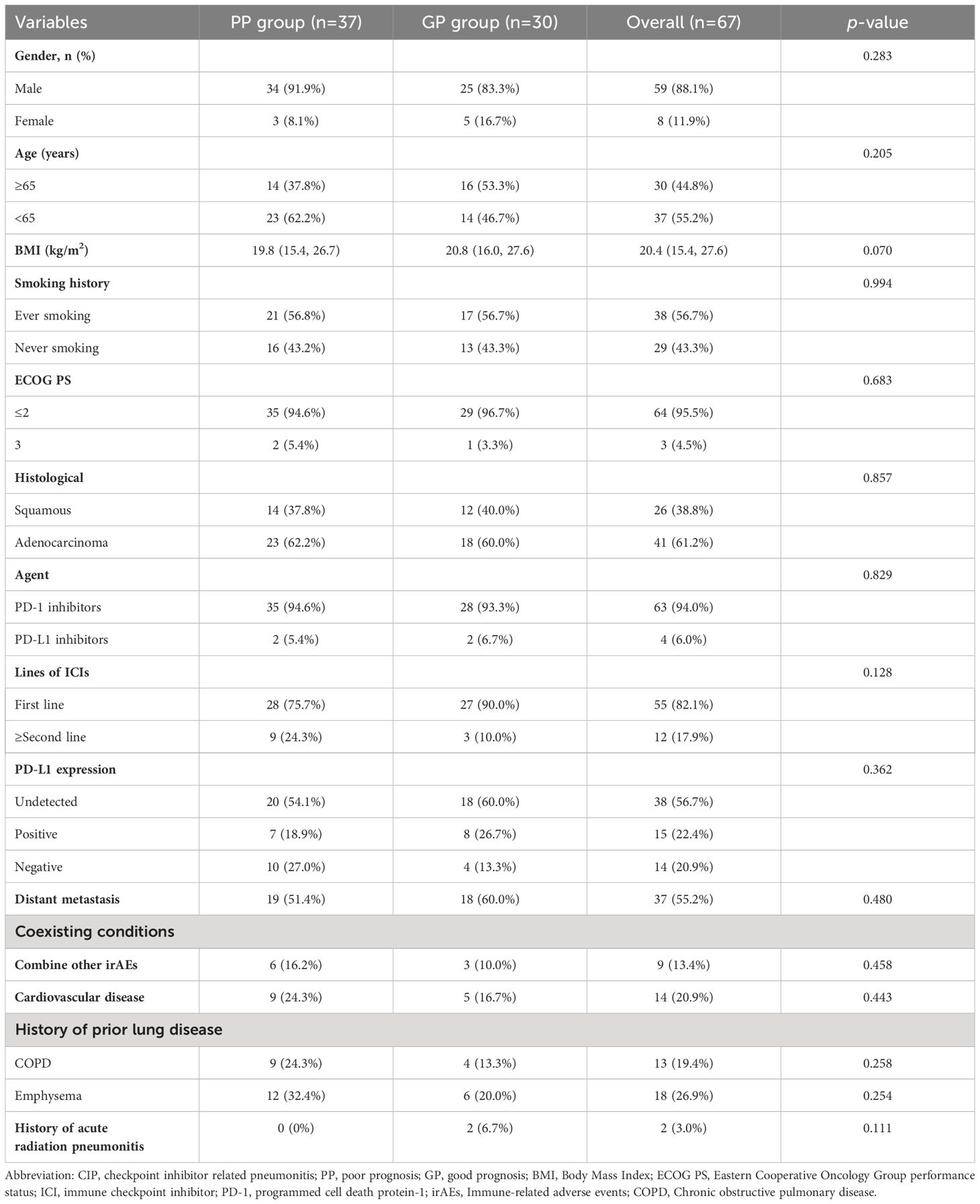
A subgroup analysis of the clinical characteristics of 67 patients in the severe CIP group in this section was further conducted. Based on whether the OS was greater than the median OS, patients in the severe CIP group were divided into the GP group (greater than the median OS) and the PP group (less than the median OS). The baseline clinical characteristics of the severe CIP group were presented in Table 2. In the severe CIP group, 59 (88.1%) patients were male, 38 (56.7%) had a history of smoking, 64 (95.5%) had an ECOG ≤ 2, and 41 (61.2%) had a histological type of lung adenocarcinoma. 55 (82.1%) patients received ICIs treatment at the time of first-line treatment, and 15 (22.4%) patients had positive PD-L1 expression. There were no significant differences in baseline clinical characteristics between the two groups except that the KL-6 levels exhibited a significant difference between the GP group and the PP group, with a statistical significance of P < 0.001. The laboratory results of the severe CIP group were presented in Table 3. There were no significant differences in laboratory data between the two groups (all p>0.05).
Causes of death in patients in severe CIP group
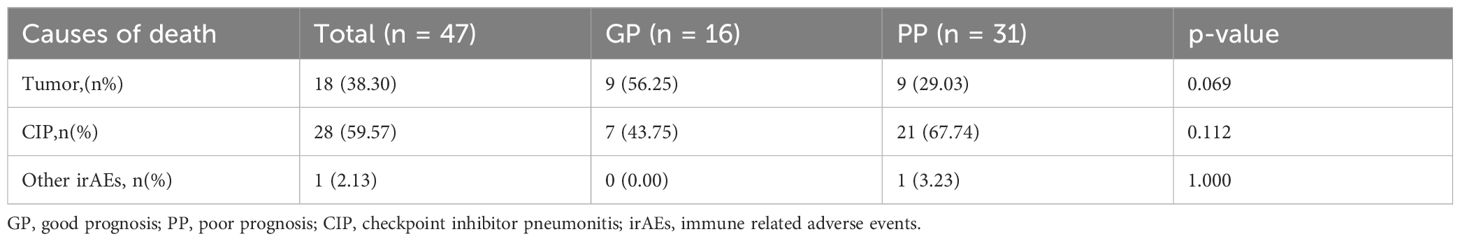
By the end of follow-up, a total of 47 patients in the severe CIP group had died. Among severe CIP patients, 28 (59.57%) died due to CIP, with 7 (43.75%) in the GP group and 21 (67.74%) in the PP group (P=0.112). Additionally, 18 (38.30%) patients died due to tumor, with 9 (56.25%) in the GP group and 9 (29.03%) in the PP group (p = 0.069). One patient (3.23%) in the PP group died from immune myocarditis. The chi-square test results for the analysis of causes of death in the severe group are shown in Table 4 and Figure 2. Swimmer plots shows the disease course of all severe immune checkpoint inhibitor related pneumonitis patients (Figure 3).
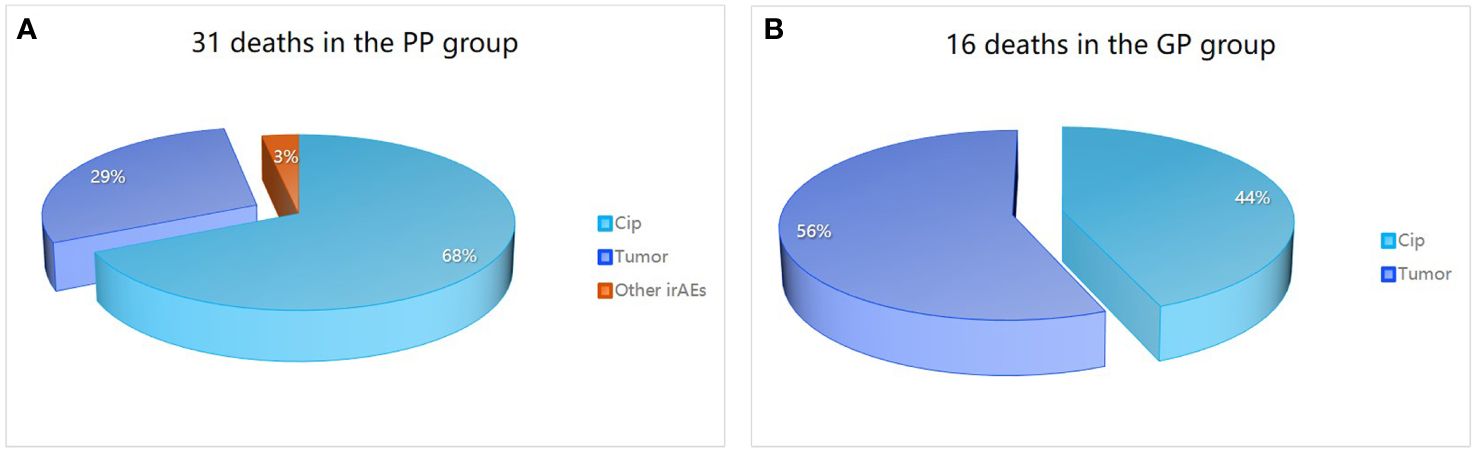
Figure 2 Causes of death in patients in severe CIP group. Causes of death in the PP group (A) is different from that in the GP group (B). PP, poor prognosis; GP, good prognosis; CIP, checkpoint inhibitor pneumonitis; irAEs, immune-related adverse events.

Figure 3 Swimmer plots of the disease course of severe immune checkpoint inhibitor related pneumonitis patients. In the GP group, the overall survival surpasses the median overall survival of 4.4 months observed among severe CIP. In the PP group, the overall survival is shorter than the median overall survival of 4.4 months observed among severe CIP. PP, poor prognosis; GP, good prognosis, CIP, checkpoint inhibitor pneumonitis.
Risk factors for poor prognosis in patients with severe CIP
The univariate and multivariate logistic regression analysis were performed to explore risk factors for poor outcomes in patients with severe CIP. In univariate regression analysis, the dependent variable was whether the patient was in the PP group, and the independent variable was the baseline clinical characteristics and laboratory data of the patient. Univariate regression analysis showed that suspension of antitumor therapy (OR=3.598, 95%CI, 1.307-9.905, p=0.013) and KL-6(OR=1.001, 95%CI, 1.001-1.002, p<0 .001) were risk factors for poor prognosis. And no other statistical difference was found in other independent variables (Table 5). According to the results of the literature review, we included the previously reported independent variables of high risk, such as IL-6, IL-10, LDH, KL-6, smoking history and suspension of anti-tumor therapy into the multivariate regression analysis (15, 17–20). Multivariate Logistic regression analysis showed that suspension of anti-tumor therapy (OR=4.247, 95%CI, 1.067-16.915, p=0.040) and KL-6 (OR=1.002,95%CI, 1.001-1.002, p<0 .001) were independent risk factors for poor prognosis (Table 6).
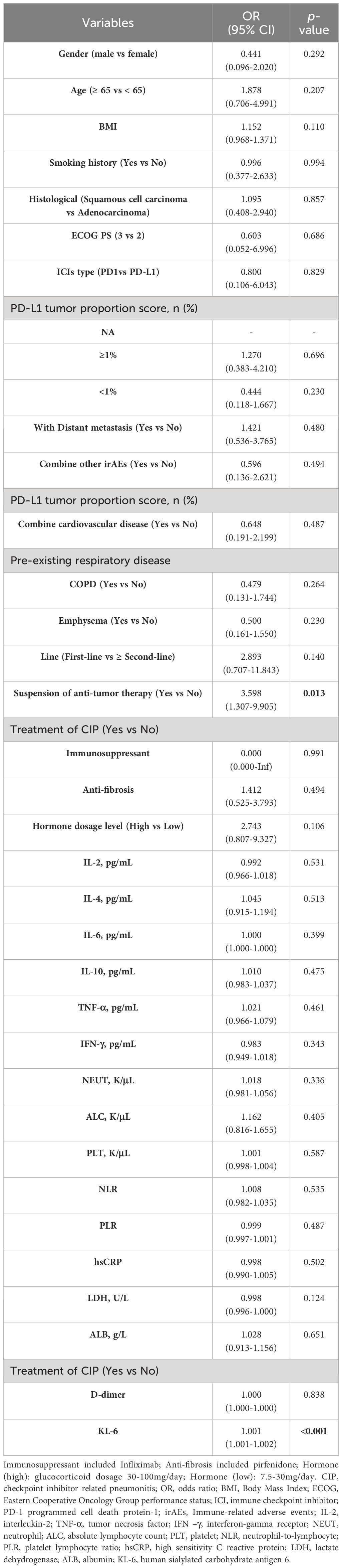
Table 5 The univariate logistic regression analysis of risk factors for poor prognosis in patients with severe CIP.
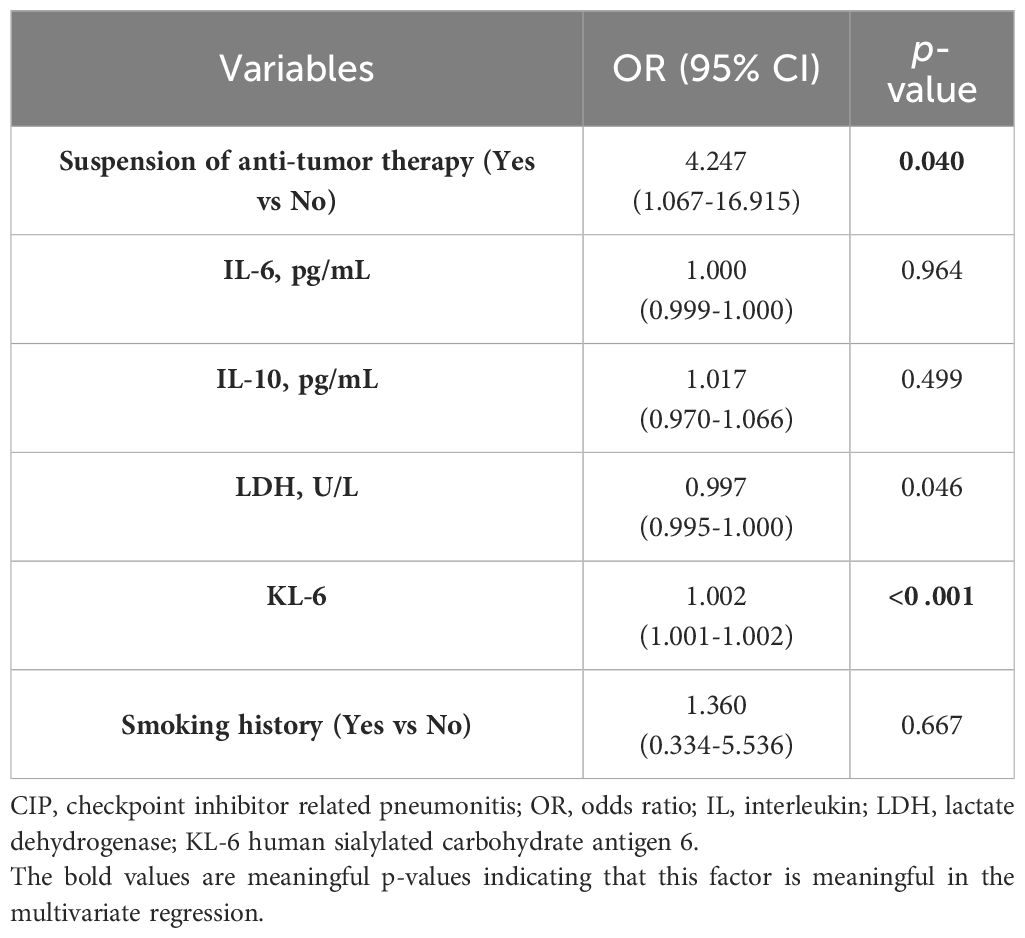
Table 6 The multivariate logistic regression analysis of risk factors for poor prognosis in patients with severe CIP.
Discussion
Accompanied by promising survival benefits, ICIs are associated with a broad spectrum of toxic effects known as irAEs, including rash, colitis, hepatitis, endocrinopathies, and pneumonitis. Another Meta-analysis revealed that both the overall incidence and the rate of severe CIP occurrence in lung cancer patients surpass those in patients with other malignancies (21, 22).
Most irAEs are mild and tolerable, some can be fatal. Severe pulmonary irAEs, notably CIP, are rare but carry a high mortality rate, often overlapping clinically and radiologically with respiratory symptoms of the primary tumor (23). Although many articles considered that the development of irAEs of any grade was significantly associated with better clinical outcomes in patients with advanced NSCLC treated with ICIs monotherapy (20, 24). However, the results of this study showed that patients with severe grade CIP had a worst prognosis than those with milder CIP, as in the previous article (9). ECOG PS scores in all patients who died due to CIP were two or worse. Moreover, many patients with severe CIP have pulmonary infections because of their poor pulmonary status may also be a risk factor for their poor prognosis.
The mortality rate of severe CIP patients in this study is relatively high. One reason is that our team started the CIP research earlier and did more research on it, with a special focus on treating immune-related adverse events, especially CIP, so we received more severe CIP patients at first diagnosis and referral. Another reason is that some patients also suffered from some basic diseases such as ILD and COPD. As a real-world study, the mortality rate is relatively high, similar to the mortality rates of 22.7% and 28% reported in two real-world studies.
Previous studies rarely reported the causes of death in patients with severe CIP. This study conducted an in-depth analysis of the patient population with severe CIP and found that The primary cause of death among all patients with severe CIP is CIP complicated with infection. However, the leading causes of mortality differ between the poorer prognosis group and the better prognosis group within the severe CIP cohort, with CIP complicated with infection being the primary cause in the PP group and tumor progression being the primary cause in the GP group. Based on this finding, we recommend further expanding the study sample to comprehensively investigate this phenomenon. This may be due to the fact that poorer prognosis group, coupled with severe infections, progresses rapidly. Although CIP was better controlled in the good prognosis group, the longer suspension of anti-tumor treatment resulted in difficult-to-control tumor progression, ultimately leading to more deaths from tumor progression. The clinicians' tendency to prioritize the treatment of CIP while overlooking antitumor therapy could be the reason behind this. Therefore, it was necessary to give patients timely anti-tumor therapy, such as continuing chemotherapy regimen or increasing anti-vascular therapy. It is also crucial to note the importance of promptly controlling infections associated with CIP.
In addition, this study showed that elevated KL-6 concentrations predicted a poor prognosis in patients with CIP. KL-6 is a high molecular weight glycoprotein encoded by Mucin gene, which is mainly distributed on the cell surface of type II alveolar epithelial cells (AECs), and the elevated KL-6 concentrations typically disrupt alveolar capillaries and type II AECs regeneration (25, 26). Articles have been reported the elevated KL-6 level indicated more severe, more progressive, and predicted the higher mortality and poor outcomes of ILD (Interstitial lung disease) (20, 24). CIP is closely associated with interstitial pneumonia (27). ICIs-associated lung injury manifests in a variety of forms, including interstitial pneumonia.
Due to insufficient preclinical research, understanding the mechanisms of CIP remains an area requiring further exploration. CIP in non-small cell lung cancer results from various contributing factors. Studies indicate that PD-1/PD-L1 inhibitors can boost T cell anti-tumor activity. Activated T cells infiltrate lung tissues in CIP patients, signaling increased anti-tumor effects. However, excessive immune responses can harm normal tissues (28–31). Past research indicates a possible link between CIP development and heightened levels of pre-existing and newly appearing autoantibodies in human immunity. Pre-existing antibodies like rheumatoid factor (RF), antinuclear antibodies, anti-thyroglobulin, and anti-thyroid peroxidase antibodies are independently associated with irAE occurrence in various organs (32, 33). Salahaldin A. Tahir and colleagues additionally discovered a significant 1.34-fold increase in autoantibodies against CD74 in patients with immune-related pneumonia after receiving immune checkpoint inhibitor therapy, suggesting a role for CD74 autoantibodies in pneumonia (34). Some research indicates that the pathophysiology of irAEs may involve cytokine mediation. In a study with melanoma patients, certain cytokines like G-CSF, GM-CSF, Fractalkine, FGF-2, IFNα2, IL-12p70, IL-1α, IL1, IL-1RA, IL-2, and IL-13 showed significant upregulation at baseline and early treatment stages, correlating with high-grade irAE occurrence (35)In a study of NSCLC patients receiving ICIs treatment, interleukin-6 (IL-6), IL-17A, IL-35, C-reactive protein (CRP), procalcitonin (PCT), surfactant protein-D (SP-D), and Krebs von den Lungen-6 (KL-6) were more frequently observed in patients with CIP compared to those without CIP (36). Several other potential mechanisms remain to be explored, with some studies suggesting that the modulation of the gut microbiome may be associated with the efficacy and toxicity of immunotherapy (37, 38) Hakozaki and colleagues noted notable distinctions in the gut microbiomes of late-stage NSCLC patients who developed low-grade versus high-grade irAEs (39). Further exploration is warranted to elucidate the involvement of non-coding RNAs, such as microRNA-146a, in regulating irAEs (40). The associated mechanisms of severe CIP and its relationship with outcomes necessitate additional investigation.
There were some limitations to our study. First, this was a single-center retrospective study with a limited sample size, so information bias cannot be ruled out. Another limitation was that we did not exclude differences in the tumor histological type and ECOG score when comparing OS in the severe CIP group and mild CIP groups, which may affect the validity of the final results. Nevertheless, we reported a strong relationship between CIP grade and prognosis, associated risk factors, and leading causes of death, which have some clinical significance. In follow-up studies, we will conduct a prospective study with a large sample size to explore prognostic risk factors in patients with CIP.
Conclusions
In conclusion, patients with severe CIP have a poor prognosis, especially those with elevated KL-6, and the main cause of death is immune checkpoint inhibitor-associated pneumonitis complicated with infection. In addition, anti-tumor therapy for severe CIP patients should be resumed in time and should not be delayed for too long.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the local Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NS: Data curation, Formal analysis, Investigation, Writing – original draft. RL: Data curation, Formal analysis, Investigation, Writing – original draft. HD: Data curation, Formal analysis, Investigation, Writing – original draft. QL: Software, Writing – original draft. JD: Supervision, Writing – original draft. YZ: Validation, Writing – original draft. WM: Visualization, Writing – original draft. WG: Investigation, Writing – original draft. MH: Data curation, Writing – original draft. ML: Software, Writing – original draft. XX: Visualization, Writing – original draft. XL: Conceptualization, Methodology, Project administration, Writing – review & editing. CZ: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. It is fund by Guangzhou Science and Technology Major Clinical Project(2023C-DZ06).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
2. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
3. Denisenko TV, Budkevich IN, Zhivotovsky B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. (2018) 9:117. doi: 10.1038/s41419-017-0063-y
4. Joseph NA, Chiou SH, Lung Z, Yang CL, Lin TY, Chang HW, et al. The role of HGF-MET pathway and CCDC66 cirRNA expression in EGFR resistance and epithelial-to-mesenchymal transition of lung adenocarcinoma cells. J Hematol Oncol. (2018) 11:74. doi: 10.1186/s13045-018-0557-9
5. Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer. (2018) 17:53. doi: 10.1186/s12943-018-0793-1
6. Chen R, Manochakian R, James L, Azzouqa AG, Shi H, Zhang Y, et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol. (2020) 13:58. doi: 10.1186/s13045-020-00881-7
7. Wang Q, Yang S, Wang K, Sun SY. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J Hematol Oncol. (2019) 12:63. doi: 10.1186/s13045-019-0759-9
8. Toi Y, Sugawara S, Kawashima Y, Aiba T, Kawana S, Saito R, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. (2018) 23:1358–65. doi: 10.1634/theoncologist.2017-0384
9. Tone M, Izumo T, Awano N, Kuse N, Inomata M, Jo T, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer. (2019) 10:2006–12. doi: 10.1111/1759-7714.13187
10. Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for Malignancies: A meta-analysis. Front Pharmacol. (2017) 8:730. doi: 10.3389/fphar.2017.00730
11. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. (2017) 5:95. doi: 10.1186/s40425-017-0300-z
12. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
13. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:iv264–6. doi: 10.1093/annonc/mdy162
14. Tjokrowidjaja A, Lord SJ, John T, Lewis CR, Kok PS, Marschner IC, et al. Pre- and on-treatment lactate dehydrogenase as a prognostic and predictive biomarker in advanced non-small cell lung cancer. Cancer. (2022) 128:1574–83. doi: 10.1002/cncr.34113
15. Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. (2021) 19:254–66. doi: 10.6004/jnccn.2021.0013
16. Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-related adverse events (irAEs): diagnosis, management, and clinical pearls. Curr Oncol Rep. (2020) 22:39. doi: 10.1007/s11912-020-0897-9
17. Lin X, Deng H, Yang Y, Wu J, Qiu G, Li S, et al. Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor-related pneumonitis in patients with lung cancer. Front Oncol. (2021) 11:698832. doi: 10.3389/fonc.2021.698832
18. Sung WW, Wang YC, Lin PL, Cheng YW, Chen CY, Wu TC, et al. IL-10 promotes tumor aggressiveness via upregulation of CIP2A transcription in lung adenocarcinoma. Clin Cancer Res. (2013) 19:4092–103. doi: 10.1158/1078-0432.CCR-12-3439
19. Chen J, Gao X, Shen S, Xu J, Sun Z, Lin R, et al. Association of longitudinal platelet count trajectory with ICU mortality: A multi-cohort study. Front Immunol. (2022) 13:936662. doi: 10.3389/fimmu.2022.936662
20. Zhang T, Shen P, Duan C, Gao L. KL-6 as an immunological biomarker predicts the severity, progression, acute exacerbation, and poor outcomes of interstitial lung disease: A systematic review and meta-analysis. Front Immunol. (2021) 12:745233. doi: 10.3389/fimmu.2021.745233
21. Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: A meta-analysis. Front Pharmacol. (2018) 9:1430. doi: 10.3389/fphar.2018.01430
22. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.2453
23. Yin J, Wu Y, Yang X, Gan L, Xue J. Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy in non-small-cell lung cancer: occurrence and mechanism. Front Immunol. (2022) 13:830631. doi: 10.3389/fimmu.2022.830631
24. Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. (2006) 11:164–8. doi: 10.1111/j.1440-1843.2006.00834.x
25. Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. (2012) 50:3–13. doi: 10.1016/j.resinv.2012.02.001
26. Kobayashi J, Kitamura S. KL-6: a serum marker for interstitial pneumonia. Chest. (1995) 108:311–5. doi: 10.1378/chest.108.2.311
27. Huang A, Xu Y, Zang X, Wu C, Gao J, Sun X, et al. Radiographic features and prognosis of early- and late-onset non-small cell lung cancer immune checkpoint inhibitor-related pneumonitis. BMC Cancer. (2021) 21:634. doi: 10.1186/s12885-021-08353-y
28. Ivanova EA, Orekhov AN. T helper lymphocyte subsets and plasticity in autoimmunity and cancer: an overview. BioMed Res Int. (2015) 2015:327470. doi: 10.1155/2015/327470
29. Naidoo J, Cottrell TR, Lipson EJ, Forde PM, Illei PB, Yarmus LB, et al. Chronic immune checkpoint inhibitor pneumonitis. J Immunother Cancer. (2020) 8:e000840. doi: 10.1136/jitc-2020-000840
30. Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest. (2019) 129:4305–15. doi: 10.1172/JCI128654
31. Suzuki K, Yanagihara T, Matsumoto K, Kusaba H, Yamauchi T, Ikematsu Y, et al. Immune-checkpoint profiles for T cells in bronchoalveolar lavage fluid of patients with immune-checkpoint inhibitor-related interstitial lung disease. Int Immunol. (2020) 32:547–57. doi: 10.1093/intimm/dxaa022
32. Kong YC, Flynn JC. Opportunistic autoimmune disorders potentiated by immune-checkpoint inhibitors anti-CTLA-4 and anti-PD-1. Front Immunol. (2014) 5:206. doi: 10.3389/fimmu.2014.00206
33. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. (2003) 9:1477–83. doi: 10.1038/nm955
34. Tahir SA, Gao J, Miura Y, Blando J, Tidwell RSS, Zhao H, et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc Natl Acad Sci U S A. (2019) 116:22246–51. doi: 10.1073/pnas.1908079116
35. Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res. (2019) 25:1557–63. doi: 10.1158/1078-0432.CCR-18-2795
36. Zhang Q, Tang L, Zhou Y, He W, Li W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: current understanding in characteristics, diagnosis, and management. Front Immunol. (2021) 12:663986. doi: 10.3389/fimmu.2021.663986
37. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. (2017) 28:1368–79. doi: 10.1093/annonc/mdx108
38. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
39. Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaïfaoui M, Mimpen I, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. (2020) 8:1243–50. doi: 10.1158/2326-6066.CIR-20-0196
Keywords: immune checkpoint inhibitor, pneumonitis, non-small cell lung cancer, prognosis, cause of death
Citation: Sun N, Li R, Deng H, Li Q, Deng J, Zhu Y, Mo W, Guan W, Hu M, Liu M, Xie X, Lin X and Zhou C (2024) The prognostic impact of severe grade immune checkpoint inhibitor related pneumonitis in non-small cell lung cancer patients. Front. Oncol. 14:1372532. doi: 10.3389/fonc.2024.1372532
Received: 18 January 2024; Accepted: 17 May 2024;
Published: 25 June 2024.
Edited by:
Jamie S. Lin, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Haitao Tao, People’s Liberation Army General Hospital, ChinaXinpei Deng, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2024 Sun, Li, Deng, Li, Deng, Zhu, Mo, Guan, Hu, Liu, Xie, Lin and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengzhi Zhou, ZG9jdG9yemN6QDE2My5jb20=; Xinqing Lin, bGlueGlucWluZzgxQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Ni Sun1,2†
Ni Sun1,2† Chengzhi Zhou
Chengzhi Zhou