- 1National Cancer Control Program, Ministry of Health, Nairobi, Kenya
- 2Field Epidemiology and Laboratory Program, Ministry of Health, Nairobi, Kenya
- 3Noncommunicable Disease Program, Clinton Health Access Initiative, Nairobi, Kenya
- 4Unit of Cancer Epidemiology, Belgian Cancer Centre, Sciensano, Brussels, Belgium
- 5Department of Human Structure and Repair, Ghent University Hospital, Ghent, Belgium
- 6Cancer Research Institute Ghent (CRIG), Ghent, Belgium
- 7Department of Obstetrics and Gynaecology, Ghent University Hospital, Ghent, Belgium
- 8Department of Obstetrics and Gynaecology, Aga Khan University, Nairobi, Kenya
Background: Cervical cancer is the leading cause of cancer deaths among women in Kenya. In the context of the Global strategy to accelerate the elimination of cervical cancer as a public health problem, Kenya is currently implementing screening and treatment scale-up. For effectively tracking the scale-up, a baseline assessment of cervical cancer screening and treatment service availability and readiness was conducted in 25 priority counties. We describe the findings of this assessment in the context of elimination efforts in Kenya.
Methods: The survey was conducted from February 2021 to January 2022. All public hospitals in the target counties were included. We utilized healthcare workers trained in preparation for the scale-up as data collectors in each sub-county. Two electronic survey questionnaires (screening and treatment; and laboratory components) were used for data collection. All the health system building blocks were assessed. We used descriptive statistics to summarize the main service readiness indicators.
Results: Of 3,150 hospitals surveyed, 47.6% (1,499) offered cervical cancer screening only, while 5.3% (166) offered both screening and treatment for precancer lesions. Visual inspection with acetic acid (VIA) was used in 96.0% (1,599/1,665) of the hospitals as primary screening modality and HPV testing was available in 31 (1.0%) hospitals. Among the 166 hospitals offering treatment for precancerous lesions, 79.5% (132/166) used cryotherapy, 18.7% (31/166) performed thermal ablation and 25.3% (42/166) performed large loop excision of the transformation zone (LLETZ). Pathology services were offered in only 7.1% (17/238) of the hospitals expected to have the service (level 4 and above). Only 10.8% (2,955/27,363) of healthcare workers were trained in cervical cancer screening and treatment; of these, 71.0% (2,097/2,955) were offering the services. Less than half of the hospitals had cervical cancer screening and treatment commodities at time of survey. The main health system strength was presence of multiple screening points at hospitals, but frequent commodity stock-outs was a key weakness.
Conclusion: Training, commodities, and diagnostic services are major gaps in the cervical cancer program in Kenya. To meet the 2030 elimination targets, the national and county governments should ensure adequate financing, training, and service integration, especially at primary care level.
Introduction
Cervical cancer is the second leading cause of cancer incidence and the leading cause of cancer deaths among women in Sub-Saharan Africa (SSA). In 2020, an estimated 117,316 cases of cervical cancer were diagnosed in Africa, and more than 76,000 women died from the disease in the continent, representing 22% of global deaths from cervical cancer (1). Majority of cervical cancer deaths occur among socio-economically disadvantaged women, especially those with poor access to quality health services (2, 3). While cervical cancer deaths continue falling in countries with organized screening programs and high human papillomavirus (HPV) vaccination coverage, the burden in SSA is increasing (4). In Kenya, cervical cancer is the leading cause of cancer deaths, with approximately 3,200 deaths reported in 2020 (1).
Cervical cancer has very effective modalities for screening, early diagnosis and treatment (5). To reduce the global burden of disease from cervical cancer, the World Health Organization (WHO) launched the Global strategy to accelerate the elimination of cervical cancer as a public health problem in 2020 (6). This strategy identifies key interventions and targets for countries globally by 2030: vaccination against HPV, screening with a high precision test and linkage to treatment. However, innovative strategies and collaborations are necessary to address low HPV vaccination coverage, low screening uptake and high loss to follow-up from screening programs, if low and middle-income countries are to move towards cervical cancer elimination (7). Health system strengthening and effective organization of cervical cancer screening programs have been identified as critical ingredients for success (8). Unfortunately, majority of SSA countries have not implemented and/or sustained high quality cervical cancer screening programs, due to health system deficiencies as well as socio-cultural influences (3, 9–11).
Cervical cancer screening coverage in Kenya was estimated at 16% in 2015 (12). One possible explanation for this low coverage is service availability; only a quarter of hospitals were offering cervical cancer screening services in 2018 (13). In order to move towards cervical cancer elimination, Kenya is implementing a national cervical cancer screening and treatment scale-up, targeting 25 priority counties since 2021. The scale-up involves healthcare workers training, supply of screening and treatment commodities and equipment as well as setting-up governance and coordination structures for the national cervical cancer program. Before the scale-up was launched, a baseline assessment of the cervical cancer screening and treatment service readiness was conducted in the 25 focus counties. The main objective of the baseline assessment was to provide an objective situational analysis of the national cervical cancer program, inform the planning of the scale-up and provide a basis for evaluating future successes of the targeted health system interventions. We present the findings from this assessment and its implications for cervical cancer elimination efforts in Kenya.
Methods
Study design and population
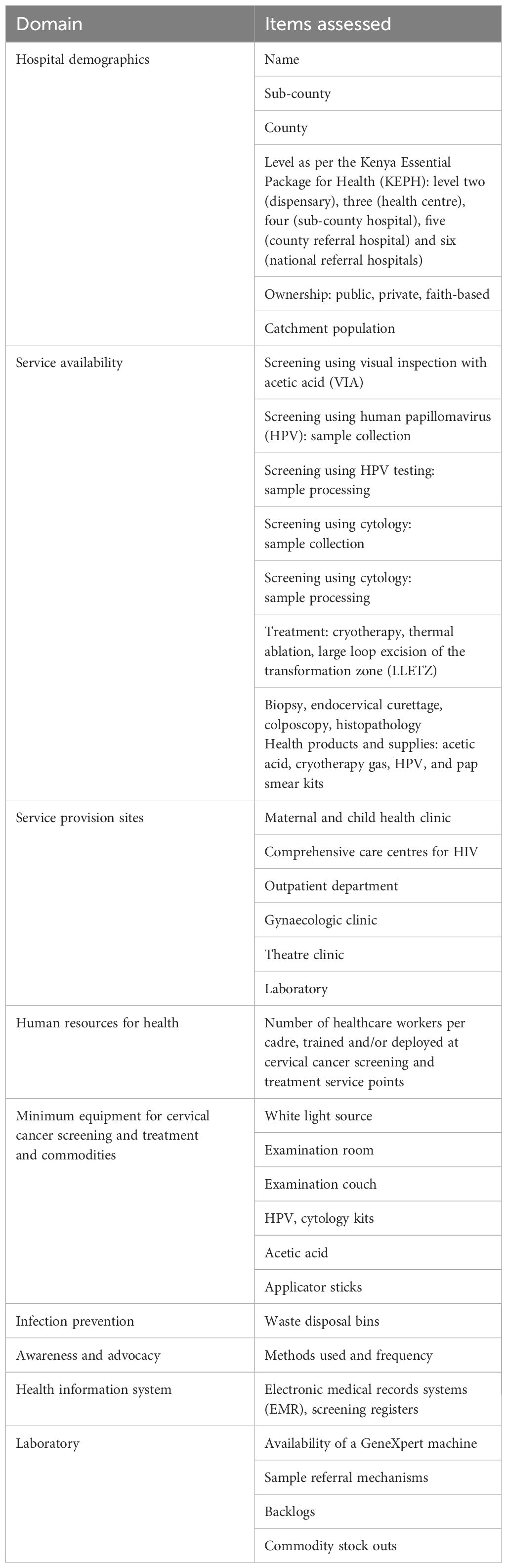
This was a cross-sectional survey, conducted in 25 of the 47 counties in Kenya, which were earmarked for the first phase of the national scale-up of cervical cancer screening and treatment. The counties were selected on the basis of HIV burden, regional representation, and sites where a previous pilot on cervical cancer screening scale-up had been carried out. The assessment was carried out over 12 months, from February 2021 to January 2022. The study population was hospitals, from level two (dispensaries) to level six (national referral hospitals) in the target counties. Screening using visual inspection with acetic acid (VIA), HPV sample collection, cryotherapy and thermal ablation are the modalities expected at level two and three hospitals; additional services like large loop excision of the transformation zone (LLETZ), HPV and cytology sample processing, biopsy and histology are expected from level four and above. All eligible hospitals in the selected counties were assessed. Two critical areas for cervical cancer screening programs were assessed in the hospitals: the screening service points and the laboratory. The specific areas assessed are shown in Table 1.
Survey procedures
Two healthcare workers from each sub-county, who had already been identified and trained as peer trainers for cervical cancer screening and treatment, were utilized as data collectors for the survey. The trainers/data collectors had been selected from a pool of nurses/clinical officers/medical officers stationed at cervical cancer screening service provision points in their respective hospitals. A module on the survey tools and procedures was part of their training of trainers (TOT); it included administration of the questions, maneuvering through the electronic tools and data transmission procedures. This approach was deemed to be both efficient and provided an opportunity for the trainers to undertake hospitals mapping before they commenced their cascaded trainings. Each pair was then required to visit and administer the survey tools to hospitals managers, screening, and laboratory staff in all hospitals in their sub-county.
Data collection and analysis
Data collection approaches included both interviewing key informants in various departments at the hospitals, as well as direct observation of hospitals/processes of interest. Data collection was conducted using two questionnaires: one for cervical cancer screening and treatment services and one for laboratory services. The questionnaires were created electronically using the SurveyCTO© application and loaded into android tablets. Data was transmitted instantaneously to a central database, domiciled at the National Cancer Control Program (NCCP), for processing. Data cleaning and analysis were conducted using Epi-Info software (US CDC, Atlanta, GA). Descriptive statistics were calculated, in terms of the availability and readiness of various components of the cervical cancer screening and treatment program, across various strata including hospitals type and KEPH level.
Findings
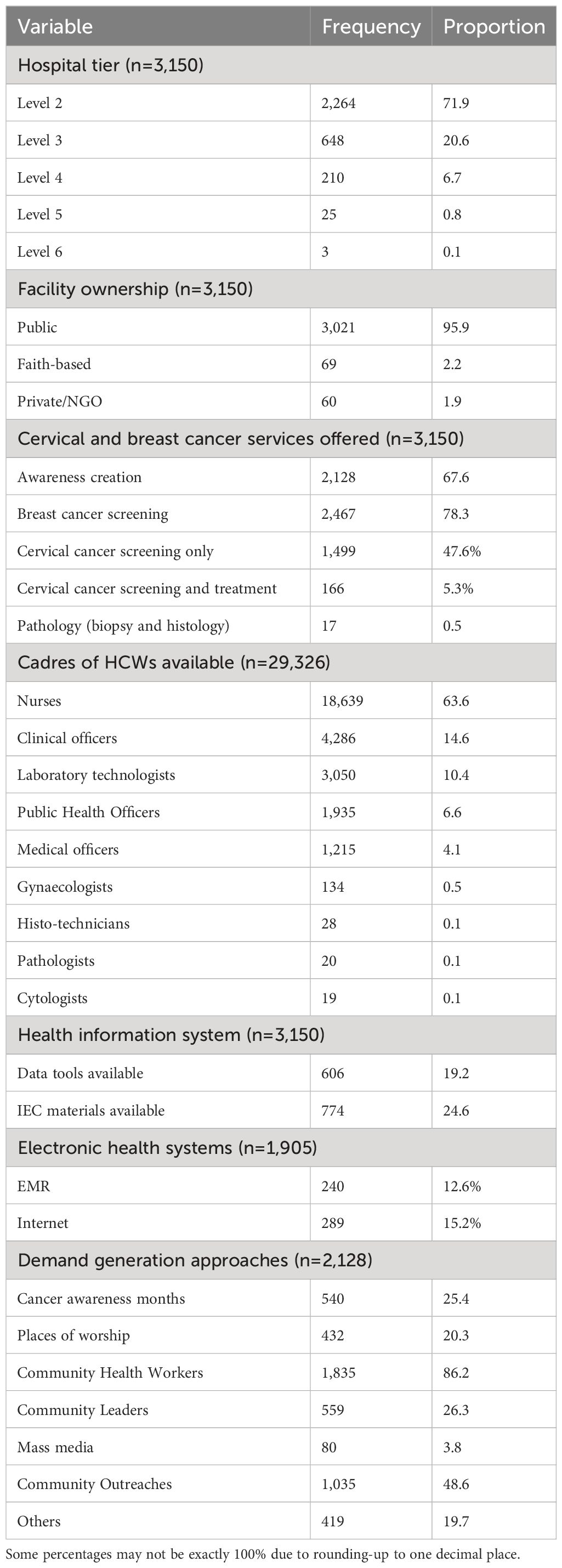
A total of 3,150 hospitals in 25 counties were assessed; majority 3,021 (95.9%) were public hospitals. Majority of the hospitals (3,122 [99.1%]) were primary health care hospitals (level 2-4). Cervical cancer screening was available in 1,665 hospitals (52.6%); however, only 166 (5.3%) were offering both screening and treatment for cervical cancer. The bulk of the health workforce available in the surveyed hospitals was made up of nurses (63.6% [18,639/29,326]). Awareness creation on cervical cancer screening services available was reported by 67.6% of the hospitals; (2,128/3,150); use of community health workers (86.2% [1,835/2,128]) and community outreaches (48.6% [1,035/2,128]) were the most popular methods for awareness creation (some facilities were using multiple approaches). Mass media was the least used approach (3.8%) even though it has the greatest capacity to reach many people. Clinical breast examination (CBE) was available at 78.3% (2,467/3,150) of the hospitals. Only 19.2% (606/3,150) had cervical cancer screening data capture and reporting tools at the time of the survey. Approximately 60% (1,905/3,150) of the hospitals had some form of EMR systems available at some service provision points; however, none had integrated cervical cancer screening data capture in the EMR. Other facility variables are shown in Table 2.
Service delivery per level of care
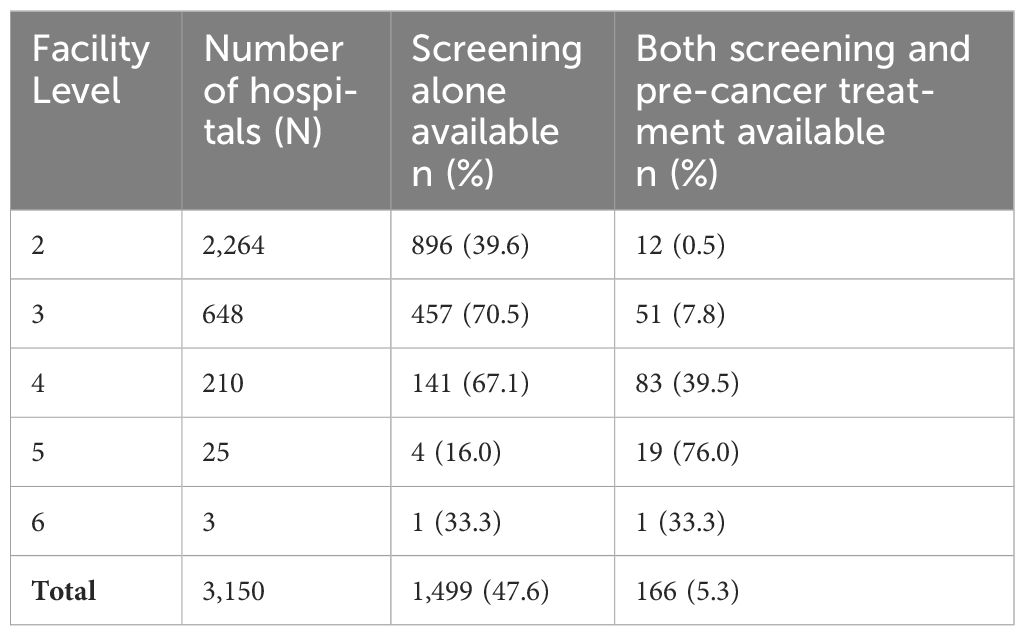
Cervical cancer screening service availability was highest at level 3 (70.5% [457/648]) and level 4 (67.1% [141/210]) (Table 3). However, availability of both screening and treatment was highest at level 5 hospitals (76% [19/25]). Majority of levels 2 and 3, which formed the bulk of the hospitals, did not have cervical pre-cancer treatment services.
The primary screening method used in most hospitals with screening services was VIA in 96.0% (1,599/1,665) of the hospitals. Among hospitals offering pre-cancer treatment, the modality commonly used was cryotherapy, available in 79.5% (132/166) of these hospitals; 63.9% (106/166) offered single visit approach. In diagnostics, cervical biopsy was available in 21.8% (52/238) of level four and above hospitals and histology in 7.1% (17/238). Among hospitals offering the service, the median cost of histopathology was $ 12.46 [IQR; 5.81–20.76]; the cost was borne by the patients in all the hospitals. Availability of other services across hospitals as per level of where the service is expected, is shown in Figure 1.

Figure 1 Proportion of assessed hospitals, offering various services along the cervical cancer screening and treatment continuum (level 2 and 3, n=2,912; level 4 and above, n=238). LLETZ: Large loop excision of the transformation zone; VIA: visual inspection with acetic acid. Single visit approach: both screening and treatment offered during the same visit.
Most of the screening, diagnostic and treatment services were offered in the maternal and child health (MCH) clinic and comprehensive clinics (CCC) for people living with HIV; for instance, 66.5% (1,108/1,665) of the hospitals offering VIA were providing it at MCH only, 1.9% (32/1,665) at CCC alone and 24.7% (412/1,665) at both MCH and CCC.
Screening and treatment health workforce
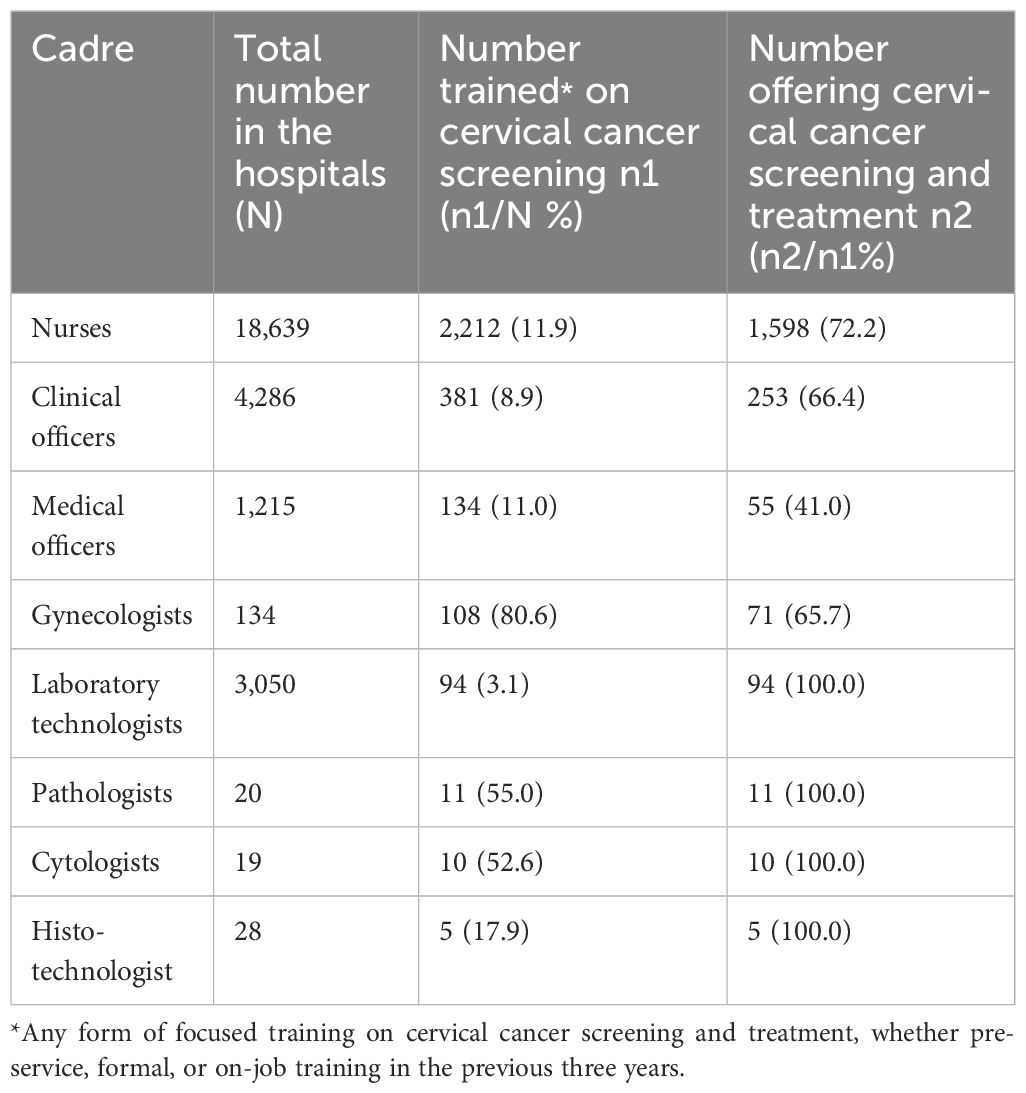
Only 10.8% (2,955/27,363) of all the HCWs were trained in cervical cancer screening and treatment, with nurses contributing 74.9% (2,212/2,955) of the trained workforce. Among those who are trained, 72.2% of nurses, 66.4% of clinical officers, 41.0% of medical officers, and 65.7% of gynecologists were deployed at cervical cancer screening and treatment service provision points at their hospitals (Table 4).
Screening commodities availability
Among hospitals that had included cervical cancer screening in their service charter, half (830/1,665) had acetic acid available while 48.9% (815/1,665) had the recommended light source for pelvic examination at the time of the assessment. Other critical commodities like HPV tests and pap smear kits were available in less than five percent of the hospitals offering screening (Figure 2).

Figure 2 Availability of critical cervical cancer screening commodities in hospitals in 25 Kenyan counties, 2022. (n=3,150).
Health system readiness
We noted some key strengths and weaknesses in the health system readiness in moving towards cervical cancer elimination (Table 5). Multiple service delivery points offer opportunities for a better reach and exploitation of efficiencies of service integration. Having multiple cadres offering cervical cancer screening and treatment offers a larger pool for service provision and skill-set strengthening for an effective cervical program. Cervical precancer lesions treatment availability is limited in the hospitals surveyed, which may reduce successful care linkage for women with positive screening results. Another major weakness is the erratic and inefficient supply chain for the screening and treatment commodities, especially cryotherapy gas that limited the number of hospitals able to offer both screening and treatment. Primary care hospitals offer free services, but are limited in service readiness for both screening and treatment.

Table 5 Strengths and weaknesses of the healthcare system to support cervical cancer screening and treatment in Kenya.
Discussion
Summary of findings
We found that primary health care (PHC) hospitals form the bedrock of cervical cancer service provision in the 25 Counties surveyed. While more than half of all the hospitals offer cervical cancer screening, only 5.0% offer both screening and treatment. Only one in 10 of HCWs in the surveyed hospitals were trained in cervical cancer screening and treatment. Less than half of the hospitals had available stock of cervical cancer screening and treatment commodities at the time of the survey. Presence of multiple screening points at the hospitals was the main health system strength, but commodity stockouts was identified as the main weakness.
Cervical cancer screening service readiness in Kenya
Majority of the surveyed hospitals were PHC level (2 and 3). This agrees with the structure of the overall health system in Kenya, where PHC hospitals form the bulk of the available public hospitals countrywide. Therefore, strengthening PHC system would be a major step in increasing access to cervical cancer screening and treatment, to make progress towards the 2030 elimination targets. Levels 2 and 3 also have service provision at no cost to patients, implying that they can be avenues for removing financial barriers to cervical cancer screening uptake. PHC, especially within the context of Universal Health Coverage (UHC), is important for increasing access to cervical screening (14).
Unfortunately, more than half of the PHC hospitals do not offer cervical cancer screening, and even those that do, fail to provide treatment. One reason may be inadequate trained and competent personnel; while some HCWs reported that they had received training in the past, some did not feel competent enough to offer treatment. Another reason could be erratic provision of screening and treatment health commodities and unavailability of treatment equipment. For instance, despite a country-wide distribution of cryotherapy equipment over a decade ago, we found that many were either broken, or had run out of cryotherapy gas and never replenished. PHC hospitals, while offering free services, have no financial planning autonomy, and rely on secondary level hospitals for procurement of supplies; in such circumstances health promotion interventions like cancer screening may be deprioritized when financial resources are very limited. Even where trained personnel were available at some point, they are lost by either transfer to other hospitals/departments or retirement from service and no regular replacements done. These findings are similar to a recent national service readiness survey in Kenya, which showed higher readiness in referral hospitals compared with PHC hospitals (15).
MCH and CCC/HIV clinics are the main cervical cancer screening service points in the surveyed hospitals. Traditionally, cervical cancer screening in Kenya was domiciled under the reproductive health services, hence services were offered either at MCH or family planning clinics. Organized cervical cancer screening also served as an integral component of HIV care, due to the epidemiological and biological linkage between HIV and cervical cancer. Integration is an efficient policy direction for increasing cervical cancer screening uptake; lessons from integration at MCH and CCC can enable incorporation of more service provision points at hospitals, including outpatient departments (OPD) and gynecological clinics. More hospitals were offering CBE than cervical cancer screening, proving another opportunity for integration. Ample evidence exists on the efficacy of integrating cervical cancer screening in reproductive, HIV and vaccination programs in SSA (16–23).
We found frequent unavailability of critical supplies for cervical cancer screening and treatment, especially acetic acid, cryotherapy gas and HPV kits. Procurement of such commodities may not be prioritized at the county level, compared with diagnostic commodities and medicines. In addition, screening commodities are not available at the Kenya Medical Supplies Authority (KEMSA), the main medical supplier for the County Departments of Health in Kenya, possibly due to policy or resource constraints. NHIF does not cover preventive or promotive health services like cancer screening, which severely limits the financing component of the national cervical cancer control program. However, this may change with the ongoing UHC reforms in the health sector. Lack of screening commodities was also identified as a key gap in an evaluation of the Zimbabwe cervical cancer program (24).
Multiple service provision points, by different cadres were identified as key strengths in the cervical cancer program in the surveyed hospitals; unavailability of treatment services, erratic commodity supply chain and few numbers of trained personnel were the major weaknesses. Availing multiple screening points at hospitals minimizes lost opportunities and increase screening uptake. Health service provision in Kenya is based on the Kenya Essential Package for Health (KEPH) levels; cervical cancer screening ideally is supposed to be offered across all the levels, but especially PHC hospitals (2–4). All the HCW cadres in these levels are eligible for training on cervical cancer screening and treatment, as guided by the respective schemes of service. Accessibility of screening and integrating with other services offered at the hospitals were noted as drivers of cervical cancer screening uptake in Malawi (25). In Uganda, building capacity among PHC health workers in cervical cancer screening and treatment has been adopted as a strategy to address unmet needs in the population (26). In addition to commodities supply chain, the Zimbabwean study also identified staffing challenges, lack of equipment, limited funding and ineffective leadership and governance structure (24). A similar approach, including training PHC personnel, adapting screening approaches to practical local contexts and enhancing local infrastructure to perform various screening tests, has been suggested for two West-African countries (27).
Strengths and limitations
A particular strength of this study was that we conducted a census of all the hospitals in the 25 Counties, spread out in the 10 regions of Kenya; therefore, the findings are likely representative of the true state of cervical cancer control service readiness. The assessment also comprehensively examined the main health system building blocks, therefore provides critical insights for areas in need of strengthening for Kenya to move towards elimination. A weakness of the study was that the survey did not undertake an exploratory angle, to find out the possible underlying reasons to some of the identified gaps. Such an undertaking would have provided more information for planning and focusing the interventions in a more effective and efficient manner and is planned for subsequent program evaluations.
Conclusion and recommendations
We identified major gaps in the service readiness for an effective cervical cancer program in the 25 Counties, but also some opportunities, which if explored can provide a path towards elimination. We recommend a more efficient supply for cervical cancer screening and treatment commodities at PHC, primarily through public financing. Since level 2 and 3 hospitals constitute the majority of the hospitals, they should be enabled to offer cervical cancer screening and treatment by ensuring adequately trained staff and essential health commodities. Availability of screening services in nearby hospitals has been identified as one of the determinants of screening uptake (28). Additional service provision points at hospitals need to integrate cervical cancer screening to their routine service provision, to reduce missed opportunities for screening when women visit for other services. A study in Ethiopia identified restricting screening to a single service point as a barrier to screening uptake (29). A cervical cancer human resource development plan is necessary to guide recruitment, training, mentorship, retention, and replacement of personnel at the county level; sustained capacity-building of HCWs is necessary for success of programs (30). Cervical cancer screening and treatment should be included in the ongoing health financing reforms, especially at PHC; recent evidence shows adequate financing will be necessary for cervical cancer elimination (31). Regular similar assessments should be conducted to inform the efficacy of ongoing investments in the strengthening of the national cervical cancer control program.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Since the unit of assessment was the hospitals, we collected data on service availability and readiness; no personal information was obtained. The study was approved by the Ministry of Health (MoH) and the respective County Departments of Health. Specifically, the assessment was modelled on a broader health system service availability and readiness assessment, usually conducted for all health services in Kenya every five years. However, in this case, we focused on readiness for the country to implement selected interventions within the cervical cancer elimination strategy. Being a routine component of health system evaluation and strengthening, this assessment did not require an Institutional Review Board (IRB) clearance process as per relevant stipulations by the Ministry of Health. All methods were carried out in accordance with the Monitoring and Evaluation guidelines of MoH. Even though the data collected was not personal in nature, safety was ensured during transmission and archiving through password-protected files.
Author contributions
VM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DM: Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. CK: Formal analysis, Writing – original draft, Writing – review & editing. J-PB: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing. PN: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. LO: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing. MN: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing. MA: Writing – original draft, Writing – review & editing. PT: Writing – original draft, Writing – review & editing. MT: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by funding from Unitaid through Clinton Health Access Initiative (grant ID UNITCANCER1). In addition, MA was supported by the Horizon 2020 Framework Programme for Research and Innovation of the European Commission, through the RISCC Network (Grant No. 847845).
Acknowledgments
We acknowledge the respective county directors of health from the 25 counties, the cervical cancer program County focal persons, the TOTs who served as the data collectors and the facility managers and service point in-charges, who were the main respondents. We also acknowledge technical support provided by the Clinton Health Access Initiative, during the conduction of this assessment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1371529/full#supplementary-material
Data Sheet 1 | Screening and treatment services assessment dataset.
Data Sheet 2 | Laboratory services assessment dataset.
Data Sheet 3 | Screening and treatment services assessment codebook.
Data Sheet 4 | Laboratory services assessment dataset codebook.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. WHO. Address inequality: prevent cervical cancer. Available online at: https://www.who.int/mediacentre/commentaries/cervical-cancer-prevention/en/.
3. Temmerman M, Bustreo F. Cervical cancer services are the next frontier for universal healthcare coverage in LMICs. BMJ opinion blogs. (2017). Available: https://blogs.bmj.com/bmj/2017/09/20/cervical-cancer-services-are-the-next-frontier-for-universal-healthcare-coverage-in-lmics/.
4. Jedy-Agba E, Joko WY, Liu B, Buziba NG, Borok M, Korir A, et al. Trends in cervical cancer incidence in sub-Saharan Africa. Br J Cancer. (2020) 123:148–54. doi: 10.1038/s41416-020-0831-9
5. Ngoma M, Autier P. Cancer prevention: cervical cancer. Ecancermedicalscience. (2019) 13:952. doi: 10.3332/ecancer.2019.952
6. WHO. A Global Strategy for elimination of cervical cancer. Available online at: https://www.who.int/activities/a-global-strategy-for-elimination-of-cervical-cancer.
7. Shin MB, Liu G, Mugo N, Garcia PJ, Rao DW, Bayer CJ, et al. A framework for cervical cancer elimination in low-and-middle-income countries: A scoping review and roadmap for interventions and research priorities. Front Public Health. (2021) 9:670032. doi: 10.3389/fpubh.2021.670032
8. Bhatla N, Nessa A, Oswal K, Vashist S, Sebastian P, Basu P. Program organization rather than choice of test determines success of cervical cancer screening: Case studies from Bangladesh and India. Int J Gynaecol Obstet. (2021) 152:40–7. doi: 10.1002/ijgo.13486
9. Pierz AJ, Randall TC, Castle PE, Adedimeji A, Ingabire C, Kubwimana G, et al. A scoping review: Facilitators and barriers of cervical cancer screening and early diagnosis of breast cancer in Sub-Saharan African health settings. Gynecol Oncol Rep. (2020) 33:100605. doi: 10.1016/j.gore.2020.100605
10. Yimer NB, Mohammed MA, Solomon K, Tadese M, Grutzmacher S, Meikena HK, et al. Cervical cancer screening uptake in Sub-Saharan Africa: a systematic review and meta-analysis. Public Health. (2021) 195:105–11. doi: 10.1016/j.puhe.2021.04.014
11. Lim JN, Ojo AA. Barriers to utilisation of cervical cancer screening in Sub Sahara Africa: a systematic review. Eur J Cancer Care. (2017) 26:1–9. doi: 10.1111/ecc.2017.26.issue-1
12. Ng'ang'a A, Nyangasi M, Nkonge NG, Gathitu E, Kibachio J, Gichangi P, et al. Predictors of cervical cancer screening among Kenyan women: results of a nested case-control study in a nationally representative survey. BMC Public Health. (2018) 18:1221. doi: 10.1186/s12889-018-6054-9
13. Ministry of Health. Kenya hospitals assessment report, 2019. Available online at: http://www.health.go.ke/wp-content/uploads/2020/01/KHFA-2018-19-Popular-version-report-Final-.pdf.
14. Woo YL, Gravitt P, Khor SK, Ng CW, Saville M. Accelerating action on cervical screening in lower- and middle-income countries (LMICs) post COVID-19 era. Prev Med. (2021) 144:106294. doi: 10.1016/j.ypmed.2020.106294
15. Ammoun R, Wami WM, Otieno P, Schultsz C, Kyobutungi C, Asiki G. Readiness of hospitals to deliver non-communicable diseases services in Kenya: a national cross-sectional survey. BMC Health Serv Res. (2022) 22:985. doi: 10.1186/s12913-022-08364-w
16. Dreyer G, Botha MH, Snyman LC, Visser C, Burden R, Laubscher N, et al. Combining cervical cancer screening for mothers with schoolgirl vaccination during human papillomavirus (HPV) vaccine implementation in South Africa: results from the VACCS1 and VACCS2 trials. Int J Gynecol Cancer. (2022) 32:592–8. doi: 10.1136/ijgc-2021-003079
17. Pfaff C, Singano V, Akello H, Amberbir A, Berman J, Kwekwesa A, et al. Early experiences in integrating cervical cancer screening and treatment into HIV services in Zomba Central Hospital, Malawi. Malawi Med J. (2018) 30:211–4. doi: 10.4314/mmj.v30i3.14
18. Davies NECG, Chersich M, Mullick S, Naidoo N, Makhoba N, Rees H, et al. Integrating Cervical Cancer Screening into Safer Conception Services to Improve Women's Health Outcomes: A Pilot Study at a Primary Care Clinic in South Africa. Sex Transm Dis. (2019) 46:91–7. doi: 10.1097/OLQ.0000000000000914
19. Tchounga B, Boni SP, Koffi JJ, Horo AG, Tanon A, Messou E, et al. Cervical cancer screening uptake and correlates among HIV-infected women: a cross-sectional survey in Côte d'Ivoire, West Africa. BMJ Open. (2019) 9:e029882. doi: 10.1136/bmjopen-2019-029882
20. Diala PC, Randa M, Odhiambo J, Ganda G, Cohen CR, Mungo C. Barriers and facilitators to integrating clinical breast examinations with cervical cancer screening programs in outpatient clinics in Western Kenya. JCO Glob Oncol. (2021) 7:1722–9. doi: 10.1200/GO.21.00272
21. Wirtz C, Mohamed Y, Engel D, Sidibe A, Holloway M, Bloem P, et al. Integrating HPV vaccination programs with enhanced cervical cancer screening and treatment, a systematic review. Vaccine. (2022) 40 Suppl 1:A116–23. doi: 10.1016/j.vaccine.2021.11.013
22. Castle PE, Einstein MH, Sahasrabuddhe VV. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA Cancer J Clin. (2021) 71:505–26. doi: 10.3322/caac.21696
23. Leno DWA, Diallo FD, Delamou A, Komano FD, Magassouba M, Niamy D, et al. Integration of family planning counselling to mass screening campaign for cervical cancer: experience from Guinea. Obstet Gynecol Int. (2018) 2018:3712948. doi: 10.1155/2018/3712948
24. Tapera O, Nyakabau AM, Simango N, Guzha BT, Jombo-Nyakuwa S, Takawira E, et al. Gaps and opportunities for cervical cancer prevention, diagnosis, treatment and care: evidence from midterm review of the Zimbabwe cervical Cancer prevention and control strategy (2016-2020). BMC Public Health. (2021) 21:1478. doi: 10.1186/s12889-021-11532-y
25. Pittalis C, Panteli E, Schouten E, Magongwa I, Gajewski J. Breast and cervical cancer screening services in Malawi: a systematic review. BMC Cancer. (2020) 20:1101. doi: 10.1186/s12885-020-07610-w
26. Jatho A, Mugisha NM, Kafeero J, Holoya G, Okuku F, Niyonzima N, et al. Capacity building for cancer prevention and early detection in the Ugandan primary healthcare hospitals: Working toward reducing the unmet needs of cancer control services. Cancer Med. (2021) 10:745–56. doi: 10.1002/cam4.3659
27. Haque A, Kouriba B, Aïssatou N, Pant A. Eliminating cervical cancer in Mali and Senegal, two sub-Saharan countries: insights and optimizing solutions. Vaccines (Basel). (2020) 8:181. doi: 10.3390/vaccines8020181
28. Atnafu DD, Khatri R, Assefa Y. Drivers of cervical cancer prevention and management in sub-Saharan Africa: a qualitative synthesis of mixed studies. Health Res Policy Syst. (2024) 22:21. doi: 10.1186/s12961-023-01094-3
29. Jemal Z, Chea N, Hasen H, Tesfaye T, Abera N. Cervical cancer screening utilization and associated factors among female health workers in public hospitals of Hossana town, southern Ethiopia: A mixed method approach. PloS One. (2023) 18:e0286262. doi: 10.1371/journal.pone.0286262
30. Moucheraud C, Kawale P, Kafwafwa S, Bastani R, Hoffman RM. Health care workers' experiences with implementation of "screen and treat" for cervical cancer prevention in Malawi: A qualitative study. Implement Sci Commun. (2020) 1:112. doi: 10.1186/s43058-020-00097-3
31. Financing for cervical cancer elimination. UICC. (2023) Available at: https://www.uicc.org/what-we-do/thematic-areas-work/cervical-cancer-elimination/financing-cervical-cancer-elimination.
Keywords: cervical cancer, screening, Kenya, baseline assessment, service readiness
Citation: Mwenda V, Murage D, Kilonzo C, Bor J-P, Njiri P, Osiro L, Nyangasi M, Arbyn M, Tummers P and Temmerman M (2024) Baseline assessment of cervical cancer screening and treatment capacity in 25 counties in Kenya, 2022. Front. Oncol. 14:1371529. doi: 10.3389/fonc.2024.1371529
Received: 24 January 2024; Accepted: 18 June 2024;
Published: 02 July 2024.
Edited by:
Manoj Menon, Fred Hutchinson Cancer Center, United StatesReviewed by:
Hiren Koshiya, Desert Valley Hospital, United StatesEdwin K. Wiredu, University of Ghana, Ghana
Copyright © 2024 Mwenda, Murage, Kilonzo, Bor, Njiri, Osiro, Nyangasi, Arbyn, Tummers and Temmerman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerian Mwenda, dmFsbXdlbmRhQGdtYWlsLmNvbQ==
 Valerian Mwenda
Valerian Mwenda David Murage1,2
David Murage1,2 Catherine Kilonzo
Catherine Kilonzo Lance Osiro
Lance Osiro Mary Nyangasi
Mary Nyangasi Marleen Temmerman
Marleen Temmerman