
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 19 March 2024
Sec. Cancer Imaging and Image-directed Interventions
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1370466
Objectives: The present study aimed to develop a radiomics nomogram based on conventional ultrasound (CUS) to preoperatively distinguish high tumor-infiltrating lymphocytes (TILs) and low TILs in triple-negative breast cancer (TNBC) patients.
Methods: In the present study, 145 TNBC patients were retrospectively included. Pathological evaluation of TILs in the hematoxylin and eosin sections was set as the gold standard. The patients were randomly allocated into training dataset and validation dataset with a ratio of 7:3. Clinical features (age and CUS features) and radiomics features were collected. Then, the Rad-score model was constructed after the radiomics feature selection. The clinical features model and clinical features plus Rad-score (Clin+RS) model were built using logistic regression analysis. Furthermore, the performance of the models was evaluated by analyzing the receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA).
Results: Univariate analysis and LASSO regression were employed to identify a subset of 25 radiomics features from a pool of 837 radiomics features, followed by the calculation of Rad-score. The Clin+RS integrated model, which combined posterior echo and Rad-score, demonstrated better predictive performance compared to both the Rad-score model and clinical model, achieving AUC values of 0.848 in the training dataset and 0.847 in the validation dataset.
Conclusion: The Clin+RS integrated model, incorporating posterior echo and Rad-score, demonstrated an acceptable preoperative evaluation of the TIL level. The Clin+RS integrated nomogram holds tremendous potential for preoperative individualized prediction of the TIL level in TNBC.
Triple-negative breast cancer (TNBC), a distinct form of breast cancer, is identified by the lack of estrogen receptor (ER), human epidermal growth factor receptor type 2 (HER2), and progesterone receptor (PR) expression (1). TNBC has a higher rate of recurrence and metastasis than non-TNBC (1, 2).
Tumor-infiltrating lymphocytes (TILs) have proven to be a dependable predictor of treatment response and prognosis in cases of breast cancer (3, 4). TILs are primarily observed in cases of TNBCs (5, 6). In several previous studies, the presence of higher TILs in TNBC tumors was found to be associated with improved outcomes (5–9). The patients with higher TILs in breast cancer were shown to have a higher responsiveness to chemotherapy compared to the patients with lower TILs in breast cancer (10–12). In the past few years, there has been a growing emphasis on the utilization of immunotherapy, particularly the application of immune checkpoint inhibitors, owing to their favorable clinical outcomes observed in both early and advanced TNBC (13–15). It is anticipated that TILs will function as a prognostic indicator for the immunotherapeutic efficacy in patients receiving immune checkpoint inhibitors. At present, TILs are often assessed in histopathological slides obtained through invasive core needle biopsy, especially for neoadjuvant chemotherapy patients (3). However, the core needle biopsy has several well-known pitfalls, including the limited tissue sampling and heterogeneity in lymphocyte distribution, which may affect the accuracy of evaluating TIL level. Hence, it is imperative to develop a non-invasive imaging method allowing for visualization of the entire tumor to serve as an important supplement to evaluate TIL level.
Breast conventional ultrasound (CUS) is a real-time, non-radiative, cost-effective and commonly used diagnostic imaging method for breast tumors in clinical settings. Radiomics, a potentially valuable quantitative technique, involves the extraction of high-throughput imaging characteristics from medical images (16, 17), which has extensive application in classifying phenotypic subtypes and making prognostic predictions for solid tumors (18, 19). CUS-based radiomics has shown the potential to differentiate malignant and benign lesions (20) and evaluate the prognosis for patients with breast cancer (21, 22). However, limited research has been conducted on the efficacy of the CUS-based radiomics approach in differentiating high TILs from low TILs in TNBC. This research aims to investigate the potential of CUS-radiomics models for preoperative assessment of TIL level in patients with TNBC.
In the present study, we retrospectively enrolled primary invasive breast cancer patients in our hospital from 1 January 2017 to 30 October 2023 with baseline breast ultrasounds available. A total of 3795 consecutive patients were initially included who underwent breast ultrasound examinations and were confirmed with pathological diagnosis of primary invasive breast cancer. The criteria for exclusion were as stated below: (1) non-TNBC breast cancer proven by immunohistochemistry (IHC) staining (n=3451), (2) without pathological evaluation of TILs (n=83), (3) pathological evaluation using the needle biopsy specimen but not surgical resection specimens (n=69), (4) preserved ultrasound images were marked with measurement lines (n=26), (5) underwent neoadjuvant chemotherapy in TNBC before surgical resection (n =12), (6) preoperative breast ultrasound images lost (n=9). After these exclusions, 145 patients with TNBC were finally included. The detailed patient inclusion is illustrated in Figure 1. The framework of the current study is presented in Figure 2.
The assessment of stromal TILs was conducted based on pathological hematoxylin and eosin (H&E) slides by pathologists according to published guidelines (23). The percentage of stroma in each breast cancer tumor was documented relative to the lymphoid cells (24). In this study, TILs refer to stromal TILs unless otherwise specified. Previous studies found that a percentage of TILs≥20% was associated with pathologic complete response in TNBC (25, 26). Thus, this study defined the percentage of TILs≥20% as a high TIL level.
Experienced board-certified radiologists performed all breast ultrasound examinations. Several ultrasound machines were used for conducting breast ultrasound examinations, including ESAOTE (MyLab 90 X-vision, Italy) and Logic E9 (GE Healthcare, USA) with high-frequency probes. Relevant images of the primary tumor were recorded during the examination, including both transverse and longitudinal views.
Two experienced radiologists with ≥ 8 years of expertise in breast ultrasound retrospectively assessed the CUS characteristics of the index tumor for each patient. They were blinded to the ultrasound and pathologic reports. In the event of a discrepancy between the two observers, a consensus was reached following deliberation. The CUS characteristics were assessed for the following CUS features: shape, margin, orientation, echo pattern, posterior echo features, vascularity, and calcification.
To begin with, the patients were randomly assigned to either the training or validation group in a 7:3 ratio. Then, the jpg file format was used to save the ultrasound images, and subsequently, they were imported into the PyCharm software (2022.2 edition). Subsequently, a radiologist with ≥ 8 years’ experience in breast ultrasound manually delineated the region of interest (ROI), which was then verified by another radiologist with ≥ 8 years’ experience in breast ultrasound utilizing the Labelme software package (3.16.7). Neither of the radiologists had knowledge of the pathological findings while annotating the images, and any discrepancies were resolved through consensus reached via discussion. Radiomics characteristics were obtained from tumors following image processing using various filters through the utilization of the Pyradiomics package (Version 3.0.1) which were subsequently categorized into distinct classes, including 1) first-order features; 2) shape-based features; 3) high-order features, including GLCM, GLDM, GLRLM, GLSZM, and NGTDM. Finally, 837 radiomics features were extracted for this study.
To begin with, we employed an independent t-test or Mann-Whitney U test to identify significant features exhibiting statistically meaningful differences (P<0.05). Afterwards, the least absolute shrinkage and selection operator (LASSO) was utilized for selecting coefficients that are not zero through cross validation of 10-fold. Lastly, the Rad-score was computed using the chosen features. The detailed formulation of the Rad-score can be found in the Supplementary Materials.
The baseline clinical features and Rad-score were compared using univariate analyses between the low TILs and high TILs groups (Table 1). Then, multivariable logistic regression models were constructed by including variables that had a significance level of p <0.05 in the univariate analyses in both the clinical features model and clinical features plus Rad-score (Clin+RS) integrated model. The model’s ability to discriminate was assessed through analysis of the receiver operating characteristic (ROC) curve, while the accuracy of the models’ predictions was measured via calculating the area under the curve (AUC) in the training dataset and validation dataset.
Statistical analyses were conducted utilizing SPSS software (version 26) and R software (version 4.2.1). Quantitative variables were expressed as mean ± SD or medians (25th percentile, 75th percentile). The normality of the data was evaluated using the Kolmogorov–Smirnov test. To analyze quantitative variables, either an independent samples t-test or a Mann–Whitney U-test was conducted, while categorical variables were analyzed using either a chi-square test or Fisher’s exact test. The models were assessed for their diagnostic accuracy using ROC analysis. To compare the ROC curves, DeLong’s test was employed. Moreover, calibration curves were performed to evaluate the predictive performance. Furthermore, decision curve analysis (DCA) was conducted to evaluate the clinical usefulness. A statistically significant result was defined as P < 0.05 (two-sided).
In this study, 145 female patients with TNBC were finally included, including 87 low TILs and 58 high TILs patients. There were no significant variations in age and tumor size observed between the low TILs group and high TILs group (P>0.05, Table 1). Compared with the low TILs, the tumors with high TILs showed a higher probability of having an oval or round shape, parallel orientation, well-defined margins, complex cystic and solid echo patterns, and posterior enhancement (all p<0.05). The calcification and vascularity exhibited no significant differences between the low TILs group and high TILs group (all p>0.05).
In our research, the individuals were randomly allocated into two separate datasets for training and validation purposes, with a distribution ratio of 7:3. No significant differences were found in clinical characteristics between the training and validation datasets (P>0.05), except for margin (P=0.045, Supplementary Table S1). There was no difference in age and tumor size between low TILs and high TILs, as observed in the training dataset (P>0.05, Supplementary Table S2) and validation dataset (P > 0.05, Supplementary Table S3). The training dataset showed that shape, posterior echo, margin, and Rad-score differed significantly (P < 0.05, Supplementary Table S2), while the validation dataset showed that echo pattern and posterior echo differed significantly between the low TILs group and high TILs group (P < 0.05, Supplementary Table S3).
For every individual, a total of 837 radiomics features were obtained. After undergoing a process of feature selection, a LASSO regression analysis (Supplementary Figure S1) identified and retained 25 radiomics features (Supplementary Table S4), which were used to construct the Rad-score in the training dataset. The high TILs group had higher Rad-score than the low TILs group in all participants (P<0.001, Table 1), training dataset (P<0.001, Supplementary Table S2), and validation dataset (P=0.002, Supplementary Table S3). No statistically difference was detected in the Rad-score between the training dataset and validation dataset (P=0.88, Supplementary Table S1).
In the clinical model, multivariable logistic regression analysis revealed that shape (oval/round shape, P=0.024) and posterior echo (posterior enhancement, P=0.007) were independent predictors for predicting high TILs in the training dataset (Table 2). In the Clin+RS integrated model, multivariable analysis showed that posterior echo enhancement (P=0.041) and Rad-score (P<0.001) were independent predictors for predicting high TILs in the training dataset (Table 2).
In the training dataset, the clinical model achieved an AUC of 0.710, while the Rad-score model showed a higher AUC of 0.835. The Clin+RS integrated model outperformed both with an even higher AUC of 0.848 (Figure 3, Table 3). Compared to the clinical model, the Rad-score model (P=0.039), and Clin+RS integrated model (P=0.007) showed better performances; compared to the Rad-score model, Clin+RS integrated model showed better performance (P=0.048). In the validation dataset, the clinical model achieved an AUC of 0.691, while the Rad-score model showed an improved performance with an AUC of 0.811. Notably, when integrating both clinical and Rad-score features in the Clin+RS model, a higher AUC of 0.847 was observed (Figure 3, Table 3). A nomogram of the Clin+RS integrated model was further developed to individually predict high TILs in the patients with TNBC (Figure 4). In the validation dataset, the prediction results and observations showed a strong correspondence in terms of calibration and DCA curves for the Clin+RS integrated model (Figure 5).
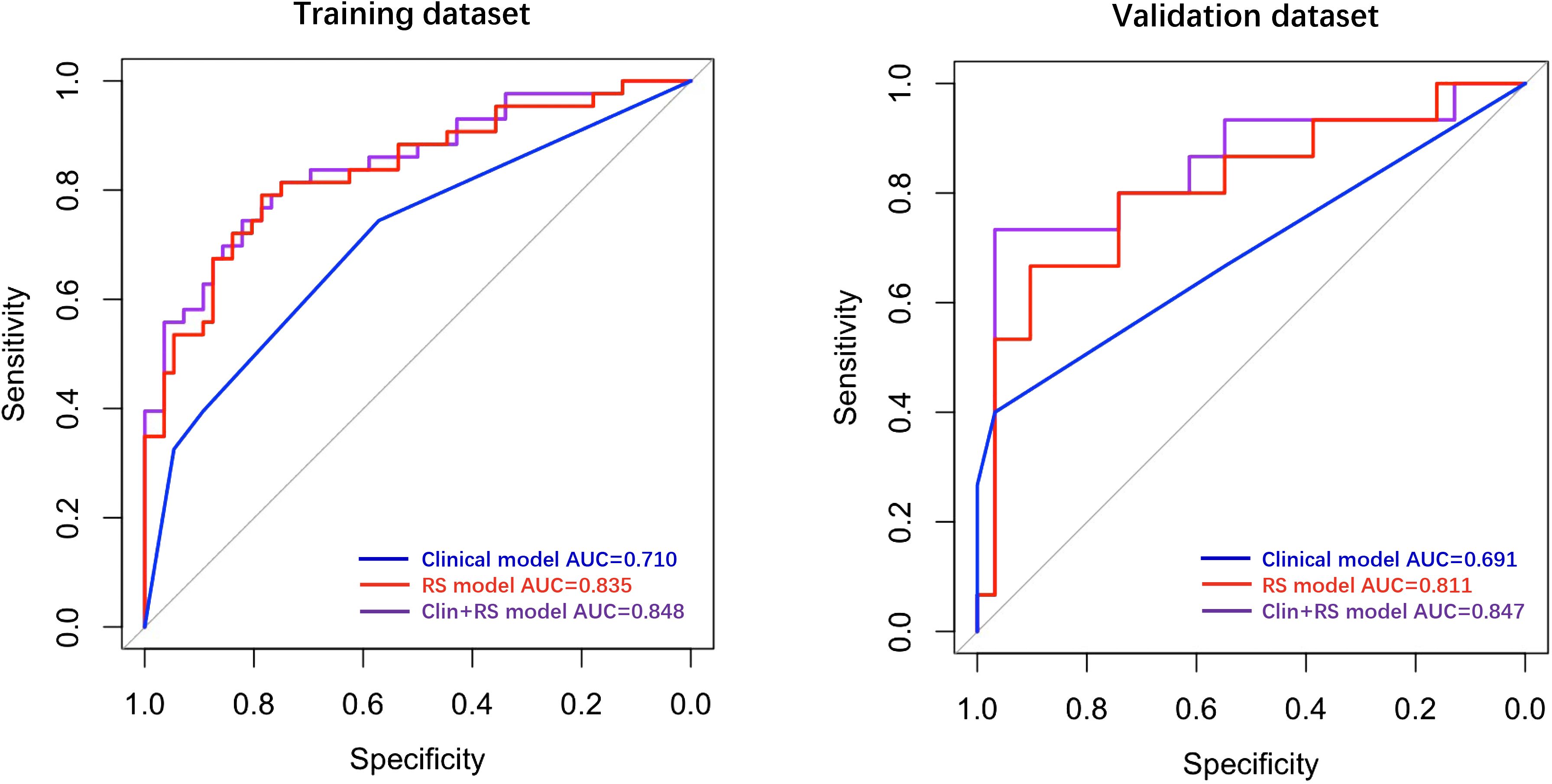
Figure 3 ROC curves for predicting high TILs in training and validation datasets. Clin+RS, clinical+Rad-score; ROC, Receiver operating characteristic; RS, Rad-score.
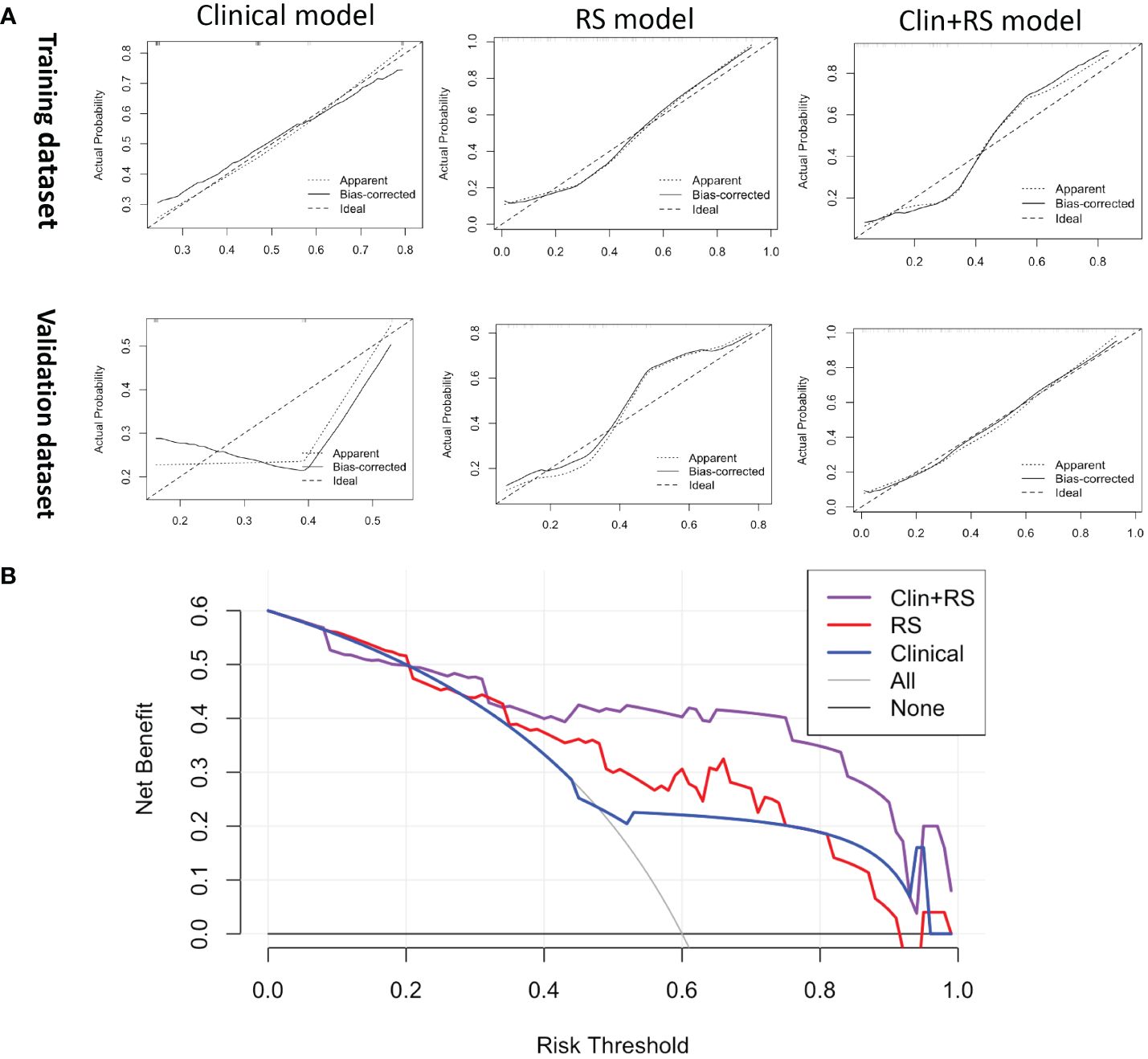
Figure 5 (A) The calibration curve of the clinical, RS, and Clin+RS models for predicting the probability of high TILs. (B) DCA for predicting the probability of high TILs in the validation dataset. Clin+RS, clinical features plus Rad-score; DCA, Decision curve analysis; RS, Rad-score.
In the current research, we effectively created and verified a nomogram that combines CUS characteristics with CUS-Radiomics features. The nomogram functions as an evaluation tool for preoperatively assessing TIL levels in patients with TNBC, offering valuable perspectives without requiring invasive procedures. Moreover, the nomogram derived from the Clin+RS integrated model demonstrated enhanced predictive accuracy and net benefit compared to the clinical or Rad-score models individually.
Current treatment strategies for individuals with breast cancer predominantly rely on the molecular categorization of this disease (27, 28). TNBC, which accounts for approximately 10% to 20% of cases with breast cancer, poses difficulties in treatment due to its heterogeneous nature and absence of clearly defined molecular targets (29, 30). Moreover, TNBC is linked to an increased likelihood of recurrence and metastasis, leading to unfavorable consequences (29). TILs have been identified as indicators of immune infiltration and prognosis in breast cancer, showing promise as potential biomarkers for predicting patient response to immunotherapy (3, 4). However, the current quantification of TILs relies on the manual assessment using pathological slides, which is restricted by the invasive nature of specimen collection and the labor-intensive analysis method. Therefore, noninvasive preoperative imaging examinations are crucial for predicting the TIL level in TNBC as a supplementary diagnostic method.
CUS has been proved to be a dependable imaging technique in the clinical diagnosis of breast cancer. The present research established a clinical model to determine the TIL level using independent predictors of tumor shape and posterior echo features. In the clinical model, we found that the tumors with high TILs had a higher probability of have an oval/round shape and posterior echo enhancement, which are the characteristics of benign breast lesions (31, 32). Indeed, many TNBC tumors are easily misdiagnosed as benign tumors because of their benign-like CUS appearances (33). Therefore, the tumors with high TILs may be more likely to be misdiagnosed as benign tumors, such as fibroadenomas. The biological mechanism of the association between these sonographic appearances and the TIL level remains to be elucidated.
Radiomics has become increasingly significant in the diagnosis and prognosis prediction, and the Rad-score model has been proposed for assessing the therapeutic outcome and prognosis of individuals diagnosed with breast cancer (21, 22). In this study, after radiomics feature selection, 25 radiomics features that described the tumor heterogeneity were associated with the TIL level (Supplementary Table S4). Then, the Rad-score was created using a set of 25 radiomics features mentioned above. Furthermore, we established three models (clinical model, Rad-score model and Clin+RS integrated model) to noninvasively predict the TIL level. Of these three models, the Clin+RS integrated model demonstrated superior performance, achieving an AUC of 0.848 and 0.847 in the training dataset and validation dataset correspondingly. Prior to this study, multiple investigations have investigated the correlation between radiomics and TILs using mammographic or MRI images in patients with breast cancer (34–38). However, CUS is the most commonly diagnostic imaging method for breast tumors in clinical settings due to the real-time, non-radiative, and cost-effective advantages. To the best of our knowledge, our research is the first to investigate the correlation between radiomics and TILs in TNBC utilizing CUS images. The validation cohort demonstrated excellent performance of the prediction model, achieving an AUC value of 0.847. Furthermore, the prediction model was validated by its high accuracy (0.891), sensitivity (0.733), and specificity (0.968). The calibration curve of the integrated model demonstrated a favorable concordance, while the DCA of the integrated model indicated a greater net benefit. Thus, the Clin+RS integrated model has the potential to serve as a reliable and valuable approach in effectively differentiating between high and low TILs in patients with TNBC. In addition, nomogram is a useful tool for multi-index joint diagnosis or prediction in breast cancers, providing substantial advantages in clinical settings offering (39, 40). The present study further constructed a Clin+RS integrated nomogram for preoperatively predicting the TIL level in TNBC.
This study has some limitations. First, our study conducted a retrospective analysis at a single institution with a restricted sample size. In the future, the model needs to be further validated by larger sample sizes from multiple centers and a prospective cohort. Second, we only analyzed CUS-based radiomics features. Future studies could further perform radiomics analyses using ultrasound elastography and contrast-enhanced ultrasound images. Lastly, the variations in ultrasound image acquisition stemming from different ultrasound equipment may also influence experimental outcomes.
In summary, the radiomics features based on CUS exhibit promising potential as a biomarker for differentiation between high and low TILs in patients with TNBC. The Clin+RS integrated model, which integrates posterior echo and Rad-score, exhibits enhanced predictive precision in comparison to the clinical or Rad-score models individually. In addition, the Clin+RS integrated nomogram holds tremendous potential for preoperative individualized prediction of the TIL level in TNBC.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the ethics consultant committee of Second Affiliated Hospital, Zhejiang University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft. PJ: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Writing – original draft. WX: Data curation, Formal analysis, Investigation, Resources, Writing – original draft. CW: Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing. PH: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Zhejiang medicine and health science and technology project (2024KY1402), the National Natural Science Foundation of China (82030048, 82230069, 82102191, 32201138, and 82001818), Key Research and Development Program of Zhejiang Province (NO. 2019C03077).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1370466/full#supplementary-material
1. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. (2010) 363:1938–48. doi: 10.1056/NEJMra1001389
2. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. (2016) 13:674–90. doi: 10.1038/nrclinonc.2016.66
3. Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. (2016) 13:228–41. doi: 10.1038/nrclinonc.2015.215
4. Byrne A, Savas P, Sant S, Li R, Virassamy B, Luen SJ, et al. Tissue-resident memory T cells in breast cancer control and immunotherapy responses. Nat Rev Clin Oncol. (2020) 17:341–8. doi: 10.1038/s41571-020-0333-y
5. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. (2013) 31:860–7. doi: 10.1200/jco.2011.41.0902
6. Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. (2014) 25:1536–43. doi: 10.1093/annonc/mdu191
7. Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. (2014) 32:2959–66. doi: 10.1200/jco.2013.55.0491
8. Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. (2014) 25:1544–50. doi: 10.1093/annonc/mdu112
9. Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, Palazzo A, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. (2016) 27:249–56. doi: 10.1093/annonc/mdv571
10. Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. (2010) 28:105–13. doi: 10.1200/jco.2009.23.7370
11. Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. (2015) 33:983–91. doi: 10.1200/jco.2014.58.1967
12. Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer–a substudy of the neoadjuvant GeparQuinto trial. PloS One. (2013) 8:e79775. doi: 10.1371/journal.pone.0079775
13. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. (2020) 382:810–21. doi: 10.1056/NEJMoa1910549
14. Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2020) 21:44–59. doi: 10.1016/s1470-2045(19)30689-8
15. Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. (2020) 396:1090–100. doi: 10.1016/s0140-6736(20)31953-x
16. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. (2016) 278:563–77. doi: 10.1148/radiol.2015151169
17. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. (2012) 48:441–6. doi: 10.1016/j.ejca.2011.11.036
18. Wang C, Li H, Jiaerken Y, Huang P, Sun L, Dong F, et al. Building CT radiomics-based models for preoperatively predicting Malignant potential and mitotic count of gastrointestinal stromal tumors. Transl Oncol. (2019) 12:1229–36. doi: 10.1016/j.tranon.2019.06.005
19. Li G, Li L, Li Y, Qian Z, Wu F, He Y, et al. An MRI radiomics approach to predict survival and tumour-infiltrating macrophages in gliomas. Brain. (2022) 145:1151–61. doi: 10.1093/brain/awab340
20. Du Y, Zha HL, Wang H, Liu XP, Pan JZ, Du LW, et al. Ultrasound-based radiomics nomogram for differentiation of triple-negative breast cancer from fibroadenoma. Br J Radiol. (2022) 95:20210598. doi: 10.1259/bjr.20210598
21. Zha HL, Zong M, Liu XP, Pan JZ, Wang H, Gong HY, et al. Preoperative ultrasound-based radiomics score can improve the accuracy of the Memorial Sloan Kettering Cancer Center nomogram for predicting sentinel lymph node metastasis in breast cancer. Eur J Radiol. (2021) 135:109512. doi: 10.1016/j.ejrad.2020.109512
22. Jiang M, Li CL, Luo XM, Chuan ZR, Lv WZ, Li X, et al. Ultrasound-based deep learning radiomics in the assessment of pathological complete response to neoadjuvant chemotherapy in locally advanced breast cancer. Eur J Cancer. (2021) 147:95–105. doi: 10.1016/j.ejca.2021.01.028
23. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. (2015) 26:259–71. doi: 10.1093/annonc/mdu450
24. Dieci MV, Radosevic-Robin N, Fineberg S, van den Eynden G, Ternes N, Penault-Llorca F, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. (2018) 52:16–25. doi: 10.1016/j.semcancer.2017.10.003
25. Abuhadra N, Sun R, Litton JK, Rauch GM, Yam C, Chang JT, et al. Prognostic impact of high baseline stromal tumor-infiltrating lymphocytes in the absence of pathologic complete response in early-stage triple-negative breast cancer. Cancers (Basel). (2022) 14:1323. doi: 10.3390/cancers14051323
26. Ruan M, Tian T, Rao J, Xu X, Yu B, Yang W, et al. Predictive value of tumor-infiltrating lymphocytes to pathological complete response in neoadjuvant treated triple-negative breast cancers. Diagn Pathol. (2018) 13:66. doi: 10.1186/s13000-018-0743-7
27. Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. (2011) 29:3885–91. doi: 10.1200/jco.2011.36.1105
28. Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. (2019) 5:66. doi: 10.1038/s41572-019-0111-2
29. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. (2007) 13:4429–34. doi: 10.1158/1078-0432.Ccr-06-3045
30. Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. (2007) 110:876–84. doi: 10.1002/cncr.22836
31. Costantini M, Belli P, Lombardi R, Franceschini G, Mulè A, Bonomo L. Characterization of solid breast masses: use of the sonographic breast imaging reporting and data system lexicon. J Ultrasound Med. (2006) 25:649–59. doi: 10.7863/jum.2006.25.5.649
32. Hong AS, Rosen EL, Soo MS, Baker JA. BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol. (2005) 184:1260–5. doi: 10.2214/ajr.184.4.01841260
33. Li JW, Zhang K, Shi ZT, Zhang X, Xie J, Liu JY, et al. Triple-negative invasive breast carcinoma: the association between the sonographic appearances with clinicopathological feature. Sci Rep. (2018) 8:9040. doi: 10.1038/s41598-018-27222-6
34. Tang WJ, Kong QC, Cheng ZX, Liang YS, Jin Z, Chen LX, et al. Performance of radiomics models for tumour-infiltrating lymphocyte (TIL) prediction in breast cancer: the role of the dynamic contrast-enhanced (DCE) MRI phase. Eur Radiol. (2022) 32:864–75. doi: 10.1007/s00330-021-08173-5
35. Xu N, Zhou J, He X, Ye S, Miao H, Liu H, et al. Radiomics model for evaluating the level of tumor-infiltrating lymphocytes in breast cancer based on dynamic contrast-enhanced MRI. Clin Breast Cancer. (2021) 21:440–9.e1. doi: 10.1016/j.clbc.2020.12.008
36. Yu H, Meng X, Chen H, Han X, Fan J, Gao W, et al. Correlation between mammographic radiomics features and the level of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer. Front Oncol. (2020) 10:412. doi: 10.3389/fonc.2020.00412
37. Yu H, Meng X, Chen H, Liu J, Gao W, Du L, et al. Predicting the level of tumor-infiltrating lymphocytes in patients with breast cancer: usefulness of mammographic radiomics features. Front Oncol. (2021) 11:628577. doi: 10.3389/fonc.2021.628577
38. Su GH, Xiao Y, Jiang L, Zheng RC, Wang H, Chen Y, et al. Radiomics features for assessing tumor-infiltrating lymphocytes correlate with molecular traits of triple-negative breast cancer. J Transl Med. (2022) 20:471. doi: 10.1186/s12967-022-03688-x
39. Xu M, Zeng S, Li F, Liu G. Utilizing grayscale ultrasound-based radiomics nomogram for preoperative identification of triple negative breast cancer. Radiol Med. (2024) 129:29–37. doi: 10.1007/s11547-023-01739-x
Keywords: radiomics, tumor-infiltrating lymphocytes (TILs), breast cancer, triple-negative breast cancer (TNBC), ultrasound
Citation: Hu L, Jin P, Xu W, Wang C and Huang P (2024) Clinical and radiomics integrated nomogram for preoperative prediction of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer. Front. Oncol. 14:1370466. doi: 10.3389/fonc.2024.1370466
Received: 14 January 2024; Accepted: 04 March 2024;
Published: 19 March 2024.
Edited by:
Chen Liu, Army Medical University, ChinaReviewed by:
Lu-Lu Zhai, University of Science and Technology of China, ChinaCopyright © 2024 Hu, Jin, Xu, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pintong Huang, aHVhbmdwaW50b25nQHpqdS5lZHUuY24=; Chao Wang, d2FuZ2NoYW9zbWFydEB6anUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.