- 1Department of Radiation Oncology, Weifang People’s Hospital, Weifang, China
- 2Department of Pathology, Weifang People’s Hospital, Weifang, China
- 3Department of Otolaryngology, Weifang People’s Hospital, Weifang, China
Background: Nuclear protein in testis (NUT) cancers, also known as midline cancers, tends to occur in organs near the midline, such as the nasal sinuses and mediastinum. NUT carcinoma is very rare and has a poor prognosis.
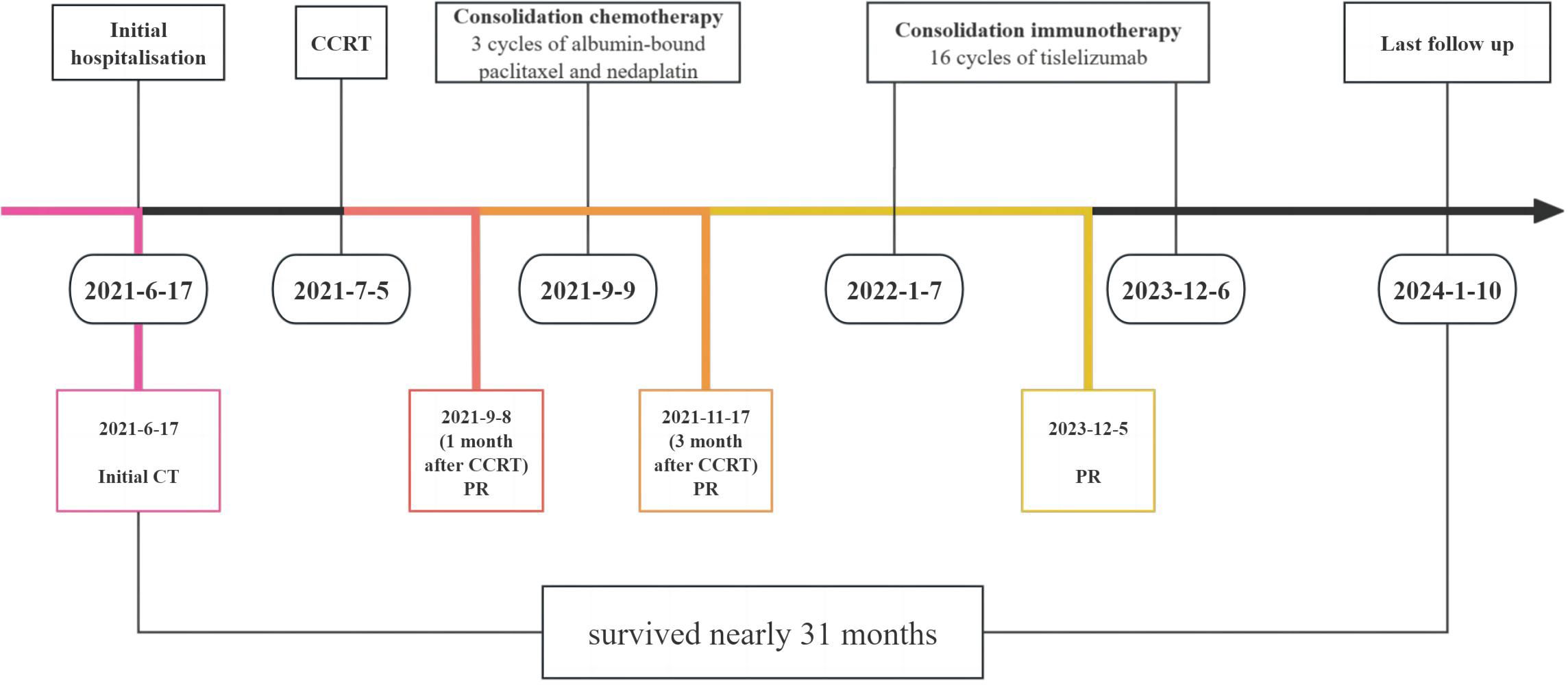
Case description: We report the case of a 44-year-old female patient with sinonasal NUT carcinoma who presented with a soft tissue mass in the left frontal sinus, ethmoid sinus, and left nasal cavity on computed tomography; the tumor was poorly demarcated from the left rectus medialis. After discussion with a multidisciplinary team with expertise on head and neck tumors, the patient was considered inoperable, and definitive concurrent chemoradiotherapy (CCRT) was recommended. The patient underwent CCRT followed by three cycles of consolidation chemotherapy with albumin-bound paclitaxel and nedaplatin. Subsequently, the patient underwent 16 cycles of consolidation therapy with the programmed death–1 (PD-1) inhibitor tislelizumab. The immune-related adverse events included grade 2 hypothyroidism. After CCRT, consolidation chemotherapy, and consolidation immunotherapy, the patient achieved a favorable outcome. The patient survived for 31 months, and there were no signs of recurrence or metastasis during follow-up.
Conclusion: At present, there is no clear consensus on the consolidation treatment plan after CCRT for sinonasal NUT cancer. We used consolidation immunotherapy for the first time and achieved good efficacy, providing an innovative and promising treatment plan for refractory sinonasal NUT cancer.
1 Introduction
Nuclear protein in testis (NUT) carcinoma is extremely rare and has mostly been reported in case studies; the main primary tumor sites are the chest and head and neck (1, 2). The main molecular feature is a rearrangement of the testicular nucleoprotein gene (NUTM1). Although NUTM1 can fuse to numerous different partner genes, it most frequently forms a BRD4-NUTM1 fusion oncogene related to NUT carcinoma (2). The prognosis is very poor, with a median survival of less than 1 year (3). The treatment options for NUT cancer include surgery, radiation therapy, and chemotherapy. Surgery is critical for the treatment of NUT cancer, and surgery combined with postoperative chemoradiotherapy or radiotherapy is associated with improved survival (4). For patients with inoperable tumors or those who refuse surgery, radical concurrent chemoradiotherapy (CCRT) is an alternative. Recent advances in treatment options for head and neck NUT cancer include induction chemotherapy, proton radiotherapy, and immunotherapy. The SINTART 1 study showed that patients with tumor shrinkage greater than or equal to 80% after induction chemotherapy for surgically resectable sinonasal tumors were given the option of radiotherapy and exemption from surgical treatment (5). The SINTART 2 study showed that the addition of induction chemotherapy to treatment regiments for inoperable sinonasal tumors did not significantly improve survival (6). The above two phase II studies of induction chemotherapy did not include NUT cancers, and only one retrospective study of NUT cancers has analyzed the value of induction chemotherapy. Ramesh et al. (7) conducted a retrospective analysis of 12 patients with sinonasal NUT cancer and concluded that induction chemotherapy may be beneficial to patients. Patients with recurrent sinonasal NUT may be considered for proton radiotherapy. Muramatsu et al. (8) reported a case of sinonasal NUT carcinoma with local recurrence followed by reirradiation using proton radiotherapy, which led to complete response. In recent years, the rapid development of immunotherapy has led to the development of new options and useful additions to treatments for NUT cancer, which is often refractory. Currently, treatment with PD-1 and programmed death–ligand 1 (PD-L1) inhibitors has been reported for a small number of patients with lung, thyroid, and parotid NUT cancer (9, 10) but has not yet been reported for sinonasal NUT cancer. Moreover, there is no standard for consolidation regimens after CCRT. This study aimed to explore new treatment options for NUT cancer and strategies for consolidation immunotherapy after CCRT: we report the treatment of one patient with sinonasal NUT cancer with immunotherapy with PD-1 inhibitors after CCRT.
2 Case presentation
The patient was a 44-year-old female from Shandong, China. She was first admitted to our hospital on 17 June 2021, with the complaint of left eye pain and headache for 3 months. She had undergone surgery for congenital heart disease 30 years prior. She was admitted to the hospital and underwent relevant examinations. Nasal endoscopy revealed a mass in the left middle nasal meatus adjacent to the left middle nasal turbinate (Figure 1). Biopsy pathology revealed that the tumor cells were blue, rounded, heterogeneous cells, some of which were naked nucleated cells with minimal cytoplasm (Figures 2A, B). Immunohistochemical (IHC) staining revealed tumor cells that were positive for NUT expression (Figure 2C). The Ki-67 mitotic index was nearly 70%. Epstein-Barr virus (EBV)-encoded RNA (EBER) in situ hybridization was negative. Based on NUT IHC, a diagnosis of NUT cancer was established. Further testing revealed positive PD-L1 expression in both tumor cells and immune cells (Figure 2D). The percentages of tumor cells and immune cells with PD-L1 positivity were 65% and 1%, respectively. Enhanced computed tomography (CT) of the sinuses revealed that most of the mass was located in the left ethmoid sinus, with the mass invading the frontal sinus upward, invading the medial orbital wall and the rectus medialis to the left, with a discontinuity of bone in the medial orbital wall on the left side of the orbital wall, invading the intracranial area upward, and breaching the wall of the floor of the sieve sinus downward into the middle nasal passages (Figures 3A–C). 18F-fluorodeoxyglucose (18F-FDG) PET/CT demonstrated hypermetabolic activity with increased fluorodeoxyglucose uptake in the ethmoid sinus mass (maximum standardized uptake value, SUVmax 27.7) (Figure 3D). Regional lymph node involvement and metastatic disease were also excluded. Considering the results of nasal endoscopy, paranasal sinus CT, and PET/CT, the final diagnosis was sinonasal NUT carcinoma (cT4bN0M0, stage IVA AJCC-8 version). After discussion with our multidisciplinary team (MDT) of experts on head and neck tumors, including the Department of Radiology, Pathology, Otolaryngology, Radiation Oncology and Medical Oncology, we concluded that the tumor was inoperable and recommended definitive CCRT. The patient agreed with the treatment plan derived from the MDT discussion and underwent definitive CCRT in our department. The gross tumor volume (GTV) contained macroscopic primary tumor detectable on CT imaging. The clinical target volume (CTV) was defined as the GTV plus a 5- to 10-mm margin to encompass the sites of microscopic extension including bilateral parapharyngeal space, bilateral retropharyngeal lymph node drainage area, left II–IV lymph drainage area, right partial II area, left cavernous sinus, bilateral sieve sinus and pterygoid sinus, frontal sinus, left maxillary sinus, nasopharyngeal, oropharyngeal, bilateral intrinsic nasal cavities, cranial base, bilateral pterygoid plate, pterygoid, medial pterygoid muscle, and pterygopalatine fossa. A planning target volume (PTV) was generated by incorporating a three-dimensional margin of 3 mm to the target volume in order to account for the uncertainties associated with treatment setup and internal organ mobility. The prescribed doses were 70 Gy and 60.06 Gy in 33 fractions, for the PTVs derived from GTV and CTV, respectively. Volume-modulated arc therapy technology was used to administer radiotherapy. During radiotherapy, the patient received three cycles of synchronized cisplatin chemotherapy (50 mg/m2 days 1–2 q3w) and sodium glycididazole (1.25 g days 1, 3, and 5 qw). According to the Response Evaluation Criteria in Solid Tumors, the efficacy of chemoradiotherapy (1 month after CCRT) was evaluated as a partial response (PR). Afterward, the patient received three cycles of consolidation chemotherapy with albumin-bound paclitaxel (260 mg/m2 day 1 q3w) and nedaplatin (80 mg/m2 day 1 q3w). The efficacy of three cycles of consolidation chemotherapy was evaluated as PR (Figure 3E). Considering that NUT cancer is a highly malignant tumor with a poor prognosis, subsequent consolidation immunotherapy was agreed upon after thorough communication with the patient. Sixteen cycles of consolidation therapy with intermittent tislelizumab (200 mg day 1) were started on 7 January 2022, and the last immunotherapy treatment was given on 6 December 2023. Details of the timing of the use of tislelizumab are given in the Supplementary File. After 16 rounds of immunotherapy, the ethmoid sinus lesions achieved a state of sustained remission, and the efficacy assessment revealed a PR (Figure 3F). Adverse effects throughout treatment are tolerable. Acute toxicity during radiotherapy is mainly characterized by localized radiodermatitis of the facial skin. According to the Radiation Therapy Oncology Group’s acute radiation morbidity scoring criteria for skin, acute radiation dermatitis was grade 1. There was no late toxicity after radiotherapy, and, to date, the patient has not experienced vision loss. The immune-related adverse events included grade 2 hypothyroidism. The recent workup on 6 December 2023 demonstrated no local recurrence or distant metastasis. The entire treatment timeline of the patient is shown in Figure 4.

Figure 1. Nasal endoscopy revealed a mass (black arrow) in the left middle nasal meatus adjacent to the left middle turbinate (white arrow).
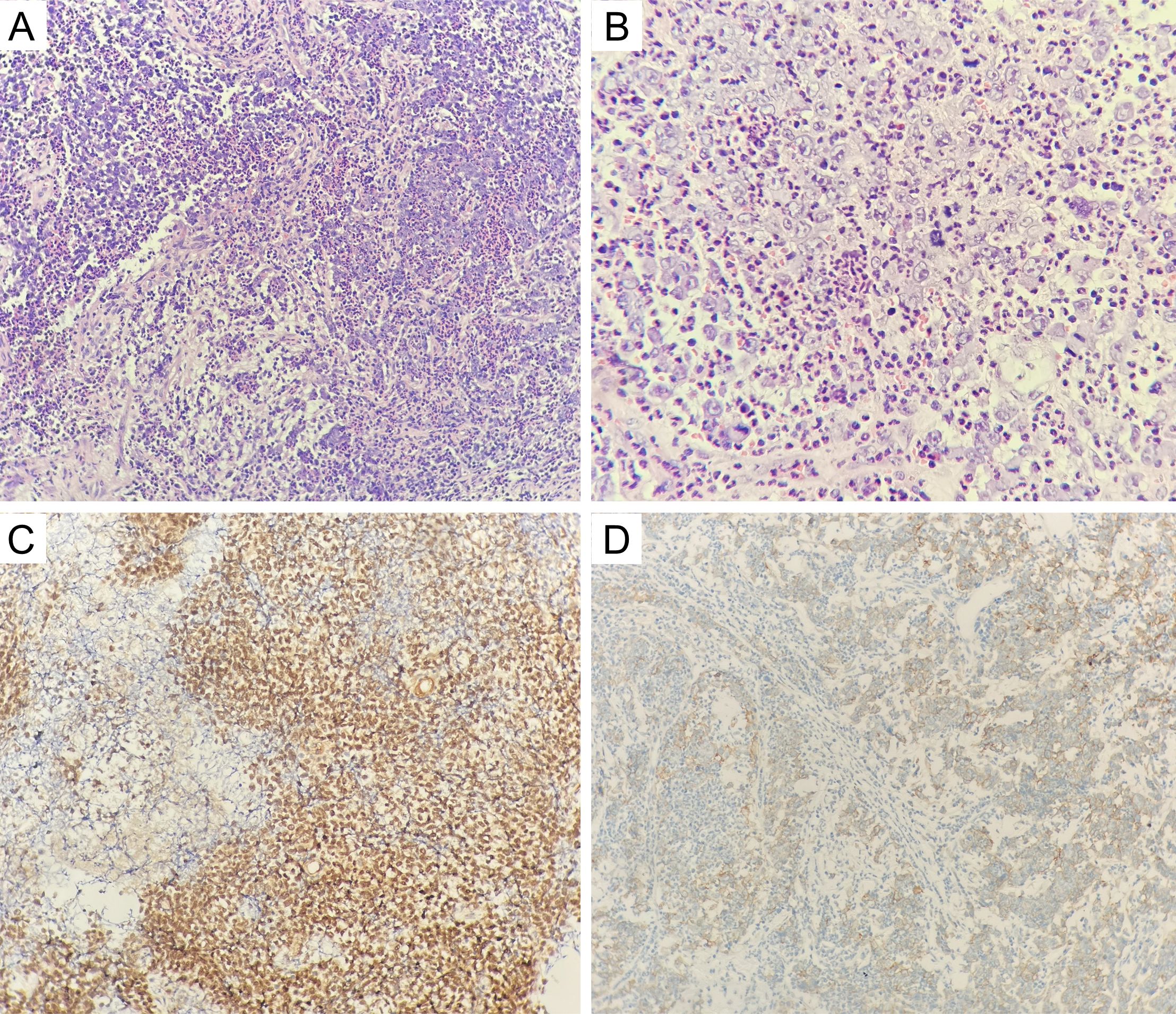
Figure 2. (A, B) The tumor cells were blue rounded heterogeneous cells, some of which were naked nucleated cells with minimal cytoplasm (hematoxylin and eosin; original magnification, ×200 and ×400); (C) positive NUT staining in the nucleus of tumor cells (hematoxylin and eosin; original magnification, ×200); (D) positive PD-L1 staining in tumor cells and immune cells (hematoxylin and eosin; original magnification, ×200).
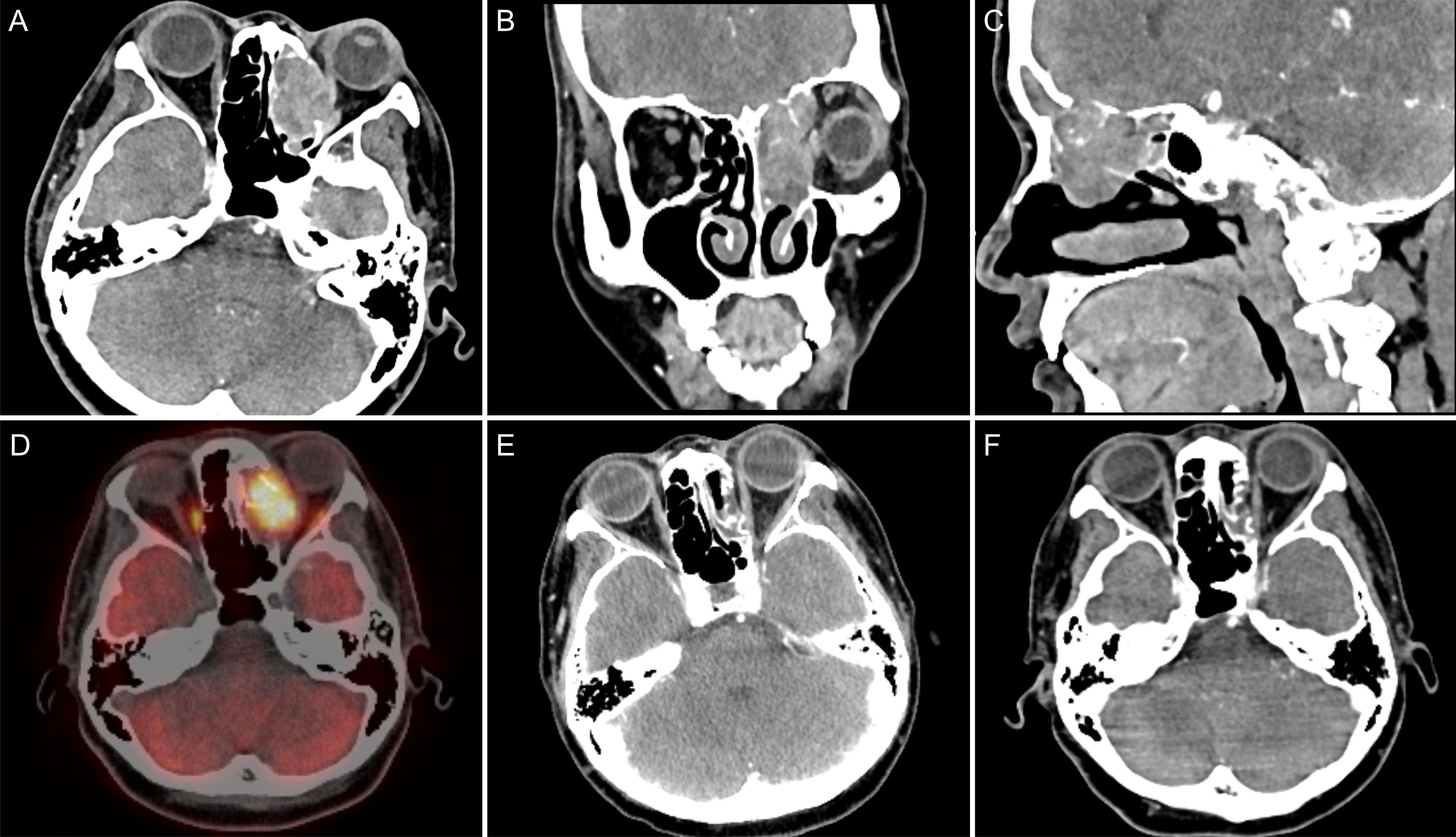
Figure 3. (A) CT axial view showed a mass in the left ethmoid sinus that was poorly demarcated from the left rectus medialis; (B) CT coronal view showed the mass invading the left orbit with intracranial invasion; (C) CT sagittal view shows the mass invading the frontal sinus with intracranial invasion; (D) PET/CT revealed a hypermetabolic mass with an SUVmax of 27.7 in the left ethmoid sinus; (E) CT after three cycles of consolidation chemotherapy revealed that the left ethmoid mass was considerably reduced compared with the previous mass, and the efficacy evaluation was PR; (F) the lesions reached a state of sustained remission after 13 cycles of immunotherapy, and the efficacy evaluation remained a PR.
3 Discussion
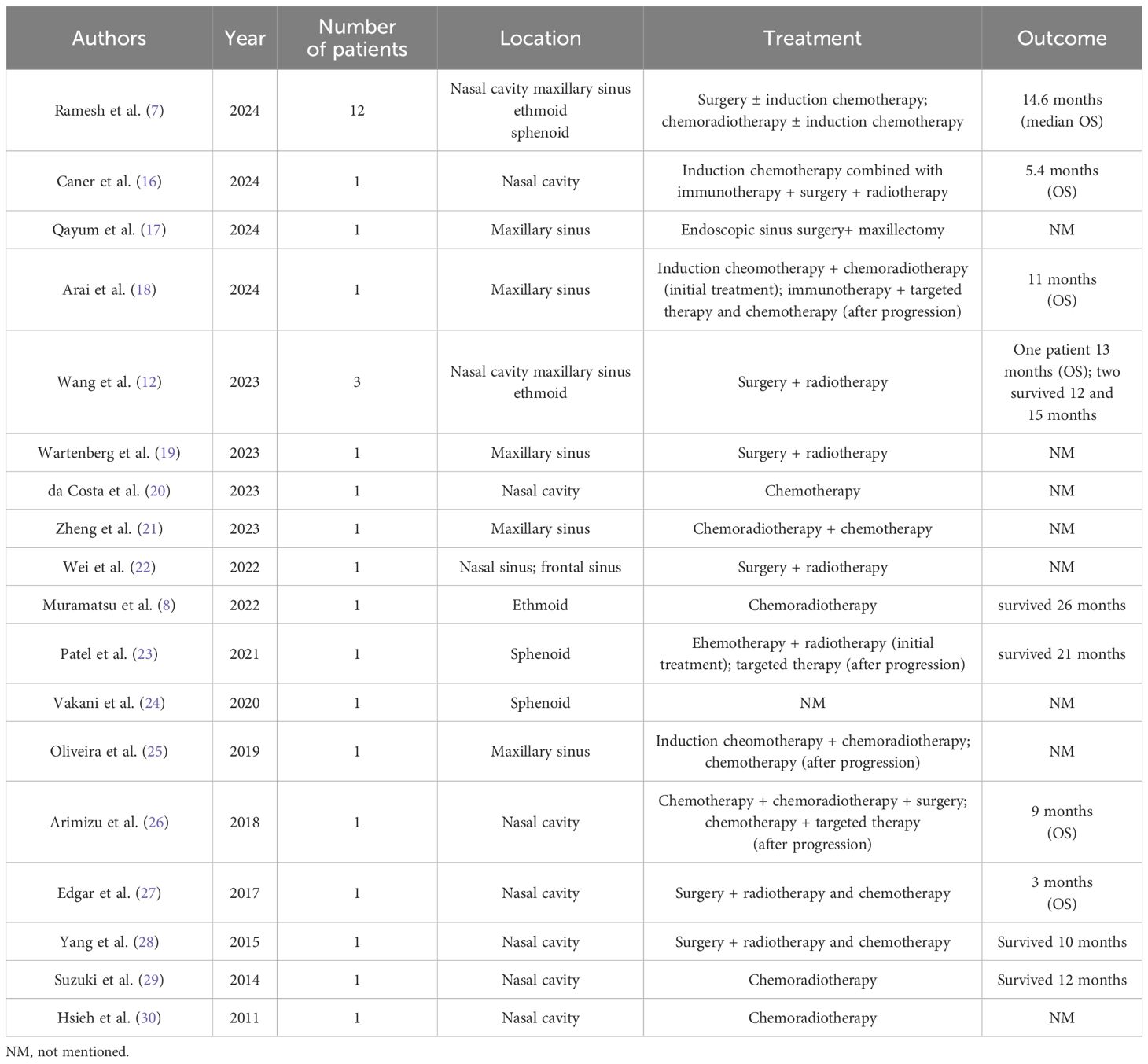
Sinonasal NUT carcinoma is very rare. There is a lack of large-scale epidemiologic studies and only a few retrospective studies with small sample sizes. A review of the literature by Lee et al. revealed that 4 of the 362 cases of poorly differentiated or undifferentiated carcinomas of the head and neck were sinonasal NUT carcinomas (11). A single-center study from China revealed that 3 of the 145 cases of sinonasal malignancies were NUT carcinomas (12). NUT has a very unfavorable prognosis. The median survival time for patients with primary NUT cancer of the chest is only 4.4 months (13). Compared to that for primary tumors in the lungs, the median survival time for primary NUT cancers of the head and neck is slightly greater, at only 9.7 months (13). In head and neck NUT cancers, survival may also vary depending on the location of the primary focus. A recent single-center study of 12 patients with sinonasal NUT carcinoma revealed median OS and median disease-specific survival times of up to 14.6 months (7).
Pathologic diagnosis of NUT cancer includes IHC and genetic testing, and genetic testing methods include fluorescence in situ hybridization (FISH), next-generation sequencing (NGS), and reverse transcription–polymerase chain reaction (RT-PCR) (14). Diffuse (>50%) positive NUT expression on IHC is sufficient for diagnosing NUT cancer (15). The diagnosis of NUTMI molecular rearrangements by FISH and NGS is not necessary, but the above two tests are helpful in determining the prognosis of NUT cancer patients (13). In this case, the patient was diffusely positive for NUT expression according to IHC, and the diagnosis was confirmed on this basis.
We reviewed the literature on sinonasal NUT cancer (7, 8, 12, 16–30) (Table 1). Treatment decisions were made on the basis of the above literature by first assessing the tumor stage. The treatments for locally advanced disease include surgery combined with postoperative adjuvant radiotherapy, definitive chemoradiotherapy, induction chemotherapy combined with surgery, and induction chemotherapy combined with radiotherapy. The treatments for metastatic disease include chemotherapy combined with immunotherapy and debulking surgery and chemotherapy combined with local palliative radiotherapy. Bromodomain and extra-terminal domain inhibitors may be an option after progression on first-line therapy for metastatic NUT cancer. In this case, tumor was considered late stage and considered to be unsuitable for surgical treatment by the MDT; ultimately, CCRT was selected as the treatment strategy. After CCRT, we first performed consolidation chemotherapy include albumin-bound paclitaxel and nedaplatin. There are few previous studies on consolidation chemotherapy after CCRT for sinonasal cancer. The consolidation chemotherapy regimen can refer to the induction chemotherapy regimen. The most commonly used induction chemotherapy regimens for sinonasal cancer are docetaxel and platinum (TP) and docetaxel, cisplatin, and fluorouracil (TPF) (31). TP includes paclitaxel and cisplatin, and TPF includes paclitaxel, cisplatin, and fluorouracil. These two consolidation chemotherapy regimens also provide a reference for consolidation chemotherapy for nasal sinus NUT cancer. At the end of CCRT and consolidation chemotherapy, the patient received consolidation immunotherapy based on three considerations. First, recent evidence has confirmed that consolidation therapy with immune checkpoint inhibitors (ICIs) can improve the survival of patients with lung and esophageal cancer after CCRT (32–34). In rare tumors, such as pulmonary sarcomatoid carcinoma (PSC) and pulmonary pleomorphic carcinoma (PPC), there are also case reports of encouraging results with consolidation immunotherapy after CCRT (35, 36). Although there is currently no evidence of survival benefits from ICI consolidation therapy after CCRT in patients with head and neck tumors, these findings in patients with lung and esophageal cancer can guide studies on head and neck tumors. A summary of clinical trials or case reports of consolidation immunotherapy after radiotherapy for malignant tumors is listed in Table 2 (32, 35–42). These clinical trials and case reports provide some reference for the selection of future consolidation ICI regimens after chemoradiotherapy for sinonasal NUT cancer patients. Second, although the patient’s treatment efficacy after radiotherapy was evaluated as PR, NUT cancer has a poor prognosis and is prone to recurrence and metastasis, and good recent treatment efficacy may not necessarily indicate a good long-term prognosis. Thus, maintenance therapy may be needed after radical treatment is completed. Maintenance therapy requires the selection of an agent that is both highly effective and less toxic, and PD-1 inhibitor immunotherapy may be an option. Third, the case in this study had high PD-L1 expression in tumor cells. High-dose anti–PD-1/anti–PD-L1 therapy is generally believed to indicate a greater response rate and clinical benefit when PD-L1 is expressed (43). According to a recent report from the NUT symposium, patients with PD-L1 positivity or a high tumor mutation load can receive ICIs in combination with chemotherapy (15). Additionally, there have been recent case reports of the combined use of immunotherapy in head and neck NUT cancers. One patient had thyroid NUT cancer combined with carelizumab immunotherapy in addition to postoperative chemotherapy (9). Another case involved parotid NUT cancer, which was treated with targeted agents combined with sintilimab immunotherapy after multiple postoperative metastases were detected (10).
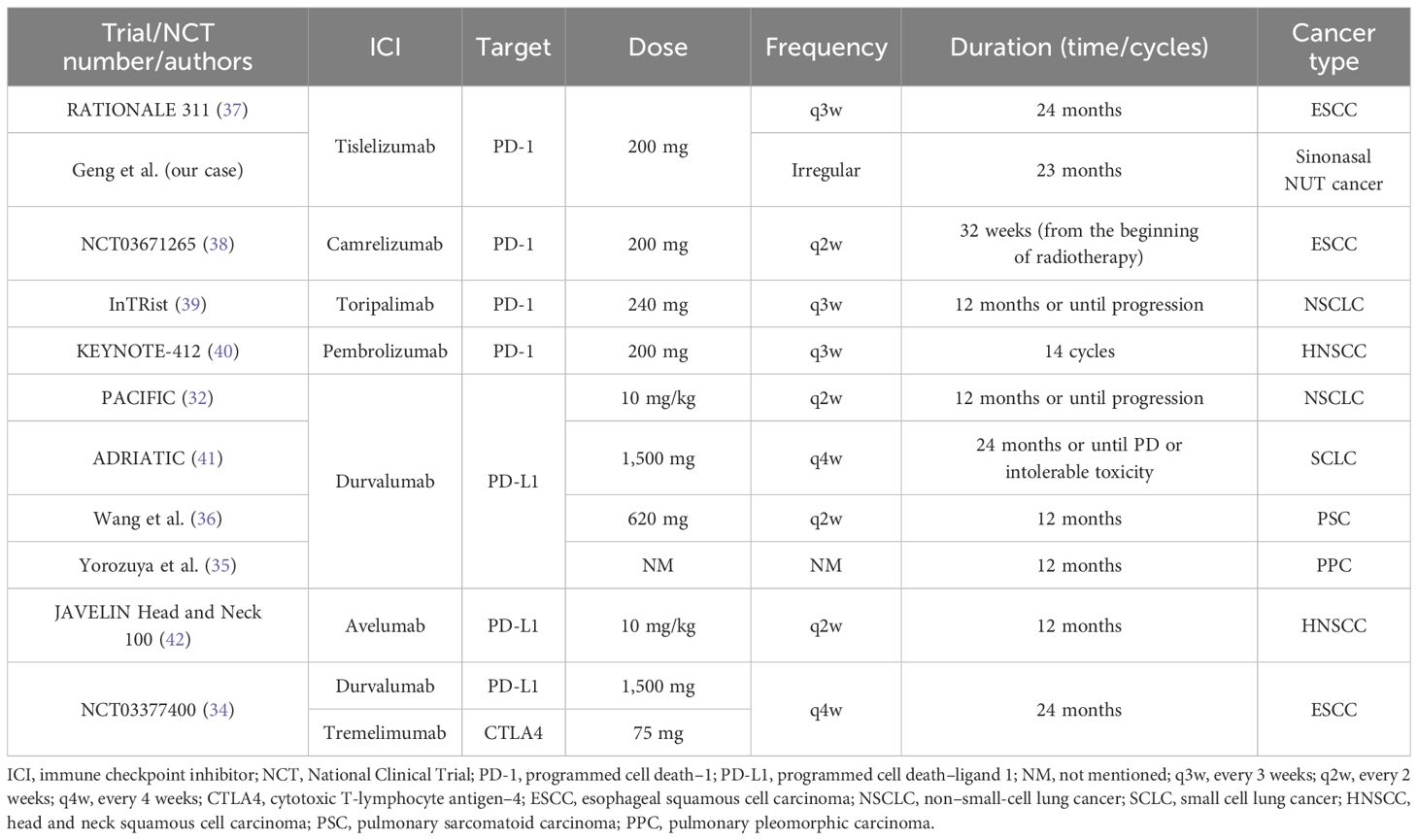
Table 2. Summary of clinical trials or case reports of consolidation ICI after chemoradiotherapy in patients with different cancer.
4 Conclusions
NUT carcinoma of the nasal cavity and sinuses is very rare, and only 50 cases have been reported in the literature. Surgery is the first choice for the treatment of NUT carcinoma, and CCRT can be chosen for patients who cannot be treated surgically. Compared with surgery, radiotherapy has the advantage of preserving organ function, thus improving the quality of life of patients. There is no consensus yet on consolidation treatment after CCRT. In this case, we studied a case of ethmoid NUT cancer that was treated with consolidation PD-1 inhibitor therapy after CCRT, which yielded a good therapeutic response. More case studies are needed in the future to validate the efficacy of consolidation immunotherapy after CCRT and to study the underlying mechanisms involved.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Weifang People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XG: Data curation, Writing – original draft, Writing – review & editing. XC: Data curation, Writing – review & editing. XW: Data curation, Writing – review & editing. SL: Writing – review & editing. GH: Data curation, Writing – review & editing. ZS: Data curation, Writing – review & editing. FH: Supervision, Writing – review & editing. JL: Conceptualization, Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Medical and Health Science and Technology Development Program Project of Shandong Province (Grant No.202109030776).
Acknowledgments
We thank the patient for her trust in us, her cooperation with the treatment, and her informed consent for the publication of this case report. We would also like to thank all the doctors and nurses who were responsible for this patient’s treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1368187/full#supplementary-material
References
1. Virarkar M, Mallery M, Saleh M, Ramani NS, Morani AC, Bhosale P. Clinical, radiographic, pathologic characterization and survival outcomes of nuclear protein of the testis carcinoma. J Comput Assist Tomogr. (2021) 45:431–41. doi: 10.1097/RCT.0000000000001163
2. Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr. (2020) 4:pkz094. doi: 10.1093/jncics/pkz094
3. French CA. The importance of diagnosing NUT midline carcinoma. Head Neck Pathol. (2013) 7:11–6. doi: 10.1007/s12105-013-0428-1
4. Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. (2016) 122:3632–40. doi: 10.1002/cncr.30242
5. Resteghini C, Castelnuovo P, Nicolai P, Vischioni B, Nicolai P, Castelnuovo P, et al. The SINTART 1 study. A phase II non-randomised controlled trial of induction chemotherapy, surgery, photon-, proton- and carbon ion-based radiotherapy integration in patients with locally advanced resectable sinonasal tumours. Eur J Cancer. (2023) 187:185–94. doi: 10.1016/j.ejca.2023.03.033
6. Bossi P, Orlandi E, Resteghini C, Vischioni B, Nicolai P, Castelnuovo P, et al. The SINTART 2 Study. A phase II non-randomised controlled trial of induction chemotherapy, photon-, proton- and carbon-ion-based radiotherapy integration in patients with locally advanced unresectable sinonasal tumours. Eur J Cancer. (2023) 187:134–43. doi: 10.1016/j.ejca.2023.03.034
7. Ramesh U, Contrera KJ, Shakibai N, Su SY, Brahimaj B, Roberts D, et al. Sinonasal NUT carcinoma: A consecutive case series and systematic review. Head Neck. (2024) 46:29–36. doi: 10.1002/hed.27553
8. Muramatsu J, Takada K, Sugita S, Tsuchiya T, Yamamoto K, Takagi M, et al. Complete response induced by concurrent chemoradiotherapy in a patient with NUT carcinoma. Intern Med. (2022) 61:1299–304. doi: 10.2169/internalmedicine.7741-21
9. Zhou J, Duan M, Jiao Q, Chen C, Xing A, Su P, et al. Primary thyroid NUT carcinoma with high PD-L1 expression and novel massive IGKV gene fusions: A case report with treatment implications and literature review. Front Oncol. (2021) 11:778296. doi: 10.3389/fonc.2021.778296
10. Fu S, Wang Z, Li C, Li Y, Zhang K, Zhong Z, et al. The whole treatment process and thinking of a patient with NUT carcinoma of the parotid gland: a case report. Front Oncol. (2023) 13:1094770. doi: 10.3389/fonc.2023.1094770
11. Lee T, Cho J, Baek CH, Son YI, Jeong HS, Chung MK, et al. Prevalence of NUT carcinoma in head and neck: Analysis of 362 cases with literature review. Head Neck. (2020) 42:924–38. doi: 10.1002/hed.26067
12. Wang L, Zhu Z, Wang W, Zha Y, Wang X, Surita A, et al. Sinonasal NUT carcinoma: A retrospective case series from a single institution. Front Surg. (2023) 10:1098704. doi: 10.3389/fsurg.2023.1098704
13. French CA, Cheng ML, Hanna GJ, DuBois SG, Chau NG, Hann CL, et al. Report of the first international symposium on NUT carcinoma. Clin Cancer Res. (2022) 28:2493–505. doi: 10.1158/1078-0432.CCR-22-0591
14. Zhang Y, Han K, Dong X, Hou Q, Li T, Li L, et al. Case report and literature review: primary pulmonary NUT-midline carcinoma. Front Oncol. (2021) 11:700781. doi: 10.3389/fonc.2021.700781
15. Bishop JA. Newly described tumor entities in sinonasal tract pathology. Head Neck Pathol. (2016) 10:23–31. doi: 10.1007/s12105-016-0688-7
16. Caner B, Orhan SO, Deligonul A, Evrensel T. Immunotherapy experience in sinonasal NUT midline carcinoma, case report. J Cancer Res Ther. (2024) 20:479–81. doi: 10.4103/jcrt.jcrt_1083_22
17. Qayum A, Khan MWZ, Arshad AR, Hasnain S, Tariq MD, Khan S, et al. A rare case of P63-negative sinonasal nut midline carcinoma in the elderly. Eur J Case Rep Intern Med. (2024) 11:4265. doi: 10.12890/2024_004265
18. Arai S, Tomioka R, Ueda Y, Shimizu A, Okamoto I, Tsukahara K. Maxillary sinus NUT carcinoma: A case report. Cancer Diagn Progn. (2024) 4:370–8. doi: 10.21873/cdp.10334
19. Wartenberg M, Hool S-L, Marrazzini A, Giger R, Rupp NJ. Differentiated papillary NUT carcinoma: an unexpected, deceptively bland presentation of a sinonasal carcinoma. Head Neck Pathol. (2023) 17:803–7. doi: 10.1007/s12105-023-01554-w
20. Everton Assunção Ribeiro da Costa R, Luz Santos I, Júlia Andrade Pereira Soares M, Dos Reis de Paula I, Gerônimo da Silva Júnior R, Eduardo Coelho de Sá C. A case of metastatic NUT carcinoma of the nasal cavity. Oral Oncol. (2023) 142:106432. doi: 10.1016/j.oraloncology.2023.106432
21. Zheng YY, Cao YY, Li JZ, Chen XM. NUT carcinoma of the maxillary sinus in a child: a case report. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2023) 58:1013–5. doi: 10.3760/cma.j.cn115330-20221202-00728
22. Wei X, Teng X, Zhang Y, Cheng M, Chen G. Case report: NUT carcinoma in an elderly woman with unique morphology and immunophenotype highlights a diagnostic pitfall. Transl Cancer Res. (2022) 11:1850–60. doi: 10.21037/tcr-22-364
23. Patel SA, Singer B, Shen C, Zanation AM, Yarbrough WG, Weiss J. A case of metastatic NUT carcinoma with prolonged response on gemcitabine and nab-paclitaxel. Clin Case Rep. (2021) 9:e04616. doi: 10.1002/ccr3.4616
24. Vakani PN, Maheshwari J, Maheshwari M, Shah B. Sinonasal NUT midline carcinoma: A new histological entity. Indian J Pathol Microbiol. (2020) 63:103–5. doi: 10.4103/ijpm.Ijpm_373_19
25. Oliveira LJC, Gongora ABL, Latancia MT, Barbosa FG, Gregorio JVAM, Testagrossa LA, et al. The first report of molecular characterized BRD4-NUT carcinoma in Brazil: a case report. J Med Case Rep. (2019) 13:279. doi: 10.1186/s13256-019-2213-6
26. Arimizu K, Hirano G, Makiyama C, Matsuo M, Sasaguri T, Makiyama A. NUT carcinoma of the nasal cavity that responded to a chemotherapy regimen for Ewing’s sarcoma family of tumors: a case report. BMC Cancer. (2018) 18:1134. doi: 10.1186/s12885-018-5087-x
27. Edgar M, Caruso AM, Kim E, Foss RD. NUT midline carcinoma of the nasal cavity. Head Neck Pathol. (2017) 11:389–92. doi: 10.1007/s12105-016-0763-0
28. Yang L, Yang S. NUT midline carcinoma of sinonasal tract: report of a case. Zhonghua Bing Li Xue Za Zhi. (2015) 44:912–3. doi: 10.3760/cma.j.issn.0529-5807.2015.12.018
29. Suzuki S, Kurabe N, Minato H, Ohkubo A, Ohnishi I, Tanioka F, et al. A rare Japanese case with a NUT midline carcinoma in the nasal cavity: a case report with immunohistochemical and genetic analyses. Pathol Res Pract. (2014) 210:383–8. doi: 10.1016/j.prp.2014.01.013
30. Hsieh M-S, French CA, Liang C-W, Hsiao C-H. NUT midline carcinoma: case report and review of the literature. Int J Surg Pathol. (2011) 19:808–12. doi: 10.1177/1066896909353600
31. Melder KL, Geltzeiler M. Induction chemotherapy for locoregionally advanced sinonasal squamous cell carcinoma and sinonasal undifferentiated carcinoma: A comprehensive review. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15153798
32. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. (2022) 40:1301–11. doi: 10.1200/JCO.21.01308
33. Zhang Y, Tian Y, Zheng L, Sun X, Zhao Z, Zheng Y, et al. Efficacy and safety of consolidation durvalumab after chemoradiation therapy for stage III non-small-cell lung cancer: a systematic review, meta-analysis, and meta-regression of real-world studies. Front Pharmacol. (2023) 14:1103927. doi: 10.3389/fphar.2023.1103927
34. Park S, Oh D, Choi Y-L, Chi SA, Kim K, Ahn MJ, et al. Durvalumab and tremelimumab with definitive chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Cancer. (2022) 128:2148–58. doi: 10.1002/cncr.34176
35. Yorozuya T, Taya T, Yasuda K, Nagano Y, Shioya M, Chiba H, et al. Long-term response with durvalumab after chemoradiotherapy for pulmonary pleomorphic carcinoma: A case report. Thorac Cancer. (2020) 11:1090–3. doi: 10.1111/1759-7714.13331
36. Wang Y, Yang L, Wang J, Gui L, Li W, Liu Z, et al. Case report: first case of consolidation immunotherapy after definitive chemoradiotherapy in mediastinal lymph node metastatic sarcomatoid carcinoma. Front Oncol. (2021) 11:788856. doi: 10.3389/fonc.2021.788856
37. Yu R, Wang W, Li T, Li J, Zhao K, Wang W, et al. RATIONALE 311: tislelizumab plus concurrent chemoradiotherapy for localized esophageal squamous cell carcinoma. Future Oncol. (2021) 17:4081–9. doi: 10.2217/fon-2021-0632
38. Zhang W, Yan C, Zhang T, Chen X, Dong J, Zhao J, et al. Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: a phase 1b study. Oncoimmunology. (2021) 10:1971418. doi: 10.1080/2162402x.2021.1971418
39. Wang Y, Deng L, Wang J, Zhang T, Wang W, Wang X, et al. Induction PD-1 inhibitor toripalimab plus chemotherapy followed by concurrent chemoradiotherapy and consolidation toripalimab for bulky locally advanced non-small-cell lung cancer: protocol for a randomized phase II trial (InTRist study). Front Immunol. (2023) 14:1341584. doi: 10.3389/fimmu.2023.1341584
40. Machiels J-P, Tao Y, Licitra L, Burtness B, Tahara M, Rischin D, et al. Pembrolizumab plus concurrent chemoradiotherapy versus placebo plus concurrent chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (KEYNOTE-412): a randomised, double-blind, phase 3 trial. Lancet Oncol. (2024) 25:572–87. doi: 10.1016/S1470-2045(24)00100-1
41. Senan S, Okamoto I, Lee GW, Chen Y, Niho S, Mak G, et al. Design and rationale for a phase III, randomized, placebo-controlled trial of durvalumab with or without tremelimumab after concurrent chemoradiotherapy for patients with limited-stage small-cell lung cancer: the ADRIATIC study. Clin Lung Cancer Mar. (2020) 21:e84–8. doi: 10.1016/j.cllc.2019.12.006
42. Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol Apr. (2021) 22:450–62. doi: 10.1016/s1470-2045(20)30737-3
Keywords: NUT, sinonasal, concurrent chemoradiotherapy, immunotherapy, PD-1 inhibitor
Citation: Geng X, Chang X, Wang X, Li S, Han G, Song Z, Hao F and Li J (2024) Consolidation immunotherapy following concurrent chemoradiotherapy in a patient with sinonasal NUT carcinoma: a case report. Front. Oncol. 14:1368187. doi: 10.3389/fonc.2024.1368187
Received: 10 January 2024; Accepted: 21 October 2024;
Published: 06 December 2024.
Edited by:
Timothy James Kinsella, Brown University, United StatesReviewed by:
Barbara Vischioni, National Center of Oncological Hadrontherapy, ItalyXinmao Song, Fudan University, China
Copyright © 2024 Geng, Chang, Wang, Li, Han, Song, Hao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianwen Li, TGp3d2YxMTJAMTYzLmNvbQ==
 Xiaotao Geng
Xiaotao Geng Xiaolong Chang1
Xiaolong Chang1 Xiaoli Wang
Xiaoli Wang Shunjia Li
Shunjia Li Furong Hao
Furong Hao