- 1Department of General Surgery, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Emergency Medicine Department, Xuanwu Hospital, Capital Medical University, Beijing, China
Background: The triple combination of programmed cell death protein–1 (PD-1) inhibitors plus anti-angiogenesis tyrosine kinase inhibitors (TKIs) with or without transarterial chemoembolization (TACE) or hepatic arterial infusion chemotherapy (HAIC) enhance the effect of treatment for unresectable hepatocellular carcinoma (uHCC). The present study compared the efficacy and safety of PD-1 plus TKI with or without transarterial chemo(embolization) for uHCC.
Methods: The meta-analysis was conducted using data acquired from PubMed, EMBASE, the Cochrane Library, Ovid, Web of Science, and Clinical Trials.gov from the inception date to December 2023. All clinical outcomes of interest included overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and adverse events (AEs). The hazard ratio (HR) and risk ratio (RR) with 95% confidence intervals (CIs) were used to measure the pooled effect. In addition, subgroup analysis was conducted to determine the specific patient population that benefited.
Results: The OS (HR = 0.47; 95% CI: 0.39–0.56, P < 0.05), PFS (HR = 0.52; 95% CI: 0.45–0.60, P < 0.05), and ORR (RR = 1.94; 95% CI: 1.60–2.35, P < 0.05) were significantly better in TACE/HAIC+TKI+PD-1(TACE/HAIC TP) group than TKI+PD-1(TP) group. The incidence of AEs was acceptable.
Conclusion: The triple therapy of TACE/HAIC TP had better efficacy for uHCC than TP, with acceptable security.
Systematic review registration: PROSPERO, identifier CRD42023475953.
1 Introduction
Hepatocellular carcinoma (HCC) is known as a leading cause of cancer-related mortality globally. The incidence and mortality of HCC still remain high, of which primary liver cancer is the most common (1, 2). The majority of patients with HCC lose the opportunity for surgery, ablation, and liver transplantation, as they are in the intermediate and late stage when diagnosed, resulting in a poor prognosis (3). So far, there is no clear guideline recommending the best treatment regime for the so-called unresectable hepatocellular carcinoma (uHCC).
According to the Barcelona Clinic Liver Cancer (BCLC) treatment strategy or Chinese National Liver Cancer (CNLC), transarterial chemoembolization (TACE) is the first globally recognized as the preferred treatment method for uHCC patients, but it also has certain limitations (4). Recently, hepatic artery infusion chemotherapy (HAIC) is usually applied for uHCC, especially for patients with poor response to TACE (5, 6). In Japan, HAIC has been recommended in treatment guidelines for HCC (7). For advanced-stage HCC, Sorafenib, a multi-kinase inhibitor, was approved be the first-line treatment by the U.S. Food and Drug Administration (FDA) in 2007 (8). In the first line of tyrosine kinase inhibitors (TKIs) in 2008, Lenvatinib was found to be non-inferior to Sorafenib for HCC (9).
Recently, immune checkpoint inhibitors (ICIs), particularly programmed death protein–1 (PD-1) inhibitors, were shown to be clinically beneficial for patients with advanced HCC, leading to more options for uHCC. The clinical trials demonstrated the combination therapy with lenvatinib and the PD-1 inhibitor nivolumab resulted in better OS and ORR in patients with intermediate or advanced-stage HCC (10). The IMbrave150 trial, the ORIENT-32 trial, and the Phase III trial of camrelizumab plus apatinib revealed that ICIs combined with anti-angiogenesis TKI also significantly improved the outcomes in patients with advanced HCC than the previous first-line treatment of TKI alone (11, 12). Therefore, new systemic therapy is the preferred option for uHCC patients currently; there is growing evidence that PD-1 inhibitors and anti-angiogenesis TKI in combination are recommended as systemic treatments in uHCC patients at present (13, 14). The reason is that multi-kinase inhibitor with potent antiangiogenic properties, which can reverse the immunosuppressive microenvironment of tumors and enhance the immune anti-tumor efficacy of PD-1. In addition, numerous clinical trials have evaluated that TKI+PD-1 can improve HAIC/TACE-induced hypoxia and modulate the immunosuppressive microenvironment of uHCC (15, 16). Nevertheless, the triple therapeutic strategies for uHCC are debatable, and the efficacy and safety of TACE/HAIC+ TKI +PD-1(TACE/HAIC TP) remain unclear.
TACE or HAIC are recommended as important treatments for uHCC. PD-1 plus anti-angiogenesis TKI has also resulted in satisfactory outcomes in the treatment of uHCC. Therefore, this meta-analysis was conducted to determine the efficacy and safety of TACE/HAIC + TKI +PD-1(TACE/HAIC TP) versus TKI +PD-1(TP) for uHCC.
2 Materials and methods
The article has been reported in line with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analyses) checklist (17). This meta-analysis was registered in PROSPERO (CRD42023475953).
2.1 Literature search strategy
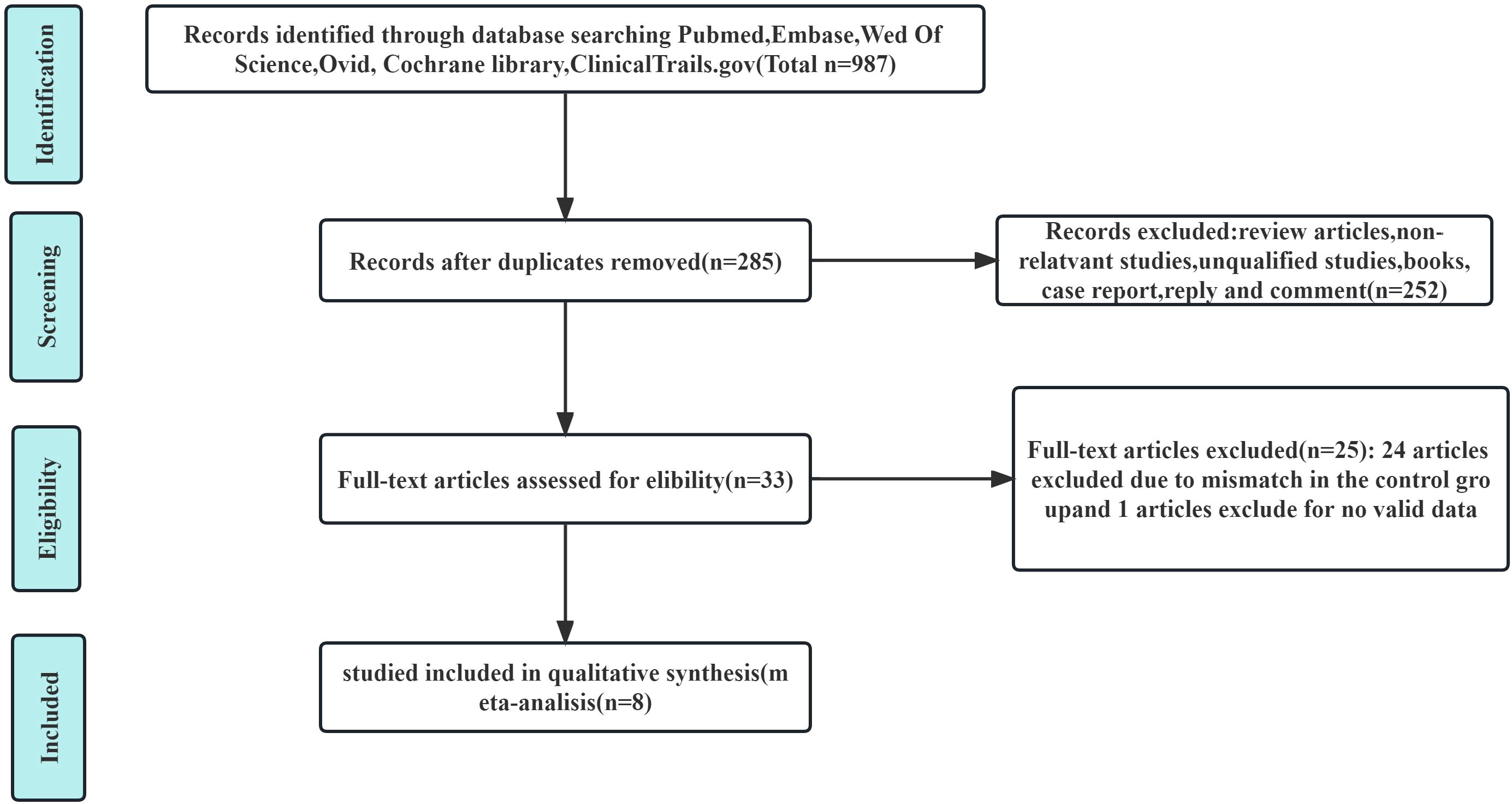
The publication time was limited to when the databases were established until December 2023. We conducted a systematic search of PubMed, EMBASE, the Cochrane Library, Ovid, Web of Science, and ClinicalTrials.gov databases to identify useful literature related to this meta-analysis. The MESH terms used in these databases included (“carcinoma, hepatocellular” or “liver cancer” or “HCC” or “liver neoplasm”), [“hepatic arterial infusion chemotherapy” (HAIC) or “TACE”], (“PD-1” or “ICI”) (“anti-angiogenesis TKIs”). There were no language limitations or other restrictions imposed in the literature search strategy. Thereafter, two authors (X.X. and X.X.) independently extracted and confirmed relevant data. The flowchart of the article screening and selection process are presented in the Figure 1.
2.2 Study selection
2.2.1 Inclusion criteria
1. The study patients with HCC in the intermediate and late stage according to BCLC and CNLC;
2. The patients with uHCC had received TACE/HAIC TP compared with TP;
3. The types of study include cohort studies and randomized controlled trials (RCTs);
4. The primary outcomes assessed were OS, PFS, ORR, and AEs, which include at least one evaluable survival outcome.
2.2.2 Exclusion criteria
1. Unable to obtain full text of literature;
2. The study types included a review, a meta-analysis, a conference abstract, a letter, and a case report;
3. The study lacked effective outcomes data or reported irrelevant outcomes;
4. Other confounding factors.
2.3 Data extraction and quality assessment
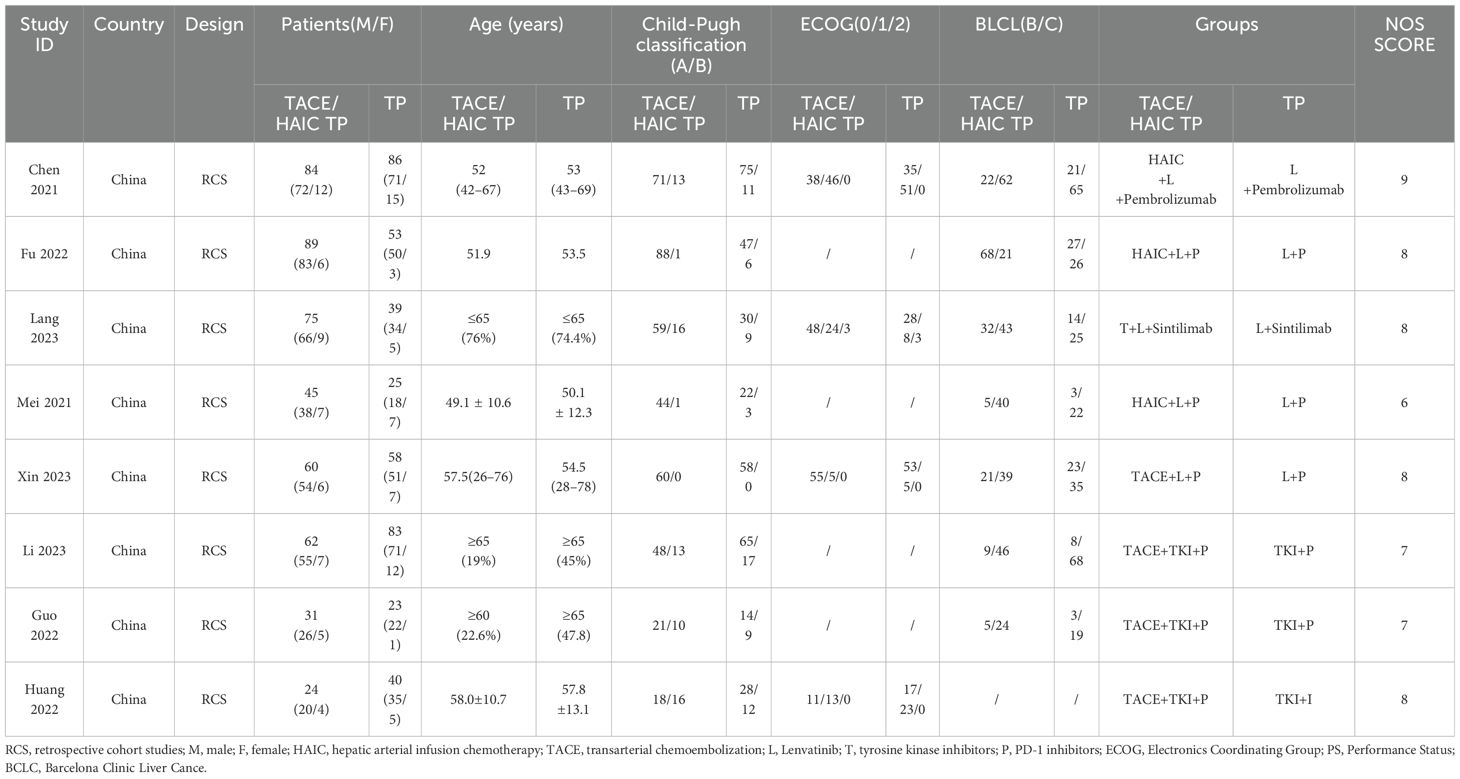
Two reviewers (X.X. and X.X.) independently screened the studies and evaluated the quality of the included studies in a standardized way. Any discrepancy was resolved through a discussion, and a third reviewer (X.X.) would decide if necessary. The data extracted from each study include the name of the first author, the year of publication, the nationality and the design of the study population, and the clinical characteristics of patients (including sex, age, and HCC stage).
Version 2 of the Cochrane tool for assessing the risk of bias in randomized trials (RoB2) was applied for RCT and the Newcastle-Ottawa Scale (NOS) was applied for cohort studies (18, 19). The quality assessment of each literature is presented in Table 1.
3 Statistical analysis
Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to analyze OS and PFS. The risk ratios (RRs) with 95% CIs were calculated to analyze ORR and AEs. A fixed effect model was used for data pooling if no significant heterogeneity among included trials was observed. Otherwise, a random effect model was used. Multiple subgroup analyses were conducted to investigate the possible sources of heterogeneity. The results of tests for trials are presented (P < 0.05 in the χ2 test suggested significant heterogeneity). The I2 statistic values of <25%, 25%–50%, and >50% were considered low, moderate, and high heterogeneity, respectively. The I2 statistic (I2 > 50% was deemed to have significant heterogeneity) and chi-square test (P < 0.10 was deemed to suggest a significant heterogeneity) were used to assess the heterogeneity among the trials.
The funnel plots were performed to detect the existence of publication bias (P < 0.10 was deemed to represent significant publication bias). All analyses were performed using the Revman5.4 software.
4 Results
4.1 Search results
A total of eight articles satisfied the inclusion and exclusion criteria, of which 285 were selected after removing duplicates (20–27). After reviewing the titles and abstracts of 285 articles, we excluded 252 articles. The full text of the remaining 33 articles were evaluated. Then 25 studies were excluded for lacking an appropriate control group or valid data. Ultimately, eight articles were included in the current meta-analysis.
All selected articles are retrospective cohort studies without RCT. There are three experimental groups with HAIC+TKI+PD-1 (HAIC TP) and five experimental groups with TACE+TKI+PD-1 (TACE TP) (20–27), while all control groups with TP.
4.2 Study characteristics
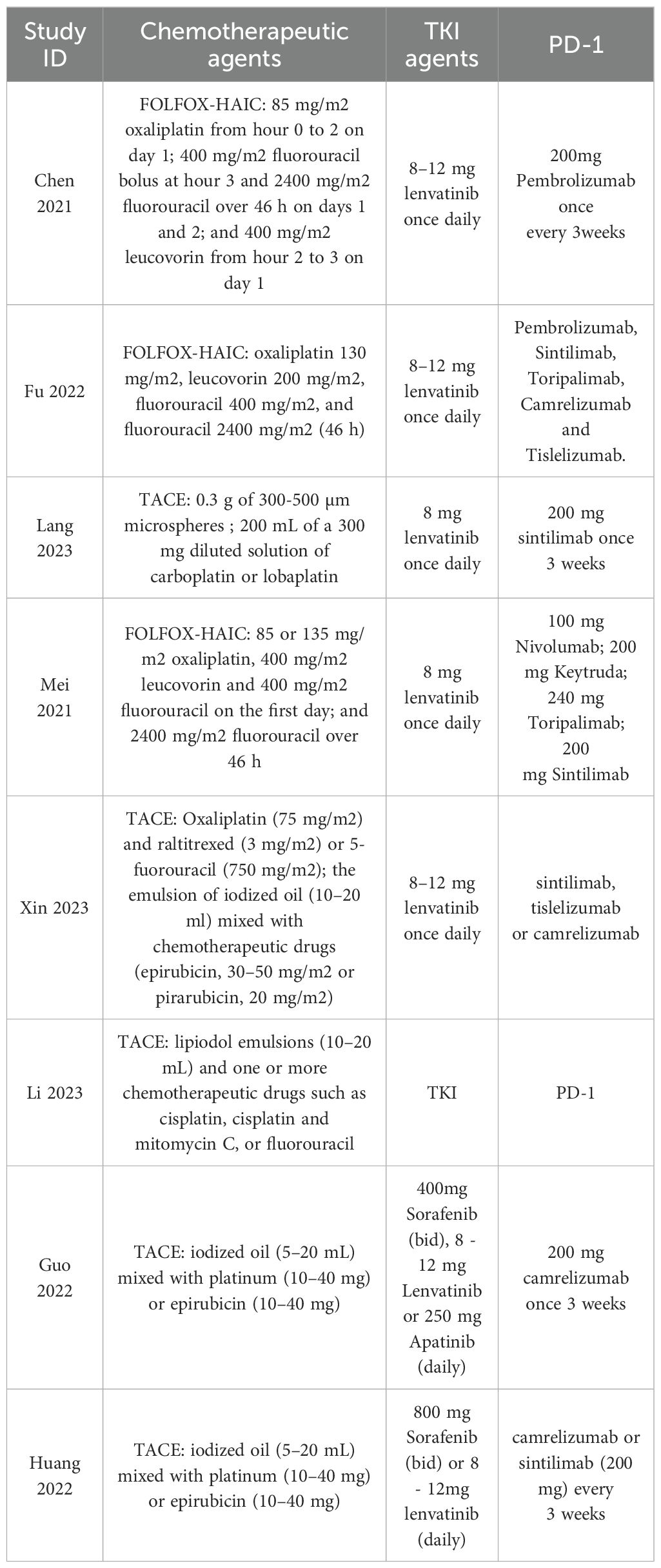
The included study characteristics are summarized in Table 1. All included articles were published in China from 2021 to 2023. Among the 877 patients included in our study, 766 were males and 111 were females. Furthermore, 470 patients with uHCC received the triple therapy of TACE/HAIC TP, whereas 407 patients received TP. The dose and duration of HAIC/TACE TP regimens are shown in Table 2.
Beneficiary populations are further identified through subgroup analyses based on the data of univariate analysis in each included trial.
4.3 Risk of bias
The results of the risk of bias analysis within studies are reported (Table 1). The methodological quality of the included studies was assessed using NOS as all included studies are retrospective studies. It contains the selection of subjects, comparability of the groups, and assessment of outcomes, with a maximum of 9 points. Studies with a score of more than 6 were determined to be high quality.
4.4 Meta-analysis outcomes
4.4.1 Overall survival, progression-free survival, and objective response rate
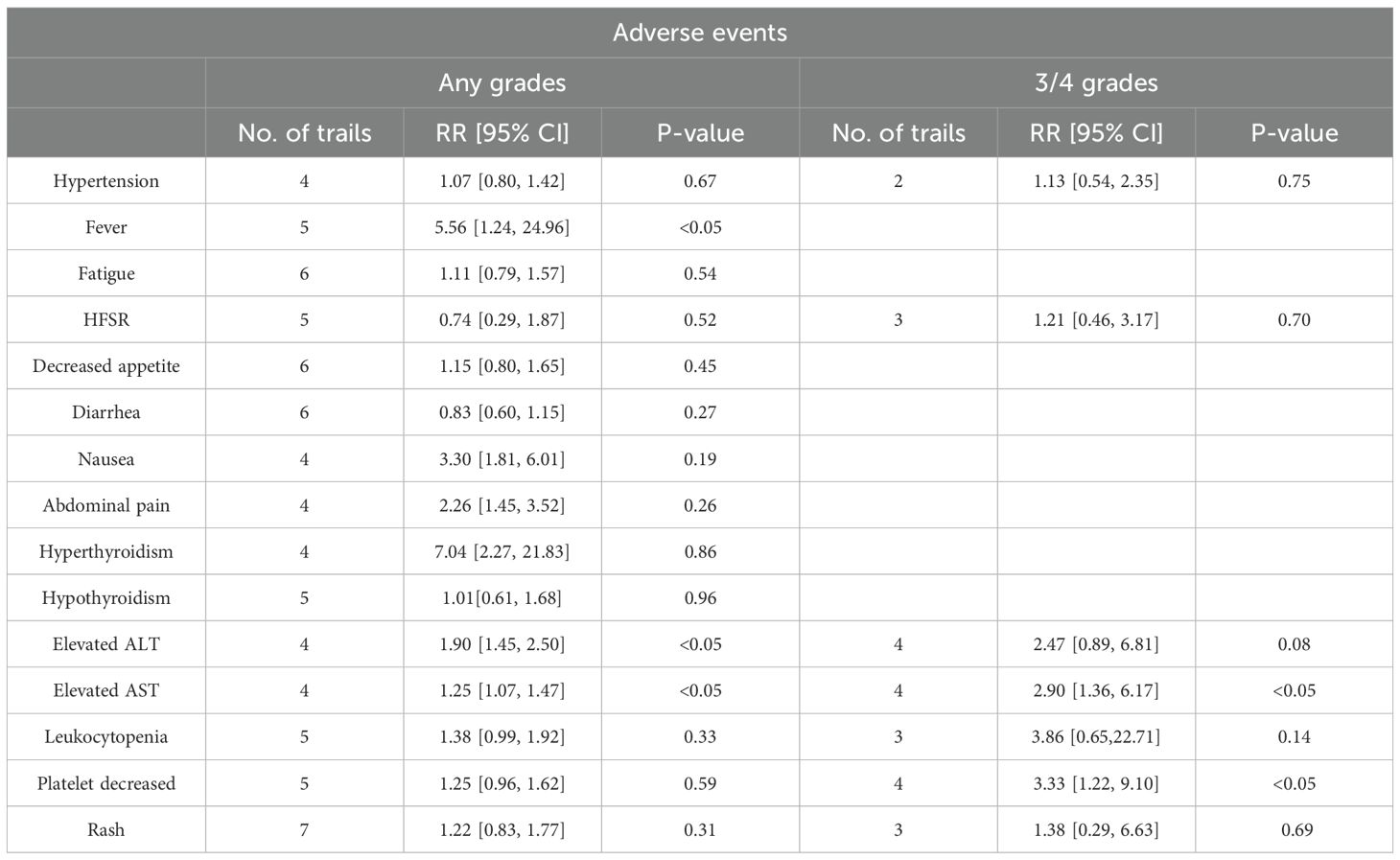
All eight articles in our study reported overall survival (OS) and PFS for the groups of TACE/HAIC TP and TP in Figure 2, including the point estimate (HR) and its 95% CI (17–24). Meta-analysis showed that triple therapy in the TACE/HAIC TP group had significantly longer OS (HR = 0.47; 95% CI: 0.39–0.56, P < 0.05) and PFS (HR = 0.52; 95% CI: 0.45–0.60, P < 0.05) than the TP group. As no significant heterogeneity was observed among the comparison of OS (I2 = 0%, P = 0.43), fixed effect models were adopted to estimate. While, for progression-free survival (PFS), significant heterogeneity was observed (P = 0.00001 < 0.1, I2 = 100%), random effect models were adopted.
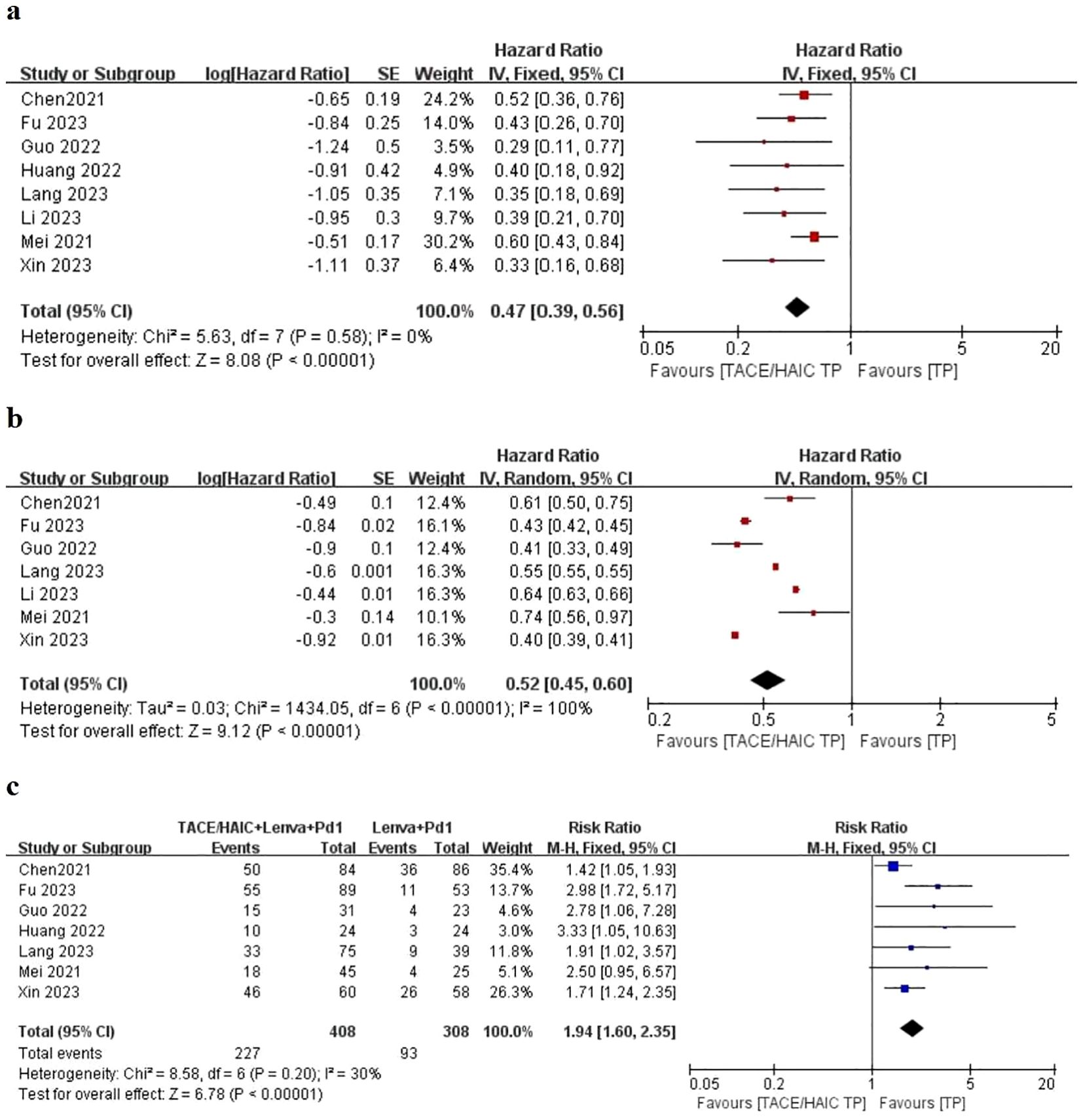
Figure 2. Forest plot about the pooled results of TACE/HAIC TP versus TP for unresectable HCC. Outcome: OS (A), PFS (B), and ORR (C) in total.
Patients in eight studies were assessed for ORR in Figure 2. The pooled analysis revealed that the objective response rate (ORR) (RR = 1.94; 95% CI: 1.60–2.35, P < 0.05) of the TACE/HAIC TP group was better than that of the TP group. A fixed effect model was used for data pooling as no significant heterogeneity among included trials was observed.
4.4.2 Subgroup analysis
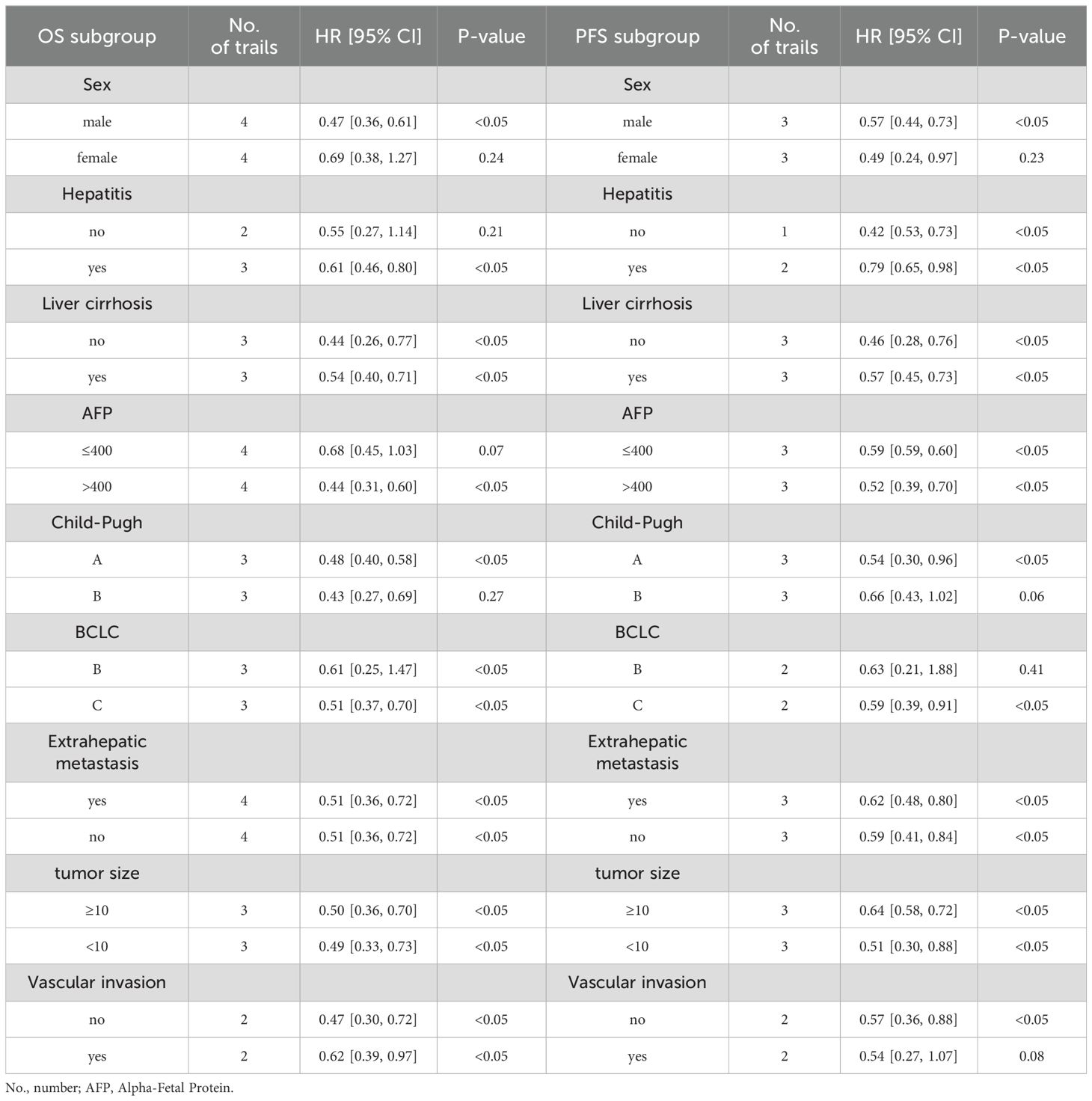
The subgroup of OS and PFS analysis based on the data of univariate analysis in each included trial showed similar better outcomes in triple therapies of TACE/HAIC TP. Across all subgroups analysis, TACE/HAIC TP was superior to TP for both PFS and OS (Table 3). As for PFS, patients without hepatitis (HR: 0.42; 95% CI 0.53–0.73; P < 0.05) had significantly better outcomes than those patients without hepatitis (HR: 0.79; 95% CI 0.65–0.98; P < 0.05).
In addition, the subgroup based on chemotherapeutic regime is shown in Figure 3. Similar outcomes of OS and PFS indicated that HAIC (HR: 0.53; 95% CI 0.43–0.67; P < 0.05 vs. HR: 0.36; 95% CI 0.26–0.49; P < 0.05), TACE (HR: 0.57; 95% CI 0.40–0.80; P < 0.05 vs. HR: 0.49; 95% CI 0.41–0.59; P < 0.05), and ORR (HR: 2.07; 95% CI 1.16–3.69; P < 0.05 vs. HR: 1.87; 95% CI 1.44–2.44; P < 0.05) were not associated with survival difference in two lines of therapy.
Figure 3. Forest plot about the pooled results of TACE TP versus HIAC TP for unresectable HCC. Outcome: OS (A), PFS (B), and ORR (C) in total.
4.4.3 Adverse events
All studies reported treatment-related AEs in the TACE/HAIC TP and TP groups. However, there is significant heterogeneity in the reporting methods and definitions used by the authors of each study, making it difficult and impossible for us to aggregate their results in one statistical method. In all studies, AEs are shown in Table 4. The incidence of any grade fever and elevated alanine aminotransferase (ALT) and elevated Aspartate aminotransferase (AST)in TACE/HAIC TP group was higher than that in the TP group (P < 0.05). Moreover, the incidence of 3–4 grades elevated AST and platelet decreased in TACE/HAIC TP group was higher than that in the TP group (P < 0.05). It is indicated that there was no obvious difference in most AEs between the TACE/HAIC TP group and the TP group.
5 Publication bias
Publication bias analysis was not performed for this meta-analysis as the number of included studies was less than 10.
6 Discussion
This study is the first systematic meta-analysis aimed at identifying almost all literature studies on the treatment of uHCC with TACE/HAIC TP versus TP, analyzing the efficacy and safety of this comparison and providing a basis for future clinical treatment of uHCC. We conducted a subgroup analysis based on the data of univariate analysis in each included trial to identify beneficial population. The results of this meta-analysis show that the TACE/HAIC TP triple combination regimen led to significantly longer OS, PFS, and better ORR compared with TP, suggesting that the addition of transarterial chemo(embolization) to TKI and PD-1 can prolong survival and improve the prognosis of patients with intermediate or advanced stage HCC. This result was further supported by subgroup analyses, which identified the triple therapy is more effective. The satisfied results may be due to a synergistic antitumor effect of the three treatments.
TACE can embolize tumor blood vessels by injecting iodine oil as well as carrying chemotherapy drugs to continuously kill cancer cells (28). In addition, HAIC refers to the insertion of a percutaneous catheter into the tumor’s blood-supplying artery to inject chemotherapy drugs directly into the liver tumor, which increases the concentration of chemotherapy drugs in tumors and avoids first-pass effects (29). With the advanced diagnosis and high recurrence rate of liver cancer, systematic treatment has become an essential choice and has made effective progress (30). In a phase II trial conducted in China, HAIC showed better efficacy and tolerable toxicity in patients with advanced HCC, which receive better ORR (40.8%) (13). TACE/HAIC involves inserting a catheter into the tumor’s blood supply artery and embolization of the target artery, causing ischemia and necrosis of the tumor tissue. However, arterial embolization cause more serious AEs. Recent evidence suggests that the use of associated liver partition and portal vein alignment (ALPPS) after arterial embolization can rapidly increase the residual volume of the liver in the future. However, due to the high incidence rate and mortality of ALPPS, there is still controversy (31).
Except for the first-line treatment of TKI for uHCC, immunotherapy is constantly breaking through, among which PD-1/PD-L1 is currently a relatively in-depth and thorough ICI in clinical research. PD-1/PD-L1 inhibitors reactivate the body’s immune response to tumor cells by blocking the interaction between PD-1 and PD-L1 (32, 33). Multiple studies have shown that triple therapy is more effective than other combination therapies or single therapy regimens, but there is currently no guidelines recommending the optimal treatment option for uHCC. Although the combination of atezolizumab and bevacizumab is a first-line systemic treatment for uHCC, considering the high cost of the dual immunotherapies and the prevalent risk of gastric bleeding due to cirrhosis, it is crucial to determine the optimal second-line treatment regime. These issues still need to be addressed through future research. Scholars are paying attention to the ongoing III phase of the immunotherapy combination experiment. The IMbrave150 trial showed significantly better OS (not reached vs. 13.2 months), PFS (6.8 vs. 4.3 months), and ORR (33.3% vs. 13.3%) with atezolizumab+bevacizumab than with Sorafenib for uHCC who had received no previous systemic treatment (14). The FDA officially approved atezolizumab+bevacizumab as the first-line treatment for uHCC in 2020, which has stimulated further clinical development of immunotherapy combination approaches for HCC (34). A subsequent randomized, open-label phase 2/3 study conducted in China to compare the efficacy of sintilimab+ IBI305 (a bevacizumab analog) with Sorafenib as a first-line treatment for uHCC, which showed significantly better OS (not reached vs. 10.4 months) and PFS (4.6 vs. 2.8 months) with sintilimab+IBI305 than with Sorafenib for uHCC (35). The CARES-310 trail demonstrated TKI+PD-1 showed a statistically significant and clinically meaningful benefit in PFS (5·6 months vs. 3·7 months) and OS (22·1 months vs. 15·2 months) compared with TKI for patients with uHCC, presenting as a new and effective first-line treatment option for this population (36). And similar outcomes presented that combining TKI and ICI provides an acceptable antitumor efficacy in first-line therapy for advanced-stage HCC patients (37).
The immunotherapy combination therapy is superior to first-line TKI treatment for HCC. The result contributed to several possible reasons as follows: (1) During TKI treatment, changes in immune cell surface antigens may affect the tumor microenvironment and promote tumor escape. Therefore, combination of ICI can restore the immune support microenvironment; (2) targeted anti-angiogenic drugs can restore blood vessel normalization and promote drug release, so a small amount of ICI can be applied to reduce the occurrence of AEs (38, 39).
We conduct this meta-analysis, which showed that TACE/HAIC TP is better than TP for uHCC, and we speculate the mechanism of synergistic anti-tumor effects of triple treatments may be as follows: (1) TKI mainly inhibits activities of vascular endothelial growth factor receptors (VEGFR1-3) and fibroblast and growth factor receptors (FGFR1-4), which the inhibition of VEGFR and FGFR can elicit antitumor immunity and enhance PD-1 checkpoint blockade in HCC (40). (2) Anti-angiogenesis normalizes tumor vessels and breaks the hypoxic microenvironment of tumors, which attenuating the activity of chemoresistance. (3) Tumor necrosis caused by TKIs and chemotherapy regimens can trigger immunogenic cell death, thereby improving the efficacy of immunotherapy (41). (4) The reason why t TACE/HAIC TP is superior to TP is that the chemotherapeutics can have synergistic effects with multiple drugs and effectively reduce the tumor burden (42). For treatment-related AEs, there were no fatal AEs in both the TACE/HAIC TP group and TP group. However, it is still necessary to pay attention to the strong toxic side effects that may be brought about by combined treatment strategies. It is essential to assess the severity of AEs, and immediately discontinue medication, and provide corresponding symptomatic support and treatment if necessary. In addition, based on subgroup analysis of chemotherapy regimens, the results of TACE regime are similar with HAIC regime may be due to the limited number of included trials. Currently, clinical trials of triple therapy based on immune drugs and targeted drugs are increasingly, which not only for the treatment of uHCC but also for patients who may undergo surgical conversion (43, 44). However, more research is needed to explore and reach consensus on the optimal period for transformation, in order to provide patients with reasonable, personalized, and more beneficial treatment regime.
There were several limitations in this meta-analysis. First, selection bias is difficult to avoid as all included studies are retrospective and there is not enough case to analyze as only eight studies were included. Second, considering that all the published studies came from China, the conclusion would not be applicable for the western populations. Last but not least, the diverse of TKI and PD-1 inhibitors affects the consistency of the trails. Additionally, the dosage and duration of HAIC or TACE regimens may cause bias in this study.
7 Conclusion
This meta-analysis demonstrated that TACE/HAIC+TKI+PD-1 was superior to TKI+PD-1 with respect to OS, PFS, ORR, and rare AEs for uHCC. Identification of a subgroup for uHCC who may be benefited most from the triple therapy of HAIC+TKI+PD-1 compared to TACE+TKI+PD-1. Further trails need to verify the conclusion.
Author contributions
YC: Writing – original draft. TL: Writing – review & editing. YL: Data curation, Writing – original draft. LJ: Data curation, Writing – original draft. WC: Data curation, Writing – original draft, Writing – review & editing. JW: Data curation, Writing – original draft. CZ: Data curation, Writing – original draft. CB: Data curation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank all teams and individuals who were involved in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. European Association for the Study of the Liver. Electronic address:ZWFzbG9mZmljZUBlYXNsb2ZmaWNlLmV1; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
3. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. (2020) 9:682–720. doi: 10.1159/000509424
4. Department of Medical Administration, National Health and Health Commission of the People’s Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition). Zhonghua Gan Zang Bing Za Zhi. (2020) 28:112–28. doi: 10.3760/cma.j.issn.1007-3418.2020.02.004
5. He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. (2017) 36:83. doi: 10.1186/s40880-017-0251-2
6. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: A randomized phase III trial. J Clin Oncol. (2022) 40:150–60. doi: 10.1200/JCO.21.00608
7. Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. (2019) 49:1109–13. doi: 10.1111/hepr.13411
8. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
9. Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. (2018) 15:599–616. doi: 10.1038/s41571-018-0073-4
10. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
11. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT- 32): a randomised, open-label, Phase 2–3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
12. Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, et al. LBA35-Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Ann Oncol. (2022) 33:S808–69. doi: 10.1016/annonc/annonc1089
13. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
14. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
15. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. (2022) 19:151–72. doi: 10.1038/s41571-021-00573-2
16. Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. (2018) 69:60–9. doi: 10.1016/j.jhep.2018.02.008
17. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
18. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
19. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
20. Chen S, Xu B, Wu Z, Wang P, Yu W, Liu Z, et al. Pembrolizumab plus lenvatinib with or without hepatic arterial infusion chemotherapy in selected populations of patients with treatment-naive unresectable hepatocellular carcinoma exhibiting PD-L1 staining: a multicenter retrospective study. BMC Cancer. (2021) 21:1126. doi: 10.1186/s12885-021-08858-6
21. Fu Y, Peng W, Zhang W, Yang Z, Hu Z, Pang Y, et al. Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and pd1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J Gastroenterol. (2023) 58:413–24. doi: 10.1007/s00535-023-01976-x
22. Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. (2021) 11:618206. doi: 10.3389/fonc.2021.618206
23. Lang M, Gan L, Ren S, Han R, Ma X, Li G, et al. Lenvatinib plus sintilimab with or without transarterial chemoembolization for intermediate or advanced stage hepatocellular carcinoma: a propensity score-matching cohort study. Am J Cancer Res. (2023) 13:2540–53.
24. Xin Y, Cao F, Yang H, Zhang X, Chen Y, Cao X, et al. Efficacy and safety of atezolizumab plus bevacizumab combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Front Immunol. (2022) 13:929141. doi: 10.3389/fimmu.2022.929141
25. Li H, Su K, Guo L, Jiang Y, Xu K, Gu T, et al. PD-1 inhibitors combined with antiangiogenic therapy with or without transarterial chemoembolization in the treatment of hepatocellular carcinoma: A propensity matching analysis. J Hepatocell Carcinoma. (2023) 10:1257–66. doi: 10.2147/JHC.S415843
26. Guo Z, Zhu H, Zhang X, Huang L, Wang X, Shi H, et al. The efficacy and safety of conventional transcatheter arterial chemoembolization combined with PD-1 inhibitor and anti-angiogenesis tyrosine kinase inhibitor treatment for patients with unresectable hepatocellular carcinoma: a real-world comparative study. Front Oncol. (2022) 12:941068. doi: 10.3389/fonc.2022.941068
27. Huang JT, Zhong BY, Jiang N, Li WC, Zhang S, Yin Y, et al. Transarterial chemoembolization combined with immune checkpoint inhibitors plus tyrosine kinase inhibitors versus immune checkpoint inhibitors plus tyrosine kinase inhibitors for advanced hepatocellular carcinoma. J Hepatocell Carcinoma. (2022) 9:1217–28. doi: 10.2147/JHC.S386672
28. Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis. (2016) 48:571–7. doi: 10.1016/j.dld.2016.02.005
29. Shao YY, Huang CC, Liang PC, Lin ZZ. Hepatic arterial infusion of chemotherapy for advanced hepatocellular carcinoma. Asia Pac J Clin Oncol. (2010) 6:80–8. doi: 10.1111/j.1743-7563.2010.01287.x
30. Meyers BM, Knox JJ, Liu DM, McLeod D, Ramjeesingh R, Tam VC, et al. The evolution of immune checkpoint inhibitor combinations in advanced hepatocellular carcinoma - A systematic review. Cancer Treat Rev. (2023) 118:102584. doi: 10.1016/j.ctrv.2023.102584
31. Romic B, Romic I, Mance M, Pavlek G, Skegro M. Successful associating liver partition and portal vein ligation after unsuccessful double TACE procedure complicated with sepsis and pancreatitis. Klin Onkol. (2016) 29:59–62. doi: 10.14735/amko201659
32. Rao Q, Li M, Xu W, Pang K, Guo X, Wang D, et al. Clinical benefits of PD-1/PD-L1 inhibitors in advanced hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int. (2020) 14:765–75. doi: 10.1007/s12072-020-10064-8
33. Harding JJ. Immune checkpoint blockade in advanced hepatocellular carcinoma: an update and critical review of ongoing clinical trials. Future Oncol. (2018) 14:2293–302. doi: 10.2217/fon-2018-0008
34. Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen YL, et al. FDA approval summary: atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin Cancer Res. (2021) 27:1836–41. doi: 10.1158/1078-0432.CCR-20-3407
35. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
36. Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. (2016) 7:12624. doi: 10.1038/ncomms12624
37. Schaaf MB, Garg AD, Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. (2018) 9:115. doi: 10.1038/s41419-017-0061-0
38. Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. (2014) 6:18. doi: 10.1186/2045-824X-6-18
39. Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. (2010) 10:505–14. doi: 10.1038/nrc2868
40. Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. (2023) 402:1133–46. doi: 10.1016/S0140-6736(23)00961-3
41. Lee SW, Yang SS, Lien HC, Peng YC, Tung CF, Lee TY. The combining of tyrosine kinase inhibitors and immune checkpoint inhibitors as first-line treatment for advanced stage hepatocellular carcinoma. J Clin Med. (2022) 11:4874. doi: 10.3390/jcm11164874
42. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. (2017) 67:999–1008. doi: 10.1016/j.jhep.2017.06.026
43. Haber PK, Puigvehí M, Castet F, Lourdusamy V, Montal R, Tabrizian P, et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002-2020). Gastroenterology. (2021) 161:879–98. doi: 10.1053/j.gastro.2021.06.008
44. Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, et al. Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. (2022) 11:227–52. doi: 10.21037/hbsn-21-328
Keywords: hepatic artery infusion chemotherapy, transarterial chemoembolization, tyrosine kinase inhibitors, PD-1 inhibitors, unresectable hepatocellular carcinoma
Citation: Chen Y, Jia L, Li Y, Cui W, Wang J, Zhang C, Bian C and Luo T (2024) Efficacy and safety of PD-1 inhibitors plus anti-angiogenesis tyrosine kinase inhibitors with or without transarterial chemo(embolization) for unresectable hepatocellular carcinoma: a meta-analysis. Front. Oncol. 14:1364345. doi: 10.3389/fonc.2024.1364345
Received: 10 May 2024; Accepted: 29 July 2024;
Published: 22 August 2024.
Edited by:
Peter Hamar, Semmelweis University, HungaryReviewed by:
Ivan Romic, University Hospital Centre Zagreb, CroatiaApurva Patel, Gujarat Cancer & Research Institute, India
Copyright © 2024 Chen, Jia, Li, Cui, Wang, Zhang, Bian and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Luo, dGFvbHVvMzVAMTI2LmNvbQ==
 Yue Chen1
Yue Chen1 Yu Li
Yu Li Jukun Wang
Jukun Wang Tao Luo
Tao Luo