- 1Division of Hematology, Department of Internal Medicine, Yonsei University College of Medicine, Gangnam Severance Hospital, Seoul, Republic of Korea
- 2Division of Haematology-Oncology, Department of Internal Medicine, Pusan National University School of Medicine, Busan, Republic of Korea
- 3Division of Haematology-Oncology, Department of Internal Medicine, Jeonbuk National University Medical School, Jeonju, Republic of Korea
- 4Division of Haematology-Oncology, Department of Internal Medicine, Chonnam National University Hwasun Hospital, Jeollanam-do, Republic of Korea
- 5Division of Hematology-Oncology, Department of Internal Medicine, Korea University Anam Hospital, Seoul, Republic of Korea
- 6Department of Internal Medicine, Dong-A University College of Medicine, Busan, Republic of Korea
- 7Division of Haematology-Oncology, Department of Internal Medicine, Inje University Busan Paik Hospital, Busan, Republic of Korea
- 8Division of Hemato-Oncology, Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, Republic of Korea
- 9Department of Internal Medicine, Ewha Women’s University College of Medicine, Seoul, Republic of Korea
- 10Division of Hematology-Oncology, Department of Internal Medicine, Korea University Guro Hospital, Seoul, Republic of Korea
- 11Division of Hematology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, Seoul, Republic of Korea
Background: Bone marrow (BM) involvement is an indicator of a poor prognosis in diffuse large B-cell lymphoma (DLBCL); however, few studies have evaluated the role of immunoglobulin gene rearrangement (IgR) in detecting BM involvement.
Methods: We evaluated the clinical characteristics and treatment outcomes of patients with DLBCL based on histological BM involvement or positive BM IgR using polymerase chain reaction or next-generation sequencing. We also investigated the role of consolidative upfront autologous hematopoietic stem cell transplantation (ASCT) in patients with DLBCL and BM involvement.
Results: Among 624 patients, 123 (19.7%) with histological BM involvement and 88 (17.5%) with positive IgR in histologically negative BM had more advanced disease characteristics. Overall (OS) and progression-free (PFS) survival was better for patients with negative BM histology and negative IgR than that in patients with histological BM involvement (P = 0.050 and P < 0.001, respectively) and positive IgR with negative BM histology (P = 0.001 and P = 0.005, respectively). Survival rates did not differ among 82 (13.1%) patients who were treated with upfront ASCT and had histological BM involvement or positive IgR with negative BM histology. The survival outcomes were worse for patients who were not treated with upfront ASCT and for those with histological BM involvement or positive IgR, than for those with negative BM histology and negative IgR.
Conclusion: Patients diagnosed with DLBCL and BM involvement based on histology or IgR had aggressive clinical features and poor survival. Upfront ASCT mitigated poor prognosis due to BM involvement.
1 Introduction
Although the treatment outcomes of diffuse large B-cell lymphoma (DLBCL) have improved with the development of new drugs, relapse is frequent and associated with dismal outcomes (1, 2). Bone marrow (BM) involvement is classified as extranodal and stage 4, which increases the international prognostic index (IPI) and is directly linked to shorter survival (3–5). The reported incidence of BM involvement is 11%-36% and the classic definition of BM involvement is abnormal lymphoma cells in BM aspirates or biopsies (3, 4, 6). However, minimal BM involvement of malignant lymphoma cells often generates false negative results because a histological diagnosis is very difficult in the absence of significant morphological changes (7–9). Bone marrow involvement can be diagnosed using 18F-FDG PET, but only within a limited range, and diagnostic rates vary depending on the lymphoma subtype (10–13). These problems have been addressed using the polymerase chain reaction (PCR) to detect immunoglobulin gene rearrangement (IgR) in BM samples because B-cell non-Hodgkin lymphoma (NHL) undergoes clonal IgR (9, 14). Clonal immunoglobulin heavy chain (IGH) and kappa chain (IGK) gene rearrangement could help the diagnostic process when histological findings are inconclusive. Moreover, gene rearrangement can be a helpful indicator during follow-up, as well as for diagnoses (15, 16). Patients with negative histological BM can be classified based on whether they test positive for IgR and negative for BM histology which indicates a more accurately determined advanced stage and a poorer prognosis (14, 17). Immunoglobulin gene rearrangement has mostly been detected using PCR; however, next-generation sequencing (NGS) has also been recently used (18). Detecting BM involvement in patients newly diagnosed with DLBCL indicates poor prognosis; to that end, IgR tests have been applied in a few studies to detect BM involvement in patients DLBCL treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemotherapy (14, 19–21).
Patients newly diagnosed with DLBCL accompanied by negative BM histology and poor outcomes of current standard treatment should be tested for IgR to precisely diagnose BM involvement. New treatment approaches should also be applied such as high-intensity chemotherapy to overcome BM involvement as a poor prognostic factor (5). Here, we investigated the clinical characteristics and treatment outcomes of upfront consolidative ASCT as part of a high-intensity chemotherapeutic regimen in patients with DLBCL and BM involvement determined by histological or molecular biological methods.
2 Materials and methods
This study enrolled patients from nine institutions in Korea who were newly diagnosed with DLBCL based on the World Health Organization classification (22) and histological BM involvement between 2010 and 2019. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Severance Hospital (4-2019-0579 Aug 5, 2019) and each institution.
The control group comprised patients from Severance Hospital who were newly diagnosed with DLBCL and were tested for IgR regardless of BM involvement status within the same period. The exclusion criteria were disease transformation from indolent follicular lymphoma, primary central nervous system lymphoma, cutaneous DLBCL, primary mediastinal B-cell lymphoma, and human immunodeficiency virus-associated DLBCL. All patients were administered with R-CHOP as first-line chemotherapy. Upfront consolidative ASCT was considered for patients with Ann Arbor stages III or IV and elevated lactic dehydrogenase (LDH) levels who achieved complete (CR) or partial (PR) remission after R-CHOP chemotherapy. The international prognostic index (IPI) score was calculated as described (23). Responses were assessed based on the Cheson criteria (24).
2.1 Histological diagnosis of bone marrow involvement
We obtained aspirates and BM biopsies from the posterior superior iliac crest from all enrolled patients before starting chemotherapy for DLBCL. Bone marrow involvement was diagnosed based on histological criteria and immunochemical staining for B-cell markers (3, 8). Concordant BM involvement was defined as BM involvement of DLBCL, while discordant involvement was defined as involvement of small and low-grade lymphoma cells (3). The present study investigated only concordant BM involvement. Thirteen patients had discordant BM involvement without DLBCL involvement, and these patients were classified as negative. Cells of origin were classified based on the Hans algorithm using immunochemical staining (25).
2.2 Immunoglobulin gene rearrangement test to diagnose bone marrow involvement
We assessed clonal gene rearrangement in BM aspirates from 504 patients. The assays included BIOMED-2 multiplex primer sets in five master mixes that targeted the IGH and two master mixes that target the IGK locus. Fragment analysis was applied to fluorescence-labeled PCR products using an ABI 3130 DNA sequencer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and GeneMapper 3.2 software (Thermo Fisher Scientific Inc.). Next-generation sequencing (NGS) was applied from April 2017 using LymphoTrack® IGH FR1 and IGK Assays (Invivoscribe Technologies Inc., San Diego, CA, USA). After PCR amplification, libraries were purified using the Agencourt AMPure XP system (Beckman Coulter, Inc., Brea, CA, USA). Quantified libraries were sequenced on a MiSeq system using MiSeq Reagent Kit v2 (Illumina Inc., San Diego, CA, USA). Bioinformatics were analyzed using LymphoTrack® Dx MiSeq Data Analysis version 2.4.3 (Invivoscribe Technologies, Inc.). The cut-offs for clonality and clonotype sequences were determined as described by the manufacturers. We assessed IgR in patients without histological BM involvement. Positive IgR was defined as positive IGH and/or IGK gene rearrangement. The sensitivity of PCR is 10-3 and that of NGS is 10-4.
2.3 Statistical analysis
Overall survival (OS) was determined as elapsed time between the dates of diagnosis and death, regardless of the cause. Surviving patients were censored at the last date of follow-up. Progression-free survival (PFS) was defined as elapsed time between the dates of diagnosis to progression, relapse, or death from any cause. Survival was analyzed using Kaplan-Meier curves, and pairs of groups were compared using log-rank tests. A Cox proportional hazard model was used for multivariate analysis. The multicollinearity of all variables in univariate analyses was assessed as tolerance and a variance inflation factor using linear regression analysis. Values with P < 0.05 in all analyses were considered statistically significant. All data were statistically analyzed using SPSS for Windows, version 23.0 (IBM Corp., Armonk, NY, USA).
Results
2.4 Patients’ characteristics
Among 624 patients newly diagnosed with DLBCL, 123 (19.7%) had histological BM involvement. Among 501 patients without histological BM involvement, 88 (17.5%) were IgR positive, 29 (5.7%) and 26 (5.1%) had positive IGH and IGK rearrangement, respectively, and 33 (6.5%) had rearranged IGH and IGK (Figure 1). Patients with histological BM involvement or positive IgR with negative BM histology tended to be older (P = 0.02 and P < 0.001, respectively). Moreover, these patients had advanced-stage DLBCL with extranodal involvement at more than one site and elevated LDH, as well as significantly higher IPI scores than patients who were negative for both (Table 1). We tested 276 (55.1%) patients for clonal IgR using PCR. Fifty (50/276, 18.1%) patients showed positive results by PCR, whereas 38 (16.9%) of 225 patients had positive results of NGS. The rates of positivity rates did not significantly differ between the two test methods (P = 0.814).
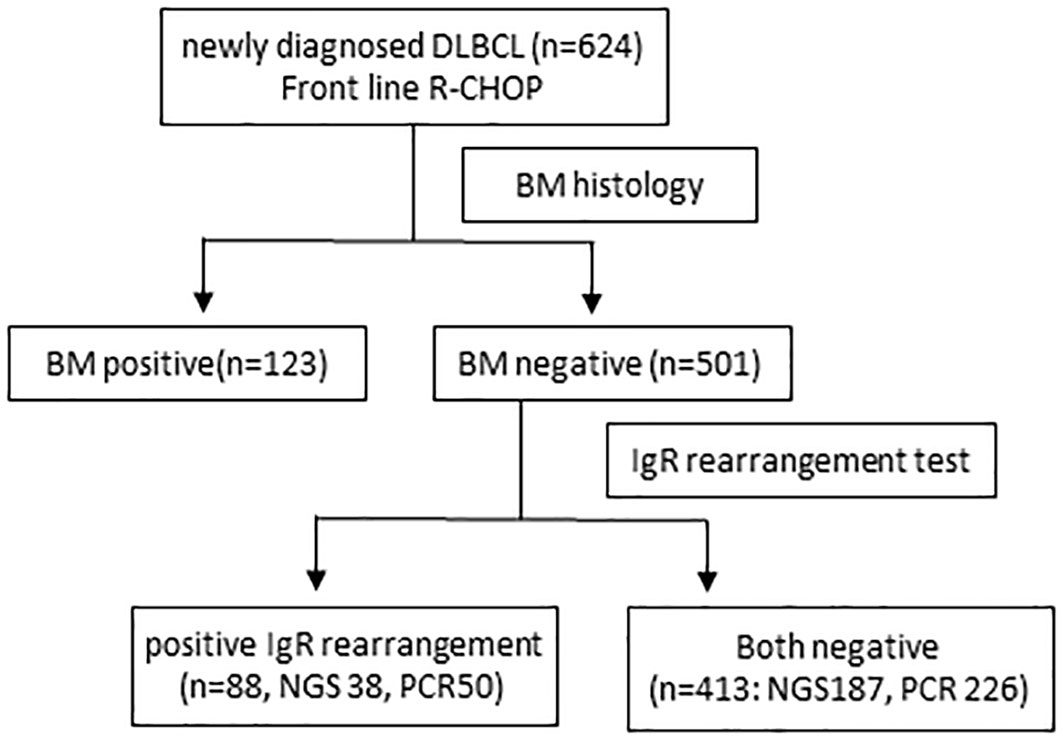
Figure 1 Flowchart of patients. BM, bone marrow; DLBCL, diffuse large B-cell lymphoma; IgR, immunoglobulin gene rearrangement R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone.
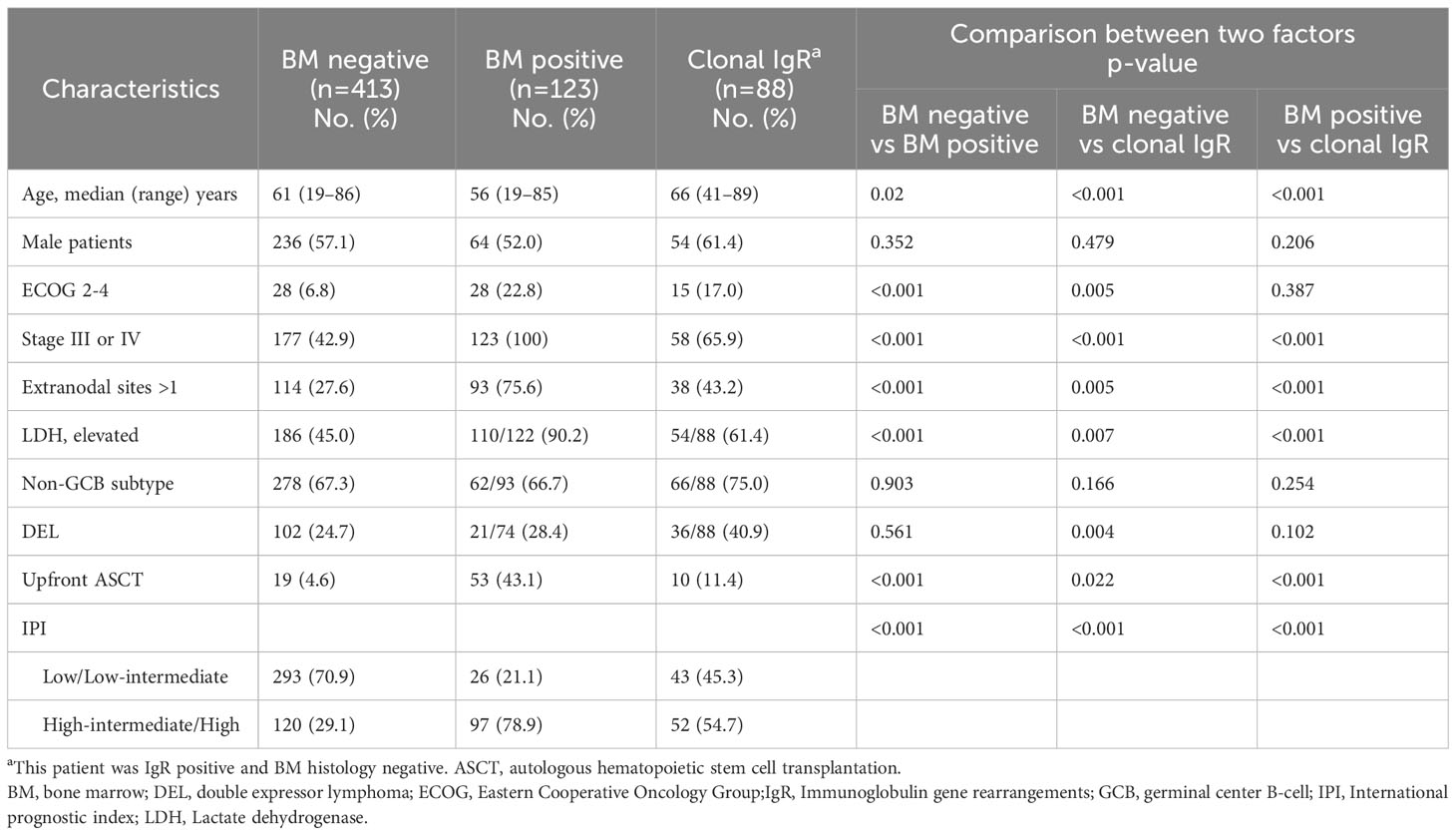
Table 1 Clinical characteristics of 624 patients according to BM involvement or immunoglobulin gene rearrangement.
2.5 Treatment outcomes according to bone marrow involvement
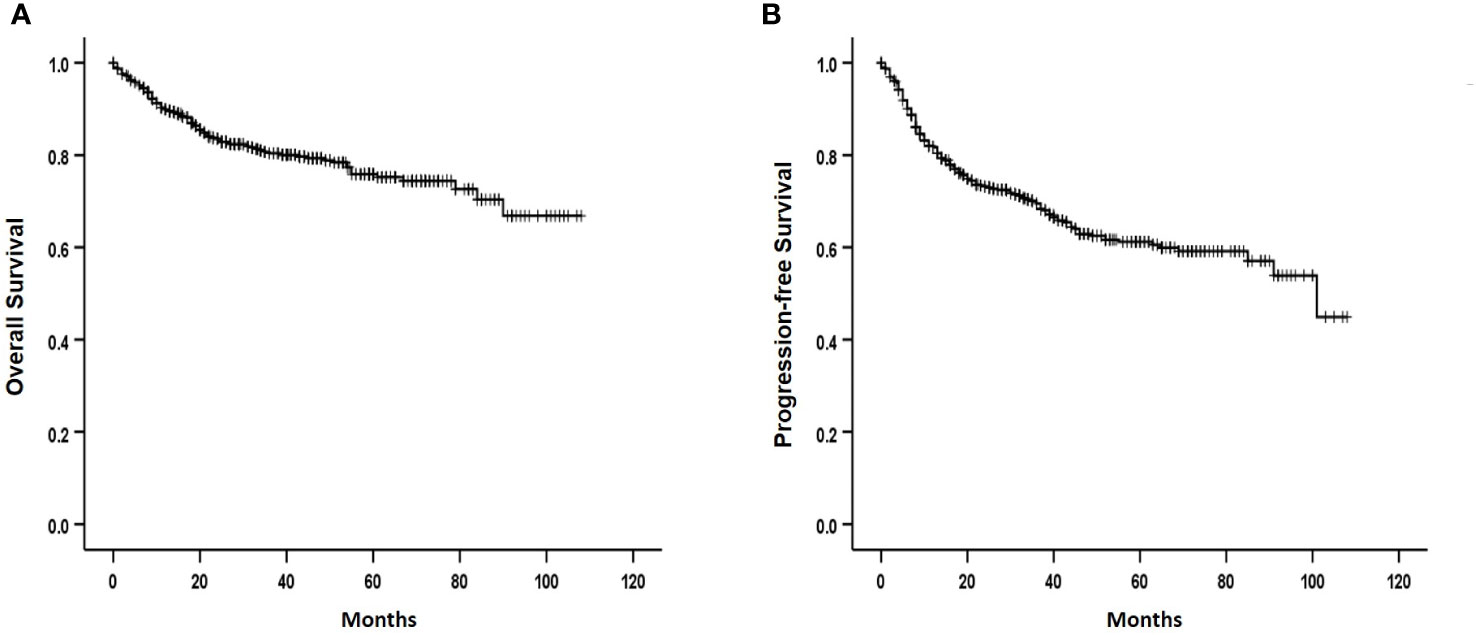
Among the registered patients who received R-CHOP chemotherapy as the first-line treatment, 587 responded. A CR was achieved in 465 (79.2%) of 587 evaluable patients, which included 93 (78.2%) of 119) with histological BM involvement. These findings did not significantly differ from those of patients without BM involvement (P = 0.428). Meanwhile, 56 (70.0%) of 80 evaluable patients with positive IgR achieved CR. This was significantly lower than the 316 (76.5%) of 388 patients without histologic and molecular BM involvement (P = 0.032). The median follow-up was 32 (range: 1-108) months, and the 3-year OS and PFS rates were 80.4% and 69.5%, respectively (Figures 2A, B). The 3-year OS and PFS were 74.9% and 56.0% in patients with histological BM involvement and 72.4% and 62.4% in those with positive IgR and negative BM histology. These were lower than the survival outcomes of patients with negative IgR and BM histology (83.9% and 75.7%, respectively; (Figures 3A, B).

Figure 3 Overall (A) and progression-free (B) survival according to bone marrow involvement by histology and immunoglobulin gene rearrangement. BM, bone marrow, IgR, immunoglobulin gene rearrangement.
2.6 Treatment outcomes according to autologous hematopoietic stem cell transplantation
Upfront consolidative ASCT was administered to 82 (13.1%) patients after they completed frontline R-CHOP chemotherapy. Among them, 53 (64.6%) had histological BM involvement, 10 (12.2%) had positive IgR and negative BM histology, and 19 (23.2%) did not have histological BM involvement and were IgR negative. Treatment outcomes were analyzed according to upfront ASCT in patients with advanced-stage and elevated LDH levels. The OS and PFS rates were better for patients who were administered upfront ASCT than for patients who were not (P = 0.010 and P = 0.004, respectively). The OS and PFS outcomes of patients who received upfront ASCT to minimize selection bias associated with treatment intensity did not significantly differ according to histological BM involvement (P = 0.388 and P = 0.663, respectively) or positive IgR (P = 0.685 and P = 0.528, respectively; Figures 4A, B). The 3-year OS and PFS rates among patients who did not receive upfront ASCT were poorer for those with BM involvement than for those without (65.0% vs. 85.1%, P = 0.001, and 49.2% vs. 77.0%, P < 0.001, respectively). The OS and PFS rates were also lower for patients with positive IgR than for those without histological BM involvement and negative IgR (72.0% vs. 85.1%, P < 0.001 and 60.6% vs. 77.0%, P < 0.001, respectively; Figures 4C, D).
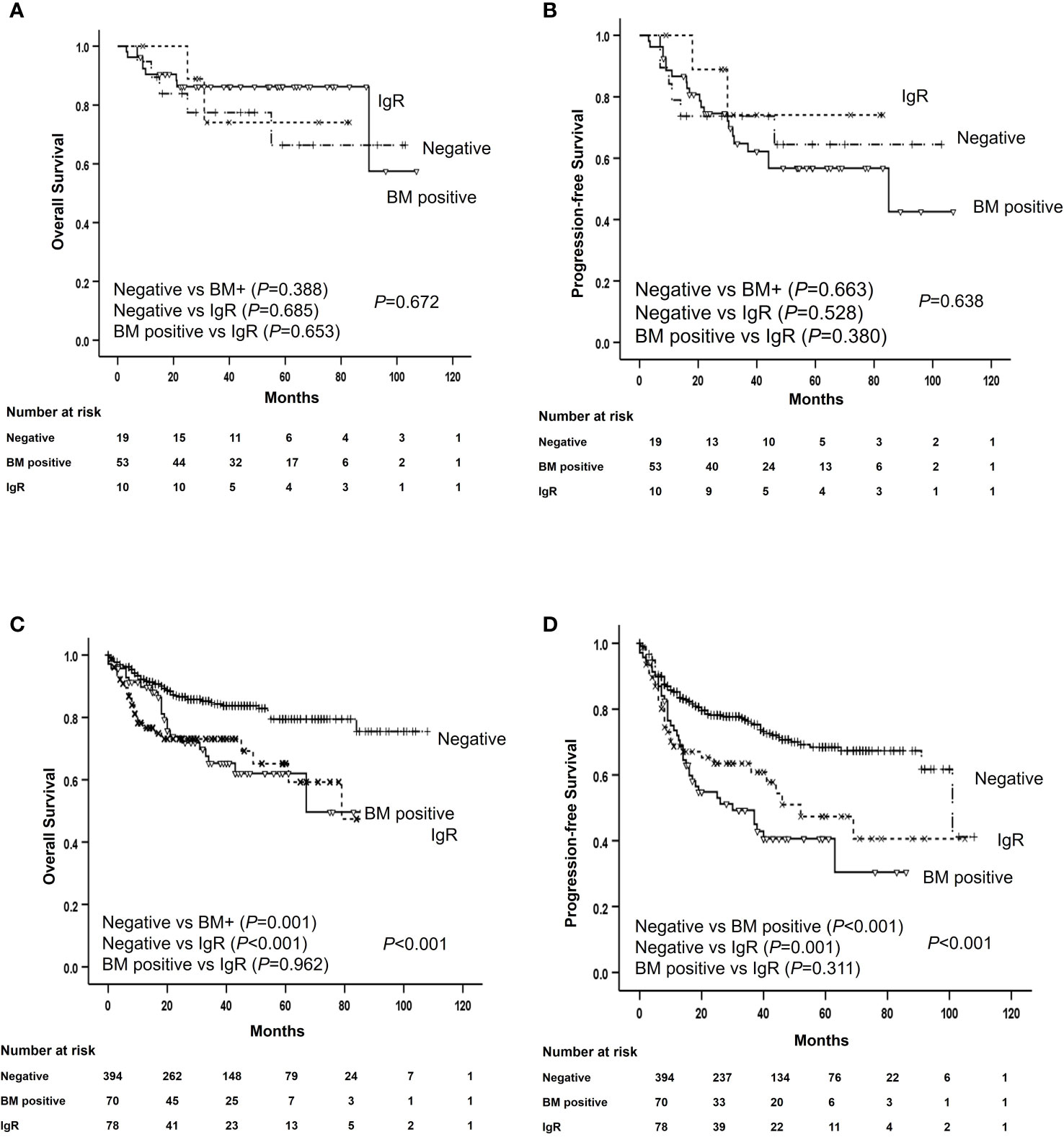
Figure 4 Survival of patients with and without ASCT according to histological bone marrow involvement and IGR. Overall and progression-free survival of patients with (A, B) and without (C, D) ASCT according to histological bone marrow involvement and immunoglobulin gene rearrangement. ASCT, autologous stem cell transplantation; BM, bone marrow; IgR, immunoglobulin gene rearrangement; OS, overall survival; PFS, progression-free survival.
2.7 Univariate analysis of prognostic factors associated with poor survival
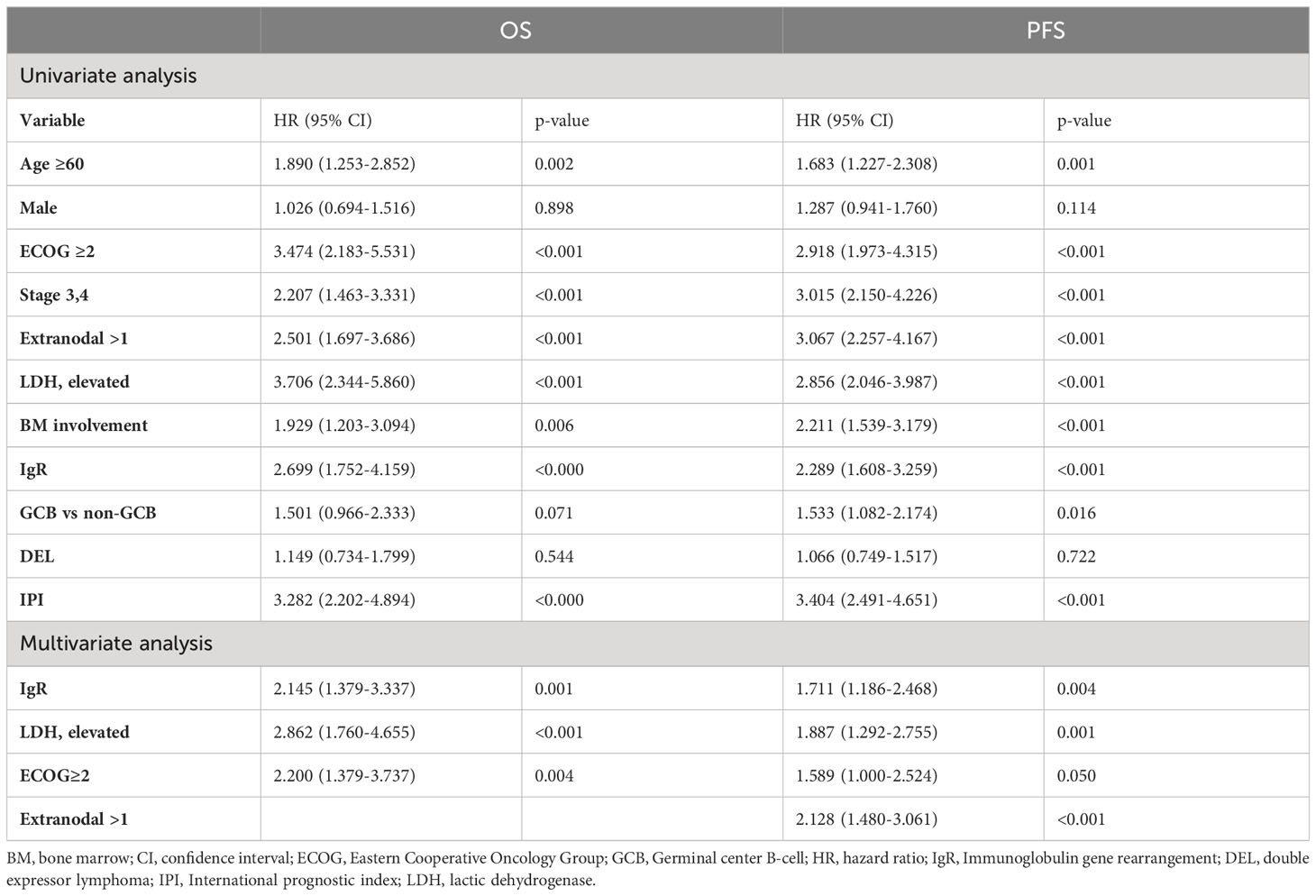
We assessed the results of the univariate analysis of patients who were not treated with upfront ASCT. The following factors were significantly associated with poor prognosis: age ≥ 60 years, poor performance status, advanced disease stage, involvement of at least one lymph node, elevated LDH, histological BM involvement, positive IgR, non-germinal center B-cell (GCB) subtype, and IPI. The multivariate analysis associated poor OS and PFS with elevated LDH (P < 0.001 and P = 0.001, respectively), poor performance status (P = 0.004 and P = 0.05, respectively), and positive IgR (P = 0.001 and P = 0.004, respectively; Table 2). In contrast, age was the only prognostic factor among patients who received upfront ASCT (P = 0.001 and P = 0.036, respectively).
3 Discussion
The present study findings revealed that the clinical characteristics of patients with DLBCL and positive IgR in BM samples (besides those with traditional histological BM involvement) who received R-CHOP chemotherapy, resembled those of patients with advanced-stage lymphoma. Moreover, these patients did not respond well to R-CHOP first-line treatment and had poor OS and PFS. Therefore, tests for IgR should be applied to precisely predict the prognosis of patients with negative BM histology.
BM involvement of DLBCL cells showed an unfavorable gene signature, which was related to tumor cell proliferation, migration, and immune escape. These could explain high-risk clinical features and poor prognosis (5). However, differentiating the histological diagnosis of BM involvement of malignant lymphoma cells can be challenging particularly in patients with small amount of lymphoma cells. The IgR test could be helpful under such circumstances. The IgR results were positive in 13%–16% of patients with DLBCL who were diagnosed with histologically normal BM and these patients did not survive for long (14, 20). Here, we found positive IgR in 17.5% of patients with negative histological BM involvement, which was similar to previous findings. Without IgR tests, these patients would have been classified as having no BM involvement and the disease stage would have been lowered. Therefore, routine IgR tests of BM samples should be recommended to evaluate the molecular BM involvement of DLBCL cells.
A higher proportion of patients with histological BM involvement had a more advanced disease stage, more frequent extranodal involvement, and more elevated LDH than patients with positive IgR and negative BM histology. Patients with histological BM involvement were classified as having stage 4 disease or a high IPI score at the time of diagnosis. However, patients with positive IgR might not be classified as having an advanced disease stage and might have been down-staged because histological BM involvement was not found. Therefore, the clinical characteristics of patients with histological BM involvement differed from those with only positive IgR with negative BM histology. Nevertheless, we found that the differences in OS and PFS between patients with histological BM involvement and those with positive IgR and negative BM histology were not significant. In addition, the multivariate analysis identified positive IgR as an important prognostic factor associated with poor OS and PFS in patients who were not treated with upfront ASCT.
The most useful tool for assessing clonality in patients with NHL until recently was BIOMED-2 PCR assays. These had been widely used as they were standardized and deemed suitable for technically routine test environments (26). However, PCR is limited by being unsuitable for samples with poor DNA quality, such as formalin-fixed paraffin-embedded (FFPE) samples, which could produce false negative results (15, 18). However, small amplicons and FFPE samples can be analyzed using NGS (18). We compared the ability of PCR and NGS to detect clonality and found no significant differences.
Although histological BM involvement is considered a poor prognostic factor, a standardized treatment approach has not yet been established. Furthermore, patients with positive IgR have not been studied. High-intensity chemotherapy, such as fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine (rituximab-hyper-CVAD/MA) or dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R) might overcome poor prognoses, and treatment outcomes are better than those of R-CHOP in high-risk patients with DLCBL (5). Consolidative upfront ASCT might also be considered as a different approach to high-intensity chemotherapy for DLBCL because it can eradicate PCR-detectable NHL cells and consequently reduce recurrence (27). Upfront ASCT in the rituximab era improves PFS in high-risk patients with DLBCL (28, 29). Based on this, we investigated whether upfront ASCT could mitigate the poor prognosis of patients with DLBCL and BM involvement. According to Korean reimbursement guidelines, consolidative upfront ASCT in clinical practice can be recommended for patients with elevated LDH and stage III/IV DLBCL at the time of diagnosis who respond to front-line R-CHOP chemotherapy. However, the present study was retrospective, and as a result, patients with good treatment response and performance might have been selected to receive upfront ASCT. Accordingly, the patients were divided into groups with and without upfront ASCT when analyzing the prognostic factors associated with survival to minimize bias associated with the intensity of treatment. Analysis of all enrolled patients showed that survival was shorter for patients with histological BM involvement or positive IgR with negative BM histology than for those without histological BM involvement and negative IgR. The results of the multivariate analysis showed that patients with poor performance status, elevated LDH, or positive IgR who did not receive upfront ASCT tended to have poor OS and PFS. These results indicated that upfront ASCT plays an important role in overcoming a poor prognosis due to histological BM involvement or positive IgR with negative BM histology. The routine application of upfront ASCT consolidation after R-CHOP is not considered standard care in all countries. However, we suggest that upfront ASCT for high-risk patients with DLBCL and BM involvement should be considered at least in those countries with access to novel target agents.
This study had the following limitations. First, this was a retrospective study and not a prospective randomized study. To overcome this limitation, we registered as many patients as possible from nine institutions in Korea. Another limitation was the absence of regular follow-up data for IgR tests, although they were applied at the time of diagnosis. Based on the concept of minimal residual disease, follow-up tests for IgR are underway and will be examined through further follow-up studies. Although IgR tests could not discriminate infiltration by a high- or low-grade component, the poor prognostic impact of IgR positivity for patients with DLBCL nevertheless generated meaningful information. Despite these limitations, our findings were meaningful insofar as we used PCR and NGS to investigate the role of IgR, in addition to histological BM involvement, in a large cohort of patients with DLBCL and analyzed their clinical characteristics and treatment outcomes.
In conclusion, tests to detect IgR BM allowed a more detailed classification of the prognosis of patients who were negative for histological BM involvement. Patients who did not receive upfront ASCT could not overcome the poor prognosis associated with BM involvement. Our results suggested that ASCT could mitigate the poor prognosis of not only patients with histological BM involvement but also those with positive IgR and negative BM histology. Accordingly, these findings require validation through future prospective studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Review Committee of Severance Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective cohort study.
Author contributions
YK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HS: Data curation, Resources, Writing – original draft, Writing – review & editing. H-YY: Data curation, Resources, Writing – original draft, Writing – review & editing. D-HY: Data curation, Resources, Writing – original draft, Writing – review & editing. YP: Data curation, Resources, Writing – original draft, Writing – review & editing. JL: Data curation, Resources, Writing – original draft, Writing – review & editing. W-SL: Data curation, Resources, Writing – original draft, Writing – review & editing. YD: Data curation, Resources, Writing – original draft, Writing – review & editing. Y-CM: Data curation, Resources, Writing – original draft, Writing – review & editing. DK: Data curation, Resources, Writing – original draft, Writing – review & editing. JK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Ministry of Science and ICT of Korea (No. NRF-2022R1A2C1013495). This study was supported by a faculty research grant of Yonsei University College of Medicine (6–2020–0092).
Acknowledgments
The authors acknowledge the patients, medical staff, and physicians who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pfreundschuh M, Kuhnt E, Trümper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol (2011) 12(11):1013–22. doi: 10.1016/S1470-2045(11)70235-2
2. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l’adulte. Blood (2010) 116(12):2040–5. doi: 10.1182/blood-2010-03-276246
3. Sehn LH, Scott DW, Chhanabhai M, Berry B, Ruskova A, Berkahn L, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol (2011) 29(11):1452–7. doi: 10.1200/JCO.2010.33.3419
4. Campbell J, Seymour JF, Matthews J, Wolf M, Stone J, Juneja S. The prognostic impact of bone marrow involvement in patients with diffuse large cell lymphoma varies according to the degree of infiltration and presence of discordant marrow involvement. Eur J Haematol (2006) 76(6):473–80. doi: 10.1111/j.1600-0609.2006.00644.x
5. Yao Z, Deng L, Xu-Monette ZY, Manyam GC, Jain P, Tzankov A, et al. Concordant bone marrow involvement of diffuse large B-cell lymphoma represents a distinct clinical and biological entity in the era of immunotherapy. Leukemia (2018) 32(2):353–63. doi: 10.1038/leu.2017.222
6. Chung R, Lai R, Wei P, Lee J, Hanson J, Belch AR, et al. Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the International Prognostic Index. Blood (2007) 110(4):1278–82. doi: 10.1182/blood-2007-01-070300
7. Wang J, Weiss LM, Chang KL, Slovak ML, Gaal K, Forman SJ, et al. Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in Malignancy. Cancer (2002) 94(5):1522–31. doi: 10.1002/cncr.10364
8. Conlan MG, Bast M, Armitage JO, Weisenburger DD. Bone marrow involvement by non-Hodgkin’s lymphoma: the clinical significance of morphologic discordance between the lymph node and bone marrow. Nebraska Lymphoma Study Group J Clin Oncol (1990) 8(7):1163–72. doi: 10.1200/JCO.1990.8.7.1163
9. Talaulikar D, Dahlstrom JE. Staging bone marrow in diffuse large B-cell lymphoma: the role of ancillary investigations. Pathology (2009) 41(3):214–22. doi: 10.1080/00313020902756295
10. Elstrom R, Guan L, Baker G, Nakhoda K, Vergilio JA, Zhuang H, et al. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood (2003) 101(10):3875–6. doi: 10.1182/blood-2002-09-2778
11. Pakos EE, Fotopoulos AD, Ioannidis JP. 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med (2005) 46(6):958–63.
12. Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM. Bone marrow 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol (2014) 89(7):726–31. doi: 10.1002/ajh.23730
13. Yoo KH. Staging and response assessment of lymphoma: a brief review of the Lugano classification and the role of FDG-PET/CT. Blood Res (2022) 57(S1):75–8. doi: 10.5045/br.2022.2022055
14. Mitterbauer-Hohendanner G, Mannhalter C, Winkler K, Mitterbauer M, Skrabs C, Chott A, et al. Prognostic significance of molecular staging by PCR-amplification of immunoglobulin gene rearrangements in diffuse large B-cell lymphoma (DLBCL). Leukemia (2004) 18(6):1102–7. doi: 10.1038/sj.leu.2403376
15. Langerak AW, van Krieken JH, Wolvers-Tettero IL, Kerkhof E, Mulder AH, Vrints LW, et al. The role of molecular analysis of immunoglobulin and T cell receptor gene rearrangements in the diagnosis of lymphoproliferative disorders. J Clin Pathol (2001) 54(7):565–7. doi: 10.1136/jcp.54.7.565
16. Arnold A, Cossman J, Bakhshi A, Jaffe ES, Waldmann TA, Korsmeyer SJ. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med (1983) 309(26):1593–9. doi: 10.1056/NEJM198312293092601
17. Kokovic I, Jezersek Novakovic B, Novakovic S. Diagnostic value of immunoglobulin kappa light chain gene rearrangement analysis in B-cell lymphomas. Int J Oncol (2015) 46(3):953–62. doi: 10.3892/ijo.2014.2790
18. Scheijen B, Meijers RWJ, Rijntjes J, van der Klift MY, Möbs M, Steinhilber J, et al. Next-generation sequencing of immunoglobulin gene rearrangements for clonality assessment: a technical feasibility study by EuroClonality-NGS. Leukemia (2019) 33(9):2227–40. doi: 10.1038/s41375-019-0508-7
19. Seo JY, Hong J, Chun K, Jeong J, Cho H, Kim KH, et al. Prognostic significance of PCR-based molecular staging in patients with diffuse large B-cell lymphoma treated with R-CHOP immunochemotherapy. Leuk Lymphoma (2017) 58(2):357–65. doi: 10.1080/10428194.2016.1190967
20. Arima H, Maruoka H, Nasu K, Tabata S, Kurata M, Matsushita A, et al. Impact of occult bone marrow involvement on the outcome of rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone therapy for diffuse large B-cell lymphoma. Leuk Lymphoma (2013) 54(12):2645–53. doi: 10.3109/10428194.2013.788697
21. Kang YH, Park CJ, Seo EJ, Huh J, Kim SB, Kang YK, et al. Polymerase chain reaction-based diagnosis of bone marrow involvement in 170 cases of non-Hodgkin lymphoma. Cancer (2002) 94(12):3073–82. doi: 10.1002/cncr.10584
22. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. International Agency for Research on Cancer (2008).
23. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med (1993) 329(14):987–94. doi: 10.1056/NEJM199309303291402
24. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
25. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood (2004) 103(1):275–82. doi: 10.1182/blood-2003-05-1545
26. Langerak AW, Groenen PJ, Brüggemann M, Beldjord K, Bellan C, Bonello L, et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia (2012) 26(10):2159–71. doi: 10.1038/leu.2012.246
27. Zwicky CS, Maddocks AB, Andersen N, Gribben JG. Eradication of polymerase chain reaction detectable immunoglobulin gene rearrangement in non-Hodgkin’s lymphoma is associated with decreased relapse after autologous bone marrow transplantation. Blood (1996) 88(9):3314–22. doi: 10.1182/blood.V88.9.3314.bloodjournal8893314
28. Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med (2013) 369(18):1681–90. doi: 10.1056/NEJMoa1301077
Keywords: diffuse large B-cell lymphoma, bone marrow involvement, immunoglobulin gene rearrangement, progression-free survival, transplantation
Citation: Kim YR, Shin HJ, Yhim H-Y, Yang D-H, Park Y, Lee JH, Lee W-S, Do YR, Mun Y-C, Kim DS and Kim JS (2024) Clinical significance of bone marrow involvement by immunoglobulin gene rearrangement in de novo diffuse large B-cell lymphoma: a multicenter retrospective study. Front. Oncol. 14:1363385. doi: 10.3389/fonc.2024.1363385
Received: 30 December 2023; Accepted: 29 January 2024;
Published: 12 February 2024.
Edited by:
Shimin Hu, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Yi Miao, Nanjing Medical University, ChinaShih-Sung Chuang, Chi Mei Medical Center, Taiwan
Copyright © 2024 Kim, Shin, Yhim, Yang, Park, Lee, Lee, Do, Mun, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Seok Kim, hemakim@yuhs.ac
 Yu Ri Kim
Yu Ri Kim