
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 05 April 2024
Sec. Cardio-Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1362347
This article is part of the Research Topic Case Reports in Cardio-Oncology: 2023 View all 19 articles
 Akihiro Nishiyama1*
Akihiro Nishiyama1* Shigeki Sato1
Shigeki Sato1 Hiroyuki Sakaguchi1
Hiroyuki Sakaguchi1 Hiroshi Kotani1
Hiroshi Kotani1 Kaname Yamashita1
Kaname Yamashita1 Koushiro Ohtsubo1
Koushiro Ohtsubo1 Keishi Mizuguchi2
Keishi Mizuguchi2 Hiroko Ikeda2
Hiroko Ikeda2 Kenji Iino3
Kenji Iino3 Hirofumi Takemura3
Hirofumi Takemura3 Shinji Takeuchi1*
Shinji Takeuchi1*In the realm of rare cardiac tumors, intimal sarcoma presents a formidable challenge, often requiring innovative treatment approaches. This case report presents a unique instance of primary intimal sarcoma in the left atrium, underscoring the critical role of genomic profiling in guiding treatment. Initial genomic testing unveiled a somatic, active mutation in PDGFRβ (PDGFRβ N666K), accompanied by MDM2 and CDK4 amplifications. This discovery directed the treatment course toward pazopanib, a PDGFRβ inhibitor, following irradiation. The patient’s response was remarkable, with the therapeutic efficacy of pazopanib lasting for 16.3 months. However, the patient experienced a recurrence in the left atrium, where subsequent genomic analysis revealed the absence of the PDGFRβ N666K mutation and a significant reduction in PDGFRβ expression. This case report illustrates the complexities and evolving nature of cardiac intimal sarcoma treatment, emphasizing the potential of PDGFRβ signaling as a strategic target and highlighting the importance of adapting treatment pathways in response to genetic shifts.
Primary cardiac tumors are rare, with an incidence of 0.001%–0.003% worldwide (1), and intimal sarcoma is the most frequent subtype of cardiac sarcoma (2). Intimal sarcoma occurs in the innermost layer of large vessels, such as the pulmonary arteries. The clinical outcome is poor, with a median survival of only 12–13 months after radical surgery (3); thus, the development of effective treatment for intimal sarcoma is an unmet need. Gene profiling analyses of intimal sarcoma revealed that MDM2 amplification and/or overexpression is frequently detected and is now considered one of the criteria for diagnosing intimal sarcoma (2). Moreover, a previous case report suggested that PDGFRβ may be involved in the tumorigenesis of intimal sarcoma (4). However, organized data on genetic findings of cardiac intimal sarcoma are limited.
A 45-year-old male patient, experiencing constitutional symptoms including palpitations and cough for four months, was admitted to Kanazawa University Hospital. Transthoracic echocardiography revealed a 5-cm-sized mass in the left atrium, possibly adhering to the mitral valve leaflets (Figure 1A). This mass resulted in pulmonary hypertension and low cardiac output. Emergent surgical excision of the mass was performed (Figure 1B), and pathological examination confirmed the diagnosis of intimal sarcoma in the left atrium, characterized by MDM2 amplification and overexpression (Figures 1C–E).
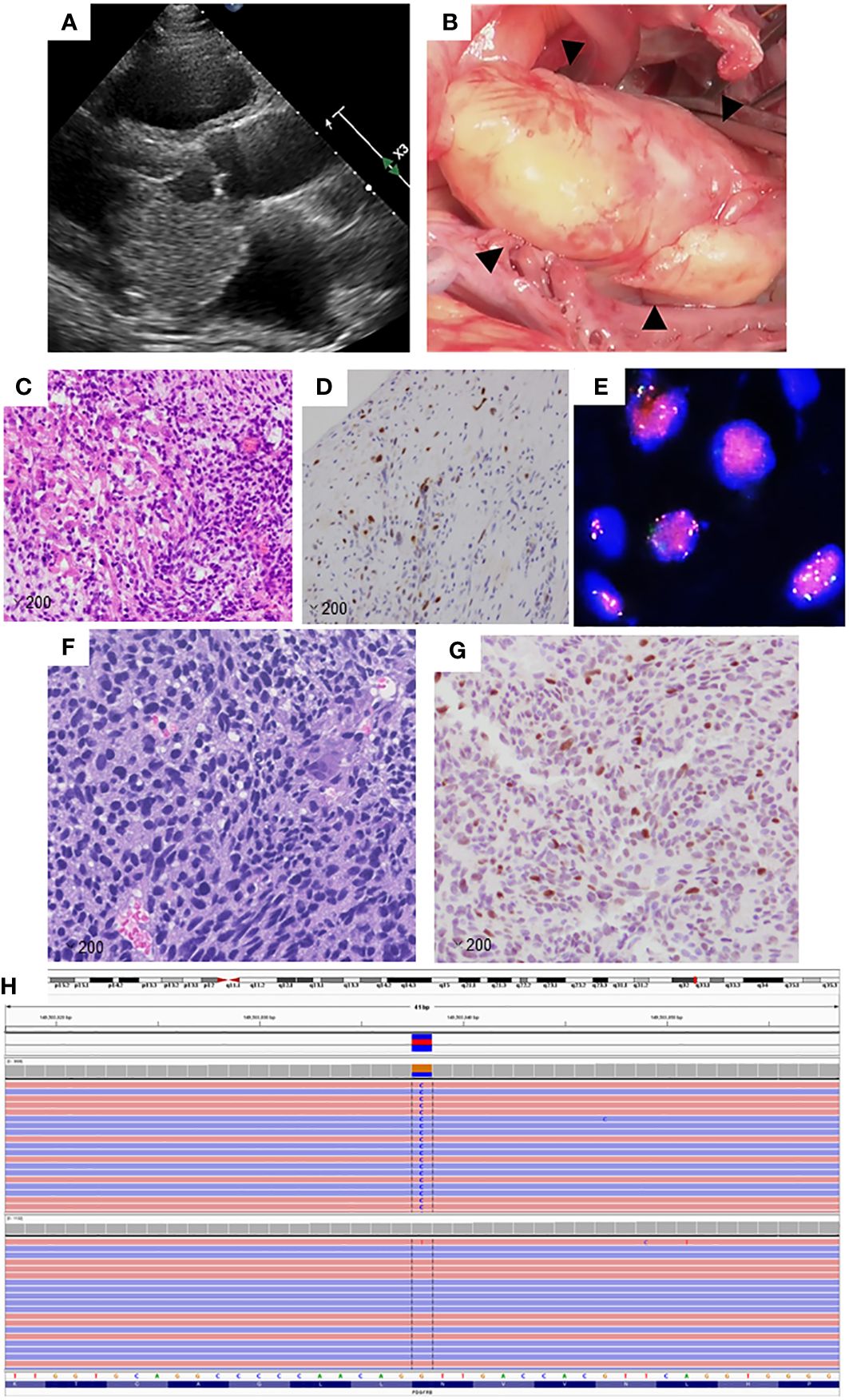
Figure 1 (A) Transthoracic echocardiography image of the primary lesion in the left atrium. (B) Perioperative image of the primary lesion in the left atrium. (C) The primary lesion shows the proliferation of spindle cells with a high nuclear-cytoplasmic ratio, magnification: ×200. (D) Tumor cells in the primary lesion showed positive immunoreactivity for MDM2 (magnification: ×200). (E) Fluorescence in situ hybridization analysis revealed the amplification of MDM2. Signals for MDM2 are presented in red, while those of CEP 12 are shown in green. (F) The left tailor muscle metastatic lesion consists of spindle cells and resembles the primary lesion (magnification: ×200). (G) The tumor cells in the metastatic lesion showed positive immunoreactivity for MDM2, magnification: ×200. (H) Illustration of PDGFRβ N666K using a next-generation sequencing platform as visualized in the Integrative Genomics Viewer.
Subsequently, the patient developed brain metastasis and underwent two sessions of Gamma Knife therapy. Despite these treatments, multiple bone metastases emerged, necessitating chemotherapy with doxorubicin, followed by eribulin. Although these regimens effectively controlled the bone metastases, a new lesion appeared in the left tailor muscle. A biopsy of this muscle mass was performed for comprehensive genomic testing. Histological analysis confirmed the left tailor muscle tumor as a metastatic intimal sarcoma, also displaying MDM2 overexpression (Figures 1F, G). The patient underwent palliative radiation therapy (60 Gy) targeting the left tailor muscle metastasis and continued with eribulin therapy. However, the thoracolumbar and sternal metastases progressed, manifesting as new lesions.
Genomic testing of the left tail muscle metastasis revealed the presence of PDGFRβ N666K mutation, along with the amplification of MDM2 and CDK4, both located on the long arm of chromosome 12 (Figure 1H). The PDGFRβ N666K had an allele frequency of 35.9%, and the copy numbers for MDM2 and CDK4 were 8.48 and 9.09, respectively. Due to pain resulting from the thoracolumbar and sternal metastases, the patient underwent external irradiation (30 Gy). Pazopanib, a sarcoma-approved drug active against PDGFRs, was initiated as a third-line treatment. Prior to the induction of pazopanib, the patient had several irradiated lesions, including metastases in the brain, left tailor muscle, sternum, and thoracolumbar spine, and a non-irradiated left adrenal metastasis. Pazopanib demonstrated a favorable and prolonged effect without requiring dose reduction, with the only side effect being a change in hair color. Unfortunately, 16.3 months after pazopanib initiation, a local recurrence in the left atrium was observed. For non-brain metastatic lesions, the relapse-free survival period was 619 days (Figure 2).
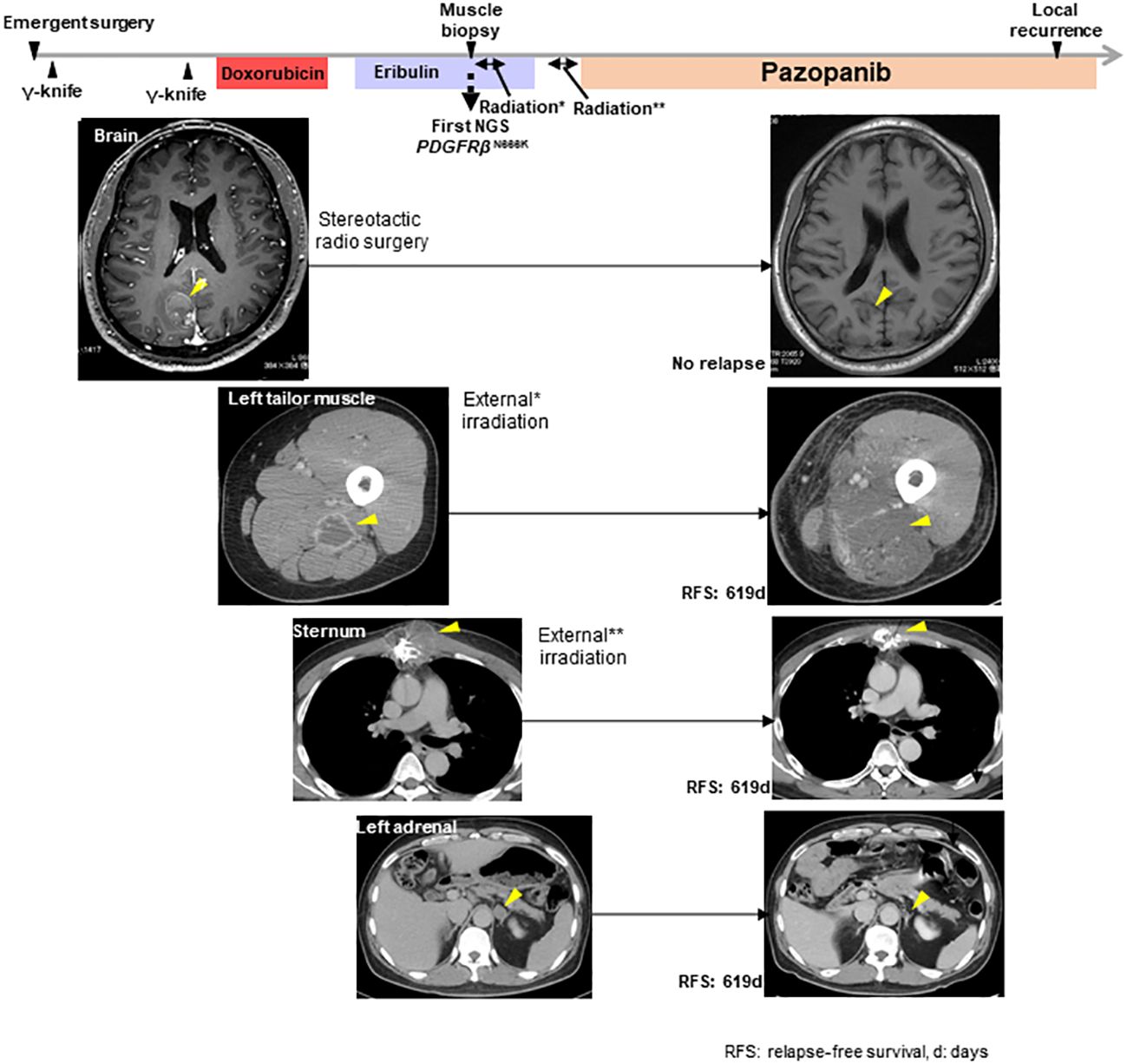
Figure 2 The clinical course from the initial operation to the local recurrence in the left atrium and the response of metastatic lesions to radiation or pazopanib. *indicated the external irradiation delivered to the left tailor muscle lesion. **indicated the external irradiation delivered to the sternum lesion. The yellow arrowheads show the target lesions.
Salvage surgery was performed to remove the recurrent lesion, but local recurrence was detected after one and a half months. Owing to the inability to undergo another surgery, the patient received palliative radiation therapy for the treatment of a recurrent lesion in the left atrium (Supplementary Figure 1A). Further genomic testing on the left atrium recurrence sample (Table 1) resulted in the establishment of a patient-derived cell line from this sample, after obtaining the ethical review approval and patient consent (Supplementary Figure 1B). The recurrent lesion lacked the PDGFRβ N666K mutation and showed minimal PDGFRβ expression, in contrast to the clear PDGFRβ positivity observed in the primary and left tail muscle metastasis lesions (Supplementary Figures 2A-C).
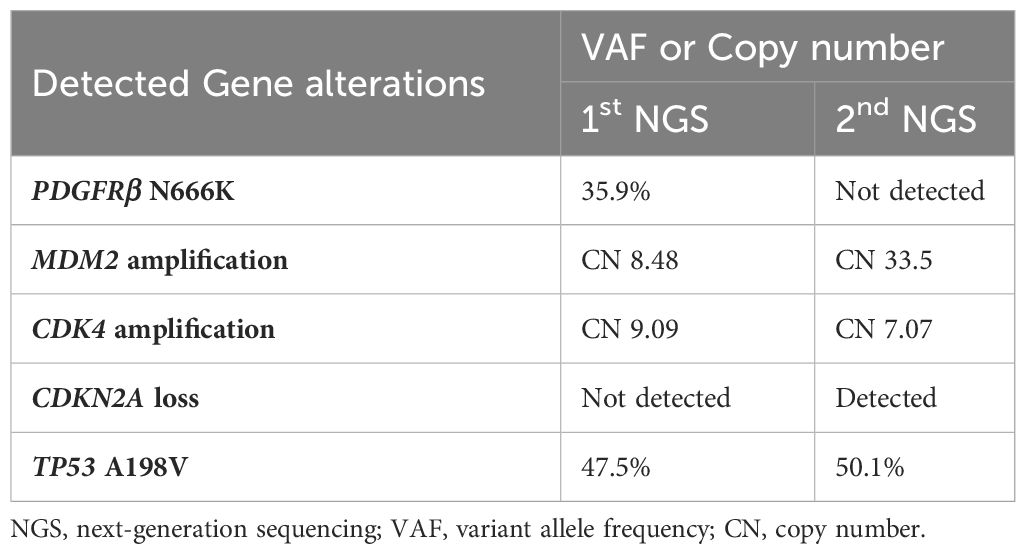
Table 1 Difference between first genomic test results and second genomic test results by next-generation sequencing.
Subsequently, new metastatic lesions developed in the right atrium and the greater curvature of the stomach. The patient received palliative radiotherapy to prevent sudden death and gastrointestinal hemorrhage. Ultimately, disease progression occurred 44.6 months after the initial surgery.
This case of cardiac intimal sarcoma with the PDGFRβ N666K mutation provides significant insights into the molecular landscape of this rare tumor type. Neuville et al.’s study on 42 cardiac intimal sarcomas reported MDM2 overexpression and MDM2/CDK4 amplification (2). Additionally, PDGFRβ mutations, found in 15.3% of patients with intimal sarcomas, have been linked to oncogenesis in mesenchymal tumors (4–6). Notably, PDGFRβ D850V, M772V, R709H, and E472D mutations in intimal sarcoma have been previously documented (4, 5, 7). However, the PDGFRβ N666K mutation detected in our patient is the first instance in intimal sarcoma, underscoring its novelty and importance.
The oncogenic role of the PDGFRβ signaling pathway is further supported by the response of PDGFRβ N666K-transfected Ba/F3 cells to inhibitors like imatinib, nilotinib, or ponatinib, and its ability to induce cancer in vivo (6). The presence of PDGFRβ N666K in the primary lesion and the left tail muscle metastasis, as evidenced by the clear PDGFRβ positivity on the cell membrane (Supplementary Figures 2A, B), suggests that these lesions were driven by this mutation. However, the recurrence’s resistance to pazopanib and the lack of PDGFRβ N666K mutation therein indicate a complex and evolving tumor biology.
Table 2 compares the efficacy of pazopanib in the present patient with those used in previous case reports (8–11). Results showed a significantly longer progression-free survival and better response, suggesting that PDGFRβ mutations may be a viable therapeutic target. However, the CDK4 amplification and loss of CDKN2A observed in the recurrent lesion, coupled with increased sensitivity to abemaciclib, a CDK4/6 inhibitor, over pazopanib (Supplementary Figure 1C), highlight the necessity for tailored treatment approaches (12, 13). Although MDM2 acts as a negative regulator of TP53 by promoting its degradation and inhibiting tumor suppressor activity, the presence of TP53 mutations complicates the therapeutic landscape. Even if MDM2 inhibitors successfully block the MDM2-TP53 interaction, they are incapable of restoring TP53 function due to these underlying TP53 alterations. Regrettably, the presence of a TP53 pathogenic variant precluded participation in studies involving MDM2 inhibitors such as milademetan (14), further emphasizing the need for a focus on alternative pathways influencing the cell cycle, specifically through CDK4 amplification and CDKN2A loss, as pivotal resistance mechanisms.
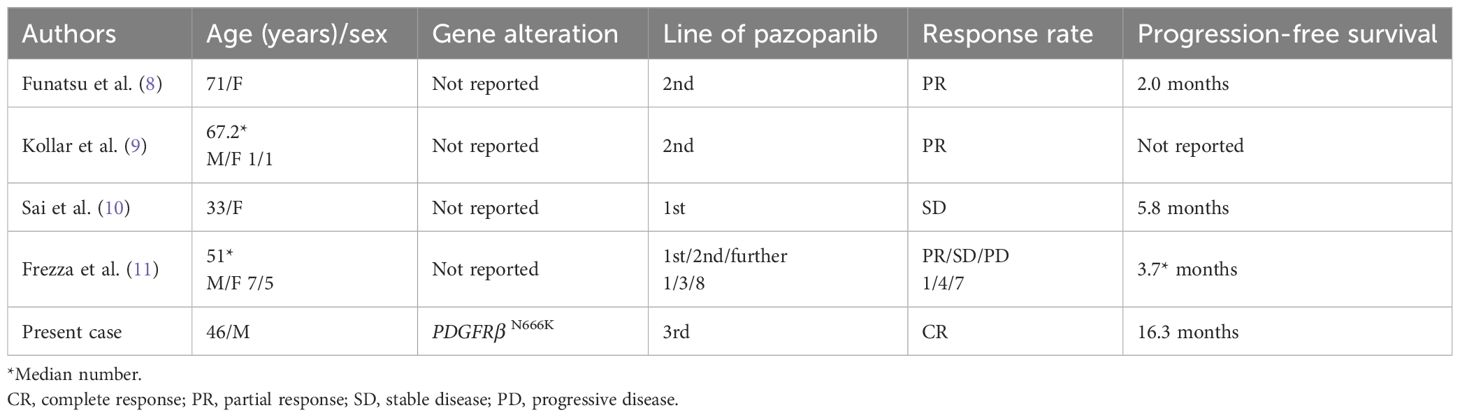
Table 2 Efficacy of pazopanib as treatment for intimal sarcoma in the present and previous case reports.
Our study has some limitations. Pazopanib is a multi-kinase inhibitor, which could obscure the specific impact of the drug on the PDGFRβ N666K mutation (10). Furthermore, as four lesions, excluding the left adrenal metastasis, had undergone irradiation before pazopanib administration, the distinct effect of the drug on these lesions remains uncertain.
This case highlights the potential of precision medicine for cardiac intimal sarcoma, showcasing the benefits of genomic testing in identifying specific alterations such as PDGFRβ N666K, MDM2 amplification, and CDK4 amplification. The prolonged efficacy observed with pazopanib following irradiation in a PDGFRβ N666K-positive patient emphasizes the potential of targeted therapies in improving the prognosis of patients with cardiac intimal sarcoma. This case report underscores the evolving nature of cancer treatment and the necessity for individualized therapeutic strategies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AN: Writing – original draft, Writing – review & editing. SS: Writing – review & editing. HS: Writing – review & editing. HK: Writing – review & editing. KY: Writing – review & editing. KO: Writing – review & editing. KM: Writing – review & editing. HI: Writing – review & editing. KI: Writing – review & editing. HT: Writing – review & editing. ST: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 20K08516 to ST.
We thank the patient and his family for providing informed consent for the processing of his data for this publication. Additionally, we thank SRL Co., Ltd. for providing the sequence data for PDGFRβ N666K.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1362347/full#supplementary-material
Supplementary Figure 1 | The radiographic findings of the local recurrence in the left atrium and the experiment using the recurrence tissue. (A) The yellow arrowheads indicated the recurrence lesions. These lesions responded to palliative radiotherapy. (B) The appearance of the cell line established from the recurrence tissue. (C) The sensitivity of the patient-derived cell line to pazopanib or abemaciclib was determined through cell viability assays.
Supplementary Figure 2 | Immunohistochemistry staining of PDGFRβ (Cell Signaling Technology; PDGF Receptor β (C82A3) Rabbit mAb #4564). (A) The primary lesion exhibited an adequate expression of PDGFRβ. (B) The left tailor muscle metastasis lesion demonstrated a high expression of PDGFRβ. (C) The local recurrence lesion in the left atrium had a low expression of PDGFRβ.
1. Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol. (2007) 19:748–56. doi: 10.1016/j.clon.2007.06.009
2. Neuville A, Collin F, Bruneval P, Parrens M, Thivolet F, Gomez-Brouchet A, et al. Intimal sarcoma is the most frequent primary cardiac sarcoma: clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. Am J Surg Pathol. (2014) 38:461–9. doi: 10.1097/PAS.0000000000000184
3. Dewaele B, Floris G, Finalet-Ferreiro J, Fletcher CD, Coindre JM, Guillou L, et al. Coactivated platelet-derived growth factor receptor α and epidermal growth factor receptor are potential therapeutic targets in intimal sarcoma. Cancer Res. (2010) 70:7304–14. doi: 10.1158/0008-5472.CAN-10-1543
4. Roszik J, Khan A, Conley AP, Livingston JA, Groisberg R, Ravi V, et al. Unique aberrations in intimal sarcoma identified by next-generation sequencing as potential therapy targets. Cancers (Basel). (2019) 11:1283. doi: 10.3390/cancers11091283
5. Ito Y, Maeda D, Yoshida M, Yoshida A, Kudo-Asabe Y, Nanjyo H, et al. Cardiac intimal sarcoma with PDGFRβ mutation and co-amplification of PDGFRα and MDM2: an autopsy case analyzed by whole-exome sequencing. Virchows Arch. (2017) 471:423–8. doi: 10.1007/s00428-017-2135-x
6. Arts FA, Chand D, Pecquet C, Velghe AI, Constantinescu S, Hallberg B, et al. PDGFRB mutants found in patients with familial infantile myofibromatosis or overgrowth syndrome are oncogenic and sensitive to imatinib. Oncogene. (2016) 35:3239–48. doi: 10.1038/onc.2015.383
7. Fu X, Niu W, Li J, Kiliti AJ, Al-Ahmadie HA, Iyer G, et al. Activating mutation of PDGFRB gene in a rare cardiac undifferentiated intimal sarcoma of the left atrium: a case report. Oncotarget. (2017) 8:81709–16. doi: 10.18632/oncotarget.20700
8. Funatsu Y, Hirayama M, Shiraishi J, Asakura T, Wakaki M, Yamada E, et al. Intimal sarcoma of the pulmonary artery treated with pazopanib. Intern Med. (2016) 55:2197–202. doi: 10.2169/internalmedicine.55.6199
9. Kollár A, Jones RL, Stacchiotti S, Gelderblom H, Guida M, Grignani G, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. (2017) 56:88–92. doi: 10.1080/0284186X.2016.1234068
10. Sai S, Imamura Y, Kiyota N, Jimbo N, Toyoda M, Funakoshi Y, et al. Relationship between PDGFR expression and the response to pazopanib in intimal sarcoma of the pulmonary artery: a case report. Mol Clin Oncol. (2021) 14:6. doi: 10.3892/mco.2020.2168
11. Frezza AM, Assi T, Lo Vullo SL, Ben-Ami E, Dufresne A, Yonemori K, et al. Systemic treatments in MDM2 positive intimal sarcoma: a multicentre experience with anthracycline, gemcitabine, and pazopanib within the World Sarcoma Network. Cancer. (2020) 126:98–104. doi: 10.1002/cncr.32508
12. Dickson MA, Schwartz GK, Keohan ML, D’Angelo SP, Gounder MM, Chi P, et al. Phase 2 trial of the CDK4 inhibitor palbociclib (PD0332991) at 125 mg dose in well-differentiated or dedifferentiated liposarcoma. JAMA Oncol. (2016) 2:937–40. doi: 10.1001/jamaoncol.2016.0264
13. Fennell DA, King A, Mohammed S, Greystoke A, Anthony S, Poile C, et al. Abemaciclib in patients with p16ink4A-deficient mesothelioma (MiST2): a single-arm, open-label, phase 2 trial. Lancet Oncol. (2022) 23:374–81. doi: 10.1016/S1470-2045(22)00062-6
14. Koyama T, Shimizu T, Kojima Y, Sudo K, Okuma HS, Shimoi T, et al. Clinical activity and exploratory resistance mechanism of milademetan, an MDM2 inhibitor, in intimal sarcoma with MDM2 amplification: an open-label phase Ib/II study. Cancer Discov. (2023) 13:1814–25. doi: 10.1158/2159-8290.CD-23-0419
Keywords: intimal sarcoma, PDGFRβ N666K mutation, precision oncology, MDM2 amplification, CDK4 amplification
Citation: Nishiyama A, Sato S, Sakaguchi H, Kotani H, Yamashita K, Ohtsubo K, Mizuguchi K, Ikeda H, Iino K, Takemura H and Takeuchi S (2024) Case report: Navigating treatment pathways for cardiac intimal sarcoma with PDGFRβ N666K mutation. Front. Oncol. 14:1362347. doi: 10.3389/fonc.2024.1362347
Received: 28 December 2023; Accepted: 25 March 2024;
Published: 05 April 2024.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Numrah Fadra, Mayo Clinic, United StatesCopyright © 2024 Nishiyama, Sato, Sakaguchi, Kotani, Yamashita, Ohtsubo, Mizuguchi, Ikeda, Iino, Takemura and Takeuchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akihiro Nishiyama, YW4wNTEwQHN0YWZmLmthbmF6YXdhLXUuYWMuanA=; Shinji Takeuchi, dGFrZXVjaGlAc3RhZmYua2FuYXphd2EtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.