- 1Department of Oncology, MZB Foundation for Cancer Research, New York, NY, United States
- 2Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL, United States
- 3Department of Radiology, University Diagnostic Imaging, Bronx, NY, United States
- 4Department of Surgery, Mount Sinai Hospital, New York, NY, United States
Introduction: Routine blood tests are prognostic tests for patients with cholangiocarcinoma. New drug regimens may produce a median overall survival of 2 years or more.
Methods: This single practice, IRB-approved, phase II trial examines prognostic tests, Kaplan-Meier survival, and univariate Cox regression analyses. Eligibility requires: intent-to-treat; signed consent; advanced measurable intrahepatic cholangiocarcinoma, with or without resistance to the test drugs; any adult age; performance status 0–2; and expected survival of ≥ 6 weeks. Biweekly treatment, with 1/3 of standard dosages in mg/M2, includes: Gemcitabine 500; 5-Fluorouracil 1200 over 24 hours; Leucovorin 180; Irinotecan 80; and on day 2, Oxaliplatin 40. On progression, drugs are added on day 2: first, Docetaxel 25 precedes Oxaliplatin, with or without Mitomycin C 6 after Oxaliplatin. The next sequential additions are day 1, Cetuximab 400 total mg, then 200 mg weekly, and then Bevacizumab 10 mg/kg is substituted for Cetuximab (FDA IND# 119005).
Results: For 35 patients, 19 with 1–2 lines of prior therapy, resistant tumors, and 16 no prior therapy, survival at 24-months is ≥ 72 and ≥ 58%, respectively. For 14 patients aged ≥ 70 years, ≥ 63% survive 24 months, P = 0.28. Validated tests that predict ≤ 6-month survivals find median survival times of 17-months through > 2-years when compared to patients with favorable tests: Neutrophils lymphocyte ratio > 3.0, HR = 6.54, P < 6.4x10–3; absolute neutrophil count > 8000/μl, HR = 4.95, P < 6.5x10–3; serum albumin < 3.5 g/dl, HR = 4.10, P < 0.03; and lymphocyte monocyte ratio< 2.1, HR = 1.6, P = 0.50. Overall, the 76 (60–90)% of patients with 0–2 out of 4 high risk tests survive ≥ 24 months, (P = 7.1x10–3). Treatments produce neither hospitalization, neutropenic fever, severe enteritis, nor severe neuropathies.
Conclusion: Two-year survival is replicable and predictable. Findings warrant phase III validation tests of sequential regimens, re-challenge with recombination, low dosages, and blood tests that are associated with lethal mechanisms that impair response and survival.
1 Introduction
Cholangiocarcinoma (CCA) is projected to become the 9th leading cause of cancer-related deaths worldwide (1). Current chemotherapy for CCA results in median survival times (MSTs) of 10.5 (6.4–14.7) and 5.3 (4.1–6.6) months for primary therapy, gemcitabine and cis platin, and a mix of secondary treatments, respectively (2, 3). A phase III trial of a FOLFOX regimen found second-line MSTs of 7.2 (6.5–8.9) months and a 25.9% rate of 12-month survivors (4). The new, phase III, standard of primary care chemo-immunotherapy, with gemcitabine-cisplatin and durvalumab produces an MST of 12.9 months and 2-year survival of 23.6% (5, 6). Investigation of second-line therapy has identified 2–10 promising forms of targeted therapy, applicable to about 40% of patients. MSTs are 21.7 months for futibatinib, 21.1 months for pemigatinib, FGFR inhibitors, and 10.8 months for ivosidenib, an IDH inhibitor (4, 7–9).
Intrahepatic CCA (IHCCA), distal bile duct cancer, and gallbladder cancer (GBC) may be distinct entities; patients with IHCCA may sometimes have MSTs of 12–14 months. Management of CCA continues to present with unresolved multidisciplinary options that includes supportive care, surgery, resection to transplant, genomic evaluations, interventional radiology, radiotherapy, regional therapy, chemotherapy, targeted therapy, and chemo-immunotherapy (3–5).
Sequential test regimens have shown promising results. In previous studies, 99 patients with stage IV CCA, many of whom had resistant tumors, achieved an overall MST of more than 3 years. Added chemotherapy and targeted therapy as part of the sequence extended the patients’ MSTs by approximately 10 (6–18) months (10, 11). Derivative sequential regimens have also shown improvement in MSTs, reaching 14.5 months in an analysis of a combined group of patients, 35with CCA, 53 with new advanced pancreatic cancer (APC), 53 with resistant APC (RAPC), and 50 with resistant third-line colorectal cancers (RCRC) (12).
The use of low dosages and dose modifications of all cytotoxins prolonged survival, minimized complication rates, and avoided hospitalization. Rates of long or limiting delays of treatment were < 5% (10, 11). In contrast, standard low or high dosages of chemotherapy with 2–3 drugs alone and with added immunotherapy results in a 20–40% and 47% rate of clinically significant adverse events (AEs), respectively (2–6).
The combination of reproducible exceptional survival and safety for patients with CCA warrants further analysis of these patients’ prognostic blood tests (PBTs). An overall evaluation of parallel trials found that PBTs identified patients who may benefit from treatment (12). Also, initial PBTs have identified high and low risk subgroups of short and long survivors, among the participants in registration trials of first-line treatment for advanced CCA. PBTs were independent surrogate markers that serve as a summary variables to improve predictions of survival in comparison to clinical characteristics alone (13–16).
For patients with CCA and primary treatment, an A.L.A.N. Score (AS) model was found: where patients with 0, 1–2, or 3–4 out of 4 “unfavorable” high risk test groups had MSTs of 22, 12, and ~ 5 months, respectively (15). The 71% of patients with a favorable, low risk Neutrophil-Lymphocyte Ratio (NLR) < 3.0 had an MST of 10.6 months (P < 0.001). With an NLR of ≥ 3.0, the MST is 6.4 months, and the 95% confidence interval (CI) is 5.0–8.2 months (15). No individual low risk nor high risk PBTs identified a group of patients with an MST of > 15 or > 9 months, respectively (13–16).
There is limited experience with either the AS or individual PBTs when patients have resistant tumors; however, both the AS and individual tests predicted the overall survival outcomes of our contemporary patients with resistant tumors who received the GFLIO regimen (12). When patients were matched based on AS, serum albumin, or NLR, the elderly and heavily treated had similar survival compared to the young and new patients, respectively (17). For patients with gastric cancer, the use of baseline PBTs may assist in the evaluation and design of trials. Tests can compare patients, evaluate stratification, predict chances of survival, and identify a new subgroup of long survivors with an ECOG performance status (PS) of 2–3 (18, 19). PBTs may serve as surrogate biomarkers because mechanisms of lethality may be modified by the number of the patients’ cells, measured with the AS and PBTs (13–17). Individual cell type, neutrophils, lymphocytes, monocytes, platelets, and their subtypes produce many cytokines and growth factors that can impact tests of immunocompetence and a broad spectrum of cancer cells’ resistance to drugs (20–26).
The development of the GFLIO treatment regimen and sequence of treatment addresses safety concerns, AEs that limit treatment, and methods to reverse or circumvent the tumors’ resistance to drugs (Schema 1, Part A). Laboratory tests found combinations of two drugs that exhibit synergism and reverse drug resistance with inhibitory concentrations (IC) of 12 (6–25), compared to single drugs (10–12, 27). The use of low dosages can also avoid potential drug-drug antagonism observed at higher concentrations and AEs which limit treatment (28). These features allow for the simultaneous use of novel and multiple combinations of > 3 drugs (11, 12, 27–30). Independent investigations have confirmed that the simultaneous use of many combinations of two drugs is sometimes necessary in order to address the heterogeneity of tumors (31).
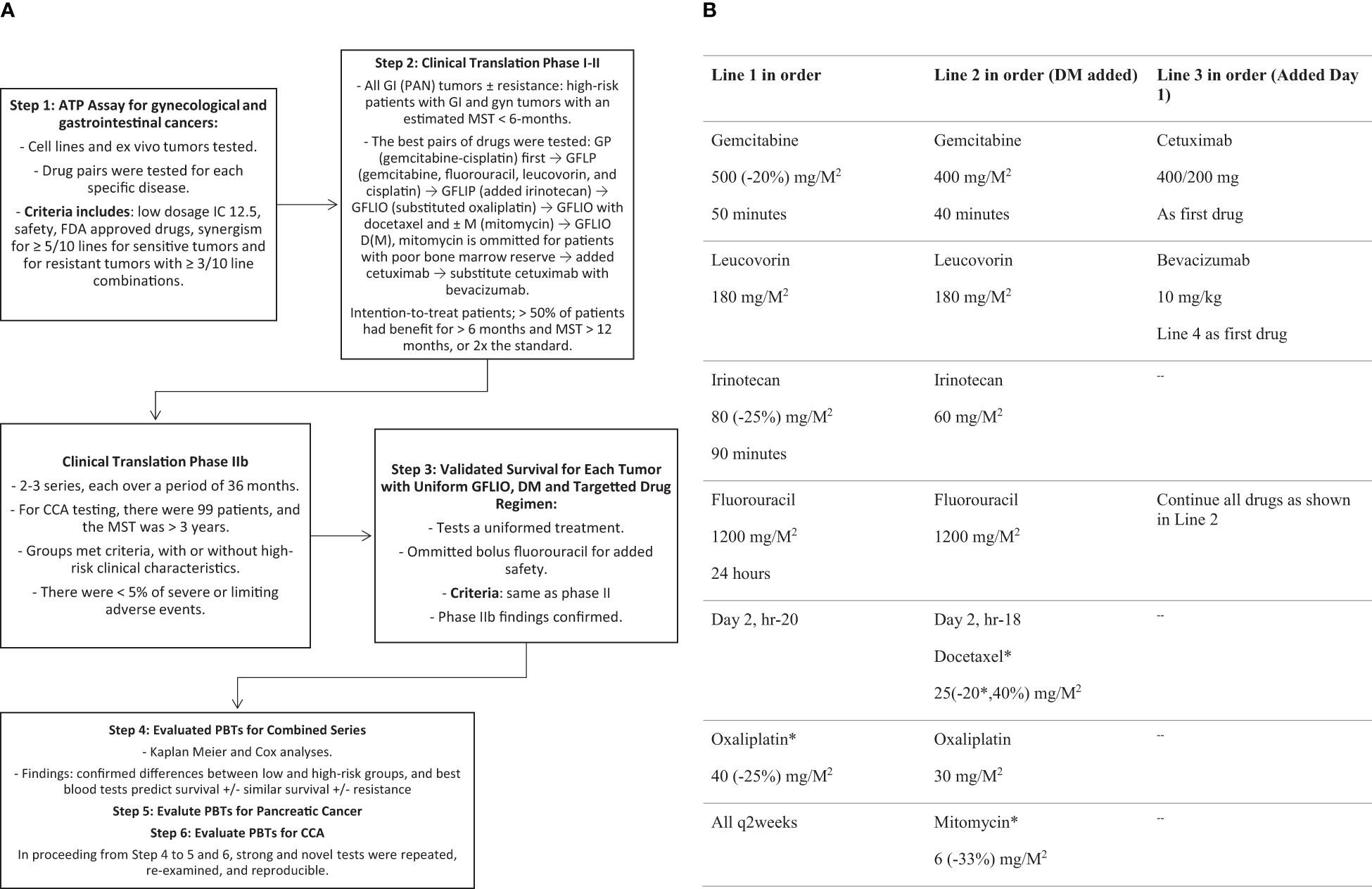
Schema 1 (A) Trial Design – Development of GFLIO ± DM ± Target Drugs. (B) Palliative Regimen. - Advanced Cholangiocarcinoma Palliative Regimen: All q2 weeks; -Parenthesis (): reference dosage reduction as needed for safety. - Bevacizumab replaces cetuximab at the time of progression. - Granulocyte Colony Stimulating Factor was added (300 mcg) on days 7 and 8 or 15 and 16 if the nadir neutrophil count is or will be <1000/µl or remains < 1250/µl, respectively.
These drug combinations may have other mechanisms of action. They improve immune function tests, increase the immunogenicity of RCRC, and produce response rates that reach nearly 66% (32–34). Metronomic therapy produces MSTs of 19.7 months for patients with stages 2–4 APCs, with or without prior therapy, when treatment consists of fluorouracil, oxaliplatin, and nab-paclitaxel (35). Simultaneous use of multiple drugs with other possible mechanisms of action can also be effective in patients with HIV, HTN, and hematologic cancers (36).
The prospective objectives of the analyses are to evaluate the performance of PBTs in the context of a treatment regimen that safely prolongs survival for aged and heavily treated patients. The secondary objective is to evaluate the number and size of well-defined subgroups of PBTs with prolonged survival in order to supplement the development of GFLIO and other regimens.
2 Methods
2.1 Statistics
Kaplan-Meier, Cox, and Greenwood’s analyses examine intention-to-treat patients and estimated 2-year survival rates starting from the first day of treatment. The effects of AS and historically validated PBTs as low risk vs. high-risk groups, age, and prior therapy were estimated using log-rank and Cox proportional hazard (PH) regression analyses with a 95% CI. Statistical software included first-round analyses with a proprietary package and validation of the analyses with open access software. Validation of the analysis utilized open access software, survminer R and python packages, scikit-learn software for survival curves and seaborn for visualization of the statistical data.
2.2 Test parameters
Variables included prior treatment, drug resistance, age, gender (Table 1), AS (Table 2), and individual PBTs as validated high vs. low-risk groups (Table 3). Survival times were estimated from the first day of treatment. Ages of 60, 65, 70, 75, and 80 were tested as cutoff points. The panel of PBTs includes favorable, low risk parameters: lymphocytes > 1.5/µl; platelets < 300,000/µl; white blood count (WBC) < 10,000/µl; absolute neutrophil count (ANC) < 8,000/µl; serum albumin > 3.5 g/dl; as well as NLR < 3.0 and the LMR > 2.1. The AS is defined by the number, 0–4/4 unfavorable high-risk tests: serum albumin < 3.5 g/dl; absolute neutrophil count >8,000/µl; neutrophil lymphocyte ratio > 3.0; and lymphocyte monocyte ratio < 2.1 (15, 16).
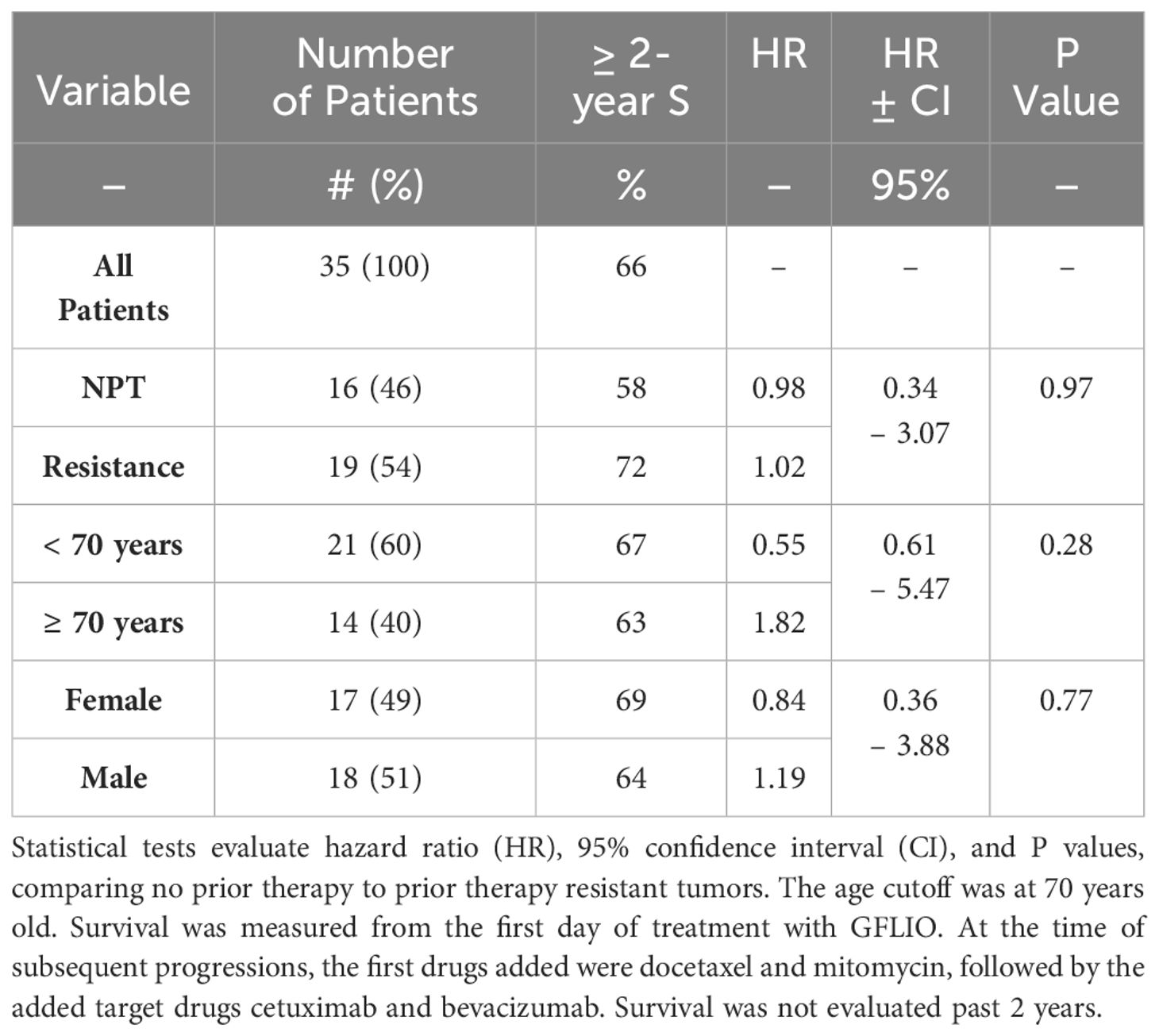
Table 1 Estimated Kaplan-Meier 2-year rates of survival and Cox regression analyses for patients with no prior treatment or resistant tumors.
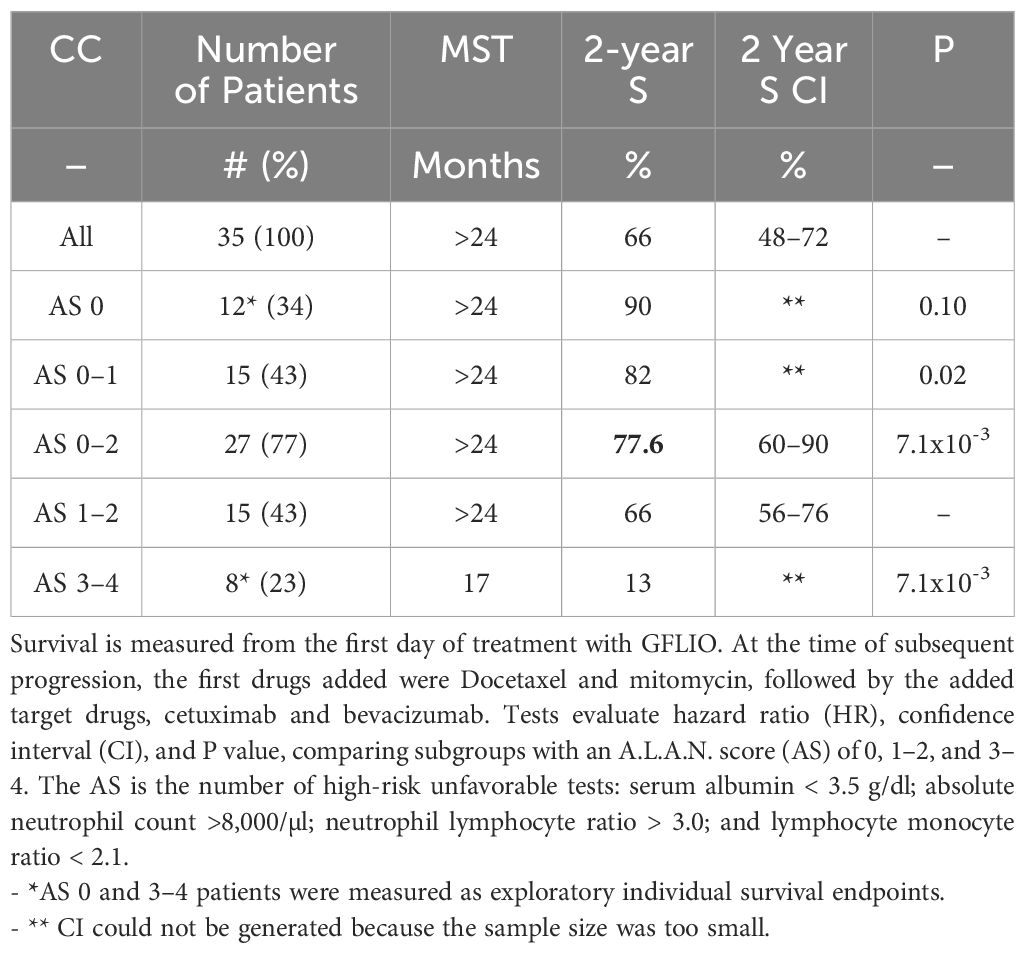
Table 2 Estimated Kaplan-Meier median survival time (MST), rate of survival at 2 years, and Cox-regression analyses for 35 patients with advanced intrahepatic cholangiocarcinoma.
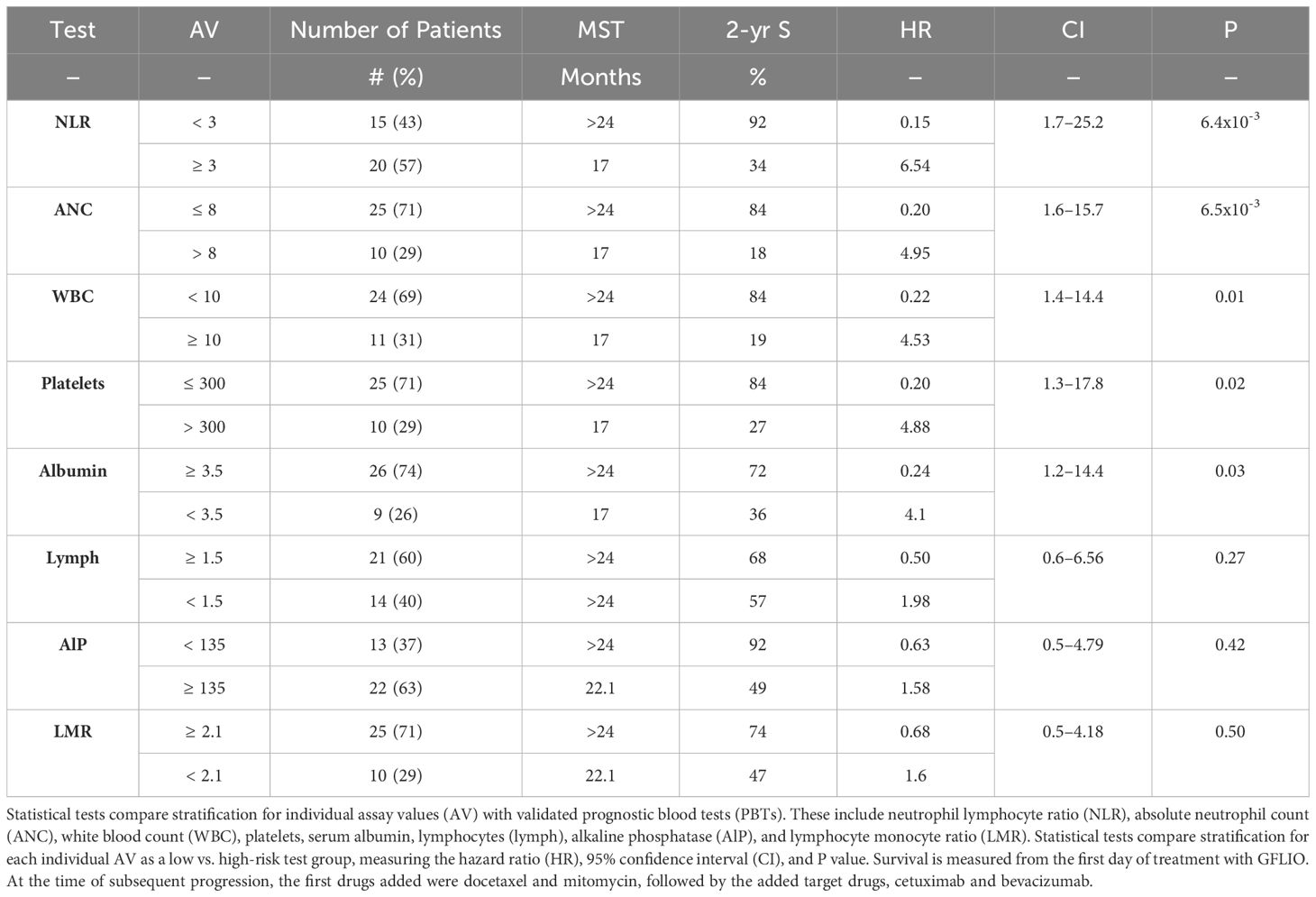
Table 3 Estimated Kaplan-Meier median survival time (MST), rate of survival at 2 years, and Cox regression analyses for 35 patients with advanced intrahepatic cholangiocarcinoma.
2.3 Patients
This study involved a compassionate extension of an IRB-approved trial. The eligibility criteria for participation included the following: intention-to-treat; active progression of disease; advanced measurable IHCCAs and Klatskin tumors; IRB written consent; Helsinki practices (37); any adult age; PS 0–2; positive biopsy; and an anticipated survival of > 6 weeks through < 52 weeks. The patients’ tumors could either be new or resistant to active standard treatment with two or more test drugs, gemcitabine, a platin (usually cisplatin), and sometimes fluorouracil, either as a 48-hour infusion or as capecitabine.
Patients were ineligible for the study if they had metastases in the CNS; recent hospitalization or IV as treatment for dehydration within the past 2 weeks; inability to reach the office; or unresolved NCI grade 3 blood tests or grade 2 co-conditions (38). The entry of patients into the study commenced in May 2016 and was closed in May 2018. Data entry closed for analysis was completed in May 2019.
2.4 IRB requirements
The IRBs’ criteria for continuation of patient accrual included a combined rate of less than 10%: grade 4 hematologic and grade 3 other complications; treatment withdrawal; hospitalization, or a forced delay of more than 3 days in treatment. Real-time monitors evaluated > 50% rates of benefit as well as safety because of both the novel low dosages and entry of patients with high-risk characteristics such as age, PS, or prior limiting AEs. This study was approved by the Western IRB and an FDA application (IND #119005).
2.5 Treatment
The sequence (Schema 1, Part B) involved biweekly administration of gemcitabine (G) at a dosage of 500 mg/M2 over 50 minutes, then irinotecan (I) at a dosage of 80 mg/M2 over 90 minutes. This was followed by continuous infusions of 5-fluorouracil (F) at a dosage of 1200 mg/M2 over 24 hours, leucovorin (L) at a dosage of 180 mg/M2, and on day two, oxaliplatin (O) at a dosage of 40 mg/M2. This drug regimen constitutes GFLIO. Drugs were added to the regimen upon disease progression, first docetaxel (D), at a dosage of 25 mg/M2, with or without mitomycin C (M) at a dosage of 6 mg/M2, a maximum of 10 mg. Mitomycin C was omitted for 1–2 cycles when patients had a prior treatment limiting cytopenia. For a second progression, cetuximab was added at a dosage of 400 mg on day 1, and then 200 mg weekly. For a third progression, bevacizumab at a dosage of 10 mg/kg on day 1, every two weeks, replacing cetuximab. The prior drugs, except for cetuximab, were continued as part of each regimen.
2.6 Serial tests
Tests conducted initially and throughout the study included a CBC every week; a physical examination, a CMP every two weeks; tumor markers monthly, and computer tomography scans at six and then every 12 weeks. Tests are repeated each time a new drug is added to the regimen and to evaluate new clinical complaints (11, 12). The latter include 1 grade change in symptoms, PS, liver function tests including bilirubin, alkaline phosphatase, AST, and ALT, and within those with a PS of 2 or a worsening Karnofsky score. Progression and resistance to prior therapy is defined by both RECIST criteria and independent referring oncologists (39). Progression during treatment with GFLIO is defined with a new full battery of tests including the best prior imaging method, CT or MRI.
2.7 Evaluation
Data was registered prospectively in real-time and in redundant electronic databases. Reviewers included dedicated data-management staff, independent oncologists, radiologists, and statisticians. Second reviewers included oncologists who re-examined the eligibility of the patients and exclusions. There were only 2 patients excluded, one with gall bladder adenocarcinoma and one who refused treatment. Preset statistical analyses were followed by similar analyses with a second software package with the use of historical cutoffs and comparison to parallel trials. Analyses are categorized as exploratory if the sample size was less than 14 patients or if the P value was > 0.01. Exploratory analyses were selected because they found possible interactions between PBTs, age and prior therapy for the overall group of contemporary patients (12).
2.8 Dose modification
Treatment with the regimen could result in brief neutropenia (1500–750) or thrombocytopenia (125,000–75,000). Granulocyte colony-stimulating factor (G-CSF) 300 mcg was given for 2 days, days 7 and 8 or 15 and 16 if the ANC was < 1000/µl or < 1250/µl on those respective days. Thereafter, 2 days of G-CSF were given with every following cycle, unless the 2 dyas of G-CSF caused the ANC > 8000/µl, in which case only 1 day of G-CSF would be given on day 7. If G-CSF produced an ANC > 1250/µl, treatment continued on day 15 or 17 without changes in the dosages of the chemotherapy. Cetuximab was administered for patients with and without KRAS mutations, in contrast to practices for patients with APC or CRC (10–12, 40, 41). Neither targeted FDFR or IDG, or targeted immunotherapy was available during the study period.
Initial dosages of cytotoxins were reduced by the percentages shown in Schema 1, Part B, for patients with: prior grade 4 hematologic or grade 3 other AEs; cytopenia needing more than 2 days of G-CSF; fragility; PS of 2; treatment delay exceeding 7 days; or (absent in this series) nadir sepsis (11, 12). Initially, on cycle 1, oxaliplatin or mitomycin C can be omitted or the dosage of docetaxel reduced to 15 mg/M2 for patients with high-risk characteristics. Subsequently, the omitted drugs could be re-introduced at 66% of level 1 dosages. Dosage can be increased by 12.5% to a maximum of level 1 dosages, in the absence of grade 4 ANC or platelet counts. Bevacizumab and irinotecan were withheld in a stop-go fashion until complete resolution of grade 1–2 enteritis. Fluorouracil was escalated monthly by 20%, no more than twice, in the absence of stomatitis and enteritis. Treatment was discontinued when the PS fell to 3, if rest or supportive care failed to improve the PS.
3 Results
Overall survival rates for 35 patients, including 19 with resistant and 16 with new, no prior chemotherapy were 88, 80, and 62% at 12, 18, and 24 months (Table 1, Figure 1). The 95% CI for the lower limit of overall MST was 21.5 months. The 95% CI for the rates of overall survival at 12 and 24 months were 70–97% and 48–85%, respectively.
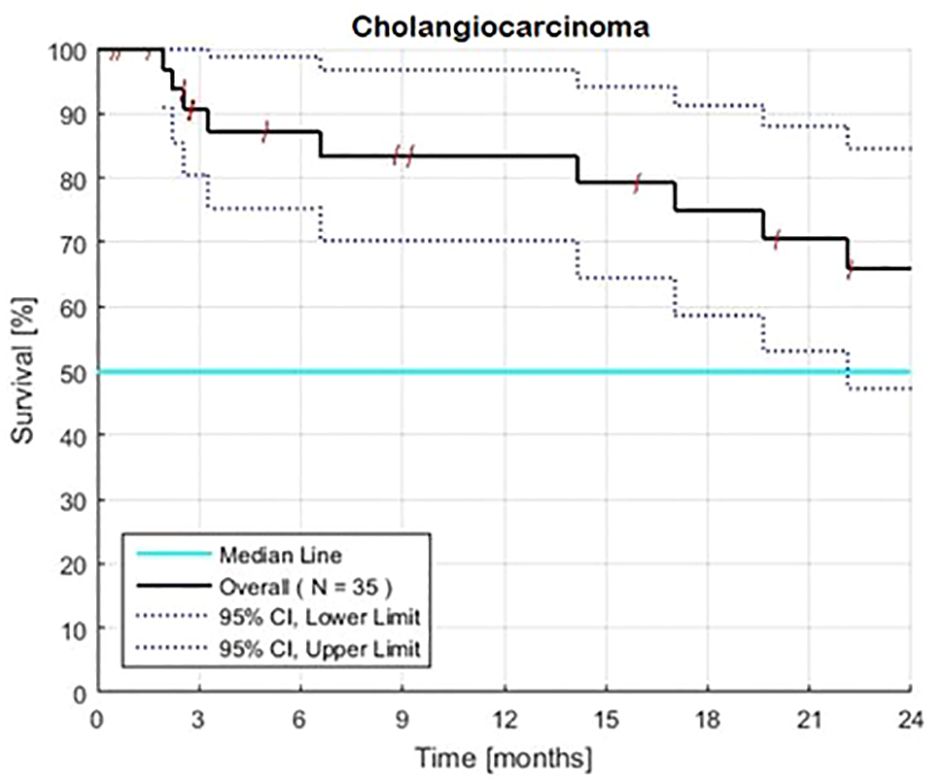
Figure 1 Estimated Kaplan-Meier overall survival of 35 patients with advanced intrahepatic cholangiocarcinoma with (N=19) and without (N=16) prior therapy. The overall 2-year rate of survival is 66% and the associated 95% confidence interval is 48–85%. Survival was calculated from the first day of treatment with GFLIO. At the time of progression, docetaxel/mitomycin C were added, and then subsequently cetuximab and then bevacizumab.
3.1 Gender and age
Both males and females had similar survival rates (Table 1). Survival rates were also similar for patients of all ages (P = 0.28) (Table 1, Figure 2). Among patients aged 70 or older (40% of the total), the HR was 1.82. An exploratory analysis of 36 months finds a modest trend, an advantage for the young compared to the elderly patients (not shown).
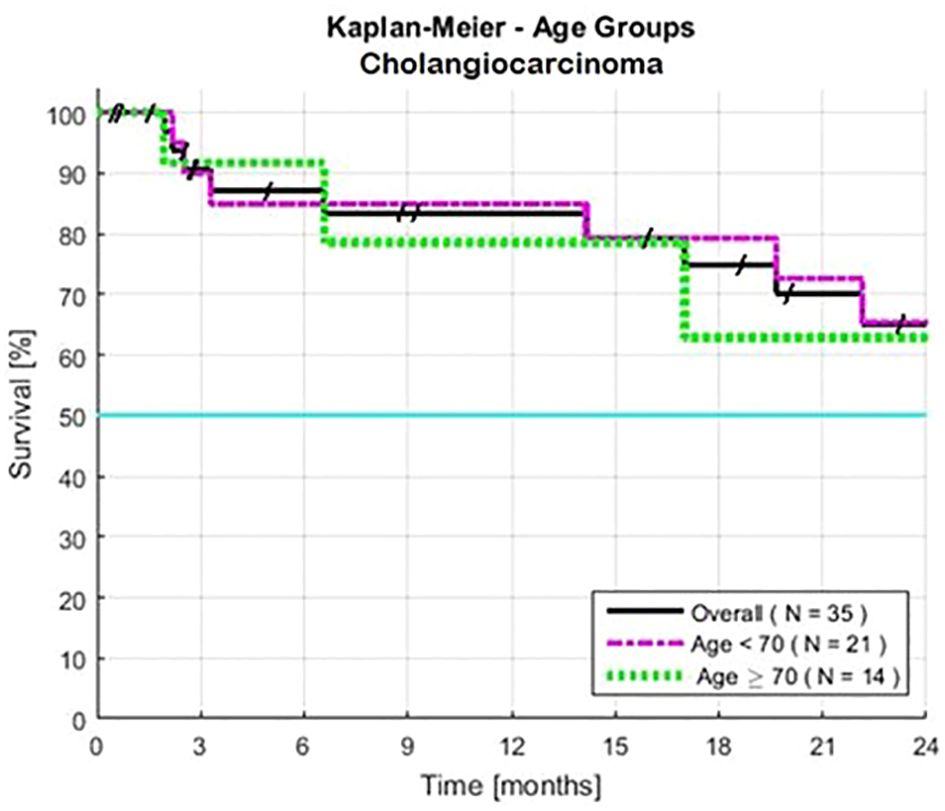
Figure 2 Estimated Kaplan-Meier and the impact of age for patients with advanced intrahepatic cholangiocarcinoma. Cutoff points were ≥ 70 years old (N=14), and < 70 years old (N=21). Two-year survivals were similar, >24 months for both subsets. Survival was calculated from the first day of treatment with GFLIO. At the time of progression, docetaxel/mitomycin C were added, and then subsequently cetuximab and then bevacizumab.
3.2 Treatment history
Survival was similar for the patients with or without prior standard therapy (HR = 1.02, P = 0.97) (Table 1, Figure 3). The frequency of low-risk tests were similar in both groups.
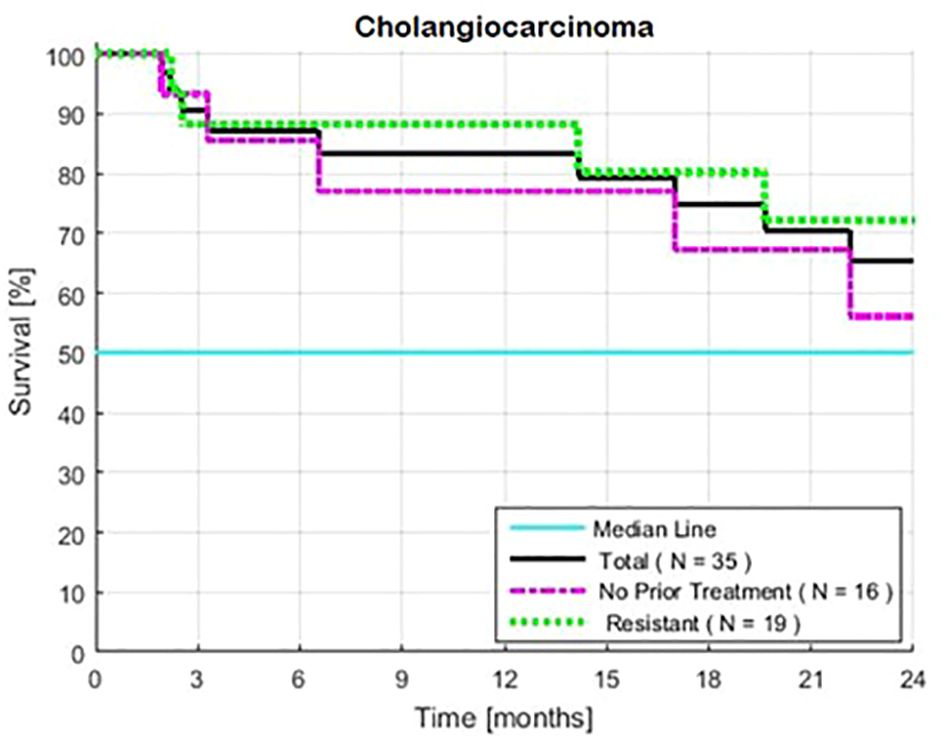
Figure 3 Estimated Kaplan-Meier and the impact of treatment (with or without 1–2 prior lines of treatment for patients with intrahepatic cholangiocarcinoma). Two-year rates of survival with and without prior treatment were 72 and 56%, respectively. Survival was calculated from the first day of treatment with GFLIO. At the time of progression, docetaxel/mitomycin C were added, and then subsequently cetuximab and then bevacizumab.
3.3 A.L.A.N. scores
Patients with an AS of 0 (no high-risk tests) (N = 12) had survival rates of 100%, 100%, and 90% at 12, 18, and 24 months, respectively (Table 2, Figure 4). Patients with an AS of 1–2 high-risk tests (N = 15) had survival rates of 76%, 66%, and 66% (CI 56–76%). Among patients with an AS of 0–2 (N = 27), 76% (CI 60–90%) survived beyond 24 months. Patients with an AS of 3–4 unfavorable tests (N = 8) had survival rates of 67, 34, and 13%. A comparison of patients with an AS of 0–2 vs. 3–4 showed an HR of 6.29 and a P value of 7x10-3.
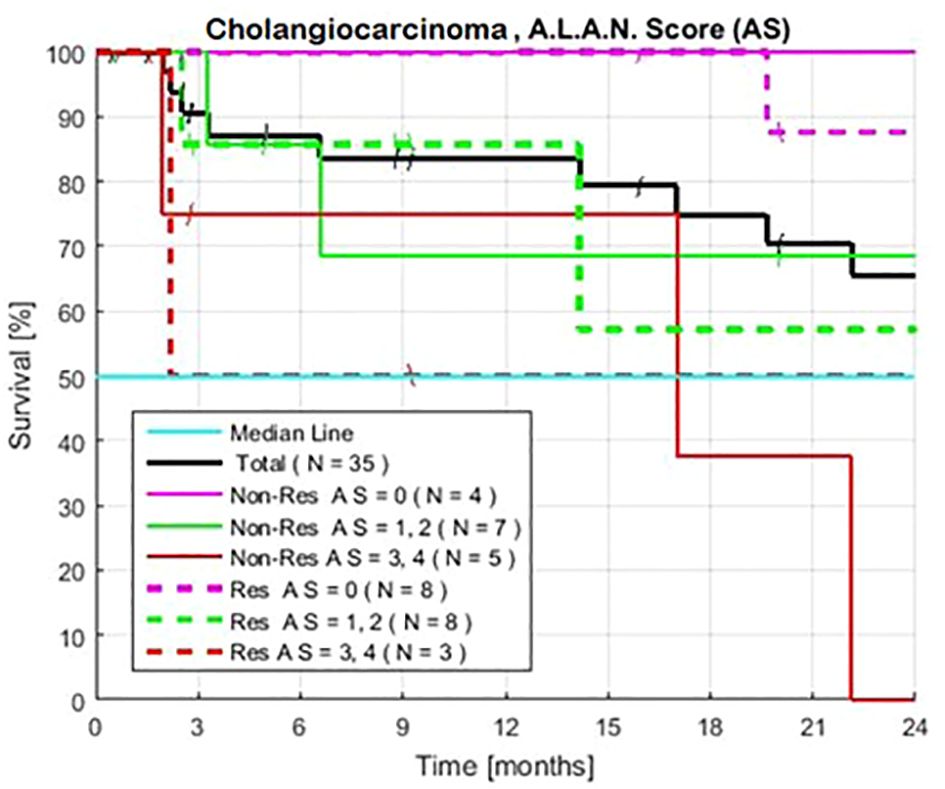
Figure 4 Exploratory estimated Kaplan-Meier survival., without and without prior treatment when patients are defined by the A.L.A.N. Score of 0, 1–2, and 3–4, the number of historically unfavorable tests: serum albumin < 3.5 g/dl; absolute neutrophil count >8,000/µl; neutrophil lymphocyte ratio > 3.0; and lymphocyte monocyte ratio < 2.1. Observed survival is similar for both groups of patients with matched AS.
3.4 Prognostic blood tests
Individual PBTs, in their descending order of statistical strength: NLR, ANC, WBC, platelet count, and serum albumin (Table 3). Groups with low-risk tests have 2-year survivals ranging from 72–92%. Groups with high-risk tests had MSTs ranging from 17 to ≥ 24 months. High-risk tests predict 2-year survival rates for high NLR,34%; high alkaline phosphates,49%; low serum albumin,36%; low lymphocytes,57%; and low LMR,47%. Tests differ in their order of statistical strength as well as their associated median and 2-year survival compared to the combined series (12).
3.5 Prior therapy
Matched groups of patients with an AS of 0 and 3–4, with and without prior therapy, showed similar long and short exploratory survival rates (Figure 4). Survival was also similar for patients with an AS of 1–2, with or without prior therapy, when these patients had similar NLR, ANC, or serum albumin levels, as observed in the combined series of patients (12).
3.6 Safety
There are neither hospitalizations, neutropenic fever, nor novel AEs due to chemotherapy. The rates of grade 1–3 AEs did not show clinically significant changes compared to reported experiences with standard therapy (2, 4).
4 Discussion
The sequential treatments replicate the MST of > 24 months in patients with IHCCA (8). Each of four PBTs (NLR, ANC, WBC, and serum albumin) divide patients into statistically significant low and high-risk groups in the expected fashion. When PBTs are the same, survival tends to be similar for both the young and elderly patients. As a new observation, it is also nearly equal for patients with or without prior therapy (12, 42–44).
Novel features of the treatment include integration of: re-challenge; continued and re-introduction of drugs; recombination with and without the addition of new synergistic drugs; simultaneous use of four or more drugs; low dosages; all reverse or bypass the tumors’ resistance to critical drugs (12). These findings, along with promising similar evidence of efficacy in parallel series support and expand upon the findings of investigations of the individual features of the treatment (12, 42, 45).
Evaluation of this series of patients with CCA finds that PBTs may independently modify and improve investigation of eligibility, small groups of patients, and mechanisms of resistance or lethality. PBTs can be screening surrogate biomarkers for inflammation, driver growth factors, cytokines, and immunosuppression (12–16, 20–26).
The prolonged survival in many low and high-risk test groups when compared to historical test groups indicates that the treatment contributes to improved survival within the known limitations of cross-trial comparisons. High-risk AS tests alone may not be a contraindication for the treatment of individual patients with CCA (16). Patients with high-risk tests can be included in trials, especially if there is a prospective plan to evaluate the PBTs and high-risk clinical characteristics in order to avoid false-negative conclusions (14). Such findings are consistent for patients with 1–2 high-risk tests; however, practice for patients with 3–4 high risk tests requires caution because patients with some other cancers have little or no benefit.
To the best of our knowledge, no combination of standard therapy and clinical characteristics can identify a group of stage IV patients with MSTs similar to those observed in this group with an AS of 0 or an AS of 1–2, or the similar prolonged MSTs compared to the expected survival of patients with APC,RAPC, and RCRC (7).
Some combination of the sequential treatments and an AS of 0–2 as eligibility criteria may minimize the poor safety outcomes resulting from age, frailty, maximum tolerated dosage, and resistance to drugs. These characteristics limit the real-world eligibility and utilization of clinically valuable drugs for the majority of patients with advanced GI cancers (2, 3, 42–44).
The use of low dosage is a promising additional approach in order to expand indications and for the development of drugs (35, 45, 46). Treatment with the regimen has shown no novel and exceptionally few occurrences of grade 4 or limiting AEs, although idiopathic and outlier events remain possible at low dosage. The slow decline of the nadir ANCs over multiple cycles allows for dose adjustments or the addition of minimal yet rapidly acting G-CSF to avoid prolonged cytopenia. Since 2020, when individual baseline PBTs are severely unfavorable, ad hoc exploratory practice includes both early recognition of relapse or impending crises, and addition of docetaxel and mitomycin (DM), with or without targeted therapy, to increase the chances of rapid response. Also, our anecdotal findings and independent work have produced long lasting response with the combinations of GFLIO-DM or single drugs, respectively, after failure of both the individual treatments with chemotherapy and immunotherapy (32–34, 47, 48). However, the patients in this series did not receive immunotherapy.
Limitations of the study are the small number of patients and the underpowered subgroups with high-risk tests. Other limitations are the absence of: a randomized comparator; multivariate tests to confirm the independence of PBTs and clinical characteristics; re-examination of our promising multi-disease experience with DM, bevacizumab, or cetuximab as sequentially added drugs (10, 11, 13–16, 49, 50).
Individual drugs may sometimes require the synergistic effects produced with the novel addition of individually ineffective irinotecan, docetaxel, or mitomycin. Target drugs may also inhibit tumor drivers that become clinically important only after multiple lines of treatment. Development efforts require both validation and caution, because the added drugs, as well as nab paclitaxel, docetaxel and liposomal irinotecan, have all shown limited or inconsistent effectiveness (51–55). Independent laboratory investigations have also found promising, sometimes broad synergy between mitomycin and the individual drugs, including gemcitabine, fluorouracil, irinotecan, oxaliplatin, and docetaxel drugs (56).
The atypical high P values of the groups with high-risk LMR and absolute lymphocyte count (ALC) in analyses of both our CCA and combined series of patients compared to earlier investigations suggests that the treatments improve the patients’ immune function.
The prolonged survival of groups with favorable tests may be evidence of unintended patient selection in referral practices. Nevertheless, the number of patients in statistically powerful subgroups with an AS of 0–2 allows for investigation of personalized PBTs both in exploratory retrospective fashion and as comparator tests for future phase III trials.
5 Conclusion
Many lines of evidence support our findings and meet the criteria for undertaking phase II-III comparisons of new and standard regimens. Survival is reproducible overall and in what may be surrogate biomarker subgroups, novel for many unfavorable subgroups, and applicable to patients with resistant tumors both in this and the large APC combined analyses (12, 41). The regimens integrate promising exploitable and infrequently examined features, safe dosages for combination chemotherapy, with or without laboratory assistance, broadly applicable and clinically promising methods to bypass resistance to drugs, improve safety of palliative care, and restore the patients’ immune functions. It also develops rechallenge-recombination, synergism with immunotherapy, target drugs, and analogues of the cytotoxins (4).
PBTs warrant investigation as supplemental comparators of treatments and as eligibility criteria to personalize the order, timely addition of drugs, and identify targetable lethal mechanisms. Addressing these objectives can benefit the majority of patients with CCA because they have historically short survival, resistant tumors, new options for immunotherapy, and concerns related to safety because of advanced age, and now avoidable high rates of AEs (31).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Western Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HB: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RD: Validation, Writing – original draft, Writing – review & editing. EK: Validation, Writing – original draft, Writing – review & editing. FB: Formal analysis, Resources, Software, Writing – review & editing. AB: Data curation, Formal analysis, Writing – original draft. DG: Resources, Supervision, Writing – original draft. VN: Data curation, Formal analysis, Writing – original draft. MS: Formal analysis, Investigation, Project administration, Resources, Writing – original draft. AH: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. MZB Center for Cancer Research, Aid L’ Shalom Foundation, and Marcus Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. (2020) 126:2666–78. doi: 10.1002/cncr.32803
2. Fiteni F, Jary M, Monnien F, Nguyen T, Beohou E, Demarchi M, et al. Advanced biliary tract carcinomas: a retrospective multicenter analysis of first and second-line chemotherapy. BMC gastroenterol. (2014) 14:1–7. doi: 10.1186/1471–230X-14–143
3. Lamarca A, Hubner RA, Ryder WD, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. (2014) 25:2328–38. doi: 10.1093/annonc/mdu162
4. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. (2021) 22:690–701. doi: 10.1016/S1470–2045(21)00027–9
5. Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM evidence. (2022) 1(8):EVIDoa2200015. doi: 10.1056/EVIDoa2200015
6. Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. Three-year survival and safety update from the phase 3 TOPAZ-1 study of durvalumab plus chemotherapy in biliary tract cancer, in: Poster presented at: 2024 CCF Conference, Salt Lake City, Utah, United States of America, April 17–19, 2024.
7. Rizzo A, Tavolari S, Ricci AD, Frega G, Palloni A, Relli V, et al. Molecular features and targeted therapies in extrahepatic cholangiocarcinoma: promises and failures. Cancers. (2020) 12:3256. doi: 10.3390/cancers12113256
8. Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. New Engl J Med. (2023) 388:228–39. doi: 10.1056/NEJMoa2206834
9. Karasic TB, Eads JR, Goyal L. Precision medicine and immunotherapy have arrived for cholangiocarcinoma: an overview of recent approvals and ongoing clinical trials. JCO Precis Oncol. (2023) 7:e2200573. doi: 10.1200/PO.22.00573
10. Bruckner H, Hirschfeld A, Buddaraju S, Stega J, Jahan M, Schwartz ME. Multidisciplinary effect of adding docetaxel and mitomycin-C to low-dose multidrug therapy for cholangiocarcinoma. J Clin Oncol. (2011) 29:e14546–. doi: 10.1200/jco.2011.29.15_suppl.e14546
11. Bruckner HW, Hirschfeld A, Schwartz M. Targeted therapy for resistant cholangiocarcinoma with bevacizumab or cetuximab added to failed cytotoxic drug cores. Anticancer Res. (2016) 36:399–402.
12. Bruckner HW, Bassali F, Dusowitz E, Gurell D, Book A, De Jager R. Actionable tests and treatments for patients with gastrointestinal cancers and historically short median survival times. PloS One. (2022) 17:e0276492. doi: 10.1371/journal.pone.0276492
13. Grenader T, Nash S, Plotkin Y, Furuse J, Mizuno N, Okusaka T, et al. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studies. Ann Oncol. (2015) 26:1910–6. doi: 10.1093/annonc/mdv253
14. Cho KM, Park H, Oh DY, Kim TY, Lee KH, Han SW, et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their dynamic changes during chemotherapy is useful to predict a more accurate prognosis of advanced biliary tract cancer. Oncotarget. (2017) 8:2329. doi: 10.18632/oncotarget.13731
15. Salati M, Caputo F, Cunningham D, Marcheselli L, Spallanzani A, Rimini M, et al. The ALAN score identifies prognostic classes in advanced biliary cancer patients receiving first-line chemotherapy. Eur J Cancer. (2019) 117:84–90. doi: 10.1016/j.ejca.2019.05.030
16. Müller L, Mähringer-Kunz A, Jungmann F, Tanyildizi Y, Bartsch F, Czauderna C, et al. Risk stratification in advanced biliary tract cancer: validation of the ALAN score. J Oncol. (2020) 53:1–8. doi: 10.1155/2020/6180613
17. Bruckner HW, Hirschfeld A, De Jager R, Bassali F, Gurell D, Nghiem V, et al. Moderate dose sequential chemotherapy (CT) for the elderly with and without resistant cancers (RC). J Geriatric Oncol. (2019) 10:39. doi: 10.1016/S1879–4068(19)31181–6
18. Bruckner HW, Lavin PT, Plaxe SC, Storch JA, Livstone EM. Absolute granulocyte, lymphocyte, and monocyte counts: useful determinants of prognosis for patients with metastatic cancer of the stomach. Jama. (1982) 247:1004–6. doi: 10.1001/jama.1982.03320320040027
19. Lavin PT, Bruckner HW, Plaxe SC. Studies in prognostic factors relating to chemotherapy for advanced gastric cancer. Cancer. (1982) 50:2016–23. doi: 10.1002/1097–0142(19821115)50:10<2016:AID-CNCR2820501007>3.0.CO;2–2
20. Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. New Engl J Med. (2012) 366:610–8. doi: 10.1056/NEJMoa1110352
21. Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. (2015) 41:971–8. doi: 10.1016/j.ctrv.2015.10.003
22. Fujii T, Tokuda S, Nakazawa Y, Kurozumi S, Obayashi S, Yajima R, et al. Implications of low serum albumin as a prognostic factor of long-term outcomes in patients with breast cancer. in vivo. (2020) 34:2033–6. doi: 10.21873/invivo.12003
23. Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. (2020) 18:1–6. doi: 10.1186/s12916–020-01817–1
24. Zhao J, Huang W, Wu Y, Luo Y, Wu B, Cheng J, et al. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: a systematic review and meta-analysis. Cancer Cell Int. (2020) 20:1–4. doi: 10.1186/s12935–020-1094–5
25. Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. (2021) 14:1–7. doi: 10.1186/s13045-021-01187-y
26. Patysheva M, Frolova A, Larionova I, Afanas' ev S, Tarasova A, Cherdyntseva N, et al. Monocyte programming by cancer therapy. Front Immunol. (2022) 13:994319. doi: 10.3389/fimmu.2022.994319
27. Kozuch P, Grossbard ML, Barzdins A, Araneo M, Robin A, Frager D, et al. Irinotecan combined with gemcitabine, 5-fluorouracil, leucovorin, and cisplatin (G-FLIP) is an effective and non-cross resistant treatment for chemotherapy refractory metastatic pancreatic cancer. Oncologist. (2001) 6:488–95. doi: 10.1081/cnv-120022357
28. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. (2010) 70:440–6. doi: 10.1158/0008–5472.CAN-09–1947
29. Bruckner HW, Hirschfeld A, Stega J, Dottino P. Abstract CT314:”Multi-metronomic” algorithms for targeted therapy to improve value of response and “failed drugs” for “resistant” women's cancers. Cancer Res. (2014) 74:CT314–. doi: 10.1158/1538–7445.AM2014-CT314
30. Bruckner HW, Gurell D, Hirschfeld A. Bevacizumab added to moderate-dose chemotherapy for refractory uterine cancer. Anticancer Res. (2018) 38:547–52. doi: 10.21873/anticanres.12257
31. Palmer AC, Chidley C, Sorger PK. A curative combination cancer therapy achieves high fractional cell killing through low cross-resistance and drug additivity. Elife. (2019) 8:e50036. doi: 10.7554/eLife.50036
32. Correale P, Cusi MG, Del Vecchio MT, Aquino A, Prete S, Tsang KY, et al. Dendritic cell-mediated cross-presentation of antigens derived from colon carcinoma cells exposed to a highly cytotoxic multidrug regimen with gemcitabine, oxaliplatin, 5-fluorouracil, and leucovorin, elicits a powerful human antigen-specific CTL response with antitumor activity in vitro. J Immunol. (2005) 175:820–8. doi: 10.4049/jimmunol.175.2.820
33. Correale P, Botta C, Cusi MG, Del Vecchio MT, De Santi MM, Gori Savellini G, et al. Cetuximab ± chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer. (2012) 130:1577–89. doi: 10.1002/ijc.26181
34. Caraglia M, Correale P, Giannicola R, Staropoli N, Botta C, Pastina P, et al. GOLFIG chemo-immunotherapy in metastatic colorectal cancer patients. A critical review on a long-lasting follow-up. Front Oncol. (2019) 9:1102. doi: 10.3389/fonc.2019.01102
35. Isacoff WH, Reber HA, Bedford R, Hoos W, Rahib L, Upfill-Brown A, et al. Low-dose continuous 5-fluorouracil combined with leucovorin, nab-paclitaxel, oxaliplatin, and bevacizumab for patients with advanced pancreatic cancer: a retrospective analysis. Targeted Oncol. (2018) 13:461–8. doi: 10.1007/s11523-018-0572-3
36. Chow CK, Atkins ER, Hillis GS, Nelson MR, Reid CM, Schlaich MP, et al. Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines versus standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomized, double-blind, active-controlled trial. Lancet. (2021) 398:1043–52. doi: 10.1016/S0140-6736(21)01922-X
37. Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. (2004) 57:695–713. doi: 10.1111/j.1365-2125.2004.02103.x
38. Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Physics. (2000) 47:13–47. doi: 10.1016/S0360–3016(99)00559–3
39. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
40. De Jager R, Bruckner HW, Hirschfeld A, Gurell D, Nghiem V, Book A, et al. Moderate sequential third-to fourth-line chemotherapy (CT) algorithms (Algo) for the elderly with resistant colorectal cancer (R-CRC). J Geriatric Oncol. (2019) 10:S47–8. doi: 10.1016/S1879-4068(19)31196-8
41. De Jager RL, Bruckner H, Bassali F, Hirschfeld A, Gurell D, Nghiem V, et al. Survival and prognostic tests in a real-world phase II experience with a safe, moderate-dose, sequential chemotherapy (CT) algorithm (ALGO) for metastatic pancreatic adenocarcinoma (PANC). J Clin Oncol. (2020) 38:15. doi: 10.1200/JCO.2020.38.15_suppl.e16763
42. Townsley C, Pond GR, Peloza B, Kok J, Naidoo K, Dale D, et al. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. (2005) 23:3802–10. doi: 10.1200/JCO.2005.06.742
43. Horgan A, Knox J, Aneja P, Le L, McKeever E, McNamara M. Patterns of care and treatment outcomes in older patients with biliary tract cancer. Oncotarget. (2015) 6:44995. doi: 10.18632/oncotarget.v6i42
44. Bruckner H, Hirschfeld A, DeJager R, Bassali F, Gurell D, Nghiem V, et al. Blood tests predict safe survival of elderly with resistant GI cancers. J Geriatric Oncol. (2021) 12:S51. doi: 10.1016/S1879-4068(21)00427-6
45. Hall PS, Swinson D, Cairns DA, Waters JS, Petty R, Allmark C, et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:869–77. doi: 10.1001/jamaoncol.2021.0848
46. Vary A, Lebellec L, Di Fiore F, Penel N, Cheymol C, Rad E, et al. FOLFIRINOX relative dose intensity and disease control in advanced pancreatic adenocarcinoma. Ther Adv Med Oncol. (2021) 13:17588359211029825. doi: 10.1177/17588359211029825
47. Chakrabarti S, Dong H, Paripati HR, Ross HJ, Yoon HH. First report of dramatic tumor responses with ramucirumab and paclitaxel after progression on pembrolizumab in two cases of metastatic gastroesophageal adenocarcinoma. oncologist. (2018) 23:840. doi: 10.1634/theoncologist.2017–0561
48. Argota IB, Romano PM, Sanmamed MF, Ruiz MR, Melero I, Resano L, et al. Chemotherapy after immunotherapy failure in patients with advanced gastrointestinal tumors. Ann Oncol. (2018) 29:vi21–22. doi: 10.1093/annonc/mdy314.002
49. Bruckner H, Meeus SI, Reilly JP, Cooperman AM. Laboratory based non-cross-resistant chemotherapy for pancreatic carcinoma. InAm S Clin Oncol Gastronintestinal Cancers Symposium. (2004).
50. Bruckner H, Simon K, Hrehorovich V. Low-dose sequential multi-drug regimens for advanced pancreatic cancer. J Clin Oncol. (2008) 26:15568–. doi: 10.1200/jco.2008.26.15_suppl.15568
51. Borbath I, Ceratti A, Verslype C, Demols A, Delaunoit T, Laurent S, et al. Combination of gemcitabine and cetuximab in patients with advanced cholangiocarcinoma: a phase II study of the Belgian Group of Digestive Oncology. Ann Oncol. (2013) 24:2824–9. doi: 10.1093/annonc/mdt337
52. Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardière C, Boucher E, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non- comparative phase 2 trial. Lancet Oncol. (2014) 15:819–28. doi: 10.1016/S1470-2045(14)70212-8
53. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. (2016) 13:261–80. doi: 10.1038/nrgastro.2016.51
54. Shroff RT, Guthrie KA, Scott AJ, Borad MJ, Goff LW, Matin K, et al. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. Journal of Clinical Oncology. (2023) 41:4. doi: 10.1200/JCO.2023.41.4_suppl.LBA49
55. Taghizadeh H, Djanani A, Eisterer W, Gerger A, Gruenberger B, Gruenberger T, et al. Systemic treatment of patients with locally advanced or metastatic cholangiocarcinoma–an Austrian expert consensus statement. Front Oncol. (2023) 13. doi: 10.3389/fonc.2023.1225154
Keywords: cholangiocarcinoma, low-dosage, chemotherapy, prognosis, survival, resistance, geriatrics
Citation: Bruckner HW, De Jager R, Knopf E, Bassali F, Book A, Gurell D, Nghiem V, Schwartz M and Hirschfeld A (2024) Cholangiocarcinoma, sequential chemotherapy, and prognostic tests. Front. Oncol. 14:1361420. doi: 10.3389/fonc.2024.1361420
Received: 26 December 2023; Accepted: 06 June 2024;
Published: 24 June 2024.
Edited by:
John Gibbs, Hackensack Meridian Health, United StatesReviewed by:
Massimiliano Salati, University Hospital of Modena, ItalyVeronika Lukacs-Kornek, University of Bonn, Germany
Copyright © 2024 Bruckner, De Jager, Knopf, Bassali, Book, Gurell, Nghiem, Schwartz and Hirschfeld. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Howard W. Bruckner, aG93YXJkYnJ1Y2tuZXJAZ21haWwuY29t; Elisheva Knopf, ZWtub3BmMjAyM0BoZWFsdGguZmF1LmVkdQ==
 Howard W. Bruckner
Howard W. Bruckner Robert De Jager
Robert De Jager Elisheva Knopf
Elisheva Knopf Fred Bassali1
Fred Bassali1