- 1Clinical Medical College of Acupuncture, Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2School of Rehabilitation Sciences, Southern Medical University, Guangzhou, China
Background: The effect of first-line complex decongestive therapy (CDT) for breast cancer-related lymphedema (BCRL) depending on various factors forces patients to seek additional treatment. Therefore, this meta-analysis was conducted to evaluate the effect of different conservative medical interventions as a complement to CDT. This is the first meta-analysis that includes various kinds of conservative treatments as adjunctive therapy to get broader knowledge and improve practical application value, which can provide recommendations to further improve BCRL patients’ health status.
Methods: RCTs published before 18 December 2023 from PubMed, Embase, Cochrane Library, and Web of Science databases were searched. RCTs that compared the effects of conservative medical intervention were included. A random-effects or fixed-effects model was used based on the heterogeneity findings. Study quality was evaluated using the Cochrane risk of bias tool.
Results: Sixteen RCTs with 690 participants were included, comparing laser therapy, intermittent pneumatic compression (IPC), extracorporeal shock wave therapy (ESWT), electrotherapy, ultrasound, diet or diet in combination with synbiotic supplement, traditional Chinese medicine (TCM), continuous passive motion (CPM), and negative pressure massage treatment (NMPT). The results revealed that conservative medical intervention as complement to CDT had benefits in improving lymphedema in volume/circumference of the upper extremity [SMD = −0.30, 95% CI = (−0.45, −0.15), P < 0.05, I2 = 51%], visual analog score (VAS) for pain [SMD = −3.35, 95% CI (−5.37, −1.33), P < 0.05, I2 = 96%], quality of life [SMD = 0.44, 95% CI (0.19, 0.69), P < 0.05, I2 = 0], and DASH/QuickDASH [SMD = −0.42, 95% CI (−0.70, −0.14), P < 0.05, I2 = 10%] compared with the control group. Subgroup analysis revealed that laser therapy and electrotherapy are especially effective (P < 0.05).
Conclusion: Combining conservative medical interventions with CDT appears to have a positive effect on certain BCRL symptoms, especially laser therapy and electrotherapy. It showed a better effect on patients under 60 years old, and laser therapy of low to moderate intensity (5–24 mW, 1.5–2 J/cm2) and of moderate- to long-term duration (≥36–72 sessions) showed better effects.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=354824, identifier CRD42022354824.
1 Background
Breast cancer (BC) is the most frequently diagnosed cancer among women around the world although the incidence rate continues to rise and the overall mortality rate has declined with the advancement of early screening and diagnosis in many high-income countries (1). As the 5-year survival rate is almost 90% (2), there is an increasing number of BC survivors suffering long-term complications brought by surgery and radiation-related therapeutic exposure. BC-related lymphedema (BCRL) is one of the most seen complications among the survivors, with up to 40% of BC survivors suffering from upper extremity complications especially if lymphedema axillary lymph node procedures are applied (3). BCRL is associated with swelling in the limbs, persistent inflammation, pain, numbness, and restricted mobility (4), causing great distress in physical and mental function, and patients consider it as a medical burden (5, 6).
Since BCRL remains both incurable and debilitating (7), it is still challenging to confirm effective and safe therapy for patients. Currently, the first-line therapy for lymphedema is complex physical therapy/complex decongestive therapy (CDT), comprised of two phases: the first phase includes manual lymphatic drainage (MLD), compression therapy (bandages/garments/pumps), remedial exercise, and skin and nail care (8); the second phase includes lifelong self-care maintenance. Even though CDT has been proven as the most widely used treatment for lymphedema, its effectiveness depends greatly upon the therapist’s experience and overall patient compliance with the complex self-care requirement (9), which may cause low adherence in the long term because of repeated and tedious procedures. Furthermore, using compression therapy alone shows limited benefit to edema over a long-term period (10). The benefits of CDT will be greater if applied in the early lymphedema stage (stage I) (11) or less than 1 year of lymphedema duration (12). The severe condition of BCRL might hinder the effect of CDT, thus forcing patients to seek additional treatment (13), e.g., surgical treatment such as lymphaticovenular anastomosis (10, 14) and various conservative treatments to better improve the overall status of BCRL patients. Due to these limitations, a multidisciplinary approach and additional treatment strategies to treat BCRL systematically are necessary to be explored to optimize treatment efficiency and contribute to a more complete and efficient treatment, improving the quality of life as well as the adherence of the survivors (15).
To date, no studies have investigated the effectiveness of combinations of various conservative medical interventions and CTD. Our study was therefore designed to evaluate the effect of conservative interventions combining CDT in improving the symptoms of BCRL patients, and the results may help practitioners choose more efficient treatments. The hypothesis is that a combination of certain conservative interventions in these patients would improve the symptoms more significantly compared with CDT alone.
2 Methods
2.1 Eligibility criteria
All available randomized controlled trials (RCTs) met the following criteria: 1) types of studies: RCTs; 2) types of participants: women patients with BCRL and no restrictions on their BC and lymphedema stage, nationality, and age; 3) types of intervention: conservative treatment including physical therapy/oral supplements. Standard care (ST) was defined as any combination of skin care, exercises, compression method, and part of CDT. Giving the patients with only health education or guidance will be defined as none; 4) types of outcomes: primary outcomes were changes in edema, such as volume/circumference of the arm (lower scores mean better effect). Secondary outcomes included quality of life (QoL); VAS for pain; disabilities of the arm, shoulder, and hand (DASH/QuickDASH); and grip strength; and 5) language: English.
The exclusion criteria were as follows: 1) case reports, reviews, study protocols, conference abstracts, commentaries, and letters; 2) duplicate articles; 3) animal experiments; 4) studies that used surgical intervention and oral medicine (such as diosmin) and compared instruments like bandages/kinesio taping/garments aiming to compare different brands; studies that compared exercises were also excluded because our focus was on medical practice rather than self-care practice and studies that used intervention in the control group other than CDT/standard care/none; 5) any study design except RCT; 6) unavailable original full text; 7) language except English; and 8) studies with outcome only shown in graphs without concrete data form and failure to contact the authors to obtain the data.
2.2 Information sources and search strategy
Four relevant English literature databases (Embase, Cochrane Library, Web of Science, PubMed) were searched for all relevant citations published until 18 December 2023. We established search strategies that combined Medical Subject Headings (MESH term) and random words related to BCRL, interventions of interest (treatment/therapy), and RCTs. Furthermore, the reference lists of the included studies were manually reviewed to look for additional relevant manuscripts. The specific search strategies are shown in Appendix 1.
2.3 Selection process
Two reviewers (CYD, ZGW) independently screened the titles and abstracts of identified citations and full texts of potentially eligible studies. Disagreements were resolved by discussion or third-party (XYZ) adjudication when necessary.
2.4 Data collection and data items
Two reviewers (CYD, ZJC) independently extracted the study data, including the name of the first author, publication year, participant characteristics (country and age), sample size, intervention and the comparison treatments, baseline, course of treatment, outcomes, adverse events (AEs), and dropout. Lymphedema volume was measured as volume calculated from the circumference, water displacement and bioimpedance spectroscopy. Two researchers (CYD, ZJC) checked the extracted data for consistency, and a third researcher (XYZ, CZT) arbitrated any dispute.
2.5 Study risk of bias assessment
The Cochrane Risk of Bias Assessment tool (Cochrane Collaboration) was used to assess the risk of bias. The following types of bias were assessed: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessment, 5) incomplete outcome data, 6) selective reporting, and 7) other bias. Each item was classified into three types: low risk, high risk, and unclear risk. The quality of the included trials was evaluated by two reviewers (CYD, ZJC) independently, and disagreements were resolved by a third researcher (ZGW).
2.6 Effect measures
Continuous data were expressed as the mean ± standard deviation (SD) and summarized as a mean difference (MD) or standardized mean difference (SMD). Considering the primary outcomes reflected by the volume (ml/cm3) or circumference (cm) of the arm, the different units can cause great differences in data size; therefore, SMD with 95% confidence intervals (CIs) was used to analyze the primary outcome.
Heterogeneity among the studies was detected using P and I2 statistics. A random-effects model was adopted when heterogeneity was observed (P < 0.05 and/or I2 > 50%); otherwise, a fixed-effects model was adopted. If the pooled result included clinical heterogeneity, subgroup analysis was performed to search for the source of heterogeneity. Clinical heterogeneity was defined as differences in participants, treatment, outcome characteristics, or research setting.
All data were analyzed using the Excel 2016 (Microsoft) and RevMan 5.3 (Cochrane Collaboration, Oxford, UK) software.
3 Results
3.1 Study selection
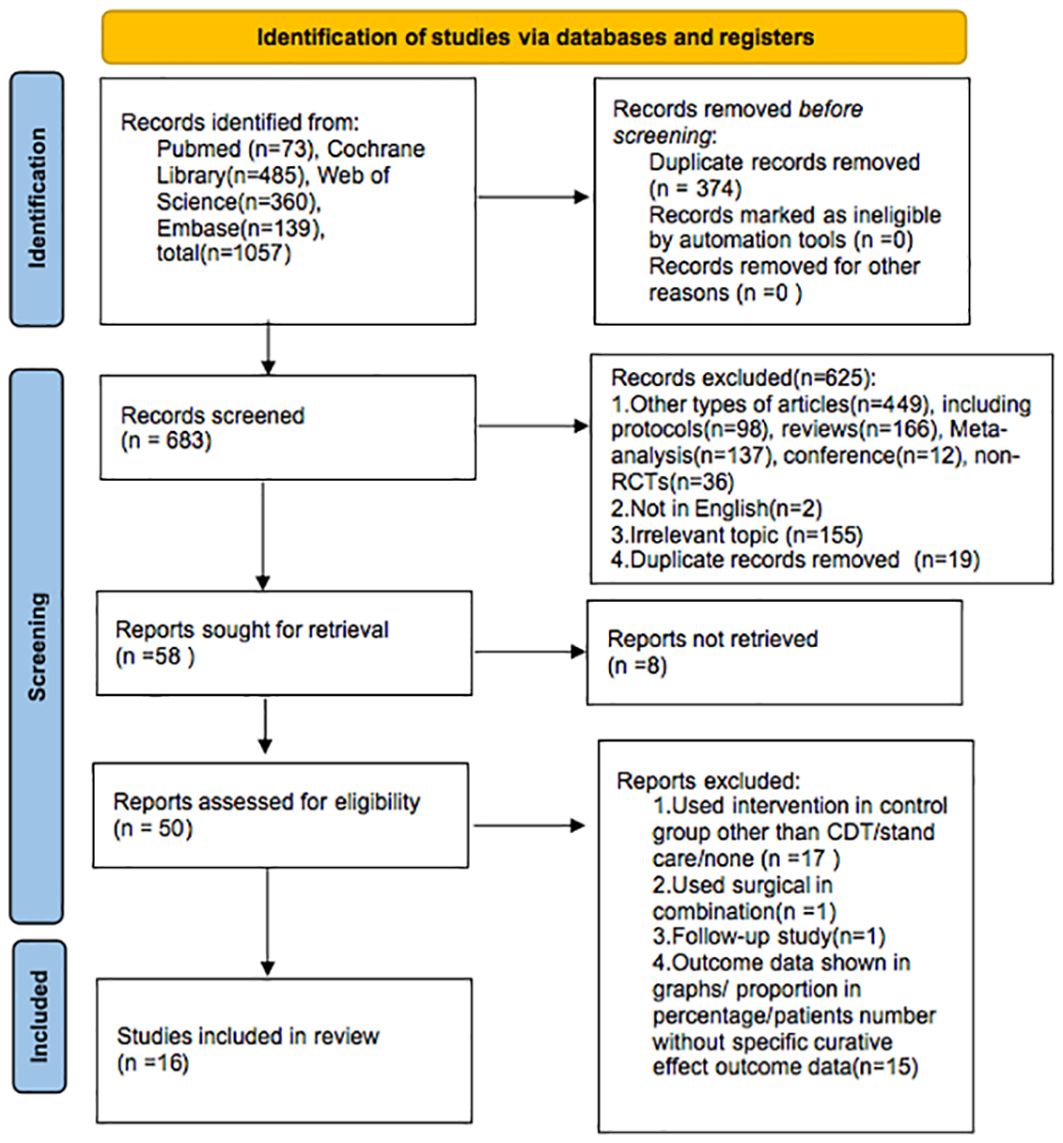
The PRISMA flow diagram (Figure 1) displays the selection process. We found 1,057 articles by searching the databases (PubMed, Cochrane Library, Web of Science, Embase). After excluding 374 duplicate articles, two researchers conducted independent review and exclusion. Based on the title and abstract, 625 articles did not meet the selection criteria and were excluded, and 8 reports could not be retrieved.
Of the remaining 50 papers, 34 were excluded for the following reasons: in 17 studies, the control intervention was not CDT/standard care/none; 1 study used surgical intervention as combination; 1 study focused on follow-up research; and 15 articles did not report relevant concrete outcomes; specifically, the outcome data were shown in graphs/proportion in percentage/patients’ number without specific curative effect outcome data. Finally, 16 articles were included in our analysis.
3.2 Study characteristics
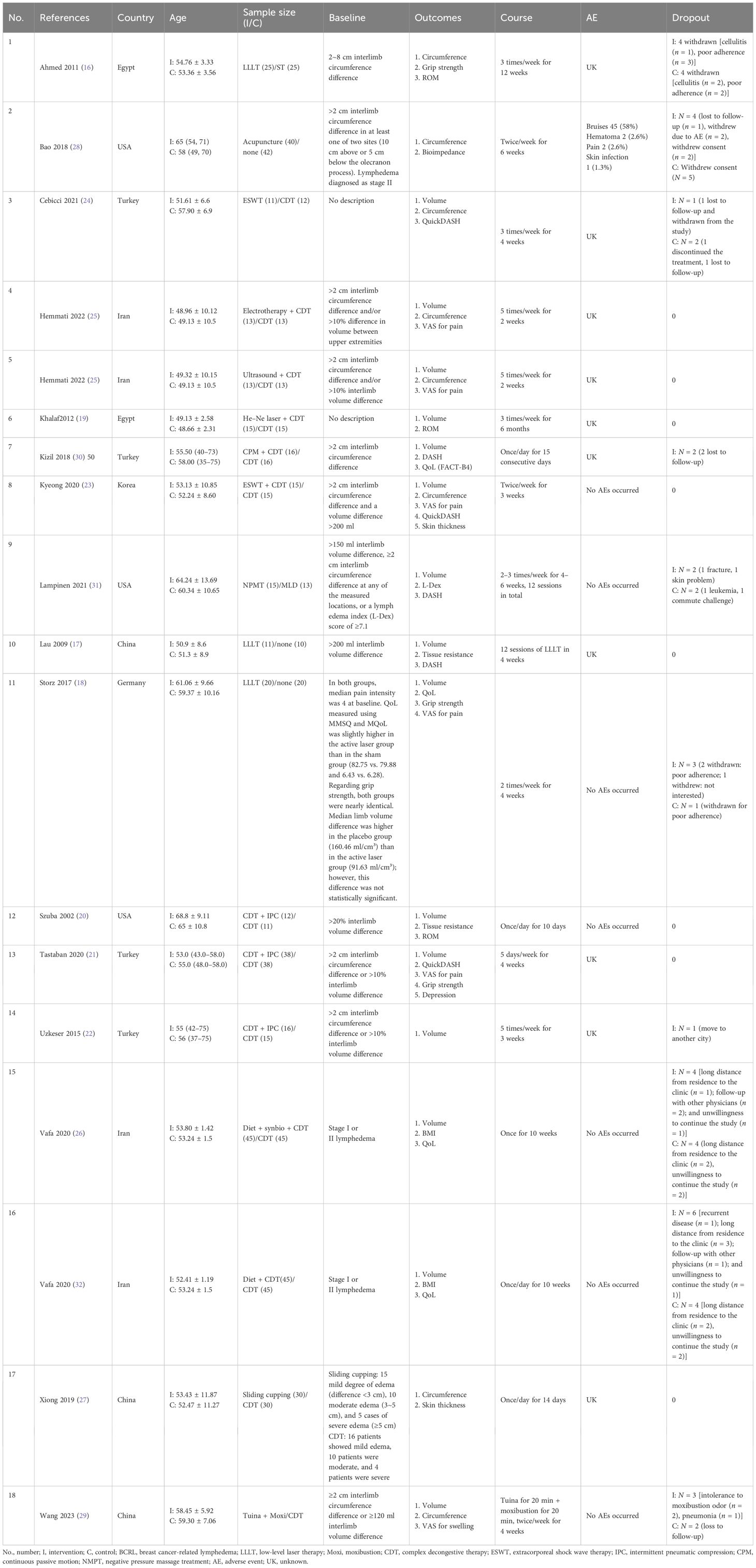
A total of 16 randomized controlled trials with 690 participants were ultimately included in this meta-analysis, of which 4 studies (16–19) adopted laser therapy; 3 studies (20–22) adopted IPC; 2 studies (23, 24) adopted ESWT; 1 study with three arms adopted electrotherapy (25) and ultrasound (25); 1 study with three arms adopted diet (26) or diet combined with a synbiotic supplement (26); 3 studies adopted the traditional Chinese medicine (TCM) method including sliding cupping (27), acupuncture (28), and Tuina in combination with moxibustion (29); 1 study adopted continuous passive motion (CPM) (30); and 1 study adopted negative pressure massage treatment (NMPT) (31).
Four RCTs (21, 22, 24, 30) were conducted in Turkey, three RCTs (20, 28, 31) were conducted in the USA, two RCTs (25, 26) were conducted in Iran, two RCTs (16, 19) were conducted in Egypt, three RCTs (17, 27, 29) were conducted in China, one RCT was conducted in Germany (18), and one RCT was conducted in Korea (23).
The results of the 16 RCTs involved changes in arm circumference (16, 23–25, 27–29) and/or volume (17–20, 22, 24–26, 29–31). The secondary outcomes in the included RCTs were VAS for pain (18, 21, 23, 25), quality of life (18, 26, 30), DASH (17, 30, 31) or QuickDASH (21, 23, 24), grip strength (16, 18, 21), bioimpedance (28, 31), BMI (26), range of motion (ROM) (16, 19, 20) of the shoulder, tissue resistance (17, 20), and skin thickness (23, 27). The summarized characteristics of the 16 RCTs are presented in Table 1.
3.3 Risk of bias in the studies
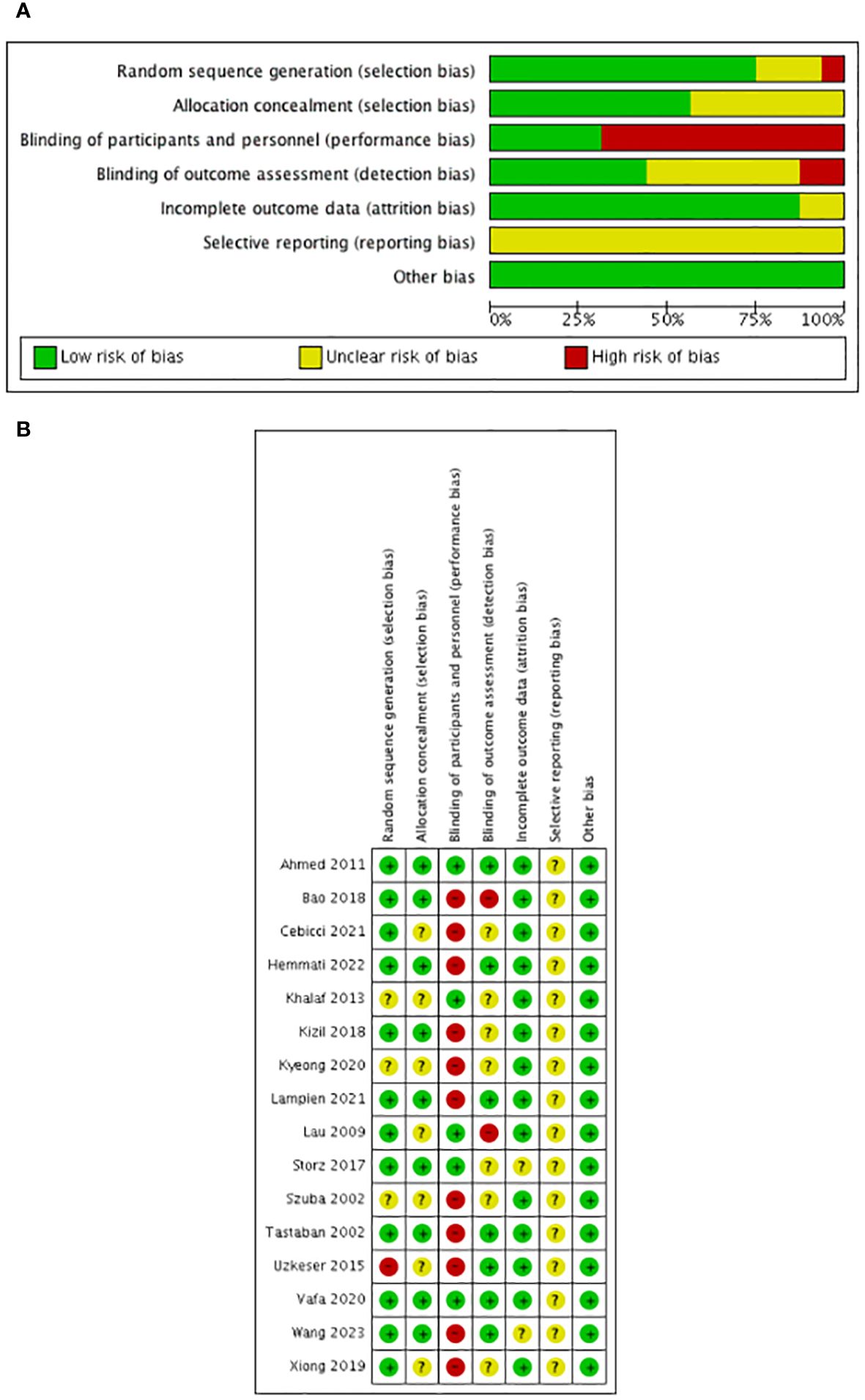
The risk of bias assessment is shown in Figure 2. All the included trials mentioned randomization: 12 studies described the randomization method in detail such as the random number table (27), block randomization (24, 26), block permutation method (25), random number table using block randomization (30), computer-generated program (16, 18, 21, 28, 29, 31), and the Bebbington method (17) and were defined as low risk of bias; 3 studies (19, 20, 23) had no concrete description of randomization and were defined as unclear risk of bias; and 1 study (22) randomized the patients according to admittance time and was defined as high risk of bias.
Furthermore, six studies (16, 18, 21, 28, 29, 31) used a computer-generated program in randomization to ensure the allocation concealment; two studies (25, 29) used sequentially numbered, sealed, and opaque envelopes; and one study (30) used an uninvolved researcher to assign patients. These studies were defined as low risk. The rest of the studies did not describe the allocation concealment and were defined as unclear risk.
As for performance blinding, blinding of the treating physiotherapist and patients in some interventions such as ESWT (24), IPC (20–22), CPM (30), NPMT (31), electrotherapy (25), ultrasound (25), acupuncture (28), sliding cupping (27), and Tuina in combination with moxibustion (29) was impossible considering the nature of the studies and according to the description of the authors; therefore, these were defined as high risk. Laser therapy (16–18, 31) (both active and placebo laser devices were similar in terms of weight, emitted sounds, and optical appearance to guarantee strictly controlled double-blinded conditions) and capsules (26) (the placebo capsule was comprised of lactose and was equal to the synbiotic capsule in terms of appearance, color, shape, size, smell, taste, and packaging) were performed in a blinded manner according to the description of the authors and were defined as low risk.
As for assessment blinding, six studies (16, 21, 22, 25, 29, 31) had a clear description of the blinding of outcome assessments and were defined as low risk. Two studies (17, 28) mentioned research staff not being blinded to the treatment group and were defined as high risk. Eight studies (18–20, 23, 24, 26, 27, 30) did not mention blinding in assessment and were defined as unclear risk.
Nine studies (16, 18, 22, 24, 26, 28–31) reported dropouts with clear reasons, and seven studies (17, 19–21, 23, 25, 27) reported no dropouts. We assessed the risk of attrition bias in all these studies as low risk.
As for selective reporting, although all the included studies reported all outcomes in the methods section, the protocol was not available and was defined as unclear risk.
Furthermore, we assessed the other risks as low considering that the whole design of most of the included studies was relatively formal.
3.4 Primary outcome: arm volume/circumference change
3.4.1 Volume/circumference change and subgroup analysis
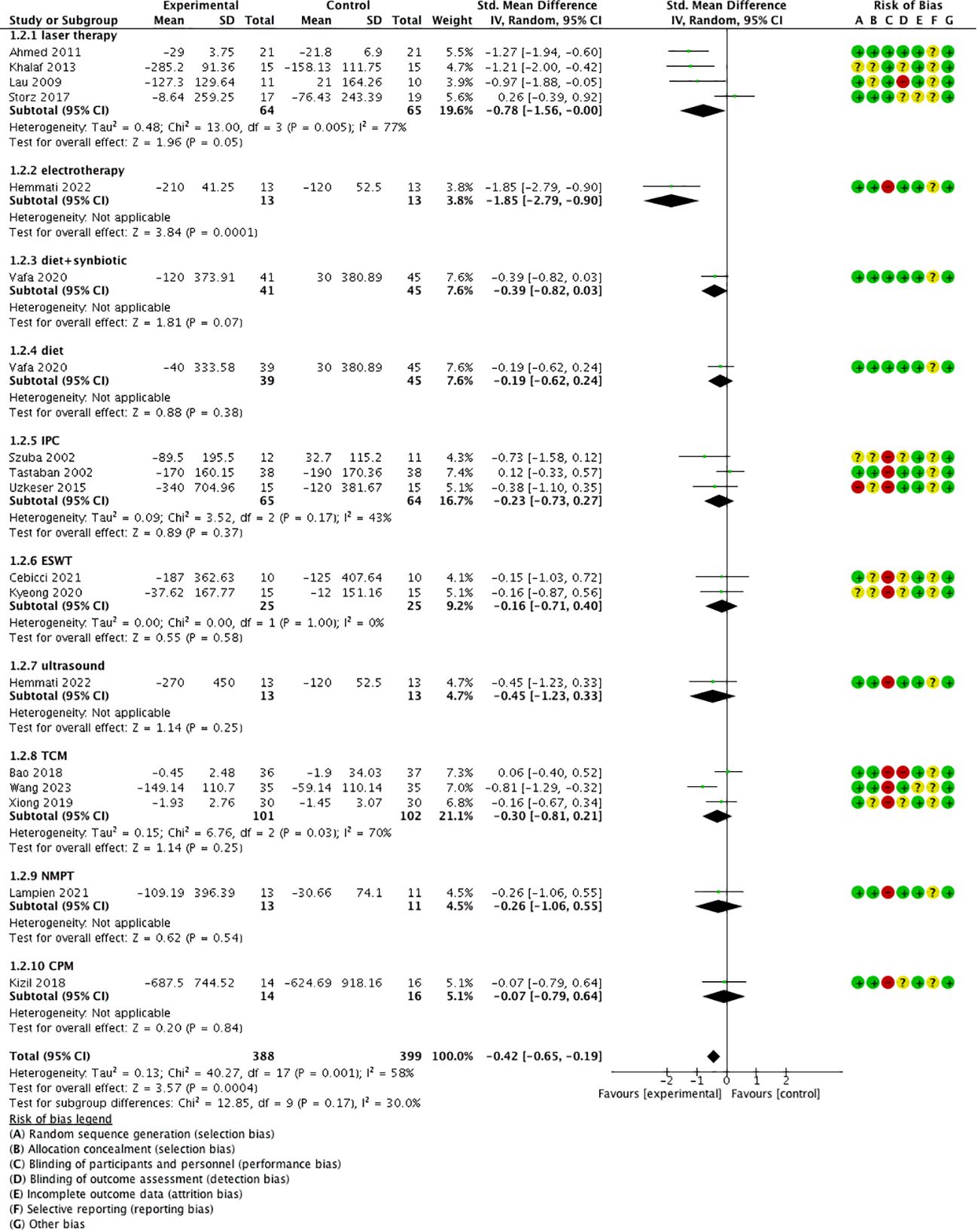
Of the 15 included studies, 4 studies (23–25, 29) reported both volume and circumference change of the limbs, 9 studies (17–22, 26, 30, 31) reported only volume change, and 3 studies (16, 27, 28) reported only circumference change. Considering the different units of volume (cm3) or circumference (cm) may result in great differences in data size; standardized mean difference (SMD) with 95% confidence intervals (CIs) was used.
The results revealed that conservative medical intervention as a complement to CDT had benefits in improving lymphedema in volume/circumference of the upper extremity compared with that of the control group [SMD = −0.40, 95% CI = (−0.62, −0.18), P < 0.05, I2 = 53%] (Figure 3).
3.4.2 Subgroup analysis
3.4.2.1 Age
According to age-grouped data, the age group <50 years old [SMD = −1.14, 95% CI = (−1.91, −0.37), P < 0.05, I2 = 61%] and between 50 and 60 years old [SMD = −0.34, 95% CI = (−0.56, −0.13), P < 0.05, I2 = 32%] showed a better effect; however, the age group >60 years old [SMD = −0.07, 95% CI = (−0.44, 0.29), P > 0.05, I2 = 20%] had a relatively poor effect. It can be found that most heterogeneities come from the age group <50 years old (Figure 4).
3.4.2.2 Different treatments
Subgroup analysis based on different treatments showed that laser therapy [SMD = −0.78, 95% CI = (−1.56, −0.00), P = 0.05, I2 = 77%] and electrotherapy [SMD = −1.85, P < 0.05, 95% CI = (−2.79, −0.90)] had better effect, and diet/diet + synbiotic, IPC, ESWT, ultrasound, TCM (sliding cupping, acupuncture, Tuina combined with moxibustion), NPMT, and CPM did not show a significantly better effect compared with the control group (P > 0.05) (Figure 5).
3.4.2.3 Dose-grouped and session-grouped laser therapy analysis
In a dose–subgroup analysis of laser therapy, we found that only low intensity (5 mW, 1.5/cm2) [SMD = −1.13, 95% CI = (−1.65, −0.62), P < 0.05, I2 = 0%] to moderate intensity (24 mW, 2 J/cm2) [SMD = −0.97, 95% CI = (−1.88, −0.05), P < 0.05] (Figure 6A) and moderate term (36 sessions) [SMD = −1.08, 95% CI = (−1.75, −0.40), P < 0.05] to long term (72 sessions) [SMD = −1.21, 95% CI = (−2.00, −0.42), P < 0.05] significantly improved lymphedema compared with those of the control group (Figure 6B).

Figure 6 Forest plot of laser therapy subgroup analysis. (A) Forest plot of the laser dose–subgroup analysis. (B) Forest plot of the laser session–subgroup analysis.
3.5 Secondary outcome 1: quality of life
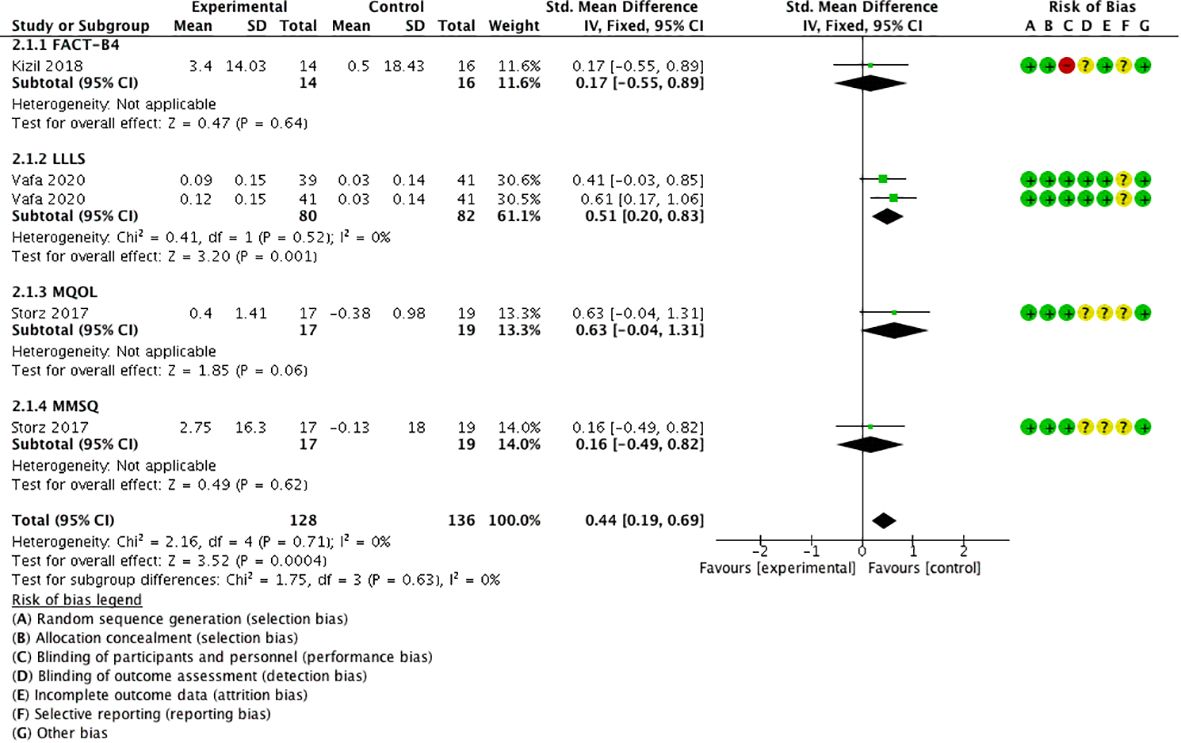
Three studies (18, 26, 30) took four comparisons using QoL as the outcome measure, using the scales of Functional Assessment of Cancer Therapy for Breast Cancer (FACT-B4) (30), Lymphedema Life Impact Scale (LLIS) (26), McGill Quality of Life Questionnaire (MQoL), and the German version of the Multidimensional Mood State Questionnaire (MMSQ) (18). The meta-analysis displayed that QoL was significantly higher in the experimental group than in the control group [SMD = 0.44, 95% CI (0.19, 0.69), I2 = 0], and a statistically significant difference was found (P < 0.05) (Figure 7).
3.6 Secondary outcome 2: DASH/QuickDASH
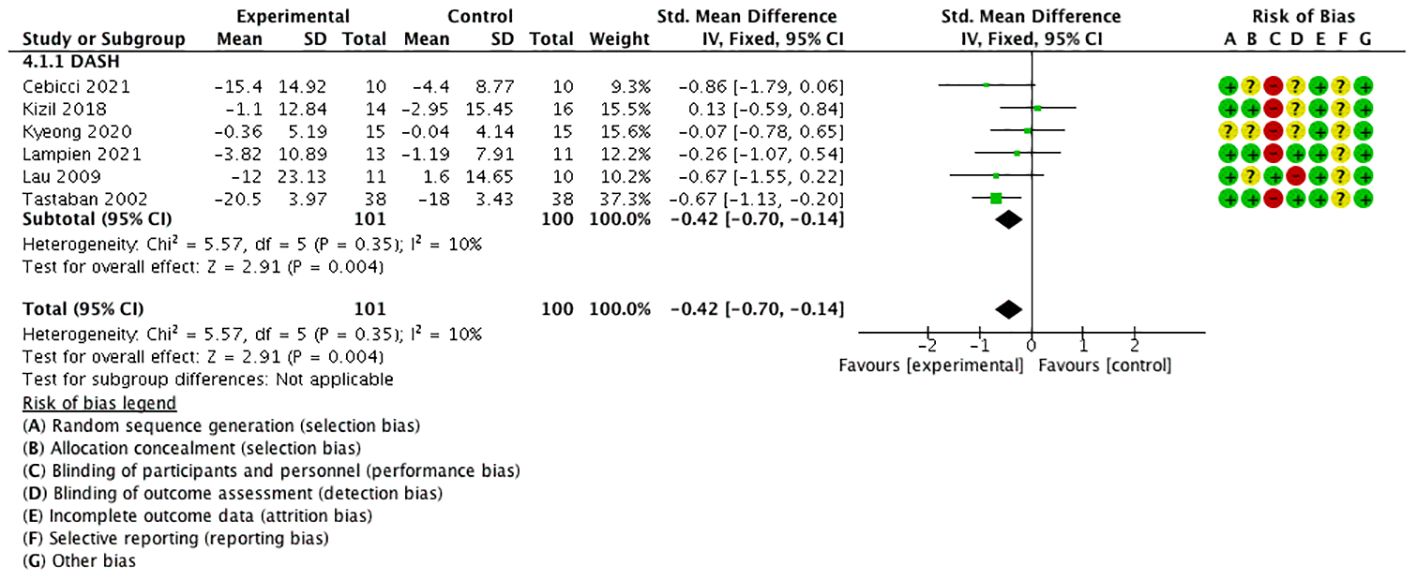
Six studies used the quick disabilities of the arm, shoulder, and hand (QuickDASH) (21, 23, 24) or DASH (17, 30, 31) questionnaire as the outcome (these are self-reported assessment tools that measure physical function and symptoms in individuals with a musculoskeletal disorder of the upper limb). The scores indicated the level of disability and severity, ranging from 0 (no disability) to 100 (most severe disability).
The meta-analysis manifested that QuickDASH/DASH was significantly lower in the experimental group than in the control group [SMD = −0.42, 95% CI (−0.70, −0.14), I2 = 10%], and a statistically significant difference was found (P < 0.05) (Figure 8).
3.7 Secondary outcome 3: VAS for pain
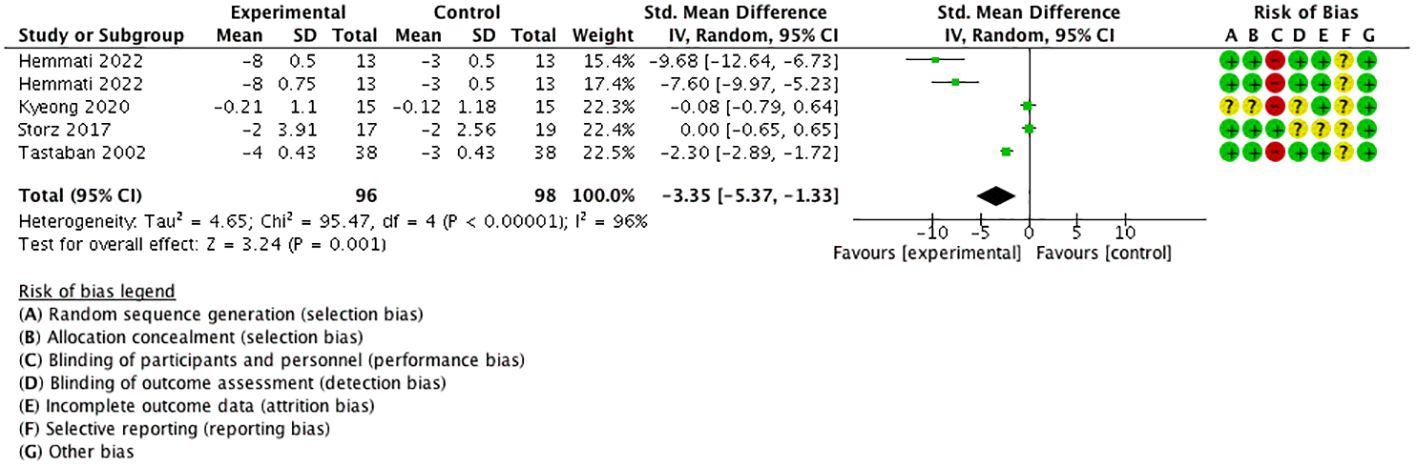
Four studies (18, 21, 23, 25) performed five comparisons using the visual analog scale (VAS) for pain as the outcome with a 0–10 numerical rating scale. The meta-analysis proved that VAS for pain was significantly decreased in the experimental group than in the control group [SMD = −3.35, 95% CI (−5.37, −1.33), I2 = 96%], and a statistically significant difference was found (P < 0.05) (Figure 9).
3.8 Adverse events
One study reported the AEs in detail (during acupuncture, bruises 45 cases, hematoma 2 cases, pain 2 cases, skin infection 1 case) and 7 studies reported no AEs (ESWT, IPC, LLLT, NPMT, diet, synbiotic). However, 10 studies did not mention AEs.
4 Discussion
4.1 Main findings
Our results showed that the addition of certain conservative medical treatments to CDT can exert a better effect in reducing the volume/circumference of the swollen limbs of BCRL patients, as well as relieve pain and disability and also improve the quality of life of the patients. Furthermore, we found among the various treatments that laser therapy and electrotherapy had the best effect in relieving swollen limbs, and we recommend laser therapy of 5–24 mW, 1.5–2 J/cm2 intensity, and 36–72 sessions for BCRL patients to achieve the best effect based on the subgroup analysis findings. Moreover, patients <60 years old may show a better effect than elderly patients, indicating the importance for younger patients to receive relative treatments as it will be more efficient and economical.
4.2 Strengths and limitations
Our review has several strengths. First, most of the included articles were of moderate to high quality with relatively standardized design, which improved the confidence of the results. Second, the entire search strategy and data analysis process were relatively formal. Third, we included 16 eligible RCTs with 655 participants investigating the additional beneficial effect of conservative medical treatments on swelling, pain, disability, and quality of life. Fourth, this study was conducted using in-depth subgroup meta-analyses to evaluate potential sources of heterogeneity.
However, this study has some limitations. First, some TCM treatments (including acupuncture/sliding cupping) did not show a significant overall effect in our study although some beneficial effects were observed (33), and this may have resulted from the lack of search of Chinese databases such as CNKI, VIP, and SinoMed, which may contain more research about TCM, and the selection and combination of acupoints also play an important role in the effectiveness of treatments. Second, this study was based on study-level data but not on patient-level data, and the analysis was based on small sample sizes, so the results should be interpreted carefully. Third, our study did not include gray literature such as some studies from the National Institutes of Health (NIH) and the World Health Organization (WHO). Fourth, this review just included RCT studies and excluded non-RCTs such as prospective pilot studies, and this might cause an underestimated effect of some treatments. Fifth, the exclusion criteria in the included studies did not mention the history of surgery to treat lymphedema, which may cause some efficacy differences.
4.3 Relation to previous studies
Several meta-analyses about BCRL treatment came to the following conclusions: one study concluded that conservative treatment interventions may not meaningfully improve lymphedema volume compared with standard care (34), and this study did not take CDT as the control group, which is the first-line treatment. Another study concluded that acupuncture and moxibustion (33) are effective in treating BCRL; however, different control methods were used; therefore, it is difficult to interpret the conclusions of the study. A third study concludes that ESWT combined with CDT could have significant effects (35); however, some non-RCT studies were included, which might affect the results. The present study, on the other hand, excluded non-RCT studies as the focus was on medical practice; furthermore, various kinds of conservative treatments were included to obtain broader knowledge, and CDT was used as a first-line treatment to improve its practical application value.
4.4 Future perspectives
Our findings indicate that it is meaningful to discover and promote conservative medical treatments in clinical practice to better help BCRL patients relieve their symptoms such as swollen limbs, pain, and disability and improve their life quality. However, there is still a lack of consensus on which patients will benefit from each treatment option, and there are no guidelines on the appropriate time to start treatment, which can lead to treatment issues (36).
Low-level laser therapy (LLLT), also called photobiomodulation (PBM), may reduce inflammation, prevent fibrosis, and stimulate lymphangiogenesis (32). Electrical stimulation reduces edema by increasing muscle contraction and can increase lymph and blood flow up to 30-fold; moreover, muscle contraction favors the removal of intercellular proteins (37). However, there are relatively fewer studies about electrotherapy, and future studies can explore more about the efficacy and safety as well as the intervention time of applying electrotherapy.
The selection and combination of conservative medical treatments may play an important role in the effectiveness of treatments. At present, CDT is widely known as the most important treatment for BCRL. Previous meta-analyses (35) concluded that ESWT combined with CDT could significantly improve the volume of lymphedema in BCRL patients but not enough to replace CDT. Our review also supported the idea that a combination of conservative treatments with CDT could provide significant clinical benefits to BCRL patients but cannot replace CDT in treating BCRL.
Moreover, different measurements of the affected limbs by circumference or volume calculated by circumference or water displacement made it difficult to aggregate analyses. In future trials, it may be a better option to report the volume changes by water displacement to uniform the measurement, and water displacement is more direct than calculation by circumference. Conservative rehabilitation interventions need to be continued to develop studies to help guide therapeutic decisions that can promote health-related quality of life in BCRL women (38).
As for safety assessment, no adverse events occur when applying ESWT, IPC, LLLT, NPMT, diet, or symbiotic treatment, and most AEs come from acupuncture, indicating that practitioners should pay more attention in performing acupuncture and poor performance may also reduce the effect of acupuncture.
5 Conclusion
In conclusion, our research supports the efficacy of combining conservative medical intervention with CDT over utilizing CDT alone in improving the health status of BCRL patients (reducing lymphedema volume, pain, and functional disability and improving quality of life), particularly with the inclusion of laser therapy and electrotherapy. This combination showed better effects on patients under 60 years old, and laser therapy of low to moderate intensity (5–24 mW, 1.5–2 J/cm2) and of moderate- to long-term duration (≥36–72 sessions) showed better effects.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
CD: Conceptualization, Data curation, Formal analysis, Software, Supervision, Writing – original draft, Writing – review & editing. ZW: Writing – original draft, Data curation, Formal analysis, Software. ZC: Writing – review & editing, Software. XZ: Writing – original draft, Funding acquisition, Supervision, Writing – review & editing. CT: Writing – review & editing, Conceptualization, Funding acquisition, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (82174479,82205245) and the Natural Science Foundation for Guangdong, China (2023A1515011143).
Acknowledgments
The authors would like to thank XZ and Wen Hao for their excellent technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1361128/full#supplementary-material
References
1. Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: A 25-year study. Asian Pac J Cancer Prev. (2019) 20:2015–20. doi: 10.31557/APJCP.2019.20.7.2015
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
3. Deutsch M, Land S, Begovic M, Sharif S. The incidence of arm edema in women with breast cancer randomized on the National Surgical Adjuvant Breast and Bowel Project study B-04 to radical mastectomy versus total mastectomy and radiotherapy versus total mastectomy alone. Int J Radiat Oncol Biol Phys. (2008) 70:1020–4. doi: 10.1016/j.ijrobp.2007.07.2376
4. Omidi Z, Kheirkhah M, Abolghasemi J, Haghighat S. Effect of lymphedema self-management group-based education compared with social network-based education on quality of life and fear of cancer recurrence in women with breast cancer: a randomized controlled clinical trial. Qual Life Res. (2020) 29:1789–800. doi: 10.1007/s11136-020-02455-z
5. Fu MR, Ridner SH, Hu SH, Stewart BR, Cormier JN, Armer JM. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology. (2013) 22:1466–84. doi: 10.1002/pon.3201
6. Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. J Clin Oncol. (2012) 30:3726–33. doi: 10.1200/JCO.2012.41.8574
7. Chen CE, Chiang NJ, Perng CK, Ma H, Lin CH. Review of preclinical and clinical studies of using cell-based therapy for secondary lymphedema. J Surg Oncol. (2020) 121:109–20. doi: 10.1002/jso.25661
8. Armer JM, Hulett JM, Bernas M, Ostby P, Stewart BR, Cormier JN. Best practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema. Curr Breast Cancer Rep. (2013) 5:134–44. doi: 10.1007/s12609-013-0105-0
9. Tiwari P, Coriddi M, Salani R, Povoski SP. Breast and gynecologic cancer-related extremity lymphedema: a review of diagnostic modalities and management options. World J Surg Oncol. (2013) 11:237. doi: 10.1186/1477-7819-11-237
10. Shimbo K, Kawamoto H, Koshima I. Comparative study of conservative treatment and lymphaticovenular anastomosis with compression therapy for early-stage breast cancer-related lymphoedema. J Plast Reconstr Aesthet Surg. (2024) 88:390–6. doi: 10.1016/j.bjps.2023.11.035
11. Keskin D, Dalyan M, Ünsal-Delialioğlu S, Düzlü-Öztürk Ü. The results of the intensive phase of complete decongestive therapy and the determination of predictive factors for response to treatment in patients with breast cancer related-lymphedema. Cancer Rep (Hoboken). (2020) 3:e1225. doi: 10.1002/cnr2.1225
12. Michopoulos E, Papathanasiou G, Krousaniotaki K, Vathiotis I, Troupis T, Dimakakos E. Lymphedema duration as a predictive factor of efficacy of complete decongestive therapy. Lymphology. (2021) 54:140–53. doi: 10.2458/lymph.4788
13. Ezzo J, Manheimer E, McNeely ML, Howell DM, Weiss R, Johansson KI, et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst Rev. (2015) 5):Cd003475. doi: 10.1002/14651858
14. Ciudad P, Bolletta A, Kaciulyte J, Losco L, Manrique OJ, Cigna E, et al. The breast cancer-related lymphedema multidisciplinary approach: Algorithm for conservative and multimodal surgical treatment. Microsurgery. (2023) 43:427–36. doi: 10.1002/micr.30990
15. Leal NF, Carrara HH, Vieira KF, Ferreira CH. Physiotherapy treatments for breast cancer-related lymphedema: a literature review. Rev Lat Am Enfermagem. (2009) 17:730–6. doi: 10.1590/S0104-11692009000500021
16. Ahmed Omar MT, Abd-El-Gayed Ebid A, El Morsy AM. Treatment of post-mastectomy lymphedema with laser therapy: double blind placebo control randomized study. J Surg Res. (2011) 165:82–90. doi: 10.1016/j.jss.2010.03.050
17. Lau RW, Cheing GL. Managing postmastectomy lymphedema with low-level laser therapy. Photomed Laser Surg. (2009) 27:763–9. doi: 10.1089/pho.2008.2330
18. Storz MA, Gronwald B, Gottschling S, Schöpe J, Mavrova R, Baum S. Photobiomodulation therapy in breast cancer-related lymphedema: a randomized placebo-controlled trial. Photodermatol Photoimmunol Photomed. (2017) 33:32–40. doi: 10.1111/phpp.12284
19. Khalaf MM, Hassan MA, Ibrahim ZM. Helium Neon laser therapy for post mastectomy lymphedema and shoulder mobility. Egyptian J Med Hum Genet. (2013) 14:195–9. doi: 10.1016/j.ejmhg.2012.11.003
20. Szuba A, Achalu R, Rockson SG. Decongestive lymphatic therapy for patients with breast carcinoma-associated lymphedema. A randomized, prospective study of a role for adjunctive intermittent pneumatic compression. Cancer. (2002) 95:2260–7. doi: 10.1002/cncr.10976
21. Tastaban E, Soyder A, Aydin E, Sendur OF, Turan Y, Ture M, et al. Role of intermittent pneumatic compression in the treatment of breast cancer-related lymphoedema: a randomized controlled trial. Clin REHABILITATION. (2020) 34:220–8. doi: 10.1177/0269215519888792
22. Uzkeser H, Karatay S. Intermittent pneumatic compression pump in upper extremity impairments of breast cancer-related lymphedema. Turkish J Med Sci. (2013) 43:99–103. doi: 10.3906/sag-1203-78
23. Lee KW. Effects of extracorporeal shockwave therapy on improvements in lymphedema, quality of life, and fibrous tissue in breast cancer-related lymphedema. Ann Rehabil Med. (2020) 44:386–92. doi: 10.5535/arm.19213
24. Aykac Cebicci M, Dizdar M. A comparison of the effectiveness of complex decongestive therapy and extracorporeal shock wave therapy in the treatment of lymphedema secondary to breast cancer. Indian J surgery. (2021) 83:749–53. doi: 10.1007/s12262-021-02769-3
25. Hemmati M, Rojhani-Shirazi Z, Zakeri ZS, Akrami M, Salehi Dehno N. The effect of the combined use of complex decongestive therapy with electrotherapy modalities for the treatment of breast cancer-related lymphedema: a randomized clinical trial. BMC Musculoskelet Disord. (2022) 23:837. doi: 10.1186/s12891-022-05780-1
26. Vafa S, Zarrati M, Malakootinejad M, Totmaj AS, Zayeri F, Salehi M, et al. Calorie restriction and synbiotics effect on quality of life and edema reduction in breast cancer-related lymphedema, a clinical trial. Breast. (2020) 54:37–45. doi: 10.1016/j.breast.2020.08.008
27. Xiong ZF, Wang T, Wang HL, Wang YY, Gan L, Lu G. Sliding-cupping along meridian for lymphedema after breast cancer surgery: a randomized controlled trial. World J acupuncture - moxibustion. (2019) 29:179–85. doi: 10.1016/j.wjam.2019.08.005
28. Bao T, Iris Zhi W, Vertosick EA, Li QS, DeRito J, Vickers A, et al. Acupuncture for breast cancer-related lymphedema: a randomized controlled trial. Breast Cancer Res Treat. (2018) 170:77–87. doi: 10.1007/s10549-018-4743-9
29. Wang C, Liu H, Shen J, Hao Y, Zhao L, Fan Y, et al. Effects of tuina combined with moxibustion on breast cancer-related lymphedema: A randomized cross-over controlled trial. Integr Cancer Ther. (2023) 22:15347354231172735. doi: 10.1177/15347354231172735
30. Kizil R, Dilek B, Şahin E, Engin O, Soylu AC, Akalin E, et al. Is continuous passive motion effective in patients with lymphedema? A randomized controlled trial. Lymphat Res Biol. (2018) 16:263–9. doi: 10.1089/lrb.2017.0018
31. Lampinen R, Lee JQ, Leano J, Miaskowski C, Mastick J, Brinker L, et al. Treatment of breast cancer-related lymphedema using negative pressure massage: A pilot randomized controlled trial. Arch Phys Med Rehabil. (2021) 102:1465–72.e2. doi: 10.1016/j.apmr.2021.03.022
32. Bensadoun RJ. Photobiomodulation or low-level laser therapy in the management of cancer therapy-induced mucositis, dermatitis and lymphedema. Curr Opin Oncol. (2018) 30:226–32. doi: 10.1097/CCO.0000000000000452
33. Gao Y, Ma T, Han M, Yu M, Wang X, Lv Y, et al. Effects of acupuncture and moxibustion on breast cancer-related lymphedema: A systematic review and meta-analysis of randomized controlled trials. Integr Cancer Ther. (2021) 20:15347354211044107. doi: 10.1177/15347354211044107
34. Lytvyn L, Zeraatkar D, Anbari AB, Ginex PK, Zoratti M, Niburski K, et al. Conservative intervention strategies for adult cancer-related lymphedema: A systematic review and network meta-analysis. Oncol Nurs Forum. (2020) 47:E171–e89. doi: 10.1188/20.ONF.E171-E189
35. Tsai YL, TJ I, Chuang YC, Cheng YY, Lee YC. Extracorporeal shock wave therapy combined with complex decongestive therapy in patients with breast cancer-related lymphedema: A systemic review and meta-analysis. J Clin Med. (2021) 10:14. doi: 10.3390/jcm10245970
36. Anuszkiewicz K, Jankau J, Kur M. What do we know about treating breast-cancer-related lymphedema? Review of the current knowledge about therapeutic options. Breast Cancer. (2023) 30:187–99. doi: 10.1007/s12282-022-01428-z
37. Cornwall MW. Electrotherapy explained: principles and practice, ed 4. Phys Ther. (2007) 8):1088. doi: 10.2522/ptj.2007.87.8.1088
38. Muñoz-Alcaraz MN, Jiménez-Vílchez AJ, Pérula-de Torres L, Serrano-Merino J, García-Bustillo Á, Pardo-Hernández R, et al. Effect of conservative rehabilitation interventions on health-related quality of life in women with upper limb lymphedema secondary to breast cancer: A systematic review. Healthcare (Basel). (2023) 11:15. doi: 10.3390/healthcare11182568
Keywords: breast cancer-related lymphedema, BCRL, meta-analysis, systematic review, adjunctive therapy
Citation: Deng C, Wu Z, Cai Z, Zheng X and Tang C (2024) Conservative medical intervention as a complement to CDT for BCRL therapy: a systematic review and meta-analysis of randomized controlled trials. Front. Oncol. 14:1361128. doi: 10.3389/fonc.2024.1361128
Received: 25 December 2023; Accepted: 10 April 2024;
Published: 26 April 2024.
Edited by:
Daniel P. Bezerra, Oswaldo Cruz Foudantion (FIOCRUZ), BrazilReviewed by:
Luigi Losco, University of Salerno, ItalyPaola Ciamarra, University of Campania Luigi Vanvitelli, Italy
Copyright © 2024 Deng, Wu, Cai, Zheng and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunzhi Tang, am9yZGFuNjY0QDE2My5jb20=; Xiaoyan Zheng, emhlbmd4aWFveWFuMTgxQDEyNi5jb20=
 Chuyu Deng
Chuyu Deng Zhiguo Wu
Zhiguo Wu Zijie Cai1
Zijie Cai1 Xiaoyan Zheng
Xiaoyan Zheng Chunzhi Tang
Chunzhi Tang