
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 13 March 2024
Sec. Breast Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1356001
This article is part of the Research Topic The Impact of Specific Environmental Exposures on Breast, Lung, and Colon Cancer: Advancing Public Health Strategies for Enhanced Outcomes View all 16 articles
 Dylan J. Jester1*
Dylan J. Jester1* Mehret T. Assefa1
Mehret T. Assefa1 Daya K. Grewal1,2†
Daya K. Grewal1,2† Abou M. Ibrahim-Biangoro1,3†
Abou M. Ibrahim-Biangoro1,3† Jennifer S. Jennings1,4
Jennifer S. Jennings1,4 Maheen M. Adamson1,5
Maheen M. Adamson1,5Background: The effects of military environmental exposures (MEE) such as volatile organic compounds (VOCs), endocrine-disrupting chemicals (EDCs), tactile herbicides, airborne hazards and open burn pits (AHOBP), and depleted uranium on health are salient concerns for service members and Veterans. However, little work has been done to investigate the relationship between MEE and risk of breast cancer.
Data sources and methods: We conducted a scoping review on MEE, military deployment/service, and risk of breast cancer among active-duty service members and Veterans. PRISMA was used. PubMed, Embase, and citations of included articles were searched, resulting in 4,364 articles to screen: 28 articles were included.
Results: Most papers on military deployment and military service found a lower/equivalent risk of breast cancer when comparing rates to those without deployment or civilians. Exposure to VOCs due to military occupation or contaminated groundwater was associated with a slightly higher risk of breast cancer. Exposure to Agent Orange was not associated with an increased risk of breast cancer. Evidence regarding EDCs was limited. No paper directly measured exposure to AHOBP or depleted uranium, but deployments with known exposures to AHOBP or depleted uranium were associated with an equivalent/lower risk of breast cancer.
Conclusions: Women are the fastest growing population within the military, and breast cancer poses a unique risk to women Veterans who were affected by MEE during their service. Unfortunately, the literature on MEE and breast cancer is mixed and limited, in part due to the Healthy Soldier Paradox and poor classification of exposure(s).
The number of women Veterans served by the Department of Veterans Affairs - Veterans Health Administration (VA) more than quintupled between 2000 and 2021 (159,810 to 870,000+) (1, 2), while the number of men grew substantially slower over the same period (2, 3). In 2020, women comprised 19% of all military branches (2, 4), which highlights an ongoing need for the expansion of women-specific health services. The 2023 Office of Women’s Health - State of Reproductive Health governmental report found that abnormal breast conditions were reported as one of the top five reproductive and sexual health concerns for women Veterans aged 45+ (5). As VA projects the resources needed to care for the expanding women Veteran population, clinical and educational efforts must consider the unique health concerns faced by women Veterans.
Breast cancer (BC) is the most prevalent cancer among women, with around 300,000 cases diagnosed in the United States (U.S.) annually (6). One out of every eight women will be diagnosed at least once in their lifetime (6). The incidence rate (IR) of BC peaks in the 60s and 70s for women and the mortality rate increases exponentially with age (7), with Black women having the highest risk of mortality out of all racial and ethnic groups in the U.S. While the IR of BC has increased over the past two decades, the mortality rate has lowered substantially following advancements in early detection and treatment (7). Conversely, less than 1% of all BC patients are men (8) but BC in men is deadlier than in women (8). Military men with BC tend to present at a higher stage and with a larger tumor size than military women with BC, though demographics or tumor characteristics do not fully explain the higher rate of mortality in men with BC (9). BC is of great concern to VA and is a presumptive condition under The Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Presumptive conditions allow Veterans to receive care for ongoing health concerns that are of unknown etiology, and can be presumed to be related to service (10). Cancer of any kind remains an ongoing concern for Veterans as they age, and especially among Veterans with military environmental exposures (MEE).
The rates of cancers differ among active-duty personnel and the general U.S. population (11). Over 800 active-duty personnel receive a cancer diagnosis yearly, and tumor etiology is often correlated with service characteristics and MEE (12). These exposures include, but are not limited to, airborne hazards and open burn pits (AHOBP), asbestos, biological and chemical warfare tests, contaminated water, chemical agent resistant coating paint, embedded substances such as depleted uranium and lead, fuels, industrial solvents, ionizing radiation, mefloquine for malaria, nerve agents, noise, pesticides, perfluoroalkyl and polyfluoroalkyl substances, pyridostigmine bromide pills for sarin gas exposure, tactile herbicides, and vaccines. Cancer among current and former military personnel with known MEE persists as a complex health concern (12–15).
The current literature on MEE and cancer is limited. For example, the tactile herbicide Agent Orange was linked to an increased incidence of several cancers, including leukemia and cancers that start in soft tissues (16), and a slightly higher rate of BC was found among military personnel when compared to civilians (17). However, higher rates of BC may be tied to confounding risk factors in military personnel, such as delayed age of first childbirth or increased use of contraceptives. Additionally, military personnel often have greater access to routine screening, resulting in quicker identification of early-stage BCs (18, 19). In other words, tying BC incidence to MEE rather than characteristics associated with service (i.e., confounding factors) is a difficult task.
Combat exposure has increased from 7% to 24% when comparing pre-1990 to post-1990 women Veterans, suggesting that MEE concerns may grow among women Veterans in the coming decades (20). However, few have investigated BC in association with specific MEE. Therefore, we conducted a scoping review to determine whether deployment/military service and MEE affect the risk of BC among active-duty personnel and Veterans.
Unlike systematic reviews that focus on a specific research question, scoping reviews ask broad research questions to characterize and understand a developing and heterogenous area within the literature (21). Search terms were compiled using PubMed’s Medical Subject Headings (MeSH) trees and through consultation with the California War Related Illness and Injury Study Center (CA WRIISC), the Women’s Operational Military Exposure Network Center of Excellence (WOMEN CoE) and Advisory Board, and staff oncologists at VA Palo Alto Health Care System. Relevant articles were searched for in PubMed and Embase and terms can be found in the notes of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Figure 1). Some articles broadly examined cancer incidence and did not mention BC in the title or abstract, but included estimates of BC within the results/tables. Therefore, four authors (AIB, DJJ, DKG, MTA) screened citations from the included articles to find these additional manuscripts.
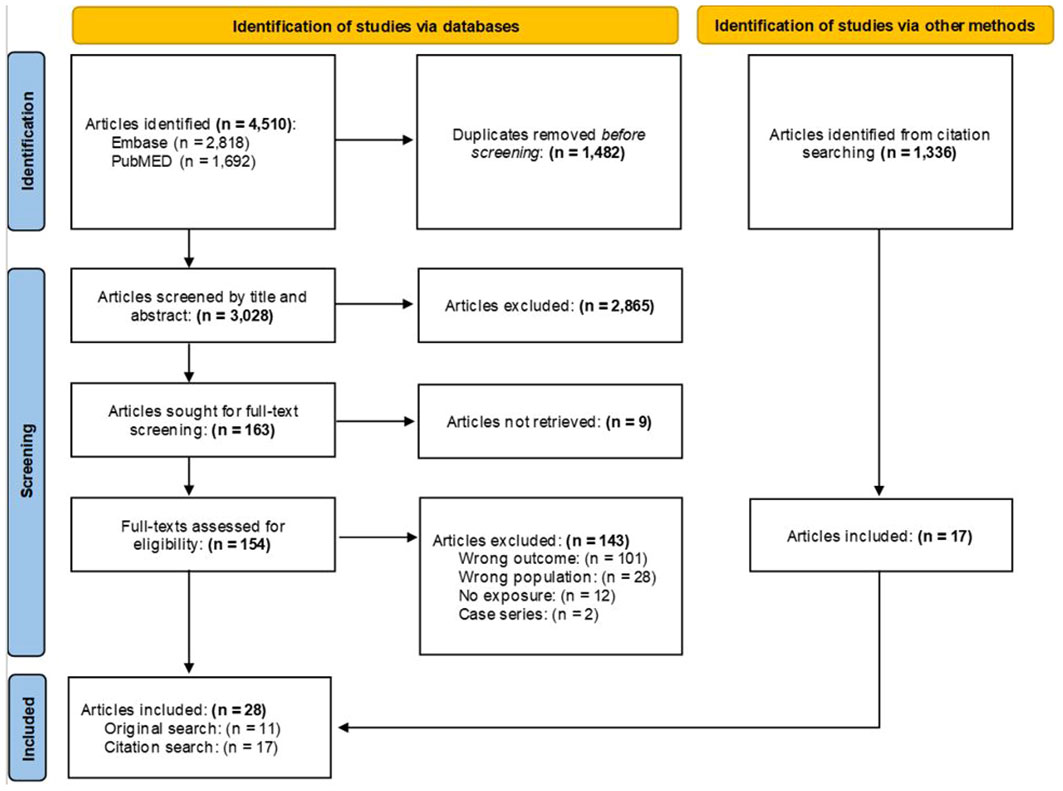
Figure 1 PRISMA flow chart. MeSH Trees used: Diseases Category ==> Neoplasms ==> Neoplasms by Site ==> Breast Neoplasms. Titles and abstracts were searched using the terms: (“breast cancer*” OR “breast neoplasm*” OR “breast tumor*” OR “breast metas*” OR “mammary metas*” OR “mammary cancer*” OR “malignant neoplasm* of the breast*” OR “malignant neoplasm* of breast*” OR “breast malignant neoplasm*” OR “malignant tumor* of breast*” OR “malignant tumor* of the breast*” OR “breast malignant tumor*” OR “cancer of breast*” OR “cancer of the breast*” OR “mammary carcinoma*” OR “mammary neoplasm*” OR “breast carcinoma*” OR “mastect*” OR “lumpect*” OR “mammogr*”) AND (Veteran* OR military OR combat OR deploy* OR undeploy* OR soldier* OR war OR wars OR warzone OR “department of defense” OR DOD OR front-line* OR duty OR enlist*). Asterisk wildcards were used to find word endings. Terms were left purposefully broad to examine the largest possible selection of the literature. Prospective or retrospective cohort, case-control, cross-sectional, ecological, or related study designs (e.g., case-cohort) were included. Case studies, case series, reviews and meta-analyses, book chapters, theses, and dissertations were excluded.
To be included, studies had to: (1) enroll active-duty personnel, Reservists, or Veterans, (2) measure MEE or military service/deployment, (3) concern BC risk (i.e., papers on BC mortality were excluded), and (4) have an English full-text.
Covidence software was used to collate and screen the articles. Four authors (AIB, DJJ, DKG, MTA) screened titles/abstracts and full-texts and met weekly to resolve disagreements through discussion. The database search was conducted on June 16, 2023, and resulted in a total of 4,510 articles. After the removal of 1,482 duplicates, 3,028 titles and abstracts were screened and 2,865 were excluded. A total of 163 full-texts were assessed, of which 11 were included. After screening an additional 1,336 citations from the included articles, 17 were retained for a final total of 28 articles.
Data extraction was completed by four authors (AIB, DJJ, DKG, MTA) with each paper receiving at least two checks for accuracy and included the following headings: author/publication year, sample characteristics, sample size, exposure, results, warfare era/service years, and diagnosis years. See Table 1 for the characteristics of each study.
In total, 28 papers were synthesized. Sample size ranged from 64 to millions. Several military conflicts were included: Malayan Emergency, Vietnam War, Israel-Lebanon Conflicts, Persian Gulf War, Kosovo War, Bosnian War, Croatian War of Independence, and post-9/11 conflicts (Operation Enduring Freedom [OEF], Operation Iraqi Freedom [OIF], Operation New Dawn [OND]). More than half of the studies used a case-control or cohort study design. Several MEE were examined: military service/deployment, volatile organic compounds (VOCs), endocrine-disrupting chemicals (EDCs), Agent Orange, and ultraviolet B radiation (Vitamin D synthesis).
Twenty-three papers measured BC among military personnel and those deployed to specific conflicts (17, 22, 23, 25–33, 37–42, 44–48).
Nine papers compared risk of BC in military personnel compared to civilians or standardized national rates. Zhu and colleagues (2009) conducted a cohort study and compared military and civilian cancer surveillance data. They found a slightly higher IRR for Black military women 1.37 [1.21, 1.55] and for White military women 1.19 [1.09, 1.30] when compared to civilians (17). Katuwal and colleagues (2018) carried out a cohort study of nearly 7.5 million Nordic women from 1961-2005 and found roughly 375,000 cases of BC. Military personnel had the greatest SIR for BC at 1.58 [1.03, 2.32] (29). Storm and colleagues (2006) followed 460 women military personnel who deployed to the Balkans and found no significantly increased risk (Standardized Incidence Ratio [SIR]=1.5 [0.3, 4.3]) (37). Yamane and colleagues (2006) compared BC IRs among 76,477 U.S. Air Force active-duty personnel to national IRs, and found the rates to be statistically equivalent (SIR=0.88 [0.76, 1.01]) (41). Yi (42) included 185,265 male Vietnam Veterans from Korea (n=8 cases), but found a statistically equivalent rate of BC when compared to the general population (SIR=1.37 [0.67, 2.83]) (42). Strand and colleagues (2015) conducted a cohort study of 21,582 Norwegian male military peacekeepers deployed to Lebanon and zero cases of BC were observed (SIR=0.00 [0.00, 2.07]) (39). Mahar and colleagues (2022) published a cohort study of 30,576 Canadian Veterans and police and 122,293 matched controls. Women Veterans had no statistically significant increased risk of BC (adjusted HR=1.19 [0.70, 2.02]) (33). Bytnar and colleagues (23) reconducted the analysis from Zhu et al. (17) using military (n=1,185 cases) and civilian (n=183,042 cases) cancer surveillance data. Black (IRR=1.06 [0.96, 1.16]) and White (IRR=1.06 [0.98, 1.13]) women military personnel had no significant increased risk of BC when compared to the general population (23). Lee and colleagues (2023) compared 250,842 Vietnam-era Korean Veterans (353 women) with 1,050,489 matched Korean civilians (1,695 women) and observed 123 cases of BC (IR=5.1 [4.2, 6.0]), but found no significantly increased rate (SIR=1.05 [0.88, 1.26]) (30).
Six papers provided estimates that differed by demographic or work characteristics. Hansen and Lassen (26) studied 218 women with BC and 899 age-matched controls from a nested cohort study of 18,551 women Danish military employees. Women with the highest tertile of cumulative night shift military work exposure had an increased odds of BC (OR=2.3 [1.2, 4.6]) and women with a morning chronotype preference (inclination to be more active during the morning) and intense night shifts had the largest risk (OR=3.9 [1.6, 9.5]) (26). The Armed Forces Health Surveillance Center (2013) published a cohort study and found that the IR of BC among active-duty service women was 40.6 per 100,000 from 2000-2012 (48). Non-Hispanic Black women, older women, senior officers, and women serving in healthcare or administrative roles had an increased risk of BC (48), and women with combat-specific duties had a mildly increased risk. Conversely, women who served in the U.S. Marine Corps, those who identified as Hispanic, younger women, junior enlistees, and women with “other” duties had a decreased risk of BC (48). Lee and colleagues (2016) examined all U.S. active-duty personnel from 2005-2014, of which 652 cases of BC were observed (IR=31.8 per 100,000). Active-duty women who were non-Hispanic Black, officers, healthcare workers, or older had an increased risk of BC (31). Zullig and colleagues (2012) found no major differences in BC incident diagnoses among Black, “Other,” and White Veterans from the 2007 Veterans Affairs Central Cancer Registry (45). Zullig and colleagues (2017) updated their 2007 analysis (Zullig et al. (45),) with data from 2010, but results did not change appreciably (46). Zullig and colleagues (2019) conducted a cross-sectional study of 1,330 incident invasive cancer cases among women Veterans in 2010; BC was the most common invasive cancer (30.23%, n=402), but it did not differ by race (47).
Four papers compared deployment characteristics. Kang and colleagues (2000) followed 3,392 Vietnam-era deployed women Veterans (n=170 cases) and 3,038 Vietnam-era women Veterans who never deployed to Vietnam (n=126). Both the crude (odds ratio [OR]=1.22 [0.96, 1.55]) and adjusted ORs (OR=1.18 [0.91, 1.51] suggested that the odds of developing BC were statistically equivalent between groups (28). Macfarlane and colleagues (2003) followed a cohort of 51,721 Gulf War Veterans and 50,755 matched service personnel and found no increased risk among Veterans that deployed in support of the Gulf War (IRR=0.59 [0.21, 1.62]) (32). Young and colleagues (2010) followed 621,902 Gulf War Veterans and 746,248 non-Gulf War Veteran controls. Gulf War Veterans had a statistically equivalent IR of BC among men (Proportional IR [PIR]=0.78 [0.39, 1.58]) and women (PIR= 1.01 [0.86, 1.20]), respectively (44). Gaffey and colleagues (2023) conducted a cohort study of 576,601 women Veterans (n=141,935 OEF/OIF-deployed). Those who deployed in support of OEF/OIF were 23% [17%, 29%] less likely to be diagnosed with BC (RR=0.77; [0.71, 0.83]) (25).
Four studies provided general incidence rates without a comparison group. Hoiberg & Ernst (27) conducted a cohort study of 364 active-duty Navy women (n=47 cases) and found an IR by age (overall 34.1 per 100,000) (27). Ajene and colleagues (22) conducted a cohort study of 78 women Naval personnel and found a BC IR of 8.53 per 100,000 persons (22). Strand and colleagues (2014) analyzed 268 Norwegian women military peacekeepers deployed to Kosovo, and one incident case of BC was observed (38). Strand and colleagues (2020) reanalyzed their 2014 cohort of Norwegian military peacekeepers deployed to Kosovo: 275 women were included and three BC cases were found (40).
Two papers measured exposure to VOCs. Rennix and colleagues (2005) conducted a cohort study of 274,596 enlisted Army women. Exposure to VOCs was tied to job titles, categorized as none, low, medium, or high (35). None of the top military occupational specialties were associated with BC risk. However, history of moderate or high exposure to VOCs was associated with a 48% higher incidence (IRR=1.48 [1.03, 2.12]) (35). Ruckart and colleagues (2015) led a case-control study of Marines stationed at the Camp Lejeune garrison who were exposed to contaminated groundwater (71 cases of male BC, 373 controls). Adjusted ORs for high residential cumulative exposures to tetrachloroethylene, t-1,2 dichloroethylene, and vinyl chloride were 1.20 [0.16, 5.89], 1.50 [0.30, 6.11], 1.19 [0.16, 5.89], respectively (36). Ever being stationed at Camp Lejeune and high cumulative exposures to VOCs were associated with an earlier age at onset for male BC, though it was not statistically significant (36).
Carran and Shaw (24) conducted a cohort study of 71 Veterans and 76 female adult children of Veterans. They found an increased risk of BC among female adult children of New Zealand Veterans deployed to Malaya who were exposed to the EDC dibutylphthalate (24).
One paper directly measured exposure to the tactile herbicide, Agent Orange. Yi & Ohrr (43) mapped each unit’s post location and tactile area of responsibility to known geographic regions of chemically treated areas. Korean Vietnam-era Veterans (n=180,251 with follow-up from 1992-2003) were given an Exposure Opportunity Index and stratified into categories (high vs. low) in a cohort study (43). Exposure to high levels of Agent Orange was not associated with an increased risk of BC (adjusted HR=0.53 [0.12, 2.26]) (43).
Mohr and colleagues (2013) examined the relationship between pre-diagnostic serum 25-hydroxyvitamin D (Vitamin D) and risk of BC among active-duty personnel using a nested case-control study with 600 incident cases and 600 controls. No statistically significant relationship was found between serum Vitamin D levels and odds of BC.
This scoping review covered 28 papers on the relationships between military service, MEE, and BC among active-duty personnel and Veterans. Unfortunately, evidence is still needed before conclusive remarks can be made. Most papers on military service or deployment reported a decreased or statistically equivalent risk of BC, while a few larger surveillance studies found an increased risk. When considering the effects of military service and deployment on risk of BC, individual and environmental risk factors should be considered (49). If risk factors are not controlled for, findings may be biased by the Healthy Soldier Paradox (25).
The Healthy Soldier Paradox occurs when healthier personnel are deployed in support of military operations and sicker personnel are not deployed or are given different military occupations (50). This form of sampling bias can lead to inaccurate associations between deployment and health outcomes, where deployed individuals appear to have a lower risk of adverse outcomes than non-deployed personnel. Recent work has called the Healthy Soldier Paradox into question for OEF/OIF/OND-era Veterans (51), as OEF/OIF/OND-era Veterans have a higher risk of mortality when compared to the general U.S. population. However, it may be that healthy solider effects vary by outcome, such that OEF/OIF/OND-era Veterans may have a higher risk of mortality but a decreased risk of BC (25). In a study of 31,548 military healthcare system users with BC and 63,096 controls with BC, the military healthcare system users had a significantly lower risk of mortality (24% [20%, 29%]) (52). This lower mortality risk was found across all ages, Stages II, III, and IV tumors, and for Black and White patients (52), suggesting that military personnel may also benefit from an equal-access healthcare system (18, 19).
Many studies have few years of follow-up, small sample sizes, lack accurate measurement, and suffer from misclassification bias. Follow-Up: Epidemiological analyses of cancer require a sufficiently long follow-up so that cases can occur. Most cases of BC occur in women aged 50+ (6), and many personnel from post-9/11 conflicts may not be in this age group yet. Small Sample Size: Women personnel and Veterans with MEE concerns are still a relatively small group from an epidemiological perspective. Accurate Measurement of Exposure: Investigators often rely on participant recall for MEE (leading to recall bias) and do not consider that most MEE are transient, may occur more than once, and may occur with varying severity. Accurate Measurement of Outcome: Measurement of BC has improved in recent decades due to advances in mammography screening (53) and modern classification codes (e.g., ICD-10). However, characteristics of the breast tumor (e.g., T-stage, N-stage, M-stage) and histological and molecular subtyping are not often explored. Misclassification Bias: Misclassification bias reflects an issue with categorizing participants by exposure/outcome status. Without accurately measuring MEE, participants may be misclassified, leading to null results (54). For many articles, exposures are generalized to entire groups, but individual-level data are needed.
Most papers in this review considered military service or military deployment as an exposure when measuring BC risk in personnel. Unlike most social and environmental exposures, military service (yes/no) is not well operationalized and leaves a lot to be desired in terms of specificity. Characteristics of military service (e.g., military occupation and job duties, deployment location, rank, military branch, number of years served, and MEE) should be measured in future studies, as these factors may improve our detection of the Healthy Soldier Paradox. Additionally, tying specific military occupations and job duties to MEE will be crucial for determining causality, and will inform policy and practice regarding the expansion of personal protective equipment and environmental toxin passive monitoring devices in the field.
With these limitations stated, several conclusions may still be found. Exposure to VOCs appears to impact the downstream risk of BC among military personnel (35, 36). Specific VOCs’ effects are largely unknown, but the risk of BC appears stronger among women than among men, and the mechanism of action may be through oxidative damage, cytotoxicity, and genotoxicity (55–57). The effects of EDCs have not been sufficiently studied in military samples, but they are known to impact risk of BC in civilians (58). Vitamin D (34, 59, 60) may not be considered an environmental exposure, but ionizing radiation from the sun would be an important MEE. Future work should measure UV exposure, heat, and drought directly. No papers directly assessed the effects of AHOBP or depleted uranium, but many Veterans who deployed in support of Gulf War and OEF/OIF/OND with MEE to AHOBP and depleted uranium are now at an age where BC is a salient concern (61). One large study looked at Agent Orange and BC risk and did not find an association. Unfortunately, no studies were found on other tactile herbicides (e.g., Agents White, Blue, Purple, Pink, Green) or pesticides. Many MEE included in this review were not specific to military populations. VOCs, EDCs, and carcinogenic airborne hazards are well-known occupational exposures in the civilian sector. Too little work has been done to understand if the effects of these generic exposures are moderated by military service. Finally, it is important to recognize that a greater number of high-quality articles will be needed to draw significant conclusions that link MEE and BC, as surveillance cohort studies are insufficient to draw causal links.
Future studies should: 1) Measure MEE in real time (e.g., dose, duration, source, route of entry) using ecological momentary assessment or passive monitoring; 2) Study specific VOCs, EDCs, and AHOBP; 3) Compare deployed to non-deployed military personnel and include a group of civilian controls when possible; 4) Recruit a diverse group of women and gender-diverse personnel, including all military branches, races/ethnicities, ranks, occupations, and deployment locations; 5) Determine warfare theater/era effects; 6) Measure BC histological/molecular subtypes; 7) Expand years of follow-up and increase recruitment; and 8) Explore biological plausibility by tying MEE to specific carcinogenic pathways.
Findings on MEE and BC are varied, in part due to the Healthy Soldier Paradox, potential misclassification of exposure(s), and modest sample sizes. The strongest evidence with reproducible findings appears to be Veterans’ increased risk of BC after being exposed to VOCs.
DJ: Writing – review & editing, Writing – original draft, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. MA: Writing – review & editing, Writing – original draft, Supervision, Software, Project administration, Investigation, Formal analysis, Data curation. DG: Writing – review & editing, Writing – original draft, Software, Investigation, Formal analysis, Data curation. AI: Writing – review & editing, Writing – original draft, Software, Investigation, Formal analysis, Data curation. JJ: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Funding acquisition. MA: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Funding acquisition.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Women’s Operational Military Exposure Network Center of Excellence (WOMEN CoE). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors would like to thank the California War Related Illness and Injury Study Center (WRIISC) staff and the Women’s Operational Military Exposure Network Center of Excellence (WOMEN CoE) staff and advisory board for their continued support of this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Maher N. Understanding sex differences in health and health care utilization among veterans can help guide future veterans administration health care planning and policy. J Women’s Health (2002). (2021) 30:916–7. doi: 10.1089/jwh.2020.9009
2. Frayne SM, Mattocks KM. Sourcebook: women veterans in the Veterans Health Administration. In: sociodemographics and use of VHA and non-VA care (fee), (Washington D. C: Department of Veterans Affairs) vol. 2. (2012).
3. Bagalman E. The number of veterans that use VA health care services: A fact sheet. Washington (DC: Congressional Research Service (2014).
4. U.S. Department of Defense. 2021 Demographics profile of the military community(2021). Available online at: https://download.militaryonesource.mil/12038/MOS/Reports/2021-demographics-report.pdf.
5. Office of Women’s Health. State of reproductive health volume II: VA reproductive health diagnoses and organization of care(2023). Available online at: https://www.womenshealth.va.gov/WOMENSHEALTH/docs/VHA-WH-Reproductive-Health-Report-2023.pdf.
6. Ban KA, Godellas CV. Epidemiology of breast cancer. Surg Oncol Clinics. (2014) 23:409–22. doi: 10.1016/j.soc.2014.03.011
7. Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistics, 2022. CA: Cancer J Clin. (2022) 72:524–41. doi: 10.3322/caac.21754
8. Konduri S, Singh M, Bobustuc G, Rovin R, Kassam A. Epidemiology of male breast cancer. Breast. (2020) 54:8–14. doi: 10.1016/j.breast.2020.08.010
9. Aggarwal A, Adepoju B, Yacur M, Maron D, Sharma MC. Gender disparity in breast cancer: a veteran population-based comparison. Clin Breast Cancer. (2021) 21:e471–8. doi: 10.1016/j.clbc.2021.01.013
10. Department of Veterans Affairs. The PACT Act and your VA benefits (2023). Available online at: https://www.va.gov/resources/the-pact-act-and-your-va-benefits/.
11. Byrnes A. VA launches national women veterans oncology system of excellence. Battling Breast Cancer. (2021). Available at: https://news.va.gov/press-room/va-creates-national-women-veterans-oncology-system-of-excellence-in-fight-against-breast-cancer/.
12. Lovejoy LA, Shriver CD, Ellsworth RE. Cancer incidence and etiology in the active duty population of US Military. Military Med. (2024) 189(1-2):e58–e65. doi: 10.1093/milmed/usac297
13. Ferras M, Dye J, Ayala GX, Schmied E. An examination of factors that influence receipt of reproductive health screenings among female veterans. Military Med. (2023) 188:42–8. doi: 10.1093/milmed/usac036
14. Braun LA, Kostas-Polston EA, Miedema J, Hoffecker L, Wilson C. A scoping review of cervical cancer risk factors, prevention, diagnosis, and treatment in US active duty military women. Women’s Health Issues. (2021) 31:S53–65. doi: 10.1016/j.whi.2021.04.003
15. Cohen BE, Maguen S, Bertenthal D, Shi Y, Jacoby V, Seal KH. Reproductive and other health outcomes in Iraq and Afghanistan women veterans using VA health care: association with mental health diagnoses. Women’s Health Issues. (2012) 22:e461–71. doi: 10.1016/j.whi.2012.06.005
16. American Cancer Society. Agent Orange and Cancer Risk (2023). Available online at: https://www.cancer.org/cancer/risk-prevention/chemicals/agent-orange-and-cancer.html.
17. Zhu K, Devesa SS, Wu H, Zahm SH, Jatoi I, Anderson WF, et al. Cancer incidence in the US military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. (2009) 18:1740–5. doi: 10.1158/1055-9965.EPI-09-0041
18. Kvasnovsky CL, Kesmodel SB, Gragasin JL, Punnoose V, Johnson PA, Goel R, et al. Expansion of screening mammography in the Veterans Health Administration: implications for breast cancer treatment. JAMA surgery. (2013) 148:999–1004. doi: 10.1001/jamasurg.2013.3738
19. Lairson DR, Chan W, Newmark GR. Determinants of the demand for breast cancer screening among women veterans in the United States. Soc Sci Med. (2005) 61:1608–17. doi: 10.1016/j.socscimed.2005.03.015
20. Patten E, Parker K. Women in the U.S. Military: Growing Share, Distinctive Profile (2011). Available online at: https://givingtogether.org/resources/Events/Education%20Event%20Veterans%20Handouts/Pew%20Survey%20of%20Women%20Veterans%20December%202011.pdf.
21. Munn Z, Peters MD, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res methodology. (2018) 18:1–7. doi: 10.1186/s12874-018-0611-x
22. Ajene A, Bohnker B, Malakooti MA, Riegodedios A, Sack DM. Neoplasms in the Navy, 1998–2000: a descriptive analysis of the Physical Evaluation Board database. Military Med. (2004) 169:707–11. doi: 10.7205/MILMED.169.9.707
23. Bytnar JA, McGlynn KA, Shriver CD, Zhu K. Cancer incidence in the US military: An updated analysis. Cancer Res. (2023) 82:20–0.doi: 10.1002/cncr.34978
24. Carran M, Shaw I. New Zealand Malayan war veterans’ exposure to dibutylphthalate is associated with an increased incidence of cryptorchidism, hypospadias and breast cancer in their children. New Z Med J (Online). (2012) 125:52–63.
25. Gaffey AE, Han L, Ramsey CM, Skanderson M, Dziura J, Driscoll M, et al. Post-9/11 deployment history and the incidence of breast cancer among women veterans. Ann Epidemiol. (2023) 77:98–102. doi: 10.1016/j.annepidem.2022.11.010
26. Hansen J, Lassen CF. Nested case–control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. (2012) 69:551–6. doi: 10.1136/oemed-2011-100240
27. Hoiberg A, Ernst J. Cancer among Navy personnel: incidence and mortality. Military Med. (1980) 145:195–200. doi: 10.1093/milmed/145.3.195
28. Kang HK, Mahan CM, Lee KY, Magee CA, Selvin S. Prevalence of gynecologic cancers among female Vietnam veterans. J Occup Environ Med. (2000) 42(11):1121–7. doi: 10.1097/00043764-200011000-00018
29. Katuwal S, Martinsen JI, Kjaerheim K, Sparen P, Tryggvadottir L, Lynge E, et al. Occupational variation in the risk of female breast cancer in the Nordic countries. Cancer Causes Control. (2018) 29:1027–38. doi: 10.1007/s10552-018-1076-2
30. Lee W, Park S, Kang SK, Ham S, Yoon JH, Choi WJ. Cancer risk in Vietnam war veterans from the Korean Vietnam war veterans’ health study cohort. Front Oncol. (2023) 13:1048820. doi: 10.3389/fonc.2023.1048820
31. Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, US Armed Forces, 2005-2014. Msmr. (2016) 23:23–31.
32. Macfarlane GJ, Biggs AM, Maconochie N, Hotopf M, Doyle P, Lunt M. Incidence of cancer among UK Gulf war veterans: cohort study. Bmj. (2003) 327:1373. doi: 10.1136/bmj.327.7428.1373
33. Mahar AL, Aiken AB, Cramm H, Cyr KS, Shellenberger J, Kurdyak P. Cancer incidence among Canadian Veterans: A matched cohort study. Cancer Epidemiol. (2022) 79:102199. doi: 10.1016/j.canep.2022.102199
34. Mohr SB, Gorham ED, Alcaraz JE, Kane CI, Macera CA, Parsons JK, et al. Serum 25-hydroxyvitamin D and breast cancer in the military: a case–control study utilizing pre-diagnostic serum. Cancer Causes Control. (2013) 24:495–504. doi: 10.1007/s10552-012-0140-6
35. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. (2005) 48:157–67. doi: 10.1002/(ISSN)1097-0274
36. Ruckart PZ, Bove FJ, Shanley E, Maslia M. Evaluation of contaminated drinking water and male breast cancer at Marine Corps Base Camp Lejeune, North Carolina: a case control study. Environ Health. (2015) 14:1–16. doi: 10.1186/s12940-015-0061-4
37. Storm HH, Jørgensen HO, Kejs AMT, Engholm G. Depleted uranium and cancer in Danish Balkan veterans deployed 1992–2001. Eur J Cancer. (2006) 42:2355–8. doi: 10.1016/j.ejca.2006.01.064
38. Strand LA, Martinsen JI, Borud EK. Cancer risk and all-cause mortality among Norwegian military United Nations peacekeepers deployed to Kosovo between 1999 and 2011. Cancer Epidemiol. (2014) 38:364–8. doi: 10.1016/j.canep.2014.04.003
39. Strand LA, Martinsen JI, Borud EK. Cancer incidence and all-cause mortality in a cohort of 21 582 Norwegian military peacekeepers deployed to Lebanon during 1978–1998. Cancer Epidemiol. (2015) 39:571–7. doi: 10.1016/j.canep.2015.04.011
40. Strand LA, Martinsen JI, Borud EK. A 5-year continued follow-up of cancer risk and all-cause mortality among norwegian military peacekeepers deployed to Kosovo during 1999–2016. Military Med. (2020) 185:e239–43. doi: 10.1093/milmed/usz179
41. Yamane GK. Cancer incidence in the US air force: 1989-2002. Aviation space Environ Med. (2006) 77:789–94.
42. Yi SW. Cancer incidence in Korean Vietnam veterans during 1992-2003: the Korean veterans health study. J Prev Med Public Health. (2013) 46:309. doi: 10.3961/jpmph.2013.46.6.309
43. Yi SW, Ohrr H. Agent Orange exposure and cancer incidence in Korean Vietnam veterans: a prospective cohort study. Cancer. (2014) 120:3699–706. doi: 10.1002/cncr.28961
44. Young HA, Maillard JD, Levine PH, Simmens SJ, Mahan CM, Kang HK. Investigating the risk of cancer in 1990–1991 US Gulf War veterans with the use of state cancer registry data. Ann Epidemiol. (2010) 20:265–272.e1. doi: 10.1016/j.annepidem.2009.11.012
45. Zullig LL, Jackson GL, Dorn RA, Provenzale DT, McNeil R, Thomas CM, et al. Cancer incidence among patients of the US Veterans Affairs health care system. Military Med. (2012) 177:693–701. doi: 10.7205/MILMED-D-11-00434
46. Zullig LL, Sims KJ, McNeil R, Williams CD, Jackson GL, Provenzale D, et al. Cancer incidence among patients of the US Veterans Affairs Health Care System: 2010 update. Military Med. (2017) 182:e1883–91. doi: 10.7205/MILMED-D-16-00371
47. Zullig LL, Goldstein KM, Sims KJ, Williams CD, Chang M, Provenzale D, et al. Cancer among women treated in the veterans affairs healthcare system. J Women’s Health. (2019) 28:268–75. doi: 10.1089/jwh.2018.6936
48. Center (AFHSC AFHS). Incident diagnoses of breast cancer, active component service women, US Armed Forces, 2000-2012. Msmr. (2013) 20(9):25–7.
49. Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer: Targets Ther. (2019) 11(9):151–64. doi: 10.2147/BCTT
50. Sullivan-Baca E, Rehman R, Haneef Z. An update on the healthy soldier effect in US Veterans. Military Med. (2023) 188:3199–204. doi: 10.1093/milmed/usac151
51. Bollinger MJ, Schmidt S, Pugh JA, Parsons HM, Copeland LA, Pugh MJ. Erosion of the healthy soldier effect in veterans of US military service in Iraq and Afghanistan. Population Health metrics. (2015) 13:1–12. doi: 10.1186/s12963-015-0040-6
52. Lin J, Hu H, Shriver CD, Zhu K. Survival among breast cancer patients: comparison of the US military health system with the surveillance, epidemiology and end results program. Clin Breast cancer. (2022) 22:e506–16. doi: 10.1016/j.clbc.2021.11.010
53. Bytnar JA, Byrne C, Olsen C, Witkop CT, Martin MB, Banaag A, et al. The impact of mammography screening guideline changes among women serving in the US Military. Military Med. (2020) 185:e2088–96. doi: 10.1093/milmed/usaa176
54. Spiegelman D. Approaches to uncertainty in exposure assessment in environmental epidemiology. Annu Rev Public Health. (2010) 31:149–63. doi: 10.1146/annurev.publhealth.012809.103720
55. Wang F, Li C, Liu W, Jin Y. Oxidative damage and genotoxic effect in mice caused by sub-chronic exposure to low-dose volatile organic compounds. Inhalation toxicology. (2013) 25:235–42. doi: 10.3109/08958378.2013.779767
56. Cavallo D, Ursini CL, Fresegna AM, Ciervo A, Maiello R, Buresti G, et al. Occupational exposure in industrial painters: sensitive and noninvasive biomarkers to evaluate early cytotoxicity, genotoxicity and oxidative stress. Int J Environ Res Public Health. (2021) 18:4645. doi: 10.3390/ijerph18094645
57. Cetintepe SP, Hazar M, Bilinmiş I, Dilsiz SA, Basaran N. Evaluation of genotoxicity, oxidative stress and immune parameters of auto-paint workers. Environ Res. (2023) 237:116970. doi: 10.1016/j.envres.2023.116970
58. Rodgers KM, Udesky JO, Rudel RA, Brody JG. Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms. Environ Res. (2018) 160:152–82. doi: 10.1016/j.envres.2017.08.045
59. Chlebowski RT. Vitamin D and breast cancer incidence and outcome. Anti-Cancer Agents Medicinal Chem (Formerly Curr Medicinal Chemistry-Anti-Cancer Agents). (2013) 13:98–106. doi: 10.2174/187152013804487362
60. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer—epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—an updated review. Cancers. (2021) 13:4287. doi: 10.3390/cancers13174287
Keywords: female, breast neoplasm, women veterans, war, environmental epidemiology
Citation: Jester DJ, Assefa MT, Grewal DK, Ibrahim-Biangoro AM, Jennings JS and Adamson MM (2024) Military environmental exposures and risk of breast cancer in active-duty personnel and veterans: a scoping review. Front. Oncol. 14:1356001. doi: 10.3389/fonc.2024.1356001
Received: 14 December 2023; Accepted: 29 February 2024;
Published: 13 March 2024.
Edited by:
Chitra Thakur, Stony Brook University, United StatesReviewed by:
Ronak Loonawat, Wuxi Advanced Therapeutics, Inc., United StatesCopyright © 2024 Jester, Assefa, Grewal, Ibrahim-Biangoro, Jennings and Adamson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dylan J. Jester, RHlsYW4uSmVzdGVyQHZhLmdvdg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.