
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 15 May 2024
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1355270
Introduction: Gastric cancer, characterized by high incidence and substantial disease burden, has drawn continuous attention regarding its occurrence and prognosis. Genetics plays a crucial role in influencing the prognosis of gastric cancer, and single nucleotide polymorphisms are closely associated with the occurrence, development, and prognosis of this malignant tumor. Our study aims to conduct survival analysis on patients carrying different single nucleotide polymorphisms, exploring the relationship between miRNA single nucleotide polymorphisms and the prognosis of gastric cancer.
Methods: Genetic data from 344 patients in Xianyou, Fujian, formed the basis of our study. We delineated the survival rate and median survival time, utilizing the log-rank test and COX regression analysis as statistical tools.
Results: Upon stratifying the data by sex or operation, it was discerned that the GG genotype at MSH2 rs17502941 independently posed a heightened risk for gastric cancer. Other stratification analyses suggested that the subsequent single nucleotide polymorphisms were correlated with patient prognosis: rs17502941, rs884225, rs1468063, rs7143252, and rs2271738.
Discussion: The outcomes of this study strongly suggest that miRNA polymorphisms significantly influence the survival time of gastric cancer patients and can serve as effective predictors for the prognosis of gastric cancer.
According to cancer incidence and mortality data for the year 2018 from the International Agency for Research on Cancer (IARC), gastric cancer constituted 8.2% of all cancer-related deaths (1). A substantial number of patients receive a diagnosis of advanced-stage cancer, indicative of a low rate of early detection (2). Simultaneously, the prognosis of gastric cancer is intricately linked to various factors, encompassing the degree of differentiation, sex, tumor microenvironment, and the epithelial-mesenchymal transition (EMT) signature associated with metastasis (3–5). Heredity stands out as a significant factor influencing the prognosis of gastric cancer, with single nucleotide polymorphism closely intertwined with the onset, progression, and prognosis of this malignancy. A comprehensive meta-analysis indicates that diminished microRNA expression is detrimental to patient prognosis and may expedite the progression of gastric cancer (6). Discrepancies in miRNA blood levels have been identified in patients with gastric cancer (GC), affirming the utility of miRNA expression as a diagnostic and prognostic biomarker for this condition (7). Moreover, investigations have proposed a close association between miRNA and multidrug resistance (8, 9). There is suggestive evidence that miR-214 can regulate the expression of FGF9, impeding the migration and invasion of gastric cancer (GC) cells. Conversely, miR-519a-3p has been implicated in promoting liver metastasis in gastric cancer and is associated with an unfavorable prognosis (10, 11). Several studies have identified miRNA-21 rs1292037 and miR-149 rs2292832C/T as potential prognostic indicators for hepatocellular carcinoma (12, 13). Additionally, investigations have linked miR-34 rs4938723 and miR-195-5p to the prognosis of colorectal cancer (14, 15). Moreover, research findings indicate that single nucleotide polymorphisms in miRNAs are correlated with the prognosis of various types of tumors (16–19). While numerous studies have established associations between miRNA single nucleotide polymorphisms and the prognosis of various types of tumors, limited attention has been directed toward exploring the connection between miRNA single nucleotide polymorphisms and the prognosis of gastric cancer. Our research complements the existing knowledge body in this specific domain.
Our study aimed to scrutinize the correlation between miRNA polymorphisms and the prognosis of gastric cancer. We meticulously selected 11 miRNA loci and assessed their impact on gastric cancer prognosis using four distinct models: Co-dominance, Allele gene, dominant model, and recessive model. Subsequently, a stratified analysis was conducted based on parameters such as sex, age, location, TNM stage, the performance of surgery, and administration of chemotherapy. Multivariate COX analysis was then applied to sites demonstrating statistical significance in the stratified analysis, thereby delving into the factors exerting independent risk effects on the prognosis of gastric cancer.
This research encompassed the distribution of 555 questionnaires, of which 96 cases posed challenges for investigation due to reasons such as removal, denial of dis-ease, and rejection of examination. (recovery rate: 82.7%) Following a thorough examination of the questionnaires, 115 incomplete and unqualified counts were excluded, and ultimately, 344 questionnaires were finally adopted. Therefore, our study included 344 patients, with peripheral blood samples collected from each patient. The inclusion criteria were as follows: (1) voluntary participation in the study and signing of informed consent by the researchers, (2) patients confirmed by endoscopic biopsy or surgical specimens to have gastric adenocarcinoma, (3) residing in Xianyou for more than 10 years, (4) confirmed between April 2013 and November 2017, with the final follow-up conducted in December 2022. Exclusion criteria were: (1) patients simultaneously diagnosed with other types of tumors besides gastric adenocarcinoma, (2) severe mental illness or poor compliance leading to inability to follow-up, (3) severe genetic diseases, including chromosomal number and structural abnormalities. The histological type of tumors was evaluated according to the standards of the World Health Organization (WHO), and tumor staging was classified according to the guidelines outlined in the American Joint Committee on Cancer Staging Manual (7th edition).
Previous studies have identified 112 loci associated with gastric cancer, including 12 miRNA polymorphic loci, 99 miRNA target gene polymorphic loci, and 1 miRNA synthesis pathway gene polymorphic locus (Figure 1). To further identify the miRNA-SNPs most closely related to gastric cancer, we selected 11 loci for this study through the following steps:
1. Initially, utilizing the dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/), we determined the minimum allele frequency (MAF) of each SNP locus in the Chinese population. Loci with MAF between 0.15 and 0.40 were chosen.
2. Subsequently, based on information from the NCBI website (https://pubmed.ncbi.nlm.nih.gov/), we selected loci associated with gastrointestinal (GI) tumors.
3. Finally, using the F-SNP website (http://compbio.cs.queensu.ca/F-SNP/), we screened for loci with corresponding functions.
The 11 selected miRNA candidate loci were examined using the Sequenom Mass ARRAY SNP method. The process involved the following steps:
1. Designing Primers:
Design PCR amplification primers and single-base extension primers for SNP locus to be tested using Genotyping Tools and Mass ARRAY Assay Design software (Table 1).
2. PCR Amplification:
Perform PCR amplification reactions for the selected loci.
3. Product Processing:
Process the PCR amplification products.
4. Mass ARRAY Dispensing:
Initiate the Mass ARRAY RS1000 dispenser and deposit the processed product onto the SpectroCHIP (Sequenom) chip.
5. SpectroCHIP Detection:
Employ Mass ARRAY MALDI-TOP to detect the SpectroCHIP, capturing the type and output of results.
6. Result Analysis:
Analyze the results using TYPER4.0 software.
This methodology allows for the precise genotyping of the selected miRNA candidate loci.
Throughout the on-site investigation, the inspectors meticulously adhered to the questionnaire requirements. Following the survey, the questionnaire data underwent a thorough examination. Any incomplete questionnaires were promptly supplemented with as much information as possible. To ensure data accuracy, a double data entry method was employed, involving logical corrections and consistency testing. Additionally, a random sample of 10% of the entries underwent further review to enhance the overall quality and reliability of the data.
Dish QC (DQC) serves as a pivotal metric for evaluating the quality of genotyping. This assessment relies on comparing the distribution of signal values with background signal values, where a greater difference signifies a more effective experimental process and higher genotyping quality for the respective sample. In this experiment, all DQC results exceeded 0.82, indicating excellent genotyping quality. Genotypes were determined in a blinded manner, and 2% of the samples underwent random selection for repeated testing. The concordance rate in these repeated tests was found to be 98.47%. This robust quality control process ensures the reliability and accuracy of the genotyping results.
Survival rates for different genotypes of the same polymorphic locus in the 1st, 3rd, and 5th years were derived from the mortality table. The Kaplan-Meier method was employed to calculate the survival time of patients with different genotypes, and corresponding survival curves were graphically represented. The log-rank test was then applied to analyze the correlation between single nucleotide polymorphism and the prognosis of gastric cancer. Hazard ratios (HRs) and 95% confidence intervals (CIs) were computed using both univariate and multivariate COX regression modeling methods.
For statistical analysis, the significance level (α) was set at 0.05, and all P values were based on two-sided tests. The statistical software utilized for these analyses was SPSS 24.0, ensuring robust and standardized statistical evaluation of the data.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Fujian Medical University, China (No. 97,2014).
Among the 344 patients included in the study, there were 252 male patients and 92 female patients, ranging in age from 36 to 96 years, with an average age of (69.15 ± 9.34) years and a median age of 69 years. The survival rates for the 344 gastric cancer patients were recorded at 73.47%, 45.71%, and 39.67% in the 1st, 3rd, and 5th years, respectively, with a median survival time of 30.00 months.
To explore the relationship between the overall condition and prognosis of patients with stomach cancer, the aforementioned statistical methods were employed. It was observed that age, TNM stage, and whether surgery was performed were all correlated with the prognosis of gastric cancer patients (P<0.05) (Table 2).
The study incorporated a total of 11 miRNA polymorphic sites, with detailed information provided in Table 3. The results of the statistical analysis revealed a significant difference in the survival time associated with the A mutant gene of the polymorphic locus miRNA-1297 rs9536676 in comparison to the wild-type gene G. Specifically, the risk of death for patients carrying the A allele was 1.258 times higher than that of patients carrying the G allele (HR=1.258, 95% CI=1.000-1.581, Log-rank P = 0.046). In the case of the polymorphic locus MSH2 rs17502941, survival times exhibited statistical significance in the recessive model (AA+AG/GG). Patients carrying the GG genotype had a 1.329 times higher risk of death compared to those carrying the AA/AG genotype (HR=1.329, 95% CI=1.006-1.756, Log-rank P=0.043) None of the other polymorphic loci were found to be associated with the prognosis of gastric cancer (Tables 4, 5).
In the dominant model, the AG and AA genotypes at miRNA-1297 rs9536676 emerge as risk factors for patients aged over 65 years, male patients, and those diagnosed with cardiac cancer. Similarly, the AG and GG genotypes at miRNA-519b rs10413288 serve as protective factors for patients with cardiac cancer and those who have undergone chemotherapy, as per the dominant model. Notably, the AG and GG genotypes at MSH2 rs11125144 act as risk factors for female patients (co-dominant and dominant models) but as a protective factor for patients who did not undergo surgery (recessive model).
In the stratified analysis, rs17502941, rs884225, rs1468063, rs7143252, and rs2271738 were all identified as significant factors linked to the prognosis of patients (Tables 6–11).
Building upon the insights garnered from the Univariate survival analysis, a comprehensive COX multifactor analysis was conducted specifically for MSH2 rs17502941 (Figures 2, 3). Stratifying the analysis by sex or operation, it was found that the GG genotype at MSH2 rs17502941 (recessive model) serves as an independent risk factor for the prognosis of gastric cancer (Tables 12, 13).
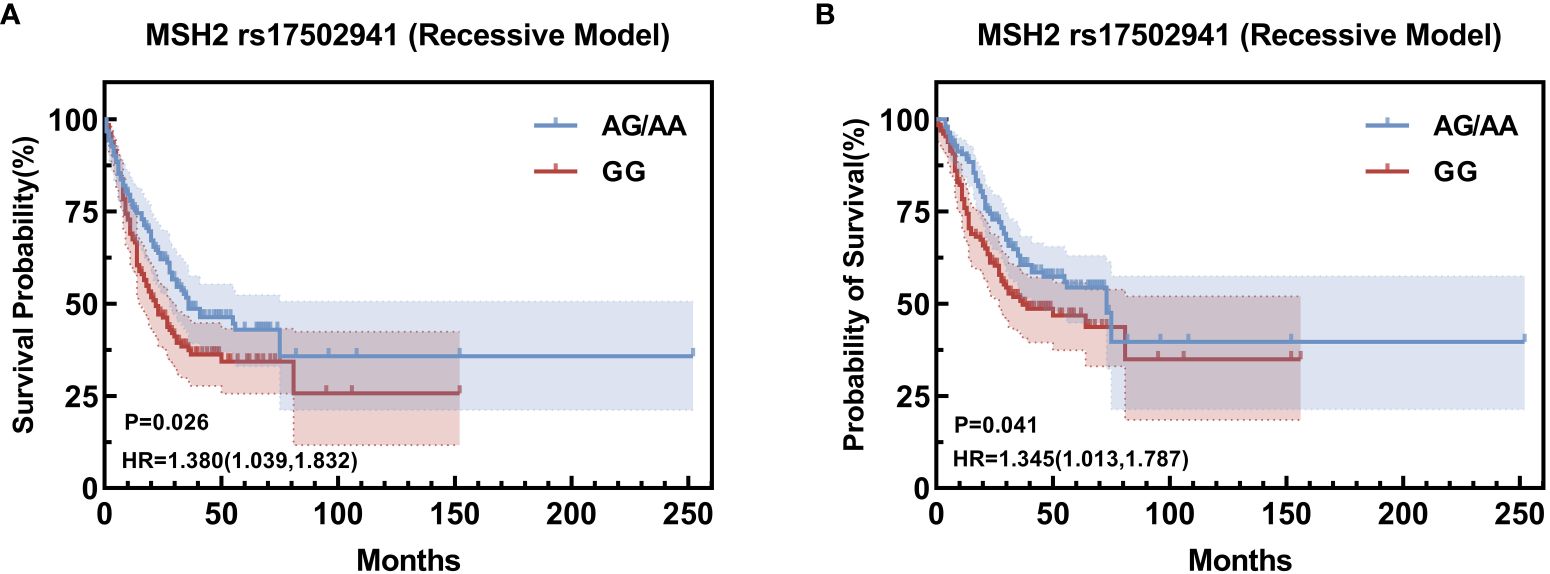
Figure 3 Kaplan-Meier Curve for rs17502941 based on comprehensive COX multifactor analysi, stratified by male (A), stratified by operation (B).
Our study uncovered a robust association between the single nucleotide poly-morphism of MSH2 rs17502941 and the prognosis of gastric cancer. Employing COX stratification analysis, we identified that the GG locus of rs17502941 was indicative of a poor prognosis in male patients and those who underwent surgery in a recessive model. Numerous studies have consistently highlighted the intricate relationship between diverse miRNAs and the prognosis of gastric cancer. Elevated expression levels of miR-203, miR-218, and miR-194 have been correlated with a favorable prognosis for gastric cancer, while diminished expression of miR-17-5p and miR-34a serves as a marker for an unfavorable prognosis (6, 20, 21). Furthermore, a diminished expression of PARP-1, a DNA damage response (DDR)-associated protein, has been identified as a prognostic indicator for patients with stage 2 and 3 gastric cancer (22). Given its role as a DDR-associated gene, the low expression of MSH2 may similarly contribute to an unfavorable prognosis. Numerous investigations have proposed associations between MSH2 and various cancers, including prostate, colorectal, and ovarian cancers (23, 24), in addition to its linkage with the risk of gastric cancer (25, 26). Additionally, a separate study identified a correlation between the TC+CC genotype of MSH2 rs2303428 and a diminished survival rate in non-cardia cancers (27). In our investigation, we observed that the GG genotype of MSH2 rs2303428 stood out as an independent risk factor for an unfavorable prognosis among male, surgical patients, aligning with previous findings. However, in multifactorial analyses, we did not discern their significant effects on the prognosis of gastric cancer.
Earlier studies had implicated these miRNAs in the prognosis of gastric cancer. A research endeavor from Hubei, China, underscored that the diminished expression of miRNA-379 served as an independent risk factor for patients’ prognosis (28). Likewise, a study conducted in Xi’an, China, revealed that miRNA-1297 exhibited low expression in gastric cancer tissues, and this reduced expression independently correlated with compromised disease-free survival for patients (29). However, our investigation sorely yielded statistically significant associations between miRNA-379 rs7143252, miRNA-1297 rs9536676, and gastric cancer prognosis. This discrepancy may be indicative of regional variations in gastric cancer genotyping, and the presence of a complex interplay of confounding factors and genes cannot be discounted. Further research is imperative, as there remains a possibility that multiple loci identified in the univariate analysis are indeed linked to the prognosis of gastric cancer.
Research endeavors have identified risk factors affecting the prognosis of gastric cancer as well as specific loci that could be associated with its prognosis. These findings offer crucial insights, laying the groundwork for further exploration. Some miRNAs related to gastric cancer risk and prognosis have already been collected and arranged by academics, including miR-499, miR-146a, and so on (30), it’s noteworthy that the loci unveiled in our study are not among them. This discrepancy suggests, on the one hand, the significance of our research, and on the other hand, underscores the vast untapped potential within this field. Global epidemiological studies on gastric cancer have unveiled intriguing patterns, emphasizing the distinct genetic landscape in Asian populations. Notably, Asians exhibit a heightened frequency of IL-10 and IL-17 gene diversity. This diversity, in con-junction with environmental factors and lifestyle habits, collectively influences the risk and prognosis of gastric cancer (31). Moreover, specific regional variations have been observed, such as the unique characteristics of gastric cancer in the Tibetan Plateau compared to other regions. This dissimilarity encompasses differences in incidence rates, mutation types, and molecular variations among patients from the highlands and Western countries, as well as Han Chinese and ethnic minorities, have different molecular variations in their mutated gene, For instance, an analysis revealed a notable discrepancy in notch2 mutations, with only one out of 12 Han Chinese exhibiting this mutation compared to seven out of 18 ethnic minorities (32). These findings underscore the imperative need for region-specific sequencing efforts, particularly in areas marked by elevated gastric cancer rates.
Gaining insight into diverse mutation types and single nucleotide polymorphisms across different regions not only establishes a theoretical framework but also lays the molecular foundation for the development of miRNA therapy in gastric cancer. This breakthrough holds the promise of tailoring treatments according to the distinct mutation profiles of patients in various regions. Furthermore, it paves the way for the creation of genetic databases encompassing patients from diverse geographical areas. These databases will serve as valuable reservoirs of background information, facilitating future investigations into the underlying molecular mechanisms. We anticipate that on-going and advanced research endeavors will propel miRNA therapy for gastric cancer from theory to reality. This therapeutic approach, already under exploration in diverse fields such as non-alcoholic liver disease, diabetes mellitus, myocardial fibrosis, and resensitization of chemotherapy-resistant cancer cells, holds immense potential (33). However, it is essential to acknowledge the limitations of our current study. Primarily, the outcomes may not be universally applicable, as our sample pool is derived from Xianyou, Fujian. Additionally, future studies necessitate larger sample sizes to ensure a more precise exploration of influencing factors.
Persisting as the primary contributor to cancer-related mortality on a global scale, gastric cancer maintains a dynamic temporal trend. It continues to be characterized by high incidence, elevated morbidity, and a substantial disease burden (34). Our research serves as a foundational reference for future investigations into molecular mechanisms, contributing to a comprehensive understanding of the involvement of single nucleotide polymorphisms (SNPs) in the pathogenesis of gastric cancer.
The data analyzed in this study is subject to the following licenses/restrictions: The data are not publicly available due to privacy. Requests to access these datasets should be directed to cGluZ3d1XzE5QDE2My5jb20=.
The studies involving humans were approved by the Ethics Committee of Fujian Medical University, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
PW: Writing – original draft. YZ: Validation, Writing – review & editing. YL: Visualization, Methodology, Writing – review & editing. JC: Formal analysis, Conceptualization, Writing – review & editing. YJ: Writing – review & editing, Data curation. JX: Writing – review & editing. BL: Writing – review & editing, Project administration. CW: Writing – review & editing, Supervision, Resources.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Fujian Natural Science Foundation (grant number 2015J01673), Fujian Natural Science Foundation (grant number 2017J01811), and Fujian Medical Innovation Project (grant number 2016-CX-41).
The authors express their gratitude to friends and teachers for their invaluable support and assistance throughout this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J OF Cancer. (2019) 144:1941–53. doi: 10.1002/ijc.31937
2. Tan Z. Recent advances in the surgical treatment of advanced gastric cancer: A review. Med Sci MONITOR. (2019) 25:3537–41. doi: 10.12659/MSM.916475
3. Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res. (2019) 7:737–50. doi: 10.1158/2326-6066.CIR-18-0436
4. Song J, Wei R, Huo S, Gao J, Liu X. Metastasis related epithelial-mesenchymal transition signature predicts prognosis and response to immunotherapy in gastric cancer. Front Immunol. (2022) 13:920512. doi: 10.3389/fimmu.2022.920512
5. Sexton RE, Hallak MNA, Uddin H, Diab M, Azmi AS. Gastric cancer heterogeneity and clinical outcomes. Technol IN Cancer Res Treat. (2020) 19:1079203125. doi: 10.1177/1533033820935477
6. Li Z, Liu Z-M, Xu B-H. A meta-analysis of the effect of microRNA-34a on the progression and prognosis of gastric cancer. Eur Rev Med Pharmacol Sci. (2018) 22:8281–7. doi: 10.26355/eurrev_201812_16525
7. Link A, Kupcinskas J. MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: Current insights and future perspectives. World J OF Gastroenterol. (2018) 24:3313–29. doi: 10.3748/wjg.v24.i30.3313
8. Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv L, et al. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. (2020) 19:62. doi: 10.1186/s12943-020-01185-7
9. Bonelli P, Borrelli A, Tuccillo FM, Silvestro L, Palaia R, Buonaguro FM. Precision medicine in gastric cancer. World J Gastrointestinal Oncol. (2019) 11:804–29. doi: 10.4251/wjgo.v11.i10.804
10. Wang R, Sun Y, Yu W, Yan Y, Qiao M, Jiang R, et al. Downregulation of miRNA-214 in cancer-associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J OF Exp Clin Cancer Res. (2019) 38:20. doi: 10.1186/s13046-018-0995-9
11. Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J OF Exp Clin Cancer Res. (2022) 41:296. doi: 10.1186/s13046-022-02499-8
12. Wu C, Tang G, Wang X, Zhang J, Chen S, Lu C, et al. Micro-RNA-21 rs1292037 A>G polymorphism can predict hepatocellular carcinoma prognosis (HCC), and plays a key role in cell proliferation and ischemia-reperfusion injury (IRI) in HCC cell model of IRI. SAUDI Med J. (2020) 41:383–92. doi: 10.15537/smj.2020.4.24994
13. Feng J, Liu Z, Yu L, Wu C, Luo X-B. MicroRNA-149 rs2292832 C/T polymorphism predicts the prognosis of hepatocellular carcinoma patients with bone metastasis. Lab Med. (2022) 53:561–9. doi: 10.1093/labmed/lmac036
14. Kassim SA, Yang X, Abbas M, Wu S, Faran MM, Meng QT, et al. Pri-miR-34b/c rs4938723 polymorphism is associated with decreased risk and better prognosis for colorectal cancer patients. Arch OF Med Res. (2019) 50:55–62. doi: 10.1016/j.arcmed.2019.05.008
15. Shokrollah N, Samadi P, Jalali A, Dalirfardouei R, Afshar S, Pourjafar M. A systems biology approach to identify novel biomarkers in progression from crohn’s disease to colorectal cancer. Asian Pac J Cancer Prev. (2023) 24:1993–2001. doi: 10.31557/APJCP.2023.24.6.1993
16. Zhang Y-K, Wu L-L, Li T-T, Cao D-Y, Zheng Q, Liu L. The POLR2E rs3787016 polymorphism is associated with susceptibility to and prognosis of gastric cancer. NEOPLASMA. (2021) 68:665–71. doi: 10.4149/neo_2021_201125N1277
17. Wu Y, Zhao T, Jia Z, Cao D, Cao X, Pan Y, et al. Polymorphism of the programmed death-ligand 1 gene is associated with its protein expression and prognosis in gastric cancer. J OF Gastroenterol AND Hepatol. (2019) 34:1201–7. doi: 10.1111/jgh.14520
18. Wang W, Li Z, Wang J, Du M, Li B, Zhang L, et al. A functional polymorphism in TFF1 promoter is associated with the risk and prognosis of gastric cancer. Int J OF Cancer. (2018) 142:1805–16. doi: 10.1002/ijc.31197
19. Sanguansin S, Saelee P, Kritsiriwuthinan K, Pongstaporn W. The association of pre-miR27a gene polymorphism and clinicopathological data in thai breast cancer patients. Asian Pac J Cancer Prev. (2023) 24:2055–9. doi: 10.31557/APJCP.2023.24.6.2055
20. Shekari N, Baradaran B, Shanehbandi D, Kazemi T. Circulating microRNAs: valuable biomarkers for the diagnosis and prognosis of gastric cancer. Curr MEDICINAL Chem. (2018) 25:698–714. doi: 10.2174/0929867324666171003123425
21. Wang J, Zhang M, Hu X, She J, Sun R, Qin S, et al. miRNA-194 predicts favorable prognosis in gastric cancer and inhibits gastric cancer cell growth by targeting CCND1. FEBS Open Bio. (2021) 11:1814–26. doi: 10.1002/2211-5463.13125
22. Park SE, Kim HS, Jung E-J, Suh JH, Min H, Chi K-C, et al. Low PARP-1 expression level is an indicator of poor prognosis in patients with stage II and III gastric cancer. J Cancer. (2022) 13:869–76. doi: 10.7150/jca.65145
23. Vietri MT, D’Elia G, Caliendo G, Resse M, Casamassimi A, Passariello L, et al. Hereditary prostate cancer: genes related, target therapy and prevention. Int J OF Mol Sci. (2021) 22. doi: 10.3390/ijms22073753
24. Moller P, Seppala TT, Bernstein I, Holinski-Feder E, Sala P, Gareth Evans D, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. GUT. (2018) 67:1306–16. doi: 10.1136/gutjnl-2017-314057
25. Usui Y, Taniyama Y, Endo M, Koyanagi YN, Kasugai Y, Oze I, et al. Helicobacter pylori, homologous-recombination genes, and gastric cancer. New Engl J OF Med. (2023) 388:1181–90. doi: 10.1056/NEJMoa2211807
26. Wang D, Zhou J, Wang T, Li X, Li S, Chen S, et al. Polymorphisms in MSH2 gene and risk of gastric cancer, and interactions with lifestyle factors in a Chinese population. Cancer Epidemiol. (2012) 36:e171–6. doi: 10.1016/j.canep.2012.02.003
27. Zhao X, Dai D, Li X, Shen B, Chen X, Shu Y, et al. A polymorphism within the mismatch repair gene predicts prognosis and adjuvant chemotherapy benefit in gastric cancer. Gastric Cancer. (2019) 22:1121–9. doi: 10.1007/s10120-019-00962-8
28. Xu M, Qin S, Cao F, Ding S, Li M. MicroRNA-379 inhibits metastasis and epithelial-mesenchymal transition via targeting FAK/AKT signaling in gastric cancer. Int J OF Oncol. (2017) 51:867–76. doi: 10.3892/ijo.2017.4072
29. Gao W, Cao Y, Guo P, Bao X, Zhu H, Zheng J, et al. Downregulation of MiR-1297 predicts poor prognosis and enhances gastric cancer cell growth by targeting CREB1. BIOMED PHARMACOTHER. (2018) 105:413–9. doi: 10.1016/j.biopha.2018.05.094
30. Pan L, Shi Y, Zhang J, Luo G. Association between single nucleotide polymorphisms of miRNAs and gastric cancer: A scoping review. Genet Testing Mol Biomarkers. (2022) 26:459–67. doi: 10.1089/gtmb.2021.0258
31. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Gastroenterol Review-Przeglad Gastroenterologiczny. (2019) 14:26–38. doi: 10.5114/pg.2018.80001
32. Rong G, Zhang Y, Ma Y, Chen S, Wang Y. The clinical and molecular characterization of gastric cancer patients in qinghai-tibetan plateau. Front Oncol. (2020) 10:1033. doi: 10.3389/fonc.2020.01033
33. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discovery. (2017) 16:203–22. doi: 10.1038/nrd.2016.246
Keywords: miRNA, single nucleotide polymorphism, prognosis, gastric cancer, rs17502941
Citation: Wu P, Zhang Y, Lyu Y, Chen J, Jiang Y, Xiang J, Liu B and Wu C (2024) MiRNA polymorphisms affect the prognosis of gastric cancer: insights from Xianyou, Fujian. Front. Oncol. 14:1355270. doi: 10.3389/fonc.2024.1355270
Received: 13 December 2023; Accepted: 22 April 2024;
Published: 15 May 2024.
Edited by:
Prasanna K. Santhekadur, JSS Academy of Higher Education and Research, IndiaReviewed by:
Fang Guo, Northern Theater Command General Hospital, ChinaCopyright © 2024 Wu, Zhang, Lyu, Chen, Jiang, Xiang, Liu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuancheng Wu, d2NjQGZqbXUuZWR1LmNu; Baoying Liu, bGJ5QG1haWwuZmptdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.