
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 07 June 2024
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1353943
This article is part of the Research TopicManagement of Rare Oncological CasesView all 62 articles
 Katarzyna Stawarz1*
Katarzyna Stawarz1* Adam Galazka1
Adam Galazka1 Anna Gorzelnik1
Anna Gorzelnik1 Monika Durzynska2
Monika Durzynska2 Karolina Bienkowska-Pluta1
Karolina Bienkowska-Pluta1 Jakub Zwolinski1
Jakub Zwolinski1Introduction: Extramedullary plasmacytoma (EMP) is an uncommon solitary tumor originating from neoplastic plasma cells located outside the bone marrow. Despite its rarity, the occurrence of EMP without a concurrent diagnosis of multiple myeloma (MM) is considered extremely rare. Approximately 80–90% of EMP cases are found in the head and neck region, with a higher incidence in men aged between 50 and 60 years. The current treatment modalities include radiotherapy (RT) as a first-line approach, with surgery or chemotherapy regarded as other therapeutic options. While RT proves effective in the majority of EMP cases, there are instances where the tumor remains refractory to radiation. In this case report, we present an unusual scenario of EMP resistant to RT without concurrent signs of multiple myeloma which was successfully treated with surgery followed by systemic therapy.
Case report: A 72-year-old male was admitted to the Head and Neck Cancer Clinic with a 6-month history of swallowing difficulties. He denied experiencing weight loss or pain on swallowing. Basic laboratory tests yielded results within normal limits, except for beta-2 microglobulin. Physical examination revealed an enlarged submandibular lymph node on the right side. Fiberoptic examination identified a soft tissue polypoid mass within the right piriform fossa, slightly protruding into the vocal slit. A CT scan displayed a well-circumscribed 2 cm polypoid, homogeneously enhancing soft tissue mass adjacent to the posterior surface of the epiglottis and the right side of the tongue base. Bone marrow biopsy revealed no abnormalities, and there were no clinical or laboratory signs of multiple myeloma. Based on the tumor biopsy results and imaging studies, a diagnosis of EMP was made. Due to the lack of response to RT, surgical removal of the tumor was pursued, followed by systemic therapy. Ultimately, the patient achieved full recovery with effective disease control.
Conclusion: In conclusion, EMP without concurrent multiple myeloma is an exceedingly rare condition that demands a multidisciplinary approach for both diagnosis and treatment. Moreover, although RT continues to be the primary standard treatment for EMP, in some cases other therapeutic regimens prove to be successful.
Extramedullary plasmacytoma (EMP) is a rare solitary tumor originating from neoplastic plasma cells located outside the bone marrow. It does not involve the bone marrow and can manifest anywhere in the body, with a predilection for soft tissues, particularly in the head and neck region (1, 2). EMP accounts for approximately one-third of solitary plasmacytoma tumors, constituting up to 5% of all plasma cell malignancies (3). The upper respiratory and digestive tracts are the most common sites for EMP, representing 80–90% of cases (4). This slow-growing submucosal malignancy typically presents its initial symptoms related to the mass effect, resulting in the obstruction of local structures and causing dysphagia, odynophagia, or voice problems. Male patients are more frequently affected, with an average age at diagnosis in their fifth to sixth decade of life (5). While EMP is regarded as an uncommon malignancy which can sometimes progresses to multiple myeloma, the occurrence of EMP without multiple myeloma remain exceedingly rare (6). The diagnosis of EMP is established through tumor and bone marrow biopsies, as well as laboratory tests and imaging studies (7). Although radiotherapy (RT) is the currently recommended treatment option due to the tumor’s radiosensitivity and excellent disease control, its efficacy may be insufficient in certain cases (8). Moreover, the challenging location of the tumor underscores the importance of individualizing the optimal therapeutic approach. In instances of resistance to RT, surgery and chemotherapy remain viable therapeutic alternatives that have proven effective in some patients (9). In this case scenario we described a patient with the EMP resistant to RT with no signs of multiple myeloma which was successfully treated with surgery and systemic therapy.
A 72-year-old male of Polish origin was admitted to the Head and Neck Cancer Clinic at the National Institute of Oncology in Poland with a 6-month history of swallowing problems. He denied weight loss but admitted occasional swallowing difficulties, with no reported hoarseness or pain on swallowing. His past medical history was notable for hypertension, and there was no family history of neoplastic, genetic, or metabolic disorders. The patient denied cigarette smoking or alcohol use disorder. On presentation, his vital signs were within normal limits (HR: 87, RR: 132/87 mm Hg, temperature: 36.6°C). Physical examination revealed no abnormalities in the mouth or nasal cavities, but an enlarged submandibular lymph node was palpated on the right side of the neck. Indirect laryngoscopy and fiberoptic exams revealed a round 2 cm, smooth polypoid mass protruding from the right piriform fossa, partially occluding the vocal slit during phonation (Figures 1A, B). Laboratory tests showed Hb 13 g/dL, calcium level of 10.5 mg/dL, free kappa chains of 18.30 mg/L, free lambda chains of 15.90 mg/L, with a Kappa/Lambda ratio of 1.15. Additionally, normal serum BUN/creatinine, sodium, potassium, albumin, and LDH levels were detected. The patient underwent a whole body CT scan, and based on the laboratory results, a hematological and oncological consultation was ordered to rule out lymphoma, multiple myeloma, or other hematological malignancies. Further tests included serum quantitative immunoglobulins (IgM: 42.3 mg/dL, IgG: 796 mg/dL, IgA: 245 mg/dL), serum protein electrophoresis, monoclonal protein immunofixation (all normal), and a slightly elevated beta-2-microglobulin level of about 3.33 mg/L. No Bence-Jones protein was detected in the 24-hour urine collection. The CT scan revealed a well-circumscribed polypoid homogeneously enhancing soft tissue mass adjacent to the posterior surface of the epiglottis and the right side of the tongue base, approximately measuring 17x11mm x 23mm. An enlarged lymph node posterior to the right submandibular gland, reaching about 23x20mm, was also noted. Subsequently, the patient underwent an open biopsy procedure of the tumor, along with a bone marrow biopsy. The tumor pathological findings confirmed the diagnosis of EMP (Figures 2A–F). Additionally, since the patient showed no signs of multiple myeloma, and the bone marrow biopsy yielded normal results, the diagnosis of multiple myeloma was excluded. Moreover, the histopathological findings helped rule out diagnoses such as lymphoma, sarcoma, or squamous cell carcinoma (SCC).Given the histopathological, laboratory, and imaging findings, the patient was scheduled for a re-consultation with a hematologic specialist and radiotherapist to initiate treatment. Subsequently, the patient underwent intensity-modulated radiation therapy (IMRT) with a total dose of 42 Gy over the next 4 weeks, comprising five sessions per week. Nevertheless, the RT turned out to be unsuccessful with no evidence of a tumor decrease on fiberoptic exam upon therapy completion. Due to the unsuccessful response to the radiotherapy, the patient underwent a PET/CT scan and was scheduled for surgical tumor removal. Whole-body PET/CT images displayed an increased FDG uptake in a soft tissue density lesion in the right throat originating from the right piriform fossa, as well as in the right cervical lymph node of IIA group, with no abnormal FDG avid uptake elsewhere suggesting a distant lesion. The maximum standardized uptake value of the tumor was 7.1 (Figures 3A, B). The surgical procedure was conducted under general anesthesia, with the use of a laryngoscope. The tumor was visualized and partially removed using microlaryngeal scissors and bipolar coagulation, as complete resection was not feasible, and only a debulking approach was applied (Figures 4A, B). Post-surgery, the patient underwent a follow-up visit after two weeks, during which a fiberoptic exam revealed a residual tumor mass (Figures 4C, D). Following this, the patient was referred for a hematological and oncological consultation to proceed with a chemotherapeutic regimen of dexamethasone and bortezomib, but due to severe side effects including numerous bleeding episodes, anemia and dizziness, and the absence of a MM diagnosis, chemotherapy was discontinued after one course. The therapeutic regimen comprised subcutaneous injections of bortezomib at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 of a 21-day treatment cycle, along with dexamethasone administered at 20 mg on the day of and the day following bortezomib injection. Regular follow-up included fiberoptic exams every 3 months during the first year, MRI every 6 months, and a PET scan once per year. It has been 18 months since the diagnosis of EMP, and the patient remains under observation at the National Institute of Oncology with no signs of multiple myeloma or tumor recurrence detected thus far. Despite initially feeling overwhelmed by the diagnosis and the lack of response to radiotherapy, the patient remained calm and optimistic about recovery. Eventually, his convalescence was uneventful, with no tumor recurrence observed after the surgical and chemotherapeutic approach.
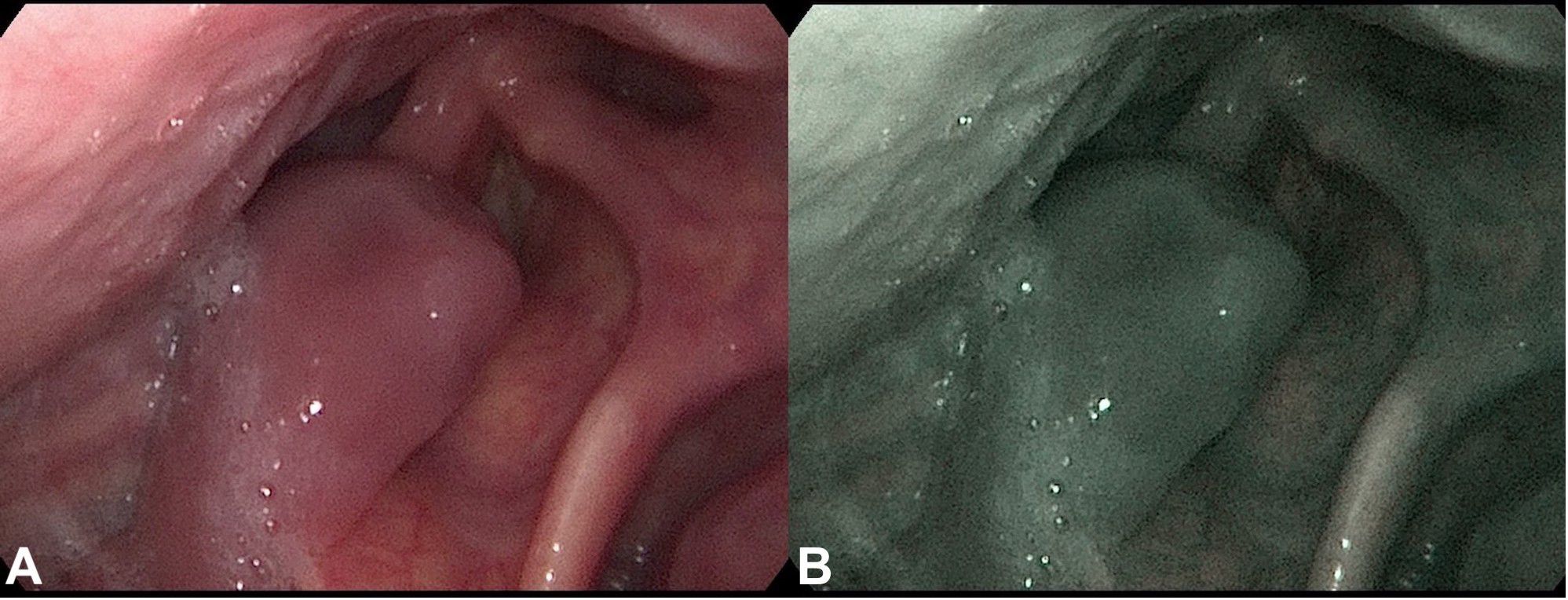
Figure 1 Fiberoptic examination of the throat tumor. Fiberoptic exam displaying a polypoid throat protruding from the right piriform fossa, partially occluding the vocal slit during phonation. (A) Conventional white-light image. (B) NBI (Narrow Band Imaging) image of the same site.
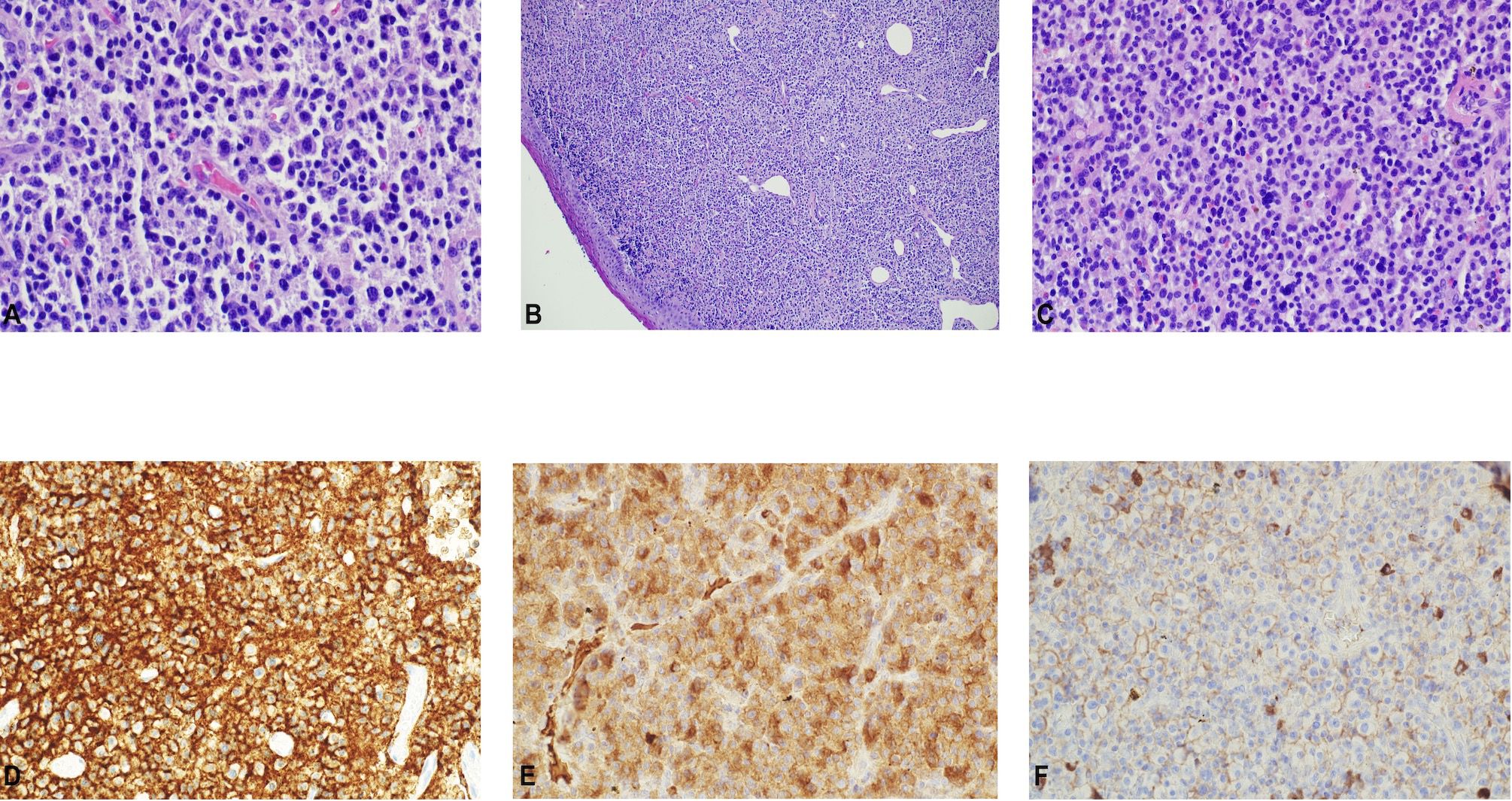
Figure 2 Histopathological assessment of the tumor. (A–C). Throat tumor biopsy showing sheets of plasma cells. H&E. (x 400). Immunohistochemistry displaying positivity for (D) CD138, (E) lamda, and the (F) absence of kappa light chain expression.
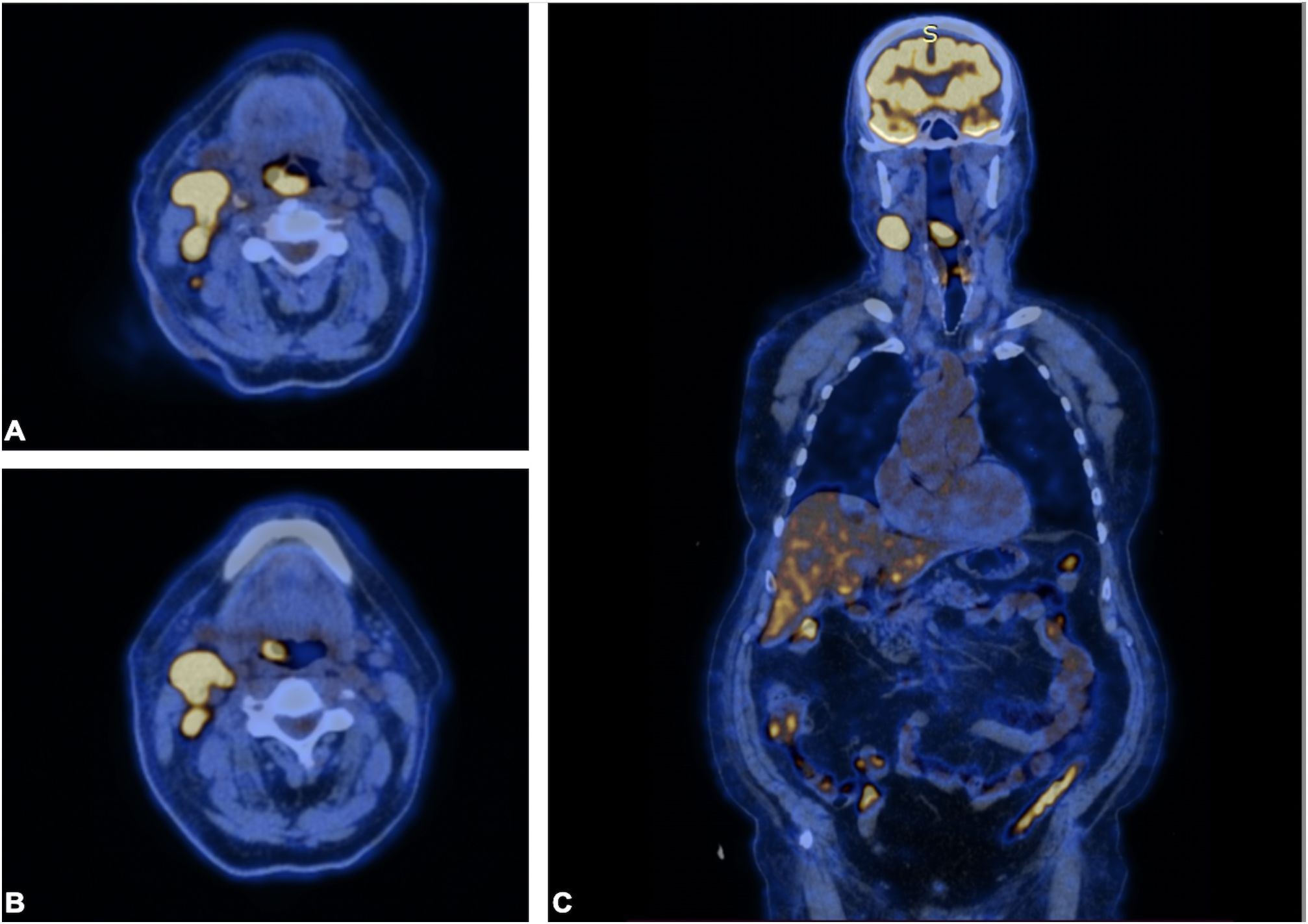
Figure 3 PET scan. PET scan showing increased FDG uptake in a (A, B) soft tissue density lesion in the right throat originating from the right piriform fossa (horizontal section) and in the (C) right cervical lymph node of IIA group (coronal section). Note that no abnormal FDG avid uptake was observed elsewhere, indicating the absence of a distant lesion.
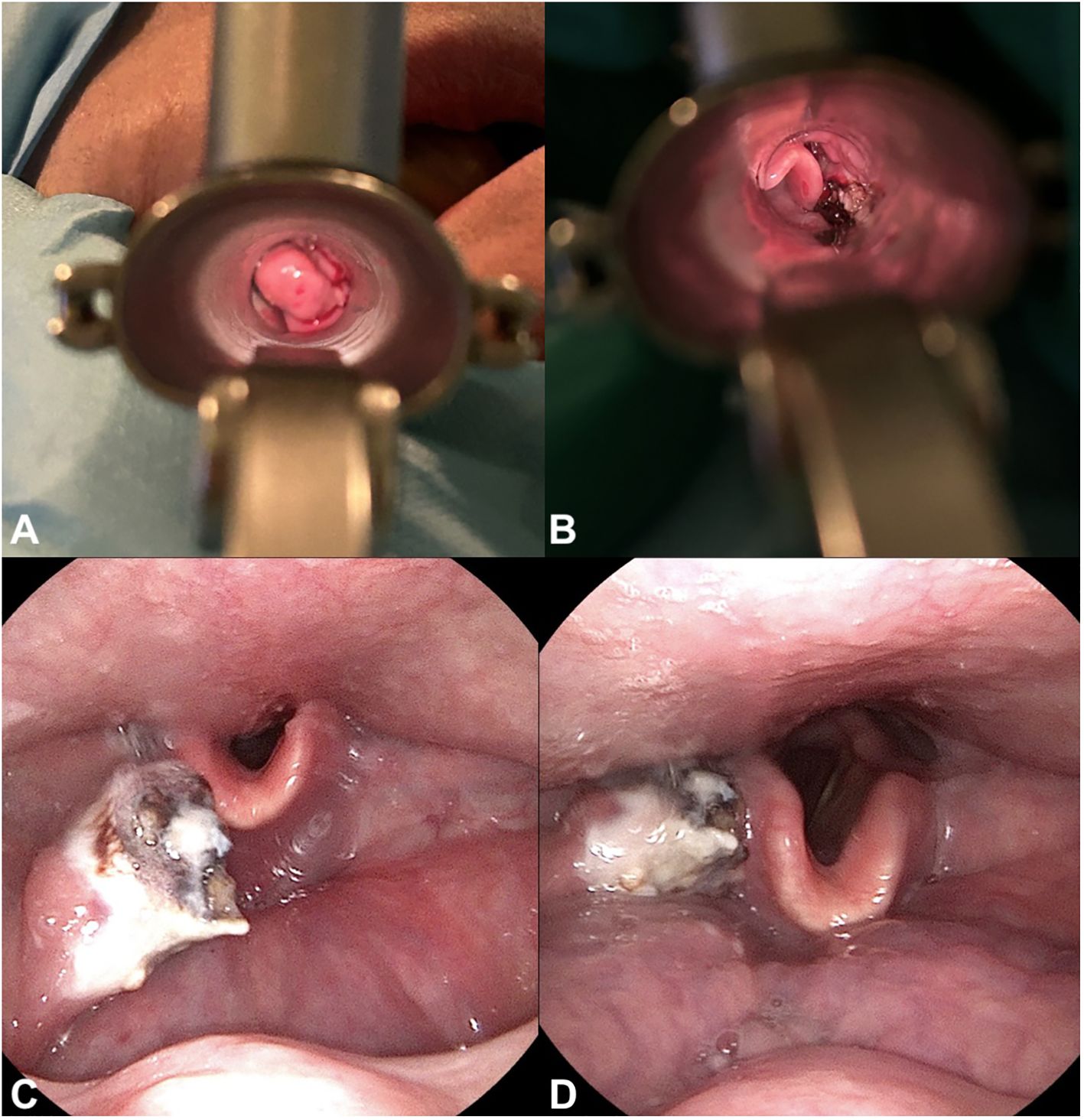
Figure 4 Surgical procedure and post-surgical fiberoptic exam. Visualization of the tumor through a laryngoscope, shown (A) during the surgical removal of the tumor and (B) post-removal. (C, D). Fiberoptic examination post-surgery revealing residual tumor tissue.
In this case scenario, we present a rare example of EMP without a concomitant diagnosis of multiple myeloma. EMP is an uncommon malignancy originating from plasma cells and can manifest in soft tissues throughout the body. Typically, EMP is characterized by a slow-growing submucosal tumor within the respiratory and digestive tract, with the head and neck region being the most common location (6, 10). Predominantly observed in nasal cavities, paranasal sinuses, nasopharynx, oropharynx, and larynx, EMP’s symptoms are location-dependent and primarily associated with the mass effect, leading to issues such as swallowing difficulties, breathing problems, epistaxis, or nasal obstruction (6, 11). EMP does not affect the bone marrow but can progress to multiple myeloma (MM) (12). Two forms of EMP are recognized: primary and secondary. Primary EMP denotes tumors present without any other plasma cell malignancies, such as multiple myeloma or monoclonal gammopathy. On the other hand, secondary EMP occurs in the presence of other plasma cell dyscrasias, most commonly MM (8). To date, numerous cases of EMP without MM have been reported in the literature, with several cases presenting the tumor in the laryngopharynx location (13–17). Given its rarity, a high index of suspicion is required for an accurate diagnosis, necessitating tumor and bone marrow biopsies to assess the level of monoclonal plasma cells (7). Histopathological examination of the tumor tissue reveals a dense infiltrate of plasmablastic or anaplastic plasma cells, which may be immature or mature (18). However, the similarities in histopathological features and the high pleomorphism of plasma cells may lead to confusion with aggressive lymphoma, rhabdomyosarcoma, or other sarcomas (19). Specifically, EMP should be distinguished from plasmablastic lymphoma, which exhibits overlapping features with myeloma and lymphoma. Plasmablastic lymphoma comprises B-cells, lymphoplasmablastic cells, and monoclonal cells with an immunohistochemical profile that typically includes CD20 expression, but not CD138 and is often associated with Epstein-Barr virus (EBV) infection (20). It is a highly aggressive lymphoma observed in immunocompromised patients, primarily affecting the oral cavity and gastrointestinal tract (17). Conversely, EMP may also be occasionally confused with angiosarcoma. However, angiosarcoma, consisting of atypical endothelial cells that may resemble plasmocytes, typically induces the destruction of surrounding tissues (21). Additionally, an extremely rare anaplastic MM may occasionally mimic EMP. Originating from immature plasma cells and presenting with extramedullary tumors, it leads to bone marrow invasion and osseous lytic lesions, which are not observed in EMP (22). Another condition that can sometimes mimic EMP is SCC, which is the most common malignancy in the head and neck region (23). Despite the similarity in location, EMP typically presents with localized growth, while SCC is more aggressive with a higher potential for metastasis. Additionally, poorly differentiated SCC cells may sometimes test positive for CD138 or plasma cell markers, making it crucial to use a comprehensive panel of antibodies to differentiate between carcinoma and EMP (24). On the other hand, large B-cell lymphoma (LBCL) cells, while they may share similar microscopic characteristics with EMP, typically express CD19, CD20, CD79a, and PAX5, but are negative for plasma cell markers such as CD138 and CD38 (25). Additionally, LBCL typically presents as a rapidly growing mass with extranodal involvement in over 50% of cases with stomach or gastrointestinal tract most commonly affected (26).
Furthermore, for adequate locoregional staging of EMP, a CT scan or MRI is essential. However, a PET scan appears to be the preferred imaging study for ruling out MM or other hematologic malignancies. The diagnostic approach should encompass the following evaluation criteria: (i) biopsy of a solitary lesion of bone or soft tissue with evidence of clonal plasma cells, (ii) bone marrow biopsy within normal limits with no clonal plasma cells, (iii) no abnormalities in the skeletal survey displayed on MRI (or CT) of the spine and pelvis (except for the primary solitary lesion), (iv) no evidence of hypercalcemia, anemia, renal insufficiency, or bone lesions (CRAB) caused by a lymphoplasmacytic cell proliferative disorder (27).The rarity of EMP and its prolonged course make it challenging to determine the optimal treatment strategy. In the presented case study a radiotherapeutic regimen was used as a primary approach as it is the preferred first-line treatment of EMP due to the tumor’s radiosensitivity (28). This approach is considered feasible, particularly when the location of the EMP presents challenges for surgical intervention, such as proximity to vital and sensory organs. According to the literature, RT is typically administered over a 4-week period with a total dose ranging from 40 to 50 Gy (29). In the majority of EMP cases, radiotherapy is effective, demonstrating excellent disease control and a low risk of recurrence (30). Given the radiosensitive nature of EMP, RTH remains the treatment of choice. However, in cases refractory to radiotherapy, other therapeutic approaches need to be employed. Depending on the tumor location and the proximity of critical structures, radical surgical resection may not be feasible. Therefore, additional treatment modalities are required. In a case report described by Karakullukcu et al., surgical debulking combined with photodynamic therapy was employed to manage residual EMP tumor resulting in a successful outcome (31). Another therapeutic approach presented in the available literature involved bronchoscopic electrocautery snare combined with CO2 cryotherapy, followed by RTH for EMP located in the trachea (32). In the presented case report, a surgical debulking approach was employed because radical resection was not feasible. Consequently, due to the risk of progression to MM, lack of response to RTH and the residual tumor, the patient underwent a subsequent chemotherapeutic regimen of dexamethasone and bortezomib. Nevertheless, existing literature suggests that the majority of cases of EMP respond well to radiotherapy, and the other treatment options should be implemented in case of radiotherapy resistance. The current publications confirm positive outcomes with surgery and/or systemic therapy, including bortezomib or cyclophosphamide alone or in combination with vincristine and prednisolone (33). In their case report on gastric EMP, Katodritou et al. were the first to present dexamethasone and bortezomib as an effective treatment approach for managing EMP without associated multiple myeloma (34). Similarly, in a case report by Wei et al., pancreatic EMP was successfully treated with a combination of bortezomib and dexamethasone after the tumor failed to respond to the traditional chemotherapy regimen of vincristine, doxorubicin, and dexamethasone (the VAD regimen) (35). Moreover, certain immunomodulatory agents, such as daratumumab, have also been found to be effective in EMP patients (36). However, the potential toxicity of chemotherapy, including risks of myelosuppression, neuropathy, and organ toxicity, are significant drawbacks that can limit its application. Decisions regarding chemotherapy should consider factors like the patient’s overall health, existing comorbidities, potential drug interactions, and the severity or progression of the disease. In our patient, poor tolerance to chemotherapy and the absence of signs of MM led to the discontinuation of the chemotherapy regimen after just one course. Moreover, it appears that the resistance to radiotherapy in EMP tumors requires further molecular research to assess the likelihood of the radiotherapy efficacy. As such, this rare case study of EMP without multiple myeloma diagnosis proves the rarity of this malignancy as well as shed some light on its possible resistance to radiotherapy. Although the patient described in this manuscript achieved recovery with no evidence of tumor recurrence, he experienced side effects of initial radiotherapy, including xerostomia and radiation-induced skin reactions. A significant limitation of this case report is the inability to generalize these findings and to establish causality. However, despite these limitations, this case report highlights a rare and unusual case of EMP that is refractory to radiotherapy, underscoring the importance of a personalized medicine approach and raising awareness among healthcare professionals about EMP. Though, the presented case underscores the need for further research on a larger group of patients with EMP to identify the most effective therapeutic approach. This case report underscores the exceptionally rare form of EMP without MM, further compounded by its resistance to radiotherapy, rendering it an even more unique case within the EMP spectrum. A high degree of clinical suspicion is necessary for diagnosing EMP. Namely, counseling patients with EMP demands a considerate and patient-centered approach, considering the rarity of the condition and its potential for progression to MM. While SCC remains the most common malignancy within the head and neck region, the diagnosis of EMP should always be considered in a patient with a tumor in this region. Consequently, there is an imperative for clinicians to maintain a high index of suspicion when assessing EMP patients and determining the most appropriate treatment plan.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
KS: Writing – review & editing. AGa: Writing – review & editing, Investigation. AGo: Writing – review & editing, Supervision. MD: Writing – review & editing, Supervision, Investigation. KB-P: Writing – review & editing, Investigation. JZ: Writing – review & editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was funded by Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1353943/full#supplementary-material
CRAB, (C) calcium elevation, (R) renal insufficiency, (A) anemia, (B) bone abnormalities; CT, computed tomography; EBV, Epstein Barr virus EMP, extramedullary plasmacytoma; Gy, Grey; HR, heart rate; IMRT, intensity-modulated radiation therapy; LBCL, large B-cell lymphoma; MM, multiple myeloma; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; RR, respiratory rate, RT, radiotherapy; SCC, squamous cell carcinoma.
1. Alexiou C, Kau RJ, Dietzfelbinger H, Kremer M, Spiess JC, Schratzenstaller B, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. (1999) 85:2305–14. doi: 10.1002/(ISSN)1097-0142
2. Reed V, Shah J, Medeiros LJ, Ha CS, Mazloom A, Weber DM, et al. Solitary plasmacytomas: outcome and prognostic factors after definitive radiation therapy. Cancer. (2011) 117:4468–74. doi: 10.1002/cncr.26031
3. Barzenje DA, Kolstad A, Ghanima W, Holte H. Long-term outcome of patients with solitary plasmacytoma treated with radiotherapy: a population-based, single-center study with median follow-up of 13.7 years. Hematol Oncol. (2018) 36:217–23. doi: 10.1002/hon.2415
4. Castro EB, Lewis JS, Strong EW. Plasmacytoma of paranasal sinuses and nasal cavity. Arch Otolaryngol. (1973) 97:326–9. doi: 10.1001/archotol.1973.00780010336009
5. Merino F, Hernández IP, Aniceto GSánchez, Ballestín C. Extramedullary plasmacytoma in the maxillofacial region: A review of the literature and case report. Oral Maxillofac Surg cases. (2018) 4:141–5. doi: 10.1016/j.omsc.2018.07.004
6. Liebross RH, Ha CS, Cox JD, Weber D, Delasalle K, Alexanian R. Clinical course of solitary extramedullary plasmacytoma. Radiother Oncol. (1999) 52:245–9. doi: 10.1016/S0167-8140(99)00114-0
7. Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, Kristinsson SY, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. (2018) 11:10. doi: 10.1186/s13045-017-0549-1
8. Holler A, Cicha I, Eckstein M, Haderlein M, Pöttler M, Rappl A, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts—a follow-up. Cancer Med. (2022) 11:4743–55. doi: 10.1002/cam4.4816
9. Pham A, Mahindra A. Solitary plasmacytoma: a review of diagnosis and management. Curr Hematol Malig Rep. (2019) 14:63–9. doi: 10.1007/s11899-019-00499-8
10. Granato L, Petitto JW, Prospero JD. Plasmocitoma extramedular do aparelho respiratório Apresentação do caso. Rev Bras Otorrinolaringol. (1977) 43:214–23.
11. Majumdar S, Raghavan U, Jones NS. Solitary plasmacytoma and extramedullary plasmacytoma of the paranasal sinuses and soft palate. J Laryngol Otol. (2002) 116:962–5. doi: 10.1258/00222150260369561
12. Hughes M, Doig A, Soutar R. Solitary plasmacytoma and multiple myeloma: adhesion molecule and chemokine receptor expression patterns. Br J Haematol. (2007) 137:486. doi: 10.1111/j.1365-2141.2007.06599.x
13. Padhi P, El-Behery R. Extramedullary solitary plasmacytoma with anaplastic features of the nasopharynx. Case Rep Hematol. (2020) 2020:8845546–4. doi: 10.1155/2020/8845546
14. Raghuram S, Faizal B, Sanjeevan KV, Eapen M, Nair IR, Philip A, et al. Recurrent extramedullary plasmacytomas without multiple myeloma: A case report with review of the literature. Cancer Treat Res Commun. (2022) 31(2022):100550. doi: 10.1016/j.ctarc.2022.100550
15. Szczepanek E, Drozd-Sokołowska J, Sokołowski J, Rzepakowska A, Moskwa A, Pachla J, et al. Solitary extramedullary plasmacytoma of the larynx and secondary laryngeal involvement in plasma cell myeloma: single-centre retrospective analysis and systematic literature review. J Clin Med. (2022) 11:4390. doi: 10.3390/jcm11154390
16. Mokhtari TE, Sadow PM, Naunheim MR. Extramedullary plasmacytoma of the larynx. Mayo Clin Proc. (2024) 99:13–4. doi: 10.1016/j.mayocp.2023.07.001
17. Tanrivermis Sayit A, Elmali M, Gün S. Evaluation of extramedullary plasmacytoma of the larynx with radiologic and histopathological findings. Radiologia (Engl Ed). (2020) 22:S0033–8338(20)30121–1. doi: 10.1016/j.rx.2020.07.006
18. Torne R, Su WP, Winkelmann RK, Smolle J, Kerl H. Clinicopathologic study of cutaneous plasmacytoma. Int J Dermatol. (1990) 29:562–6. doi: 10.1111/j.1365-4362.1990.tb03469.x
19. Jacevičiūtė E, Rudžianskienė M, Dambrauskienė R, Vajauskas D, Gerbutavičius R. Anaplastic extramedullary plasmacytoma resistant to novel therapies: a case report. Oncologie (De Gruyter). (2023) 25:327–32. doi: 10.1515/oncologie-2023-0054
20. Zuze T, Painschab MS, Seguin R, Kudowa E, Kaimila B, Kasonkanji E, et al. Plasmablastic lymphoma in Malawi. Infect Agent Cancer. (2018) 13:22. doi: 10.1186/s13027-018-0195-4
21. Gaballah AH, Jensen CT, Palmquist S, Pickhardt PJ, Duran A, Broering G, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. (2017) 90:20170039. doi: 10.1259/bjr.20170039
22. Wu J, Chu E, Chase CC, Choi T, Gasparetto C, Young K, et al. Anaplastic multiple myeloma: case series and literature review. Asploro J BioMed Clin Case Rep. (2022) 5:1–11. doi: 10.36502/asjbccr
23. Barsouk A, Aluru JS, Rawla P, Saginala K, Barsouk A. Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med Sci (Basel). (2023) 11:42. doi: 10.3390/medsci11020042
24. Zuo Z, Tang Y, Bi CF, Zhang WY, Zhao S, Wang XQ, et al. Extraosseous (extramedullary) plasmacytomas: a clinicopathologic and immunophenotypic study of 32 Chinese cases. Diagn Pathol. (2011) 6:123. doi: 10.1186/1746–1596-6–12323
25. Fan J, Thanedar S, Ovechko V, Jana B. Diffuse large B-cell lymphoma with aberrant coexpression of CD5 and CD8 T-cell markers. Case Rep Oncol. (2024) 17:517–23. doi: 10.1159/000536571
26. Conlan MG, Bast M, Armitage JO, Weisenburger DD. Bone marrow involvement by non-Hodgkin's lymphoma: the clinical significance of morphologic discordance between the lymph node and bone marrow. Nebraska Lymphoma Study Group J Clin Oncol. (1990) 8:1163–72. doi: 10.1200/JCO.1990.8.7.1163
27. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. (2014) 15:e538–48. doi: 10.1016/S1470–2045(14)70442–5
28. Kilciksiz S, Celik OK, Pak Y, Demiral AN, Pehlivan M, Orhan O, et al. Clinical and prognostic features of plasmacytomas: a multicenter study of Turkish Oncology Group-Sarcoma Working Party. Am J Hematol. (2008) 83:702–7. doi: 10.1002/ajh.21211
29. Wen G, Wang W, Zhang Y, Niu S, Li Q, Li Y. Management of extramedullary plasmacytoma: Role of radiotherapy and prognostic factor analysis in 55 patients. Chin J Cancer Res. (2017) 29:438–46. doi: 10.21147/j.issn.1000-9604.2017.05.08
30. Gaube A, Nica SG, Dobrea C, Neicu SA, Moldoveanu VG, Serbanescu M, et al. Radiation response of soft-tissue extramedullary plasmacytoma in multiple myeloma-A case report. Clin Case Rep. (2021) 9:e05084. doi: 10.1002/ccr3.5084
31. Karakullukcu B, De Boer JP, Van Veen R, Wegman J, Tan B. Surgical debulking combined with photodynamic therapy to manage residual extramedullary plasmacytoma of the nasopharynx. Photodiagnosis Photodyn Ther. (2011) 8:264–6. doi: 10.1016/j.pdpdt.2011.03.340
32. Zhang X, Su L, Ran YG, Qie S, Zhang X, Liu C, et al. Extramedullary plasmacytoma of the trachea: A case report and review of the literature. Med (Baltimore). (2018) 97:e9594. doi: 10.1097/MD.0000000000009594
33. Wiltshaw E. Chemotherapy in the management of extramedullary plasmacytoma. Cancer Chemother Pharmacol. (1978) 1:167–75. doi: 10.1007/BF00253117
34. Katodritou E, Kartsios C, Gastari V, Verrou E, Mihou D, Banti A, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. (2008) 32:339–41. doi: 10.1016/j.leukres.2007.04.016
35. Wei JY, Tong HY, Zhu WF, Liu H, Zhang FJ, Yu WJ, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. (2009) 8:329–31.
Keywords: extramedullary plasmacytoma, multiple myeloma, case report, radiotherapy, surgery
Citation: Stawarz K, Galazka A, Gorzelnik A, Durzynska M, Bienkowska-Pluta K and Zwolinski J (2024) Case report: An uncommon presentation of extramedullary plasmacytoma without a concurrent diagnosis of multiple myeloma. Front. Oncol. 14:1353943. doi: 10.3389/fonc.2024.1353943
Received: 11 December 2023; Accepted: 20 May 2024;
Published: 07 June 2024.
Edited by:
Lidia Castagneto Gissey, Sapienza University of Rome, ItalyReviewed by:
Joselle Cook, Mayo Clinic, United StatesCopyright © 2024 Stawarz, Galazka, Gorzelnik, Durzynska, Bienkowska-Pluta and Zwolinski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katarzyna Stawarz, a2F0YXJ6eW5hLnN0YXdhcnpAY29pLnBs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.