- 1Public Health Department, Yanet-Liyana College of Health Sciences, Hawassa, Ethiopia
- 2School of Public Health, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Background: The incidence of breast cancer (BC) is rampantly increasing in developing countries particularly Ethiopia. Unfortunately, the morbidity and mortality rates are sharply increasing, and because of this, families are suffering from socioeconomic crises. Despite this, there is limited evidence on the determinants of breast cancer in Ethiopia as well as in the study area.
Objective: To identify the determinants of BC among women attending oncology units in selected hospitals in Hawassa City, Ethiopia, in 2023.
Method: A hospital-based, case-control study with 300 patients (75 cases and 225 controls) was carried out in Hawassa from June to July 2023. A simple random sampling technique was used to select cases and controls. Data were collected via pretested and structured digitally installed questionnaires with Kobo collection/smartphones. The data were exported from the server to SPSS version 27 for analysis. Descriptive analysis of univariate, bivariate, and multivariable logistic regression data was conducted to determine the associations between breast cancer incidence and independent factors.
Results: A total of 300 women participated in this study for a response rate of 100%. The mean ( ± SD) ages of the respondents were 37.2 ( ± 14.8) and 36.6 ( ± 15.1) years for the cases and controls, respectively. According to the multivariate logistic regression model, postmenopausal status [AOR: 2.49; 95% CI (1.18, 5.23)], family history of cancer [AOR: 2.33; 95% CI (1.12, 4.82)], oral contraceptives [AOR: 2.74; 95% CI (1.34, 5.99)], overweight and/or obesity [AOR: 2.29; 95% CI: (1.14, 4.59)], and consumption of solid oil [AOR: 2.36; 95% CI (1.20, 4.67)] were independently associated with BC risk.
Conclusion: This study revealed important risk factors for BC. Therefore, women should adopt healthier lifestyles through healthy nutrition and regular exercise to reduce the risk of developing BC. In addition, early detection and regular screening are proactive approaches for detecting BC.
1 Introduction
Breast cancer (BC) is the most prevalent cancer in women globally accounting for 2.3 million new cases and one in six cancer deaths in 2020 (1). Globalization has led to a greater incidence of BC in high-income countries (571/100,000) than in low-income countries (95/10,000) (2). Due to globalization, risk factors for cancer are appearing with our modern diet, which emphasizes super grain and more processed food; the use of addictive substances, toxic and pharmaceutical products; and waste exposure. However, the prevention of cancer still depends on the recognition and elimination of risks from carcinogens (3). The five most prevalent nations were all in Europe. The number of new cases in Africa is estimated to have been 92,600 in 2008 and 133,900 in 2012 (1). In 2020, 2.3 million women were diagnosed with BC, and 685,000 women died from BC worldwide (4, 5).
In Africa, 8% of all BC cases are diagnosed, but the mortality rate is far higher than expected (1). Moreover, sub-Saharan Africa has the highest BC incidence and mortality rates attributed to Westernized lifestyles, food changes, and reduced physical activity among the African population (6). In 2020, there was significant geographical variation in the major cancers in sub-Saharan Africa (7).
In Ethiopia, BC is the most prevalent cancer accounting for 17.7% of cancer deaths and 22.6% of all cancer cases annually (8, 9). In addition, every year, approximately 60,000 new cases of BC are diagnosed. Similarly, evidence from the Addis Ababa cancer registry report shows that BC is responsible for 23% of all cancer cases and 33% of all cancer cases in women (10). Moreover, it is the most common malignancy in Ethiopia with increased rates of mortality and morbidity. For instance, of the 4,139 new cancer patients diagnosed between 2012 and 2013, 67% were female, and 31.5% had BC. The age-standardized incidence rate of BC was 43.3 per 100,000 (11) females, and a yearly average of 216 incident cases of BC were reported between 1997 and 2012 (11).
Multiple factors, such as alcohol consumption, tobacco use, obesity, inactivity, diet, family history, early menarche, late menopause, late age, null parity, and nonbreastfeeding practices, are known to increase the risk of BC (8). In contrast, lifestyle modifications, targeted prevention programs, and population-based screening can significantly reduce BC incidence (12).
Unfortunately, even if all of the potentially modifiable risk factors could be mitigated, this would only reduce the risk of developing BC by at most 30% (13). In addition, 20%–30% o BCs can be ascribed to controllable characteristics, and 5%–10% of BCs can be related to factors such as genetic mutations and family history (14). Thus, the healthcare system should be strengthened, and the gradual adoption of universal health coverage should be encouraged especially for low- and middle-income nations where cancer and noncommunicable disease programs are frequently inaccessible and limited-resource citizens are critical (10).
The World Health Organization launched the Global BC Initiative to address the increasing burden of BC, which is estimated to kill 685,000 women globally in 2020 (1). Similarly, the Federal Ministry of Health of Ethiopia (FMOHE) developed and amended a strategy to reduce risk factors and encourage a healthy lifestyle to prevent and control noncommunicable diseases (NCDs), including BC (15). On the other hand, the population of Ethiopia is diverse, representing a wide range of lifestyles, cultures, socioeconomic statuses, and breastfeeding practices. These elements may have an impact on overall health, which may include BC risk (15).
However, to the best of our knowledge, most of the existing studies are cross-sectional, and little is known about BC determination in Ethiopia or in the study area. Therefore, this study aimed to identify determinants of BC among women attending oncology units in the Sidama Region in selected hospitals.
2 Materials and methods
2.1 Study design
A hospital-based unmatched case-control study design was employed at Hawassa City from 1 June– to 30 July 2023.
2.2 Study area and period
The study was conducted in the public and private health facilities of Hawassa City. The Hawassa City Administration is located in Sidama Region, Ethiopia, approximately 275 km south of Addis Ababa. The city had a total of 385,257 populations with male dominance in number. Of the total population, 89,765 were females of reproductive age.
Cities are administrative structures with dense grid populations of more than 1,500 people per km2. Within a city, there are small units of administrative structure called subcities. Therefore, in Hawassa City, there are eight small administrative units composed of the smallest administrative units called kebeles. Based on the above explanation, Hawassa City had eight subcities (small administrative units), and each subcity had a different number of smallest administrative units called kebeles accounting for 32 kebeles. Of these smallest administrative units, 11 were rural and 21 were urban kebeles. Hawassa City also has 8 hospitals (3 public and 5 private), 12 public health centers, and 18 health posts. The three public hospitals are Hawassa University Comprehensive Specialized Hospital (HUCSH), Adare General Hospital (AGH), and Hawela Tula Primary Hospital (HTPH) (11). According to the 2022 Sidama Regional State Health Bureau report, a flow of approximately 177 patients per month is expected. The study was performed from 1 June to 30 July 2023.
2.3 Population
2.3.1 Source population and study population
All women who were more than 15 years old and who presented at Hawassa City health facilities were the source population. All women with confirmed BC visiting the selected health facilities for cases and all women without BC visiting the selected health facilities for other services during the study period composed the study population.
2.4 Inclusion and exclusion criteria
The patients were all women over the age of 15 years who had BC that had been confirmed and were receiving chemotherapy. The controls were all women over the age of 15 years who visited selected hospitals for other unrelated to cancer disease in the period of the study. However, women who were mentally incompetent or seriously ill during the course of data collection were excluded from both the case and control groups.
2.5 Sample size determination
Using the statistical software Epi-info version 7, the sample size for unmatched case-control studies was calculated. The following assumptions were used: 95% confidence level, 80% power, a case-to-control ratio of 1:3, a percentage of exposure among control-exposed women (i.e., the percentage of overweight and obese women without BC), and a percentage of exposure among cases (i.e., the percentage of obese women with BC).
The proportion of obese individuals among the controls was 25%, and the proportion of obese individuals among the patients was 31.9%, based on research performed in Addis Ababa, Ethiopia. Based on the above assumptions, the sample size was 272 (66 cases and 204 controls), and after adding a 10% nonresponse rate, the final sample size for the study was 300 (75 cases and 225 controls) (Table 1).
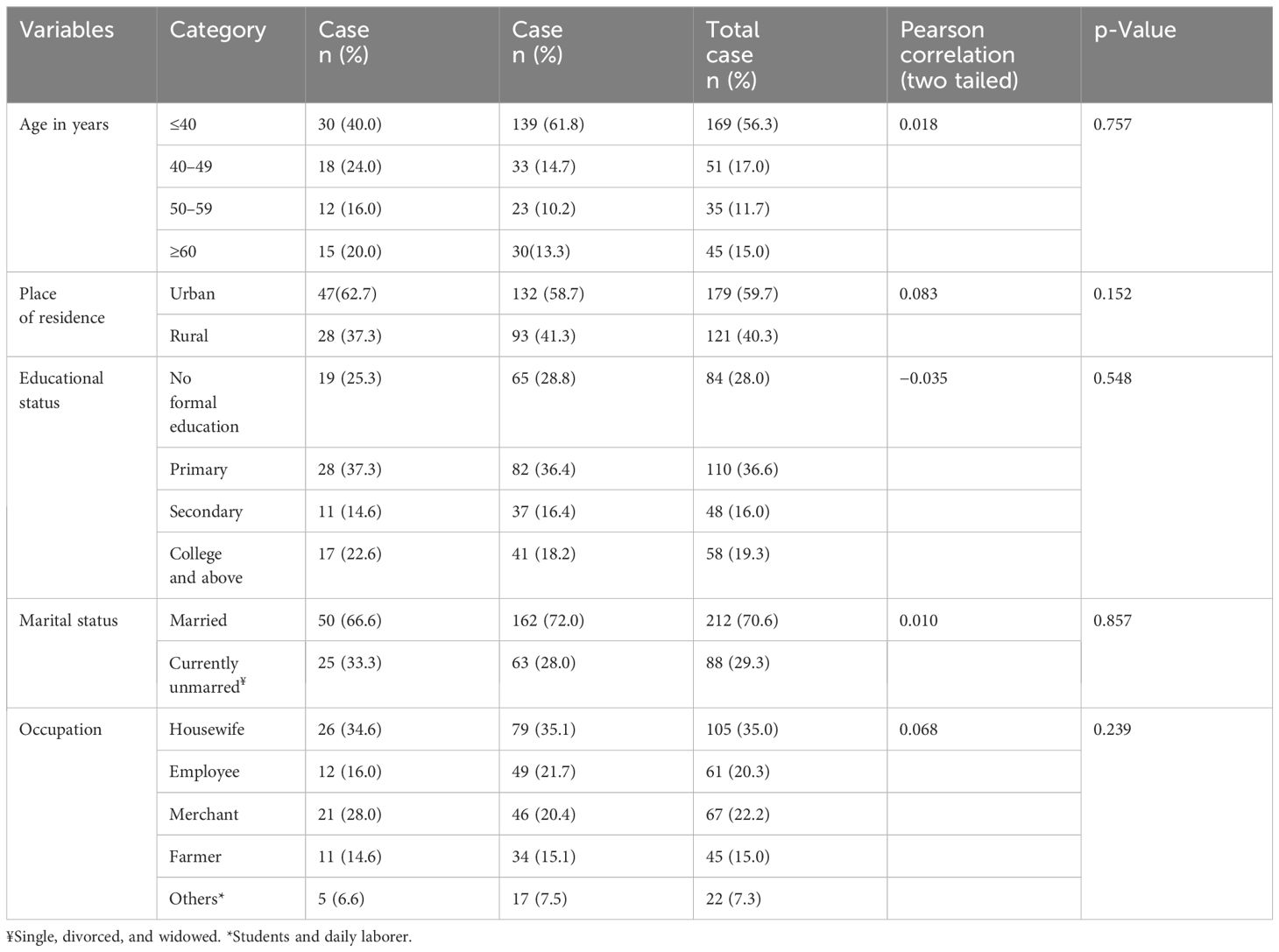
Table 1 Sociodemographic characteristics of the study participants in selected health facilities in Hawassa City, 2023.
2.6 Sampling technique and procedures
The study was performed in two hospitals, namely, Hawassa University Comprehensive (HUCSH) and Specialized Hospital and Yanet Internal Medicine Specialized Center (YIMSC), which provide chemotherapy services. The final calculated sample size was proportionally allocated to each hospital based on the number of patients who received chemotherapy services for BC treatment within the last year’s data from the health management information system (HMIS) report. Hence, 46 cases and 138 controls from HUCSH and 29 cases and 87 controls from YIMSC were included in the study. Since the cases are rare, we included all cases sequentially, but controls were included using a systematic random sampling technique. Therefore, once we included one patient, the next three controls were included in the study until the sample size was met.
2.7 Study variables
2.7.1 Dependent variable: breast cancer
Dependent variable is breast cancer.
2.7.2 Independent variables
Sociodemographic variables such as age, educational level, occupation, place of residence, and family history of cancer; behavior and/or alcohol intake, smoking status, fatty diet intake, and body mass index; reproductive variables such as age at first delivery, age at menarche, parity, breastfeeding practice, and use of hormonal contraceptives; diet and lifestyle variables such as vegetable intake, exercise, and fruit intake; and environmental and health-related variables such as pesticide contact, radiation exposure, breast trauma, and history of breast infection.
2.8 Data collection instruments and procedures
2.8.1 Data collection instruments
Primarily, the tool was developed in English after reviewing different literature from previous studies (16–18). Then, the questionnaire was translated into the local languages Sidamagna Afoo and Amharic by an expert and translated back to English by another expert to maintain consistency.
2.8.2 Data collection procedures
One day of training was given for two data collectors and one supervisor on the instrument. The interviewer administered a questionnaire supplemented with a checklist to collect data using the Kobo toolbox. Hence, during the interview, the data collector interviewed study participants about their sociodemographic, behavioral, clinical, and reproductive matters for both cases and controls. In addition, other pertinent determinant factors were identified from patients’ medical records. Pretests were performed on 5% of the sample before the actual data collection, and the necessary amendments were made.
The patient’s interview and the review of the patient’s records were used to gather information about the patient’s sociodemographic (age, residence, marital status, and level of education) and behavioral (smoking, BMI, alcohol consumption, and physical activity) BMI results. Patients were classified as underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), or obese (>30). Reproductive (parity, OC use, menopausal status, breastfeeding practices) characteristics. Before the real data collection process, a pretest was conducted using the collected data.
2.9 Data quality assurance
To ensure data quality, first, the data collection tools were prepared in English, translated to Amharic, and then returned to English to ensure consistency. Appropriate training was given to the data collectors and supervisors. The training included a briefing on the general objectives of the study, approach to accessing study participants, clarity on each item in the instrument, data collection procedure, including or excluding the target data source, timeliness of data submission, data handling, and time management. Pretests were performed outside the study health institution on 5% of the sample before the actual data collection, and the necessary correction was made based on the pretest results to avoid any confusion and for better completion of the questions. Every day, the collected data were reviewed and cross-checked for completeness.
2.10 Data processing and analysis
The data were collected via Kobo data collection and exported to SPSS version 27 for analysis. Descriptive statistics, such as frequency and percent distribution, were used to present categorical variables. Means and standard deviations were used for continuous variables. We conducted an independent sample t test to evaluate the equality of variance and mean difference among cases and controls to exposure variables to evaluate the mean difference in exposure between cases and controls. To identify factors related to BC, a binary logistic regression analysis was performed. According to the bivariate analysis, variables with a p-value of 0.25 were candidates for multivariate analysis. To demonstrate the strength of the link, an adjusted odds ratio with a 95% confidence interval was calculated. The association of the dependent and independent variables was considered significant at a p-value of less than 0.05. The goodness of fit was checked by the Hosmer and Lemeshow test (p-value = 0.86). Moreover, multicollinearity was assessed for each variable considering variance inflation factor (VIF) values less than 10.
2.11 Operational definitions
BC: Women who had a confirmed BC diagnosis according to histological examination. Case: Women with BC and histologically confirmed cases (8). Controls: Women who visited gynecological OPD in selected hospitals for noncancer care. Cancer: This is a condition in which body cells multiply uncontrollably and become contagious to other parts of the body (19). BMI: Somebody’s mass divided by the square of height in meters (20). For menopausal status, women who met any of the following criteria were classified as postmenopausal: 1) had menstruation for no less than 1 year (any age) and 2) had undergone bilateral oophorectomy or estrogen deprivation therapy (21). Active smoking is a condition in which people (including former smokers) have a history of smoking within 6 months (22). Passive smoking: People who are not smokers but are exposed to tobacco smoke for more than 15 min at least 1 day per week (22).
3 Results
3.1 Sociodemographic characteristics
A total of 300 women (75 cases and 225 controls) participated in the study for a response rate of 100%. Thirty (40%) of the cases and 139 (61.78%) of the controls were aged less than 40 years with the mean ( ± SD) age of the women being 37.2 ( ± 14.8) and 36.6 ( ± 15.1) for the cases and controls, respectively. More than half of the participants [47 (62.67%) cases and 132 (58.67%) controls] were urban dwellers. Seventeen (22.67%) patients and 41 (18.22%) controls had a college education or above. Regarding occupational status, 26 (34.67%) patients and 79 (35.11%) controls were housewives (1).
3.2 Reproductive health-related characteristics
Of the total respondents, 34.67% (n = 26) of the patients and 22.22% (n = 50) of the controls had experienced their first menstrual period before the age of 12 years, and the mean ( ± SD) ages of the women who experienced menarche were 13.5 ( ± 2.1) and 14 ( ± 1.92) years for the cases and controls, respectively. Approximately 10 (13.33%) patients and 8.44% (n = 19) of the controls had a history of abortion. Forty-two (56%) patients and 26.22% (n = 59) of the controls were premenopausal women. More than a quarter (29.33%) of the patients and 16.44% (n = 37) of the women had irregular menstrual periods. More than half (58.67%) of the patients and 36.89% (n = 83) of the controls used oral contraceptives. In the majority of the patients, 66.6% (n = 50) and 73% (n = 162) of the controls had never breastfed their infants. One-quarter 25.33%, n = 19) of the patients and 27.11% (n = 61) of the controls had a history of breast infection (Table 2).
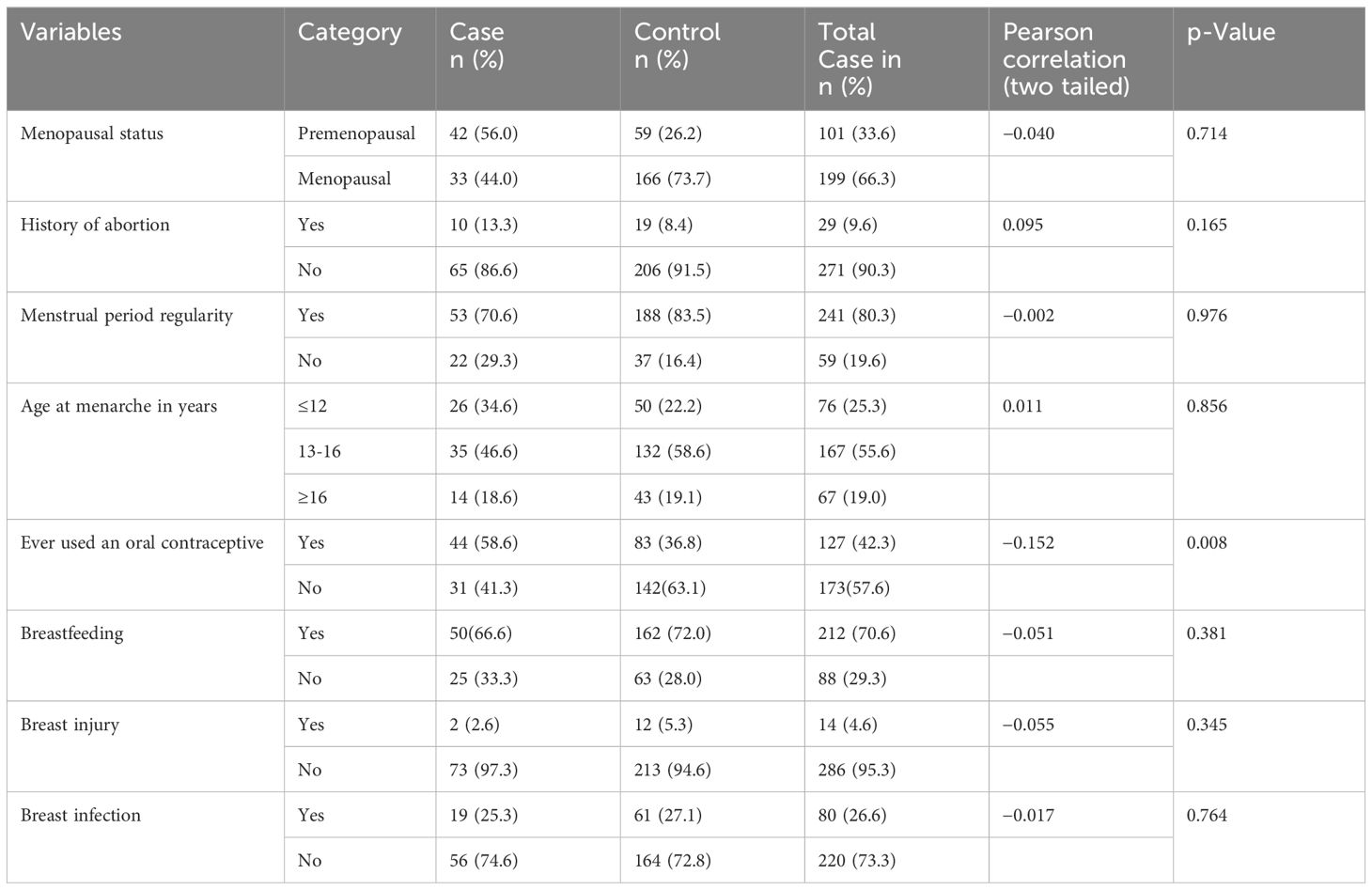
Table 2 Reproductive health-related characteristics of the women included in the study in selected health facilities in Hawassa City, 2023 (n = 300).
3.3 Behavioral and biological-related characteristics
Among the patients, 30.67% (n = 23) and 16.00% (n = 36) of the controls had a family history of cancer. Forty percent (n = 30) of the patients and 25.33% (n = 57) of the controls were overweight and/or obese. In the majority of the patients, 77.33% (n = 58) and 70.22% (n = 158) of the controls did not participate in regular physical exercise. In the majority of the participants, 88% (n = 66) of the patients and 82.22% (n = 185) of the controls ate fewer than seven servings of fruit per week. Regarding exposure to smoking, 18.67% (n = 14) of the patients and 22.22% (n = 50) of the controls were exposed. Approximately 40% (n = 30) of the patients and 30.22% (n = 68) of the controls used packed food or drinks. Approximately 50.67% (n = 38) of the patients and 33.78% (n = 76) of the controls consumed solid oil. Among the participants, 9.33% (n = 7) of the patients and 8.8% (n = 8) of the controls had ever undergone a mammogram. Approximately 45.33% (n = 34) of the patients and 44.4% (n = 100) of the controls had practiced self-breast examinations (Table 3).
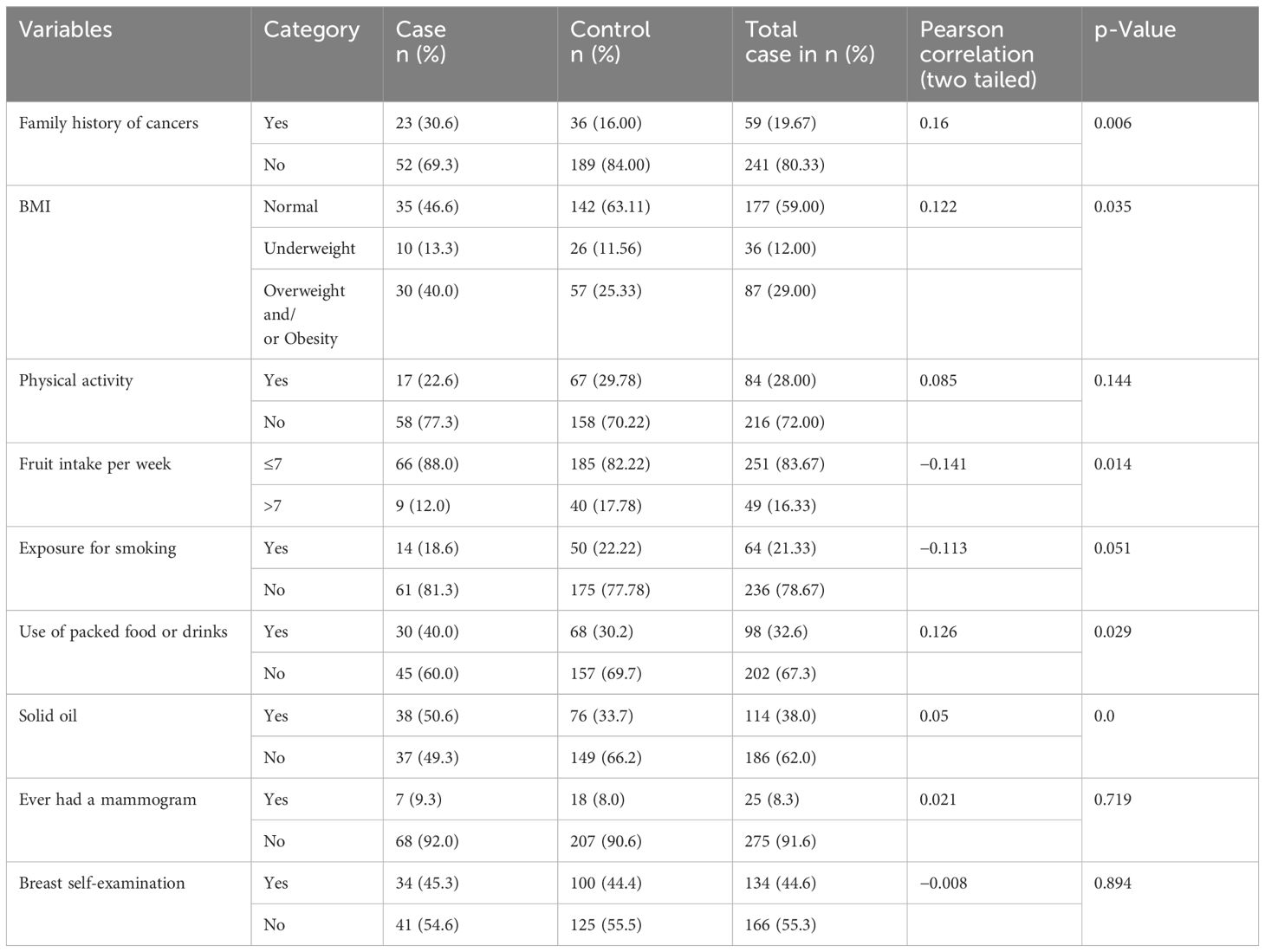
Table 3 Behavioral and biological characteristics of the study participant women in selected health facilities in Hawassa City, 2023.
3.4 The mean difference in exposure between cases and controls
In this study, we conducted an independent-sample t-test to evaluate the equality of variance and mean difference among cases and controls to exposure variables. To check the similarity of variance among cases and controls, Levene’s test for equality of variances was used (i.e., equal variances, p > 0.05, and unequal variances p < 0.05). Similarly, to identify the mean difference among cases and controls, a t-test for the equality of means with respective p-values was used [p < 0.05 indicates a significant difference in the means of the two sample populations tested (cases and controls)]. Since the variance is greater than 4 for almost all of the test variables, we assumed unequal variance and used a one-sample t-test (23). The factors associated with an increased risk of BC among women were family history of cancer (p < 0.014), consumption of packed food (p < 0.03), BMI (p < 0.04), fruit consumption (p < 0.03), menopausal status (p < 0.01), and smoking status (p < 0.01) (Table 4).
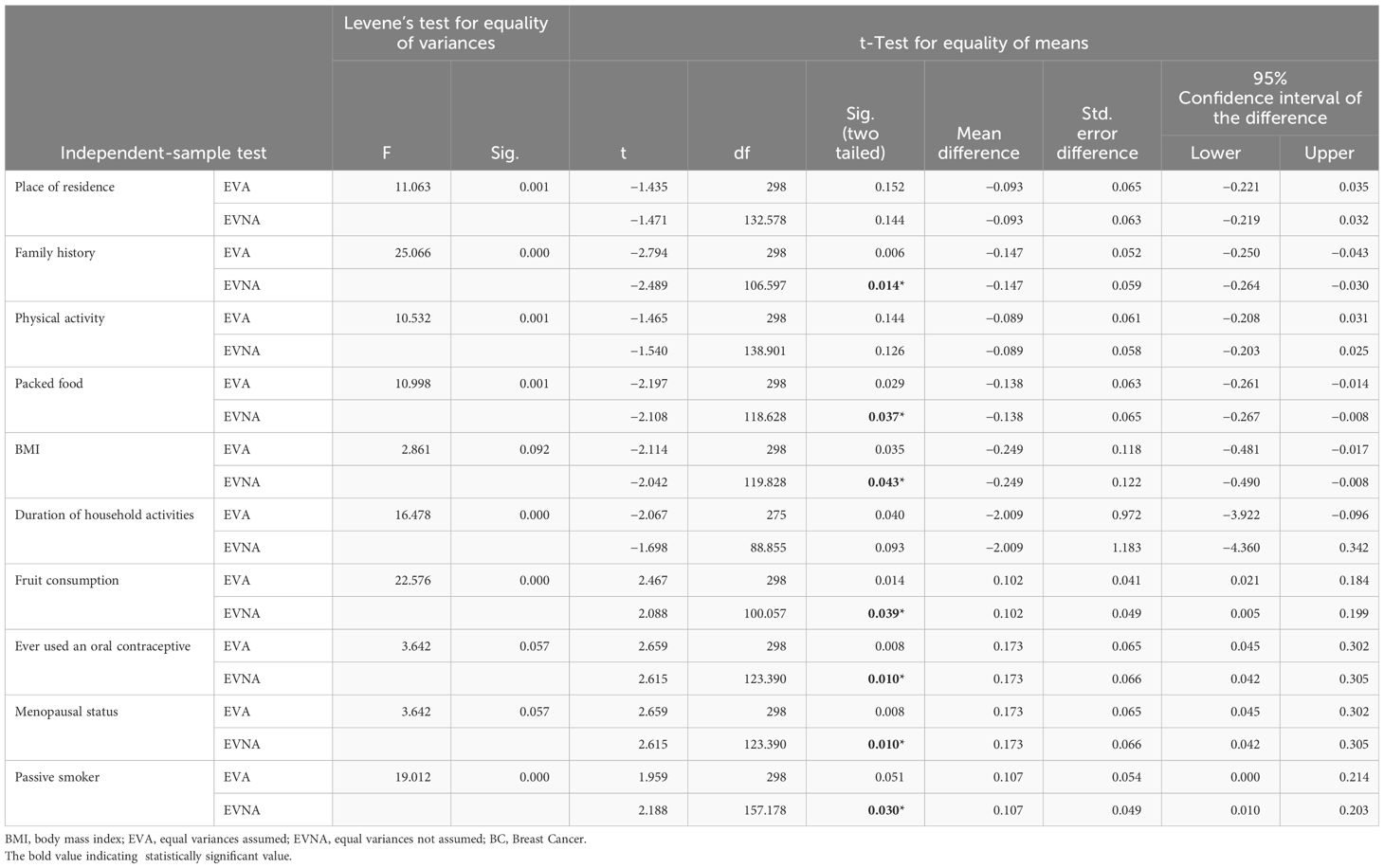
Table 4 Independent-sample t-test of respondents for determinants of BC among women attending oncology units at selected health facilities of Hawassa City, Sidama Region, Ethiopia, 2023 (n = 75 cases and n = 225 controls).
3.5 Factors associated with BC
According to the bivariate logistic regression analysis, age, menopausal status, use of packed foods or drinks, history of abortion, regularity of menstruation, age at menarche, body mass index (BMI), family history of cancer, use of oral contraceptives, regular physical exercise, and consumption of solid oil were independently associated with BC. However, in the multivariate logistic regression analysis, menopausal status, overweight and obesity status, use of oral contraceptives, family history of cancer, and consumption of solid oil were independently associated with BC.
The odds of having BC were almost three times greater among postmenopausal women than among their male counterparts [adjusted odds ratio (AOR) = 2.5; 95% CI: 1.18–5.2, p = 0.01]. Women with a family history of cancer were 2.33 times more likely to develop BC than women without a family history of cancer (AOR = 2.33; 95% CI: 1.12–4.82, p = 0.02). The odds of developing BC were almost three times greater among oral contraceptive users than among their counterparts (AOR = 2.74; 95% CI: 1.34–5.99, p = 0.005). Similarly, the odds of having BC were almost two times greater among overweight women than among normal-weight women (AOR = 2.29; 95% CI: 1.14–4.59, p = 0.01). Finally, women who used palm/solid oil were 2.4 times more likely to have BC than were their male counterparts (AOR = 2.36; 95% CI: 1.20–4.67, p = 0.01) (Table 5).
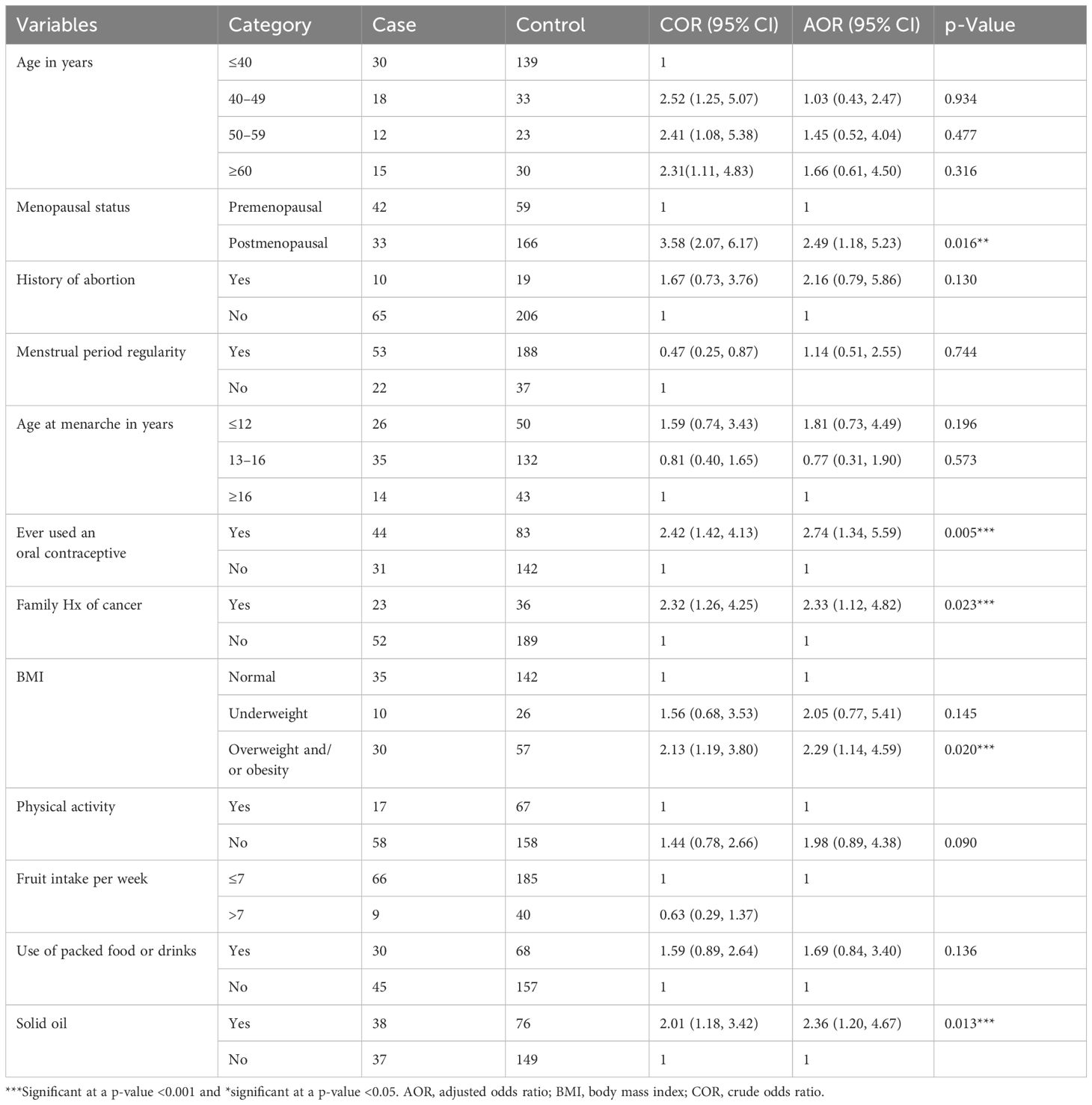
Table 5 Bivariate and multivariate logistic regression analysis of factors associated with BC risk among women who attended selected health facilities in Hawassa City, 2023.
4 Discussion
In this facility-based study, we evaluated the determinants of BC among women attending oncology health facilities in Hawassa City, Sidama Region, Ethiopia. Postmenopausal status (p < 0.016), BMI (p < 0.020), family history of cancer (p < 0.023), use of oral contraceptives (p < 0.02), and consumption of saturated fat (p < 0.013) were independently associated with BC.
Our study showed that menopausal status significantly increases BC risk with postmenopausal women having almost three times greater odds than premenopausal women. This finding is similar to that of a study in Addis Ababa (24). Another study from Malaysia showed that postmenopausal women had a 52% greater risk of BC (25). A meta-analysis from India comparable with this study showed that postmenopausal women have a 35% greater risk of developing BC than premenopausal women (26). A possible explanation could be that postmenopausal women face an increased risk of BC due to decreased estrogen levels, obesity, and a sedentary lifestyle, which can increase the risk of developing the disease. Thus, a systematic review and meta-analysis of evidence suggested that postmenopausal women should have regular physical activity and a healthy diet to prevent overweight and obesity to address BC (27).
Similarly, a family history of cancer significantly increases the risk of BC, and women with a history of BC are 2.3 times more likely to develop BC. This result is in line with research in Addis Ababa (8), which demonstrated that women with a family history of BC are substantially more likely to develop BC than other women. Early detection and screening could be key for BC progression. Families with a history of BC may also be more aware of the risks and symptoms leading them to seek regular screening and early detection. While this proactive approach to healthcare is important, it can create a perception of greater risk due to increased awareness and monitoring within the family.
The study revealed that oral contraceptives significantly increased BC risk with women using oral contraceptives having 2.74 times greater odds of developing BC than nonusers. This result is in line with a study conducted in Jordan and Ethiopia (28, 29). Naturally occurring estrogen and progesterone stimulate the development and growth of some cancers (e.g., cancers that express the receptor for these hormones, such as BC). Birth control pills contain synthetic versions of these female hormones. In contrast, oral contraceptives and BC were not significantly linked in a study in Ethiopia (24). The possible reason for this slight increase in risk could be related to the hormonal components of oral contraceptives. Oral contraceptives contain synthetic versions of the hormones estrogen and progestin, which are used to prevent pregnancy by suppressing ovulation and altering the cervical mucus and the lining of the uterus. Therefore, maintaining regular BC screenings and self-exams is crucial for the early detection and treatment of BC regardless of whether a woman is using oral contraceptives.
Women’s nutritional status significantly impacts BC risk with overweight or obese women having a 2.29-fold greater risk than normal-weight women. This finding is comparable to that of a study conducted in Jordan and two other studies in Ethiopia (8, 30, 31). The possible justification could be unhealthy eating patterns that are marked by high consumption of refined carbohydrates, sugar, and saturated fats (32). This increased risk can be attributed to the higher levels of estrogen produced by adipose tissue and the fact that obese women are more physically inactive, both of which can promote the growth of BC cells.
Furthermore, consuming saturated oil significantly increases BC risk. Women consuming solid oil have 2.36 times greater odds of developing BC than nonusers. This result is in line with a study performed in the USA showing that eating saturated fat increases the risk of developing BC (33). The possible reason could be that saturated fatty acids increase low-density lipoprotein cholesterol, obesity, and free radicals, which increase the risk of BC (34). A meta-analysis revealed that a high-fat diet is a risk factor for BC (35). Another systematic review showed that an unhealthy high-fat diet may contribute to obesity and affect BC (36).
The strengths of this study included the use of an observational study design with a 1:3 case-to-control ratio, which enhances its ability to identify BC determinants and its validity due to its multicenter nature. The possible study limitations include the small number of patients in the health facility, recall bias, and over- or underreporting of self-reported data on dietary consumption and physical activity.
5 Conclusion
BC is a public health problem both globally and in Ethiopia. This study aimed to identify the determinants of BC. Therefore, postmenopausal status, BMI, family history of cancer, being overweight, using oral contraceptives, and consuming solid oil were identified as risk factors for BC. Therefore, women should adopt healthier lifestyles through healthy nutrition and regular physical exercise, which might contribute to reducing the risk of developing BC in women. In addition, early detection and regular screening are proactive approaches for identifying BC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical clearance was obtained from the Ethical Review Committee of Yanet-Liyana College of Health Science (with Ref # of LHC/YLCHS/OGL/981/15 and Date: 29/05/2023 GC). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. TA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. AM: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to extend our sincere gratitude to Yanet-Liyana College of Health Science for their ethical letter provision. We would also like to acknowledge all our data collectors and supervisors who participated in the data collection at the field level. We would like to extend our gratitude to all the study participants for their important insights and contributions in providing information in this research process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. (2022) 66:15–23. doi: 10.1016/j.breast.2022.08.010
2. Kashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, et al. Global increase in breast cancer incidence: risk factors and preventive measures. BioMed Res Int. (2022) 2022. doi: 10.1155/2022/9605439
3. Sasco AJ. Cancer and globalization. BioMed Pharmacother. (2008) 62:110–21. doi: 10.1016/j.biopha.2007.10.015
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
5. Nguyen D, Yu J, Reinhold WC, Yang SX. Association of independent prognostic factors and treatment modality with survival and recurrence outcomes in breast cancer. JAMA Netw Open. (2020) 3:e207213–e. doi: 10.1001/jamanetworkopen.2020.7213
6. Mekonnen BD. tffBreast self-examination practice and associated factors among female healthcare workers in Ethiopia: a systematic review and meta-analysis. PloS One. (2020) 15:e0241961. doi: 10.1371/journal.pone.0241961
7. Bray F, Parkin DM. Cancer in sub-Saharan Africa in 2020: a review of current estimates of the national burden, data gaps, and future needs. Lancet Oncol. (2022) 23:719–28. doi: 10.1016/S1470-2045(22)00270-4
8. Tolessa L, Sendo EG, Dinegde NG, Desalew A. Risk factors associated with breast cancer among women in addis ababa, Ethiopia: Unmatched case–control study. Int J Women’s Health. (2021) 13:101–10. doi: 10.2147/IJWH.S292588
9. Bacha RH, Jabir YN, Asebot AG, Liga AD. Risk factors affecting survival time of breast cancer patients: The case of southwest Ethiopia. J Res Health Sci. (2021) 21:e00532. doi: 10.34172/jrhs.2021.65
10. Anderson BO, Abdel-Wahab M, Fidarova E. The Global Breast Cancer Initiative: a strategic collaboration to strengthen health care for noncommunicable diseases. Lancet Oncol. (2021) 22(5):578–81. doi: 10.1016/S1470-2045(21)00071-1
11. Bejitual K, Fikre R, Ashegu T, Zenebe A. Determinants of neonatal sepsis among neonates admitted to the neonatal intensive care unit of public hospitals in Hawassa City Administration, Sidama Region, Ethiopia, 2020: an unmatched, case–control study. BMJ Open. (2022) 12:e056669. doi: 10.1136/bmjopen-2021-056669
12. Mertens E, Barrenechea-Pulache A, Sagastume D, Vasquez MS, Vandevijvere S, Peñalvo JL. Understanding the contribution of lifestyle in breast cancer risk prediction: a systematic review of models applicable to Europe. BMC Cancer. (2023) 23(1):687. doi: 10.1186/s12885-023-11174-w
13. WHO. Breast cancer fact sheet (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
14. Liu H, Shi S, Gao J, Guo J, Li M, Wang L. Analysis of risk factors associated with breast cancer in women: a systematic review and meta-analysis. Trans Cancer Res. (2022) 11:1344. doi: 10.21037/tcr
15. Khalis M, Charbotel B, Chajès V, Rinaldi S, Moskal A, Biessy C, et al. Menstrual and reproductive factors and risk of breast cancer: A case−control study in the Fez region, Morocco. PloS One. (2018) 13:e0191333. doi: 10.1371/journal.pone.0191333
16. Duche H, Tsegay AT, Tamirat KS. Identifying risk factors of breast cancer among women attending selected hospitals of Addis Ababa city: hospital-based unmatched case−Control study. Breast Cancer (Dove Med Press). (2021) 13:189–97. doi: 10.2147/BCTT.S293867
17. Tolessa L, Sendo EG, Dinegde NG, Desalew A. Risk factors associated with breast cancer among women in Addis Ababa, Ethiopia: unmatched case−Control study. Int J Womens Health. (2021) 13:101–10. doi: 10.2147/IJWH.S292588
18. Tekeste Z, Berhe N, Arage M, Degarege A, Melaku YA. Cancer signs and risk factors awareness in Addis Ababa, Ethiopia: a population-based survey. Infect Agent Cancer. (2023) 18:1. doi: 10.1186/s13027-022-00477-5
19. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
20. García-Estévez L, Cortés J, Pérez S, Calvo I, Gallegos I, Moreno-Bueno G. Obesity and breast cancer: a paradoxical and controversial relationship influenced by menopausal status. Front Oncol. (2021) 11:705911. doi: 10.3389/fonc.2021.705911
21. Rothschild HT, Abel MK, Patterson A, Goodman K, Shui A, van Baelen K, et al. Obesity and menopausal status impact the features and molecular phenotype of invasive lobular breast cancer. Breast Cancer Res Treat. (2022) 191:451–8. doi: 10.1007/s10549-021-06453-8
22. Fakhri N, Chad MA, Lahkim M, Houari A, Dehbi H, Belmouden A, et al. Risk factors for breast cancer in women: an update review. Med Oncol. (2022) 39:197. doi: 10.1007/s12032-022-01804-x
23. Zimmerman DW. Teacher’s corner: A note on interpretation of the paired-samples t test. J Educ Behav Stati. (1997) 22:349–60. doi: 10.3102/10769986022003349
24. Hassen F, Enquselassie F, Ali A, Addissie A, Taye G, Tsegaye A, et al. Association of risk factors and breast cancer among women treated at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia: a case–control study. BMJ Open. (2022) 12:e060636. doi: 10.1136/bmjopen-2021-060636
25. Durham DD, Abraham LA, Roberts MC, Khan CP, Smith RA, Kerlikowske K, et al. Breast cancer incidence among women with a family history of breast cancer by relative’s age at diagnosis. Cancer. (2022) 128:4232–40. doi: 10.1002/cncr.34365
26. Vishwakarma G, Ndetan H, Das DN, Gupta G, Suryavanshi M, Mehta A, et al. Reproductive factors and breast cancer risk: A meta-analysis of case–control studies in Indian women. South Asian J cancer. (2019) 8:080–4. doi: 10.4103/sajc.sajc_317_18
27. Dehesh T, Fadaghi S, Seyedi M, Abolhadi E, Ilaghi M, Shams P, et al. The relation between obesity and breast cancer risk in women by considering menstruation status and geographical variations: a systematic review and meta-analysis. BMC Womens Health. (2023) 23:392. doi: 10.1186/s12905-023-02543-5
28. Bardaweel SK, Akour AA, Al-Muhaissen S, AlSalamat HA, Ammar K. Oral contraceptive and breast cancer: do benefits outweigh the risks? A case–control study from Jordan. BMC women’s Health. (2019) 19:1–7. doi: 10.1186/s12905-019-0770-x
29. Das D. Breast cancer: Risk factors and prevention strategies. World J Biol Pharma Health Sci. (2022) (12)(3):265280. doi: 10.30574/wjbphs.2022.12.3.0253
30. Ayoub NM, Yaghan RJ, Abdo NM, Matalka II, Akhu-Zaheya LM, Al-Mohtaseb AH. Impact of obesity on clinicopathologic characteristics and disease prognosis in preand postmenopausal breast cancer patients: a retrospective institutional study. J Obes. (2019) 2019:1–11. doi: 10.1155/2019/3820759
31. Duche H, Tsegay AT, Tamirat KS. Identifying risk factors for breast cancer among women attending selected hospitals of Addis Ababa city: Hospital-based unmatched case−control study. Breast Cancer: Targets Ther. (2021) 13:189–97. doi: 10.2147/BCTT.S293867
32. Seiler A, Chen MA, Brown RL, Fagundes CP. Obesity, dietary factors, nutrition, and breast cancer risk. Curr Breast Cancer Rep. (2018) 10:14–27. doi: 10.1007/s12609-018-0264-0
33. Dandamudi A, Tommie J, Nommsen-Rivers L, Couch S. Dietary patterns and breast cancer risk: a systematic review. Anticancer Res. (2018) 38:3209–22. doi: 10.21873/anticanres.12586
34. Xia H, Ma S, Wang S, Sun G. Meta-analysis of saturated fatty acid intake and breast cancer risk. Medicine. (2015) 94:e2391. doi: 10.1097/MD.0000000000002391
35. Uhomoibhi TO, Okobi TJ, Okobi OE, Koko JO, Uhomoibhi O, Igbinosun OE, et al. High-fat diet as a risk factor for breast cancer: a meta-analysis. Cureus. (2022) 14:e32309. doi: 10.7759/cureus.32309
Keywords: breast cancer, case–control, determinates, oncology units, Sidama Region Ethiopia
Citation: Kebede S, Alemu T and Mekonnen A (2024) Determinants of breast cancer among women attending oncology units in selected health facilities of Hawassa City, Sidama Region, Southern Ethiopia, 2023: case-control study. Front. Oncol. 14:1352191. doi: 10.3389/fonc.2024.1352191
Received: 07 December 2023; Accepted: 25 March 2024;
Published: 12 April 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Till Kaltofen, University Medical Center Regensburg, GermanyDinesh Pendharkar, Sarvodaya Hospital and Research Centre, India
Copyright © 2024 Kebede, Alemu and Mekonnen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Selamawit Kebede, c2VsYW1rZWJlZGU3MEBnbWFpbC5jb20=; Tsegaye Alemu, dHNlZ2F5ZWE0OUBnbWFpbC5jb20=; Ashenafi Mekonnen, YXNoZW13QGdtYWlsLmNvbQ==
†ORCID: Selamawit Kebede, orcid.org/0009-0000-1980-0938
Tsegaye Alemu, orcid.org/0000-0001-9511-9028
Ashenafi Mekonnen, orcid.org/0000-0001-5272-5829
 Selamawit Kebede1*†
Selamawit Kebede1*† Tsegaye Alemu
Tsegaye Alemu