- 1Department of Pathology, West China Second University Hospital, Sichuan University, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Sichuan, China
- 3National Health Commission (NHC) Key Laboratory of Chronobiology, Sichuan University, Sichuan, China
Polypoid lesions in the uterus are very common. There are a lot of benign lesions and malignant tumor should be considered. Adenosarcoma, one of the differential diagnoses, is a rare mixed epithelial and mesenchymal tumor consisting of benign epithelial components and sarcoma stroma. Here, we present a case of atypical adenosarcoma that has never been reported. This tumor was composed of benign endometrial epithelium and hyperplastic stroma with extensive bizarre stromal cells. But the cleft-like spaces and papillary stomal fronds which were the typical histological images of adenosarcoma were absent. These stomal cells, including bizarre cells, were positive for vimentin, CD10, ER, PR, cyclin D1 and P16 but were immunonegative for caldesmon. Furthermore, this tumor harbored amplification of MDM2, as revealed by fluorescence in situ hybridization testing (FISH) and next-generation DNA sequencing (NGS).
Introduction
Polypoid lesions in the uterus are very common. Such as benign endometrial and endocervical polyps and adenofibroma, as well as various malignant tumors, including endometrial stromal sarcoma, adenosarcoma, carcinosarcoma, botryoid rhabdomyosarcoma, and other uterine sarcomas should be distinguished. In addition to malignant tumors, bizarre stromal cells are occasionally present in benign polyps, but these appear to represent a degenerative phenomenon, as the cells show smudged nuclear chromatin and are not mitotically active (1). Here, we present a case of atypical adenosarcoma with bizarre stromal cells accompanied by amplification of MDM2.
Müllerian adenosarcomas, were initially reported in 1974, are uncommon biphasic epithelial-mesenchymal tumors with benign epithelial elements and a malignant mesenchymal component (2). The lesion most often occurs in the endometrium, but rare tumors in the cervix, ovary, fallopian tube, and vagina have been reported (3, 4). The median age is 50-59 years old. Adenosarcoma is usually treated by hysterectomy and bilateral salpingo-oophorectomy; more conservative resection is occasionally performed for young patients. Most patients have good clinical outcomes. Moreover, aggressive clinical behavior often includes deep myometrial invasion and a pure sarcoma component comprising ≥25% of the tumor (5). The pathogenesis of uterine adenosarcomas is poorly understood. Molecular studies to date have revealed mutations in genes such as MDM2/CDK4, TP53, ATRX, FGFR2, KMT2C and DICER1 (6–9).
Case report
A 44-year-old woman who was premenopausal presented with abnormal vaginal bleeding for one month. Physical examination indicated a polypoid mass (1.2 cm × 1.1 cm) protruding into the cervix. Then hysteroscopy and biopsy were performed. The pathological investigation was considered to be a lesion of endometrial stromal hyperplasia. The patient came to our hospital for consultation for accurate diagnosis and further treatment. Pathology consultation result revealed endometrial polyps with endometrial stromal hyperplasia, and malignment endometrial stromal tumor could not be excluded. Complete resection of the lesion was recommended. Furthermore, ultrasound showed a mass in the lower segment of the uterine body and the junction of the cervix (Figure 1). Therefore, the patient underwent hysterectomy and bilateral salpingo-oophorectomy, because the patient has completed the fertility.
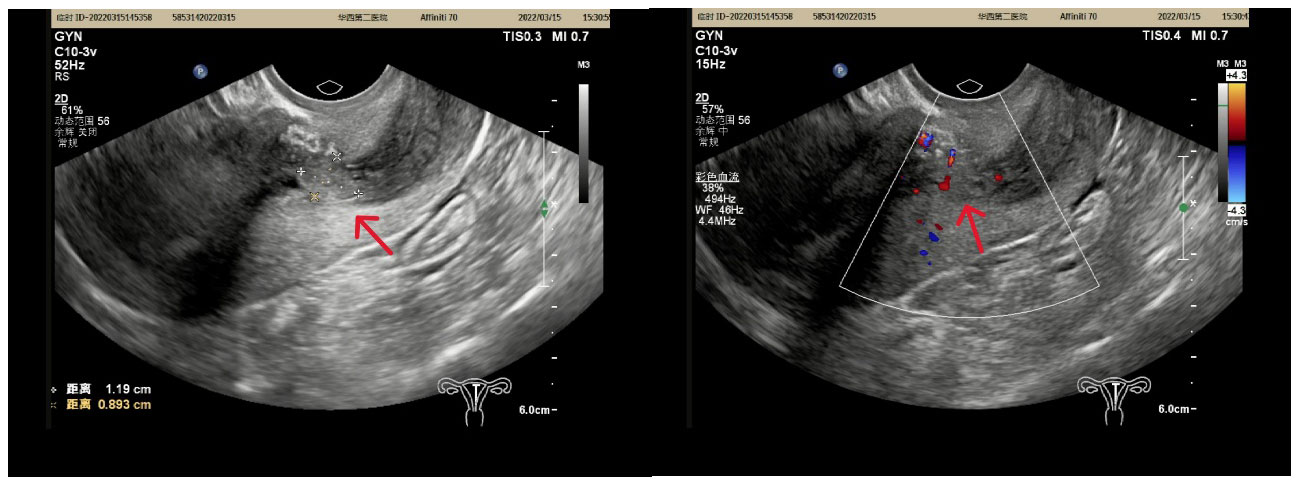
Figure 1 A slightly stronger echo (red arrow) of 1.2 cmx0.9 cmx1.3 cm in size was found in the lower part of the uterine cavity, with a clear border and point-like blood flow signals peripheral and inward from the echo.
We located the lesion in the lower segment of the uterine body, and it was confined to the endometrium through gross examination. Histologically, the surface and glandular epithelium resembled proliferative endometrial epithelium, and the stroma was hyperplastic. Focally, hypercellular stromal cuffs around glands or band-like hypercellular zones beneath the surface epithelium could be seen. However, cleft-like spaces and papillary stomal fronds were absent. Tumer necrosis and myometrial invasion were not been seen. Nuclear atypia and mitotic figures could be seen in the mesenchymal cells (>2 MF/10 HPF). Extraordinarily, there were numerous bizarre stromal cells that were pleomorphism, multinucleate and hyperchromatic (Figure 2).
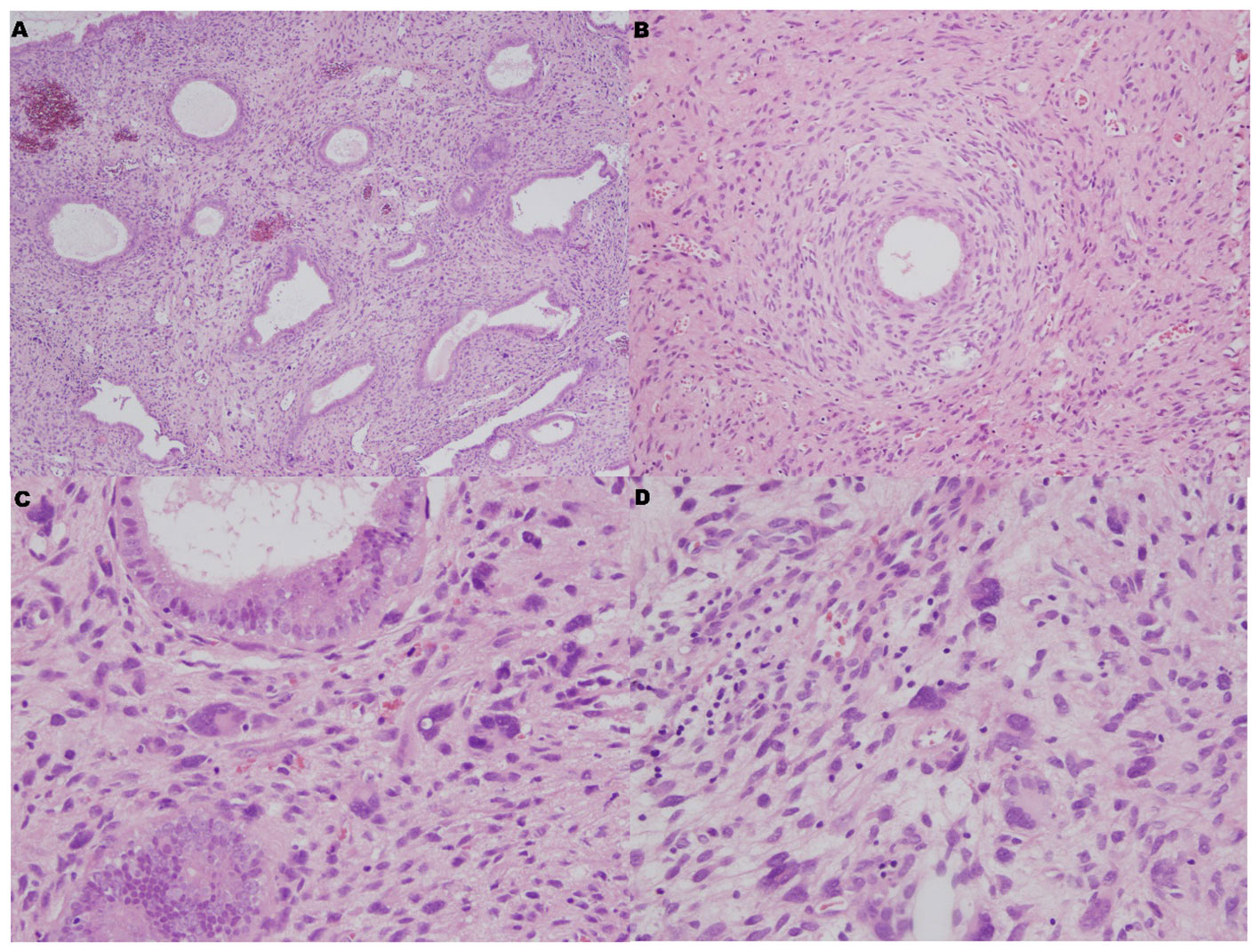
Figure 2 Microscopic features. (A) The surface and glandular epithelium was benign, but the stroma was hyperplastic (HE×40). (B) Focally, hypercellular stromal cuffs were observed around glands (HE×100). (C, D) There were numerous bizarre stromal cells that were pleomorphism, multinucleate and hyperchromatic (HE×400).
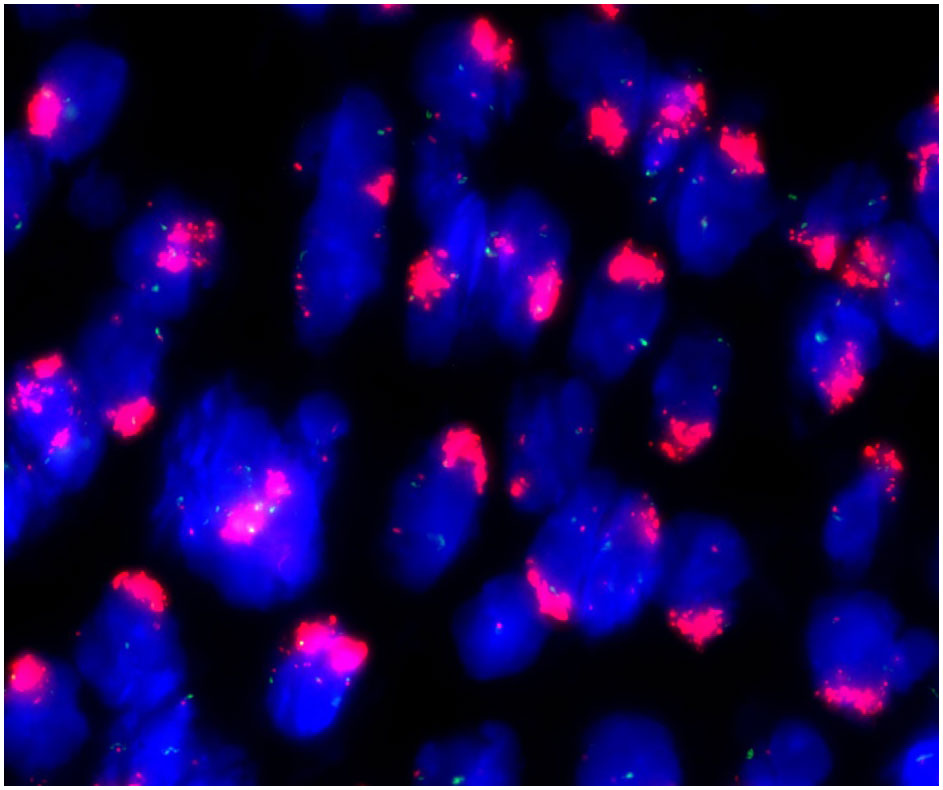
These stromal tumor cells, including the bizarre cells, stained positively for vimentin, CD10, ER, PR, cyclin D1 and P16 but were immunonegative for caldesmon. The Ki-67 index was approximately 10%-20% (Figure 3). Whole exome sequencing (WES) was performed to analyze mutations. Seven mutations were detected in the tumor, including amplification of MDM2, CDK6, CDK4, KMT2D, HGF and GRM3 and rearrangement of KMT2D, with amplification of MDM2, CDK6 and CDK4 being most prominent (Figure 4). FISH (fluorescence in situ hybridization) was performed to verify the prominent amplification of MDM2 (Figure 5). At the same time, we also detected rearrangement of JAZF1, YWHAE and BCOR through FISH. These gene mutations which are frequently associated with endometrial stromal sarcoma were not found.
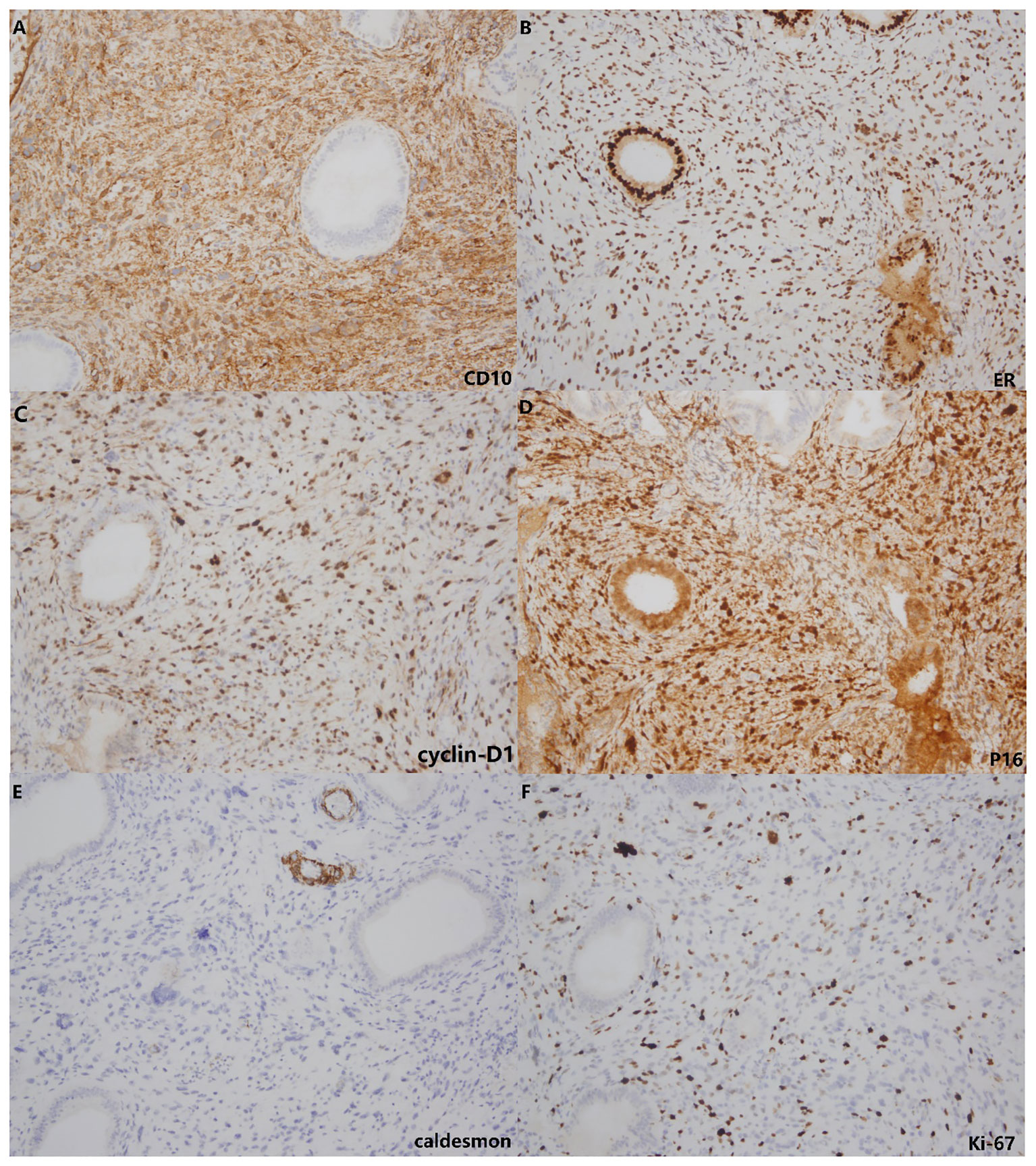
Figure 3 Immunohistochemistry. The stromal cells showed positive staining for CD10 (A), ER (B), cyclin-D1 (C) and P16 (D) but lacked staining for caldesmon (E). The Ki-67 index (F) was approximately 10%-20%.
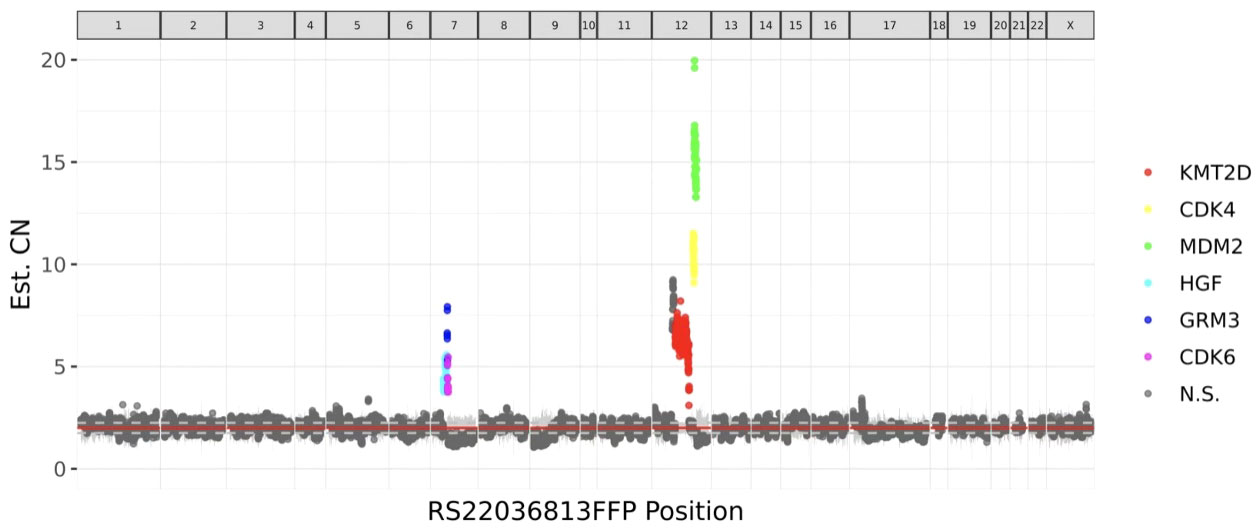
Figure 4 Whole-exome sequencing (WES) detected 7 mutations in the tumor, including amplification of MDM2, CDK6, CDK4, KMT2D, HGF and GRM3 and rearrangement of KMT2D. The most significant mutation was MDM2 amplification.
In conclusion, we diagnosed this polypoid lesion as adenosarcoma. The tumor was composed of a benign epithelium and malignant stroma. The interstitial substance with numerous bizarre cells was immunoreactive for CD10, ER and cyclin-D1 and showed amplification of MDM2.
By the telephone follow-up, the patient has been disease free for one year, without recurrence or metastasis.
Discussion
As previously reported, atypical stromal cells of the female gynecologic tract have been described and reported in the vagina, vulva, and cervix; rare cases have been documented in the endometrium, especially hyperplastic polyps of the endometrium (10). Most of these findings are considered degenerative and benign, and some have postulated that these cells are of fibroblastic origin. However, the etiology and precise nature of multinucleated giant cells and atypical stromal cells in the endometrium remain unclear.
Tai and Tavassoli described a series of 15 cases of atypical stromal cells in the endometrium, 13 in endometrial polyps and 2 within the endometrial stroma, but without endometrial polyps (11). The authors suggested that these cells represent a primitive cell population originating from multipotent mesenchymal cells that have the ability to differentiate toward either smooth muscle or endometrial stromal lineages because of immunohistochemical findings of positivity of the atypical stromal cells for endometrial stromal and smooth muscle markers. In their study, all cases (100%) stained positive for vimentin, estrogen receptor, progesterone receptor, and androgen receptor, and 37% were positive for CD10. Some other case reports show a similar viewpoint. Bizarre stromal cells of an endometrial polyp that are similar to the cells of vulvovaginal polyps, as well as at nongynecologic sites, have been reported; caution to avoid mistaking these cells for malignancy has highlighted (12). Alp Usubütün et al. described a polypoid leiomyoma with bizarre nuclei and atypical endometrial stromal cells adjacent to a leiomyoma. They regarded these atypical cells as degenerative in nature and stated that they should not be mistaken for malignant cells on histologic examination, especially when examining small endometrial samples (13).
Our case, however, is different from these reports. First, rather than being distributed under the surface epithelium only, these atypical bizarre stromal cells extended wildly in the tumor, and the atypical hypercellular stromal cuffs around glands appeared focally. The Ki-67 proliferation index was approximately 10%-20%, and mitotic figures could be seen (>2 MF/10 HPF).
What’s more, whole-exome sequencing (WES) revealed amplification of MDM2, CDK4 and CDK6. Mouse double minute 2 (MDM2) is a critical negative regulator of the tumor suppressor p53, playing a key role in controlling its transcriptional activity, protein stability, and nuclear localization. Analysis of broader panels of sarcoma samples confirmed the presence of MDM2 amplification in many histological subtypes of sarcoma, including osteosarcoma, liposarcoma, lipoma, leiomyosarcoma, rhabdomyosarcoma, malignant schwannoma, fibrosarcoma, hemangiopericytoma, and malignant fibrous histiocytoma (14). The amplification of MDM2 was also validated by FISH in our case. CDK4/6 complex is a key mediator of cell cycle progression through the G1 phase, the time when a cell prepares to initiate DNA synthesis. This gene plays a key role in mammalian development and cancer (15, 16). Furthermore, previous molecular studies of adenosarcoma have revealed mutations in genes such as MDM2/CDK4, TP53, ATRX, FGFR2, KMT2C and DICER1 (6–9). Therefore, we believe that these bizarre stromal cells formed a real tumor. This atypical adenosarcoma with extensive bizarre stromal cells is first reported here.
Additionally, differential diagnosis of adenosarcoma, including benign entities such as endometrial and endocervical polyps and adenofibroma, as well as various malignant tumors, including endometrial stromal sarcoma, leiomyosarcoma, and botryoid rhabdomyosarcoma in young patients, should be considered. Based on the morphological atypia and molecular changes observed, our case did not involve a benign lesion. Immunohistochemistry suggested the tumor to be of endometrial stromal origin. However, rearrangement of JAZF1, YWHAE and BCOR, which are frequently associated with endometrial stromal sarcoma, was not found by FISH. Hence, endometrial stromal sarcoma was not diagnosed.
In summary, endometrial polyps that occur in women of childbearing age need to be considered when the mesenchymal component is significantly hyperplasic. Such a finding should not simply be considered benign when encountered bizarre nuclear stromal cells. Müllerian adenosarcoma and endometrial stromal sarcoma should be considered and differentiated through IHC, FISH and even gene sequencing. Extended clinical observation is necessary because it is unclear whether the prognosis is similar to that of a typical adenosarcoma.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Ethics statement
The requirement of ethical approval was waived by Ethics Committee of West China the Second Hospital of Sichuan University for the studies involving humans because this research study was conducted retrospectively according to medical record data obtained during hospitalization. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because This research study was conducted retrospectively according to medical record data obtained during hospitalization. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XX: Writing – original draft. YH: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Supported by Sichuan Science and Technology Program (2022NSFSC0708).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tai LH, Fattaneh A. Tavassoli. Endometrial polyps with atypical (Bizarre) stromal cells. Am J Surg Pathol. (2002) 26:505–9. doi: 10.1097/00000478-200204000-00014
2. Clement PB, Scully RE. Müllerian adenosarcoma of the uterus. A clinicopathologic analysis of ten cases of a distinctive type of müllerian mixed tumor. Cancer. (1974) 34(4):1138–49. doi: 10.1002/(ISSN)1097-0142
3. Eichhorn JH, Young RH, Clement PB, Scully RE. Mesodermal (mullerian) adenosarcoma of the ovary: a clinicopathologic analysis of 40 cases and a review of the literature. Am J Surg Pathol. (2002) 26:1243–58. doi: 10.1097/00000478-200210000-00001
4. Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol. (1990) 21:363–81. doi: 10.1016/0046-8177(90)90198-E
5. Clement PB. Mullerian adenosarcomas of the uterus with sarcomatous overgrowth. A clinicopathological analysis of 10 cases. Am J Surg Pathol. (1989) 13:28–38. doi: 10.1097/00000478-198901000-00004
6. Hodgson A, Amemiya Y, Seth A, Djordjevic B, Parra-Herran C. High-grade mullerian adenosarcoma: genomic and clinicopathologic characterization of a distinct neoplasm with prevalent TP53 pathway alterations and aggressive behavior. Am J Surg Pathol. (2017) 41:1513–22. doi: 10.1097/PAS.0000000000000907
7. Howitt BE, Sholl LM, Dal Cin P, Jia Y, Yuan L, MacConaill L, et al. Targeted genomic analysis of mullerian adenosarcoma. J Pathol. (2015) 235:37–49. doi: 10.1002/path.4442
8. Piscuoglio S, Burke KA, Ng CK, Papanastasiou AD, Geyer FC, Macedo GS, et al. Uterine adenosarcomas are mesenchymal neoplasms. J Pathol. (2016) 238:381–8. doi: 10.1002/path.4675
9. Bean GR, Anderson J, Sangoi AR, Krings G, Garg K. DICER1 mutations are frequent in müllerian adenosarcomas and are independent of rhabdomyosarcomatous differentiation. Modern Pathol. (2019) 32:280–9. doi: 10.1038/s41379-018-0132-5
10. Abdul-Karim FW, Cohen RE. Atypical stromal cells of lower female genital tract. Histopathology. (1990) 17:249–53. doi: 10.1111/j.1365-2559.1990.tb00715.x
11. Tai LH, Tavassoli FA. Endometrial polyps with atypical (bizarre) stromal cells. Am J Surg Pathol. (2002) 26:505–9. doi: 10.1097/00000478-200204000-00014
12. Heller D, Barrett T. Bizarre stromal cells in an endometrial polyp. Int J Surg Pathol. (2016) 24(4):320–1. doi: 10.1177/1066896916632589
13. Usubütün A, Karaman N, Ayhan A, Küxcükali Türkan. Atypical endometrial stromal cells related with a polypoid leiomyoma with bizarre nuclei: A case report. Int J Gynecol Pathol. (2005) 24:352–4. doi: 10.1097/01.pgp.0000166996.57196.cb
14. Oliner JD, Saiki AY, Caenepeel S. The role of MDM2 amplification and overexpression in tumorigenesis. Cold Spring Harb Perspect Med. (2015) 6:a026336. doi: 10.1101/cshperspect.a026336
15. Baker SJ, Poulikakos PI, Irie HY, Parekh S, Reddy EP. CDK4: a master regulator of the cell cycle and its role in cancer. Genes Cancer. (2022) 13:21–45. doi: 10.18632/genesandcancer.v13
Keywords: polypoid lesions, adenosarcoma, bizarre stromal cells, MDM2, uterus
Citation: Xiao X and He Y (2024) Case report: An atypical case of uterine adenosarcoma with extensive bizarre stromal cells and amplification of MDM2. Front. Oncol. 14:1351646. doi: 10.3389/fonc.2024.1351646
Received: 06 December 2023; Accepted: 26 February 2024;
Published: 14 March 2024.
Edited by:
Luigi Tornillo, University of Basel, SwitzerlandReviewed by:
José Manuel Lopes, University of Porto, PortugalGiuseppe Scibilia, Gynecology and Obstetrics Department, Italy
Copyright © 2024 Xiao and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying He, aGV5aW5nNjI2QDE2My5jb20=
 Xiaoling Xiao
Xiaoling Xiao Ying He
Ying He