
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 06 May 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1347742
This article is part of the Research TopicAdvances in the use of EGFR TKIs in the Treatment of NSCLCView all 21 articles
 Petros Christopoulos1,2
Petros Christopoulos1,2 Franziska Herster3
Franziska Herster3 Petra Hoffknecht4
Petra Hoffknecht4 Markus Falk5,6
Markus Falk5,6 Markus Tiemann5,6
Markus Tiemann5,6 Hans-Georg Kopp3
Hans-Georg Kopp3 Andre Althoff7
Andre Althoff7 Anja Stammberger8
Anja Stammberger8 Eckart Laack9*
Eckart Laack9*Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) represent first-line standard of care in unresectable EGFR mutation-positive (EGFRm+) non-small cell lung cancer (NSCLC). However, 10–20% of patients with EGFRm+ NSCLC have uncommon EGFR variants, defined as mutations other than L858R substitutions or exon 19 deletions. NSCLC harboring uncommon EGFR mutations may demonstrate lower sensitivity to targeted agents than NSCLC with L858R or exon 19 deletion mutations. Prospective clinical trial data in patients with NSCLC uncommon EGFR mutations are lacking. Afatinib is a second-generation TKI and the only Food and Drug Administration-approved drug for some of the more prevalent uncommon EGFR mutations. We present a series of seven case reports describing clinical outcomes in afatinib-treated patients with NSCLC harboring a diverse range of extremely rare mutations with or without co-mutations affecting other genes. EGFR alterations included compound mutations, P-loop αC-helix compressing mutations, and novel substitution mutations. We also present a case with NSCLC harboring a novel EGFR::CCDC6 gene fusion. Overall, the patients responded well to afatinib, including radiologic partial responses in six patients during treatment. Responses were durable for three patients. The cases presented are in line with a growing body of clinical and preclinical evidence that indicating that NSCLC with various uncommon EGFR mutations, with or without co-mutations, may be sensitive to afatinib.
Activating epidermal growth factor receptor (EGFR) mutations occur in 14–38% of non-small cell lung cancer (NSCLC) (1). EGFR tyrosine kinase inhibitors (TKIs) represent first-line standard of care in unresectable EGFR mutation-positive (EGFRm+) disease (2). Most EGFRm+ NSCLC is driven by the so-called classical or common EGFR mutations: L858R or exon 19 deletions (Del19) (3). Approximately 10–20% of EGFRm+ NSCLC cases harbor uncommon EGFR mutations, defined as activating EGFR mutations other than L858R and Del19 (3–5). Different variants demonstrate varying sensitivity to EGFR TKIs, and uncommon EGFR mutations show lower sensitivity to many targeted agents than classical EGFR mutations; therefore, precise characterization of uncommon EGFR mutations is important to optimize treatment strategies (4).
The most prevalent uncommon EGFR variants in NSCLC are S768I, L861Q, and G719X, for which the preferred first-line treatments in advanced disease are afatinib or osimertinib (6–9). Afatinib is the only U.S. Food and Drug Administration-approved drug against S768I, L861Q, and G719X EGFR mutations with demonstrated efficacy in prospective clinical studies (10). Little prospective data exist for uncommon mutations; however, retrospective studies (7, 11–15) and databases (11, 16–18) provide some insight. Novel mutations continue to be identified that have no available clinical data to guide treatment decisions.
A recent preclinical investigation defined a structure-based classification system that permitted prediction of sensitivity to different generations of EGFR TKIs (first generation: erlotinib, gefitinib; second generation: afatinib, dacomitinib; third generation: osimertinib) (4). “Classical-like” mutations (e.g. L858R; Del19; S720P; L861Q/R) were predicted to be sensitive to all generations of EGFR TKI; “T790M-like” mutations (e.g. T790M; certain T790M-containing compound mutations) were predicted to be sensitive to third-generation EGFR TKIs; exon 20 insertions (e.g. S768dupSVD; A767dupASV) were predicted to be sensitive to exon 20 insertion-targeted compounds and second-generation EGFR TKIs; and P-loop αC-helix compressing (PACC) mutations (e.g. G719X; S768I; delE709_T710insD, and other uncommon EGFR mutations were predicted to be particularly sensitive to second-generation EGFR TKIs (4). PACC mutations occur across exons 18–21 and alter the orientation of the P-loop or αC-helix of EGFR, affecting interactions with certain TKIs. Second-generation TKIs do not interact with the P-loop of EGFR and are therefore predicted to have greater activity against PACC mutations than other generations of EGFR TKI (4). Some retrospective data support this prediction (4).
Treatment decisions can be very challenging in patients with NSCLC with multiple EGFR mutations (compound mutation) or uncommon EGFR mutations co-occurring with other gene alterations in the tumor. Treatment might be dependent on which mutation has the higher allele frequency (19) or which other cancer-related genes have co-occurring mutations (20, 21). For example, TP53, the most commonly mutated gene in NSCLC, co-occurs in ~65% of cases of EGFRm+ NSCLC, and has been associated with poor prognosis and primary/acquired resistance to EGFR TKIs (22–27).
This case series describes outcomes of patients with NSCLC harboring uncommon EGFR mutations who received afatinib. Cases were collected during routine clinical treatment across six centers in Germany between 2017 and 2023.
After presenting with a cough, a 58-year-old female with a history of paroxysmal atrial fibrillation and hemangioma was diagnosed with lung adenocarcinoma (stage IB) in May 2017, and underwent resection of the left upper lobe and lymphadenectomy. In August 2017, a single symptomatic metastasis to the third lumbar spine vertebrae with infiltration of the major psoas was detected. The patient received radiotherapy and began treatment with denosumab (120 mg every 4 weeks; Figure 1A). However, in September 2017, a new abdominal lesion next to the left lobe of the liver was reported. The patient refused biopsy to confirm diagnosis of distant metastases. Hybrid capture next-generation sequencing (NGS) of the initially resected tumor tissue identified a novel compound EGFR mutation, comprising two substitution mutations on exons 18 (G719A, a PACC mutation) and 21 (L833F, a classical-like mutation). A TP53 point inactivating mutation (p.T140 frame shift [non-activating mutation]) was also detected.

Figure 1 Cases 1 and 2 (PACC/classical compound EGFR mutation). (A) I Timeline of Case 1. II, III. May 2017. Tumor staging pT2a pN0 M0, St. IIA UICC 8. IV. August 2017. A single symptomatic metastasis and infiltration of the major psoas was detected. V. January 2020. Adrenal metastases detected. VI. August 2020. Disease progression was observed in the area of the former adrenal gland. (B) Timeline of Case 2. *Single symptomatic metastasis and infiltration of the major psoas. †488.12 mg carboplatin AUC 80%; 796 mg pemetrexed 80%. ABCP, atezolizumab plus bevacizumab plus carboplatin plus paclitaxel; AE, adverse event; DOR, duration of response; G, grade; NGS, next generation sequencing; NSCLC, non-small cell lung cancer; PACC, P-loop and αC-helix compressing; PD, progressive disease; Q4W, every 4 weeks; RT, radiotherapy; UICC, Union for International Cancer Control.
The patient began treatment with first-line afatinib, 40 mg once per day (QD), in September 2017. In November 2017, following grade 3 diarrhea, grade 2–3 stomatitis, and rhagades of the fingers, the dose of afatinib was reduced to 30 mg QD.
The patient achieved complete remission of the abdominal lesion, with the response lasting 28 months. Metastases were detected in the left adrenal gland in January 2020. In February 2020, the patient underwent adrenalectomy (R1) followed by radiotherapy and continued afatinib treatment. Disease was stable until June 2020.
In August 2020, disease progression was observed in the area of the former adrenal gland. Urinary retention was treated with a double J-tumor stent and the patient experienced urosepsis (Proteus mirabilis, two events) and nephroptosis. Following local progression and subsequent left nephrectomy in October 2020, afatinib therapy was terminated in December 2020, and the patient received second-line therapy with carboplatin/paclitaxel, atezolizumab, and bevacizumab. The total duration of afatinib treatment was 35 months.
A 71-year-old male with a history of polymyalgia rheumatica consulted his general practitioner with concerns relating to a family history of cancer. In May 2021, elevated serum tumor markers were reported, and the patient was subsequently diagnosed with NSCLC (stage IVB; programmed death-ligand 1 [PD-L1]: 1%; pulmonary and bone metastases) (Figure 1B). NGS (QIAseq Custom Lung Panel, Qiagen) identified a novel compound mutation comprising substitution mutations on exon 18 (G719A, PACC) and exon 21 (L861R, classical-like).
The patient began treatment in May 2021, with first-line afatinib (30 mg QD) plus denosumab (120 mg every 4 weeks). Following presentation with exanthem (June/July 2021, treated with topical corticosteroid) and diarrhea (August 2021, treated with loperamide), the dose of afatinib was reduced to weekly alternation of 20/30 mg.
Partial responses (PRs) were reported in June, July, and August 2021. After approximately 5 months on treatment, stable disease (SD) was reported. However, afatinib was terminated in October 2021 owing to intolerable adverse events (AEs), and osimertinib (80 mg QD) was initiated. Progressive disease (PD) was reported in January 2022, which resulted in discontinuation of osimertinib and initiation of pemetrexed and carboplatin treatment.
A 64-year-old male presented with persistent cough. A computerized tomography (CT) scan revealed pulmonary nodules on both sides of the lung, and following a biopsy by bronchoscopy the patient was diagnosed with thyroid transcription factor-1 (TTF1)-positive adenocarcinoma (stage IA) in May 2010, with resection in the same month (Figure 2A). Relevant comorbidities included arterial hypertension, treated with candesartan (32 mg). In June 2019, aged 73 years, he was diagnosed with bilateral pulmonary metastases (TTF1-positive adenocarcinoma), following biopsy by bronchoscopy. NGS detected an uncommon EGFR exon 18 deletion insertion mutation (delE709_T710insN), classified as PACC (4).
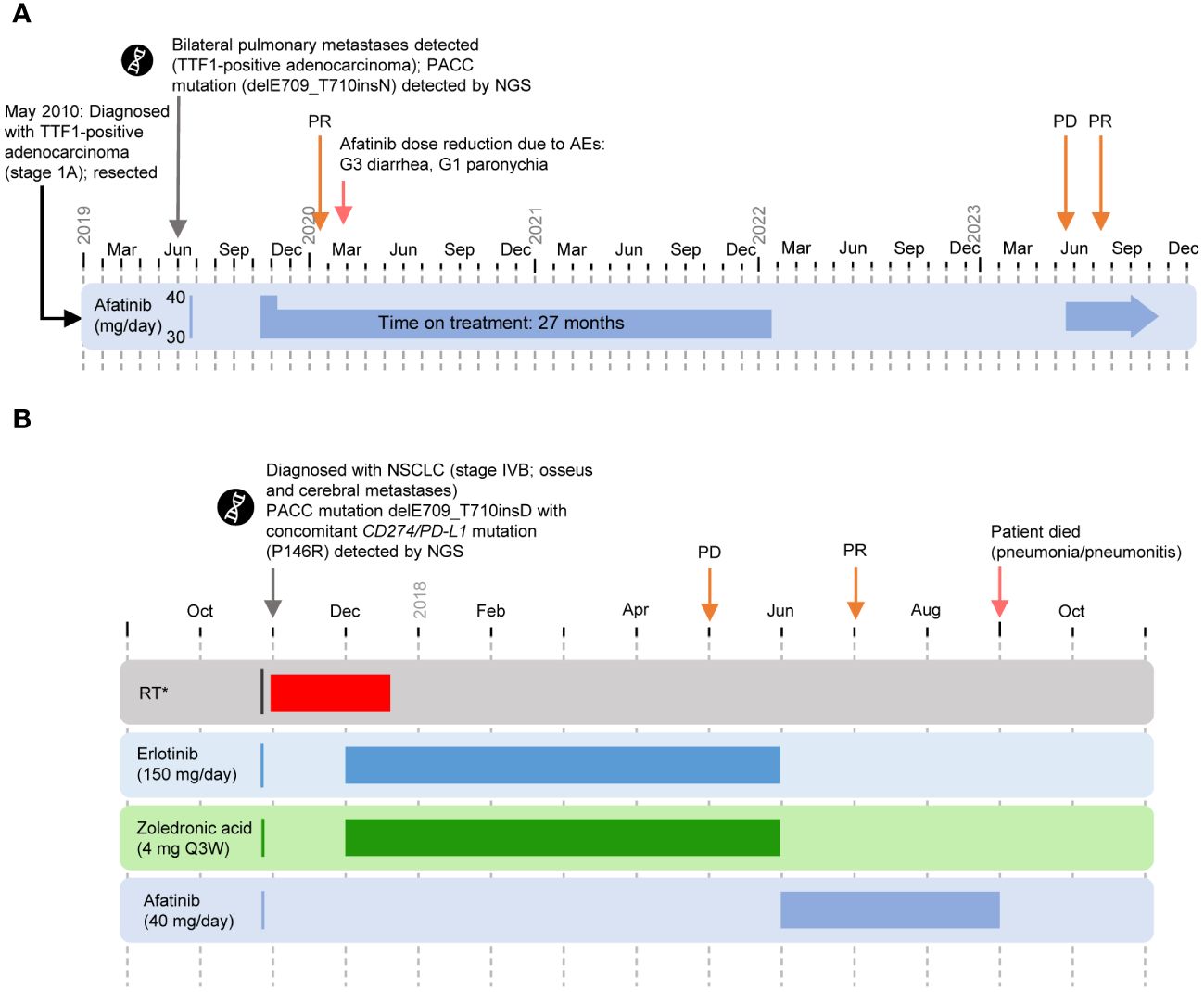
Figure 2 Cases 3 and 4. (PACC exon 18 deletion insertion mutation) (A) Timeline of Case 3. Afatinib was discontinued in January 2022 at the patient’s request. In May 2023, progression of the lung metastases was observed following a CT scan. Afatinib was resumed and PR was observed in July 2023. As of September 2023, the patient remains symptom free. (B) Timeline of Case 4. *Nov 2017: palliative RT (thoracic spine, lumbar, Os pubis left [5 Gy/Fraction]. WBRT: Nov–Dec 2017. Zoledronic acid: Dec 2017–Jun 2018. First-line treatment with erlotinib: Dec 2017–Jun 2018. Second-line treatment with afatinib: Jun–Sep 2018. AE, adverse event; G, grade; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; PACC, P-loop and αC-helix compressing; PD, progressive disease; PR, partial response; Q3W, every 3 weeks; RT, radiotherapy; TTF-1, thyroid transcription factor-1; WBRT, whole brain radiotherapy.
The patient began first-line afatinib (30 mg QD) in October 2019. Following emergence of grade 3 diarrhea, afatinib was paused and the patient was treated with loperamide and hydration until symptoms had resolved. Following grade 1 paronychia, the dose of afatinib was reduced to 20 mg QD. No further AEs occurred.
The patient achieved a best response of PR after 3 months of treatment with afatinib. The patient reported good quality of life with no clinical symptoms of disease. Afatinib was continued for 27 months and was discontinued in January 2022 at the patient’s request. In May 2023, progression of the lung metastases was observed following a CT scan. Afatinib was resumed and PR was observed in July 2023. As of September 2023, the patient remains symptom free.
A 64-year-old female was diagnosed with NSCLC (stage IVB) in November 2017 during workup of a painful pathologic fracture in the 8th thoracic vertebra (Figure 2B). Radiologic imaging revealed a central tumor of the left lower lung lobe, as well as additional bone and brain metastases. Relevant comorbidities included arterial hypertension, chronic bronchitis, osteoporosis, and gastritis. NGS identified delE709_T710insD with a co-occurring CD274/PD-L1 mutation (P146R).
The patient received whole-brain radiotherapy from November to December 2017, with palliative radiotherapy (thoracic spine, lumbar, 20 Gy, 5 Gy/fraction) in November 2017. The patient began first-line erlotinib (150 mg QD) plus intravenous zoledronic acid (4 mg every 3 weeks) in December 2017. Following PD in May 2018, treatment was discontinued in June. The patient received second-line afatinib (40 mg QD) starting in June 2018, and achieved a PR in July 2018. Subsequently, the patient experienced pneumonitis probably related to preceding radiotherapy of the thoracic spine, leading to death in September 2018 after approximately 3 months on treatment.
A 74-year-old male presenting with weight loss was diagnosed with NSCLC (stage IVA, with pulmonary and pleural metastases) in February 2018 (Figure 3A). Relevant comorbidities included arterial hypertension, non-erosive reflux disease, chronic hepatitis C infection, hiatal hernia, and other gastrointestinal conditions. NGS testing confirmed a rare EGFR exon 25 mutation, H988R, with co-occurring TP53 and CDKN2A mutations.
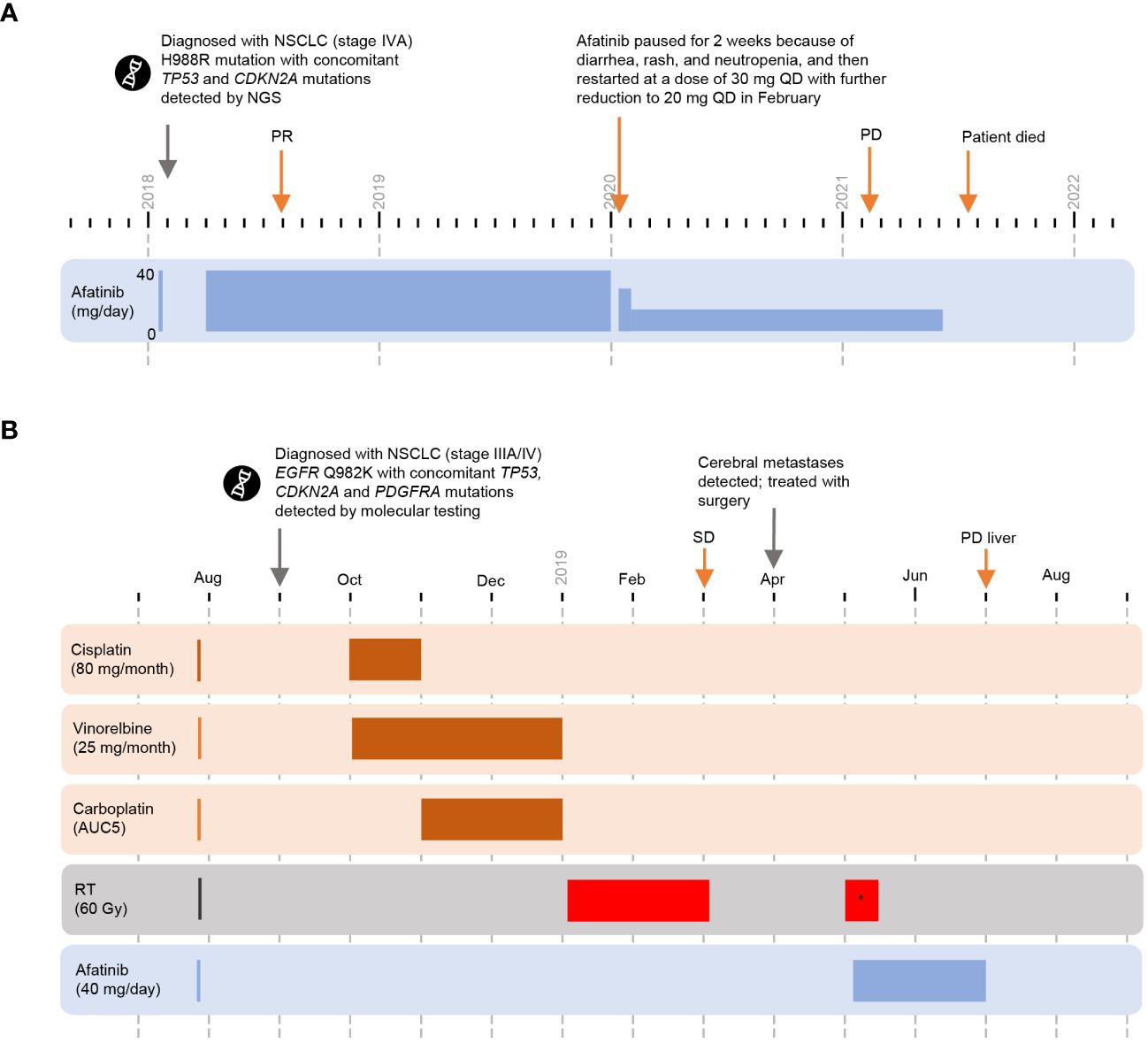
Figure 3 Cases 5 and 6 (novel substitution mutations) (A) Timeline of Case 5. (B) Timeline of Case 6. *whole brain radiotherapy. CDKN2A, cyclin-dependent kinase inhibitor 2A; EGFR, epidermal growth factor receptor; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; QD, each day; RT, radiotherapy; SD, stable disease; TP53, tumor protein 53.
First-line treatment with afatinib (40 mg QD) started April 2018 and a PR was reported in August 2018. In January 2020, afatinib treatment was paused for 2 weeks because of diarrhea, rash, and neutropenia, and then restarted at a dose of 30 mg QD. The dose of afatinib was further reduced to 20 mg QD in February 2020. PR was maintained until at least February 2021 (date of last imaging). The patient deteriorated clinically without tumor progression and died in July 2021. Total duration on afatinib was 39 months; no other treatment was reported.
In September 2018, an asymptomatic 65-year-old male with a history of arterial hypertension, latent diabetes mellitus, and degenerative spinal syndrome, was diagnosed with NSCLC (stage IIIA/IV) following magnetic resonance imaging examination of the cervical spine (Figure 3B); suspicious enlargement of the left adrenal gland was also observed. Positron emission tomography-CT standardized uptake values were 11–15 for the primary lung tumor and mediastinal lymph nodes, and four for the left adrenal gland. After discussion with the interdisciplinary tumor board, it was agreed to treat the patient according to stage III disease management practice and continue to monitor the left adrenal gland with serial imaging. Molecular testing confirmed a novel point mutation in EGFR exon 24 (Q982K) with co-occurring TP53, CDKN2A, and PDGFRA mutations.
Cisplatin (80 mg/m2 day 1 every 3 weeks) plus vinorelbine (25 mg/m2 day 1 and day 8 every 3 weeks), was initiated in October 2018. An episode of tinnitus prompted a switch from cisplatin to carboplatin (AUC 5) from cycle 2. Sequential radiotherapy (60 Gy) began in January 2019. Best overall response to chemoradiotherapy was SD in March 2019.
Cerebellar metastases were detected in April 2019, and were resected in the same month, followed by whole-brain radiotherapy in May 2019. The patient received second-line afatinib (40 mg QD) starting in May 2019. Despite a stable thoracic tumor, new liver lesions were detected in July 2019 and afatinib treatment was terminated. The total duration of afatinib treatment was 2 months.
A 56-year-old female ex-smoker (until 2005, 20 pack-years) with a history of bronchial asthma who presented with pleural effusion affecting the left thorax was diagnosed with stage IVB (UICC) adenocarcinoma NSCLC (primary lesion left lower lobe) in November 2022 (TTF-1-positive, CK7 positive, Ki-67 score: 70%; PD-L1 Tumor Proportion Score 0%, PD-L1 immune cells 0%; cT3 pN2 cM1a [pleural, pulmonary, osseus, and cervical lymph nodes]). First-line treatment (carboplatin/pemetrexed/pembrolizumab ± vibostolimab/denusomab) as part of the KeyVibe-007 trial (EUDRA-CT: 2021-004564-94) began in January 2023 (Figure 4). At restaging in May 2023, multiple new bilateral pulmonary metastases were detected and participation in the trial ended.
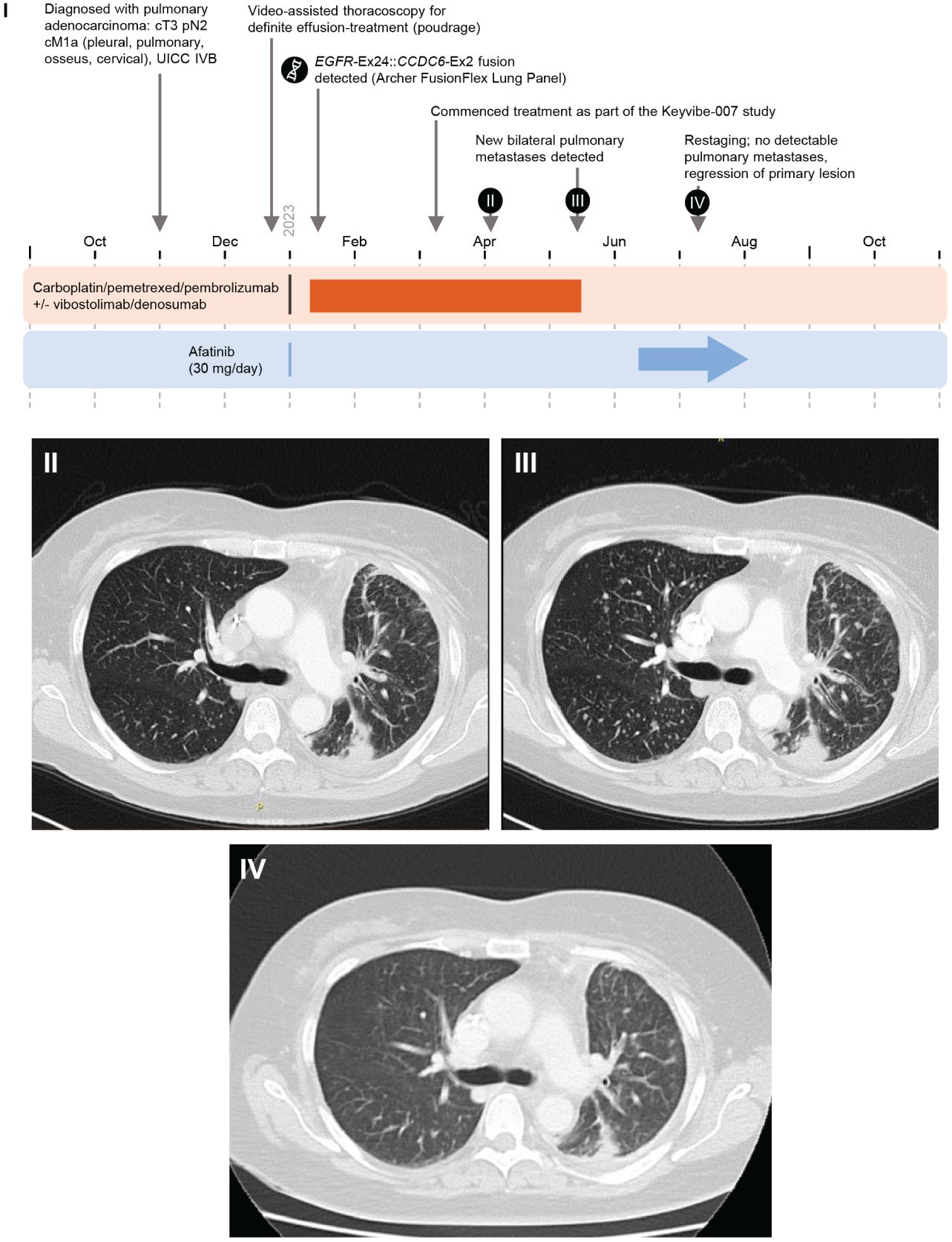
Figure 4 Case 7 (novel EGFR::CCDC6 fusion) I. Timeline of Case 1. II. April 2023. III. May 2023, New bilateral pulmonary metastases detected. IV. July 2023. Restaging: no detectable pulmonary metastases, regression of primary lesion. Ex, Exon; UICC, Union for International Cancer Control.
Molecular testing (Archer FusionPlex Lung Panel) in January 2023 detected an EGFR exon 24::CCDC6 (coiled-coil domain containing 6) exon 2 fusion. Treatment with second-line afatinib 30 mg QD began in June 2023. Regression of the primary lesion and complete resolution of pulmonary metastases were observed after 4 weeks. Treatment and response are ongoing.
This report describes outcomes with afatinib in NSCLC with a diverse range of extremely rare EGFR alterations found in routine clinical practice (Supplementary Figure 1). Five patients harbored rare aberrations that, to the best of our knowledge, have not been previously described in literature. Four patients had known PACC mutations either in isolation or as part of a compound EGFR mutation. Two patients had PACC mutations with co-occurring mutations in TP53 or PDGFRA. One patient had an EGFR gene fusion, a rare type of driver event. Overall, these patients responded well to afatinib (Supplementary Table 1), consistent with preclinical modelling (4) and previous studies of afatinib treatment in patients with uncommon mutations (11, 28).
Cases 1 and 2 involved compound mutations comprising PACC and classical-like mutations which both responded to afatinib. Case 1 had a durable response to afatinib despite the presence of a co-occurring TP53 mutation plus a novel compound EGFR mutation, that included substitution mutations on exons 18 (G719A; a PACC mutation) and 21 (L833F; a classical-like mutation). Cases 3 and 4 exhibited PACC exon 18 deletion insertion mutations, and both patients had a clinical response to afatinib treatment, including a long (>2 years) response reported for Case 3. Case 4 also harbored a concomitant CD274/PD-L1 mutation, which we believe has not previously been described. Accumulating evidence indicates that delE709_T710insD is sensitive to afatinib and does not appear to be affected by the concomitant mutation. Cases 5 and 6 had EGFR substitution mutations in exon 25 (H988R) and exon 24 (Q982K), respectively; situated in the cytoplasmic region C-terminal domain, beyond the tyrosine kinase domain. Mutations here may destabilize receptor conformation, potentially causing upregulation of kinase activity and irregular downstream signaling (29). Both had co-occurring mutations in other genes, which are known to be prognostic biomarkers (23, 30–32). In Cases 5 and 6, prolonged survival was observed with first-line afatinib. In Case 7, the patient with the fusion, a dramatic response was observed in response to second-line afatinib. It is currently unknown how these rare mutations, and the EGFR::CCDC6 fusion, align with the structure-based classification system (4), highlighting the difficulty associated with making treatment decisions for patients with novel mutations.
The selection of optimal treatment for patients with rare or compound EGFR mutations is often complex. Previous case reports describe compound mutations comprising substitution mutations classified as PACC and classical-like that respond to TKIs (33, 34). We found only one other report of a L833F-containing mutation, in which a patient with an L833F/L861Q mutation also achieved durable PR in response to first-line afatinib (progression-free survival [PFS]: 10 months) and clinical benefit to later-line osimertinib (34). A previous review briefly mentions the patient described in Case 5 with the H988R substitution (35). The review also mentions an additional patient with an H988R mutation who did not respond to afatinib treatment (35). While the recent structure-based classification system (4) has provided helpful information regarding predicted sensitivities of uncommon mutations, the sensitivities of rare compound mutations and the influence of co-occurring mutations remain difficult to predict in the absence of prospective clinical trials. Treatment decisions for these patients requires careful consideration.
Previous case reports of EGFR PACC insertion deletion mutations in NSCLC indicate sensitivity to afatinib and other EGFR TKIs (gefitinib, erlotinib) (36–38), including a 23-month PFS response with afatinib (37). Case 4 achieved a PR with afatinib after PD on erlotinib, which is also consistent with a previous case study where clinical benefit with afatinib following prior erlotinib treatment was reported (39). We have identified 16 reports of patients with a delE709_T710insD mutation who received EGFR TKIs (16), and consistent with the preclinical modelling (4), delE709_T710insD-mutated NSCLC appears to be more sensitive to afatinib than first-generation TKIs. In a review of 14 cases, PFS was significantly improved with afatinib compared with first-generation TKIs (median 7.0 vs. 3.1 months; p = 0.005) and all patients receiving afatinib achieved a PR (36).
Although EGFR gene fusions are rare, clinical responses in EGFR fusion-driven tumors have been reported with EGFR TKIs (40, 41). The EGFR::CCDC6 fusion is novel, to our knowledge; however CCDC6-tyrosine kinase fusions (for example with ALK, ROS1, or RET), are recognized–and druggable–driver events in lung cancer (42). The durable response in this patient reinforces the importance of testing for fusion driver events, as this important class of somatic alteration can underly disease sensitive to targeted agents.
The interplay between EGFR alterations and co-occurring mutations in different genes represents a new frontier for NSCLC clinical research. In our case series, three patients had co-mutations in TP53, two had co-mutations in CDKN2A, and one had overexpression of PD-L1, plus a co-mutation affecting, CD274/PD-L1. These alterations occur commonly in patients with EGFRm+ NSCLC and have been associated with poor prognosis and resistance to TKIs (23, 30–32). However, in our case series, co-mutations did not prevent patients gaining benefit from afatinib treatment. Two patients with co-occurring TP53 mutations exhibited prolonged time on treatment (35 and 39 months), durable response to afatinib was observed in one of the two patients with CDKN2A mutations (both patients with substitution mutations), and a PR was observed in the patient with a CD274/PD-L1 mutation. Decisions about the initial treatment of NSCLC with uncommon EGFR mutations have key importance for the subsequent course and should be made carefully based on published evidence about TKI efficacy, as this can vary widely according to the specific mutation (43), and real-world data indicate that approximately 30–35% of patients do not receive treatment after the first line (44).
Reports of NSCLC with TKI-sensitive uncommon EGFR mutations may also prompt further clinical trial research in this setting; however, if clinical practice is to evolve, obstacles in terms of mutation testing must also be overcome. Current guidelines recommend that broad molecular profiling should be carried out for all patients diagnosed with NSCLC, which generally means NGS-based testing (9, 45). Globally, rates of molecular profiling in lung cancer patients are below 50% with a wide regional variation (46); financial constraints, quality and standardization of testing, access to testing, awareness, and turnaround times have all been cited as barriers to testing. Furthermore, some commonly used testing panels may miss uncommon mutations or those occurring outside of Exons 19, 20, and 21 (47, 48). Advances in testing strategies and methodologies have the potential to improve molecular profiling in NSCLC; these include the adoption of the structure-based classification system into testing panels and the use of liquid biopsy as a rapid, non-invasive means of assaying genomic profiles (49). Liquid biopsy may be particularly useful for monitoring temporal changes in mutation and biomarker status and is already in use to detect resistance to EGFR TKIs (50).
Integration of classification systems and real-world evidence may support future treatment decisions in patients with uncommon EGFRm+ NSCLC. Better understanding of the impact of co-occurring mutations is required. Patients with uncommon EGFR mutations are now being included in several ongoing randomized clinical trials assessing the efficacy and safety of EGFR TKIs in NSCLC (51–54). In a recent analysis of the phase III ACHILLES trial in treatment-naive patients with uncommon or compound EGFR mutations, PFS with afatinib (10.6 months) was significantly longer than with platinum-based chemotherapy (5.7 months; hazard ratio: 0.422, p = 0.0007), supporting the use of first-line EGFR TKIs in this setting (55).
These cases corroborate the clinical and preclinical evidence that certain uncommon EGFR mutations are sensitive to afatinib. This series illustrates the importance of further study in this area and the need for publicly available mutation databases to support prescribing decisions in the absence of prospective clinical trial data for patients with rare mutations.
The datasets generated and analyzed during the study are available from author AS on reasonable request. Requests to access these datasets should be directed to AS: anja.stammberger@boehringer-ingelheim.com.
Ethical approval was not required for the studies involving humans because Manuscript details a collection of case studies from patients treated in routine practice, not results for a formal study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
PC: Data curation, Formal analysis, Writing – review & editing. FH: Data curation, Formal analysis, Writing – review & editing. PH: Data curation, Formal analysis, Writing – review & editing. MF: Data curation, Formal analysis, Writing – review & editing. MT: Data curation, Formal analysis, Writing – review & editing. H-GK: Data curation, Formal analysis, Writing – review & editing. AA: Data curation, Formal analysis, Writing – review & editing. AS: Data curation, Formal analysis, Writing – review & editing. EL: Data curation, Formal analysis, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The case series report was supported and funded by Boehringer Ingelheim.
We thank the patients, their families, and all the investigators who participated in these studies. Medical writing support was provided by Jim Sinclair, PhD, of Ashfield Medcomms, an Inizio company, and funded by Boehringer Ingelheim.
AS is a current employee of Boehringer Ingelheim. PC reports research funding from Amgen, AstraZeneca, Boehringer Ingelheim, Novartis, Roche, Takeda, and advisory board/lecture fees from AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Gilead, Janssen, Novartis, Pfizer, Roche, Takeda, and Thermo Fisher.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1347742/full#supplementary-material
1. Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. (2016) 7:78985–93. doi: 10.18632/oncotarget.12587
2. Hsu WH, Yang JC, Mok TS, Loong HH. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. (2018) 29:i3–9. doi: 10.1093/annonc/mdx702
3. Zhang T, Wan B, Zhao Y, Li C, Liu H, Lv T, et al. Treatment of uncommon EGFR mutations in non-small cell lung cancer: new evidence and treatment. Transl Lung Cancer Res. (2019) 8:302–16. doi: 10.21037/tlcr.2019.04.12
4. Robichaux JP, Le X, Vijayan RSK, Hicks JK, Heeke S, Elamin YY, et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature. (2021) 597:732–7. doi: 10.1038/s41586-021-03898-1
5. Evans M, O'Sullivan B, Smith M, Hughes F, Mullis T, Trim N, et al. Large-scale EGFR mutation testing in clinical practice: analysis of a series of 18,920 non-small cell lung cancer cases. Pathol Oncol Res. (2019) 25:1401–9. doi: 10.1007/s12253-018-0460-2
6. Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. (2015) 16:830–8. doi: 10.1016/S1470-2045(15)00026-1
7. Cho JH, Lim SH, An HJ, Kim KH, Park KU, Kang EJ, et al. Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-LU15-09). J Clin Oncol. (2020) 38:488–95. doi: 10.1200/JCO.19.00931
8. Leitlinienprogramm Onkologie. S3-leitlinie prävention, diagnostik, therapie und nachsorge des lungenkarzinoms (2022). Available at: https://www.leitlinienprogramm-onkologie.de/leitlinien/lungenkarzinom/ (Accessed February, 2023).
9. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, Version 3.2022, NCCN Clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:497–530. doi: 10.6004/jnccn.2022.0025
10. Boehringer Ingelheim International GmbH. GILOTRIF® (afatinib) tablets, for oral use. Highlights of prescribing information (2018). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/201292s014lbl.pdf (Accessed February, 2022).
11. Yang JC, Schuler M, Popat S, Miura S, Park K, Passaro A, et al. Afatinib for the treatment of non-small cell lung cancer harboring uncommon EGFR mutations: an updated database of 1023 cases brief report. Front Oncol. (2022) 12:834704. doi: 10.3389/fonc.2022.834704
12. Eide IJZ, Stensgaard S, Helland A, Ekman S, Mellemgaard A, Hansen KH, et al. Osimertinib in non-small cell lung cancer with uncommon EGFR-mutations: a post-hoc subgroup analysis with pooled data from two phase II clinical trials. Transl Lung Cancer Res. (2022) 11:953–63. doi: 10.21037/tlcr-21-995
13. Bar J, Peled N, Schokrpur S, Wolner M, Rotem O, Girard N, et al. UNcommon EGFR mutations: International Case series on efficacy of osimertinib in Real-life practice in first liNe setting (UNICORN). J Thorac Oncol. (2023) 18(2):169–180. doi: 10.1016/j.jtho.2022.10.004
14. Pizzutilo EG, Cerea G, Oresti S, Agostara AG, Signorelli D, Stabile S, et al. 996P - Activity of osimertinib in NSCLC with uncommon EGFR mutations: retrospective observational multicenter study (ARTICUNO). Ann Oncol. (2022) 33:S448–554. doi: 10.1016/annonc/annonc1064
15. Janning M, Süptitz J, Albers-Leischner C, Delpy P, Tufman A, Velthaus-Rusik JL, et al. Treatment outcome of atypical EGFR mutations in the German National Network Genomic Medicine Lung Cancer (nNGM). Ann Oncol. (2022) 33:602–15. doi: 10.1016/j.annonc.2022.02.225
16. Boehringer Ingelheim International GmbH. Uncommon EGFR mutations. Available at: https://www.uncommonegfrmutations.com (Accessed January, 2023).
17. Vanderbilt-Ingram Cancer Center. My cancer genome (R) genetically informed cancer medicine. Available at: https://www.mycancergenome.org/ (Accessed January, 2023).
18. Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. (2017) 1:1–16. doi: 10.1200/PO.17.00011
19. Friedlaender A, Tsantoulis P, Chevallier M, De Vito C, Addeo A. The impact of variant allele frequency in EGFR mutated NSCLC patients on targeted therapy. Front Oncol. (2021) 11:644472. doi: 10.3389/fonc.2021.644472
20. Rosell R, Karachaliou N. Co-mutations in EGFR driven non-small cell lung cancer. EBioMedicine. (2019) 42:18–9. doi: 10.1016/j.ebiom.2019.03.037
21. Zhang Y, Li S, Lyu Z, Cai J, Zheng N, Li Y, et al. The co-mutation of EGFR and tumor-related genes leads to a worse prognosis and a higher level of tumor mutational burden in Chinese non-small cell lung cancer patients. J Thorac Dis. (2022) 14:185–93. doi: 10.21037/jtd-21-1921
22. Jiao XD, Qin BD, You P, Cai J, Zang YS. The prognostic value of TP53 and its correlation with EGFR mutation in advanced non-small cell lung cancer, an analysis based on cBioPortal data base. Lung Cancer. (2018) 123:70–5. doi: 10.1016/j.lungcan.2018.07.003
23. Canale M, Andrikou K, Priano I, Cravero P, Pasini L, Urbini M, et al. The role of TP53 mutations in EGFR-mutated non-small-cell lung cancer: clinical significance and implications for therapy. Cancers (Basel). (2022) 14:1143. doi: 10.3390/cancers14051143
24. Roeper J, Falk M, Chalaris-Rissmann A, Lueers AC, Ramdani H, Wedeken K, et al. TP53 co-mutations in EGFR mutated patients in NSCLC stage IV: a strong predictive factor of ORR, PFS and OS in EGFR mt+ NSCLC. Oncotarget. (2020) 11:250–64. doi: 10.18632/oncotarget.27430
25. Jung S, Kim DH, Choi YJ, Kim SY, Park H, Lee H, et al. Contribution of p53 in sensitivity to EGFR tyrosine kinase inhibitors in non-small cell lung cancer. Sci Rep. (2021) 11:19667. doi: 10.1038/s41598-021-99267-z
26. Qin K, Hou H, Liang Y, Zhang X. Prognostic value of TP53 concurrent mutations for EGFR- TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: a meta-analysis. BMC Cancer. (2020) 20:328. doi: 10.1186/s12885-020-06805-5
27. Heredia D, Mas L, Cardona AF, Oyervides V, Motta Guerrero R, Galvez-Nino M, et al. A high number of co-occurring genomic alterations detected by NGS is associated with worse clinical outcomes in advanced EGFR-mutant lung adenocarcinoma: data from LATAM population. Lung Cancer. (2022) 174:133–40. doi: 10.1016/j.lungcan.2022.11.002
28. Yang JC, Schuler M, Popat S, Miura S, Heeke S, Park K, et al. Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: a database of 693 cases. J Thorac Oncol. (2020) 15:803–15. doi: 10.1016/j.jtho.2019.12.126
29. Amelia T, Kartasasmita RE, Ohwada T, Tjahjono DH. Structural insight and development of EGFR tyrosine kinase inhibitors. Molecules. (2022) 27:819. doi: 10.3390/molecules27030819
30. Chang SC, Lai YC, Chang CY, Huang LK, Chen SJ, Tan KT, et al. Concomitant genetic alterations are associated with worse clinical outcome in EGFR mutant NSCLC patients treated with tyrosine kinase inhibitors. Transl Oncol. (2019) 12:1425–31. doi: 10.1016/j.tranon.2019.07.008
31. Bai Y, Chen X, Hou L, Qian J, Jiang T, Zhou C, et al. PD-L1 expression and its effect on clinical outcomes of EGFR-mutant NSCLC patients treated with EGFR-TKIs. Cancer Biol Med. (2018) 15:434–42. doi: 10.20892/j.issn.2095-3941.2018.0223
32. Christopoulos P, Kirchner M, Roeper J, Saalfeld F, Janning M, Bozorgmehr F, et al. Risk stratification of EGFR(+) lung cancer diagnosed with panel-based next-generation sequencing. Lung Cancer. (2020) 148:105–12. doi: 10.1016/j.lungcan.2020.08.007
33. Berge EM, Aisner DL, Doebele RC. Erlotinib response in an NSCLC patient with a novel compound G719D+L861R mutation in EGFR. J Thorac Oncol. (2013) 8:e83–4. doi: 10.1097/JTO.0b013e31829ceb8d
34. Zhang Y, Shen JQ, Shao L, Chen Y, Lei L, Wang JL. Non-small-cell lung cancer with epidermal growth factor receptor L861Q-L833F compound mutation benefits from both afatinib and osimertinib: a case report. World J Clin cases. (2021) 9:8220–5. doi: 10.12998/wjcc.v9.i27.8220
35. Volckmar AL, Christopoulos P, Kirchner M, Allgauer M, Neumann O, Budczies J, et al. Targeting rare and non-canonical driver variants in NSCLC - An uncharted clinical field. Lung Cancer. (2021) 154:131–41. doi: 10.1016/j.lungcan.2021.02.022
36. Rubiera-Pebe R, Hicks JK, Tanvetyanon T. Efficacy of tyrosine kinase inhibitors against lung cancer with EGFR exon 18 deletion: case report and pooled analysis. Cancer Treat Res Commun. (2021) 28:100407. doi: 10.1016/j.ctarc.2021.100407
37. Wei Y, Cui Y, Guo Y, Li L, Zeng L. A lung adenocarcinoma patient with a rare EGFR E709_T710delinsD mutation showed a good response to afatinib treatment: a case report and literature review. Front Oncol. (2021) 11:700345. doi: 10.3389/fonc.2021.700345
38. An N, Wang H, Zhu H, Yan W, Jing W, Kong L, et al. Great efficacy of afatinib on a patient with lung adenocarcinoma harboring uncommon EGFR delE709_T710insD mutations: a case report. Onco Targets Ther. (2019) 12:7399–404. doi: 10.2147/OTT.S221638
39. Kobayashi Y, Togashi Y, Yatabe Y, Mizuuchi H, Jangchul P, Kondo C, et al. EGFR exon 18 mutations in lung cancer: molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first- or third-generation TKIs. Clin Cancer Res. (2015) 21:5305–13. doi: 10.1158/1078-0432.CCR-15-1046
40. Copia Sperandio R, Luiza Teixeira Tostes F, Vidal Campregher P, Ribeiro Paes V, Moura F, Schvartsman G. EGFR-RAD51 fusion in lung adenocarcinoma with systemic and intracranial response to osimertinib: a case report and review of the literature. Lung Cancer. (2022) 166:94–7. doi: 10.1016/j.lungcan.2022.02.006
41. Geoerger B, Marshall LV, Nysom K, Makin G, Bouffet E, Defachelles AS, et al. Afatinib in paediatric patients with recurrent/refractory ErbB-dysregulated tumours: results of a phase I/expansion trial. Eur J Cancer. (2023) 188:8–19. doi: 10.1016/j.ejca.2023.04.007
42. Cerrato A, Visconti R, Celetti A. The rationale for druggability of CCDC6-tyrosine kinase fusions in lung cancer. Mol Cancer. (2018) 17:46. doi: 10.1186/s12943-018-0799-8
43. Kohsaka S, Nagano M, Ueno T, Suehara Y, Hayashi T, Shimada N, et al. A method of high-throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med. (2017) 9:eaan6566. doi: 10.1126/scitranslmed.aan6566
44. Magios N, Bozorgmehr F, Volckmar AL, Kazdal D, Kirchner M, Herth FJ, et al. Real-world implementation of sequential targeted therapies for EGFR-mutated lung cancer. Ther Adv Med Oncol. (2021) 13:1–13. doi: 10.1177/1758835921996509
45. Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. (2018) 13:323–58. doi: 10.1016/j.jtho.2017.12.001
46. Smeltzer MP, Wynes MW, Lantuejoul S, Soo R, Ramalingam SS, Varella-Garcia M, et al. The International Association for the Study of Lung Cancer global survey on molecular testing in lung cancer. J Thorac Oncol. (2020) 15:1434–48. doi: 10.1016/j.jtho.2020.05.002
47. FoundationOne CDx. Available at: https://www.foundationmedicine.com/test/foundationone-cdx.
48. . Oncomine Dx. Available at: https://assets.thermofisher.com/TFS-Assets/CSD/Flyers/odxtt-eu-oncologist-flyer.pdf.
49. Leighl NB, Page RD, Raymond VM, Daniel DB, Divers SG, Reckamp KL, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res. (2019) 25:4691–700. doi: 10.1158/1078-0432.CCR-19-0624
50. Guibert N, Pradines A, Favre G, Mazieres J. Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages. Eur Respir Rev. (2020) 29:190052. doi: 10.1183/16000617.0052-2019
51. Study of furmonertinib in patients with advanced or metastatic non-small cell lung cancer (NSCLC) with activating, including uncommon, epidermal growth factor receptor (EGFR) or human epidermal growth factor receptor 2 (HER2) mutations (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05364073 (Accessed May, 2023).
52. Dacomitinib in lung cancer with uncommon EGFR mutations (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT04504071 (Accessed May, 2023).
53. Almonertinib as upfront treatment for uncommon EGFR mutation harboring non-small-ell lung cancer patients: a multicenter, open-label, phase II trial (AUTUMN) (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04553887 (Accessed May, 2023).
54. Lazertinib for patients with NSCLC harboring uncommon EGFR mutations (LU21-16) (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05277701 (Accessed May, 2023).
55. Miura S, Tanaka H, Misumi T, Yoshioka H, Kurata T, Tokito T, et al. LBA66 Afatinib versus chemotherapy for treatment-naiive non-small cell lung cancer with a sensitizing uncommon epidermal growth factor receptor mutation: a phase III study (ACHILLES/TORG1834). Ann Oncol. (2023) 34:S1310–S1. doi: 10.1016/j.annonc.2023.10.067
Keywords: EGFR, non-small cell lung cancer (NSCLC), afatinib, uncommon mutation, tyrosine kinase inhibitor
Citation: Christopoulos P, Herster F, Hoffknecht P, Falk M, Tiemann M, Kopp H-G, Althoff A, Stammberger A and Laack E (2024) Activity of afatinib in patients with NSCLC harboring novel uncommon EGFR mutations with or without co-mutations: a case report. Front. Oncol. 14:1347742. doi: 10.3389/fonc.2024.1347742
Received: 01 December 2023; Accepted: 18 April 2024;
Published: 06 May 2024.
Edited by:
Santiago Viteri, UOMI Cancer Center. Clínica Mi Tres Torres, SpainReviewed by:
Sarayut Lucine Geater, Prince of Songkla University, ThailandCopyright © 2024 Christopoulos, Herster, Hoffknecht, Falk, Tiemann, Kopp, Althoff, Stammberger and Laack. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eckart Laack, ZS5sYWFja0BoYWVtYXRvLW9ua29sb2dpZS1oaC5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.