
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 25 March 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1347282
This article is part of the Research Topic Treatment Response and Resistance to Targeted Therapies for NSCLC View all 16 articles
 Zhulin Wang1,2
Zhulin Wang1,2 Chunyao Huang2
Chunyao Huang2 Wenbo Fan2
Wenbo Fan2 Shaowu Sun2
Shaowu Sun2 Kaiyuan Li2
Kaiyuan Li2 Xu Liu2
Xu Liu2 Jiangtao Pu1*
Jiangtao Pu1* Guoqing Zhang2*
Guoqing Zhang2* Xiangnan Li2*
Xiangnan Li2*Given their good antitumor effects, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are standard first-line therapy for EGFR-sensitive mutations, including exon 19 deletions and exon 21 L858R mutations. EGFR fusion mutations and EGFR amplification are very rare in non-small cell lung cancer (NSCLC). We describe 2 patients with NSCLC harboring EGFR fusion mutations (EGFR-MACF1 and EGFR-GNAT3) combined with EGFR amplification. Both patients received EGFR-TKI treatment, and 1 of them showed an antitumor response.
Lung cancer is one of the leading causes of cancer-related death worldwide (1), and EGFR-TKIs are the standard first-line treatment for patients with NSCLC with sensitive EGFR mutations (2). EGFR gene fusion mutations are rare, and currently reported EGFR fusion mutations include EGFR-RAD51, EGFR-PURB, EGFR-ANXA2, EGFR-IGR, etc. EGFR gene fusion mutations combined with EGFR amplification are even rarer. Therefore, the optimal treatment for lung cancer patients with EGFR fusion mutations and EGFR amplification is unclear. We previously reported a patient (3) with an EGFR fusion mutation (EGFR-IGR) with EGFR amplification. After 2 months of treatment with gefitinib and cetuximab, the tumor shrank significantly, followed by right upper lobectomy and mediastinal lymph node dissection. The patient’s last follow-up was on March 4, 2023, with an OS > 30 months (Supplementary Figure 1). Previous preclinical and cell studies have shown that NSCLC patients with EGFR fusion mutations benefit from EGFR-TKI treatment (4, 5). In addition, patients with EGFR-sensitive mutations combined with EGFR amplification seem to have better antitumor responses to treatment with EGFR-TKIs (6). Therefore, we try to treat patients with EGFR fusion mutations combined with EGFR amplification with EGFR-TKI therapy. We describe two patients with EGFR fusion mutations (EGFR-MACF1 and EGFR-GNAT3) combined with EGFR amplification and provide detailed information, including the gene fusion location and response to TKI therapy.
Patient 1, a 65-year-old female, was admitted to the hospital due to chest pain and shoulder and back pain on April 27, 2022. A chest computed tomography (CT) scan showed a 2.4 cm mass in the upper lobe of the right lung, multiple right lung metastases, and mediastinal lymph node metastasis. CT-guided biopsy of the right lung lesion revealed that the mass was lung adenocarcinoma, and the patient was subsequently diagnosed with lung adenocarcinoma (T4N3M0 stage IIIC, AJCC8TH). On May 9, 2022, 86 cancer-related genes were detected in tissue samples by next-generation sequencing (NGS). The EGFR gene was fused with the MACF1 gene at the RNA level (mutation abundance: 17%), and EGFR was amplified (copy number: 24.15). The EGFR-MACF1 gene included EGFR exons 1–23 and MACF1 exons 59–93 (Figure 1A). After discussing the patient’s condition, the Lung Cancer Multidisciplinary Team (MDT) recommended treatment with almonertinib (110 mg/day) on May 11, 2022. After 1 month of treatment, a chest CT showed significant shrinkage of the mass in the patient’s right upper lobe. Afterward, the patient was re-examined every 3 months. Re-examination by chest CT on January 31, 2023, revealed that the tumor continued to respond to the EGFR-TKI (Figure 1B). According to the RECIST guidelines, the patient was considered to have a partial response to almonertinib, and the patient’s progression-free survival (PFS) was >9 months (Figure 1C).
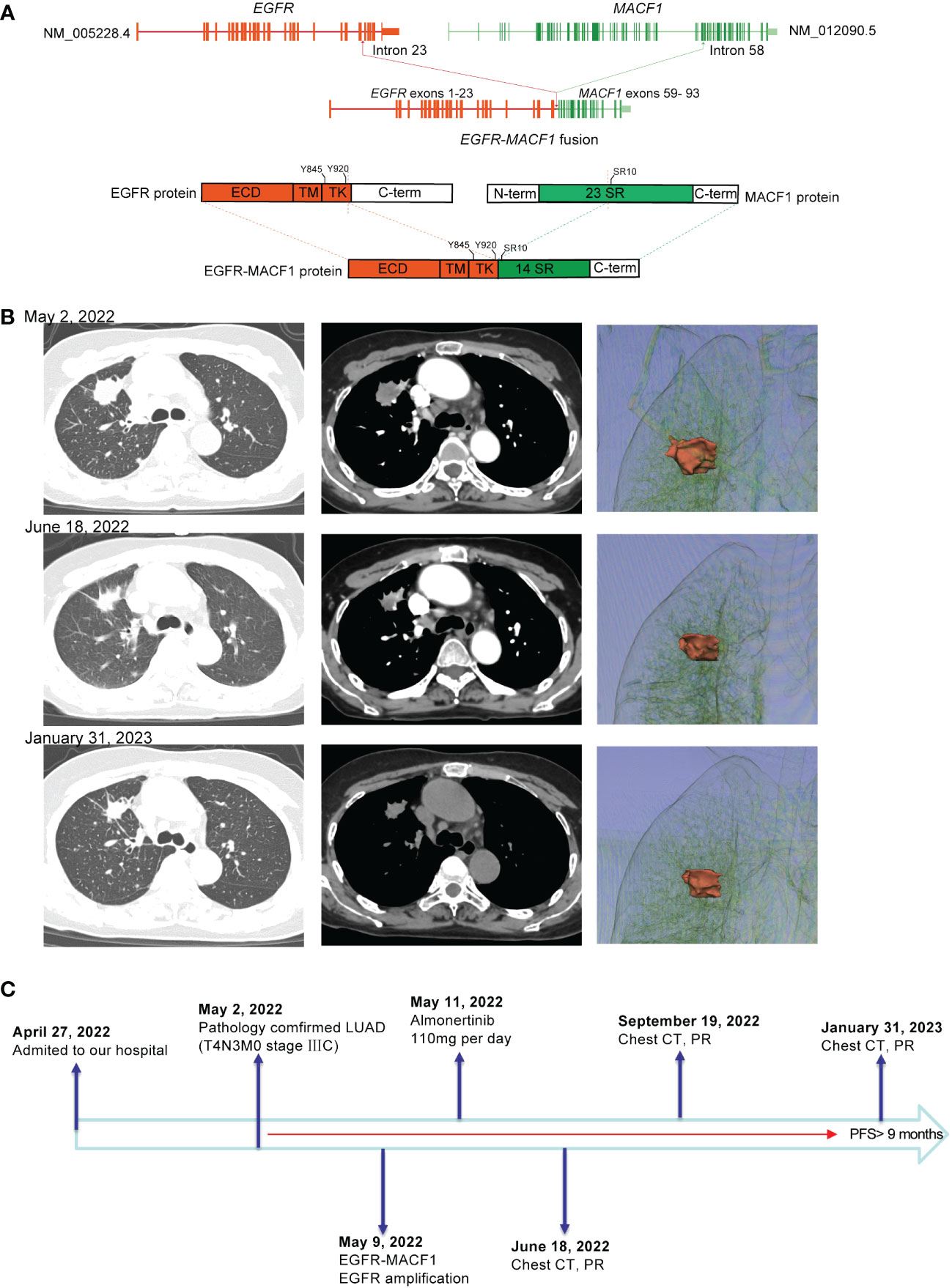
Figure 1 (A) Schematic diagram of the domain structure of the EGFR-MACF1 fusion at the RNA and protein levels. (B) Computed tomography (CT) scan and three-dimensional reconstruction before and after EGFR-TKI treatment and at the latest follow-up. (C) The entire treatment procedure.
Patient 2, a 58-year-old male, experienced cough, sputum, chest tightness, and back pain on December 3, 2021. Chest CT and positron emission tomography (PET)-CT scans revealed masses in the right upper lobe and right hilum of the patient, with the larger mass measuring 3.2 cm, multiple intrapulmonary metastases, multiple lymph node metastases throughout the body (including the mediastinum, hilar, bilateral neck, bilateral clavicle region, left armpit, etc.) and multiple bone metastases (right 7th rib, left 9th rib, 9th thoracic vertebra, etc.). CT-guided biopsy of the right lung lesion revealed that the mass was lung adenocarcinoma, which was diagnosed as lung adenocarcinoma (T4N3M1c stage IVB, AJCC8TH). On December 23, 2021, 14 cancer-related genes were detected in tissue samples using NGS. EGFR gene fusion with the GNAT3 gene (mutation abundance: 76.3%) and EGFR amplification (copy number: 8.1). The EGFR-GNAT3 gene included EGFR exons 1–24 and GNAT3 exon 8 (Figure 2A). The patient initially underwent arterial chemoembolization (protocol: pemetrexed disodium and nedaplatin), which resulted in mass shrinkage. However, due to physical reasons specific to the patient, the drug was suspended for 2 months, after which the tumor progressed after 1 month of treatment with almonertinib (110 mg/day) (Figure 2B). On June 24, 2022, the patient died of severe lung infection and systemic multiple organ failure, with an overall survival (OS) <7 months (Figure 2C). Additional information regarding the 2 patients is summarized in Table 1.
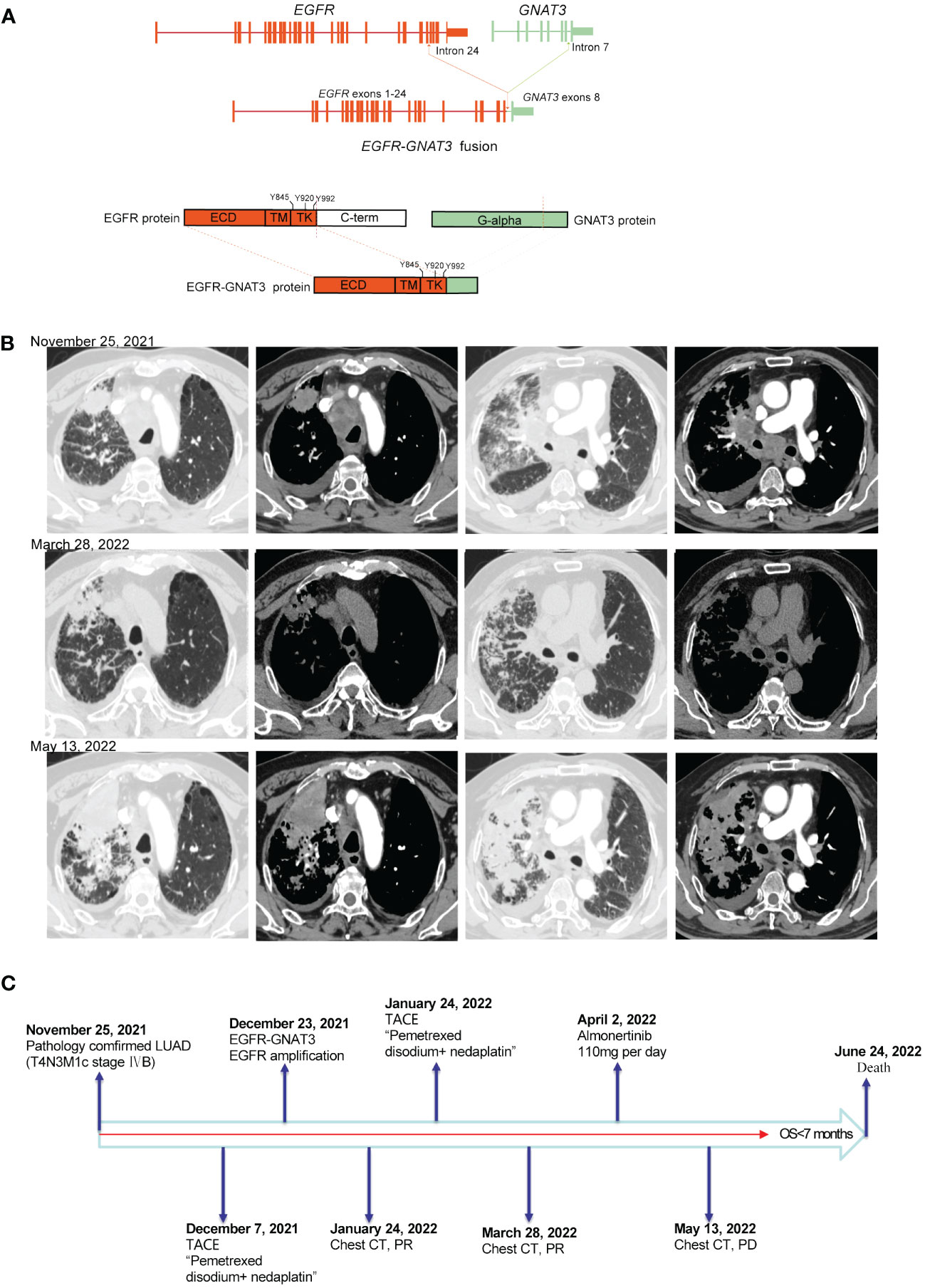
Figure 2 (A) Schematic diagram of the domain structure of the EGFR-GNAT3 fusion at the DNA and protein levels. (B) Computed tomography (CT) scan before and after treatment and after tumor progression. (C) The entire treatment procedure.
EGFR activation is a dimerization reaction that results in a transformation from an inactive to an active conformation as the local concentration of the receptor increases (7). EGFR activation occurs due to the formation of an asymmetric dimer (7). In addition, EGFR contains several autophosphorylation sites in the C-terminal tail of the receptor (including tyrosines 992, 1068, and 1173) (8, 9). Dimerization leads to phosphorylation of tyrosine residues in the C-terminal tail, which in turn activates the PI3K/AKT and MAPK oncogenic pathways.
In the model constructed by Kartik et al. (10), the EGFR-RAD51 fusion protein was shown to contribute an oligomerization domain through RAD51 to promote kinase activation. However, among other fusion partners, such as EGFR-IGR, EGFR-ANXA2, EGFR-SEPTIN14, and EGFR-SHC1, no studies have shown that the fusion partners involve oligomerization domains. In addition, upon EGFR fusion, a subset of phosphorylation sites critical for intact EGFR function and transformation may be preserved, and these phosphorylation sites may be oncogenic (8). In our study, although the fusion sites of the three patients were different, they all retained phosphorylation sites that may cause cancer (patient 1: tyrosine 845, tyrosine 920; patient 2: tyrosine 845, tyrosine 920, tyrosine 992; patient 3: tyrosine 845, tyrosine 920, tyrosine 992).
Previous studies have shown that patients with tumors harboring EGFR fusions can benefit clinically from EGFR-TKI therapy (3, 11, 12). In cell experiments, EGFR-TKIs inhibited the growth of BA/F3 cells harboring the EGFR fusion protein to varying degrees (10).
According to the case series in this study, we found that EGFR gene fusions are often accompanied by EGFR amplification. The results of Shigenari et al (13) show that the amplification of EGFR wild-type (rather than mutant EGFR) alleles may induce acquired drug resistance to third-generation EGFR-TKIs through activation induced by EGFR ligands. We recently reported on the combined targeted therapy-”sandwich” regimen (14). This strategy was successfully applied in a patient with EGFR-IGR combined with EGFR amplification (3). The fundamental principle involves using EGFR monoclonal antibodies to target EGFR amplification and EGFR-TKIs to target EGFR fusion. However, there is evidence that primary EGFR amplification may be effective for EGFR-TKI targeted therapy. Ruiz-Patiño et al. (15) and Shan et al. (6) found that patients with EGFR mutations and EGFR amplification exhibited significant antitumor responses when treated with EGFR-TKIs and had better survival than patients without amplification. Furthermore, treatment with the first-generation TKI larotrectinib resulted in significant antitumor activity in patients with advanced ESCC with EGFR overexpression or amplification (16). However, some previous studies have shown that EGFR amplification in untreated patients after TKI treatment may lead to drug resistance in patients receiving TKIs. Nitin et al (17) reported 5 patients (35.7%) had EGFR amplification in patients with drug resistance after treatment with oxitinib. These results suggest that EGFR amplification in untreated patients after TKI treatment may lead to drug resistance to TKIs. In the study of Helman et al (18), 17 of the 58 patients who progressed when they were treated with rociletinib had EGFR amplification. Taken together, these findings indicate that primary EGFR amplification may be effective for TKI therapy, while secondary EGFR amplification mediates TKI resistance. Therefore, discussions among the Lung Cancer Multidisciplinary Team (MDT) resulted in the recommendation for the use of single-drug TKI therapy in patients with rare EGFR fusion mutations and EGFR amplification. The data presented in these case studies were obtained with informed consent from each patient, and the study was approved by the Zhengzhou University Institutional Review Board.
Patient 1 achieved good clinical efficacy. Patient 2 was under the care of another medical team and was discovered when reviewing cases for this study; this was a negative case. The patient inappropriately received local intervention as a first-line therapy without systemic therapy, which led to progression of the systemic disease and a decline in the patient’s physical condition. Although a TKI was chosen for treatment in the later stage, the optimal time for treatment was missed. After the patient received targeted therapy for one month, the results showed that it was ineffective. These findings also show the importance of early systemic treatment for patients with advanced lung cancer. Additionally, we do not know whether new mutations that appeared after the previous treatment led to the poor efficacy of EGFR-TKI therapy observed in this patient because additional NGS testing was not performed after the disease had progressed.
Here, we present 2 patients with NSCLC with EGFR fusions combined with EGFR amplification, both of whom represented rare cases. One of the patients showed a significant antitumor response after EGFR-TKI treatment. Future studies should involve basic research on these rare mutations to explore their cancer-causing mechanisms.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of Zhengzhou University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. We confirm that written informed consent has been obtained from the participant/patient(s) for the publication of this case report. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
ZW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CH: Formal analysis, Methodology, Project administration, Writing – original draft. WF: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing. SS: Formal analysis, Investigation, Project administration, Writing – original draft. KL: Data curation, Formal analysis, Investigation, Writing – review & editing. XL: Conceptualization, Formal analysis, Writing – review & editing. JP: Resources, Writing – review & editing, Conceptualization, Data curation. GZ: Resources, Writing – review & editing, Formal analysis, Supervision. XNL: Funding acquisition, Methodology, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (32070623) and the Henan Province Engineering Research Center of Molecular Pathology and Clinical Experiments on Thoracic Diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1347282/full#supplementary-material
Supplementary Figure 1 | A Schematic diagram of the domain structure of the EGFR-IGR fusion at the DNA and protein levels. ECD: extracellular domain, TM: transmembrane domain, TK: tyrosine kinase domain; Y845: tyrosine 845, Y920: tyrosine 920, Y992: tyrosine 992. 1B, The entire treatment procedure.
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd−generation EGFR−TKI resistance in advanced non−small cell lung cancer (Review). Int J Oncol. (2021) 59. doi: 10.3892/ijo.2021.5270
3. Zhang G, Xia P, Zhao S, Yuan L, Wang X, Li X, et al. Gefitinib combined with cetuximab for the treatment of lung adenocarcinoma harboring the EGFR-intergenic region (SEC61G) fusion and EGFR amplification. Oncologist. (2021) 26:e1898–902. doi: 10.1002/onco.13921
4. Copia Sperandio R, Luiza Teixeira Tostes F, Vidal Campregher P, Ribeiro Paes V, Moura F, Schvartsman G. EGFR-RAD51 fusion in lung adenocarcinoma with systemic and intracranial response to osimertinib: A case report and review of the literature. Lung Cancer. (2022) 166:94–7. doi: 10.1016/j.lungcan.2022.02.006
5. Guan Y, Song Z, Li Y, Guo H, Shi J, Zhang X, et al. Effectiveness of EGFR-TKIs in a patient with lung adenocarcinoma harboring an EGFR-RAD51 fusion. Oncologist. (2019) 24:1027–30. doi: 10.1634/theoncologist.2018-0732
6. Shan L, Wang Z, Guo L, Sun H, Qiu T, Ling Y, et al. Concurrence of EGFR amplification and sensitizing mutations indicate a better survival benefit from EGFR-TKI therapy in lung adenocarcinoma patients. Lung Cancer. (2015) 89:337–42. doi: 10.1016/j.lungcan.2015.06.008
7. Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. (2006) 125:1137–49. doi: 10.1016/j.cell.2006.05.013
8. Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. (2003) 284:31–53. doi: 10.1016/s0014-4827(02)00098-8
9. Kwon Y, Kim M, Jung HS, Kim Y, Jeoung D. Targeting autophagy for overcoming resistance to anti-EGFR treatments. Cancers (Basel). (2019) 11. doi: 10.3390/cancers11091374
10. Konduri K, Gallant JN, Chae YK, Giles FJ, Gitlitz BJ, Gowen K, et al. EGFR fusions as novel therapeutic targets in lung cancer. Cancer Discovery. (2016) 6:601–11. doi: 10.1158/2159-8290.Cd-16-0075
11. Zhu YC, Wang WX, Xu CW, Song ZB, Du KQ, Chen G, et al. EGFR-RAD51 fusion variant in lung adenocarcinoma and response to erlotinib: A case report. Lung Cancer. (2018) 115:131–4. doi: 10.1016/j.lungcan.2017.12.001
12. Di Federico A, Filetti M, Palladini A, Giusti R, Piras M, De Giglio A, et al. EGFR-RAD51 gene fusion NSCLC responsiveness to different generation EGFR-TKIs: two cases and review of the literature. Transl Lung Cancer Res. (2022) 11:497–503. doi: 10.21037/tlcr-21-888
13. Nukaga S, Yasuda H, Tsuchihara K, Hamamoto J, Masuzawa K, Kawada I, et al. Amplification of EGFR wild-type alleles in non-small cell lung cancer cells confers acquired resistance to mutation-selective EGFR tyrosine kinase inhibitors. Cancer Res. (2017) 77:2078–89. doi: 10.1158/0008-5472.Can-16-2359
14. Zhang G, Yan B, Guo Y, Yang H, Li J. "Sandwich" Strategy to intensify EGFR blockade by concurrent tyrosine kinase inhibitor and monoclonal antibody treatment in highly selected patients. Front Oncol. (2022) 12:952939. doi: 10.3389/fonc.2022.952939
15. Ruiz-Patiño A, Castro CD, Ricaurte LM, Cardona AF, Rojas L, Zatarain-Barrón ZL, et al. EGFR amplification and sensitizing mutations correlate with survival in lung adenocarcinoma patients treated with erlotinib (MutP-CLICaP). Target Oncol. (2018) 13:621–9. doi: 10.1007/s11523-018-0594-x
16. Liu R, Liu L, Zhao C, Bai Y, Zheng Y, Zhang S, et al. Larotinib in patients with advanced and previously treated esophageal squamous cell carcinoma with epidermal growth factor receptor overexpression or amplification: an open-label, multicenter phase 1b study. BMC Gastroenterol. (2021) 21:398. doi: 10.1186/s12876-021-01982-4
17. Roper N, Brown AL, Wei JS, Pack S, Trindade C, Kim C, et al. Clonal evolution and heterogeneity of osimertinib acquired resistance mechanisms in EGFR mutant lung cancer. Cell Rep Med. (2020) 1. doi: 10.1016/j.xcrm.2020.100007
Keywords: EGFR fusion, EGFR amplification, rare mutations, lung adenocarcinoma, targeted therapy
Citation: Wang Z, Huang C, Fan W, Sun S, Li K, Liu X, Pu J, Zhang G and Li X (2024) Case report: EGFR fusion mutation combined with EGFR amplification responds to EGFR-TKI therapy. Front. Oncol. 14:1347282. doi: 10.3389/fonc.2024.1347282
Received: 30 November 2023; Accepted: 07 March 2024;
Published: 25 March 2024.
Edited by:
Shiyou Wei, Sichuan University, ChinaReviewed by:
Sousuke Kubo, Yokohama City University, JapanCopyright © 2024 Wang, Huang, Fan, Sun, Li, Liu, Pu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangtao Pu, cHVqaWFuZ3RhbzE5NzJAMTYzLmNvbQ==; Xiangnan Li, bHhuLTIwMDBAMTYzLmNvbQ==; Guoqing Zhang, ZHJ6aGFuZ2d1b3FpbmdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.