- 1Department of Hematology, Stem Cell Transplant and Cellular Therapy, Oncology Centre, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 2Department of Medicine, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 3College of Medicine, Imam Mohammad Ibn Saud Islamic University, Riyadh, Saudi Arabia
Azacitidine, a hypomethylating agent, has caused a paradigm shift in the outcomes of patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) who are not eligible for stem cell transplantation, particularly in combination with BCL2 and IDH inhibitors. Azacitidine and Azacitidine-based combinations have been widely considered a safe low-intensity therapy when compared to traditional conventional treatments. The development of lung toxicity from azacitidine is not a well-characterized adverse event. However, if it happens, it can be fatal, especially if not recognized and treated promptly. In this review, we aim to familiarize the reader with the presentation of azacitidine-induced lung injury, provide our suggested approach to management based on our experience and the current understanding of its mechanism, and review the literature of 20 case reports available on this topic.
1 Introduction
Myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) are clonal hematological diseases with immunological, genetic, and epigenetic heterogenicity. MDS and AML both result in ineffective hematopoiesis and dysmorphism of cells, leading to cytopenias and their complications, including infections and bleeding (1). Additionally, the progressive impairment in growth and differentiation through the hypermethylation of the tumor suppressor gene, such as p15INK4B, can promote disease progression from MDS to AML (2–5).
Epigenetic abnormalities, such as hypermethylation of CpG island, are believed to play an essential role in leukemogenesis, which can lead to the silencing of tumor suppressor genes, which, in turn, drives the neoplastic transformation to AML (3, 6–9). MDS and AML tend to occur more often in people over the age of 60 years, with a male predominance (10, 11). Since most patients are of older age, the chances of having more comorbidities and being unfit increase, which, in turn, limits the ability to use curative therapies, such as stem cell transplant (1).
5-azacytidine, an analog of the pyrimidine nucleoside cytidine, has been studied to treat acute leukemia in the United States since the early 1970s. Clinical trials have primarily focused on patients with an illness resistant to conventional chemotherapy. The findings of these studies showed that 5-azacytidine is effective in treating AML. Following that, clinical trials looked at 5-azacytidine’s impact on other hematological diseases including MDS, non-hematological neoplasms, such as solid tumors, hereditary hematologic diseases, and hemoglobinopathies (thalassemia and sickle cell anemia). The Cancer and Leukemia Group B (CALGB) started a series of clinical trials with 5-azacytidine in MDS patients in 1984. These studies, and other supporting evidence, led to the US FDA approval of 5-azacytidine to treat MDS in May 2004. By blocking DNA methyltransferase, 5-azacytidine prevents the methylation of newly produced DNA (DNMT), restoring normal function to genes involved in differentiation and proliferation (12–16). Since then, azacytidine-based combinations have also been tested and developed in MDS, although with limited success, and in AML, where it led to a number of subsequent US-FDA approvals in patients ineligible for high-intensity therapy (10, 17–24).
Azacitidine is considered to have a relatively safe toxicity profile, with the majority being cytopenia and gastrointestinal symptoms. However, there has been growing evidence of lung toxicity complicating treatment with azacitidine. In our review, we aim to shed light on the manifestations and management of this rare but potentially fatal event and further add to the accumulative knowledge published by providing our experience in managing azacitidine-induced pneumonitis in an AML patient.
2 Case report
A 63-year-old male was referred to our institute with a diagnosis of acute leukemia after presenting with fatigue, weight loss, and bruising for two weeks. He was not known to have any past medical illnesses. He had a history of heavy smoking with a pack-year smoking index of 40 but stopped smoking almost five years prior to being diagnosed with leukemia. His initial investigations showed pancytopenia on his complete blood count (CBC) and around 50% blasts on the peripheral blood smear. Bone marrow aspirate and biopsy showed features suggestive of AML, with 56% myeloid blasts detected by flow cytometry. Based on the mentioned investigations along with cytogenetic and molecular testing, he was diagnosed with AML with myelodysplastic-related changes according to the World Health Organization (WHO) classification and stratified as having an adverse-risk disease based on the European Leukemia Network (ELN) risk stratification. The patient refused intensive chemotherapy and stem cell transplant, so he was started on cycle 1 of azacitidine 75 mg/m2 IV for seven days and venetoclax for 21 days. Before starting azacitidine and venetoclax, a high aspergillus galactomannan antigen (14.18) was incidentally found. He was asymptomatic.
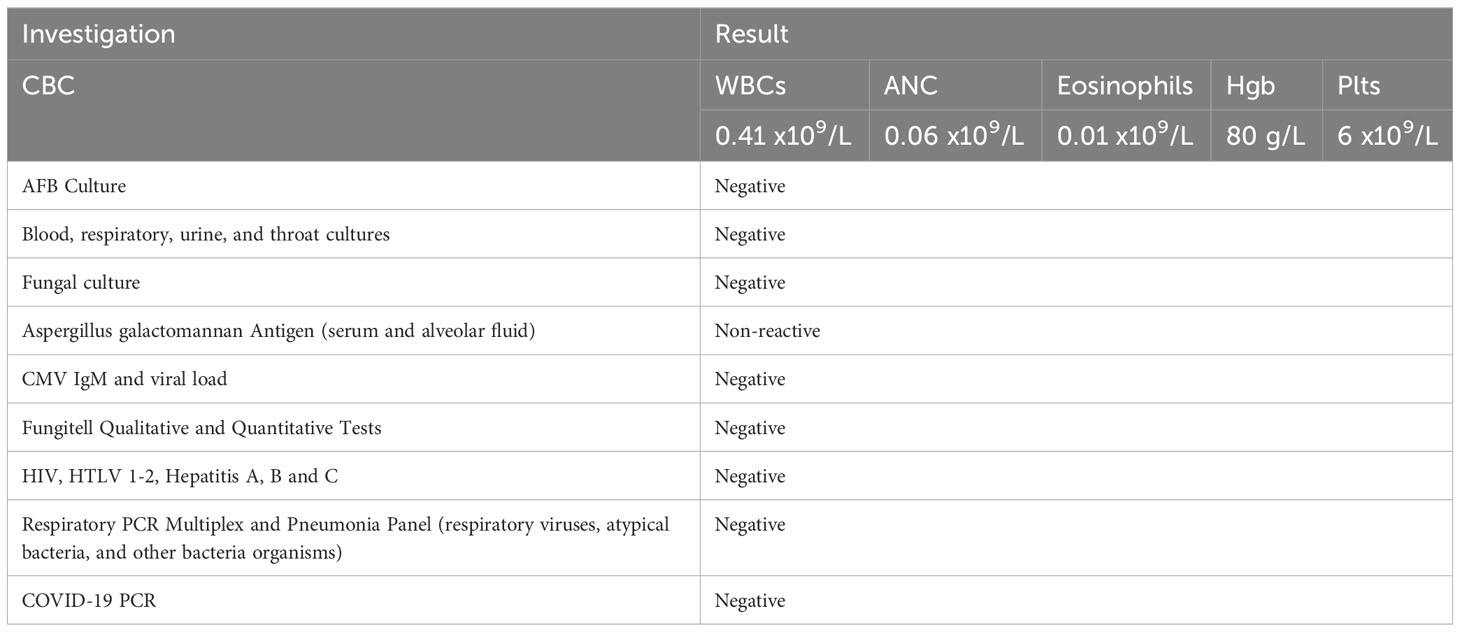
Further investigations with imaging of the chest and paranasal sinuses with computed tomography (CT) were done; the chest CT showed multiple bilateral peri-broncho-vascular rounded central consolidative nodules and cavities representing an inflammatory/infectious process. He was started on Posaconazole. Bronchoscopy and bronchoalveolar lavage (BAL) were negative for infections, including aspergillus galactomannan antigen. However, he was continued on antifungal treatment for six weeks as he was labeled to have a probable invasive fungal infection. While on antifungal treatment, he was started on cycle 1 of azacitidine and venetoclax and achieved remission, and a repeat CT chest after completion of antifungal therapy showed resolution of the previous findings. He initially refused to receive further cycles; however, after three months, he had a relapse with 80% circulating blasts, so he was restarted on azacitidine and venetoclax. On day 2 of the second cycle of azacitidine and venetoclax, he developed a fever and tachypnea. Chest examination was normal, and chest x-ray did not reveal any significant abnormality and had neutropenia on CBC. A microbiological work-up was sent, and he was started on empirical antibiotics with meropenem as he was kept on prophylactic levofloxacin, according to the institute’s febrile neutropenia protocol. Despite empirical antibiotics, he continued to have fever and worsening symptoms. Antimicrobial therapy was upgraded with antifungal treatment, vancomycin, and later sulfamethoxazole-trimethoprim, yet there was no significant improvement. CT chest showed bilateral diffuse ground-glass opacities, smooth interlobular septal thickening, severe emphysematous changes, and small bilateral pleural effusions with no evidence of pulmonary embolism. The patient was kept on empirical antibiotics and completed seven days of azacitidine. On day 11, he became more tachypneic and hypoxic and was transferred to the intensive care unit (ICU) and was started on oxygen therapy with a high-flow nasal cannula. He continued empirical antimicrobial therapy despite all infectious work-ups returning negative (see Table 1). A bronchoscopy with bronchoalveolar lavage was done and was negative for malignant cells and infectious causes. BAL fluid was bloody with 24,500 x 106/L of RBCs. He was started on Methylprednisolone 40 mg IV BID for five days for possible drug-induced pneumonitis while continuing antibiotics. He improved clinically, was weaned off oxygen therapy, and was discharged from the hospital. He was re-admitted for the third cycle of azacitidine and venetoclax. On day 5 of cycle 3 of azacitidine, he developed a fever, shortness of breath, and hypoxia. Chest x-ray showed bilateral diffuse reticulonodular opacities. An infectious work-up was sent, and he was started on empirical antimicrobial therapy. CT chest showed diffuse bilateral ground glass densities with smooth interlobular septal thickening, centrilobular nodules, and right upper lobe ground glass opacity with focal subpleural infiltrate (Figure 1). Azacitidine-induced pneumonitis was our top differential diagnosis as the symptoms reoccurred with rechallenging with azacitidine, and the Naranjo score was 8, which makes the diagnosis probable (Table 2). Azacitidine was discontinued, and he was started on dexamethasone 4 mg IV BID for three days, then tapered down and changed to prednisone orally, which was stopped after six weeks with clinical and radiological improvement. Treatment of his AML was changed to cladribine, low-dose cytarabine with venetoclax with no similar lung toxicity. The patient achieved complete remission, however he refused further treatment, relapsed 4 months later and died within a month of his relapse.
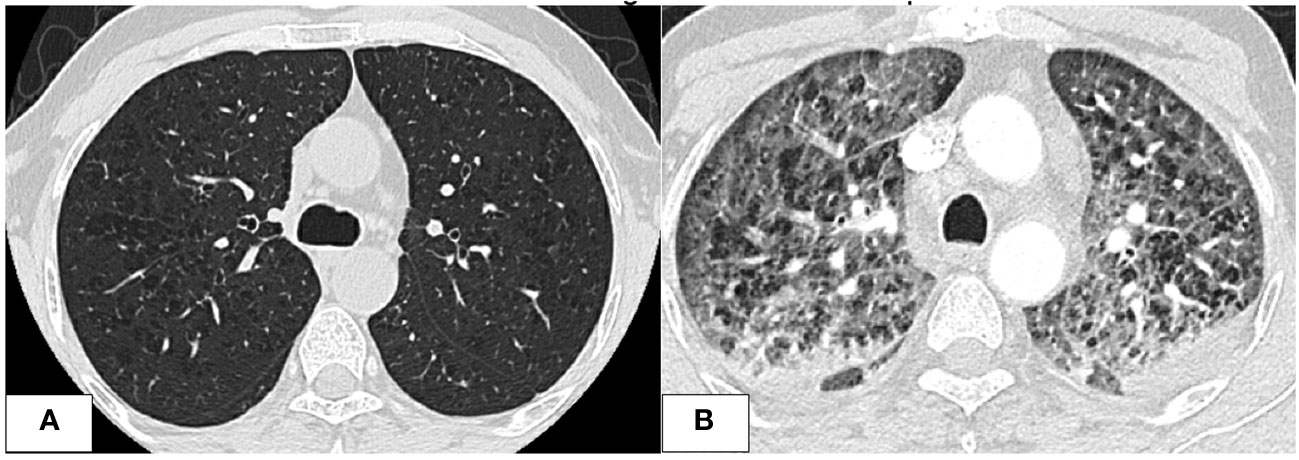
Figure 1 CT chest images. (A) After the first cycle of azacitidine, at that time, the patient was treated for invasive fungal infection. Imaging shows significant improvement of previous bilateral ill-defined nodular opacities with residual GGOs and micronodules. (B) Imaging was done at the time of the development of respiratory symptoms after the second cycle of azacitidine. Imaging showed worsening diffuse GGOs with smooth interlobular septal thickening and centrilobular nodules with bilateral bronchial wall thickening and small bilateral pleural effusions.
Table 2 The Naranjo scale results’ classifications (25).
3 Discussion
3.1 Azacitidine
3.1.1 Introduction and mechanism of action
Azacitidine, also known as 5-azacitidine, is a ring analog of the pyrimidine nucleoside of cytidine with a nitrogen atom instead of carbon at the fifth position of the heterocyclic ring (3, 26, 27). It is a first-in-class hypomethylating agent. It has a dose-dependent action, which leads to dysregulation of the RNA and DNA.
It works on the RNA by being phosphorylated to azacitidine triphosphate and then incorporated into RNA, inhibiting RNA and disturbing protein synthesis (3). Azacitidine hypomethylates the replicating DNA and inhibits its function through the dephosphorylation of azacitidine to 5-aza-2’ V-deoxycytidine diphosphate by ribonucleotide reductase (6). The 5-aza-2’ V-deoxycytidine diphosphate then is phosphorylated into triphosphate, which binds stoichiometrically to DNA methyltransferase and inhibits it irreversibly which hypomethylates the DNA regulatory sequences and increase gene transcription restoring the normal function of tumor suppressor genes and cell maturation (3, 6, 10, 26–28).
In 1982, azacitidine was used in treating thalassemia as it was found to induce the production of fetal hemoglobin through the hypomethylation of the Y globin suppressor gene (12, 29, 30). Afterwards, it showed an improvement in the survival of AML and high-risk MDS patients who cannot undergo stem cell transplant, which led to its approval in these patients (22, 30–32). Azacitidine can be cytotoxic at high doses, but at lower doses, it results in hypomethylation of DNA and differentiation of cells (33). The usual dosing in MDS/AML is 75 mg/m (2)/day for seven days every four weeks (32, 34, 35).
3.1.2 Adverse effects
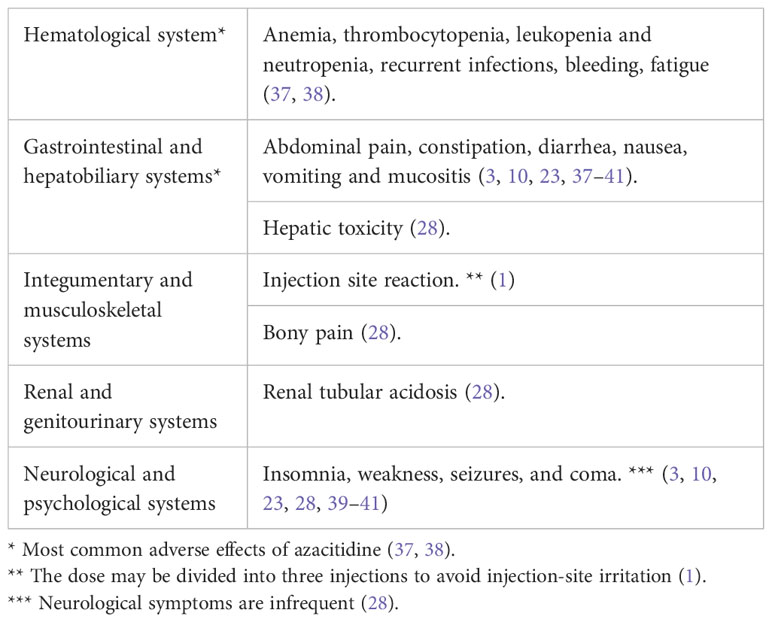
In general, medications cause adverse effects in 0.07% of admitted patients and can be fatal in 0.003% (36). One way to assess the correlation between medications and toxicities is using the Naranjo scale, one of the most used tools to detect medications’ toxicities (10). It uses a scored systemic questionnaire, which classifies the possibility of drug-induced toxicity into four categories (Table 2) (25). Azacitidine usage in hematological malignancy is considered a low-intensity treatment that is generally well-tolerated and can be administered in the outpatient setting (1). The commonly reported side effects are usually uncritical (Table 3). Nevertheless, there has been recent scattered evidence of severe side effects, including lung toxicity, reported in <0.1% of cases (10).
3.2 Azacitidine-induced lung toxicity
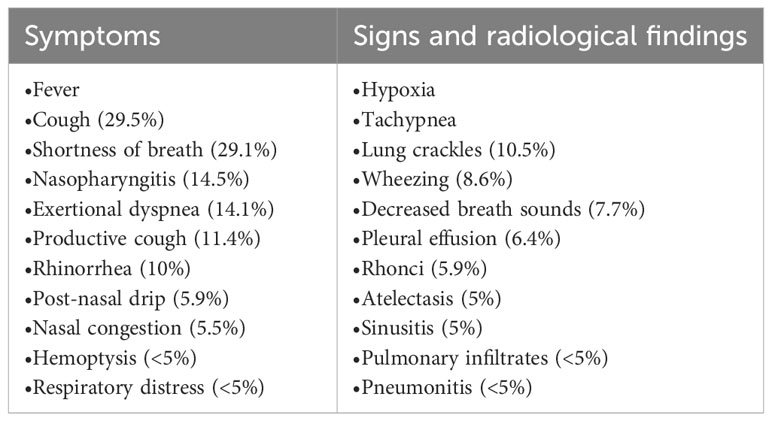
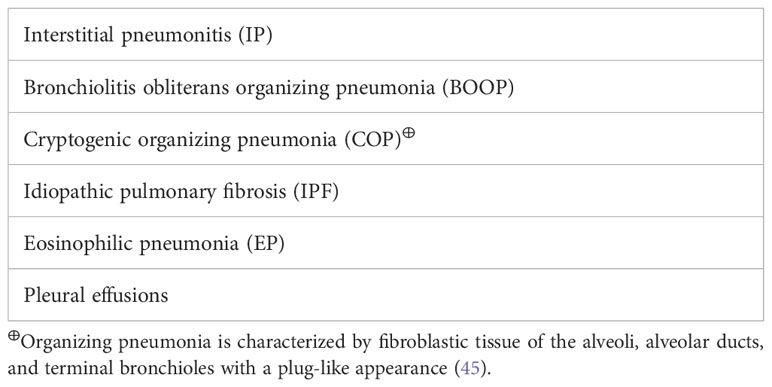
Although azacitidine-induced lung toxicity is rare, it can have dreadful outcomes, which makes early recognition and treatment initiation crucial for reversibility and survival (37). Azacitidine-induced lung toxicity comes in different forms with various presentations (Tables 4, 5). Fever and respiratory symptoms were the most common symptoms reported, commonly mistaken for an infection (42). The reported cases have occurred with the usual dose of azacitidine in MDS/AML, 75 mg/m (2)/day for 5-7 days, and can occur at any time after starting azacitidine (2, 3). Toxicity from azacitidine is irrelevant to its cumulative dose (46). Most cases occurred after eight weeks of initiation, i.e., the second cycle of azacitidine, but others have been widely inconstant (4, 10, 37, 38, 47, 48). The severity of lung injury is highly variable, but in most cases, the longer it takes to recognize it and start steroids, the higher the risk of serious complications, including mortality (49).
3.3 Pathogenesis of azacitidine-induced lung injury
The underlying mechanism of azacitidine-induced lung injury is not entirely understood. There have been multiple suggested mechanisms (Figure 2), including:
(1) Direct cytotoxicity from the drug is similar to gemcitabine-induced toxicity because of the molecular similarities between azacitidine and gemcitabine (5, 6, 30, 43). Gemcitabine damages the capillary endothelial cells, causing fluid leakage and pulmonary edema, leading to respiratory distress syndrome and interstitial pneumonitis (50–53). Also, cytidine analogs can alter the synthesis of surfactants by disturbing the production of essential phospholipids (54). In cases of delayed presentations, i.e., after multiple cycles of azacitidine, toxicity can be related to the cumulative dose of azacitidine (55).
(2) Inflammatory and immune-mediated injury, supported by the findings of lymphocytosis on pleural and alveolar fluid analysis and the improvement following immunosuppressive medications, such as steroids (56). Some of the possible immune-related mechanisms include:
a. Neutrophil-induced parenchymal injury: in patients with recovered hematopoiesis, neutrophils sequestrate in the lungs and overexpress neutrophil elastase, which increases collagen content and fibrosis (42). This thesis might explain lung injury in patients with recovered counts (43).
b. Cytokine overexpression through azacitidine’s ability to augment intracellular INF-ɣ increasing macrophage activation and changing the chromatin configuration to increase the transcription of pro-inflammatory genes, helping in tumor control but can cause collateral damage to the surrounding tissues, including the lungs (57, 58). The activation of cytokines, e.g., interleukin-5 (IL-5), by the helper T-cells, triggered by the medication leads to the build-up of eosinophils in the lungs (59, 60).
c. Azacitidine-induced autophagy; lysosome-dependent degradation of cells resulting in acute and chronic inflammation of the lungs (61). This is particularly true in hypersensitivity pneumonitis (62).
d. Oxidative stress inhibits the ERK pathway signaling in T-cells (30).
(3) Hypersensitivity reactions (30): both type I and type IV hypersensitivity reactions have been described to be the underlying mechanisms of azacitidine-induced lung injuries.
a. Type I hypersensitivity reaction; associated with high IgE levels and broncho-centric granuloma as described by Nair et al. (28) This can explain why the toxicity occurs within a few days after the exposure to azacitidine with features of eosinophilic pneumonitis (5, 55). Drug-induced eosinophilic pneumonitis usually happens within the first eight weeks after the drug initiation (28).
b. Type IV delayed hypersensitivity reaction; this is especially true in the immune reconstitution phase, where CD8+ T-lymphocytes are activated by interleukin-2 (IL-2) and interferon-gamma (IFN- ɣ), which leads to the formation of sarcoid-like granulomas with epithelioid giant cells surrounded by a ring of fibroblasts (30, 47, 63, 64).
(4) Impaired repair by type II pneumocytes (30).
(5) Lineage reprogramming of different cells due to epigenetic priming by azacitidine; however, this theory is questionable (65).
(6) Upregulation of type I collagen synthesis leads to pulmonary fibrosis through the DNA hypomethylation feature of azacitidine (6, 30, 66). However, Parker et al. were unsuccessful in activating type I procollagen genes in the human embryonic lung fibroblasts when they exposed them to azacitidine (67).
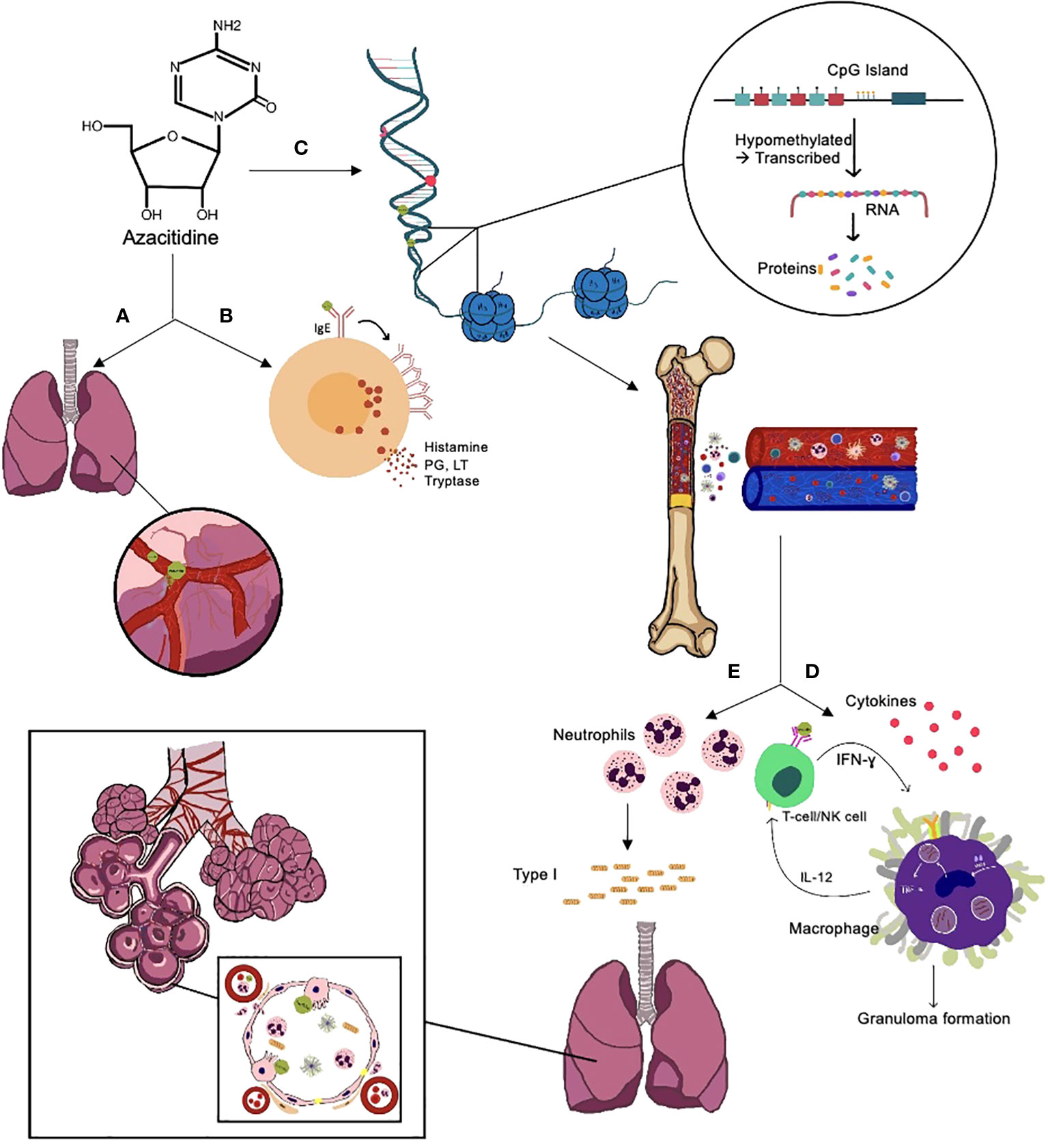
Figure 2 Suggested mechanisms of azacitidine-induced lung injury. (A) Direct toxicity to the lungs through damage to the capillary endothelial cells, causing fluid leakage and pulmonary edema, leading to respiratory distress syndrome and interstitial pneumonitis, disturbs the normal production of surfactant. (B) Type I hypersensitivity reaction; azacitidine activates the IgE on mast cells, leading to IgE aggregation and release of histamines, leukotrienes (LT), prostaglandins (PG), and tryptase, which lead to vasodilation, increased vascular permeability, and tissue damage. (C) Hypomethylates DNA, leading to a recovery in normal hematopoiesis in the bone marrow and immune system recovery. (D) Type IV hypersensitivity reaction and azacitidine's ability to augment IFN- ɣ intracellularly, which increases macrophage activation and, as a result, granuloma formation. (E) Recovery of hematopoiesis leads to increased neutrophil production, which increases neutrophil elastase and collagen type I production, leading to fibrosis, and can also damage the type II pneumocytes responsible for the repair process.
The consequence of the different suggested mechanisms is the damage to the alveolar epithelium and disequilibrium of the activity and inhibition of metalloproteinase, leading to intracellular and plasma protein leak and, subsequently, an inflammatory response in the alveolar airspaces, which stimulates repair and promotes fibrosis (45). In cases resulting in organizing pneumonia, further plug formation against the pores of Kohn due to the production of fibro-myxoid material occurs, leading to the characteristic features of organizing pneumonia (68, 69).
3.4 Risk factors of azacitidine-induced lung injury
To our knowledge, there is no definite way to predict which patient will develop this toxicity. Nevertheless, upon reviewing the cases described in the literature (Table 6), along with our case, we listed some possible factors that might increase the risk of developing lung injury after exposure to azacitidine, which include:
● History of previous or active cigarette smoking (71).
● Previous lung infections (72, 73).
● Underlying airway or parenchymal lung disease.
● Exposure to environmental toxins and medications that have a toxic effect on the lungs (74, 75).
● Chronic gastric-content aspiration (76).
● High neutrophils count (42, 77).
● Leukemic lung infiltration.
3.5 Differential diagnoses
Multiple diseases have similar features to azacitidine-induced lung injury and need to be considered when patients develop the features of azacitidine-induced lung injury mentioned above. Examples of these differentials include:
▪ Infections are one of the most critical differentials to consider, as it is a common cause of morbidity and mortality in this group of patients and have very similar features to azacitidine-induced lung injury (10). The diagnosis of azacitidine-induced lung injury is commonly delayed because infections have a similar presentation, and the diagnosis of chemotherapy-induced pulmonary toxicity is less common and requires the exclusion of infections (37, 78, 79).
▪ Malignancy, i.e. leukemic infiltrates. In these cases, it is helpful to do a bone marrow examination to evaluate the disease status and, as needed, further diagnostic studies, for example, a lung biopsy (5, 38, 78).
▪ Autoimmune diseases and vasculitis with lung involvement (2, 28, 37, 80).
▪ Extramedullary hematopoiesis.
▪ Sweet syndrome, hyper-eosinophilic syndrome, and pulmonary alveolar proteinosis (28, 38).
▪ Pulmonary hemorrhage, particularly in the setting of low platelet counts or anticoagulation/antiplatelet therapy (10).
▪ Cardiac-related pulmonary edema, i.e., heart failure (10, 78).
▪ Other medications or interventions that can cause lung toxicity (Table 7).
▪ Exacerbation of airway diseases or interstitial lung diseases (28).
3.6 Investigations and diagnostics
The diagnosis of azacitidine-induced lung injury can be challenging and requires a high index of suspicion. The pillars of the diagnosis mainly rely on the clinical assessment and ruling out other causes. The diagnosis can be delayed and is usually considered after no significant improvement following the start of empirical antibiotics and when no other cause is identified (2).
Following are the main navigating steps, we believe, that help reach the diagnosis of azacitidine-induced lung injury:
(1) Detailed history and physical examination. This is the first and one of the most crucial steps in the diagnosis process. It can establish the causative relationship between azacitidine and lung toxicity and helps rule out other causes (59). Some crucial aspects to focus on while attaining the clinical assessment include, but are not limited to:
✓ Detailed presenting illness history.
✓ Details on exposure history to azacitidine or other cytidine analogues and previous complications.
✓ History of lung, cardiac or autoimmune/rheumatological diseases.
✓ Smoking exposure; active or previous history of smoking, pack-years, type of smoking, and complications of smoking; many of the patients were either current or previous smokers.
✓ History of allergies.
✓ Medications and previous therapy exposure (Table 7).
✓ Occupation, environmental exposures, and use of herbal remedies.
✓ Traveling history.
✓ History of exposure to TB.
✓ History of malignancies
✓ Assessing for features of autoimmune diseases and allergic reactions.
✓ Careful physical examination.
(2) Diagnostic tests. Azacitidine-induced lung injury is a diagnosis of exclusion (10, 30, 56). Some of the most important investigations to be considered to exclude other causes and further support the diagnosis are listed in Table 8.
Because azacitidine-induced lung injury is a diagnosis of exclusion, recognition and management is usually delayed, which subsequently causes progression to respiratory failure and significant lung damage (28, 49). We suggest using criteria to help increase the suspicion index and possibly establish the diagnosis in a timely manner, especially in cases where obtaining a biopsy is not feasible. We suggest having 8/10 of the following factors of the criteria present to consider the diagnosis of azacitidine-induced lung injury:
(1) Fever and respiratory symptoms mimicking pneumonia in patients with AML or MDS who have been exposed to azacitidine (2, 4, 28).
(2) No improvement within 48-36 hours of the use of empirical antibiotics following local guidelines and according to suspected infection (2).
(3) Negative extensive microbiological investigations (2). In some cases, coincidental findings of positive cultures can be confusing and delay diagnosing and treating azacitidine-induced lung injury, which can have dreadful ramifications (4).
(4) Other causes of lung injury are ruled out, including infections, malignancy, exacerbation of underlying lung disease, and other medications or toxins (5, 55).
(5) Clinical, imaging, and pathological patterns in compliance with previous features described features in the literature of azacitidine-induced lung injury (Table 8) (5, 55).
(6) Histopathological proof of no active infectious cause.
(7) Naranjo score ≥ 5 (probable-definite) (6).
(8) Clinical improvement following discontinuation of azacitidine (5, 30). Radiological improvement might be slower.
(9) Favorable clinical and radiological response to early initiation of steroids (55).
(10) Recurrence of features upon reintroduction of azacitidine (5, 30, 37, 49). In cases where azacitidine-induced lung injury remains questionable, the benefits of re-introducing azacitidine outweigh the risks. Suppose similar features of lung toxicity recur with the reintroduction. In that case, this can be a distinguishing feature to support the diagnosis and lead to permanent discontinuation of azacitidine and use of alternative medications (2, 37).
3.7 Treatment and outcomes
The optimal management remains to be discovered. However, the main treatment strategy established in most reported cases is the clampdown of the inflammatory response, usually established by using corticosteroids (1, 2, 88). The data available needs to be more comprehensive to establish which specific agent is superior in management. Corticosteroids are the most used category of drugs. Other less commonly used agents in interstitial lung diseases include other immunosuppressive medications, cytotoxic agents, cyclophosphamide, and antifibrotic agents, alone or combined. However, there has not been enough evidence for their use in azacitidine-induced lung injury (1, 38, 88).
The timely introduction of steroids and the discontinuation of azacitidine have led to the reversibility of the lung injury, both clinically and radiologically, within days (10, 37). The type of corticosteroid and dosing has yet to be unified, but most cases used high-dose steroids with a slow tapering plan (Table 6) (2). Further studies are needed to establish the optimal type, dosing, and tapering plan in such cases (2).
It is reasonable to be hesitant with the use of steroids or other immunosuppressive medications in this scenario, especially early during the presentation, because of the immunocompromised state of the patients and the mimicking picture of other more common infectious causes (2, 42). We must acknowledge the critical consequences of delaying management, with mortality reaching 19% (10, 38). For that reason, we endeavored to create an algorithm that might help ease this difficult decision (Figure 3).
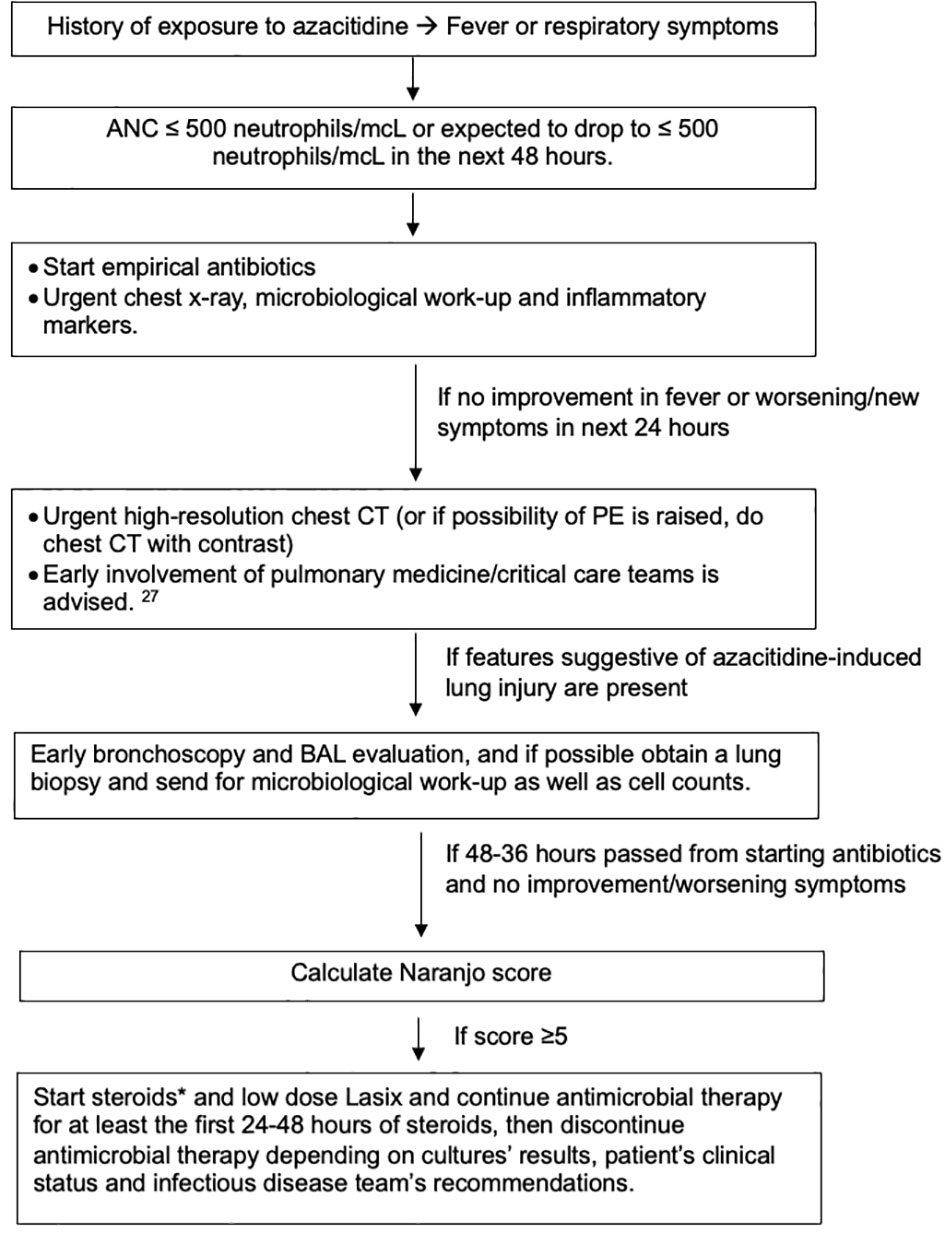
Figure 3 Suggested algorithm for managing azacitidine-induced lung injury. *Steroids type and dose depends on availability, severity of illness and comorbidities that can limit the use of steroids. We generally recommend 1mg/kg as starting dose. Gradual tapering of steroids is preferred if feasible. While patient is on steroids, especially if will be on it for a prolonged time, prophylaxis against pneumocystis jiroveci pneumonia (PJP) and fungal infections, along with protein-pump inhibitors (PPIs) as prophylaxis against gastric ulcers if indicated and vitamin D replacement.
In cases of azacitidine-induced lung injury, recurrence of the toxicity can occur with the reintroduction of azacitidine, such as the scenario in our case (37, 49). For that reason, if the possibility of azacitidine-induced lung injury is high, it is better to avoid the use of azacitidine and consider alternative medications, especially if the risk does not outweigh the benefit (37). Sekhri et al. suggested the use of decitabine as an alternative to azacitidine, as it did not cause lung toxicity and allowed for the continuation of therapy (6). Nevertheless, there have been reported cases of lung injury with the use of decitabine, which would make us extra cautious if decitabine is used as an alternative, and close monitoring with early discontinuation of the drug if respiratory symptoms develop is needed (6, 43).
4 Conclusion
Azacitidine-induced lung injury is uncommon, occurring in <0.1% of patients (37), but can have terminal outcomes. This makes it an important differential diagnosis when dealing with unexplained fever and respiratory symptoms after exposure to azacitidine. A vigilant evaluation and well-timed management are needed to establish the diagnosis, undo the injury, and prevent atrocious outcomes from happening (30). Several attempts to understand the underlying mechanism have been undertaken, but this remains an area that needs further tackling to establish the predictive factors before starting azacitidine and optimizing the management (38, 49).
Author contributions
RA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AA: Writing – review & editing. MAlm: Writing – review & editing. MAly: Writing – review & editing. MAlf: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Author MA was employed by Honoraria: Johnson & Johnson, Pfizer, Astellas, Novartis, Amgen, AstraZeneca, AbbVie, Advisory board: Johnson & Johnson, Biologix, Eli Lilly. Research support: Abbvie, AstraZeneca.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. Adams CD, Szumita PM, Baroletti SA, Lilly CM. Azacitidine-induced interstitial and alveolar fibrosis in a patient with myelodysplastic syndrome. Pharmacotherapy (2005) 25(5 I):765–8. doi: 10.1592/phco.25.5.765.63579
2. Makita S, Munakata W, Watabe D, et al. Azacitidine-induced acute lung injury in a patient with therapy-related myelodysplastic syndrome. J Int Med Res (2017) 45(2):886–93. doi: 10.1177/0300060517698331
3. Alnimer Y, Salah S, Abuqayas B, Alrabi K. Azacitidine-induced cryptogenic organizing pneumonia: A case report and review of the literature. J Med Case Rep (2016) 10(1). doi: 10.1186/s13256-016-0803-0
4. Ahrari A, Sabloff M, Bredeson C, Pakhale S, Souza C, Zwicker J, et al. Rare respiratory and neurologic adverse reactions to azacitidine in the treatment of myelodysplastic syndrome of patients treated at the ottawa hospital. J Hematol (2015) 4(4):231–4. doi: 10.14740/jh227w
5. Hayashi M, Takayasu H, Tada M, Yamazaki Y, Tateno H, Tazawa S, et al. Azacitidine-induced pneumonitis in a patient with myelodysplastic syndrome: First case report in Japan. Internal Med (2012) 51(17):2411–5. doi: 10.2169/internalmedicine.51.8167
6. Sekhri A, Palaniswamy C, Kurmayagari K, Kalra A, Selvaraj DR. Interstitial Lung Disease Associated with Azacitidine Use: A Case Report. Available at: www.americantherapeutics.com.
7. Rüter B, Wijermans PW, Lübbert M. DNA methylation as a therapeutic target in hematologic disorders: recent results in older patients with myelodysplasia and acute myeloid leukemia. Int J Hematol (2004) 80(2):128–35. doi: 10.1532/IJH97.04094
8. Jiang Y, Dunbar A, Gondek LP, Mohan S, Rataul M, O'Keefe C, et al. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood (2009) 113(6):1315–25. doi: 10.1182/blood-2008-06-163246
9. Hattori N, Ushijima T. Compendium of aberrant DNA methylation and histone modifications in cancer. Biochem Biophys Res Commun (2014) 455(1-2):3–9. doi: 10.1016/j.bbrc.2014.08.140
10. Nguyen P, Safdar J, Mohamed A, Soubani A. Azacitidine-induced pneumonitis and literature review. BMJ Case Rep (2020) 13(10). doi: 10.1136/bcr-2020-236349
11. Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes. Cancer (2007) 109(8):1536–42. doi: 10.1002/cncr.22570
12. Jones PA, Taylor SM, Wilson VL. Inhibition of DNA methylation by 5-azacytidine. In: Modified Nucleosides and Cancer. Berlin: Springer Berlin Heidelberg (1983). p. 202–11. doi: 10.1007/978-3-642-81947-6_15
13. Santi DV, Garrett CE, Barr PJ. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell (1983) 33(1):9–10. doi: 10.1016/0092-8674(83)90327-6
14. Gabbara S, Bhagwat AS. The mechanism of inhibition of DNA (cytosine-5-)-methyltransferases by 5-azacytosine is likely to involve methyl transfer to the inhibitor. Biochem J (1995) 307(1):87–92. doi: 10.1042/bj3070087
15. Silverman LR. Targeting hypomethylation of DNA to achieve cellular differentiation in myelodysplastic syndromes (MDS). Oncologist (2001) 6(S5):8–14. doi: 10.1634/theoncologist.6-suppl_5-8
16. Robertson KD, A.Jones P. DNA methylation: past, present and future directions. Carcinogenesis (2000) 21(3):461–7. doi: 10.1093/carcin/21.3.461
17. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. New Engl J Med (2020) 383(7):617–29. doi: 10.1056/nejmoa2012971
18. Van de Louw A, Lewis AM, Yang Z. Autopsy findings in patients with acute myeloid leukemia and non-Hodgkin lymphoma in the modern era: a focus on lung pathology and acute respiratory failure. Ann Hematol (2019) 98(1):119–29. doi: 10.1007/s00277-018-3494-3
19. Meadors M, Floyd J, Perry MC. Pulmonary toxicity of chemotherapy. Semin Oncol (2006) 33(1):98–105. doi: 10.1053/j.seminoncol.2005.11.005
20. Oki Y, Issa JP. Treatment options in advanced myelodysplastic syndrome, with emphasis on epigenetic therapy. Int J Hematol (2007) 86(4):306–14. doi: 10.1532/IJH97.07034
21. Gangat N, Patnaik MM, Tefferi A. Myelodysplastic syndromes: Contemporary review and how we treat. Am J Hematol (2016) 91(1):76–89. doi: 10.1002/ajh.24253
22. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol (2009) 10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8
23. Ornstein MC, Sekeres MA. Combination strategies in myelodysplastic syndromes. Int J Hematol (2012) 95(1):26–33. doi: 10.1007/s12185-011-0987-4
24. Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, et al. Ivosidenib and azacitidine in IDH1 -mutated acute myeloid leukemia. New Engl J Med (2022) 386(16):1519–31. doi: 10.1056/NEJMoa2117344
25. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther (1981) 30(2):239–45. doi: 10.1038/clpt.1981.154
26. Sullivan M, Hahn K, Kolesar JM. Azacitidine: A novel agent for myelodysplastic syndromes. Am J Health-System Pharmacy. (2005) 62(15):1567–73. doi: 10.2146/ajhp040385
28. Nair GB, Charles M, Ogden L, Spiegler P. Eosinophilic pneumonia associated with azacitidine in a patient with myelodysplastic syndrome. Respir Care (2012) 57(4):631–3. doi: 10.4187/respcare.01338
29. Friedman S. The inhibition of DNA(Cytosine-5) methylases by 5-azacytidine: the effect of azacytosine-containing DNA. Mol Pharmacol (1981) 20(2):451.
30. Misra SC, Gabriel L, Nacoulma E, Dine G, Guarino V. How to diagnose early 5-azacytidine-induced pneumonitis: A case report. Drug Saf Case Rep (2017) 4(1). doi: 10.1007/s40800-017-0047-y
31. Gurion R, Vidal L, Gafter-Gvili A, Belnik Y, Yeshurun M, Raanani P, et al. 5-azacitidine prolongs overall survival in patients with myelodysplastic syndrome - a systematic review and meta-analysis. Haematologica (2010) 95(2):303–10. doi: 10.3324/haematol.2009.010611
32. Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol (2002) 20(10):2429–40. doi: 10.1200/JCO.2002.04.117
33. Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene (2002) 21(35):5483–95. doi: 10.1038/sj.onc.1205699
34. Gryn J, Zeigler ZR, Shadduck RK, Lister J, Raymond JM, Sbeitan I, et al. Treatment of myelodysplastic syndromes with 5-azacytidine. Leuk Res (2002) 26(10):893–7. doi: 10.1016/S0145-2126(02)00028-0
35. Pharmion. Vidaza (azacitidine) Package Insert. Boulder, CO. (2004). Available at: https://www.accessdata.fda.gov
36. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. JAMA (1998) 279(15):1200. doi: 10.1001/jama.279.15.1200
37. Litvin R, Dasgupta M, Saad Eldin M, Shah M, Fakhran S. Azacitidine-induced pneumonitis in a patient with acute myeloid leukemia and hyperleukocytosis. Cureus (2022) 14(7). doi: 10.7759/cureus.26758
38. Verriere B, Ferreira V, Denis E, et al. Azacitidine-induced Interstitial Pneumonitis (2015). Available at: www.americantherapeutics.com.
39. Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol (2006) 24(24):3895–903. doi: 10.1200/JCO.2005.05.4346
40. Kaminskas E, Farrell AT, Wang YC, Sridhara R, Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, vidazaTM) for injectable suspension. Oncologist (2005) 10(3):176–82. doi: 10.1634/theoncologist.10-3-176
41. Vigil C, Garcia-Manero, Martin-Santos. Safety and efficacy of azacitidine in myelodysplastic syndromes. Drug Des Devel Ther (2010) 221:221–9. doi: 10.2147/DDDT.S3143
42. Pillai AR, Sadik W, Jones PAH, Thachil J. Interstitial pneumonitis—An important differential diagnosis for pulmonary sepsis in haematology patients. Leuk Res (2012) 36(1):e39–40. doi: 10.1016/j.leukres.2011.09.024
43. Molina M, Yellapragada S, Mims M, Rahman E, Rivero G. Pulmonary complications of azanucleoside therapy in patients with myelodysplastic syndrome and acute myelogenous leukemia. Case Rep Hematol (2015) 2015:1–5. doi: 10.1155/2015/357461
44. Vasu TS, Cavallazzi R, Hirani A, Marik PE. Case report A 64-year-old Male with Fever and Persistent Lung Infiltrate. Respir Care (2009) 54:1263–5.
45. Raghu G, Meyer KC. Cryptogenic organising pneumonia: current understanding of an enigmatic lung disease. Eur Respir Review. (2021) 30(161):210094. doi: 10.1183/16000617.0094-2021
46. San Miguel Amigo L, Franco Osorio R, Mercadal Vilchez S, Martínez-Francés A. Azacitidine adverse effects in patients with myelodysplastic syndromes. Adv Ther (2011) 28(S4):6–11. doi: 10.1007/s12325-011-0024-2
47. Oka S, Ono K, Nohgawa M. Delayed onset of azacitidine-associated pneumotitis: A case report. Am J Ther (2019) 26(5):e617–20. doi: 10.1097/MJT.0000000000000824
48. Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official american thoracic society/european respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med (2013) 188(6):733–48. doi: 10.1164/rccm.201308-1483ST
49. Kuroda J, Shimura Y, Mizutani S, Nagoshi H, Kiyota M, Chinen Y, et al. Azacitidine-associated acute interstitial pneumonitis. Internal Med (2014) 53(11):1165–9. doi: 10.2169/internalmedicine.53.1971
50. Barlési F, Villani P, Doddoli C, Gimenez C, Kleisbauer J. Gemcitabine-induced severe pulmonary toxicity. Fundam Clin Pharmacol (2004) 18(1):85–91. doi: 10.1046/j.0767-3981.2003.00206.x
51. Pavlakis N, Bell DR, Millward MJ, Levi JA. Fatal pulmonary toxicity resulting from treatment with gemcitabine. Cancer (1997) 80(2):286–91. doi: 10.1002/(SICI)1097-0142(19970715)80:2<286::AID-CNCR17>3.0.CO;2-Q
52. Umemura S, Yamane H, Suwaki T, Katoh T, Yano T, Shiote Y, et al. Interstitial lung disease associated with gemcitabine treatment in patients with non-small-cell lung cancer and pancreatic cancer. J Cancer Res Clin Oncol (2011) 137(10):1469–75. doi: 10.1007/s00432-011-1013-1
53. Sabria-Trias J, Bonnaud F, Sioniac M. Severe interstitial pneumonitis related to Gemcitabine. Rev Mal Respir (2002) 19:645–7.
54. Pacheco Y, Douss T, Pujol B, Revol A, Vergnon JM, Biot N, et al. Respiratory function and alveolar biological changes under the effect of CDP-choline in pulmonary interstitial pathology: pulmonary fibrosis and sarcoidosis. Rev Pneumol Clin (1985) 41(2):91–100.
55. Hutchinson KA, Hébert CA, Rajaram A, Fiset PO. Schwartzman K. A case of organizing pneumonia following azacitidine treatment for myelodysplastic syndrome. McGill J Med (2023) 21(1). doi: 10.26443/mjm.v21i1.983
56. Cabral SM, Ferreira P. Azacytidine-induced pneumonitis in acute myeloid leukaemia. Pulmonology (2023) 29(2):165–6. doi: 10.1016/j.pulmoe.2022.06.010
57. Ivashkiv LB. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol (2018) 18(9):545–58. doi: 10.1038/s41577-018-0029-z
58. Kotsianidis I, Spanoudakis E, Nakou E, Miltiades P, Margaritis D, Tsatalas C, et al. Hypomethylating therapy and autoimmunity in MDS: An enigmatic relationship. Leuk Res (2012) 36(4):e90–2. doi: 10.1016/j.leukres.2011.12.013
59. Allen JN. Drug-induced eosinophilic lung disease. Clin Chest Med (2004) 25(1):77–88. doi: 10.1016/S0272-5231(03)00141-2
60. Kita H, Sur S, Hunt LW, Edell ES, Weiler DA, Swanson MC, et al. Cytokine production at the site of disease in chronic eosinophilic pneumonitis. Am J Respir Crit Care Med (1996) 153(4):1437–41. doi: 10.1164/ajrccm.153.4.8616578
61. Romano A, Giallongo C, La Cava P, Parrinello NL, Chiechi A, Vetro C, et al. Proteomic analysis reveals autophagy as pro-survival pathway elicited by long-term exposure with 5-azacitidine in high-risk myelodysplasia. Front Pharmacol (2017) 8:204. doi: 10.3389/fphar.2017.00204
62. Cabrera S, Rodríguez-Bobadilla C, Vázquez-Morales D, Gaxiola M, Maciel M, Selman M, et al. Identification of autophagy-related proteins in lungs from hypersensitivity pneumonitis patients. J Histochem Cytochemistry. (2020) 68(6):365–76. doi: 10.1369/0022155420932103
63. Timmermans WMC, van Laar JAM, van Hagen PM, van Zelm MC. Immunopathogenesis of granulomas in chronic autoinflammatory diseases. Clin Transl Immunol (2016) 5(12):1–12. doi: 10.1038/cti.2016.75
64. Hunt BM, Vallières E, Buduhan G, Aye R, Louie B. Sarcoidosis as a benign cause of lymphadenopathy in cancer patients. Am J Surgery. (2009) 197(5):629–32. doi: 10.1016/j.amjsurg.2009.01.004
65. Mirakhori F, Zeynali B, Kiani S, Baharvand H, Brief BH. Brief azacytidine step allows the conversion of suspension human fibroblasts into neural progenitor-like cells citation. Cell J (2015) 17(1):153–8. doi: 10.22074/cellj.2015.522
66. Sanders YY, Ambalavanan N, Halloran B, et al. Altered DNA methylation profile in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med (2012) 186(6):525–35. doi: 10.1164/rccm.201201-0077OC
67. Parker MI, Gevers W. Demethylation of the type I procollagen genes in transformed fibroblasts treated with 5-azacytidine. Biochem Biophys Res Commun (1984) 124(1):236–43. doi: 10.1016/0006-291X(84)90942-2
68. Choi KH, Lee HB, Jeong MY, Rhee YK, Chung MJ, Kwak YG, et al. The role of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in cryptogenic organizing pneumonia. Chest (2002) 121(5):1478–85. doi: 10.1378/chest.121.5.1478
69. Lappi-Blanco E, Soini Y, Pääkkö P. Apoptotic activity is increased in the newly formed fibromyxoid connective tissue in bronchiolitis obliterans organizing pneumonia. Lung (1999) 177(6):367–76. doi: 10.1007/PL00007654
70. Hueser CN, Patel AJ. Azacitidine-associated hyperthermia and interstitial pneumonitis in a patient with myelodysplastic syndrome. Pharmacotherapy (2007) 27(12):1759–62. doi: 10.1592/phco.27.12.1759
71. Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med (1997) 155(1):242–8. doi: 10.1164/ajrccm.155.1.9001319
72. Jakab GJ. Sequential virus infections, bacterial superinfections, and fibrogenesis. Am Rev Respir Disease. (1990) 142(2):374–9. doi: 10.1164/ajrccm/142.2.374
73. Egan J, Woodcock A, Stewart J. Viruses and idiopathic pulmonary fibrosis. Eur Respir J (1997) 10(7):1433–7. doi: 10.1183/09031936.97.10071433
74. Hubbard R, Venn A, Smith C, Cooper M, Johnston I, Britton J. Exposure to commonly prescribed drugs and the etiology of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med (1998) 157(3):743–7. doi: 10.1164/ajrccm.157.3.9701093
75. Baumgartner KB, Samet JM, Coultas DB, Stidley CA, Hunt WC, Colby TV, et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: A multicenter case-control study. Am J Epidemiol. (2000) 152(4):307–15. doi: 10.1093/aje/152.4.307
76. Tobin RW, Pope CE, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med (1998) 158(6):1804–8. doi: 10.1164/ajrccm.158.6.9804105
77. Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil'The paradox of the neutrophs role in tissue injury. J Leukoc Biol (2011) 89(3):359–72. doi: 10.1189/jlb.0910538
78. Chaoui D, Legrand O, Roche N, Cornet M, Lefebvre A, Peffault de Latour R, et al. Incidence and prognostic value of respiratory events in acute leukemia. Leukemia (2004) 18(4):670–5. doi: 10.1038/sj.leu.2403270
79. Sekeres MA, Guyatt G, Abel G, Alibhai S, Altman JK, Buckstein R, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv (2020) 4(15):3528–49. doi: 10.1182/bloodadvances.2020001920
80. Cordier JF. Cryptogenic organising pneumonia. Eur Respir J (2006) 28(2):422–46. doi: 10.1183/09031936.06.00013505
81. Carvalho JG, Fernandes C, França M. Organizing pneumonia secondary to amiodarone treatment. Heliyon (2022) 8(9):e10630. doi: 10.1016/j.heliyon.2022.e10630
82. Fawcett IW. BOOP associated with nitrofurantoin. Thorax (2001) 56(2):161–1. doi: 10.1136/thorax.56.2.161
83. Tomari S, Homma K, Noguchi T, Aiba T, Matsuki T, Suzuki R, et al. Development of interstitial lung disease after initiation of apixaban anticoagulation therapy. J Stroke Cerebrovascular Diseases. (2016) 25(7):1767–9. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.036
84. Limper AH. Chemotherapy-induced lung disease. Clin Chest Med (2004) 25(1):53–64. doi: 10.1016/S0272-5231(03)00123-0
85. Souza CA, Müller NL, Johkoh T, Akira M. Drug-induced eosinophilic pneumonia: high-resolution CT findings in 14 patients. Am J Roentgenology. (2006) 186(2):368–73. doi: 10.2214/AJR.04.1847
86. Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: radiologic and pathologic manifestations. RadioGraphics (2000) 20(5):1245–59. doi: 10.1148/radiographics.20.5.g00se081245
87. Li H, Groshong SD, Lynch D, Brown KK, Frankel SK. Eosinophilic lung disease. Clin Pulm Med (2010) 17(2):66–74. doi: 10.1097/CPM.0b013e3181d2a1d6
Keywords: azacitidine induced lung injury myelodysplastic syndrome, acute myeloid leukemia, azaciditine, hypomethylating agents, acute lung injury, pneumonitis
Citation: Alyamany R, Alnughmush A, Almutlaq M, Alyamany M and Alfayez M (2024) Azacitidine induced lung injury: report and contemporary discussion on diagnosis and management. Front. Oncol. 14:1345492. doi: 10.3389/fonc.2024.1345492
Received: 27 November 2023; Accepted: 17 January 2024;
Published: 09 February 2024.
Edited by:
Carlo Finelli, Sant’Orsola-Malpighi Polyclinic, ItalyReviewed by:
Andrea Castelli, Azienda Sanitaria Locale di Biella, ItalyMichele Malagola, University of Brescia, Italy
Copyright © 2024 Alyamany, Alnughmush, Almutlaq, Alyamany and Alfayez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruah Alyamany, YXJ1YWhAa2ZzaHJjLmVkdS5zYQ==
 Ruah Alyamany
Ruah Alyamany Ahmed Alnughmush1
Ahmed Alnughmush1 Mansour Alfayez
Mansour Alfayez