- 1Department Of Hematology, Hangzhou First People’s Hospital, Hangzhou, China
- 2Fourth Clinical College, Zhejiang Chinese Medical University, Hangzhou, China
Hepatosplenic T cell lymphoma (HSTCL) is a particularly difficult-to-treat form of lymphoma, with many patients exhibiting primary resistance to chemotherapy. At present, no effective strategy for treating relapsed and refractory HSTCL has been established, with treatment being hampered by questions of how best to overcome chemoresistance to allow patients to attain more durable therapeutic benefits. While there have been marked advances in immunotherapy, allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains one of the primary approaches to curing HSTCL. Of patients who undergo immunochemotherapeutic treatment, many are resistant to conventional chemotherapeutic drugs yet remain sensitive to radiotherapy. We selected to employ a transplant pretreatment regimen consisting of total -body irradiation (TBI) and administered this regimen to two patients with HSTCL. Both patients achieved complete remission (CR) after transplantation, demonstrating extended periods without disease recurrence. We systematic reviewed previously published instances involving allo-HSCT in patients with HSTCL. We have found a total of 67 patients who have received allo-HSCT. In general, age<45 and the status of CR at HSCT may have a more favorable prognosis. Although the impact of TBI on prognosis was not found to be substantial, patients in the TBI group had higher 3-year overall survival (66.7% vs. 71.1%) and 5-year overall survival (58.4% vs. 71.1%) compared to patients in the non-TBI group. In addition, the relapse rate of the TBI group is approximately half that of the non-TBI group. This regimen is well tolerated and associated with low recurrence rates or complications, suggesting that it represents a viable pretreatment regimen for young HSTCL patients undergoing allogeneic HSCT.
Introduction
Hepatosplenic T cell lymphoma (HSTCL) is a rare and highly aggressive form of peripheral T cell lymphoma that is most commonly diagnosed in younger males, resulting in a disease characterized by systemic symptoms, thrombocytopenia, and hepatosplenomegaly without corresponding lymphadenopathy (1). Pathological examination of affected patients often revealed neoplastic cells in the splenic red pulp with infiltration of the hepatic, splenic, and bone marrow sinusoids (2). While γδ T cell receptor (TCR) expression is observed in most cases, some HSTCL cases exhibit αβ TCR expression, with both subtypes exhibiting a similar clinical course such that αβ HSTCL is generally regarded as an immuno-phenotypic variant (3). Approximately 20% of HSTCL cases develop in patients with a history of immunosuppression, and this disease is not related to infection with Epstein-Barr virus (EBV), in contrast with other forms of immune-mediated lymphoma (4, 5).
HSTCL exhibits a very aggressive clinical course, with poor chemotherapy response rates, an extremely low 7% 5-year survival rate, and a median overall survival duration of just 10 months across age groups (6). While there have been a few case reports documenting long-term survivors diagnosed with HSTCL, no standardized strategy for treating affected patients has yet been established (7). Most published data comprise case reports or case series in which CHOP (cyclophosphamide/doxorubicin/vincristine/prednisone)-based regimens have been linked to poor long-term outcomes (6). Even among patients who achieve complete remission (CR) after induction, the median overall survival duration tends to be short as the duration of disease remission tends to be short (8). The only curative treatment option for HSTCL patients is allogeneic hematopoietic stem cell transplantation (allo-HSCT). This report describes two cases of patients diagnosed with HSTCL who underwent allo-HSCT with a TBI combined precondition regimen. In addition, a systematic review of literature on adults with HSTCL who underwent allo-HSCT is also provided.
Case presentation
Patient 1
In 2020, a 29-year-old man with ecchymosis of both lower limbs showed up with no obvious explanation. Despite corticosteroids, the patient continued experiencing splenomegaly and increasing thrombocytopenia, increasing the possibility of an ITP diagnosis. In February 2021, the patient reported to our hospital. At that time, physical examination revealed that the lower pole of the spleen was located two finger widths above the umbilicus and was palpable below the left costal margin. After a thorough assessment (Table 1), a splenectomy was performed, ensuring that there were no contraindications. Significant splenic enlargement was observed during the surgery (Figures 1A, B). The histological findings revealed cells of moderate size with oval-shaped nuclei, exhibiting abnormalities in their nuclear structure and small nucleoli. Immunohistochemistry results of spleen revealed these cells were CD2-, CD3+, CD5-, CD7+, CD20- PAX5-, CD79α-, CD23-, TIA-1+, CD56-, granzyme B-, CD4-, CD8-, CD30-, CD138-, ALK-, Ki-67 + 60%. Cells were positive for TCRγ rearrangement. The splenic flow cytometry analysis was performed, which demonstrated abnormal T cells accounted for ~22.94% of total cells, and these cells were CD7++, CD16++, CD33-, CD13-, CD38-, CD117-, CD19-, CD34-, HLA-DR dim, CD56-, CD5-, CD 5-, CD25-, CD3++, CD99dim, CD2 minimal expression, CD4++, CD8-, TCRαβ-, TCRγδ+, CD335-, CD28dim, CD24, CD57-, CD94+, CD337-, CD158a,h-, CD158f-, CD158e1/e2-, CD158b1/b2j+, CD158i (46.00%). Therefore, a definitive diagnosis of stage IE Hepatosplenic T-cell Lymphoma (HSTCL) was made.
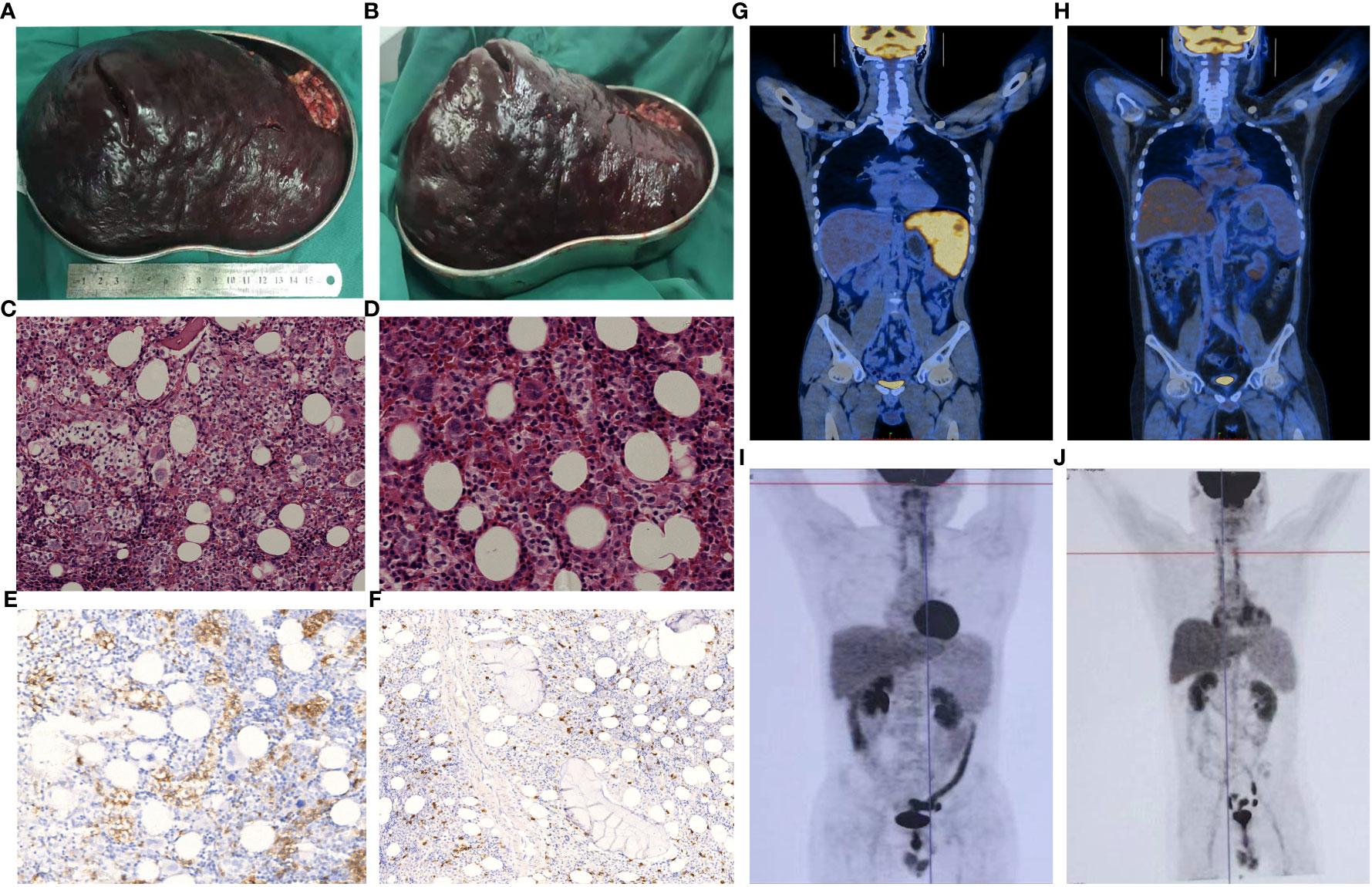
Figure 1 Clinicopathological features of the two patients. In patient 1, the splenectomy specimen revealed significant splenic enlargement, as demonstrated in images (A, B). Bone marrow histology of patient 2, stained with HE (C), x200; (D), x400), exhibited infiltration of lymphoma cells. These cells appeared as small to intermediately sized lymphoid cells within the medullary sinus, displaying slight irregularities, loose chromatin, and inconspicuous nucleoli. Immunostaining indicated that these cells were positive for CD3 (E), x400) and negative for CD5 (F), x200). A PET-CT scan performed on patient 1 before (G), 2/1/2021) and after (H), 1/26/2022) allo-HSCT demonstrated complete remission. Post allo-HSCT, patient 2 showed no abnormalities on PET-CT scans conducted on 5/13/2021 (I) and 1/26/2022 (J).
At approximately 3 weeks after surgery, the patient’s platelet levels had recovered to within the normal range. Chemotherapy consisting of an ICE regimen (ifosfamide 2.7 g d1-3, etoposide 0.15 g d1-3. carboplatin 0.5 g d2) was administered on 2/23/2021, 3/15/2021, 4/12/2021, and 5/12/2021, and after treatment PET-CT results were negative and the patient was considered to have achieved CR. As HSCTL is an aggressive disease with a poor prognosis, it was recommended that the patient undergo allo-HSCT. A FACT conditioning regimen was initiated on 6/18/2021 consisting of TBI (6 Gy, d -10), Fludarabine (150 mg/m2 in 5 days, d -9 to -6), Cytarabine (10 g/m2 in 5 days, d -9 to -5), Cyclophosphamide (2 g/m2 in 2 days, d -4 to -3), T -cell depletion with anti-human thymoglobulin (10 mg/kg in 4 days, d -4 to d -1). GVHD prophylaxis consisted of cyclosporine, mycophenolate mofetil, and low -dose methotrexate (MTX). Between 6/29/2021 and 6/30/2021, haplotypic hematopoietic stem cells were transfused (HLA matched 6/12 with blood type donor A+ for recipient A+, with mononuclear cell (MNC) counts of 8.08x108/kg and CD34+ cell count of 2.54x106/kg. On 6/28/2021, an auxiliary transfusion of cord blood was performed (HLA 7/10, O+ for A+), with a total nucleated cell (TNC) count of 2.2x107/kg and a CD34+ cell count of 1.34x105/kg. Leukocyte and platelet were engrafted on days +10 and +11, perspectively. Acyclovir was used as a precaution to prevent cytomegalovirus (CMV) activation. On day +46, the patient’s CMV DNA levels had risen to 7.82x103 copies/mL but improved with ganciclovir antiviral treatment. On day +85, the patient developed nausea and vomiting after excluding CMV or EBV infection. This was considered an instance of grade I/II gastrointestinal graft-versus-host response. Glucocorticoid and cyclosporine were used for anti-rejection treatment and were effective. On day +443, the patient developed herpes zoster infection, which improved with valaciclovir antiviral therapy. PET-CT scans (Figures 1G, H) revealed a substantially reduced number of lesions compared to the prior scans. Currently, the patient remains free from disease and has survived for 732 days following the transplantation procedure. The patient’s overall health was good, as shown in Figure 2A.
Patient 2
A 30-year-old male received medical treatment at our hospital in July 2020 due to a 3-year history of an enlarged spleen and abdominal swelling without any other associated symptoms. Recently, he has been suffering from inexplicable fatigue, decreased appetite, increased abdominal distension, and a significant weight loss of approximately 10 kg in just 3 months. Systemic examinations were performed, as indicated in Table 1. Bone marrow biopsy revealed apparent hyperplasia, with 24.0% lymphocytes, some loose nuclear chromatin, and villi or pseudo-like processes at the margins of small lymphocytes. Bone marrow flow cytometry revealed T lymphocytes that were CD45+, CD3+, CD5-in accounted for ~11.2% of nucleated cells, and these cells were found to express CD2, CD3, CD7, CD45RO, and TCR γδ but not CD4, CD5, CD8, CD16, CD30, CD57, TCR αβ, or CD45RA. Bone marrow biopsy revealed small- to intermediately-sized lymphoid cells in the medullary sinus, with slight irregularities, loose chromatin, and inconspicuous nucleoli. Immunohistochemistry results showed that the lymphoid cells were MPO-, TDT-, CD34-, CD117-, CD10-, CD3+, CD5-, CD2 weak+, CD7+, CD4-, CD8-, CD43 weak+, CD20—, CD30–, CD56–, granzyme B–, TIA-1++, Ki-67 + 10-15%+, CD42b megakaryocytes+, Gomori 1+. The T cell receptor (TCR) rearrangement results showed TCRGA+, TCRGB+, and TCRD+. The diagnosis of HSTCL was conclusively confirmed through a bone marrow biopsy (Figures 1C-F). The PET-CT scan was excluded because of its excessive cost. The diagnosis of HSTCL was considered based on these data.
After excluding contraindications, a P-Gemox chemotherapeutic regimen (gemcitabine needle [1.6 g d1, oxaliplatin [0.16 g d1], and Pegaspargase [3750 U d2]) was initiated on 8/11/2020. A P-Gemox regimen was repeated beginning on 9/2/2020, and after treatment, B ultrasonography revealed splenomegaly with localized infarction. In addition, the patient experienced a fever associated with the tumor, suggesting that the earlier chemotherapy was ineffective. An ICE chemotherapy regimen was administered on 10/16/2020, 11/14/2020, and 12/5/2020 consisting of etoposide (0.16 g d1-3), ifosfamide (2 g d1-3), and carboplatin (450 mg d1). After treatment, the spleen shrank, consistent with partial remission.
On 1/11/2021, allo-HSCT treatment was initiated using a FACT conditioning regimen. The drugs used for GVHD prophylaxis include cyclosporine, mycophenolate mofetil, and low-dose MTX. Between 1/21/2021 and 1/22/2021, haplotypic hematopoietic stem cells were transfused (HLA matched 6/12, blood type donor O+ for recipient O+) with MNC counts of 10.72x108/kg and a CD34+ cell count of 7.63x106/kg. Leukocytes and platelets were engrafted on days +16. Acyclovir was used to protect against CMV infection after transplantation. On day +59, blood tests for CMV DNA revealed a titer of 3.10x103 copies/mL, and sodium foscarnet was provided for antiviral therapy. Due to the high risk of relapse, Donor lymphocyte infusion (DLI) was treated at day +84 and day +140.
On day +119, B ultrasonography of the liver revealed enlargement and echoic changes with an increase in splenic volume (18.1 x 6.9 cm), and the lower splenic pole was located two finger-widths above the umbilicus. The PET-CT scan (Figure 1I) showed no abnormal glucose uptake by the spleen. On day +182, the patient exhibited skin and oral mucosa grade I GVHD, which improved with glucocorticoid therapy. On day +243, Alanine aminotransferase levels were significantly elevated and improved following hormone administration and hepatoprotective treatment. On day +431, B ultrasonography suggested the presence of multiple hypoechoic nodules in the bilateral axilla, which were more prominent on the right side, being about 4.1x2.2x2.8 cm in size. Substantial splenic enlargement was noted (~4.8 cm thick). An axillary lymph node biopsy was performed, and pathology did not reveal any evidence of lymphoma. Repeat PET-CT (Figure 1J) scans were negative. The patient remains disease-free at day 902 after transplant (Figure 2B).
Systematic review
In order to provide a complete overview of the clinical features and outlook for patients with HSTCL who received HSCT, we systematically assessed the available literature. The included Supplementary Table S1 provides a comprehensive overview of our search methodology, with retrieval and outcome analyses being conducted by two independent researchers. Due to the primary statistical focus on the prognosis of transplant patients, material that did not include precise survival data, especially overall survival (OS), was excluded. We incorporated 30 research papers encompassing 67 patients (including two participants from this study). In cases where articles or attachments did not expressly include precise clinical details, we used the terms ‘Not Available’ or ‘Not Defined’ to indicate the absence of data. This was accomplished to avoid any later statistical analysis in survival assessments.
Among the individuals who underwent transplants were 41 males and 20 females. The majority of them, precisely 44 out of 61, were under the age of 45. 9 individuals suffered from having spleens removed, and 58 patients were classified as stage IV. During the transplantation process, 25 patients were found to be in a state of complete remission, 26 patients were in a state of partial remission, and 11 patients were not. The donor selection process consisted of 17 instances involving sibling donors, 7 cases involving haploidentical transplants, 18 cases involving unrelated donors, and 4 cases involving cord blood. The origins of the transplants consisted of 12 cases of bone marrow, 16 cases of peripheral blood stem cells, and 4 cases of cord blood. The transplant intensity was classified as MAC in 25 cases, RIC in 11 cases, and NMAC in 1. A total of 33 patients received transplant conditioning regimens involving TBI, with the majority being under the age of 45 (29 out of 33, P=0.002). Fourteen of these cases reported the dose and method of TBI application. Seven received >6GY, and 7 received ≤6GY. The majority (10/12) underwent fractionated radiation therapy. The predominant protocols implemented for GVHD prevention were CSA or FK506, and the GVHD incidence was 72.9%(27/37) (Table 2).
Eleven patients suffered a recurrence, accounting for 18.6% of the total (11/59). Before transplantation, patients who achieved complete remission (CR) appeared to have a decreased relapse rate (4.7% vs 25.7%, P=0.072). The number of recorded deaths was 22, resulting in a mortality rate of 32.8% (22 out of 67). Patients under 45 exhibited a reduced mortality rate (27.2% vs 52.9%, P=0.076). In addition, patients who achieved CR before transplantation had a significantly lower mortality rate compared to those who did not (16% vs 45.9%, P=0.027). Out of the 19 patients whose reasons for death were recorded, only 4 died as a result of disease progression (Tables 2, 3). No indicators were identified as affecting GVHD (Supplementary Table S2).
There were 53 patients with complete data documenting the use of TBI as a component of the transplant conditioning regimen. The groups had no substantial statistical disparities in prognosis or adverse outcomes. Patients who had TBI as part of their transplant preparation showed higher expected 3-year OS rates and 5-year OS rates compared to those who did not get TBI (71% vs 67%, 71% vs 58%). The group of individuals with TBI exhibited a decreased rate of relapse in comparison to the group without TBI (25% vs 40%). The incidence of GVHD was 66.7% in the TBI group and 83.3% in the non-TBI group. The group of individuals with TBI demonstrated a substantially lower rate of relapse in comparison to the group without TBI (12.9% vs 27.8%). The mortality rate was 24.2% among individuals with TBI and 40% among those without TBI.
We conducted additional studies of patients’ OS and PFS (Supplementary Table S3). All patients who were enrolled in the study had OS data available. However, there were challenges in estimating PFS. We attempted to replace PFS nodes with post-transplant progression-free survival time, but only 25 patients reported explicit PFS durations. Prognosis may be affected by factors such as age (Figures 3A, D), the state of remission before a transplant (Figures 3B, E) and relapse after transplantation (Figures 3C, F) when considering the operating system. Before transplantation, attaining CR may affect patients’ PFS, and after the transplant, the recurrence of the disease seems to be associated with a lower PFS (as shown in the Table). Furthermore, the prognosis does not appear to be affected by either splenectomy or the transplant conditioning regimen.
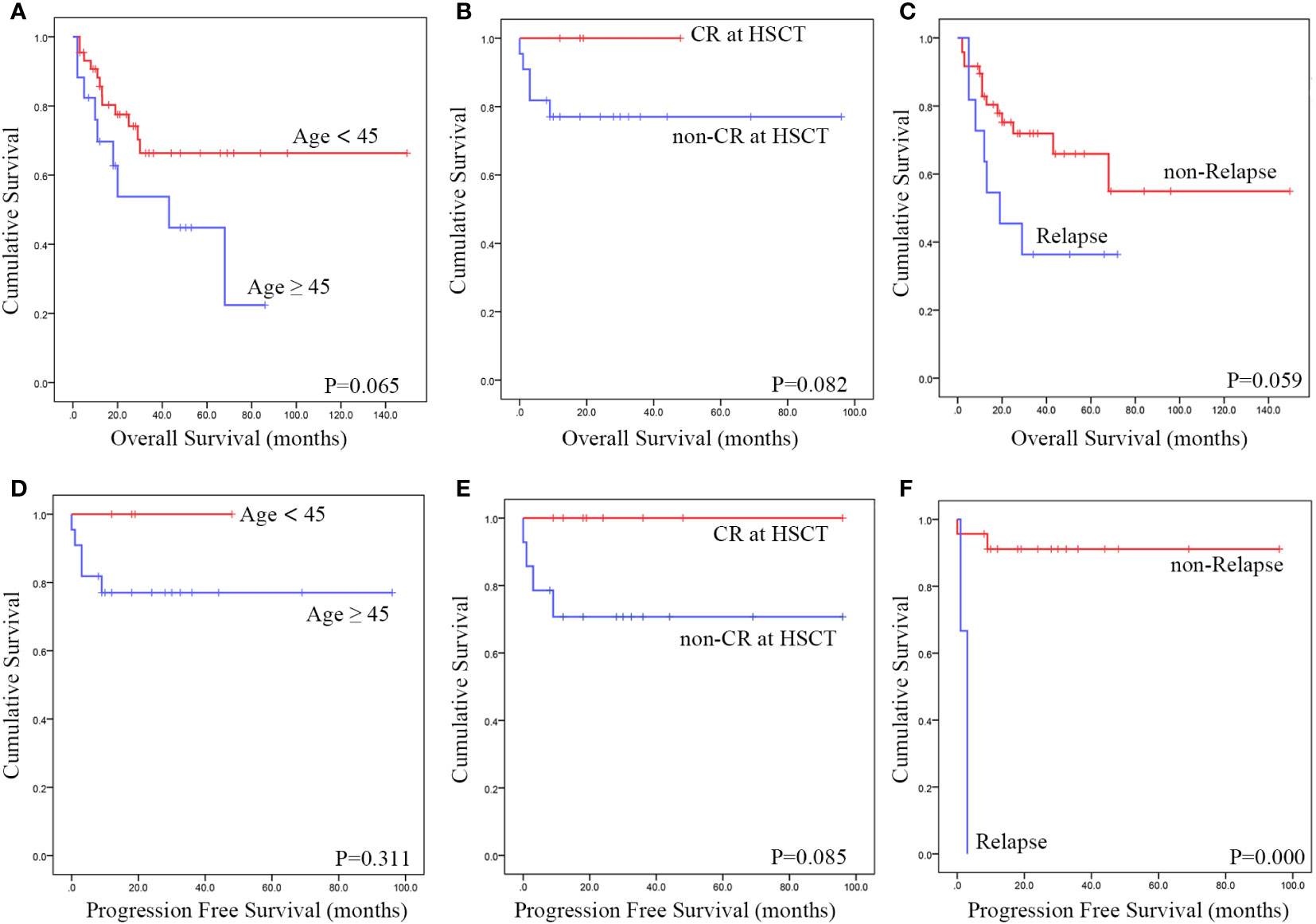
Figure 3 Using data from published cases, a Kaplan-Meier survival analysis was conducted to assess the impact of various risk factors. The findings revealed that Patients under 45 years of age (A, D), transplant status of CR (B, E) and relapse after transplantation (C, F) exhibited a improved OS and PFS.
Discussion
HSTCL is a rare and aggressive type of peripheral T cell lymphoma (PTCL) that most frequently affects young males, with a median age at diagnosis of 32 years. First reported in 1990 (9), HSTCL accounts for < 1% of all PTCL cases and is a rapidly progressive disease that is poorly responsive to chemotherapy (8). An estimated 20% of patients with HSTCL exhibit underlying immunosuppression, and viral infections (including EBV and hepatitis B virus) are rarely reported to be associated with HSTCL onset (10). Greater HSTCL incidence rates among inflammatory bowel disease patients undergoing TNF inhibitor treatment have been reported. Bernstein et al. (11) noted that the risk of this form of lymphoma was elevated among individuals with Crohn’s disease. Kandiel et al. (12) conducted a meta-analysis in which they found that lymphoid malignancy risk was increased four-fold among patients receiving biologics. Ochenrider et al. (13) additionally surveyed reports about 28 HSTCL patients with Crohn’s disease and found that all of these patients underwent azathioprine or thiopurine treatment. At the same time, 79% were treated with the biological infliximab.
HSTCL presents in a manner distinct from other more common lymphomas, with affected patients often exhibiting hepatosplenomegaly and prominent systemic symptoms without substantial lymph node involvement. Routine blood analyses may detect cytopenia without significant bone marrow infiltration (8). Other common presentations include Coombs-negative autoimmune hemolytic anemia and moderately elevated transaminase levels. Mild myeloid cell pathological hematopoiesis may be evident in the bone marrow, and significantly elevated lactate dehydrogenase levels are often detected. Both patients in this report exhibited thrombocytopenia when initially diagnosed, consistent with the fact that over 90% of patients reportedly develop thrombocytopenia that may be indicative of disease progression (14). Case 1 was initially diagnosed with immune thrombocytopenia, and the initial efficacy of hormonal treatment may have been attributable to a partial effect of this therapeutic intervention on lymphoma cells before the onset of hormone resistance.
HSTCL patients exhibit a 5-year OS rate of under 7%, with a median OS interval of just 10 months. Early induction therapy is essential when managing these patients, and while some patients undergo chemotherapeutic treatment, the response duration is generally short (8, 15). Thus, optimal patient outcomes depend on diagnosing patients and planning to perform HSCT early. In patients necessitating radical treatment, high-intensity chemotherapy is recommended, and given the absence of definitive guidelines for first-line chemotherapeutic regimens for these patients, approaches similar to those utilized for PTCL, such as CHOP or ECHOP regimens, can be implemented as initial treatments. In their single-center study of 14 HSTCL patients, Voss et al. (6) found that the median patient age was 36 years, and all patients presented with stage IV disease. Non-CHOP induction therapies were used for most patients, with most receiving ifosfamide-based induction regimens and 8 receiving a non-CHOP regimen, of whom 5 achieved remission and underwent subsequent auto- or allo-HSCT. The prognosis of CHOP regimens was reportedly poor in two studies by Belhadj et al. (14) and Falchook et al. (16). As such, the two patients in the present report were initially treated with non-CHOP induction chemotherapy. In Case 2, the patient responded poorly to a GDP regimen but achieved therapeutic remission after switching to an ICE regimen and currently remains disease-free after bridging transplantation. In Case 1, initial ICE induction chemotherapy led to CR, and the patient remained disease-free after bridging allogeneic HSCT. These outcomes align well with prior evidence that ifosfamide-based regimens are associated with better patient treatment responses.
The value of HSCT as a form of consolidation therapy in HSTCL patients has yet to be established. As such, transplantation has the potential to be curative. Patients may experience better outcomes than those who undergo other forms of disease management. While CR can be achieved following induction therapy in some cases, given the short remission duration in most HSTCL cases, early transplantation is generally recommended. In a study of seven HSTCL patients at the Memorial Sloan-Kettering Cancer Center, of whom six had undergone autologous or allogeneic HSCT, the median OS was 65.5 months (6). In the European Bone Marrow Transplant Lymphoma Working Group Study of 25 HSTCL patients (17), 2/18 of the patients who underwent all-HSCT experienced subsequent relapse compared to 5/7 patients who underwent auto-HSCT. The graft-versus-lymphoma effects associated with allo-HSCT may thus provide benefits to treated patients.
Given that HSTCL primarily affects younger patients and is associated with poor outcomes, this suggests that allo-HSCT should be considered as an option for consolidation treatment in appropriate patients. Our comprehensive evaluation revealed that patients under 45 years who have a complete response at the time of transplantation may experience a more favorable prognosis than older patients. Although the impact of TBI on prognosis was insignificant, patients in the TBI group had improved 3-year and 5-year OS rates compared to those in the non-TBI group. Post-transplant relapse has a significant impact on OS. The prognosis was also not impacted by disease stage at the time of transplantation, indicating that even patients with advanced disease at the time of transplantation may attain benefits from HSCT.
One of the two patients in this report underwent allogeneic HSCT while in CR. In contrast, the other underwent this procedure in a stable disease state, with good efficacy in both instances. Limited data from prior studies suggest that myeloablative preconditioning (MAC) is not associated with any improvements in efficacy relative to reduced intensity conditioning (RIC), suggesting that the graft-versus-lymphoma effect is the primary advantage of allogeneic transplant preconditioning. Treatment regimens incorporating TBI offer advantages for the management of relapsed and refractory lymphatic tumor patients, mainly as they provide a means of more effectively targeting sheltered tumor cells. The treatment regimen employed in this case report was between that of RIC and MAC (18), and these patients achieved deep remission following FACT pretreatment without any significant pretreatment-associated side effects. Patient 2 exhibited splenomegaly on the initial diagnosis, and while splenic enlargement was still evident on PET-CT scanning, the corresponding SUV value was low, and the patient exhibited no cytopenia or evidence of recurrent disease. This patient thus achieved CR, which continues to be accurate as of the most recent follow-up. In another report focused on HSTCL (19), four patients were identified as long-term survivors, with three of these patients having undergone splenectomy alone, including the patient with the longest survival duration of 137 months. Splenectomy may thus represent a viable alternative treatment strategy for patients with disease confined to the spleen who are refractory to chemotherapy or transplantation.
Conclusion
In conclusion, HSTCL has been associated with a very unfavorable prognosis, highlighting the necessity for further clinical trials to elucidate the significance and ideal timing of allo-HSCT in affected individuals. Younger patients can benefit significantly from allogeneic transportation due to the disease’s tendency for rapid progression and brief remission periods; therefore, it is recommended that this procedure be executed shortly after remission is achieved, with a pretreatment regimen that includes TBI constituting a viable strategy. Even in patients with relapsed and refractory HSTCL, allo-HSCT can provide benefits. Nonetheless, considering that high treatment -related mortality may be evident even in the early phases, the patient’s overall condition should be considered when determining the most suitable transplantation protocol.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
CC: Writing – original draft, Writing – review & editing. FY: Data curation, Investigation, Writing – review & editing. PM: Data curation, Formal analysis, Writing – review & editing. PS: Investigation, Writing – original draft. SQ: Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Hangzhou Science and Technology Major Project (Grant Number: 202004A15), Hangzhou Medical Health Science and Technology Major Project (Grant Number: Z20210039), and Zhejiang Province Traditional Chinese Medicine Science and Technology Project (Grant Number: 2023ZR122).
Acknowledgments
The authors express their gratitude to the Department of Pathology colleagues for their contribution to the pathological images included in this paper. Furthermore, We appreciate the understanding and cooperation provided by these two patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1345464/full#supplementary-material
Abbreviations
HSTCL, Hepatosplenic T cell lymphoma; allo-HSCT, allogeneic hematopoietic stem cell transplantation; TBI, total body irradiation; CR, complete remission; TCR, T cell receptor; EBV, Epstein-Barr virus; CHOP, chemotherapy regimen consisting of cyclophosphamide, doxorubicin, vincristine, prednisone; CR, complete remission; PET-CT, positron emission tomography-computed tomography; FACT, conditioning regimen consisting of TBI, Fludarabine, Cytarabine, and Cyclophosphamide; HLA, human leukocyte antigen; ICE, a chemotherapy regimen consisting of ifosfamide, carboplatin, and etoposide; MTX, methotrexate; MNC, mononuclear cell; TNC, total nucleated cell; CMV, cytomegalovirus; DLI, Donor lymphocyte infusion; PTCL, peripheral T cell lymphoma; MAC, myeloablative preconditioning; RIC, reduced intensity conditioning.
References
1. Farcet JP, Gaulard P, Marolleau JP, Le Couedic JP, Henni T, Gourdin MF, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood (1990) 75:2213–9.
2. Cooke CB, Krenacs L, Stetler-Stevenson M, Greiner TC, Raffeld M, Kingma DW, et al. Hepatosplenic T-cell lymphoma: a distinct clinicopathologic entity of cytotoxic gamma delta T-cell origin. Blood (1996) 88:4265–74.
3. Catania G, Zallio F, Monaco F, Corsetti MT, Trincheri N, Bonello L, et al. Successful HLA haploidentical myeloablative stem cell transplantation for aggressive hepatosplenic alpha/beta (αβ) T-cell lymphoma. Leuk Res Rep (2014) 3:90–3. doi: 10.1016/j.lrr.2014.09.001
4. Alsohaibani F, Abdulla MA, Fagih MM. Hepatosplenic T-cell lymphoma. Indian J Hematol Blood Transfus (2011) 27(1):39–42. doi: 10.1007/s12288-010-0051-1
5. Vega F, Medeiros LJ, Bueso-Ramos C, Jones D, Lai R, Luthra R, et al. Hepatosplenic gamma/delta T-cell lymphoma in bone marrow: a sinusoidal neoplasm with blastic cytologic features. Am J Clin Pathol (2001) 116(3):410–9. doi: 10.1309/BM40-YM6J-9T3X-MH8H
6. Voss MH, Lunning MA, Maragulia JC, Papadopoulos EB, Goldberg J, Zelenetz AD, et al. Intensive induction chemotherapy followed by early high-dose therapy and hematopoietic stem cell transplantation results in improved outcome for patients with hepatosplenic T-cell lymphoma: A single institution experience. Clin Lymphoma Myeloma Leuk (2013) 13(1):8–14. doi: 10.1016/j.clml.2012.09.002
7. McThenia SS, Rawwas J, Oliveira JL, Khan SP, Rodriguez V. Hepatosplenic γδ T-cell lymphoma of two adolescents: Case report and retrospective literature review in children, adolescents, and young adults. Pediatr Transplant (2018) 22(5):e13213. doi: 10.1111/petr.13213
8. Krishnan M, Lunning M. Hepatosplenic γ-δ T-cell lymphoma: who is on your speed dial? J Oncol Pract (2019) 15(6):307–12. doi: 10.1200/JOP.18.00594
9. Tripodo C, Iannitto E, Florena AM, Pucillo CE, Piccaluga PP, Franco V, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol (2009) 6(12):707–17. doi: 10.1038/nrclinonc.2009.169
10. Jaffe ES, Swerdlow SH, Campo E, Pileri S, Thiele J, Harris N, et al. WHO Classification of Tumors of the Hematopoietic and Lymphoid Tissues. Lyon: IARC (2008).
11. Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer (2001) 91(4):854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z
12. Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut (2005) 54(8):1121–5. doi: 10.1136/gut.2004.049460
13. Ochenrider MG, Patterson DJ, Aboulafia DM. Hepatosplenic T-cell lymphoma in a young man with Crohn's disease: case report and literature review. Clin Lymphoma Myeloma Leuk (2010) 10(2):144–8. doi: 10.3816/CLML.2010.n.021
14. Belhadj K, Reyes F, Farcet JP, Tilly H, Bastard C, Angonin R, et al. Hepatosplenic gamma-delta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood (2003) 102(13):4261–9. doi: 10.1182/blood-2003-05-1675
15. Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol (2008) 26(25):4124–30. doi: 10.1200/JCO.2008.16.4558
16. Falchook GS, Vega F, Dang NH, Samaniego F, Rodriguez MA, Champlin RE, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol (2009) 20(6):1080–5. doi: 10.1093/annonc/mdn751
17. Tanase A, Schmitz N, Stein H, Boumendil A, Finel H, Castagna L, et al. Allogeneic and autologous stem cell transplantation for hepatosplenic T-cell lymphoma: a retrospective study of the EBMT Lymphoma Working Party. Leukemia (2015) 29(3):686–8. doi: 10.1038/leu.2014.280
18. Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in the current era: a study in acute myeloid leukemia patients [published correction appears in Bone Marrow Transplant]. Bone Marrow Transplant (2020) 55(6):1114–25. doi: 10.1038/s41409-020-0803-y
Keywords: hepatosplenic T cell lymphoma, allogeneic hematopoietic stem cell transplantation, total body irradiation, preconditioning, prognosis, systematic review
Citation: Chen C, Yang F, Miu P, Shi P and Qian S (2024) Allo-HSCT with TBI-based preconditioning for hepatosplenic T-cell lymphoma: two case reports and systematic review of literature. Front. Oncol. 14:1345464. doi: 10.3389/fonc.2024.1345464
Received: 27 November 2023; Accepted: 11 January 2024;
Published: 29 January 2024.
Edited by:
Jacopo Mariotti, Humanities Research Hospital, ItalyReviewed by:
Xiao-Dong Mo, Peking University People’s Hospital, ChinaYifan Pang, Levine Cancer Institute, United States
Copyright © 2024 Chen, Yang, Miu, Shi and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengfei Shi, c3lwZnNoaUAxNjMuY29t; Shenxian Qian, c3hxaWFuMTAyOEBqdS5lZHUuY24=
†These authors have contributed equally to this work
 Can Chen
Can Chen Fan Yang1
Fan Yang1