
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 12 June 2024
Sec. Radiation Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1343324
Objective: This study aims to explore the clinical application of bronchial artery chemoembolization (BACE) in managing refractory central lung cancer with atelectasis.
Methods: The retrospective case series includes patients diagnosed with refractory central lung cancer and atelectasis who underwent BACE treatment at Yueyang Integrated Traditional Chinese and Western Medicine Hospital, affiliated with Shanghai University of Traditional Chinese Medicine, from January 2012 to December 2021.
Results: All 30 patients with lung cancer successfully underwent BACE procedures. Their ages ranged from 62 to 88 years, with an average age of 67.53. The treatment interval was 21 days, and the treatment cycle ranged from 2 to 12 times, averaging 4.13 times. During the BACE procedures, the Karnofsky Performance Status (KPS) score after 2 to 3 BACE cycles showed a significant improvement (82.0 ± 10.1 vs 68.3 ± 14.0, P < 0.001) than that of before BACE. Only nutritional support and symptomatic treatment were performed after BACE, and no major hemoptysis were observed. During follow-up, 23 cases resulted in mortality, while seven survived. The median progression-free survival (PFS) and overall survival (OS) were 7.0 (95% CI: 4.6–9.4) and 10.0 (95% CI: 6.2–13.8) months, respectively, with 1-, 2-, and 3-year survival rates of 84.0%, 53.5%, and 11.3%, respectively. Eight cases exhibited bronchial recanalization and relief of atelectasis. According to the RECIST scale, there were 4 cases of complete response (CR), 16 cases of partial response (PR), 9 cases of stable disease (SD), and 1 case of progressive disease (PD). No serious adverse events were reported.
Conclusion: BACE might be a safe intervention for refractory central lung cancer accompanied by atelectasis. The procedure exhibits satisfactory outcomes in tumor control, atelectasis relief, and enhancement of quality of life, warranting further investigation.
This study investigates the clinical application of bronchial artery chemoembolization (BACE) in managing refractory central lung cancer with atelectasis. The retrospective case series, conducted at Yueyang Integrated Traditional Chinese and Western Medicine Hospital, includes 30 patients diagnosed with this condition between January 2012 and December 2021. The BACE procedures were successfully performed on all patients, leading to significant short-term symptom improvements and Karnofsky performance status (KPS) scores. The median progression-free survival (PFS) and overall survival (OS) were 7 months and 10 months, respectively, with encouraging 1-, 2-, and 3-year survival rates.
The findings suggest that BACE is a safe intervention, offering favorable outcomes in tumor control, atelectasis relief, and quality of life enhancement. The observed increase in KPS scores indicates an improvement in overall patient well-being. Importantly, no serious adverse events were reported. Given these promising results, further investigation is warranted. The manuscript is well-suited for submission to a medical journal specializing in interventional oncology, respiratory medicine, or integrative medicine. The study’s focus on a novel intervention for refractory central lung cancer addresses a critical clinical challenge. It contributes valuable insights to the field, making it a strong fit for journals interested in innovative approaches to cancer management.
Central lung cancer denotes lung cancer that originates in the trachea, bronchial tree, major blood vessels, esophagus, or heart (1). Central lung cancers are usually ineligible for surgery, and the treatment options include radiotherapy and systemic therapies (chemotherapy, targeted therapy, and immunotherapy) (2–6). Central lung cancer is frequently accompanied by atelectasis (1). Atelectasis primarily arises from tumor obstruction and compression by metastatic lymph nodes, often coexisting with obstructive pneumonia (7). Patients facing malignant central airway obstruction not only contend with an unfavorable overall prognosis but also endure severe respiratory symptoms and a diminished quality of life (8–10). The management of the case under discussion was particularly arduous due to the disease’s advanced stage and the patient’s deteriorating clinical state, marked by recurrent pulmonary infections and severe respiratory distress stemming from central airway obstruction (11, 12). While bronchoscopy and stent placement represent the primary approaches for addressing atelectasis, they do not constitute a cure for the underlying tumor (13–15).
Ever since Viamonte’s pioneering bronchial artery angiography in 1964, a growing array of local intervention treatments, including bronchial artery embolization and chemoembolization, have emerged, instilling hope for the survival of lung cancer patients (16–18). In recent years, interventional therapy technologies have been widely applied in lung cancer treatment, offering advantages such as minimally invasive procedures, minimal adverse reactions, precise therapeutic outcomes, and repeatability. These advancements have significantly enhanced the survival rates and quality of life for individuals with lung cancer (19–21).
However, existing research focuses predominantly on bronchial arterial chemoembolization (BACE) and lung cancer centers on non-small cell lung cancer (NSCLC). For instance, in 2023, Yu et al. compared the efficacy and safety of drug-eluting bead BACE against conventional BACE in NSCLC (22). Similarly, in 2021, Liu et al. investigated the effectiveness and safety of drug-eluting bead BACE in combination with oral anlotinib for treating NSCLC (23). The application of BACE in addressing central lung cancer with atelectasis remains notably underreported. Recognizing the inherent challenges in treating central lung cancer, our center has, over the past decade, employed transcatheter BACE for central lung cancer complicated by atelectasis, yielding discernible therapeutic outcomes. Thus, this study aims to explore the clinical application of BACE in managing refractory central lung cancer with atelectasis.
We conducted a retrospective analysis of patients diagnosed with locally advanced (IIIB, IIIC) and advanced (IVA, IVB) refractory central lung cancer complicated with atelectasis who underwent BACE treatment at Yueyang Integrated Traditional Chinese and Western Medicine Hospital affiliated with Shanghai University of Traditional Chinese Medicine from January 2012 to December 2021. The inclusion criteria were: 1) Histopathological confirmation of lung cancer; 2) Completion of at least one BACE treatment; 3) Availability of complete follow-up information. The study was approved by the ethics committee of Yueyang Integrated Traditional Chinese and Western Medicine Hospital affiliated with the Shanghai University of Traditional Chinese Medicine. The approval number is 2021–008.
Patients underwent successful BACE procedures conducted under local anesthesia. Using the modified Seldinger technique, the right or left femoral artery was punctured, followed by transaortic selective arterial cannulation of the left or right bronchial arteries. Two micocatheter models were used. One was Stride Micocatheter (Asahi STD125–26S) with an outer diameter of 2.6Fr (0.88mm) at the head end and a Micro Guidewire with an outer diameter of 0.018Fr (0.46mm). Straight head guide wire, can be molded into a J shape. Another Merit Maestro Micocatheter (Merit Medical Syetem, 29MC29130ST), head end outer diameter is 2.9Fr (0.96mm), Swan neck shape, Inside Steerable Guidewire (Merit Medical Tenor, TNR2811), outer diameter 0.018Fr (0.46mm), straight head guide wire, can be molded into J shape according to demand. Digital subtraction angiography (DSA) confirmed the bronchial artery’s blood supply to the target tumor and ruled out abnormal shunting to the spinal cord and brain. Paclitaxel (60–80 mg/m2) and cisplatin (30–40 mg/m2) or carboplatin (dose calculated as AUC=5) were diluted to 50ml-100ml with 0.9% sodium chloride solution and slowly infused into the tumor area through the bronchial artery, taking at least 20 minutes. Subsequently, the bronchial artery endings were embolized using 150–350-560 µm gelatin sponge particles. Following the procedure, the catheter was removed, the puncture site was pressed for hemostasis and bandaged, and the patient remained in bed for 8 hours.
The efficacy evaluation was grounded in patient responsiveness and disease status, employing the Response Evaluation Criteria in Solid Tumors (RECIST) scale (24). This scale categorizes outcomes as follows: complete response (CR), denoting the disappearance of all target lesions, with any pathological lymph nodes showing a reduction in short axis to <10 mm; partial response (PR), indicating at least a 30% decrease in the sum of diameters of target lesions, using the baseline sum diameters as a reference; stable disease (SD), characterized by neither sufficient shrinkage for PR nor sufficient increase for progressive disease (PD), with the smallest sum diameters taken as reference during the study; and PD, involving at least a 20% increase in the sum of diameters of target lesions, using the smallest sum on study as reference (including the baseline sum if that is the smallest on study). Moreover, the sum must demonstrate an absolute increase of at least 5 mm, and the appearance of one or more new lesions is also considered progression. Patient outcomes were used to determine progression-free survival (PFS), overall survival (OS), Karnofsky performance status (KPS) scores, and adverse events. PFS and OS were calculated from the first BACE procedure.
All statistical analyses were performed using SPSS 25.0 for Windows (IBM, Armonk, NY, USA). Continuous and categorical variables were presented as mean ± standard deviation and n (%), respectively. PFS and OS were assessed using the Kaplan-Meier method and log-rank test. Two-sided P-values <0.05 were considered statistically significant.
The clinical characteristics of all 30 lung cancer patients are summarized in Table 1. Their ages ranged from 62 to 88 years, with an average age of 68. PD-L1 expression was <1% in all included patients, and no immunotherapy was performed. Seventeen patients (56.7%) underwent radiotherapy, chemotherapy, and molecular-targeted therapy before BACE.
The BACE procedure was successfully performed on all subjects. The treatment interval was 21 days, and the treatment cycle ranged from 2 to 12 times, averaging 4.1 cycles. The Karnofsky Performance Status (KPS) score showed a significant improvement from 68.3 ± 14.0 before BACE to 82.0 ± 10.1 after 2 to 3 BACE cycles (P < 0.001). Only nutritional support and symptomatic treatment were performed after BACE. There were no cases of major hemoptysis. Since the cases of hemoptysis were mild, the procedure was the same.
During follow-up, 23 cases resulted in mortality, while seven survived. The median progression-free survival (PFS) and overall survival (OS) were 7.0 (95% CI: 4.6–9.4) and 10.0 (95% CI: 6.2–13.8) months, respectively (Figure 1), with 1-, 2-, and 3-year survival rates of 84.0%, 53.5%, and 11.3%, respectively. Eight cases exhibited bronchial recanalization and relief of atelectasis. According to the RECIST scale, there were 4 cases of complete response (CR), 16 cases of partial response (PR), 9 cases of stable disease (SD), and 1 case of progressive disease (PD). No serious adverse events related to the procedure were reported. Patients with progressive disease were transitioned to radiotherapy, best supportive treatment, or hospice care. One patient died due to an accident, who had complications, including cough, respiratory failure, heart failure, and dysphoria.
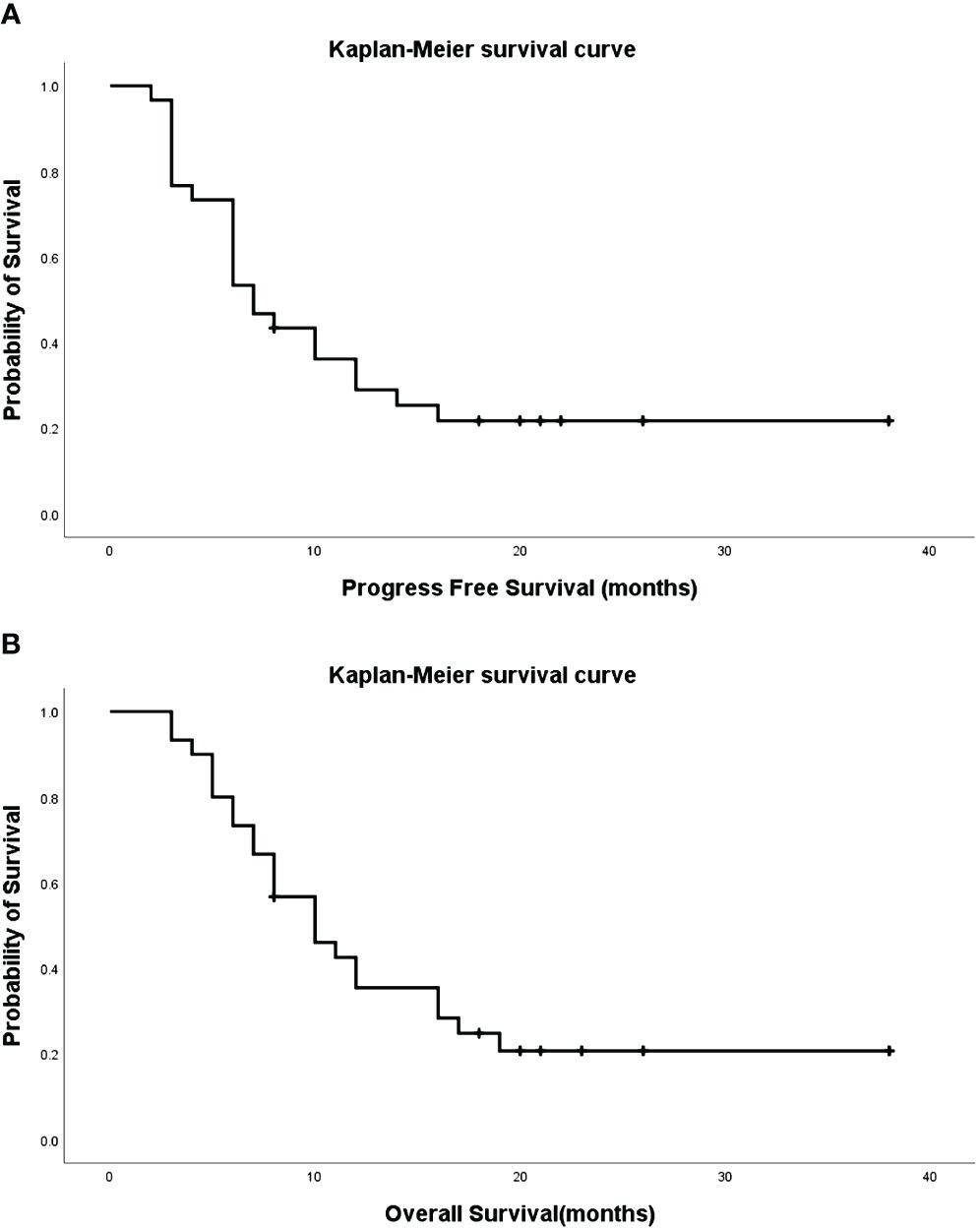
Figure 1 Progression-Free Survival (PFS) [(A), median=7.0, 95%CI: 4.6–9.4] and Overall Survival (OS) [(B), median=10.0, 95%CI: 6.2–13.8] of patients.
One patient, diagnosed by biopsy with squamous cell carcinoma (T4N3M0), exhibited a right upper lung tumor with atelectasis, hilar, and mediastinal lymph node metastasis (Figure 2A). In Figure 2B, right bronchial artery angiography revealed blood supply to the right lung tumor, amenable to chemotherapy and embolization. After six courses of BACE, the CT coronal plane image displayed the disappearance of the right upper lung tumor and atelectasis (Figure 2C).
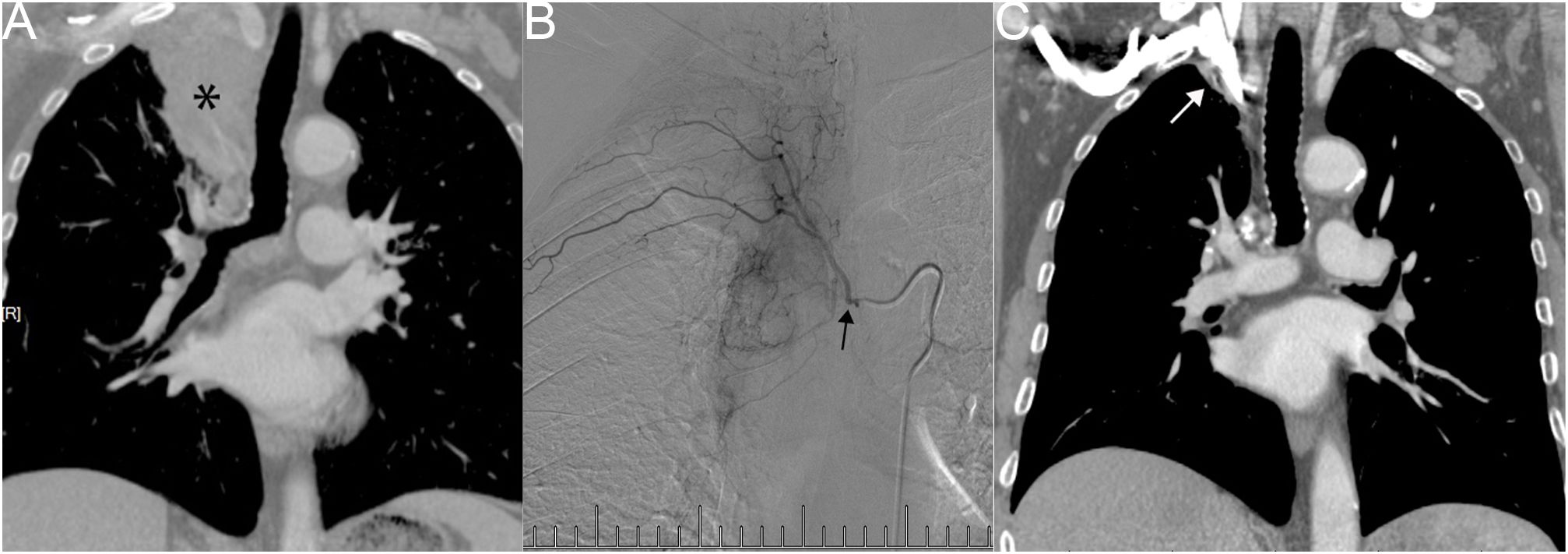
Figure 2 Male, 83 years old, with a right upper lung tumor, discovered due to chest pain, confirmed by biopsy as squamous cell carcinoma (T4N3M0). (A) CT Coronal plane image showing right upper lung tumor with atelectasis of the right upper lobe, hilar, and mediastinal lymph node metastasis (*). (B) A right bronchial artery angiography indicating blood supply to the right lung tumor, which can be perfused with chemotherapy and embolization (→). (C) CT Coronal plane image after six courses of BACE, demonstrating that the right upper lung tumor and atelectasis have basically disappeared (→).
Another patient with left lung squamous cell carcinoma, experiencing chest tightness and difficulty breathing post-systemic chemotherapy, demonstrated complete consolidation of the left lung on chest plain film, CT cross-section, and CT coronal plane (Figures 3A–C). Two bronchial arteriography images revealed the tumor’s blood supply (Figures 3D, E). After two BACE courses, the left lung was recruited, left hilar tumor necrosis occurred, and a cavity formed (Figures 3F–H).
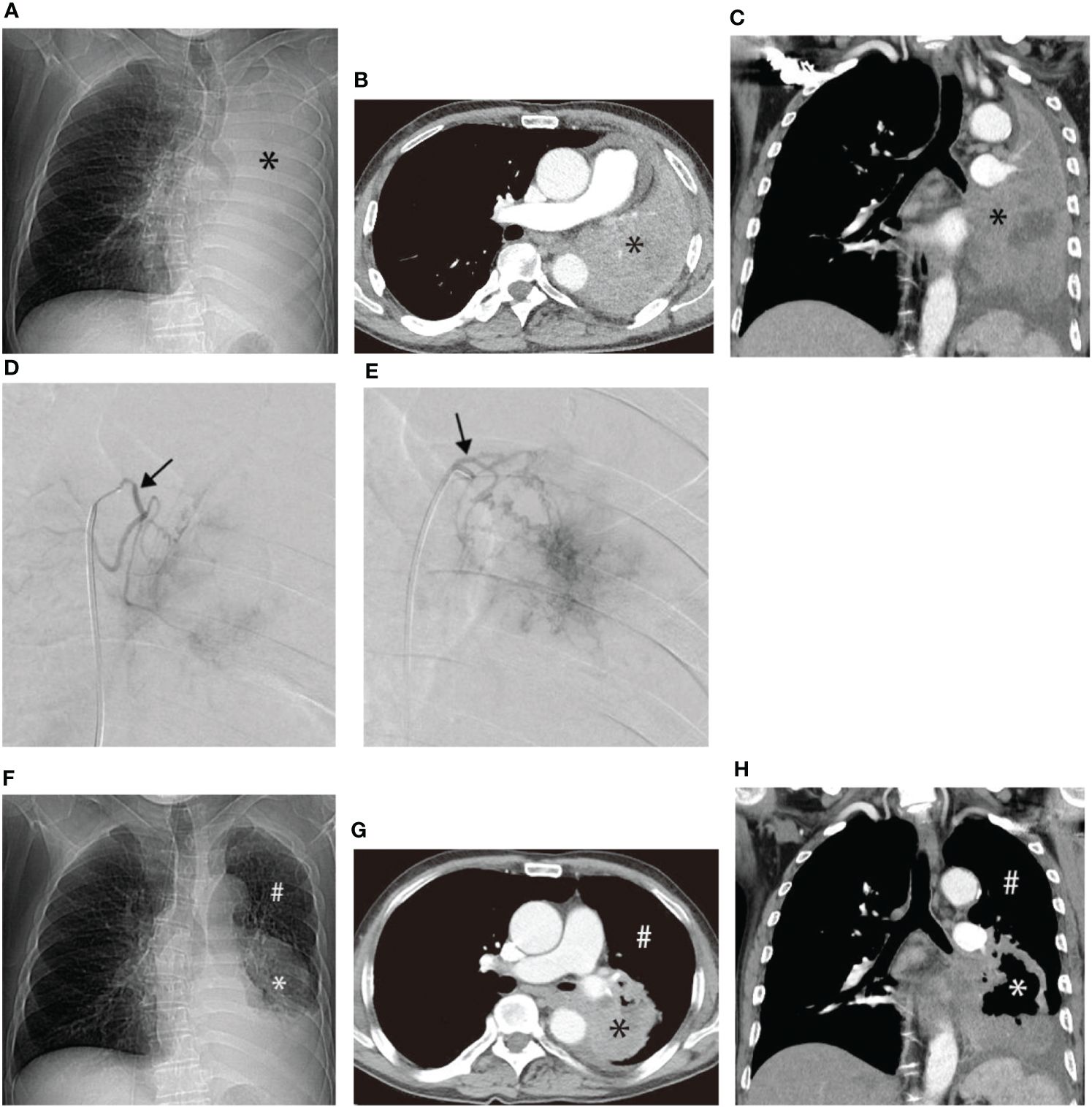
Figure 3 Male, 62 years old, experiencing progression of left lung squamous cell carcinoma after systemic chemotherapy, resulting in chest tightness and difficulty breathing after activity. (A–C) display chest plain film, CT cross-section, and CT Coronal plane, respectively, showing complete consolidation of the left lung (*). (D, E) illustrate the blood supply to the tumor through two bronchial arteriography images, respectively (→). (F–H) represent chest plain film, CT cross-section, and CT Coronal plane after two BACE courses, respectively, showing left lung recruitment (#), left hilar tumor necrosis, and cavity formation (*).
Similar improvements were observed in two other patients before and after treatment, as illustrated in Figures 4, 5. Figure 4 showcases an 84-year-old man with left lung squamous cell carcinoma who developed cough, phlegm, and chest tightness after chemotherapy. Figures 4A, B depicts left upper lung consolidation with minor effusion, while 4C reveals left bronchial artery angiography supplying the tumor. In Figures 4D, E, the left upper lung dilated with a small residual lesion, and effusion disappeared. Figure 5 displays a 58-year-old man with left lung squamous cell carcinoma experiencing atelectasis and breathing difficulty after radiotherapy, chemotherapy, and the placement of a left main bronchial stent. Figures 5A–C shows complete consolidation of the left lung and left main bronchial stent. Figures 5D, E reveals the tumor’s blood supply through two bronchial arteries. Figures 5F–H shows a partially dilated left lung and reduced dyspnea.
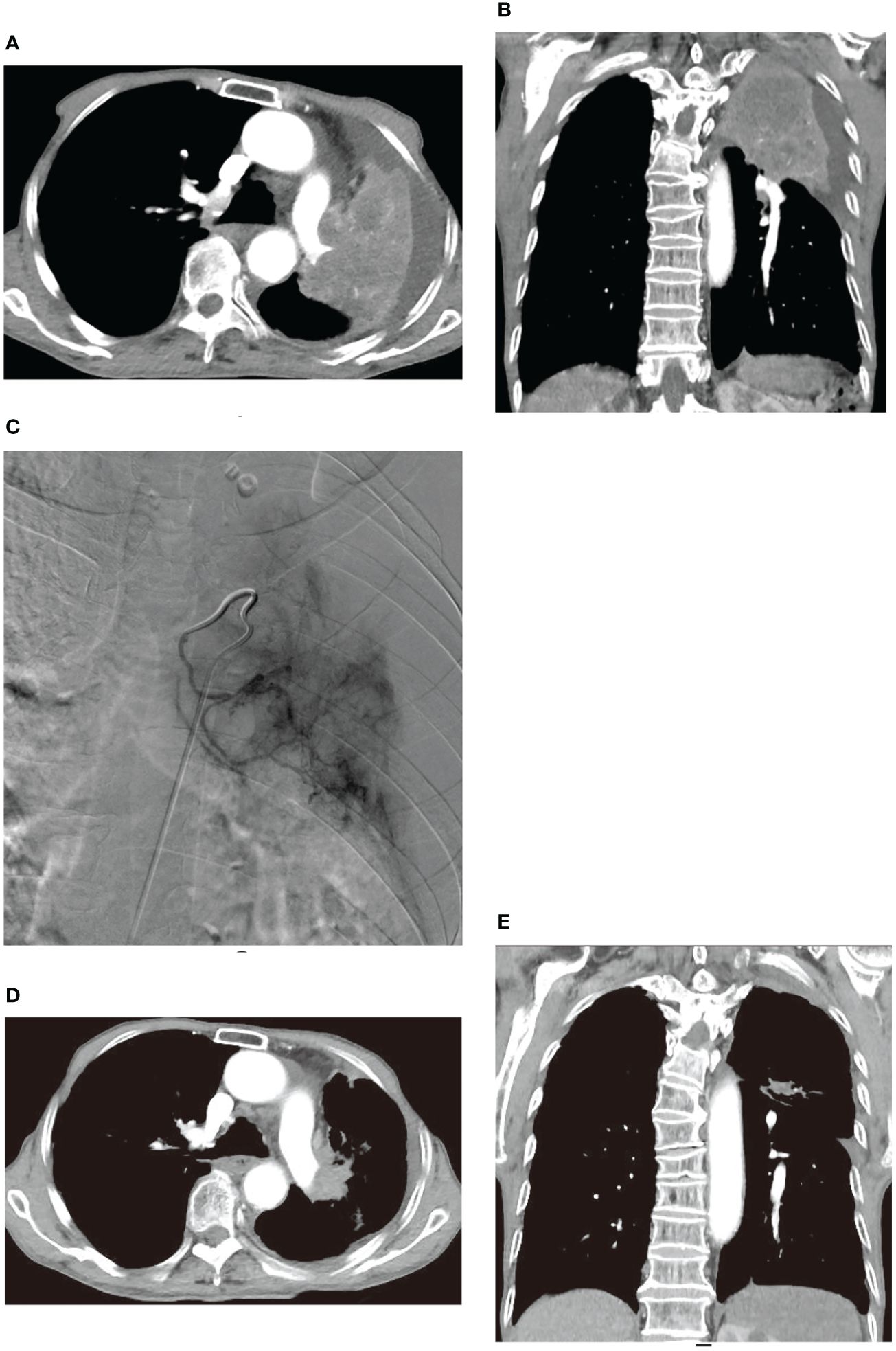
Figure 4 An 84-year-old male developed cough, bloody sputum, and chest tightness after chemotherapy for left lung squamous cell carcinoma. (A, B) are CT transverse and Coronal plane images, respectively, showing left upper lung consolidation with a small amount of effusion. (C) displays a left bronchial artery angiography, revealing a tumor supplying the left lung with abundant blood supply to the cancer. (D, E) are CT transverse and Coronal plane images, respectively, illustrating that the left upper lung is dilated, with a small amount of residual lesions and effusion disappeared.
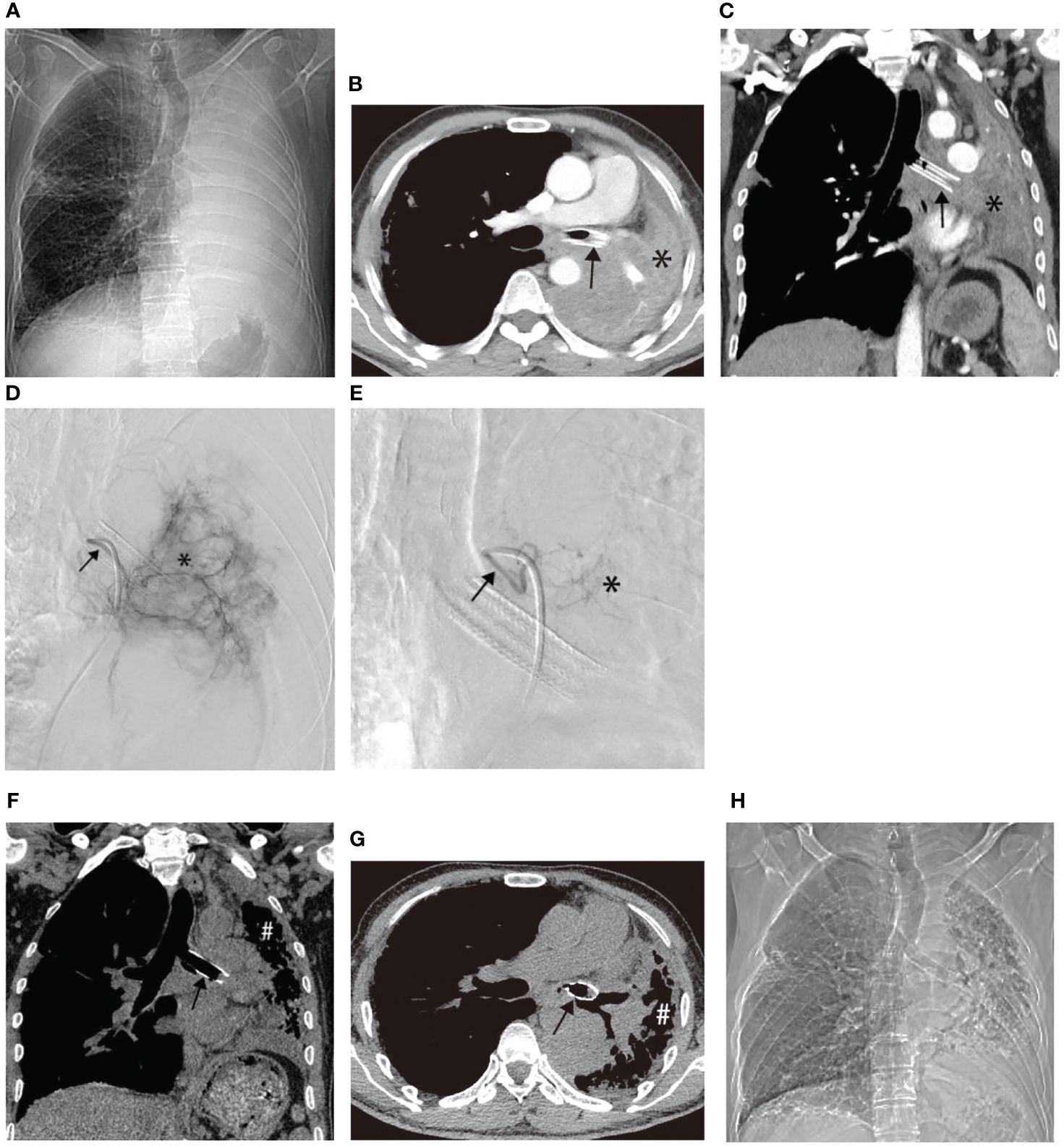
Figure 5 A 58-year-old male with left lung squamous cell carcinoma experienced atelectasis and difficulty breathing after radiotherapy, chemotherapy, and placement of a left main bronchial stent. (A–C) show chest plain film, CT cross-section, and CT coronal plane, respectively, displaying complete consolidation of the left lung (*) and the left main bronchial stent (→). (D, E) illustrate the blood supply to the tumor through two bronchial arteries, respectively (→). (F–H) exhibit chest plain films, CT cross-section images, and CT coronal images after one BACE, respectively, revealing partial dilation of the left lung (#) and relief of dyspnea in the patient.
There were some complications related to BACE treatment (Table 2). Most of them were grade 1–2. Grade 1–2 cough and chest pain after BACE usually resolved within a few hours. The hematopoietic system was impaired in some patients. Fatigue, hair loss, and constipation were common, but mostly 1–2 grades. Occasionally, there was subcutaneous ecchymosis at the femoral artery puncture point, which was probably a failure of hemostasis due to pressure.
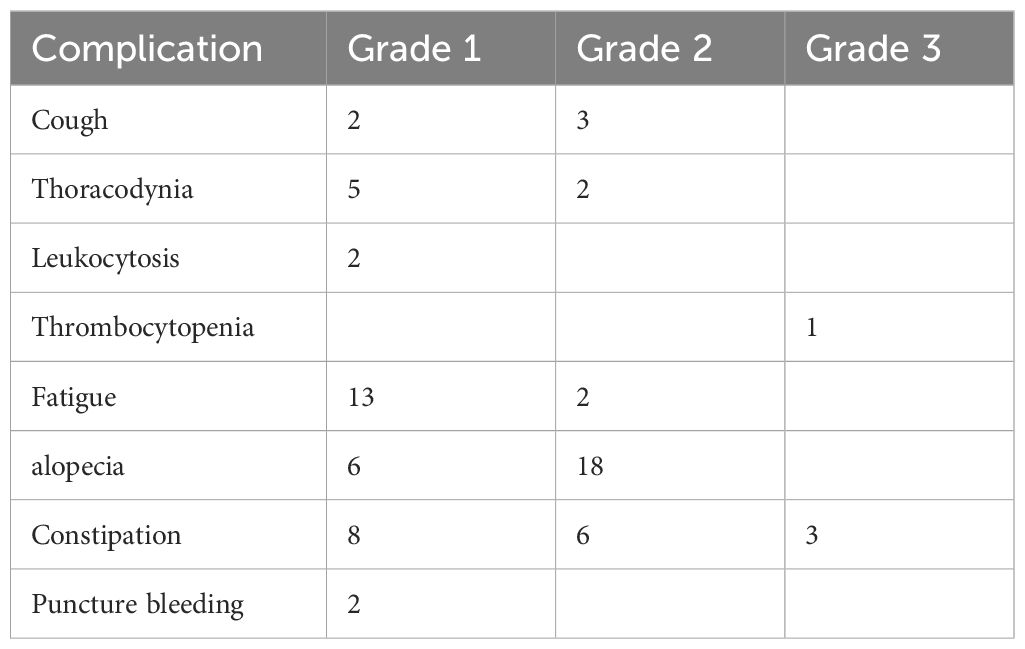
Table 2 Complications of BACE, according to the WHO standards (25).
Central lung cancers are often ineligible for surgery, and the treatment options are limited to radiotherapy and systemic therapies (chemotherapy, targeted therapy, and immunotherapy) (2–6). Still, central lung cancer carries a poor prognosis (26), and exploring novel approaches to improve prognosis is important. In this study, we conducted a retrospective analysis of 30 cases of refractory central lung cancer complicated with atelectasis treated by BACE at our center over the past decade. The results demonstrated that the short-term curative effect of BACE significantly alleviated symptoms such as cough, sputum, hemoptysis, dyspnea, and hypoxia, improved the KPS score, and showed no serious adverse events. BACE-related toxic side effects were mild, and the patients easily accepted BACE, especially those with poor physical status. These findings offer valuable insights into diagnosing and treating central lung cancer.
As acknowledged, angiogenesis plays a crucial role in tumor growth and serves as the anatomical foundation for regional therapy via blood vessels (27, 28). The primary blood supply arteries for lung cancer involve systemic circulation arteries, including the bronchial artery, intercostal artery, and phrenic artery (29, 30). BACE infusion chemotherapy through the bronchial artery maintains a high local drug concentration in tumor tissue, enhancing the effective eradication of tumor cells. This localized approach minimizes drug concentration in vital organs, consequently reducing systemic adverse reactions (31, 32), aligning with our study results. Following BACE treatment in our study, there was a significant short-term efficacy, improvement in KPS score, and no serious adverse events. Furthermore, bronchial recanalization and atelectasis were relieved in 8 patients, with enhanced lung function observed post-treatment. This improvement could be attributed to the gradual shrinkage or necrosis of the tumor after interventional treatment, alleviating dyspnea and venous return obstruction caused by tumor invasion and bronchial compression, ultimately enhancing the lung function of patients (33).
Additionally, bronchial artery embolization, by blocking local blood flow, prolongs the residence time of chemotherapy drugs in tumor tissue, thereby exerting a more potent anti-tumor effect (34). Wang et al. observed that BACE for lung cancer exhibited fewer adverse reactions, a shorter treatment course, and superior curative effects compared to systemic intravenous chemotherapy. For patients ineligible or unwilling to undergo surgery, it effectively improves the quality of life, extends survival periods, and, in some cases, offers the opportunity for secondary surgical eradication (35), representing a clinically valuable and comprehensive therapy. Moreover, Shang et al. reported an objective response rate of 69.45% and 58.33% at three and six months, respectively, after DEB-BACE in advanced non-small cell lung cancer, with disease control rates of 88.89% and 83.33% (36). While these studies confirm the value of BACE in lung cancer, our study is the first to explore the efficacy of BACE in central lung cancer with atelectasis.
Self-expandable metallic stents can be used for malignant obstructive atelectasis (37), but their use is relatively recent, and they have only recently been approved for that use in China. Some patients received stents in the present study but no self-expandable metallic stents. In addition, embolization microspheres are available, but the authors used gelatine sponge granule embolization for BACE mainly for patient safety. Recently, the use of drug-eluting beads (DEBs) has been reported. Shang et al. (36) reported an ORR of 69.45% and 58.33% at 3 and 6 months, respectively, after DEB-BACE in patients with advanced non-small cell lung cancer, with DCRs of 88.89% and 83.33%. On the other hand, serious side effects were also reported, including massive hemoptysis and death by asphyxia due to hemoptysis (38). Therefore, it is the authors’ opinion that such materials should be used with caution and only in selected patients.
The study has several limitations that need to be acknowledged. Firstly, due to its retrospective and observational nature, the study is inherently susceptible to retrospective bias and cannot establish causation (39). Secondly, the study’s small sample size and the fact that all participants came from a single center could introduce bias into the outcomes. Therefore, it is important to approach the findings of this study with caution. Thirdly, two patients with small cell lung cancer were included; this study did not focus on any specific type of lung cancer, only on refractory central lung cancer complicated with atelectasis and treated using BACE, and BACE was effective in these two cases. Although no conclusion can be drawn about the type of lung cancer, those two cases were kept. Fourthly, many patients were initially diagnosed and treated at other hospitals and referred only for BACE. Some data were missing in such cases and could not be analyzed in the present study. Consequently, validating these results requires a large-scale, randomized study conducted across multiple centers.
In conclusion, it is anticipated that the role of BACE in lung cancer treatment will gain increasing recognition and refinement. However, the value of BACE in treating central lung cancer with atelectasis requires further substantiation.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The study received approval from the ethics committee of Yueyang Integrated Traditional Chinese and Western Medicine Hospital affiliated with Shanghai University of Traditional Chinese Medicine. The approval number is 2021-008. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study is retrospective and does not require informed consent.
YL: Conceptualization, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. XZ: Conceptualization, Formal analysis, Methodology, Resources, Writing – original draft, Writing – review & editing. FZ: Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. WS: Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Shanghai Municipal Science and Technology Commission’s “Science and Technology Innovation Action Plan” Medical Innovation Research Special Project (20Y11913900).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yao K, Qiu X, Hu H, Han Y, Zhang W, Xia R, et al. Pulmonary cryptococcosis coexisting with central type lung cancer in an immuocompetent patient: a case report and literature review. BMC Pulm Med. (2020) 20:161. doi: 10.1186/s12890-020-01200-z
2. NCCN Clinical Practice Gyidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. Version 1.2024. Fort Washington: National Comprehensive Cancer Network (2023).
3. NCCN Clinical Practice Gyidelines in Oncology (NCCN Guidelines). Small Cell Lung Cancer. Version 2.2024. Fort Washington: National Comprehensive Cancer Network (2023).
4. Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143:e314S–40S. doi: 10.1378/chest.12–2360
5. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv1–iv21. doi: 10.1093/annonc/mdx222
6. Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. (2019) 30:171–210. doi: 10.1093/annonc/mdy554
7. Tennyson N, Weiss E, Sleeman W, Rosu M, Jan N, Hugo GD. Effect of variations in atelectasis on tumor displacement during radiation therapy for locally advanced lung cancer. Adv Radiat Oncol. (2017) 2:19–26. doi: 10.1016/j.adro.2016.12.001
8. Mudambi L, Miller R, Eapen GA. Malignant central airway obstruction. J Thorac Dis. (2017) 9:S1087–s1110. doi: 10.21037/jtd.2017.07.27
9. Shaller BD, Filsoof D, Pineda JM, Gildea TR. Malignant central airway obstruction: what’s new? Semin Respir Crit Care Med. (2022) 43:512–29. doi: 10.1055/s-0042–1748187
10. Serino M, Freitas C, Saleiro S, Cabrita B, Conde M, Fernandes MGO, et al. Airway stents in Malignant central airway obstruction. Pulmonology. (2021) 27:466–9. doi: 10.1016/j.pulmoe.2021.02.003
11. Yoon SM, Suh R, Abtin F, MoGhanaki D, Genshaft S, Kamrava M, et al. Outcomes with multi-disciplinary management of central lung tumors with CT-guided percutaneous high dose rate brachyablation. Radiat Oncol. (2021) 16:99. doi: 10.1186/s13014–021-01826–1
12. Shrestha P, Madan K, Hadda V, Upadhyay A, Mittal S, Tiwari P, et al. Therapeutic bronchoscopic interventions for nonmalignant central airway obstruction provide rapid and sustained improvement in symptoms and functional status. Lung India. (2020) 37:295–9. doi: 10.4103/lungIndia.lungIndia_476_19
13. Verma A, Phua CK, Wu QM, Sim WY, Rui AW, Goh SK, et al. Our clinical experience of self-expanding metal stent for Malignant central airway obstruction. J Clin Med Res. (2017) 9:58–63. doi: 10.14740/jocmr2811w
14. Pritchett MA, Lau K, Skibo S, Phillips KA, Bhadra K. Anesthesia considerations to reduce motion and atelectasis during advanced guided bronchoscopy. BMC Pulm Med. (2021) 21:240. doi: 10.1186/s12890–021-01584–6
15. Li X, Yin M, Xie P, Liu Y, Li X, Qi Y, et al. Self-expandable metallic stent implantation combined with bronchial artery infusion chemoembolization in the treatment of lung cancer with complete atelectasis. Front Oncol. (2021) 11:733510. doi: 10.3389/fonc.2021.733510
16. Fu Z, Wang C, Wei W, Xiang G, Guan L, Zhan M, et al. Efficacy and safety of drug-eluting beads bronchial arterial chemoembolization versus conventional bronchial arterial chemoembolization in lung cancer patients with hemoptysis. Future Oncol. (2022) 18:2805–15. doi: 10.2217/fon-2021–1515
17. Yamamoto S, Kamei S, Kondo Y, Hiraiwa S, Hasebe T, Sakamaki F. Bronchial artery embolization for haemothorax and haemoptysis caused by primary lung cancer. Respirol Case Rep. (2020) 8:e00529. doi: 10.1002/rcr2.529
18. Bi Y, Li F, Ren J, Han X. The safety and efficacy of oxaliplatin-loaded drug-eluting beads transarterial chemoembolization for the treatment of unresectable or advanced lung cancer. Front Pharmacol. (2022) 13:1079707. doi: 10.3389/fphar.2022.1079707
19. Giuliani M, Mathew AS, Bahig H, Bratman SV, Filion E, Glick D, et al. SUNSET: stereotactic radiation for ultracentral non-small-cell lung cancer-A safety and efficacy trial. Clin Lung Cancer. (2018) 19:e529–32. doi: 10.1016/j.cllc.2018.04.001
20. Chan MV, Huo YR, Cao C, Ridley L. Survival outcomes for surgical resection versus CT-guided percutaneous ablation for stage I non-small cell lung cancer (NSCLC): a systematic review and meta-analysis. Eur Radiol. (2021) 31:5421–33. doi: 10.1007/s00330–020-07634–7
21. Men T, Cui Q, Liu Y, Zhang S. Efficacy and safety of super-selective bronchial arterial infusion chemotherapy in the treatment of advanced non-small cell lung cancer. Chemotherapy. (2022) 67:123–31. doi: 10.1159/000522456
22. Yu G, Shen Y, Chen L, Xu X, Yang J. Drug-eluting beads bronchial arterial chemoembolization vs. conventional bronchial arterial chemoembolization in the treatment of advanced non-small cell lung cancer. Front Med (Lausanne). (2023) 10:1201468. doi: 10.3389/fmed.2023.1201468
23. Liu J, Zhang W, Ren J, Li Z, Lu H, Sun Z, et al. Efficacy and safety of drug-eluting bead bronchial arterial chemoembolization plus anlotinib in patients with advanced non-small-cell lung cancer. Front Cell Dev Biol. (2021) 9:768943. doi: 10.3389/fcell.2021.768943
24. Lalchandani UR, Sahai V, Hersberger K, Francis IR, Wasnik AP. A radiologist’s guide to response evaluation criteria in solid tumors. Curr Probl Diagn Radiol. (2019) 48:576–85. doi: 10.1067/j.cpradiol.2018.07.016
25. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. (1981) 47:207–14. doi: 10.1002/(ISSN)1097-0142
26. Xie X, Li X, Tang W, Xie P, Tan X. Primary tumor location in lung cancer: the evaluation and administration. Chin Med J (Engl). (2021) 135:127–36. doi: 10.1097/CM9.0000000000001802
27. Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. (2018) 21:425–532. doi: 10.1007/s10456–018-9613-x
28. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. (2017) 20:409–26. doi: 10.1007/s10456–017-9562–9
29. Deng L, Tang H, Qiang J, Wang J, Xiao S. Blood supply of early lung adenocarcinomas in mice and the tumor-supplying vessel relationship: A micro-CT angiography study. Cancer Prev Res (Phila). (2020) 13:989–96. doi: 10.1158/1940–6207.Capr-20–0036
30. Jiang Z, Chen J. [Anti-angiogenesis in lung cancer: current situation, progress and confusion]. Zhongguo Fei Ai Za Zhi. (2022) 25:278–86. doi: 10.3779/j.issn.1009–3419.2022.101.16
31. Zeng Y, Yin M, Zhao Y, Liu Y, Li X, Qi Y, et al. Combination of bronchial arterial infusion chemotherapy plus drug-eluting embolic transarterial chemoembolization for treatment of advanced lung cancer-A retrospective analysis of 23 patients. J Vasc Interv Radiol. (2020) 31:1645–53. doi: 10.1016/j.jvir.2020.06.007
32. Bi Y, Shi X, Ren J, Yi M, Han X, Song M. Clinical outcomes of doxorubicin-eluting CalliSpheres® beads-transarterial chemoembolization for unresectable or recurrent esophageal carcinoma. BMC Gastroenterol. (2021) 21:231. doi: 10.1186/s12876–021-01816–3
33. Jin SQ, Zhao HY, Bai B, Ma CH, Cao HL. Transcatheter arterial chemoembolization improves clinical efficacy and life quality of patients with lung cancer and reduces adverse reactions. Am J Transl Res. (2021) 13:10396–403.
34. Gutwillig A, Santana-Magal N, Farhat-Younis L, Rasoulouniriana D, Madi A, Luxenburg C, et al. Transient cell-in-cell formation underlies tumor relapse and resistance to immunotherapy. Elife. (2022) 11. doi: 10.7554/eLife.80315
35. Wang K, Liu X, Lian XL, Bao X, Li K. Editorial: Bioengineered gene and cell therapy for treating cardiovascular diseases. Front Bioeng Biotechnol. (2023) 11:1250175. doi: 10.3389/fbioe.2023.1250175
36. Shang B, Li J, Wang X, Li D, Liang B, Wang Y, et al. Clinical effect of bronchial arterial infusion chemotherapy and CalliSpheres drug-eluting beads in patients with stage II-IV lung cancer: A prospective cohort study. Thorac Cancer. (2020) 11:2155–62. doi: 10.1111/1759–7714.13522
37. Bi Y, Zhu X, Yu Z, Yi M, Han X, Ren J. Clinical outcomes of self-expandable metallic stents for Malignant obstructive atelectasis. Sci Rep. (2020) 10:3600. doi: 10.1038/s41598–020-60566–6
38. Ma X, Zheng D, Zhang J, Dong Y, Li L, Jie B, et al. Clinical outcomes of vinorelbine loading CalliSpheres beads in the treatment of previously treated advanced lung cancer with progressive refractory obstructive atelectasis. Front Bioeng Biotechnol. (2022) 10:1088274. doi: 10.3389/fbioe.2022.1088274
Keywords: bronchial artery chemoembolization, lung cancer, atelectasis, treatment effects, case series
Citation: Liu Y, Zhang X, Zhang F and Song W (2024) Bronchial artery chemoembolization in the treatment of refractory central lung cancer with atelectasis. Front. Oncol. 14:1343324. doi: 10.3389/fonc.2024.1343324
Received: 23 November 2023; Accepted: 31 May 2024;
Published: 12 June 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Yonghua Bi, First Affiliated Hospital of Zhengzhou University, ChinaCopyright © 2024 Liu, Zhang, Zhang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujin Liu, eWpsaXVAYmptdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.