
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 30 April 2024
Sec. Cancer Epidemiology and Prevention
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1338325
This article is part of the Research TopicExtending Precision Medicine to Cancer SurvivorshipView all 6 articles
Objective: Cancer seriously endangers human health and represents a global public health issue. Cancer-related fatigue (CRF) is a distressing and persistent sense of exhaustion caused by cancer or cancer treatment, widely prevalent among cancer patients. This study aims to summarize emerging trends and provide directions for future research of CRF through bibliometric and visualization analyses.
Methods: A systematic search in the Web of Science Core Collection database from 2001-01-01 to 2023-05-18 were conducted. Only reviews and articles written in English were considered. CiteSpace and the R were used for bibliometric and visualization analyses.
Results: The analysis revealed that 2,566 studies on CRF have been published by 1,041 institutions in 70 countries so far. The number of articles published and cited annually have been steadily increasing. Eduardo Bruera published the most articles, and Julienne E Bower is the most co-cited author. The University of Texas System is the leading institution in cancer-related fatigue research. The United States and China have the largest number of publications. Supportive Care in Cancer published the most articles, and Journal of Clinical Oncology is the most co-cited journal. “Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis”, authored by Mustian KM et al. and published in JAMA Oncology was the most co-cited document. Keyword analysis indicated that research focus had shifted from “epoetin alpha” and “anemia” to “risk factors”, “systematic review”, “acupuncture”, “anxiety”, “traditional Chinese medicine” and “guidelines”.
Conclusion: In conclusion, this analysis provides comprehensive research trends and knowledge network maps of CRF. Clinical physicians should concurrently focus on the anemia, insomnia, anxiety, and depression status of patients when assessing or managing CRF. Improvements in related risk factors also contribute to alleviating fatigue. Furthermore, it is essential to pay attention to authoritative CRF guidelines. Acupuncture and traditional Chinese medicine also have therapeutic potential, which merits further investigation. Researchers should draw attention to the crucial roles of inflammation, hypoxia, and mitochondrial dysfunction, which could be the frontiers.
Cancer-related fatigue (CRF) is a subjective feeling of tiredness or exhaustion associated with cancer or cancer treatment, encompassing physical, emotional, and/or cognitive aspects (1). In a meta-analysis of 144,813 participants conducted in 2020, the estimated diagnostic yield for CRF was 52% (2). Up to 80% of patients treated with chemotherapy and/or radiotherapy experience CRF (3). Chemotherapy and radiotherapy are the main factors that contribute to CRF. Other therapeutic modalities, such as targeted therapy, immunotherapy, and surgical treatments, o cancer itself, are also associated with CRF.
Cancer survivors may experience fatigue for months, years, or even up to a decade (4). CRF is more distressing and long-lasting than general fatigue and cannot be alleviated by resting. It often causes patients with cancer unbearable suffering, severely affects quality of life, disrupts daily functioning, interferes with subsequent therapies, and influences prognosis. Therefore, alleviating CRF is crucial for improving the overall quality of life of patients with cancer.
Since the 20th century, many studies have revealed the occurrence of fatigue symptom in cancer patients. Consequently, studies have been conducted to elucidate the assessment, treatment, and possible mechanisms of CRF, providing new perspectives and hotspots in the field of cancer research. Nevertheless, effective measures for reducing CRF are still limited, which are primarily non-pharmacological treatments, such as exercise and yoga. This is partly due to the obscure mechanisms underlying the occurrence of CRF. Currently, the proposed possible hypotheses for CRF include the dysregulation of inflammatory cytokines and dysfunction of skeletal muscles and mitochondria. It is important for physicians and researchers to understand the progression and characteristics of research related to CRF, which can facilitate the identification of future research directions and more efficient approaches to treating CRF.
Bibliometric analysis is an emerging analytical technique that is used to summarize advances in a certain field, reveal research hotspots, and quantitatively outline the contributions of authors, journals, institutions, and countries (5). However, no bibliometric or visualization analyses of studies on CRF have been conducted to date. CiteSpace (6) is a bibliometric tool that is commonly used to visualize information extracted from articles. The aim of this study was to conduct bibliometric and visualization analyses of CRF research published in the Web of Science Core Collection (WoSCC) database from 2001 to 2023, to summarize emerging trends and provide directions for future research.
Two investigators (PJL and QW) conducted a systematic search in the WoSCC database, covering the period from January 1st, 2001 to May 18th, 2023. In cases of disagreement, a third reviewer (ZGD) provided the final decision. The search queries used were TS = (cancer-related fatigue), and the publication date range was set from 2001-01-01 to 2023-05-18. The search was limited to the Social Sciences Citation Index (SSCI) and Science Citation Index Expanded (SCI-EXPANDED) editions. Only reviews and articles written in English were considered, while other article types such as conference abstracts and editorials were excluded. This study was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (7) (Supplementary Table 1). The systematic search yielded 3,154 records, and 2,566 studies were included in the final analysis (Figure 1). All relevant publications were downloaded on May 18th, 2023. The results were stored in download_results.txt format, and the content included “full record and cited references.”
All data were imported into CiteSpace 6.2.R3 and duplicate entries were eliminated. The publication year was analyzed using WoSCC. The collaboration among authors, institutions, and countries, as well as the co-citation of references and journals, and the co-occurrence of keywords were visualized. The R package “bibliometrix” (8) was employed to analyze the geographical distribution of countries. The findings were presented in figures and tables using techniques such as time slicing, thresholding, and pruning. We set the time span as 2001.01–2023.12 and the years per slice as 1, and chose the suitable pruning methods (minimum spanning tree, pathfinder, or no) according to the analysis results. The size of nodes in the various network maps was indicative of the frequency of co-occurrence or co-citation, while the color denoted the year. The citation bursts of references and keywords were analyzed to find the emerging themes in the CRF field. In the journal co-citation analysis, we merged the same journals (“cancer” and “cancer-am cancer soc”) to an alias list. The synonyms in keyword analysis were also merged, including “cancer-related fatigue” and “cancer related fatigue,” “cancer survivors” and “survivors,” etc.
2566 articles and review articles pertaining to CRF were published from January 2001 to May 2023. As depicted in Figure 2, there has been a stable growth trend in the number of annual publications and citations since 2001, as heightened interests among scholars in this domain. Notably, the most number of publications reached 271 in 2021 and 2022, and the highest increasing number reached 82 from 2019 to 2020. The citations ranged from 69 in 2002 to a substantial 11,731 in 2022, reflecting increasing attention to the CRF field.
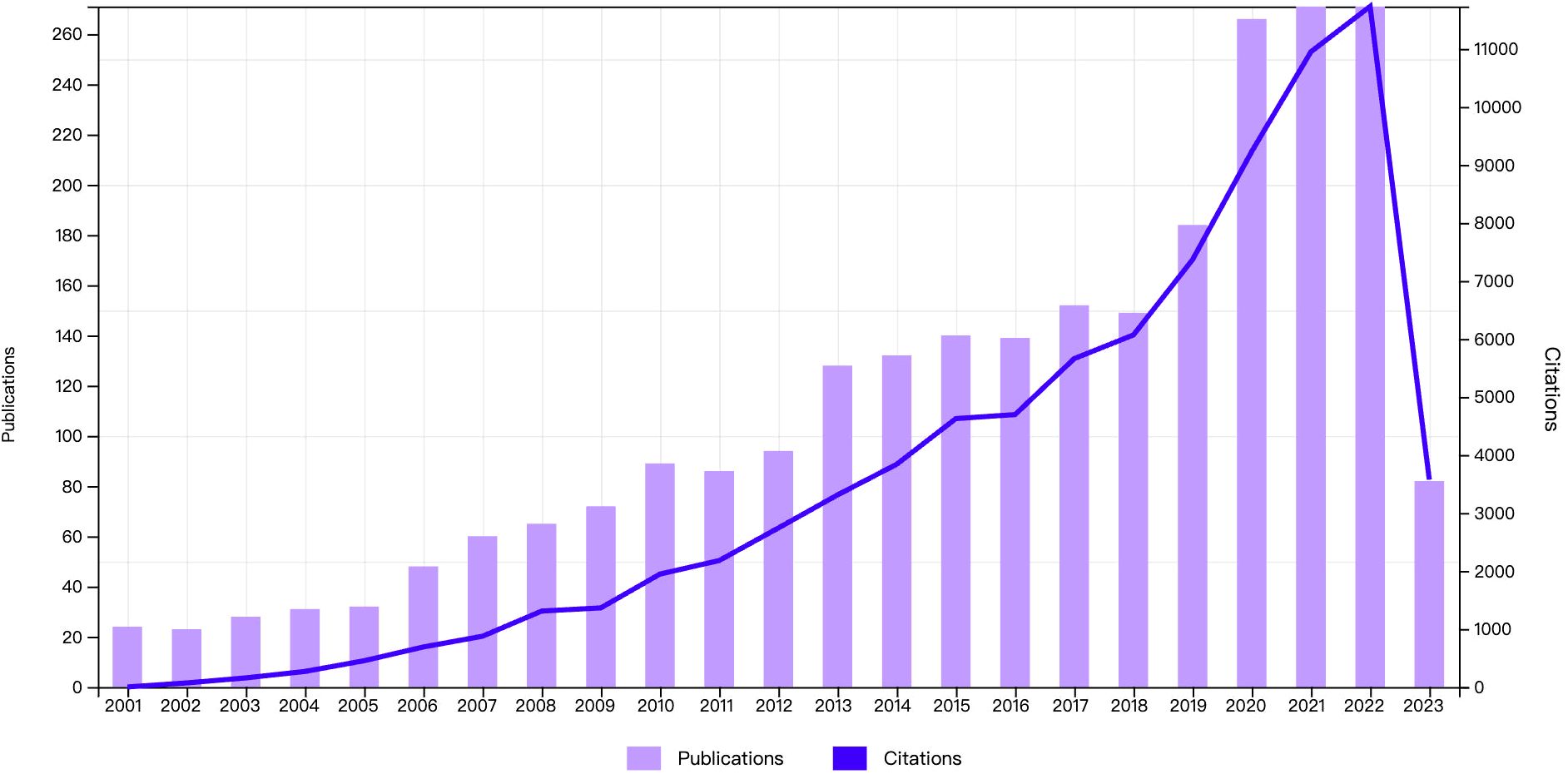
Figure 2 Annual distribution of publications. The bar graph represents the number of publications and the line graph represents the number of citations.
A total of 10,649 authors published studies about CRF according to CiteSpace. Eduardo Bruera published the most articles (n = 35), followed by Christine Miaskowski (n = 30), Leorey N Saligan (n = 29), Sriram Yennurajalingam (n = 25), and Julienne E Bower (n = 23) (Table 1). However, the density of author collaboration was relatively low, reflecting that scholars need to enhance further cooperation in this field. The network map outlines the potential collaboration among authors, including 10,170 connections (Figure 3A). Some authors have exhibited close collaborative relationships, such as Julienne E Bower and Patricia A Ganz. The degree of centrality can help us identify hubs in the map, and Paul B Jacobsen has the highest centrality (= 0.04), showing a lack of close cooperation.
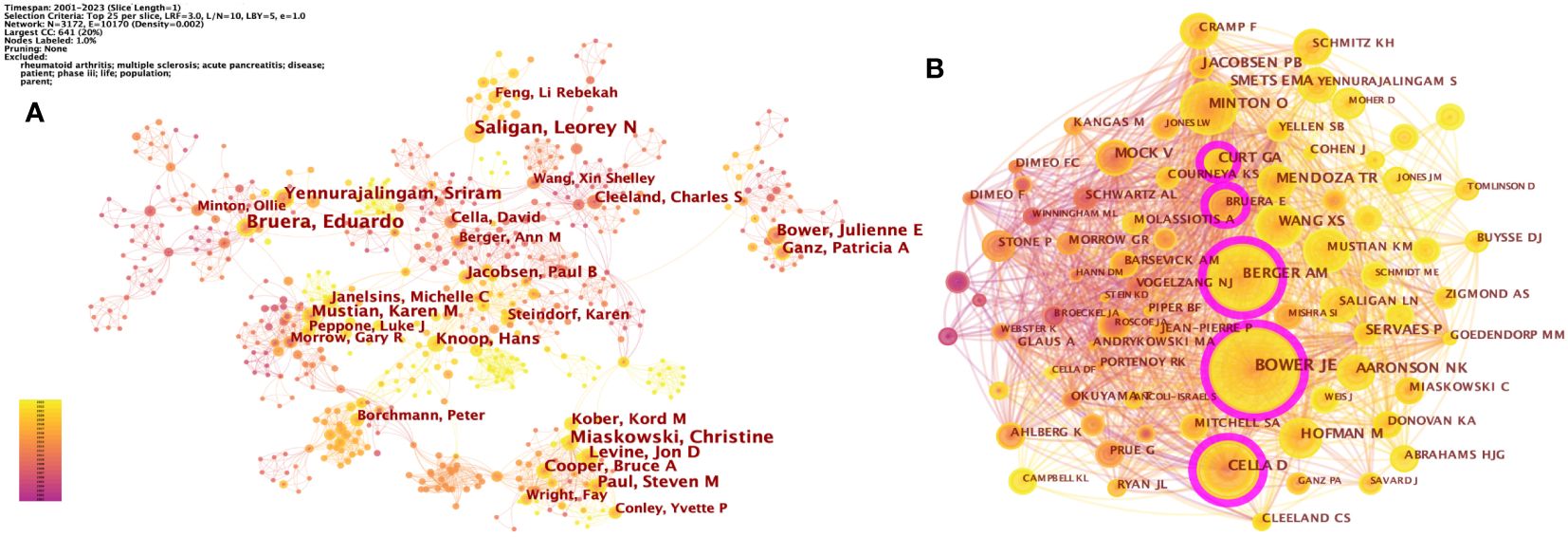
Figure 3 (A) The network of co-authorship. (B) The network of co-cited authors. Each node and the size of it represent an author/co-cited author and his number of studies/co-citations. Each line represents author collaboration. The different colors indicate the different years of publications. The purple nodes represent that the centrality of co-cited authors is > 0.10.
A co-citation relationship was established among the authors when they were cited simultaneously in one or more articles. The core authors can be shown intuitively through the network map (Figure 3B). CiteSpace identified 1,007 co-cited authors, with 25 of them being co-cited over 200 times and 66 being co-cited no less than 100 times. The most co-cited authors were Julienne E Bower (897 co-citations), Ann M Berger (582 co-citations), David Cella (520 co-citations), Ollie Minton (360 co-citations), and G A Curt (355 co-citations) (Table 1).
From 2001 to the present, 1,041 institutions from 70 countries had published studies about CRF. The top 5 productive institutions were the University of Texas System (178, 2.77%), UTMD Anderson Cancer Center (159, 2.48%), University of California System (141, 2.19%), Research Libraries UK (131, 2.04%), and Harvard University (84, 1.31%), four of which were from the USA (Table 2). Research Libraries UK exhibited the highest centrality (= 0.36). As can be seen from the pruning network map of co-institution (Figure 4A), the collaboration among some institutions is relatively close, such as Anderson Cancer Center and University of Texas System, as well as Research Libraries UK and Harvard University.
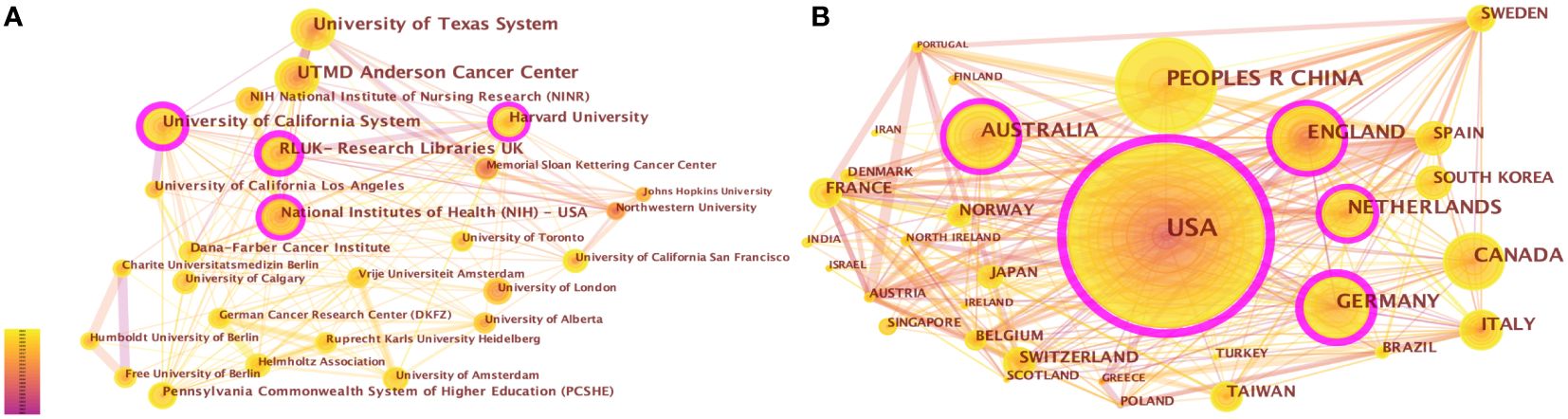
Figure 4 (A) The network of co-institutions. (B) The network of co-countries. Each node and the size of it represent an institution/country and its number of publications. The thickness of each line represents the strength of collaboration. The different colors indicate the different years of publications. The purple nodes represent that the centrality of co-cited authors is > 0.10.
The United States (1065, 31.26%) had the largest number of publications on CRF, followed by China (314, 9.22%), Germany (206, 6.05%), England (193, 5.66%), and Canada (186, 5.46%) (Supplementary Table 2). 70 nodes and 471 connections made up the network map of countries co-occurrence with a density of 0.195 (Figure 4B). The USA serves as a pivotal intermediary in the CRF area, exemplifying substantial contributions to the CRF area and assuming the central roles. Figure 5 visually represents the global distribution of publications, highlighting the cooperation among countries.
650 journals were identified through WoSCC. Supportive Care in Cancer published the most studies (212, 8.26%), followed by Journal of Pain and Symptom Management (116, 4.52%), Cancer (71, 2.77%), Cancer Nursing (62, 2.42%), and Integrative cancer therapies (58, 2.26%) (Table 3). Of the top 10 journals publishing the most articles in the CRF field, Cancer had the highest impact factor (IF) of 6.16, while no journals with an IF > 10 were observed.
We analyzed the co-cited journals with k = 5 from time slicing of 1 year, and 286 co-cited journals were identified. The co-citations of six journals were more than 1,000, and 20 journals were more than 500. The most co-cited journals were the Journal of Clinical Oncology (1900, 4.41%), Cancer (1806, 4.19%), Supportive Care in Cancer (1674, 3.89%), Journal of Pain and Symptom Management (1562, 3.63%), Annals of Oncology (1080, 2.51%), and Oncology Nursing Forum (1025, 2.38%) (Supplementary Table 2). Of these, Annals of Oncology had the highest IF (= 50.45), followed by the Journal of Clinical Oncology (IF = 45.27). 286 nodes and 2,324 connections made up the network map of countries co-occurrence with a density of 0.057 (Figure 6). Supportive Care in Cancer had the highest centrality (= 0.11), followed by PLOS ONE (= 0.09), Psycho-oncology (= 0.07), Medicine & Science in Sports & Exercise (= 0.07), and Integrative cancer therapies (= 0.07).
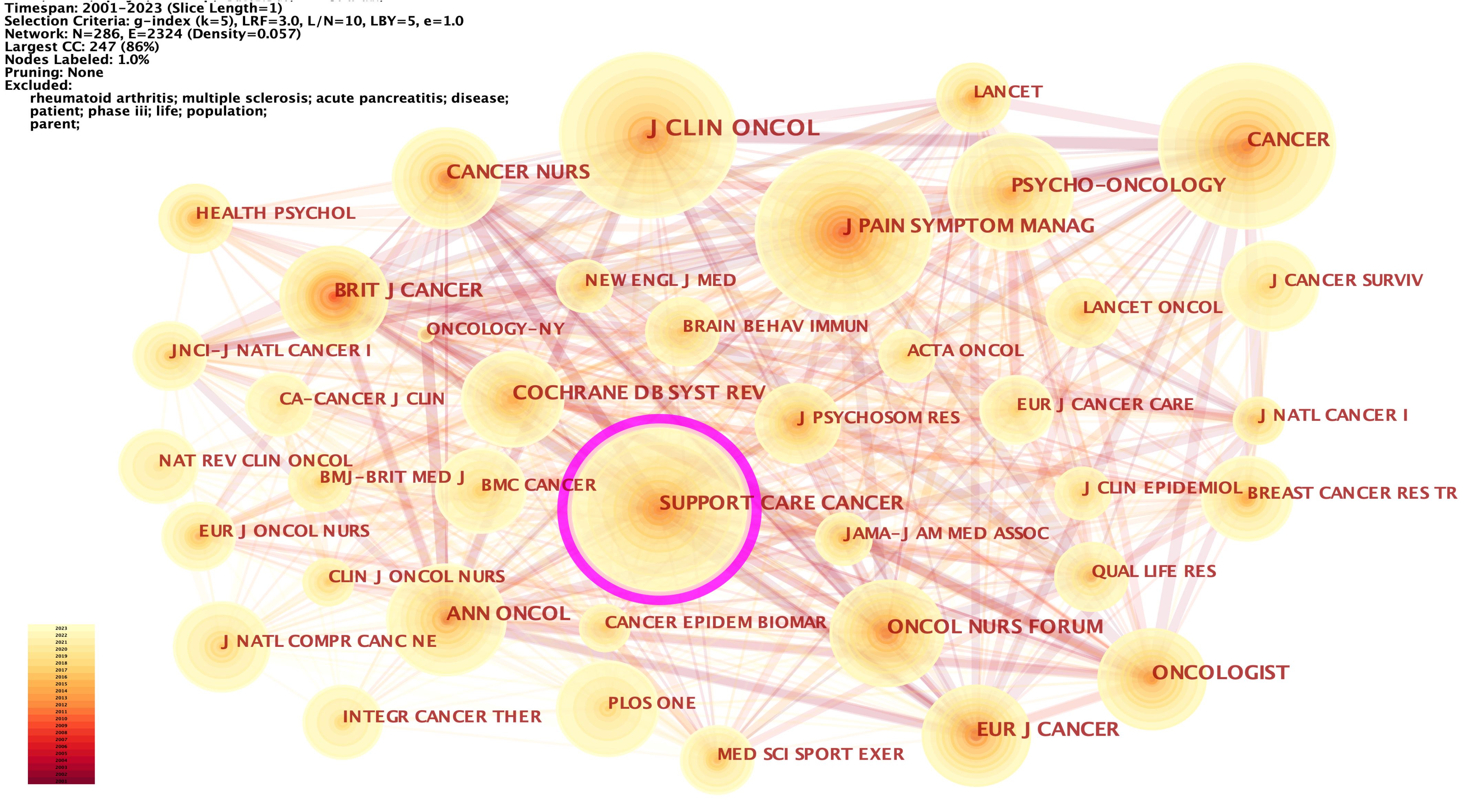
Figure 6 The network of co-cited journals. Each node and the size of it represent a co-cited journal and its number of co-citations. The thickness of each line represents the strength of collaboration. The different colors indicate the different years of publications.
The dual-map overlay of the journals revealed the relationship distribution of subject categories among the journals (Figure 7). Three predominant citation paths colored with green were identified, which means that the studies published in the Medicine/Medical/Clinical journals were mainly cited by those published in the Molecular/Biology/Genetics (z = 4.39, f = 11,470), Health/Nursing/Medicine (z = 7.10, f = 18,100), and Psychology/Education/Social journals (z = 2.43, f = 6,698).
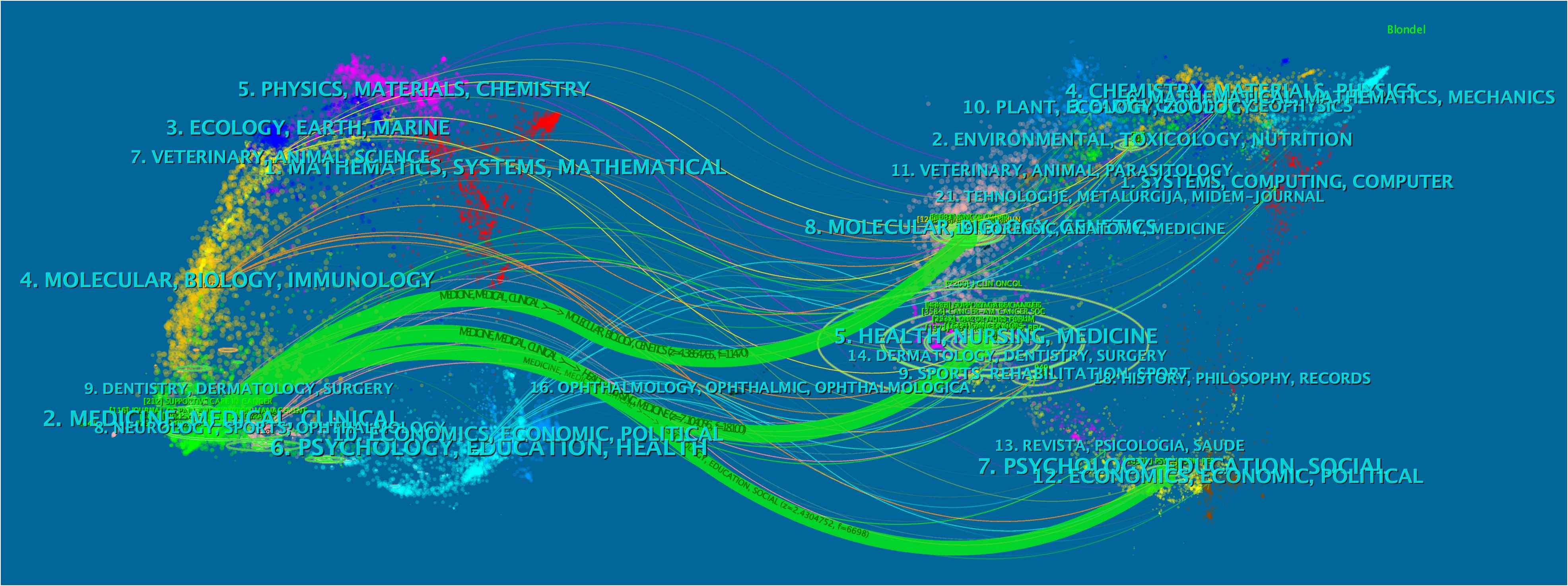
Figure 7 The dual-map overlay of journals. The citing journals were on the left of the map, and the cited ones were on the right. The length of the ellipse represents the number of authors, and the width indicates the count of publications.
When the documents were cited simultaneously, a co-citation relationship was established among them. We analyzed the most co-cited references (k = 25) with a time slicing of 1 year. The core references can be displayed visually through the network map (Figure 8), containing 6,980 links with a density of 0.0076. Of 1,360 co-cited references identified through CiteSpace, 13 were co-cited no less than 50 times, and 117 were co-cited no less than 20 times. Table 4 displays the top 10 co-cited references. “Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis”, authored by Mustian KM et al. and published in JAMA Oncology was the most co-cited document in the CRF area, which indicated that exercise and psychological treatments were more effective than pharmaceutical interventions for CRF through meta-analyses (9). Berger AM et al. provided recommendations for screening and treatments of CRF (10). Bower JE et al. summarized the possible mechanisms, risk factors, and treatments for CRF (4). Campbell KL et al. provided a consensus statement about exercise recommendations for cancer survivors (11). Hilfiker R et al. (12) evaluated the effects of non-pharmaceutical interventions on CRF. Overall, 6 of the top 10 most co-cited documents were reviews, and 4 were guidelines.
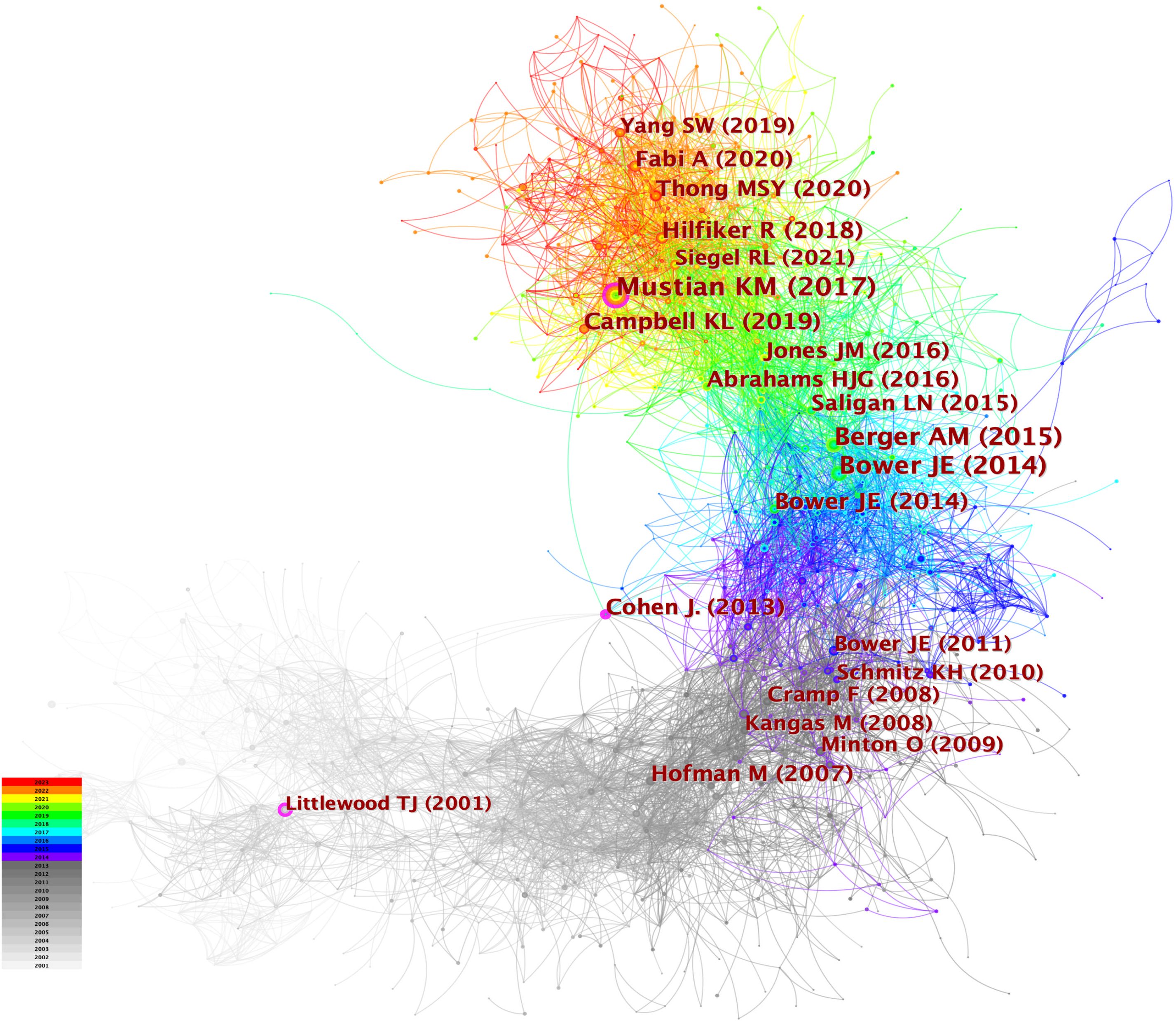
Figure 8 The network of co-cited references. Each node and the size of it represent a co-cited reference and its number of co-citations. The different colors indicate the different years of publications.
Figure 9 displays the top 25 documents with the strongest citation bursts, showing growing interest in the emerging field. The earliest beginning year of citation burst of reference was from 2002 to 2005 (strength = 19.63), entitled “Impact of Cancer-Related Fatigue on the Lives of Patients: New Findings From the Fatigue Coalition” (18) by Curt GA et al. and published in Oncologist. “Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis” (9) published by Mustian KM et al. had the strongest strength of 50.32, which was also the most co-cited reference.
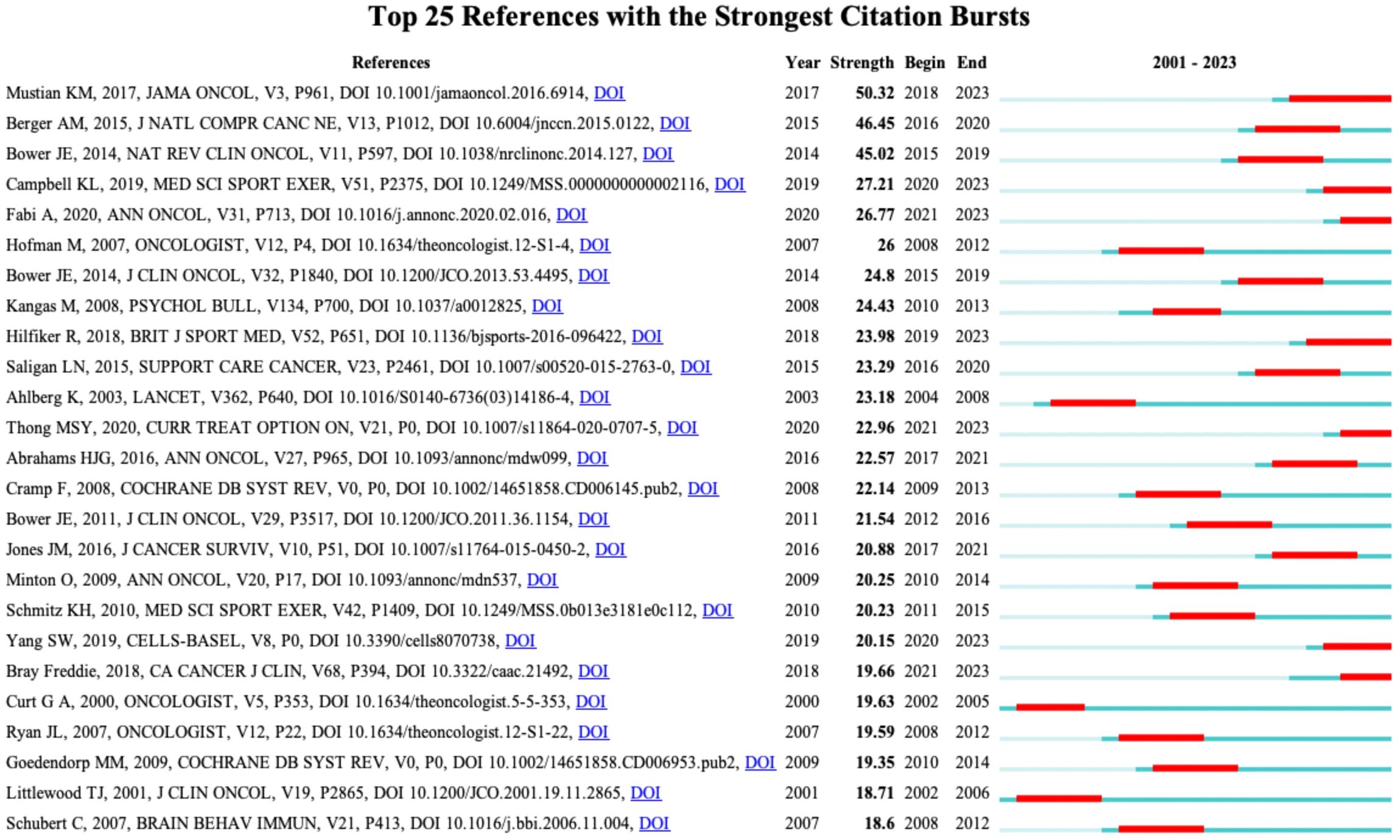
Figure 9 The top 25 references with strongest citation bursts. Red lines represent the duration of citation bursts. The figure was arranged in descending order based on the strength of citation bursts.
Keywords represent a high generalization of the central theme of an article. Analyzing keywords can help to identify the research hot spots in the CRF field over a period of time. We analyzed the top 50 highest-frequency keywords in each time slice. 565 nodes and 4,178 connections made up the network map of keywords co-occurrence with a density of 0.0262 (Figure 10). Of 565 keywords identified through CiteSpace, 31 appeared over 100 times, and 111 appeared no less than 10 times. Supplementary Table 3 presents the top 20 keywords in CRF research. The main contents of the CRF field were identified as “Quality of life” (1315), “breast cancer” (841), “survivors” (614), “management” (564), “chemotherapy” (471), “physical activity” (370), and “depression” (355). 10 clusters were revealed in the cluster analysis of keywords through CiteSpace (Figure 10), including survivorship, assessment, yoga, proinflammatory cytokines, validation, cognitive impairment, inflammation, anemia, exercise, hypoxia, and chemotherapy.
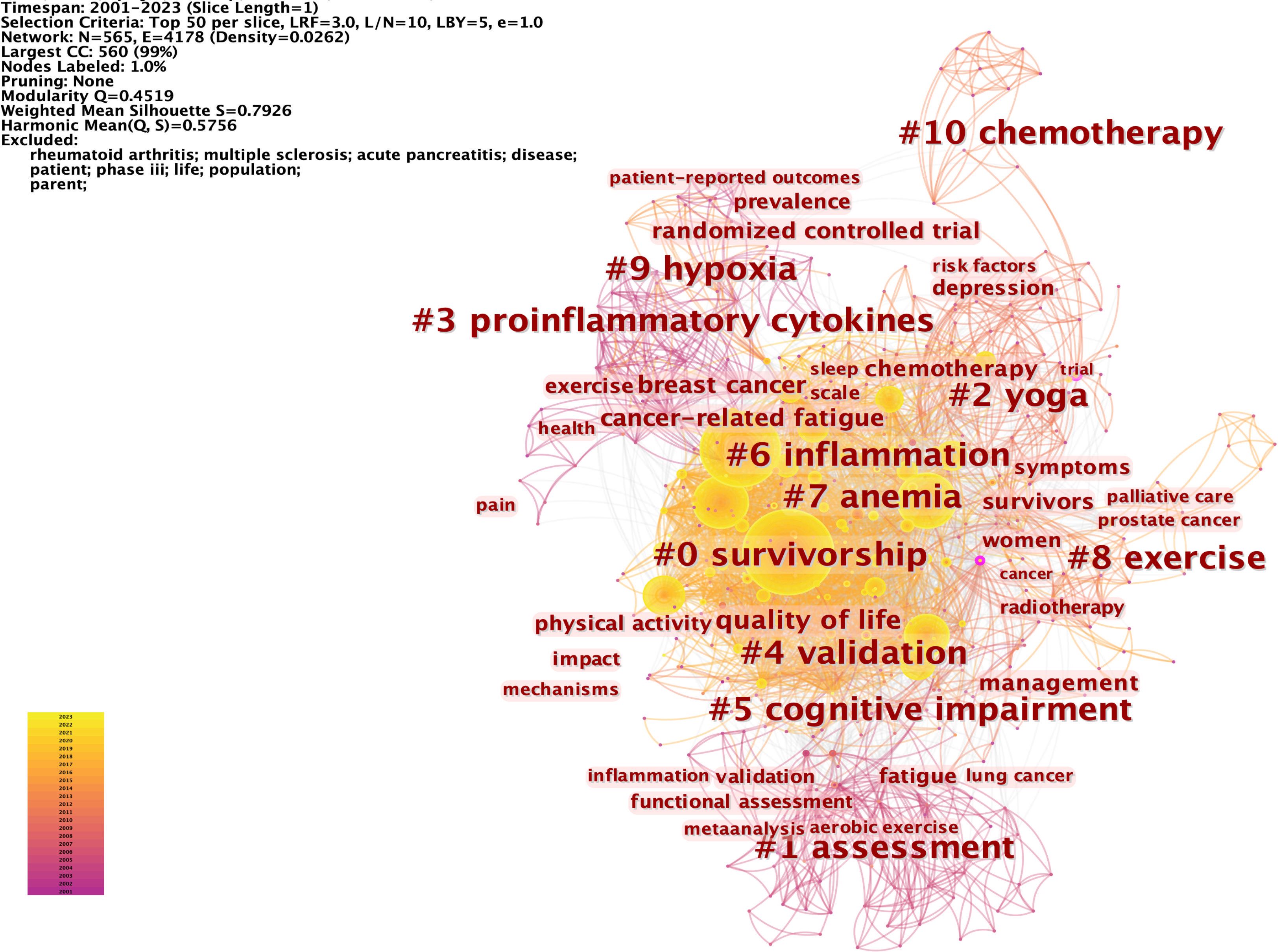
Figure 10 The network of the top 50 keywords co-occurrence and the cluster analysis. Each node and the size of it represent a co-cited reference and its number of co-citations. The different colors indicate the different years of publications. The phrases in # represent the clusters of keywords.
Figure 11 displays the top 50 keywords with the strongest citation bursts, which elucidates the evolving patterns of focal points in the CRF area. Notably, the keyword “epoetin alpha” was the strongest burst (strength = 26.78) and also has the earliest year of citation bursts (2001). The keywords “risk factors,” “systematic review,” “acupuncture,” “anxiety,” and “traditional Chinese medicine” appeared in 2019, while “guidelines” occurred in 2021, which were the most recent citation bursts. Moreover, “insomnia,” “colorectal cancer,” “risk factors,” “systematic review,” and “guidelines” have continued to be of ongoing interest until the year 2023.
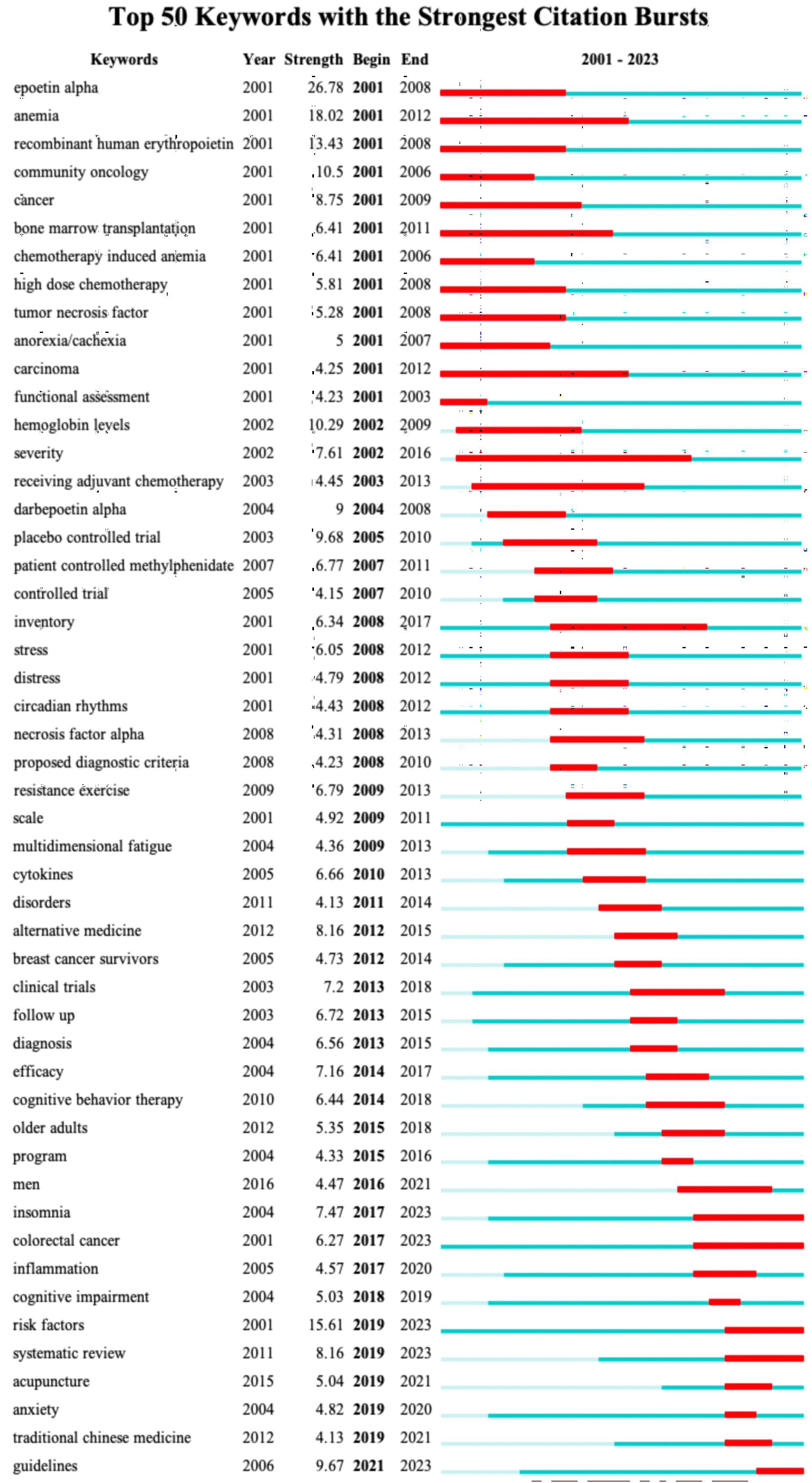
Figure 11 The top 50 keywords with strongest citation bursts. Red lines represent the duration of citation bursts. The figure was arranged in ascending order based on the beginning year of burst.
This study conducted bibliometric and visualization analyses using CiteSpace to examine 2,566 scientific articles published between 2001 and 2023, providing comprehensive knowledge network maps of CRF. The findings indicate that CRF has garnered extensive attention since 2001, with a notable surge in interest observed in 2019, showing sustained attention towards CRF over the coming years. The most prolific author is Eduardo Bruera from the UTMD Anderson Cancer Center, and the most co-cited author is Julienne E Bower from the University of California. Additionally, the University of Texas System is the most productive institution. Nine countries (the USA, Germany, England, Canada, Netherlands, Italy, France, Japan, and Greece) have embarked on comprehensive research on CRF since 2001. Here in this article the United States and China made the most contributions to this field. However, the low centrality of China highlights the need for Chinese scholars to enhance international association and collaboration.
Supportive Care in Cancer published the most studies on CRF, while Journal of Clinical Oncology has the highest number of co-citations. Most-cited references generally address the possible mechanisms and treatments of CRF. The top ten keywords with the highest frequency of occurrence are quality of life, cancer-related fatigue, breast cancer, survivors, management, chemotherapy, randomized controlled trial, fatigue, physical activity, and depression. Our analysis of keywords with the strongest citation bursts have revealed that “yoga,” “inflammation,” “hypoxia,” “anemia,” “exercise,” “acupuncture,” “anxiety,” “traditional Chinese medicine,” and “insomnia” might be emerging hotspots in the CRF area.
We summarized the hotspots in CRF research based on the results obtained from CiteSpace, primarily encompassing risk factors (anemia, insomnia, depression, and anxiety), treatments (exercise, yoga, acupuncture, and traditional Chinese medicine), and mechanisms (inflammation and hypoxia) of CRF.
Previous reports have indicated that several factors, including anemia, insomnia, anxiety, and depression, are essential for the occurrence and development of fatigue in patients with cancer (19–21).
Patients with cancer often have anemia, especially during or after chemotherapy. Low hemoglobin levels often cause a decrease in blood oxygen levels, leading to the development of some fatigue-related symptoms. These findings have prompted attempts to investigate the correlations between cancer-related anemia and CRF. Some evidence shows that administration of epoetin or darbepoetin to increase hemoglobin level is an effective therapy for reducing CRF (19). However, this treatment may be limited to patients with cancer-related anemia caused by hematological malignancies, such as lymphoma or multiple myeloma, or cancer treatments, such as chemotherapy (22, 23). The analysis conducted in the present study revealed that the keywords “epoetin alpha” and “anemia” have the earliest year of citation bursts (2001); however, this surge only lasted until 2012, indicating that the effect of anemia on CRF has not been a topic of interest in recent years.
Approximately 25-59% of patients with cancer experience insomnia. Insomnia is a prevalent type of sleep disorder characterized by poor sleep quality and difficulties with sleep initiation and maintenance (24). The National Comprehensive Cancer Network (NCCN) has identified that insomnia has a critical influence on CRF (1). A meta-analysis of 144,813 patients with cancer indicated that insomnia has a significant effect on CRF (OR = 2.83), and that improved insomnia is associated with decreased levels of CRF (2). Several possible mechanisms, such as alterations in the circadian rhythm, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, inflammatory and immune responses, and abnormal neurotransmitter levels, may be implicated in the correlation between insomnia and CRF (25).
Anxiety and depression are common mental health problems that affect approximately 10-20% of patients with cancer. The correlation between CRF and anxiety/depression is undeniable. However, the association between CRF and depression seems to receive more attention than that between CRF and anxiety (26–28). A systematic review of 59 studies showed that the correlation between anxiety (0.46) and CRF is weaker than that between depression and CRF (0.56) (29). However, several studies have indicated that anxiety may have a more pronounced effect on CRF than depression in the early stages of cancer, which may be attributed to the predominance of anxiety as the primary emotional response in this stage (30). It is believed that anxiety/depression may contribute to loss of appetite, decreased engagement in physical activities, muscle tension, or disrupted sleep, all of which are associated with the occurrence or increased levels of CRF. The precise mechanisms underlying the relationship between anxiety/depression and CRF are multifaceted and not completely understood; however, they may involve dysregulation of the HPA axis, alterations in neurotransmitter levels, and inflammatory responses (31).
In addition to anxiety and depression, there are many psychosocial risk factors of CRF, such as childhood stress, loneliness, and “catastrophize” of cancer. Conversely, CRF can also impact social, cognitive, and emotional functioning (4). The effects of CRF on social, cognitive, and emotional functioning is an integral component in various CRF scales. Moreover, recent research indicates that CRF, along with multiple cancer-related symptoms such as depression, anxiety, insomnia, poor concentration, and other cognitive impairments, is increasingly being reported as symptom clusters (32), which indicates that CRF and these symptoms may share similar mechanisms and interactions. The amelioration of one symptom is likely to improve others. Studying of associated risk factors contributes to early assessment, diagnosis, and management of CRF. Improving social, cognitive, and emotional functioning status of patients aids in reducing CRF, and avoiding the vicious cycle of “fatigue-impairment in social/cognitive/emotional functioning-fatigue exacerbation.”
Over the past two decades, multiple randomized controlled trials have been conducted with CRF as a primary or secondary outcome measure. These studies have demonstrated diverse effective management strategies for CRF (4), especially non-pharmacological interventions such as exercise, yoga, and acupuncture.
Existing evidence suggests that exercise is the most effective therapeutic option for preventing and improving CRF and can be performed by patients with cancer of any stage (8, 15). The exercise guidelines (11) for cancer survivors recommend that engaging in moderately intense aerobic exercise (three times per week) for at least 12 weeks can significantly reduce CRF. A meta-analysis (12) of 245 studies revealed that among all types of exercise, relaxation exercises may be preferable for patients with CRF during cancer treatment. However, another study (33) concluded that a combination of aerobic and resistance exercises yields the most improvements in CRF, either during or after cancer treatment. In addition, it has been reported that aerobic exercise for improving CRF is more beneficial for patients with solid tumors than for those with hematological malignancies (34). It is important to consider variations in the type, duration, and intensity of exercise for different populations, such as patients with cancer who have bone metastases, anemia, or fever (1). Further research is warranted to provide evidence of the benefits of exercise in different populations of patients with CRF.
Yoga, a type of mind-body exercise, is widely performed to relieve cancer-related symptoms, particularly fatigue. Current guidelines recommend performing yoga to reduce CRF during and after cancer treatment (1, 15). A Cochrane review (35) of 24 studies provided moderate-quality evidence that yoga improves CRF in patients with breast cancer compared with psychosocial/educational interventions. A meta-analysis (36) of 2,183 patients with breast cancer showed that an eight-week yoga regimen significantly mitigated the physical fatigue of the patients but had little effects on cognitive/mental fatigue. Yoga can regulate the dysregulation of the HPA axis, decrease plasma cortisol levels, and increase the release of endogenous dopamine and γ-aminobutyric acid levels (37–39). Some studies have been conducted to investigate the potential mechanisms underlying the improvement effect of yoga on CRF (40, 41), which may involve the modulation of the inflammatory processes, such as regulating the levels of inflammatory cytokines (e.g., IL-1β, IL-10), or the NF-κB pathway. Moreover, yoga may enhance glucocorticoid signal transduction and reduce sympathetic nervous system signaling through β-adrenergic receptors to influence inflammatory processes.
The American Society of Oncology and NCCN guidelines recommend acupuncture, an essential component of traditional Chinese medicine (TCM), for attenuating CRF in patients after cancer treatment (1, 13). A meta-analysis (42) of 1,084 patients with breast cancer revealed that acupuncture can significantly reduce CRF compared with sham acupuncture or usual care. A minimum of six acupuncture sessions (e.g., weekly for 6 weeks, three times per week for 3 weeks), each lasting at least 20 minutes, has significant effects on CRF (43). Two studies (44, 45) demonstrated that acupuncture can inhibit neuroinflammation and gut inflammation by regulating the gut microbiota-gut-brain axis, decrease serum ghrelin, leptin, and insulin levels, and improve the dysfunction of the HPA axis to improve CRF. However, as further studies are still needed to verify the effects of acupuncture on CRF, the European Society for Medical Oncology panel has not yet reached a consensus on the recommendation of acupuncture for reducing CRF.
In addition to acupuncture, Chinese herbal medicines and Chinese medicine compound prescriptions have been shown to reduce CRF. Ginseng, a commonly used Chinese herbal medicine, tonifies the qi, nourishes the blood, and strengthens the spleen. A randomized trial (N07C2) (46) provided evidence to support the effects of American ginseng (Panax quinquefolius) (2000 mg/day) on CRF, and no discernible treatment-related toxicities are reported. Three systematic reviews (47–49) have demonstrated that ginseng, including Asian, red, and American ginseng, can alleviate CRF. However, no clear criteria regarding the duration and dose of ginseng that should be administered to alleviate CRF have been established. Moreover, the effects of ginseng in reducing CRF may be influenced by various factors such as the type of ginseng, method of preparation, and ginsenoside content. In addition, huangqi (Radix Astragali), baizhu (Atractylodes macrocephala Koidz), and shanyao (Chinese yam) may regulate the levels of cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and inhibit oxidative stress to reduce CRF (50, 51).
Shengqi Fuzheng injection, which mainly consists of dangshen (Radix Codonopsis) and huangqi, improves mitochondrial dysfunction through the PI3K/Akt and AMPK signaling pathways, inhibits the levels of pro-inflammatory cytokines, and enhances immune function by downregulating multiple targets (PD-L1, TIM3, and FOXP3) to alleviate CRF (52, 53). Moreover, the effects of the Buzhong Yiqi decoction, Jianpi Shengsui Gao, and Shiquan Dabu decoction have been validated in animal studies and clinical trials (54–56). However, the evidence supporting these findings is of low quality; as such, TCM is not recommended for the treatment of CRF in any guideline. Therefore, further research on the effects of TCM on CRF is required.
The analysis conducted in this study indicated that inflammation is a hot topic in studies on the mechanisms of CRF (4). There are large variations in the symptoms experienced by both animals and humans after infections (57). These symptoms, including fatigue, significant changes in sleep patterns, and loss of appetite, are largely related to the effects of pro-inflammatory cytokines on the central nervous system. Persistent inflammation also plays an important role in CRF (58–60), primarily because of the activation of a network of pro-inflammatory cytokines by anti-tumor therapies. A study (61) demonstrated that 65% of patients with acute myelogenous leukemia/myelodysplastic syndrome experience CRF before treatment, which is associated with IL-1RA, IL-6, and TNF-α levels. Chemotherapy and radiotherapy are commonly administered to treat cancer. Chemotherapy with platinum- or anthracycline-based agents may increase IL-8 and IL-6 levels, thereby leading to CRF (62, 63). Increased serum levels of C-reactive protein (CRP) and IL-1RA are associated with increased CRF in patients undergoing radiotherapy (64). This correlation has also been observed in patients with advanced cancer (65, 66). Most breast cancer survivors with fatigue show upregulated expression of genes encoding pro-inflammatory cytokines compared with non-fatigued patients. In addition, cancer survivors with fatigue show increased expression of transcripts with response elements for NF-κB (67).
Other factors, such as psychological distress and insufficient sleep, can also affect inflammatory responses. Stress can activate peripheral inflammatory cytokines and related signaling pathways, such as the NF-κB signaling pathway, which are mediated by the activation of the sympathetic nervous system and the release of catecholamines (68–70). A meta-analysis (71) showed that acute stress may lead to marked increase in circulating IL-1β, IL-6, IL-10, and TNF-α levels. In addition, insufficient sleep can lead to elevated CRP, IL-6, and TNF-α levels, which may be associated with reduced cortisol secretion and disrupted circadian rhythm (72–74). Peripheral pro-inflammatory cytokines can access the brain through various pathways, activating microglia and astrocytes and contributing to neuroinflammation, which is a significant reason for the depression-like behaviors experienced by patients with CRF. Multiple inflammatory biomarkers, including CRP, IL-1RA, IL-6, sTNFR2, TNF-α, have been shown to be strongly associated with CRF. In addition, some of these biomarkers have been assessed as independent risk factors for CRF (17, 75–78). Moreover, some studies have confirmed that improvements in the levels of certain inflammatory markers can ameliorate CRF (40, 79–81).
Although the specific mechanism by which inflammation affects CRF remains elusive, it has been established that inflammation is intricately linked to neurotransmitter metabolism, HPA axis regulation, and skeletal muscle and mitochondrial dysfunction. Pro-inflammatory cytokines, such as IL-1β, IL-6, IFN-α, IFN-γ, and TNF-α, can activate the indoleamine 2,3-dioxygenase (IDO) pathway, which increases the degradation of tryptophan into kynurenine through the kynurenine (KYN) pathway, leading to reduced serotonin levels (82). Serotonin, an essential neurotransmitter, influences depression and sleep; however, its effect on CRF remains controversial (83, 84). The activation of the KYN pathway can also affect the functions of dopamine and norepinephrine. Kynurenic acid, generated from the degradation of kynurenine, can inhibit glutamatergic function, which may suppress the release of dopamine and result in CRF (82, 85). Methylphenidate, one of the few pharmacological therapies recommended for CRF in current clinical guidelines, acts by elevating dopamine levels (86).
Pro-inflammatory cytokines can influence the function of the HPA axis. The HPA axis regulates the generation of cytokines to exert anti-inflammatory effects through negative feedback control mechanisms (4). Cortisol, a glucocorticoid released by the HPA axis in response to factors such as inflammation, exhibits a clear circadian rhythm in healthy individuals. The diurnal cortisol rhythm appears flatter in cancer survivors with fatigue, indicating that patients with CRF experience disruptions in the circadian cortisol rhythm (87, 88). Notably, the flattened cortisol secretion slope in cancer survivors with fatigue is attributed to elevated cortisol levels in the afternoon and evening. Furthermore, a study demonstrated that breast cancer survivors with fatigue exhibit diminished expression of glucocorticoid receptor anti-inflammatory transcription factors, indicating the presence of functional resistance to glucocorticoids, which may lead to the persistence of inflammatory responses (89).
Inflammation mediates mitochondrial dysfunction through various signaling pathways. For instance, IL-6 can activate STAT3 to disrupt homeostasis in skeletal muscles, whereas TNF-α can promote protein degradation in skeletal muscles by activating the NF-κB signaling pathway (90, 91). Studies have shown that inflammation increases the mitochondrial localization of NLRP3 and enhances the production of mitochondrial reactive oxygen species (ROS) (92). Dysfunctional mitochondria release excessive ROS and diminish adenosine triphosphate generation (93). Long-term elevation of the levels of ROS induces DNA damage, protein oxidation, and cellular apoptosis. Additionally, ROS can affect skeletal muscle function by interfering with the Akt/mTOR signaling pathway and augmenting protein hydrolysis (94). Mitochondrial and skeletal muscle dysfunction are important factors that influence CRF.
Metabolic activities in immune cells change after inflammatory stimulation, such as increased oxygen consumption by activated neutrophils and elevation of glycolytic rates by activated macrophages and lymphocytes, leading to hypoxia (95). Studies show that mitochondrial dysfunction can exacerbate hypoxia within the tumor microenvironment. Under anoxic conditions, the hypoxia-inducible factor (HIF) is activated and serves as the dominant regulator of oxygen homeostasis (96). The HIF-α and HIF-β subunits induce transcription of multiple genes, such as NF-κB and toll-like receptors, promote the release of chemokines and cytokines, and stimulate the activation and recruitment of phagocytes and leukocytes to regulate the function of immune cells (97, 98). HIF-1 also has the capacity to upregulate the expression of NADPH oxidase, augmenting the generation of ROS within the cytoplasm to promote oxidative stress (99, 100). In turn, ROS can enhance the stability of HIF-1 and facilitate the activation of both HIF-1 and NF-κB. Jianpi Shengsui Gao, a kind of TCM, enhances antioxidant stress via the HIF-1 pathway to mitigate CRF (55).
Hypoxia-induced oxidative damage alters mitochondrial morphology, metabolism, and respiration (101). Mitochondrial autophagy is an adaptive metabolic response and a crucial mechanism for eliminating oxidatively damaged mitochondria, which contributes to the maintenance of energy metabolism. HIF induces mitochondrial autophagy by modulating the expression of BNIP3/Beclin-1 (102, 103). PHD2 is a key enzyme involved in the regulation of HIF-1α stability. A study showed that acteoside from Cistanche tubulosa can promote mitochondrial autophagy by suppressing PHD2 to reduce CRF (104). Existing evidence indicates that the HIF pathway may serve as a pivotal target in the treatment of chronic inflammation, which also plays a role in immune regulation and antitumor activity (105–108). Although research on the HIF pathway is still in its nascent stage, particularly in the context of CRF, the HIF pathway represents an exciting therapeutic target that warrants further investigation.
Whether CRF originated in the central or peripheral remains controversial. Previous studies examining electroencephalogram (EEG), fatigue-related muscle contractile properties, and electromyogram (EMG) supported the findings of central origin of CRF (109–113). Another study quantifying EEG-EMG coherence indicated significant and robust weakening of corticomuscular signal coupling in CRF patients, which may be caused by central and peripheral neuropathies (114). Although the mechanism of neuroelectric activity of CRF is still unclear, improving neuromuscular transmission may also be an important method for CRF treatment. Certainly, more research is needed. On the other hand, whether CRF is of central or peripheral origin, inflammation may play a central role. Pro-inflammatory cytokines can promote neuroinflammation through multiple pathways, affecting central nervous system function and neurotransmitter metabolism. Inflammation is also related to skeletal muscle mitochondrial function. The joint action of inflammation and skeletal muscle mitochondria can lead to hypoxia and abnormal oxidative stress response, which in turn aggravates inflammation and skeletal muscle mitochondrial dysfunction. This study indicates the importance of inflammation in the CRF field, and hypoxia is a possible research direction in the future. Targeting related pathways may be a new method to reduce CRF.
The results of this analysis indicate that recent research on the risk factors for CRF has predominantly been focused on insomnia, anxiety, and depression. Anemia remains a risk factor for CRF, and erythropoietin is recommended for patients with CRF and concomitant anemia (1). The main potential mechanisms by which insomnia, anxiety, and depression cause CRF include inflammation, HPA axis dysregulation, neurotransmitter alterations, and disturbances in circadian rhythms. Analysis of the keywords with the strongest citation bursts highlights inflammation as current hotspot in research on the mechanisms underlying the development of CRF. Inflammation is closely associated with HPA axis dysfunction, alterations in neurotransmitters, disruptions in circadian rhythms, and skeletal muscle and mitochondrial dysfunction, all of which collectively exert a synergistic effect on the onset and progression of CRF. Several studies have indicated that modulating the levels of pro-inflammatory cytokines, such as IL-1RA, IL-6, and TNF-α, to ameliorate systemic and neural inflammation can effectively alleviate CRF (79–81).
Cluster analysis of keywords indicated that hypoxia is a potential mechanism underlying the development of CRF, possibly representing an emerging topic for future studies in the field of CRF research. HIF-related pathways are activated in hypoxic conditions, which can modulate the functions of immune cells, release of inflammatory cytokines, oxidative stress status, and mitochondrial function. Targeting HIF-related pathways has been proven to be a possible therapeutic strategy for various chronic inflammatory and neurodegenerative diseases (115) and may potentially aid the improvement of CRF (55, 104). However, further substantiation through more evidence is warranted to determine whether targeting HIF-related pathways can ameliorate CRF.
The findings of this study highlight the current research hotspots regarding the risk factors, treatments, and mechanisms of CRF, and provide novel insights into future therapeutic directions for CRF. Notably, the results of this study indicated that hypoxia, which is closely associated with inflammation, is one of the less explored mechanisms underlying the development of CRF. Further studies are needed to clarify whether hypoxia-associated pathways can be crucial targets in the treatment of CRF.
Treatment strategies for CRF have been evaluated in previous studies. According to existing guidelines, exercise is the most effective intervention for improving CRF. However, there is no consensus on the optimal type, frequency, and intensity of exercise suitable for various types and stages of cancer. Future in-depth studies on this topic are needed to aid clinicians in designing optimal treatment strategies for patients with CRF. Acupuncture has demonstrated significant efficacy in ameliorating cancer-related symptoms. However, future extensive, large-scale, clinical studies are needed to verify its potential in improving CRF.
This study has some limitations. First, we only retrieved English literature related to CRF from the SSCI and SCI-Expanded databases through the WoSCC. Unpublished articles and articles published in other languages were not included. Second, the literature search was limited to articles with “cancer-related fatigue” in their titles, abstracts, or keywords, potentially leading to the data being insufficiently comprehensive.
This study is the first and visualization analyses of cancer-related fatigue research published worldwide over the past two decades. The collaboration among researchers, institutions, and countries is limited. In addition, the results provides a comprehensive overview for global researchers, clinical practitioners, and decision-makers regarding the research focal points on risk factors, treatments, and potential mechanisms of cancer-related fatigue. Notably, the research findings indicate that hypoxia is a potential mechanism of cancer-related fatigue that merits further investigation. The HIF-related pathways could potentially serve as novel targets for the treatment of cancer-related fatigue.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
PL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. QW: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. LF: Conceptualization, Supervision, Writing – review & editing. ZD: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. WF: Conceptualization, Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Capital Health Research and Development of Special (Grant Number 2020-2-4026), the Traditional Chinese medicine “double-chain integration” young and middle-aged scientific research and innovation team (Grant Number 2022-SLRH-LG-005), the Qin Chuangyuan “scientist + engineer” team construction of Shaanxi Province (Grant Number 2022KXJ-019), and the Key research and development projects of Shanxi Province (Grant Number 2023ZDLSF-56). The funders had no role in the design and performance of the study; extraction, synthesis, and analysis of the data; preparation, editing, and approval of the manuscript; and the decision to publicize the manuscript.
We appreciate Home-for-Researchers and Editage for language editing for us.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1338325/full#supplementary-material.
1. NCCN Clinical Practice Guidelines in Oncology. Cancer-related fatigue version 2.2023 (2023). Available online at: https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1424 (Accessed January 30, 2023).
2. Ma Y, He B, Jiang M, Yang Y, Wang C, Huang C, et al. Prevalence and risk factors of cancer-related fatigue: A systematic review and meta-analysis. Int J Nurs Stud. (2020) 111:103707. doi: 10.1016/j.ijnurstu.2020.103707
3. Horneber M, Fischer I, Dimeo F, Rüffer JU, Weis J. Cancer-related fatigue: epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. (2012) 109:161–72. doi: 10.3238/arztebl.2012.0161
4. Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. (2014) 11:597–609. doi: 10.1038/nrclinonc.2014.127
5. Agarwal A, Durairajanayagam D, Tatagari S, Esteves SC, Harlev A, Henkel R, et al. Bibliometrics: tracking research impact by selecting the appropriate metrics. Asian J Androl. (2016) 18:296–309. doi: 10.4103/1008-682X.171582
6. Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 57:359–77. doi: 10.1002/asi.20317
7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
8. Aria M, Cuccurullo C. bibliometrix: An R-tool for comprehensive science mapping analysis. J Informetrics. (2017) 11:959–75. doi: 10.1016/j.joi.2017.08.007
9. Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol. (2017) 3:961–8. doi: 10.1001/jamaoncol.2016.6914
10. Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. (2015) 13:1012–39. doi: 10.6004/jnccn.2015.0122
11. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. (2019) 51:2375–90. doi: 10.1249/MSS.0000000000002116
12. Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. (2018) 52:651–8. doi: 10.1136/bjsports-2016-096422
13. Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. (2014) 32:1840–50. doi: 10.1200/JCO.2013.53.4495
14. Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen CAHHVM, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. (2016) 27:965–74. doi: 10.1093/annonc/mdw099
15. Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. (2020) 31:713–23. doi: 10.1016/j.annonc.2020.02.016
16. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. (2007) 12:4–10. doi: 10.1634/theoncologist.12-S1-4
17. Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Yennurajalingam S, et al. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. (2015) 23:2461–78. doi: 10.1007/s00520-015-2763-0
18. Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. (2000) 5:353–60. doi: 10.1634/theoncologist.5-5-353
19. Holzner B, Kemmler G, Greil R, Kopp M, Zeimet A, Raderer M, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol. (2002) 13:965–73. doi: 10.1093/annonc/mdf122
20. DSilva F, Singh P, Javeth A. Determinants of cancer-related fatigue among cancer patients: A systematic review. J Palliat Care. (2022) 2022:8258597221131133. doi: 10.1177/08258597221131133
21. Huang ST, Ke X, Yu XY, Wu YX, Huang YX, Liu D. Risk factors for cancer-related fatigue in patients with colorectal cancer: a systematic review and meta-analysis. Support Care Cancer. (2022) 30:10311–22. doi: 10.1007/s00520-022-07432-5
22. Gascón P, Arranz R, Bargay J, Ramos F. Fatigue- and health-related quality-of-life in anemic patients with lymphoma or multiple myeloma. Support Care Cancer. (2018) 26:1253–64. doi: 10.1007/s00520-017-3948-5
23. Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst. (2008) 100:1155–66. doi: 10.1093/jnci/djn250
24. Zhang J, Zhang Z, Huang S, Qiu X, Lao L, Huang Y, et al. Acupuncture for cancer-related insomnia: A systematic review and meta-analysis. Phytomedicine. (2022) 102:154160. doi: 10.1016/j.phymed.2022.154160
25. Zee PC, Ancoli-Israel S, Workshop Participants. Does effective management of sleep disorders reduce cancer-related fatigue? Drugs. (2009) 69:29–41. doi: 10.2165/11531140-000000000-00000
26. Ruiz-Casado A, Álvarez-Bustos A, de Pedro CG, Méndez-Otero M, Romero-Elías M. Cancer-related fatigue in breast cancer survivors: A review. Clin Breast Cancer. (2021) 21:10–25. doi: 10.1016/j.clbc.2020.07.011
27. Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. (2018) 361:k1415. doi: 10.1136/bmj.k1415
28. Lobefaro R, Rota S, Porcu L, Brunelli C, Alfieri S, Zito E, et al. Cancer-related fatigue and depression: a monocentric, prospective, cross-sectional study in advanced solid tumors. ESMO Open. (2022) 7:100457. doi: 10.1016/j.esmoop.2022.100457
29. Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics. (2009) 50:440–7. doi: 10.1016/S0033-3182(09)70835-7
30. Ho RT, Kwan TT, Cheung IK, Chan CK, Lo PH, Yip PS, et al. Association of fatigue with perceived stress in chinese women with early stage breast cancer awaiting adjuvant radiotherapy. Stress Health. (2015) 31:214–21. doi: 10.1002/smi.2548
31. Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat Rev. (2017) 52:58–70. doi: 10.1016/j.ctrv.2016.11.004
32. Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, et al. Cancer-related fatigue and sleep disorders. Oncologist. (2007) Suppl 1:35–42. doi: 10.1634/theoncologist.12-S1-35
33. Dong B, Qi Y, Lin L, Liu T, Wang S, Zhang Y, et al. Which exercise approaches work for relieving cancer-related fatigue? A network meta-analysis. J Orthop Sports Phys Ther. (2023) 0:1–10. doi: 10.2519/jospt.2023.11251
34. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. (2012) 11:CD006145. doi: 10.1002/14651858.CD006145.pub3
35. Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. (2017) 1:CD010802. doi: 10.1002/14651858.CD010802.pub2
36. Dong B, Xie C, Jing X, Lin L, Tian L. Yoga has a solid effect on cancer-related fatigue in patients with breast cancer: a meta-analysis. Breast Cancer Res Treat. (2019) 177:5–16. doi: 10.1007/s10549-019-05278-w
37. Streeter CC, Gerbarg PL, Saper RB, Ciraulo DA, Brown RP. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. (2012) 78:571–9. doi: 10.1016/j.mehy.2012.01.021
38. Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. (2011) 23:135–42. doi: 10.1111/j.1745-7599.2010.00573.x
39. Streeter CC, Whitfield TH, Owen L, Rein T, Karri SK, Yakhkind A, et al. Effects of yoga versus walking on mood, anxiety, and brain GABA levels: a randomized controlled MRS study. J Altern Complement Med. (2010) 16:1145–52. doi: 10.1089/acm.2010.0007
40. Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. (2014) 43:20–9. doi: 10.1016/j.psyneuen.2014.01.019
41. Jain M, Mishra A, Yadav V, Shyam H, Kumar S, Mishra SK, et al. Long-term yogic intervention decreases serum interleukins IL-10 and IL-1β and improves cancer-related fatigue and functional scale during radiotherapy/chemotherapy in breast cancer patients: a randomized control study. Support Care Cancer. (2022) 31:6. doi: 10.1007/s00520-022-07487-4
42. Choi TY, Ang L, Jun JH, Alraek T, Birch S, Lu W, et al. Acupuncture for managing cancer-related fatigue in breast cancer patients: A systematic review and meta-analysis. Cancers (Basel). (2022) 14:4419. doi: 10.3390/cancers14184419
43. Zhang Y, Lin L, Li H, Hu Y, Tian L. Effects of acupuncture on cancer-related fatigue: a meta-analysis. Support Care Cancer. (2018) 26:415–25. doi: 10.1007/s00520-017-3955-6
44. Lv Z, Liu R, Su K, Gu Y, Fang L, Fan Y, et al. Acupuncture ameliorates breast cancer-related fatigue by regulating the gut microbiota-gut-brain axis. Front Endocrinol (Lausanne). (2022) 13:921119. doi: 10.3389/fendo.2022.921119
45. Li J, Xie J, Guo X, Fu R, Wang Y, Guan X. Effects of mind-regulation acupuncture therapy on serum ghrelin, gastric inhibitory polypeptide, leptin, and insulin levels in breast cancer survivors with cancer-related fatigue: A randomized controlled trial. Int J Gen Med. (2023) 16:1017–27. doi: 10.2147/IJGM.S405977
46. Barton DL, Liu H, Dakhil SR, Linquist B, Sloan JA, Nichols CR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst. (2013) 105:1230–8. doi: 10.1093/jnci/djt181
47. Najafi TF, Bahri N, Tohidinik HR, Feyz S, Bloki F, Savarkar S, et al. Treatment of cancer-related fatigue with ginseng: A systematic review and meta-analysis. J Herbal Med. (2021) 28:100440. doi: 10.1016/j.hermed.2021.100440
48. Luo WT, Huang TW. Effects of ginseng on cancer-related fatigue: A systematic review and meta-analysis of randomized controlled trials. Cancer Nurs. (2023) 46:120–7. doi: 10.1097/NCC.0000000000001068
49. Sadeghian M, Rahmani S, Zendehdel M, Hosseini SA, Zare Javid A. Ginseng and cancer-related fatigue: A systematic review of clinical trials. Nutr Cancer. (2021) 73:1270–81. doi: 10.1080/01635581.2020.1795691
50. Zhang C, Guo W, Yao X, Xia J, Zhang Z, Li J, et al. Database mining and animal experiment-based validation of the efficacy and mechanism of Radix Astragali (Huangqi) and Rhizoma Atractylodis Macrocephalae (Baizhu) as core drugs of Traditional Chinese medicine in cancer-related fatigue. J Ethnopharmacol. (2022) 285:114892. doi: 10.1016/j.jep.2021.114892
51. Wang Y, Liu Y, Zhang Y, Huo Z, Wang G, He Y, et al. Effects of the polysaccharides extracted from Chinese yam (Dioscorea opposita Thunb.) on cancer-related fatigue in mice. Food Funct. (2021) 12:10602–14. doi: 10.1039/D1FO00375E
52. Guo W, Liu S, Zheng X, Xiao Z, Chen H, Sun L, et al. Network pharmacology/metabolomics-based validation of AMPK and PI3K/AKT signaling pathway as a central role of shengqi fuzheng injection regulation of mitochondrial dysfunction in cancer-related fatigue. Oxid Med Cell Longev. (2021) 2021:5556212. doi: 10.1155/2021/5556212
53. Zhu G, Zhang B, Jiang F, Zhao L, Liu F. ShenQi FuZheng Injection ameliorates fatigue-like behavior in mouse models of cancer-related fatigue. BioMed Pharmacother. (2019) 111:1376–82. doi: 10.1016/j.biopha.2019.01.042
54. Li G, Ding J, Zhang Y, Wang X. The clinical application and pharmacological mechanism of Bu-Zhong-Yi-Qi decoction for treating cancer-related fatigue: An overview. BioMed Pharmacother. (2022) 156:113969. doi: 10.1016/j.biopha.2022.113969
55. Xiao M, Guo W, Zhang C, Zhu Y, Li Z, Shao C, et al. Jian Pi Sheng Sui Gao (JPSSG) alleviation of skeletal myoblast cell apoptosis, oxidative stress, and mitochondrial dysfunction to improve cancer-related fatigue in an AMPK-SIRT1- and HIF-1-dependent manner. Ann Transl Med. (2023) 11:156. doi: 10.21037/atm
56. Lee JY, Kim EH, Yoon JH, Eo W, Yoon SW. Traditional herbal medicine, sipjeondaebo-tang, for cancer-related fatigue: A randomized, placebo-controlled, preliminary study. Integr Cancer Ther. (2021) 20:15347354211040830. doi: 10.1177/15347354211040830
57. Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, et al. Cytokine-induced sickness behavior. Brain Behav Immun. (2003) 17 Suppl 1:S112–8. doi: 10.1016/S0889-1591(02)00077-6
58. O’Higgins CM, Brady B, O’Connor B, Walsh D, Reilly RB. The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer. (2018) 26:3353–64. doi: 10.1007/s00520-018-4318-7
59. Yang S, Chu S, Gao Y, Ai Q, Liu Y, Li X, et al. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells. (2019) 8:738. doi: 10.3390/cells8070738
60. Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. (2007) 21:863–71. doi: 10.1016/j.bbi.2007.03.013
61. Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. (2005) 104:788–93. doi: 10.1002/cncr.21234
62. Zhang Y, Huang X, Feng S, Chen C, Guo D, Fang L. Platinum accumulation and cancer-related fatigue, correlation with IL-8, TNF-α and hemocytes. Front Pharmacol. (2021) 12:658792. doi: 10.3389/fphar.2021.658792
63. Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. (2012) 26:706–13. doi: 10.1016/j.bbi.2012.02.001
64. Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. (2009) 15:5534–40. doi: 10.1158/1078-0432.CCR-08-2584
65. de Raaf PJ, Sleijfer S, Lamers CH, Jager A, Gratama JW, van der Rijt CC. Inflammation and fatigue dimensions in advanced cancer patients and cancer survivors: an explorative study. Cancer. (2012) 118:6005–11. doi: 10.1002/cncr.27613
66. Reyes-Gibby CC, Wang J, Spitz M, Wu X, Yennurajalingam S, Shete S. Genetic variations in interleukin-8 and interleukin-10 are associated with pain, depressed mood, and fatigue in lung cancer patients. J Pain Symptom Manage. (2013) 46:161–72. doi: 10.1016/j.jpainsymman.2012.07.019
67. Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. (2011) 25:147–50. doi: 10.1016/j.bbi.2010.09.010
68. Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U.S.A. (2003) 100:1920–5. doi: 10.1073/pnas.0438019100
69. Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. (2006) 163:1630–3. doi: 10.1176/ajp.2006.163.9.1630
70. Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. (2005) 135:1295–307. doi: 10.1016/j.neuroscience.2005.06.090
71. Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun. (2017) 64:208–19. doi: 10.1016/j.bbi.2017.01.011
72. Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. (2004) 43:678–83. doi: 10.1016/j.jacc.2003.07.050
73. Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. (2004) 89:2119–26. doi: 10.1210/jc.2003-031562
74. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. (2008) 26:971–82. doi: 10.1200/JCO.2007.10.7805
75. Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres MA. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol Med. (2017) 47:1733–43. doi: 10.1017/S0033291717000150
76. Maurer T, Jaskulski S, Behrens S, Jung AY, Obi N, Johnson T, et al. Tired of feeling tired - The role of circulating inflammatory biomarkers and long-term cancer related fatigue in breast cancer survivors. Breast. (2021) 56:103–9. doi: 10.1016/j.breast.2021.02.008
77. Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. (2007) 21:413–27. doi: 10.1016/j.bbi.2006.11.004
78. Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer AM, et al. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. (2013) 34:108–19. doi: 10.1016/j.bbi.2013.07.177
79. Norden DM, McCarthy DO, Bicer S, Devine RD, Reiser PJ, Godbout JP, et al. Ibuprofen ameliorates fatigue- and depressive-like behavior in tumor-bearing mice. Life Sci. (2015) 143:65–70. doi: 10.1016/j.lfs.2015.10.020
80. Inglis JE, Lin PJ, Kerns SL, Kleckner IR, Kleckner AS, Castillo DA, et al. Nutritional interventions for treating cancer-related fatigue: A qualitative review. Nutr Cancer. (2019) 71:21–40. doi: 10.1080/01635581.2018.1513046
81. Huang WC, Kuo KT, Bamodu OA, Lin YK, Wang CH, Lee KY, et al. Astragalus polysaccharide (PG2) Ameliorates Cancer Symptom Clusters, as well as Improves Quality of Life in Patients with Metastatic Disease, through Modulation of the Inflammatory Cascade. Cancers (Basel). (2019) 11:1054. doi: 10.3390/cancers11081054
82. Kim S, Miller BJ, Stefanek ME, Miller AH. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer. (2015) 121:2129–36. doi: 10.1002/cncr.29302
83. Morrow GR, Hickok JT, Roscoe JA, Raubertas RF, Andrews PL, Flynn PJ, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. (2003) 21:4635–41. doi: 10.1200/JCO.2003.04.070
84. Norden DM, Devine R, Bicer S, Jing R, Reiser PJ, Wold LE, et al. Fluoxetine prevents the development of depressive-like behavior in a mouse model of cancer related fatigue. Physiol Behav. (2015) 140:230–5. doi: 10.1016/j.physbeh.2014.12.045
85. Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. (2010) 15:393–403. doi: 10.1038/mp.2009.116
86. Stone PC. Methylphenidate in the management of cancer-related fatigue. J Clin Oncol. (2013) 31:2372–3. doi: 10.1200/JCO.2013.50.0181
87. Schmidt ME, Semik J, Habermann N, Wiskemann J, Ulrich CM, Steindorf K. Cancer-related fatigue shows a stable association with diurnal cortisol dysregulation in breast cancer patients. Brain Behav Immun. (2016) 52:98–105. doi: 10.1016/j.bbi.2015.10.005
88. Cuneo MG, Schrepf A, Slavich GM, Thaker PH, Goodheart M, Bender D, et al. Diurnal cortisol rhythms, fatigue and psychosocial factors in five-year survivors of ovarian cancer. Psychoneuroendocrinology. (2017) 84:139–42. doi: 10.1016/j.psyneuen.2017.06.019
89. Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. (2008) 64:266–72. doi: 10.1016/j.biopsych.2008.03.017
90. Carson JA, Hardee JP, VanderVeen BN. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin Cell Dev Biol. (2016) 54:53–67. doi: 10.1016/j.semcdb.2015.11.005
91. VanderVeen BN, Fix DK, Carson JA. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: A role for inflammation. Oxid Med Cell Longev. (2017) 2017:3292087. doi: 10.1155/2017/3292087
92. Eggelbusch M, Shi A, Broeksma BC, Vázquez-Cruz M, Soares MN, de Wit GMJ, et al. The NLRP3 inflammasome contributes to inflammation-induced morphological and metabolic alterations in skeletal muscle. J Cachexia Sarcopenia Muscle. (2022) 13:3048–61. doi: 10.1002/jcsm.13062
93. McLean JB, Moylan JS, Andrade FH. Mitochondria dysfunction in lung cancer-induced muscle wasting in C2C12 myotubes. Front Physiol. (2014) 5:503. doi: 10.3389/fphys.2014.00503
94. Hyatt HW, Powers SK. Mitochondrial dysfunction is a common denominator linking skeletal muscle wasting due to disease, aging, and prolonged inactivity. Antioxidants (Basel). (2021) 10:588. doi: 10.3390/antiox10040588
95. Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. (2017) 17:774–85. doi: 10.1038/nri.2017.103
96. Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. (2008) 30:393–402. doi: 10.1016/j.molcel.2008.04.009
97. Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. (2011) 364:656–65. doi: 10.1056/NEJMra0910283
98. Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PloS One. (2007) 2:e1364. doi: 10.1371/journal.pone.0001364
99. Korbecki J, Simińska D, Gąssowska-Dobrowolska M, Listos J, Gutowska I, Chlubek D, et al. Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: A review of the molecular mechanisms. Int J Mol Sci. (2021) 22:10701. doi: 10.3390/ijms221910701
100. Diebold I, Petry A, Hess J, Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell. (2010) 21:2087–96. doi: 10.1091/mbc.e09-12-1003
101. Fuhrmann DC, Brüne B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. (2017) 12:208–15. doi: 10.1016/j.redox.2017.02.012
102. Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. (2008) 283:10892–903. doi: 10.1074/jbc.M800102200
103. Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. (2009) 29:2570–81. doi: 10.1128/MCB.00166-09
104. Zhang S, Gong F, Liu J, Liu T, Yang J, Hu J. A novel PHD2 inhibitor acteoside from Cistanche tubulosa induces skeletal muscle mitophagy to improve cancer-related fatigue. BioMed Pharmacother. (2022) 150:113004. doi: 10.1016/j.biopha.2022.113004
105. Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol. (2013) 13:646–53. doi: 10.1016/j.coph.2013.04.009
106. Chan MC, Holt-Martyn JP, Schofield CJ, Ratcliffe PJ. Pharmacological targeting of the HIF hydroxylases–A new field in medicine development. Mol Aspects Med. (2016) 47-48:54–75. doi: 10.1016/j.mam.2016.01.001
107. Tyrakis PA, Palazon A, Macias D, Lee KL, Phan AT, Veliça P, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. (2016) 540:236–41. doi: 10.1038/nature20165
108. Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. (2019) 18:157. doi: 10.1186/s12943-019-1089-9
109. Allexandre D, Seyidova-Khoshknabi D, Davis MP, Ranganathan VK, Siemionow V, Walsh D, et al. EEG correlates of central origin of cancer-related fatigue. Neural Plast. (2020) 2020:8812984. doi: 10.1155/2020/8812984
110. Kisiel-Sajewicz K, Davis MP, Siemionow V, Seyidova-Khoshknabi D, Wyant A, Walsh D, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage. (2012) 44:351–61. doi: 10.1016/j.jpainsymman.2011.08.007
111. Kisiel-Sajewicz K, Siemionow V, Seyidova-Khoshknabi D, Davis MP, Wyant A, Ranganathan VK, et al. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PloS One. (2013) 8:e83636. doi: 10.1371/journal.pone.0083636
112. Yavuzsen T, Davis MP, Ranganathan VK, Walsh D, Siemionow V, Kirkova J, et al. Cancer-related fatigue: central or peripheral? J Pain Symptom Manage. (2009) 38:587–96. doi: 10.1016/j.jpainsymman.2008.12.003
113. Cai B, Allexandre D, Rajagopalan V, Jiang Z, Siemionow V, Ranganathan VK, et al. Evidence of significant central fatigue in patients with cancer-related fatigue during repetitive elbow flexions till perceived exhaustion. PloS One. (2014) 9:e115370. doi: 10.1371/journal.pone.0115370
114. Jiang C, Yang Q, Chen T, Siemionow V, Ranganathan VK, Yan AF, et al. Functional corticomuscular signal coupling is weakened during voluntary motor action in cancer-related fatigue. Neural Plast. (2019) 2019:2490750. doi: 10.1155/2019/2490750
Keywords: cancer-related fatigue, bibliometric analysis, CiteSpace, inflammation, hypoxia, exercise
Citation: Li P, Wang Q, Feng L, Ding Z and Fan W (2024) Bibliometric and visualization analyses of cancer-related fatigue research published worldwide from 2001 to 2023. Front. Oncol. 14:1338325. doi: 10.3389/fonc.2024.1338325
Received: 29 November 2023; Accepted: 17 April 2024;
Published: 30 April 2024.
Edited by:
Radhika Gangaraju, University of Alabama at Birmingham, United StatesReviewed by:
Mellar Pilgrim Davis, Geisinger Health System, United StatesCopyright © 2024 Li, Wang, Feng, Ding and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Feng, ZmVuZ2xpNjYzQDEyNi5jb20=; Zhiguo Ding, ZGluZ3poaWd1b18xQDE2My5jb20=; Weijing Fan, ZmFuMTg4MTEwMjMyMDJAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.