- 1BASMA Pediatric Oncology Unit, Damascus, Syria
- 2Department of Oncology, Albairouni University Hospital, Faculty of Medicine, Damascus University, Damascus, Syria
- 3Faculty of Medicine, Damascus University, Damascus, Syria
- 4Department of Biochemistry and Microbiology, Faculty of Pharmacy, Damascus University, Damascus, Syria
- 5National Commission for Biotechnology (NCBT), Damascus, Syria
Introduction: Polymorphisms in NUDT15 may result in differences in mercaptopurine-induced toxicity. This study aimed to identify the frequency of the NUDT15 (c.415C>T; rs116855232) polymorphism and investigate the effect of this polymorphism on mercaptopurine-induced toxicity in a population of Syrian patients with childhood acute lymphoblastic leukemia (ALL).
Methods: This is a retrospective study that included children with ALL reaching at least 6 months of maintenance therapy. The NUDT15 genotyping was determined using standard targeted sequencing of polymerase chain reaction products. The odds ratio (OR) for the association between toxicity and genotype was evaluated.
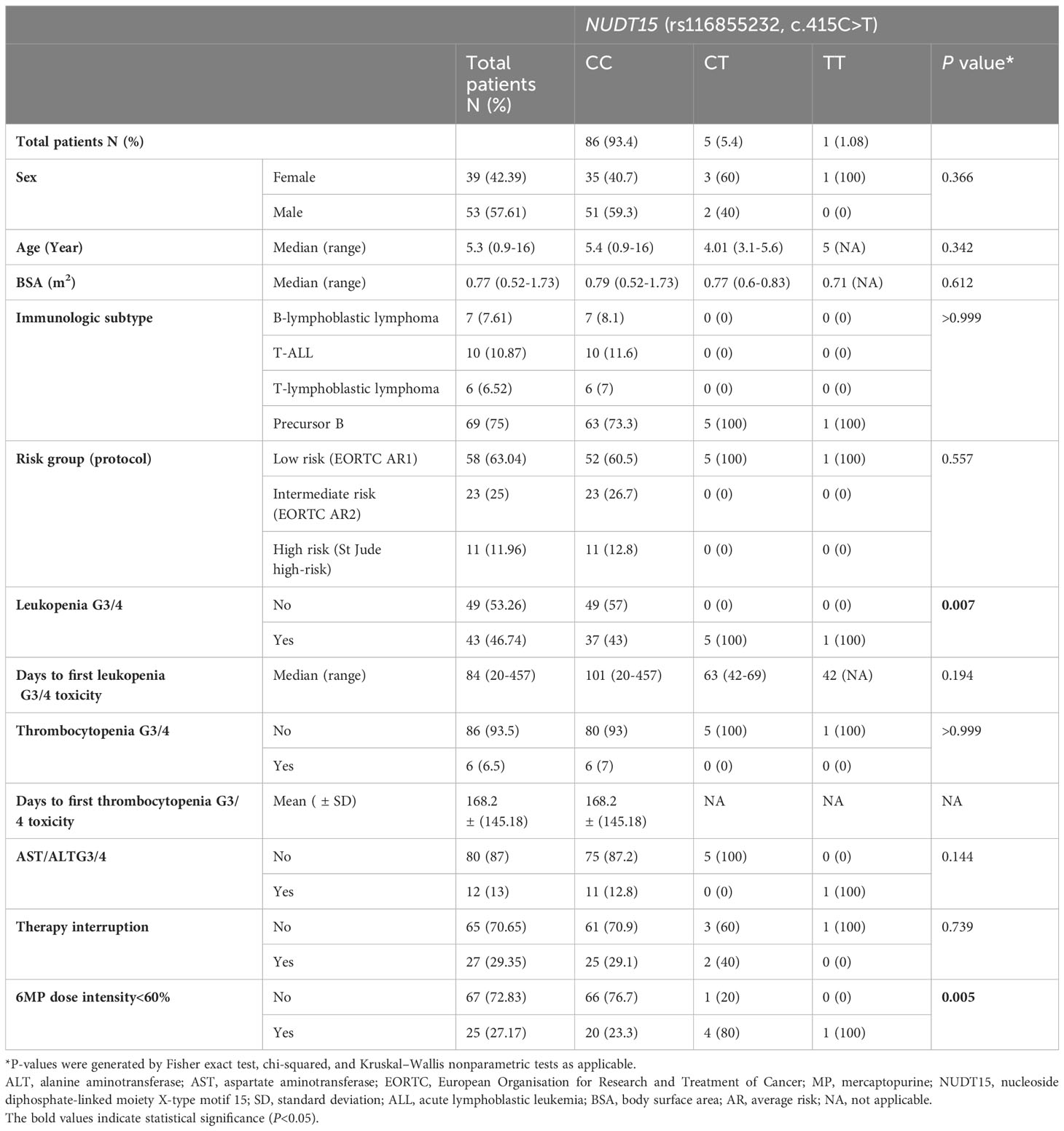
Results: A total of 92 patients were enrolled. The majority of the patients in the study population were low-risk (63.04%), followed by intermediate-risk (25%), and high-risk (11.96%). There were 5 patients (5.4%) with NUDT15 (c.415C>T; rs116855232) CT genotype, and 1 patient (1.08%) with NUDT15 TT genotype, with allele frequencies of C=0.962 and T=0.038. The mercaptopurine median dose intensity was 100%, 54.69%, and 5% for the genotypes CC, CT, and TT, respectively (P=0.009). Early onset leukopenia was significantly associated with the NUDT15 polymorphism (OR: 6.16, 95% CI: 1.11-34.18, P=0.037). There was no association between the NUDT15 genotype and hepatotoxicity.
Conclusion: Approximately 6.5% of the study population exhibited reduced NUDT15 activity. The mercaptopurine dose intensity was considerably low in NUDT15 rs116855232 TT genotype compared with CT and CC. The dosage of mercaptopurine should be adjusted according to the NUDT15 genotype in pediatric patients with ALL.
1 Introduction
Approximately one third of all cancers in children are acute lymphoblastic leukemia (ALL) (1–3), which can be cured by chemotherapy alone (4).
In pediatric patients with ALL, the antimetabolite prodrug 6-mercaptopurine (6MP) is a key chemotherapeutic agent for maintenance therapy. In addition, it is widely used in various phases of ALL treatment (5). 6MP needs to be metabolized to thioguanosine triphosphate (TGTP) which is incorporated into DNA to form a DNA-TG compound, causing futile DNA damage that leads to cell apoptosis (6, 7).
Specific polymorphisms in genes involved in the metabolism of thiopurine can directly affect toxicity and effectiveness. There is evidence that several single nucleotide polymorphisms (SNPs) can inhibit thiopurine metabolism enzyme activity, causing thioguanine nucleotide accumulation and hematologic toxicity. Nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) is a negative regulator nucleotide di-phosphatase that converts TGTP to thioguanosine monophosphate (TGMP), thereby inactivating thiopurine metabolites (8–11).
NUDT15 enzyme is encoded by the NUDT15 gene, which is located on chromosome 13q14.2 and contains three exons. NUDT15 is a 164 amino acid protein, a member of the Nudix hydrolase superfamily. The NUDT15 gene was previously sequenced in 6MP-treated patients to identify the reason for significant variability in tolerated dose in patients with similar clinical characteristics treated with the same treatment plan. More than 20 variants of the NUDT15 gene have been reported to date (12). Polymorphism in NUDT15 rs116855232 (c.415C>T), which is the core allele for NUDT15*3 leads to changing arginine 139 to cysteine (R139C). This change causes protein instability and decreases NUDT15 activity, leading to accumulation of thioguanine and increased 6MP-related toxicity (9, 13).
Previous studies concluded that the presence of NUDT15 rs116855232 polymorphism increased the chance of severe leukopenia related to 6MP treatment (14, 15). Therefore, the NUDT15 genotype can be used to guide the 6MP dose for ALL therapy. This illustrates how pharmacogenetics is driving precision medicine in cancer.
Syria is located on the eastern shore of the Mediterranean Sea in the Middle East. Syria’s location has led to the extensive admixture of its inhabitants with other populations, this has resulted in a complex ethnic, and genetic diversity. The allele frequency of NUDT15 variants differs between populations. It is 10% in East Asians, 7% in South Asians and Latinos and less than 1% in non-Finnish Europeans and Africans (16, 17). Many studies have examined the impact of NUDT15 allelic variants in East Asians and other ethnic groups, but they have been scarce in Arabs and the Middle East (18). This study aimed to investigate the frequency of the NUDT15 (c.415C>T; rs116855232) low-function variant in a population of Syrian children with ALL and to assess the association of this variant with 6MP-induced toxicity and tolerated dose.
2 Materials and methods
2.1 Ethics statement
The study protocol was approved by the Institutional Review Board of Damascus University, and the committee’s reference number was 514 on December 1, 2019. As per the ages and conceptual abilities of the patients, informed consent was obtained either from the patient’s parents or guardians or from the patients themselves.
2.2 Study design and patient eligibility
This is a retrospective cohort study performed at two recruitment sites; BASMA Pediatric Oncology Unit and Pediatric Department at Albairouni University Hospital. Both centers are located in Damascus, Syria. These two centers receive more than 50% of pediatric patients with ALL in Syria. The inclusion criteria were as follows: age less than 19 years, all pediatric patients with ALL who reached at least 6 months of maintenance therapy between January 2018 and May 2020. Patients with hepatic or renal failure, life-threatening diseases, or cancers other than ALL were excluded. A total of 92 patients met the inclusion criteria. Most patients were still receiving maintenance therapy when blood samples were collected, while the rest had finished treatment and were being monitored.
2.3 Treatment protocol and measurement of clinical factors
Patients with ALL are classified into three risk categories: Low-risk patients include patients aged from 1 to 9.9 years with B-cell precursor and white blood cell count less than 50 × 109/L and more than or equal to 1.16 DNA index, or TEL-AML1 fusion, and patients with bone marrow blasts less than 5% at days 19 or 26 or minimal residual disease (MRD) less than 0.01% at the end of induction. The intermediate-risk patients were aged more than or equal to 10 years with T cell or more than 50 × 109/L or B cell with CNS or testicular leukemia, less than 45 chromosomes (hypodiploid), MLL rearrangement, or E2A-PBX1 fusion or patient with bone marrow blasts ≥ 5 at days 19 or 26 or MRD < 1 ≥ 0.01% at the end of induction and patients that did not meet low- or high-risk criteria. The high-risk group includes those with t(9;22) BCR-ABL fusion or patients with induction failure (bone marrow blasts more or equal 5%) or MRD ≥1% at the end of induction or MRD ≥ 0.1% at week 7 (19).
Children with low and intermediate risk disease were treated using the European Organization for Research and Treatment of Cancer (EORTC) 58951, average risk-1 (AR1) and AR2 protocols, respectively (20). Children with high-risk disease were treated using the St Jude total XV protocol (21).
The standard 6MP oral dosage during the maintenance phase was 50 mg per m2 per day in the EORTC 58951 protocols. In the St Jude Total XV protocol, the standard 6MP was 50 mg per m2 per day during the first 20 weeks of maintenance, and 75 mg per m2 per day as of week 21 of maintenance.
Clinical history and examination, AEs according to the common terminology criteria for adverse events (CTCAEs) v5.0 (22), and blood tests for blood cell count (CBC), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were recorded at each visit. 6MP dose intensity, early-onset leukopenia, and treatment interruption due to chemotherapy toxicity were also collected.
As per the protocol, the 6MP dose intensity was determined by dividing the final tolerated 6MP dose by the prescribed 6MP maintenance dose. The 6MP dose was adjusted to maintain the white blood cell (WBC) above 2000 per μL (less than CTCAE G3) and the platelet count above 50.000 per μL (less than CTCAE G3). 6MP treatment interruption was defined as the cessation of chemotherapy administration resulting from infections with or without neutropenia. CTCAEs G3/4 were defined as severe toxicity at any time during maintenance treatment. When leukopenia occurs within 60 days of a maintenance therapy regimen, it is classified as early-onset leukopenia.
2.4 Genetic analyses
The total genomic DNA from peripheral blood was extracted using a blood DNA preparation kit (Jena Bioscience, Germany) as instructed by the manufacturer. DNA was stored at -20°C until sequencing. The total genomic DNA concentration was determined using a spectrophotometer (MAESTROGEN, MaestroNano, ProMN-913A, Taiwan). PCR and Sanger sequencing were used to determine the genotype of rs116855232. The sequences of the forward and reverse primers reported in previous publications were 5’-AAGCAAATGCAAAGCATCAC-3’ and 5’-GGCTGAAAGAGTGGGGGATA-3’, respectively (9). Each PCR amplification reaction contained approximately 20 ng DNA, 0.5 µM final concentration of each primer, 10 µl of direct PCR 2 X Master Mix (KAPABIOSYSTEMS, KAPA2G, KR0374-v11.23) and nuclease-free water to a final volume of 20 µl. The reactions were amplified using a DNA thermocycler (Labcycler SensoQuest, Germany). The PCR was performed under the following conditions: initial denaturation at 95°C for 5 minutes, 35 cycles of denaturation at 95°C for 30 seconds, annealing at 52°C for 30 seconds and extension at 72°C for 1 minute, followed by a final extension at 72°C for 10 minutes. An amplicon of 450 bp that contained the rs116855232 site was amplified. DNA extraction and PCR were performed at the Department of Pharmaceutical Biotechnology of the National Commission for Biotechnology, Damascus, Syria. The specimens were stored at -20°C until sequencing at Macrogen Inc. (Seoul, Republic of Korea). Sequencing of the PCR product allowed for the investigation of 4 adjacent polymorphisms to the target SNP (Table 1). The results were analyzed using Chromas 2.6 software (Technelysium, Australia) and verified by manual inspection of at least two individuals to ensure accuracy.

Table 1 NUDT15 variants investigated in 450 bp-amplicon PCR product throughout sequencing in 92 pediatric acute lymphoblastic leukemia cases.
2.5 Statistical analysis
For numeric variables, data were collected and summarized by descriptive statistics and provided as the mean and standard deviation (SD) for normally distributed data variables or as median and range for nonnormally distributed data variables. For nominal variables, data were summarized using descriptive statistics and presented as percentages and frequencies. The frequency of each genotype was calculated using the Hardy-Weinberg equation (HWE) and was determined using the chi-square test. The chi-squared or Fisher’s exact test was used to determine whether there is a statistical association between categorical variables, such as the frequency of dose reductions or interruptions, and SNPs. Genotypes of NUDT15 were used to categorize patients. For comparing 6MP dose intensity within NUDT15 genotype categories (independent samples), Kruskal–Wallis nonparametric tests were performed. The effect of rs116855232 on 6MP-induced toxicity was investigated by calculating the odds ratio (OR) and confidence interval (95% CI) by performing logistic regression analyses. SPSS (version 24) was used to perform all statistical analyses. Differences were considered statistically significant if the two-sided P<0.05.
3 Results
3.1 Patient characteristics and genetic polymorphisms
The inclusion criteria were met by 92 pediatric patients with ALL. The patients’ ages at diagnosis ranged from 0.9 to 16 years, and the median age was 5.3 years. Most patients were male (53 patients, 57.61%). Precursor B was the most common immunologic subtype (69 patients, 75%). Low risk was the most common risk group (58 patients, 63.04%). There are statistically significant differences between the NUDT15 genotypes with regards to leukopenia G3/4 [(37 patients (43%) for CC vs 5 patients (100%) for CT vs 1 patient (100%) for TT; P=0.007] and the dose intensity<60% [(20 patients (23.3%) for CC vs 4 patients (80%) for CT vs 1 patient (100%) for TT; P=0.005], but there are no differences between them in the other characters. The BASMA Pediatric Oncology Unit and Pediatric Department at Albairouni University Hospital receive more than 50% of children with cancer in Syria. Therefore, the patients who participated in this study came from all provinces around Syria. The demographics and clinical characteristics of the patients are summarized in Table 2. The patients in the study population were distributed according to the NUDT15 c.415C>T (rs116855232) among the majority of wild-type (CC) (86 patients, 93.4%), followed by heterozygous (CT) (5 patients, 5.4%), and a minority (1 patient, 1.08%) of homozygous for the variant (TT). The minor allele frequency (MAF) of NUDT15 c.415C>T (rs116855232) was 3.8% (Table 3). The frequency of the other investigated SNPs is zero.
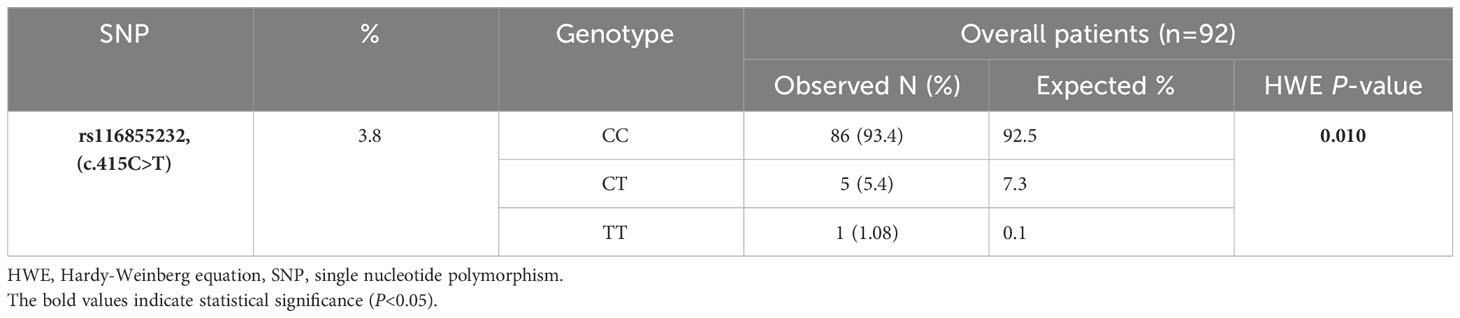
Table 3 Frequencies of the identified SNP, genotype data and results of Hardy-Weinberg Equilibrium (HWE) in 92 pediatric acute lymphoblastic leukemia cases.
A chi-square test of HWE was performed to compare the observed and predicted frequencies of the genotypes based on the investigated allelic frequency. The balance showed a deviation in the observed frequency of the genotypes of the NUDT15 c.415C>T (rs116855232) polymorphism from the expected frequency, as there was an increase in the frequency of the heterozygous genotype against a decrease in the frequency of the WT and homozygous genotypes (P=0.010) (Table 3).
3.2 Adverse events during maintenance therapy
At least one AE occurred in most patients (84 patients, 91.3%). Severe AE (G3/4) occurred in half of the patients, most of which were leukopenia (43 patients, 46.7%) and elevated liver enzymes (12 patients, 13%). Treatment was discontinued until toxicity improved in more than a quarter of patients (27 patients, 29.3%) (Table 4).
3.3 The relationship between NUDT15 genetic variants and 6MP dose intensity
The 6MP dose intensity was considerably lower in the TT genotype than in the CT and CC genotypes (P=0.009) (Table 5, Figure 1).
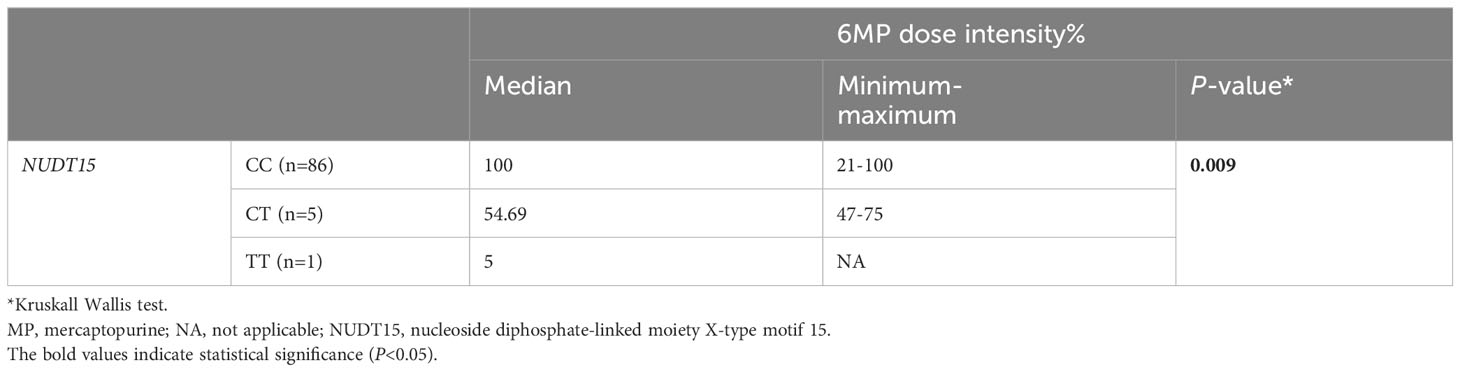
Table 5 Comparison of 6MP dose intensity % by NUDT15 genotype in 92 pediatric acute lymphoblastic leukemia cases.
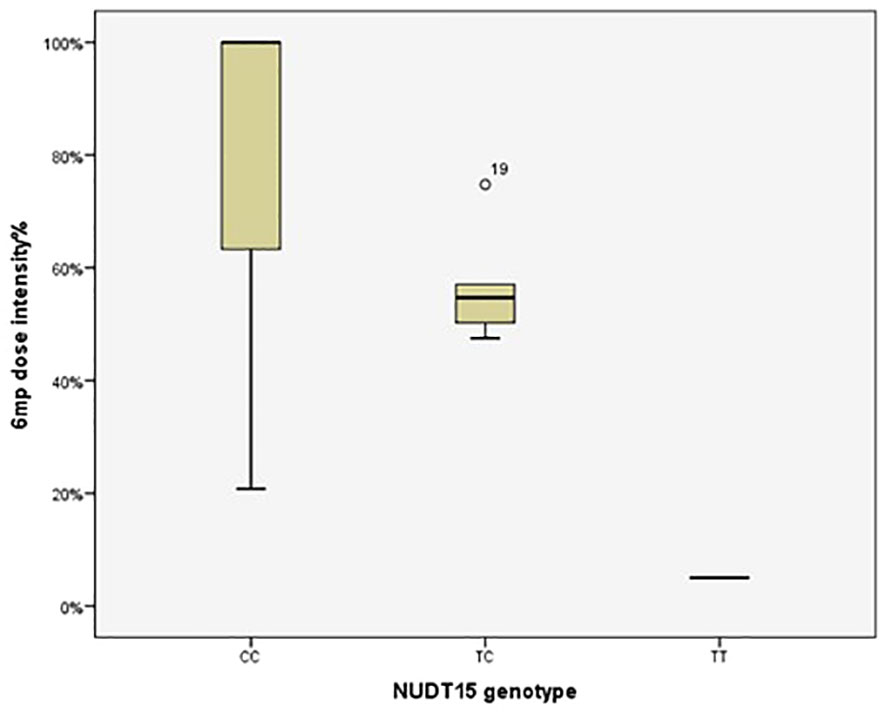
Figure 1 Therapeutic dose of 6-mercaptopurine during maintenance therapy according to NUDT15 rs116855232 genotype in 92 pediatric acute lymphoblastic leukemia cases. The median 6MP dose intensity was 5%, 54.69% and 100% for the genotypes TT (n=1), CT (n=5) and CC (n=86), respectively (P=0.009). P value was estimated by the Kruskal–Wallis test.
3.4 Association between NUDT15 variants and 6MP toxicity
Early onset leukopenia was observed in 15 patients (16.3%). Leukopenia G3/4 (<2000/mm3) at any stage of maintenance therapy was noticed in 43 patients (46.7%). NUDT15 T allele carriers (CT and TT combined) had a 6.16-fold increased risk of early-onset leukopenia G3/4 compared to patients with WT (CC) (P=0.037) (Table 6).
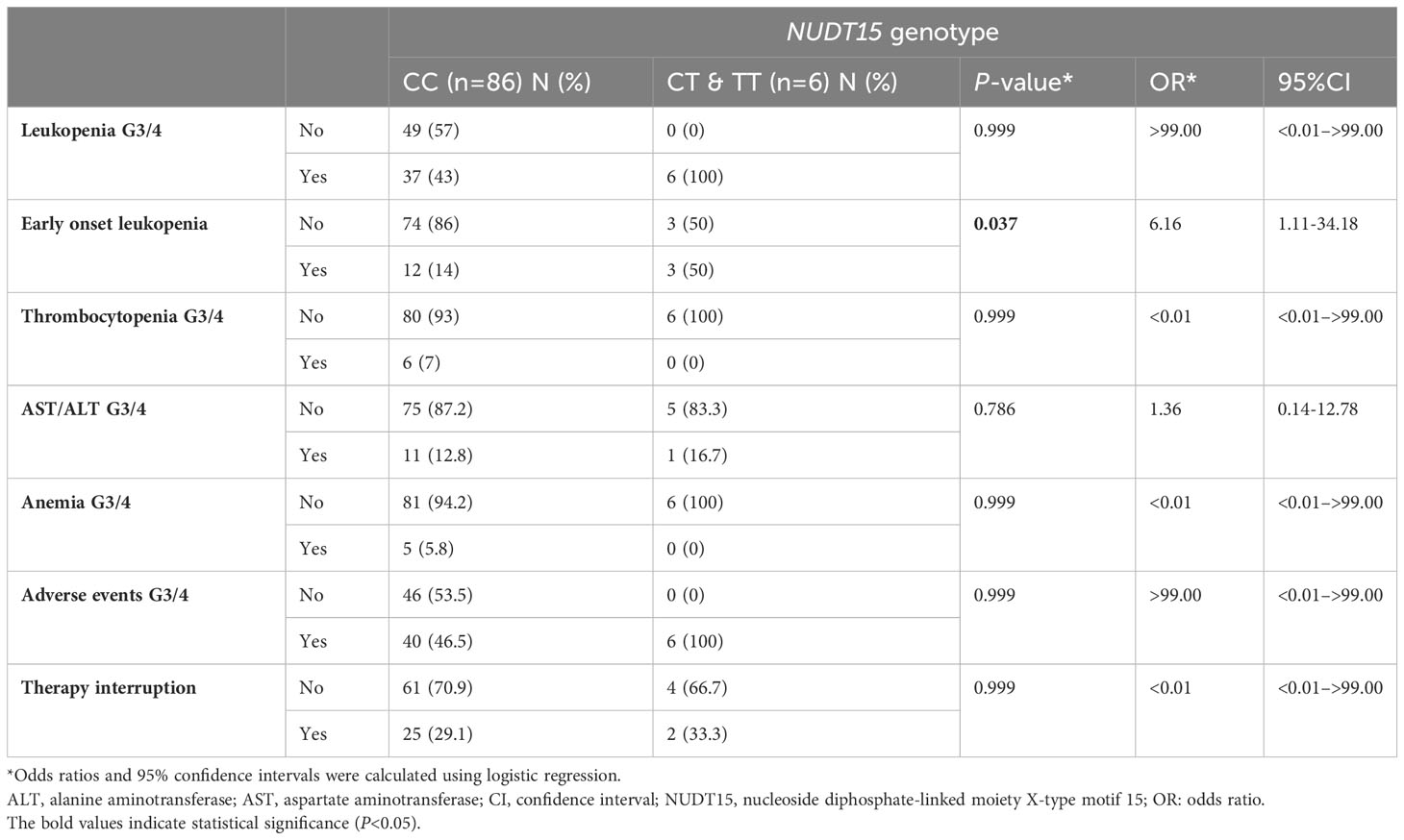
Table 6 Associations between NUDT15 genotype and risk of severe adverse events and therapy interruption in 92 pediatric acute lymphoblastic leukemia cases.
In contrast, there was no statistically significant association between the genetic variants and other 6MP-induced severe toxicity (Table 6).
Twenty-seven patients (29.35%) needed 6MP interruption due to infections but there was no significant association between the genetic variants and 6MP interruptions due to infections (P=0.999) (Table 6).
4 Discussion
This study assessed the association of the NUDT15 (c.415C>T; rs116855232) polymorphism with 6MP dose intolerance in Syrian patients with childhood ALL. We have shown that the evaluated SNP is not common in this population, and that the 6MP dose intensity was considerably lower in the TT genotype than in the CT and CC genotypes.
Pharmacogenetics has contributed to explaining the molecular mechanisms that lead to the interindividual variation in drug response. Thus, it is widely considered as an essential part of personalized medicine. Pharmacogenetics provides a significant benefit in hematology-oncology because of the narrow therapeutic index of chemotherapy drugs and the high incidence of life-threatening AEs. 6MP is the backbone of maintenance chemotherapy in ALL protocols in children. The NUDT15 polymorphism was confirmed to be associated with 6MP toxicity in multiple populations (13). A few studies have investigated this association in Middle Eastern or Arab children with ALL (10, 23).
Accordingly, the Clinical Pharmacogenetics Implementation Consortium (CPIC) is considering integrating NUDT15 preemptive genotyping into its current recommendations on thiopurine dosing. Depending on each individual’s NUDT15 allele combination, NUDT15 genotypes are classified into three distinct phenotypes. These are poor metabolizers (PMs), intermediate metabolizers (IMs), and normal metabolizers (NMs). The recent CPIC Guidelines for thiopurine categorize an individual harboring one normal function allele plus one NUDT15*3 allele as an IM, while an individual harboring two no-function alleles as PM (24).
The minor allele frequency (MAF) of the NUDT15 rs116855232 genotype was 3.8% in our population, which is higher than the MAF in the neighboring countries, as it was 0.4%, 0.6%, 1.8%, and 0% in Lebanese, Jordanian, Saudi, and Iranian Kurdish populations, respectively (10, 23, 25, 26). The differences in the frequencies of NUDT15 polymorphism between the neighboring populations could be attributed to demographic or genetic diversity. Moreover, allele frequency varies among other ethnic groups from 2 to 16%. The frequency of NUDT15 polymorphism in Chinese patients with ALL is 15.7%, 16% in Japanese, and 11.6% in Taiwan Chinese patients (13, 27, 28). In other populations, the risk allele is less common, occurring at 2% in an admixed American (8), 8.8% in Uruguayan (29), and 5% in Thais (30).
The tolerated 6MP dose in our cohort was 54.69% of the planned dose when there was a NUDT15 heterozygous (CT) genotype, which is similar to the tolerated dose in the St Jude cohort (63%) (11), lower than Chinese (83.83%) and Japanese (73.2%) (27, 28) but higher than the tolerated dose in the Lebanese cohort (33.3%) and Thai (48.8%) (10, 30). The high percentage of our population compared with the neighboring countries can be explained by the low frequency of the variant in the Lebanese cohort. There was no CT or TT genotypes in the Kurdistan cohort. In case of the homozygous (TT) genotype, the tolerated 6MP dose in our cohort was significantly low (5%), which is close to the tolerated dose in the St Jude study (8.3%) (11) but significantly lower than the tolerated dose in Chinese (60.27%), Japanese (20%), and Thai (16.6%) populations (27, 28, 30), and there was no TT genotype in the Lebanese cohort. Differences between tolerated doses between different populations could be explained by the presence of other genetic/environmental factors that might affect the absorption, metabolism, or excretion of 6MP.
Although the NUDT15 c.415C>T polymorphism is a risk factor for early onset leukopenia during maintenance therapy for children with ALL (31, 32), NUDT15 polymorphism was not associated with hepatotoxicity in our cohort; which is similar to the studies by Tanaka et al. (27), Zhou et al. (28) and Moradveisi et al. (23) in a Japanese, Chinese, and Middle Eastern cohorts, respectively. Hepatotoxicity might have other factors that play a role in cooperation with NUDT15.
Studying the frequency of polymorphisms of the NUDT15 not only helps in the dosing of 6MP in patients with ALL, but it may also give an idea of its frequency in patients with inflammatory bowel diseases (IBD) and rheumatoid arthritis to avoid thiopurine-induced toxicity. However, Studies on patients with the other diseases are needed to determine the exact frequency and impact.
There are some limitations to our study. First, due to the small sample size (n=92) and retrospective design of this study, the power to detect differences between small groups is low. Second, the treatment protocols used were not homogenous, which might affect other factors linked to the same toxicity output we looked for. Third, the present study examined the most clinically relevant allele of NUDT15 and did not investigate all variants. Furthermore, according to the US Food and Drug Administration-approved label for 6MP, patients with severe myeloid toxicity are advised to be tested for TPMT or NUDT15 deficiency, and PMs for TPMT or NUDT15 are recommended to reduce the 6MP dose (33); this study did not examine other genes encoding enzymes of 6MP metabolism, such as TPMT and inosine triphosphate pyrophosphatase (ITPA). The limitations of our study are partially related to the limited resources and financial support available in our low-income country (Syria) and because of this, we did not examine a larger sample size or examine another gene involved in 6MP metabolism. Therefore, these results should be cautiously interpreted.
In conclusion, our study found that the NUDT15 polymorphism (c.415C>T; rs116855232) is associated with mercaptopurine-induced early-onset leukopenia but not with hepatotoxicity in a population of Syrian children with ALL. It appears that NUDT15 polymorphism (c.415C>T; rs116855232) is slightly more frequent in our population than in neighboring countries. The dosage of mercaptopurine should be adjusted based on the genotype of NUDT15 in patients with ALL.
Data availability statement
The datasets presented in this article are not readily available because of ethical approval restrictions, this article’s genomic sequencing data cannot be deposited in a public database. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The study protocol was approved by the Institutional Review Board of Damascus University, and the committee’s reference number was 514 on December 1, 2019. All procedures involving human participants were performed under the ethical standards of the institutional and/or national research committee and in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. As per the ages and conceptual abilities of the patients, informed consent was obtained either from the patient's parents or guardians or from the patients themselves.
Author contributions
MM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. MS: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing. MaA: Conceptualization, Methodology, Resources, Software, Supervision, Validation, Writing – review & editing. MoA: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. KG: Conceptualization, Data curation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Damascus University-funder No. 501100020595, and the Oubari Habboush Pharma Company. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Acknowledgments
Our sincere gratitude goes out to the National Commission for Biotechnology for hosting us in its laboratories.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALL, acute lymphoblastic leukemia; AEs, adverse effects; AR, average risk; AS, activity score; CI, confidence interval; CPIC, Clinical Pharmacogenetics Implementation Consortium; CTCAE, common terminology criteria for adverse events; EORTC, European Organisation for Research and Treatment of Cancer; HWE, Hardy-Weinberg equation; IM, intermediate metabolizer; ITPA: Inosine triphosphate pyrophosphatase; MRD, minimal residual disease; 6MP, 6-mercaptopurine; NUDT15, nucleoside diphosphate-linked moiety X-type motif 15; NM, normal metabolizer; OR, odds ratio; PharmGKB: Pharmacogenomics Knowledge Base; PharmVar, Pharmacogene Variation; PM, poor metabolizer; PCR, polymerase chain reaction; SNP, single-nucleotide polymorphism; TGTP, thioguanosine triphosphate; TGMP, thioguanosine monophosphate; WT, wild type. WBC, white blood cell.
References
1. Hunger SP, Loh ML, Whitlock JA, Winick NJ, Carroll WL, Devidas M, Raetz EA, et al. Children's oncology group's 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. (2013) 60(6):957–63. doi: 10.1002/pbc.24420
2. Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J Clin Oncol. (2011) 29:551–65. doi: 10.1200/JCO.2010.30.7405
3. Lopez-Lopez E, Gutierrez-Camino A, Bilbao-Aldaiturriaga N, Pombar-Gomez M, Martin-Guerrero I, Garcia-Orad A. Pharmacogenetics of childhood acute lymphoblastic leukemia. Pharmacogenomics. (2014) 15:1383–98. doi: 10.2217/pgs.14.106
4. Narayanan S, Shami PJ. Treatment of acute lymphoblastic leukemia in adults. Crit Rev Oncol Hematol. (2012) 81:94–102. doi: 10.1016/j.critrevonc.2011.01.014
5. Yi ES, Choi YB, Choi R, Lee NH, Lee JW, Yoo KH, et al. NUDT15 variants cause hematopoietic toxicity with low 6-TGN levels in children with acute lymphoblastic leukemia. Cancer Res Treat. (2018) 50:872–82. doi: 10.4143/crt.2017.283
6. Moriyama T, Yang YL, Nishii R, Ariffin H, Liu C, Lin TN, et al. Novel variants in NUDT15 and thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry. Blood. (2017) 130:1209–12. doi: 10.1182/blood-2017-05-782383
7. Dean L, Kane M. Mercaptopurine Therapy and TPMT and NUDT15 Genotype. Bethesda (MD: National Center for Biotechnology Information (US (2012). Available at: http://europepmc.org/abstract/MED/28520348.
8. Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. (2014) 46:1017–20. doi: 10.1038/ng.3060
9. Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet [Internet]. (2016) 48:367–73. doi: 10.1038/ng.3508
10. Zgheib NK, Akika R, Mahfouz R, Aridi C, Ghanem KM, Saab R, et al. NUDT15 and TPMT genetic polymorphisms are related to 6-mercaptopurine intolerance in children treated for acute lymphoblastic leukemia at the Children’s Cancer Center of Lebanon. Pediatr Blood Cancer. (2017) 64:146–50. doi: 10.1002/pbc.26189
11. Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. (2015) 33:1235–42. doi: 10.1200/JCO.2014.59.4671
12. PharmVar. (2023). Available online at: https://www.pharmvar.org/gene/NUDT15.
13. Yin D, Xia X, Zhang J, Zhang S, Liao F, Zhang G, et al. Impact of NUDT15 polymorphisms on thiopurines-induced myelotoxicity and thiopurines tolerance dose. Oncotarget. (2017) 8:13575–85. doi: 10.18632/oncotarget.14594
14. Tatsumi G, Kawahara M, Imai T, Nishishita-Asai A, Nishida A, Inatomi O, et al. Thiopurine-mediated impairment of hematopoietic stem and leukemia cells in Nudt15 R138C knock-in mice. Leukemia. (2020) 34:882–94. doi: 10.1038/s41375-019-0583-9
15. Kakuta Y, Kawai Y, Okamoto D, Takagawa T, Ikeya K, Sakuraba H, et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. (2018) 53:1065–78. doi: 10.1007/s00535-018-1486-7
16. Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. (2016) 536(7616):285–91. doi: 10.1038/nature19057
17. Ban H, Andoh A, Tanaka A, Tsujikawa T, Sasaki M, Saito Y, et al. Analysis of thiopurine S-methyltransferase genotypes in Japanese patients with inflammatory bowel disease. Internal Med. (2008) 47:1645–8. doi: 10.2169/internalmedicine.47.1268
18. NUDT15 (2023). Available online at: https://www.pharmgkb.org/gene/PA134963132/variantAnnotation.
19. Omar AA, Basiouny L, Elnoby AS, Zaki A, Abouzid M. St. Jude Total Therapy studies from I to XVII for childhood acute lymphoblastic leukemia: a brief review. J Egypt Natl Canc Inst. (2022) 34:25. doi: 10.1186/s43046-022-00126-3
20. Mondelaers V, Suciu S, De Moerloose B, Ferster A, Mazingue F, Plat G, et al. Prolonged versus standard native E. coli asparaginase therapy in childhood acute lymphoblastic leukemia and non-Hodgkin lymphoma: Final results of the EORTC-CLG randomized phase III trial 58951. Haematologica. (2017) 102:1727–38. doi: 10.3324/haematol.2017.165845
21. Muwakkit S, Al-Aridi C, Samra A, Saab R, Mahfouz RA, Farra C, et al. Implementation of an intensive risk-stratified treatment protocol for children and adolescents with acute lymphoblastic leukemia in Lebanon. Am J Hematol. (2012) 87:678–83. doi: 10.1002/ajh.23222
22. Common Terminology Criteria for Adverse Events (CTCAE). In: Protocol Development. CTEP. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
23. Moradveisi B, Muwakkit S, Zamani F, Ghaderi E, Mohammadi E, Zgheib NK. ITPA, TPMT, and NUDT15 genetic polymorphisms predict 6-mercaptopurine toxicity in Middle Eastern children with acute lymphoblastic leukemia. Front Pharmacol. (2019) 10. doi: 10.3389/fphar.2019.00916
24. Relling MV, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui CH, Stein CM, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. (2019) 105:1095–105. doi: 10.1002/cpt.1304
25. Jarrar YB, Ghishan M. The nudix hydrolase 15 (NUDT15) gene variants among Jordanian Arab population. Asian Pacific J Cancer Prev. (2019) 20:801–8. doi: 10.31557/APJCP.2019.20.3.801
26. Goljan E, Abouelhoda M, ElKalioby MM, Jabaan A, Alghithi N, Meyer BF, et al. Identification of pharmacogenetic variants from large scale next generation sequencing data in the Saudi population. PloS One. (2022) 17. doi: 10.1371/journal.pone.0263137
27. Tanaka Y, Kato M, Hasegawa D, Urayama KY, Nakadate H, Kondoh K, et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol. (2015) 171:109–15. doi: 10.1111/bjh.13518
28. Zhou H, Li L, Yang P, Yang L, Zheng JE, Zhou Y, et al. Optimal predictor for 6-mercaptopurine intolerance in Chinese children with acute lymphoblastic leukemia: NUDT15, TPMT, or ITPA genetic variants? BMC Cancer. (2018) 18:516. doi: 10.1186/s12885-018-4398-2
29. Soler AM, Olano N, Méndez Y, Lopes A, Silveira A, Dabezies A, et al. TPMT and NUDT15 genes are both related to mercaptopurine intolerance in acute lymphoblastic leukaemia patients from Uruguay. Br J Haematol. (2018) 181:252–5. doi: 10.1111/bjh.14532
30. Puangpetch A, Tiyasirichokchai R, Pakakasama S, Wiwattanakul S, Anurathapan U, Hongeng S, et al. NUDT15 genetic variants are related to thiopurine-induced neutropenia in Thai children with acute lymphoblastic leukemia. Pharmacogenomics. (2020) 21:403–10. doi: 10.2217/pgs-2019-0177
31. Sutiman N, Chen S, Ling KL, Chuah SW, Leong WF, Nadiger V, et al. Predictive role of NUDT15 variants on thiopurine-induced myelotoxicity in Asian inflammatory bowel disease patients. Pharmacogenomics. (2018) 19:31–43. doi: 10.2217/pgs-2017-0147
32. Khaeso K, Udayachalerm S, Komvilaisak P, Chainansamit SO, Suwannaying K, Laoaroon N, et al. Meta-analysis of NUDT15 genetic polymorphism on thiopurine-induced myelosuppression in asian populations. Front Pharmacol. (2021) 12. doi: 10.3389/fphar.2021.784712
33. Table of Pharmacogenetic Associations. (2022). FDA. Available online at: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations.
Keywords: NUDT15, genotype, acute lymphoblastic leukemia, mercaptopurine, toxicity, Syria, pediatric, pharmacogenetics
Citation: Muhammad M, Saifo M, Aljamali M, Alali M and Ghanem KM (2024) The frequency of NUDT15 rs116855232 and its impact on mercaptopurine-induced toxicity in Syrian children with acute lymphoblastic leukemia. Front. Oncol. 14:1334846. doi: 10.3389/fonc.2024.1334846
Received: 07 November 2023; Accepted: 27 February 2024;
Published: 18 March 2024.
Edited by:
Antonio Ruggiero, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Azza Mahmoud Kamel, Cairo University, EgyptLucia Guadalupe Taja Chayeb, National Institute of Cancerology (INCAN), Mexico
Copyright © 2024 Muhammad, Saifo, Aljamali, Alali and Ghanem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Muhammad, bXVoMTk4Ny5tdWhhbWRAZGFtYXNjdXN1bml2ZXJzaXR5LmVkdS5zeQ==; bW10aWdlcjE5ODdAZ21haWwuY29t
 Muhammad Muhammad
Muhammad Muhammad Maher Saifo2,3
Maher Saifo2,3 Mousa Alali
Mousa Alali Khaled M. Ghanem
Khaled M. Ghanem