- 1Qi-Huang Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2College of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun, China
- 3Department of Endocrinology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: The prognostic value of lymph node ratio (LNR) has been proved in several cancers. However, the potential of LNR to be a prognostic factor for thyroid cancer has not been validated so far. This article evaluated the prognostic value of LNR for thyroid cancer through a meta-analysis.
Methods: A systematic search was conducted for eligible publications that study the prognostic values of LNR for thyroid cancer in the databases of PubMed, EMBASE, Cochrane, and Web of Science up until October 24, 2023. The quality of the eligible studies was evaluated by The Newcastle-Ottawa Assessment Scale of Cohort Study. The effect measure for meta-analysis was Hazard Ratio (HR). Random effect model was used to calculate the pooled HR and 95% confidence intervals. A sensitivity analysis was applied to assess the stability of the results. Subgroup analysis and a meta-regression were performed to explore the source of heterogeneity. And a funnel plot, Begg’s and Egger’s tests were used to evaluate publication bias.
Results: A total of 15,698 patients with thyroid cancer from 24 eligible studies whose quality were relatively high were included. The pooled HR was 4.74 (95% CI:3.67-6.11; P<0.05) and a moderate heterogeneity was shown (I2 = 40.8%). The results of meta-analysis were stable according to the sensitivity analysis. Similar outcome were shown in subgroup analysis that higher LNR was associated with poorer disease-free survival (DFS). Results from meta-regression indicated that a combination of 5 factors including country, treatment, type of thyroid cancer, year and whether studies control factors in design or analysis were the origin of heterogeneity.
Conclusion: Higher LNR was correlated to poorer disease free survival in thyroid cancer. LNR could be a potential prognostic indicator for thyroid cancer. More effort should be made to assess the potential of LNR to be included in the risk stratification systems for thyroid cancer.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=477135, identifier CRD42023477135.
1 Introduction
Thyroid cancer is one of the most common cancers worldwide according to the GLOBOCAN 2020 database of cancer incidence and mortality by the World Health Organization (1). In the past decades, the incidence of thyroid cancer has been increased globally (2–4). And by the year 2030, thyroid cancer will be likely to become the second most common cancer in female and the ninth most common cancer in male (1, 5–7). The three broad histological categories of thyroid cancer are differentiated thyroid cancer, medullary thyroid cancer and anaplastic thyroid cancer, of which papillary thyroid cancer, a subtype of differentiated thyroid cancer, accounts for nearly 85% of patients (8–10). The treatment for thyroid cancer is diverse and systematic, including minimally invasive interventions, surgery, radioactive iodine, oral multi-targeted tyrosine kinase inhibitors and so on (10–14). Although advances in treatment have been made in recent years, surgery remains to be the major and initial treatment in patients with differentiated and medullary thyroid cancer (9, 10).
The survival rate of thyroid cancer tend to be relatively high comparing with other types of cancers. According to the data from Surveillance, Epidemiology, and End Results(SEER) database, the 5-year relative survival of patients with thyroid cancer was 98.5% from 2013 to 2019 (15). Despite a high survival rate, postoperative recurrence is an emerging problem during thyroid cancer treatment[9]. Study results shows that 5%-20% of patients with differentiated thyroid cancer suffers from local or regional recurrence, and about 10%-15% of patients develop distant metastases (16–19). Thus, exploring reliable predictors for recurrence of thyroid cancer becomes an emerging issue.
Cumulative evidence suggests that lymph node status is a key prognostic factor in thyroid cancer (20–22). Currently, 2015 American Thyroid Association (ATA) Management Guidelines has recommended the number of involved lymph nodes to be a prognostic factor of thyroid cancer (9). However, studies have already shown that the number of metastasis lymph nodes is likely to be affected by surgical factors especially the number of lymph nodes dissected since inadequate lymph node dissection, which sometimes happens in surgery, may possibly make the number of metastasis lymph nodes lower than actual amount (23, 24). A study have even mentioned that the small number of lymph nodes removed increases the risk of recurrence in thyroid cancer (25). Therefore, the prognostic accuracy of the number of lymph nodes examined remains to be imperfect (25, 26).
Lymph node ratio (LNR) is an indicator defined as the number of metastatic lymph nodes divided by the number of lymph nodes checked. Differing from the number of metastasis lymph nodes, LNR takes the influence of both number of metastasis lymph nodes and number of lymph nodes examined into account. Thus, it is regarded as a prognostic factor in several cancers including gastric, breast, and colorectal cancers (27–29). Nevertheless, the prognostic value of LNR for thyroid cancer still remains to be controversial. Conflicting results may arise from differences in study design and sample size. Although systematic review has reported the prognostic significance of LNR for thyroid cancer, there has been no formal meta-analysis so far (30). Thus, we conducted the first comprehensive meta-analysis based on Hazard Ratios to investigate the prognostic value of LNR for thyroid cancer, and attempt to discuss the best cut-off value of LNR.
2 Materials and methods
2.1 Study selection
Inclusion criteria were defined as follows: (1)study design: retrospective and prospective cohort study reported the prognostic value of LNR for thyroid cancer (2)study content: the relationship between LNR and disease-free survival (DFS) (3)participants: patients with thyroid cancer who were standardized diagnosed and underwent thyroid surgery (4) outcome: disease-free survival (DFS) and other necessary survival data including Hazard ratios (HRs) and 95% confidence intervals (CIs) which could be extracted from the original literature directly or indirectly.
The exclusion criteria were as follows: (1) case reports, reviews, letters, conference records (2) studies conducted on animals (3) studies without necessary survival data for statistical analysis (4) studies which were based on non-original data (5) studies analyzed data from the same population.
Eligibility assessment was performed in a blinded standardized manner by 2 independent reviewers, and disagreement between reviewers were solved by consensus.
2.2 Search strategy
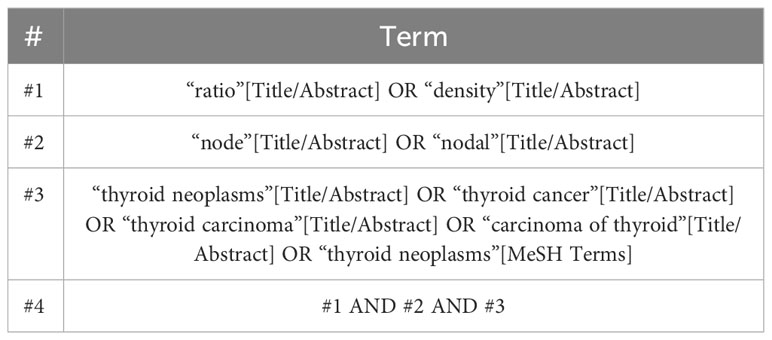
A systematic search was performed using databases including PubMed, Embase, Cochrane, and Web of Science in order to identify relevant studies from inception to October 24, 2023. Relevant medical subject heading terms, key words or word variants for “thyroid cancer” and “Lymph node ratio” were used as search items and a detailed search strategy was shown in Table 1. The guidelines for Preferred Reporting Items for Systemic Reviews and Meta-Analyses(PRISMA) were followed throughout the whole study.
2.3 Data extraction
Data extracted from eligible studies consisted of basic information including first author name, country where the study was conducted and year of publication, population-specific information including number of patients, age, gender, and study-specific details including duration of follow-up, treatment for patients, tumor size, TN staging of cancer, number of checked nodes, number of positive nodes, cut-off value of LNR estimated and selected using Receiver Operating Characteristic curve based on the data from each study by investigators, HRs and 95% CIs. Two independent reviewers extracted data using a standardized data extraction form, and any discrepancy between the reviewers was resolved by consensus.
2.4 Quality assessment
The Newcastle-Ottawa Assessment Scale of Cohort Study was applied to the quality assessment of eligible studies. This tool evaluates the quality of studies according to 9 items across 3 dimensions: selection, comparability and outcome. A study can be awarded a maximum of one score for each numbered item within the selection and outcome categories and a maximum of two scores can be given for comparability category. 2 reviewers assessed the quality of included studies independently, and any discrepancy between the reviewers was resolved by consensus.
2.5 Data synthesis and statistical analysis
This meta-analysis was performed using pooled HRs and 95% CIs to evaluate the prognostic value of LNR for thyroid cancer, and results were presented using forest plots. Missing hazard ratios would be approximated using the method from Guyot et al. if Kaplan Meier curves were provided (31). A random-effects model was applied rather than a fixed-effects model to determine the robustness of the results. Heterogeneity between studies was calculated using I2 statistics which indicated the percentage of heterogeneity that is beyond chance (I2 < 25%, low heterogeneity; I2 = 25-50%, moderate heterogeneity; and I2 > 50%, strong heterogeneity) (32). A sensitivity analysis was conducted to assess the stability and robustness of the results by omitting every individual study and calculating new HRs. Moreover, we performed subgroup analysis on the basis of histological types of thyroid cancer, treatment, location, ranges of the cut-off value of LNR, whether studies control factors in design or analysis to ensure the comparability of cohorts and whether cases with extra thyroid extension (ETE) were excluded. And a meta-regression which is a joint test for all covariates was conducted to assess the potential impact of multiple factors on heterogeneity. Finally, we used a funnel plot, Begg’s test and Egger’s test to evaluate the publication bias of included studies.
All statistic analysis were performed using STATA 11.0 software. And results were considered significant statistically if the p value was less than 0.05. The protocol of this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) system (ID: 42023477135).
3 Results
3.1 Study selection, characteristics, and quality assessment
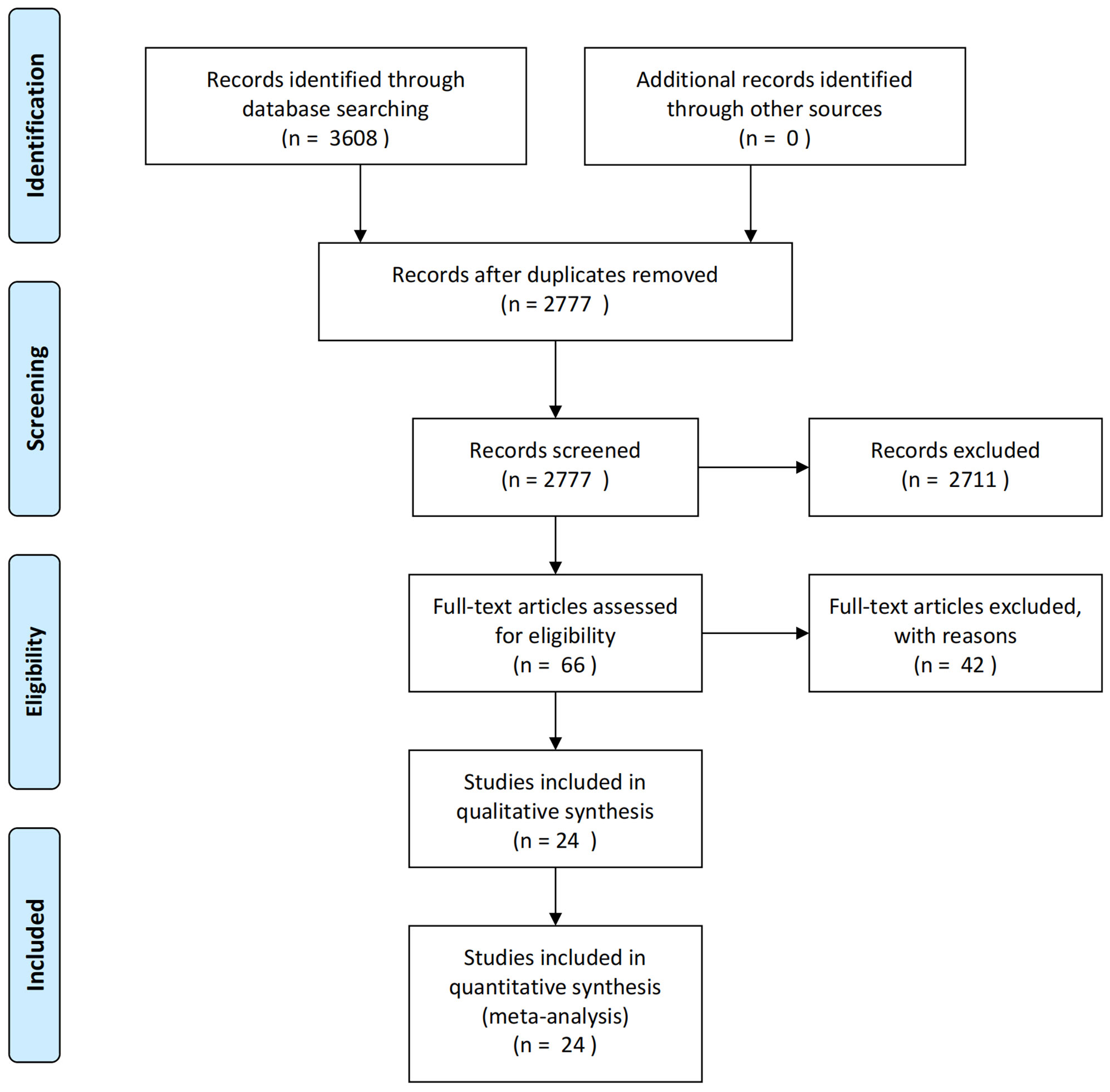
3608 articles were yielded by database searching. After reduplicates removed, 2777 articles remained for records screening. Eventually, 24 eligible articles were included after screening full texts of 66 articles according to the inclusion and exclusion criteria. And a flow diagram for study selection is shown in Figure 1.
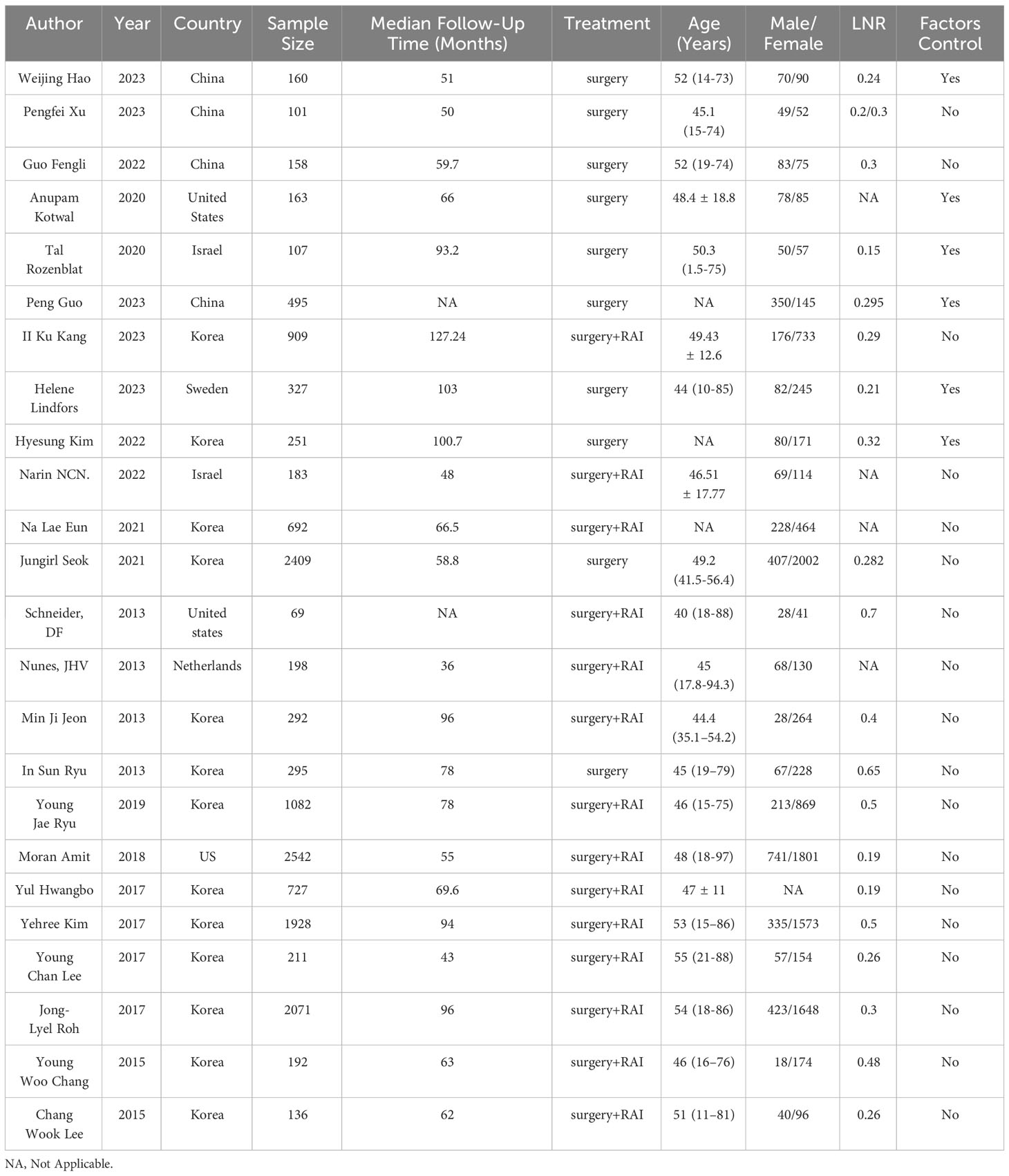
All eligible studies were retrospective cohort studies, with 13 studies reported data from Korea, 4 studies from China, 2 from the United States, 2 from Israel, and 1 from Netherlands. Among these studies, a total of 15,698 patients were included, and the median age of patients ranged from 40 to 55 years old. All patients underwent radical surgery for thyroid cancer, and available median follow-up time of studies ranged from 43 to 127.24 months. The cut-off values of LNR in included studies were diverse, and only 3 studies reported 0.3 as a common cut-off values of LNR. Main characteristics of included studies were summarized in Table 2.
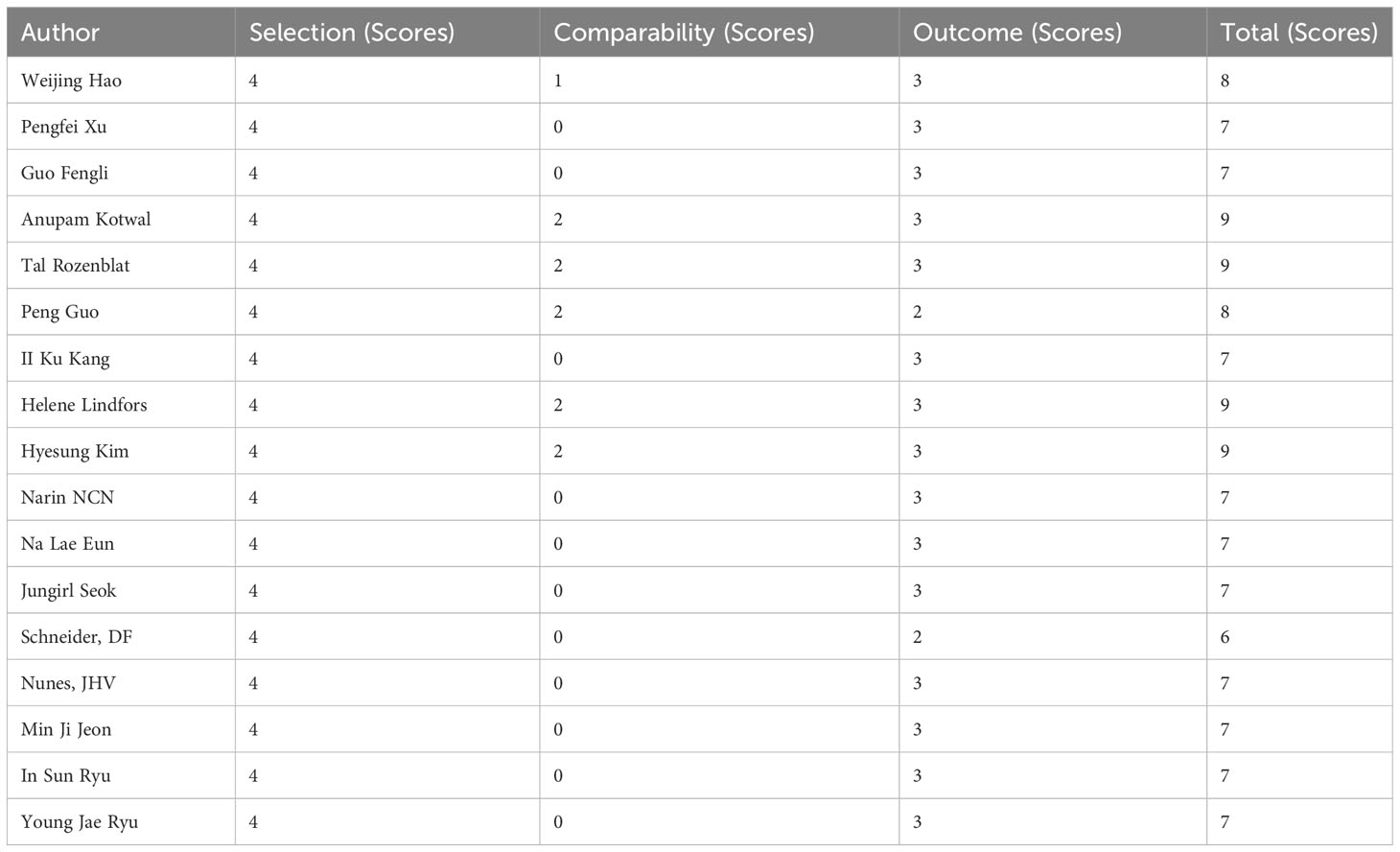
All included studies got no less than 6 scores in Newcastle-Ottawa scale and 4 studies got full score, indicating relatively high quality of eligible studies (Table 3).
3.2 Meta-analysis
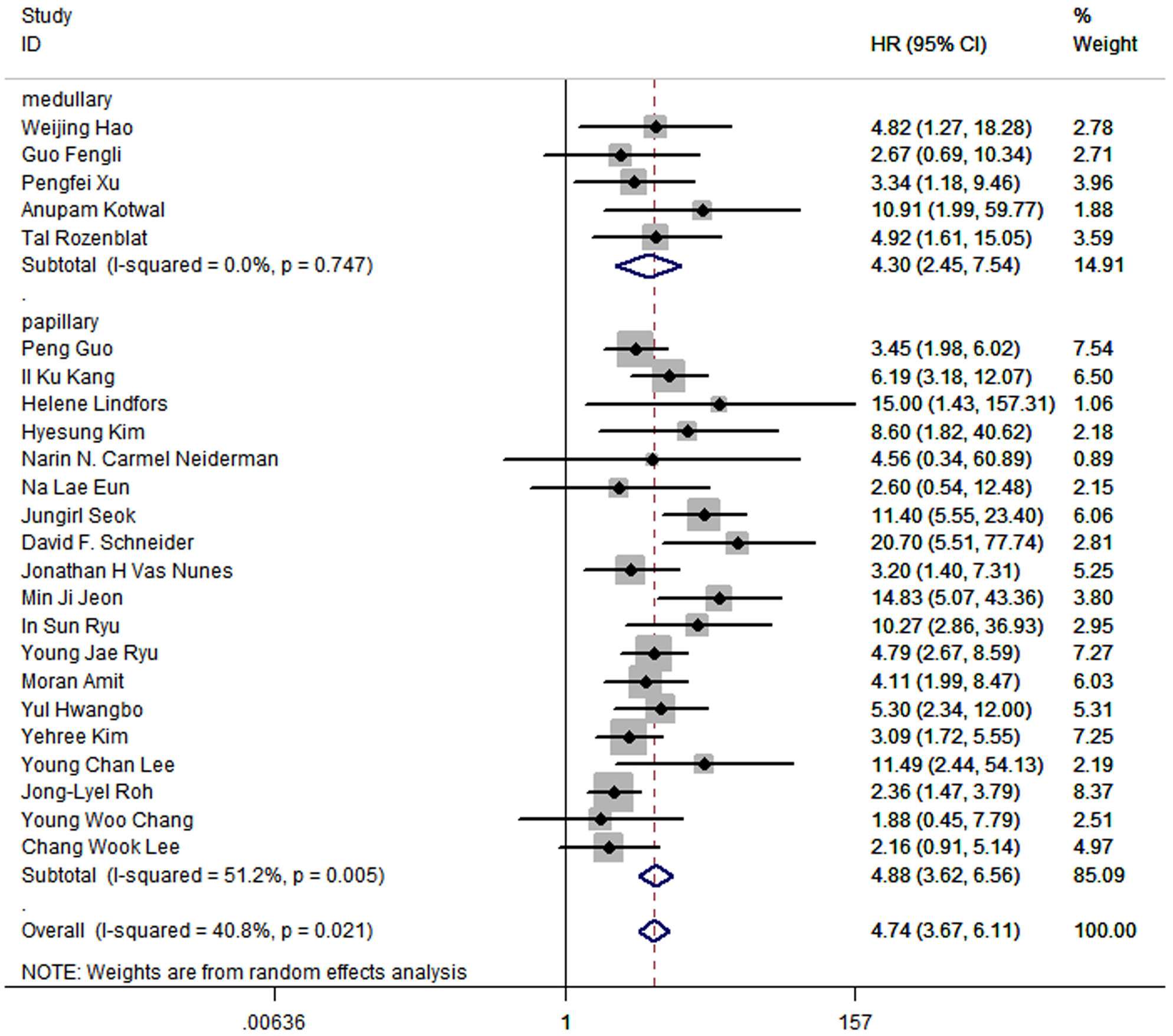
The results of meta-analysis of all 24 studies indicated that a higher LNR was associated with worse DFS (pooled HR: 4.74; 95% CI:3.67-6.11; P<0.05) and a moderate heterogeneity of 40.8% was reported (Figure 2).
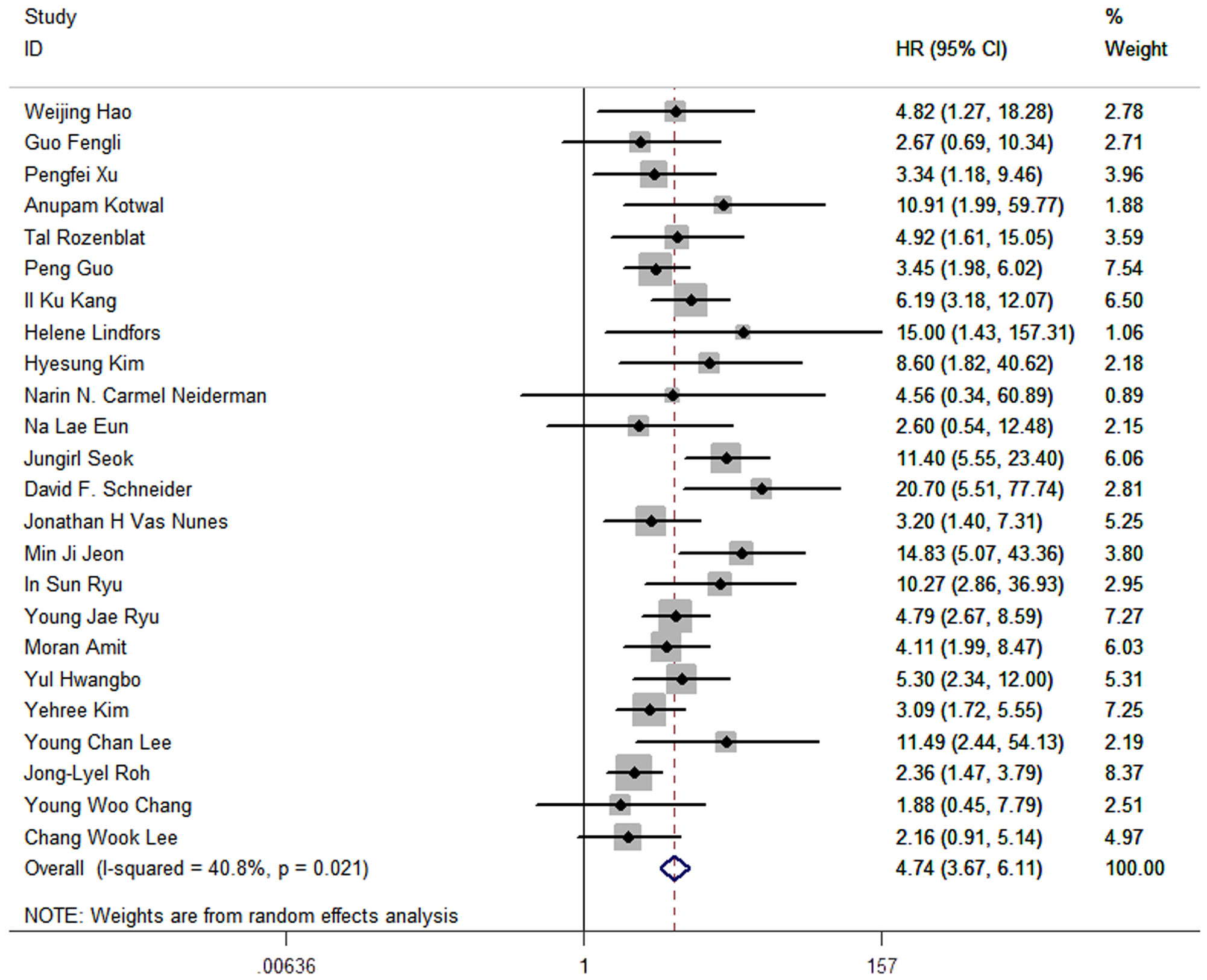
Figure 2 Forest plots and pooled estimates of the effect for meta-analysis of the association between LNR and disease-free survival in patients with thyroid cancer.
3.3 Sensitivity analysis
A sensitivity analysis was performed to assess the stability of the outcomes of meta-analysis. And the results intuitively showed the robust of the outcomes (Figure 3).
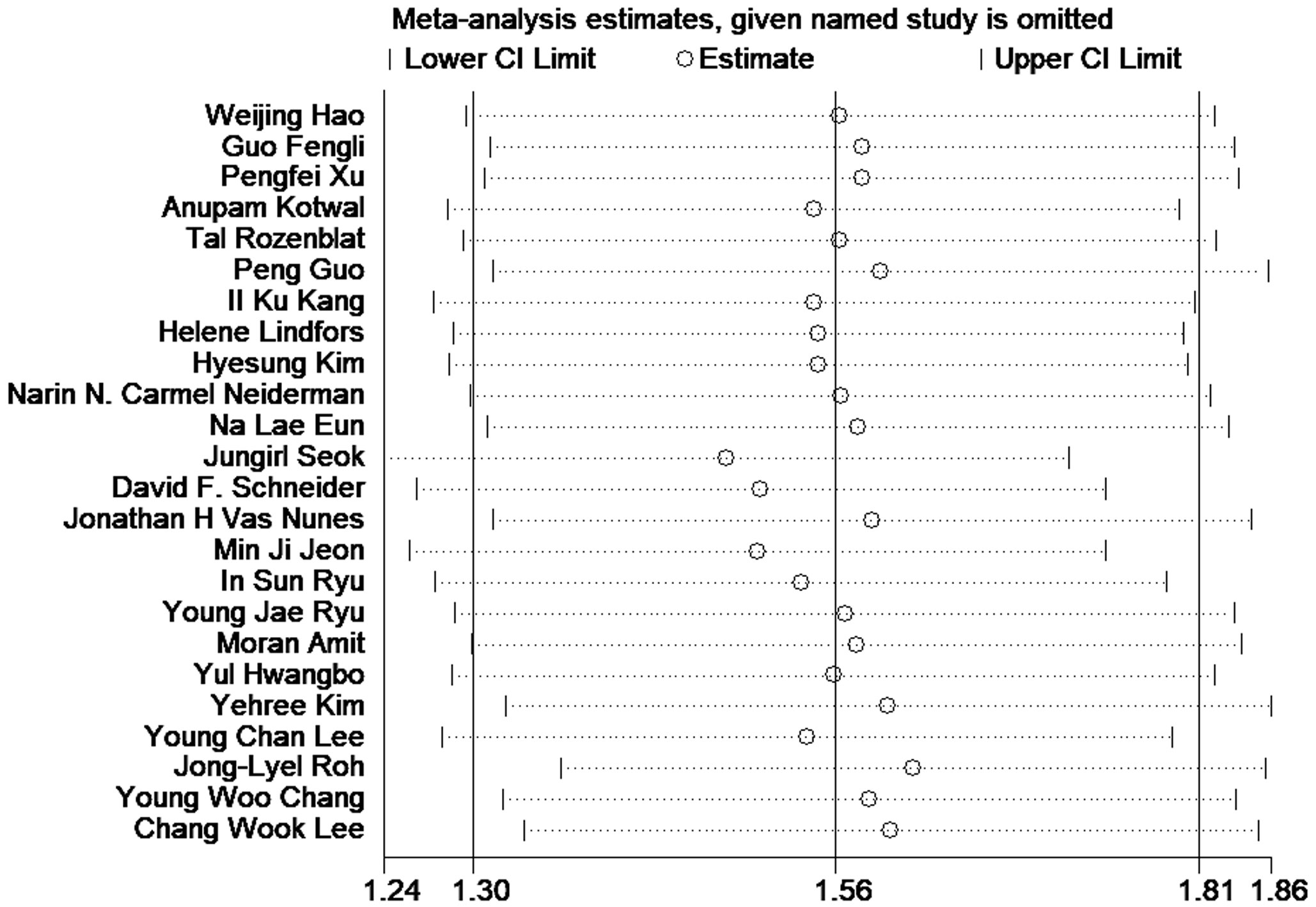
Figure 3 Sensitivity analysis of the association between LNR and disease-free survival in patients with thyroid cancer.
3.4 Subgroup analyses and meta-regression
We performed subgroup analyses based on factors including histological types of thyroid cancer, treatment, location, ranges of cut-off value of LNR and whether studies control factors in design or analysis.
The results showed that higher LNRs were correlated to poor DFS in every subgroup.
In all 24 articles, 5 of them studied medullary thyroid cancer, and the other 19 studied papillary thyroid cancer. The outcomes in medullary thyroid cancer (HR:4.30; 95%CI: 2.45-7.54; P<0.05; I2 = 0.0%) were similar to the outcomes in papillary thyroid cancer (HR: 4.88, 95%CI: 3.62-6.56; P<0.05; I2 = 51.2%), while the prognostic value of LNR was slightly higher in papillary thyroid cancer (Figure 4).
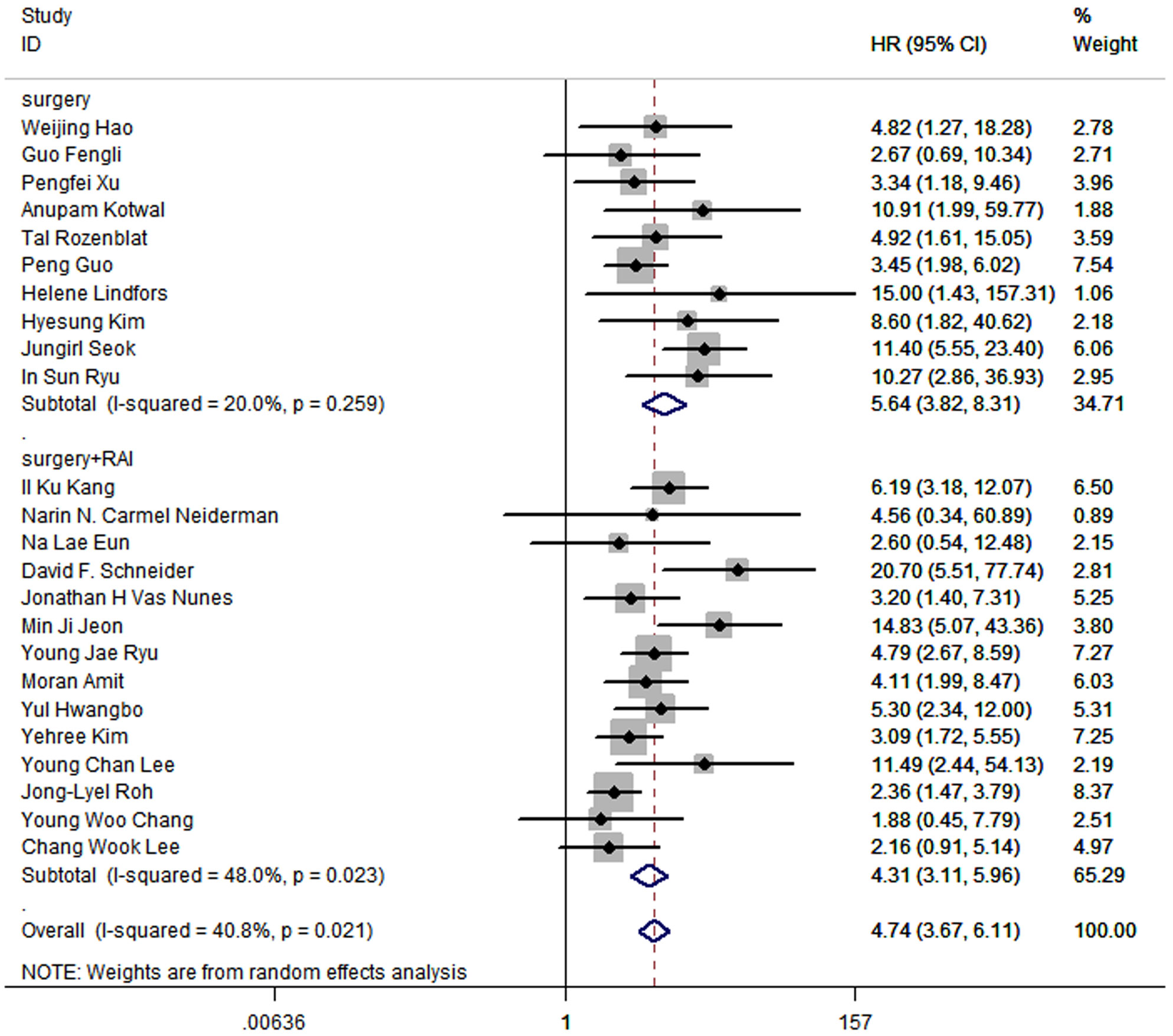
In terms of treatment, 15 studies included patients who received radical surgery and RAI, while patients in other 9 studies underwent radical surgery. The pooled HR of surgery group was 5.64 (95% CI: 3.82-8.31; P<0.05; I2 = 20.0%), and the pooled HR of surgery and RAI group was 4.31 (95%CI: 3.11-5.96; P<0.05; I2 = 48.0%), which suggested that the prognostic value of LNR is significant regardless of whether RAI was used (Figure 5).
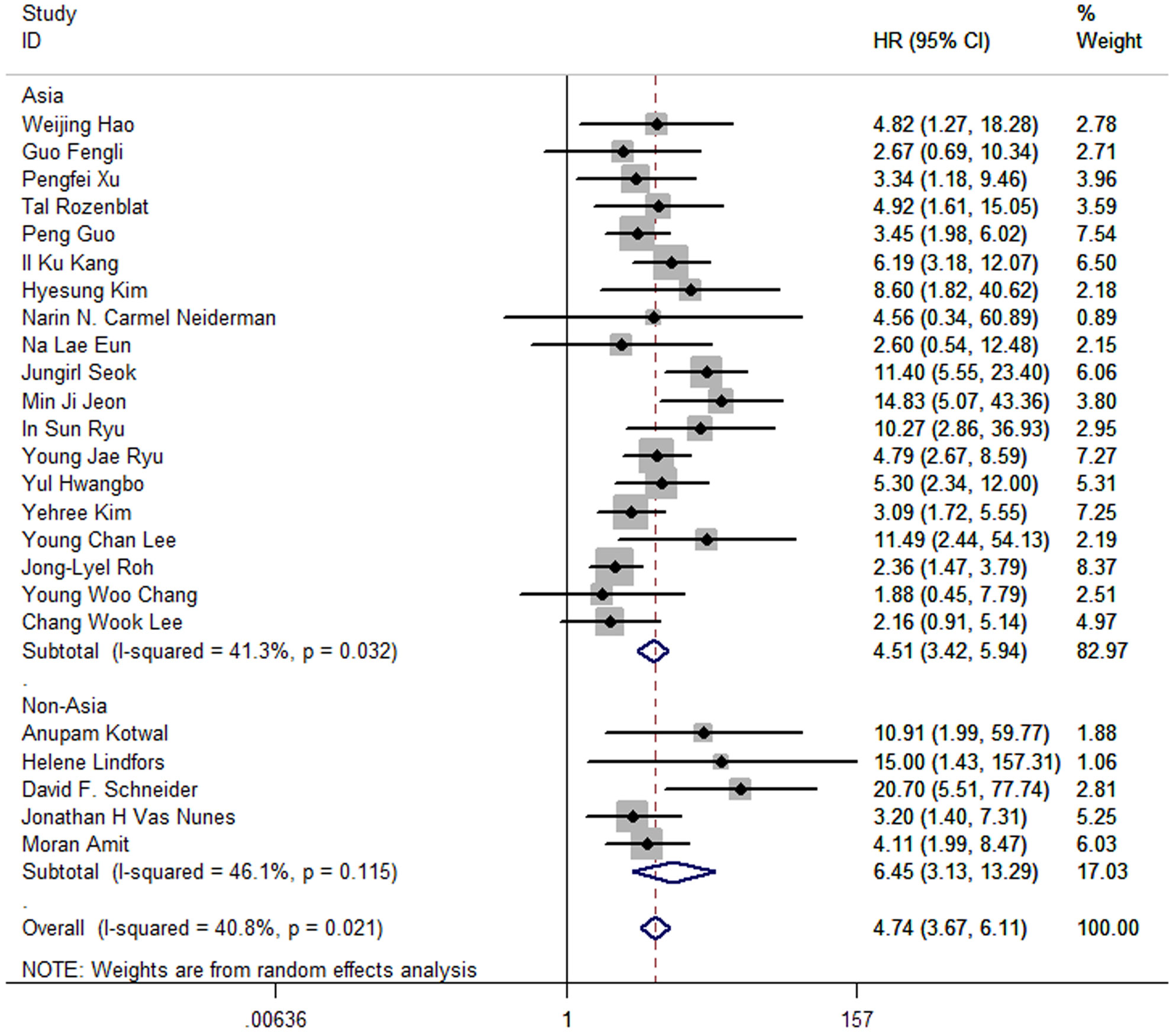
Moreover, the pooled HR of 19 Asia studies was 4.51 (95%CI: 3.42-5.94; P<0.05; I2 = 41.3%), and the pooled HR of 5 studies conducted in America and Europe was 6.45 (95%CI: 3.13-13.29; P<0.05; I2 = 46.1%) (Figure 6).
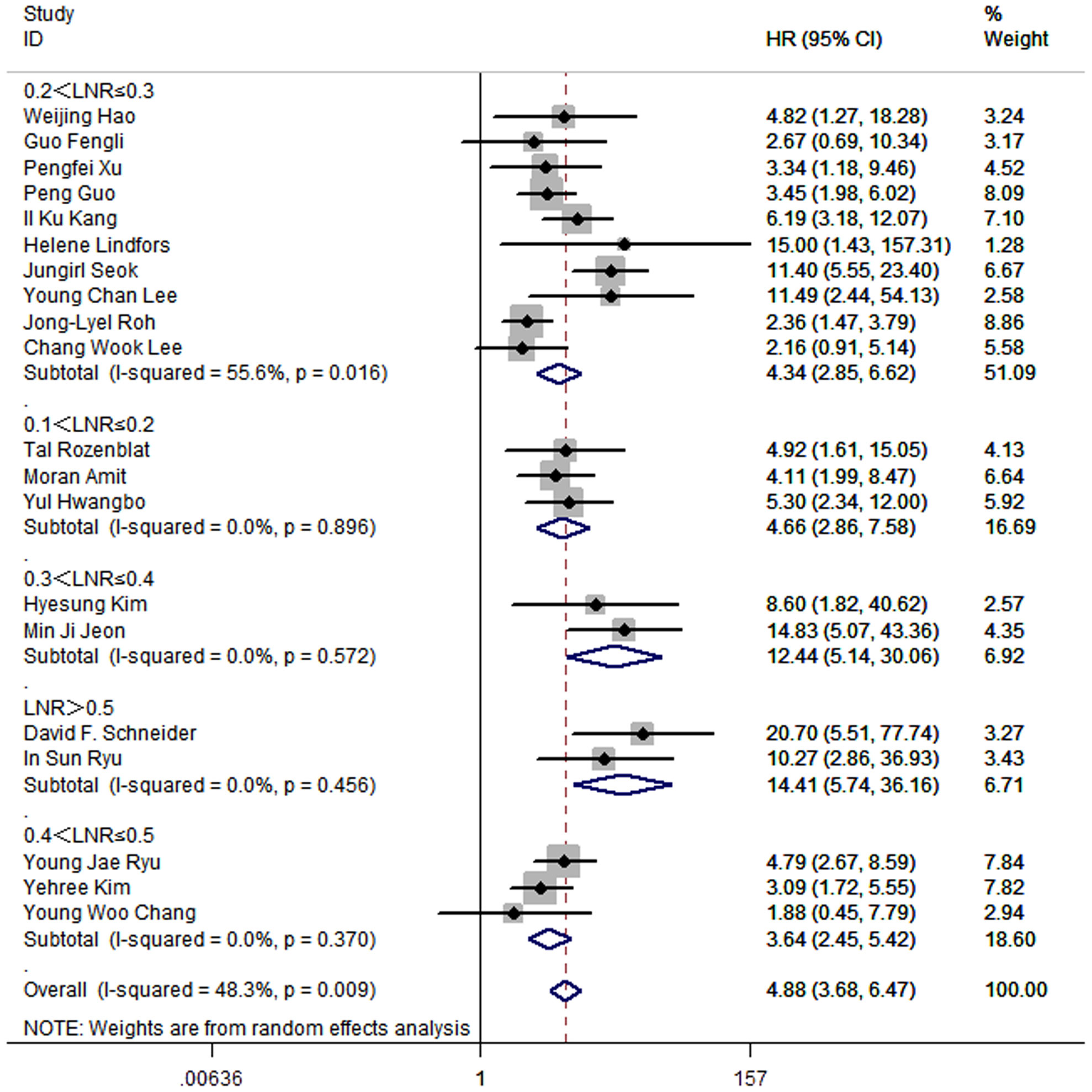
To investigate optimal range of cut-off value of LNR, studies were divided into 5 subgroups. For the 10 studies that set the cut-off value of LNR between 0.2 and 0.3, the pooled HR was 4.34 (95%CI: 2.85-6.62; P<0.05; I2 = 55.6%). For the 3 studies that set the cut-off value of LNR between 0.1 and 0.2, the pooled HR was 4.66 (95%CI: 2.86-7.58; P<0.05; I2 = 0.0%). 2 studies set the cut-off value between 0.3 and 0.4, and the pooled HR was 12.44 (95%CI: 5.14-30.06; P<0.05; I2 = 0.0%). 3 studies set the cut-off value between 0.4 and 0.5, and the pooled HR was 3.64 (95%CI: 2.45-5.42; P<0.05; I2 = 0.0%). 2 studies made the cut-off value of LNR greater than 0.5, and the pooled HR was 14.41 (95%CI: 5.74-36.16; P<0.05; I2 = 0.0%) (Figure 7).
Furthermore, the pooled HR in subgroup of which studies control factors in design or analysis to ensure the comparability of cohorts was 4.11 (95%CI: 2.84-5.96; P<0.05; I2 = 25.7%), while the pooled HR in subgroup that did not control any factors was 5.00 (95%CI:3.58-6.99; P<0.05; I2 = 45.8%) (Figure 8).
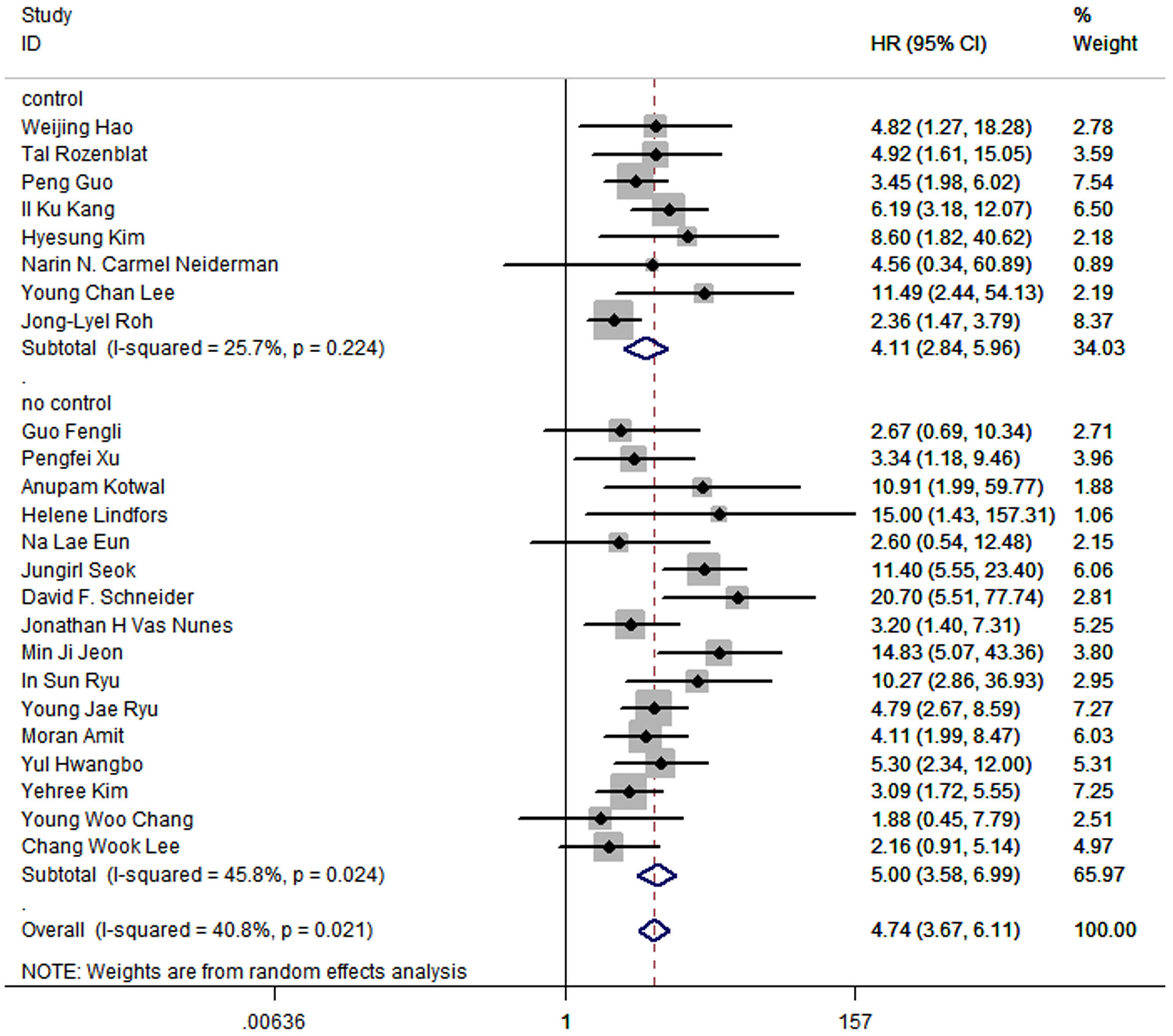
Figure 8 Forest plot for subgroup analysis by whether studies control factors in design or analysis to ensure the comparability of cohorts.
Additionally, 22 studies included patients with ETE, while other 2 studies did not. The pooled HR of group with ETE was 4.56 (95%CI: 3.51, 5.92; P<0.05; I2 = 42.1%), and the pooled HR of group without ETE was 9.56 (95%CI: 3.56, 25.66; P<0.05; I2 = 0.0%) (Figure 9).
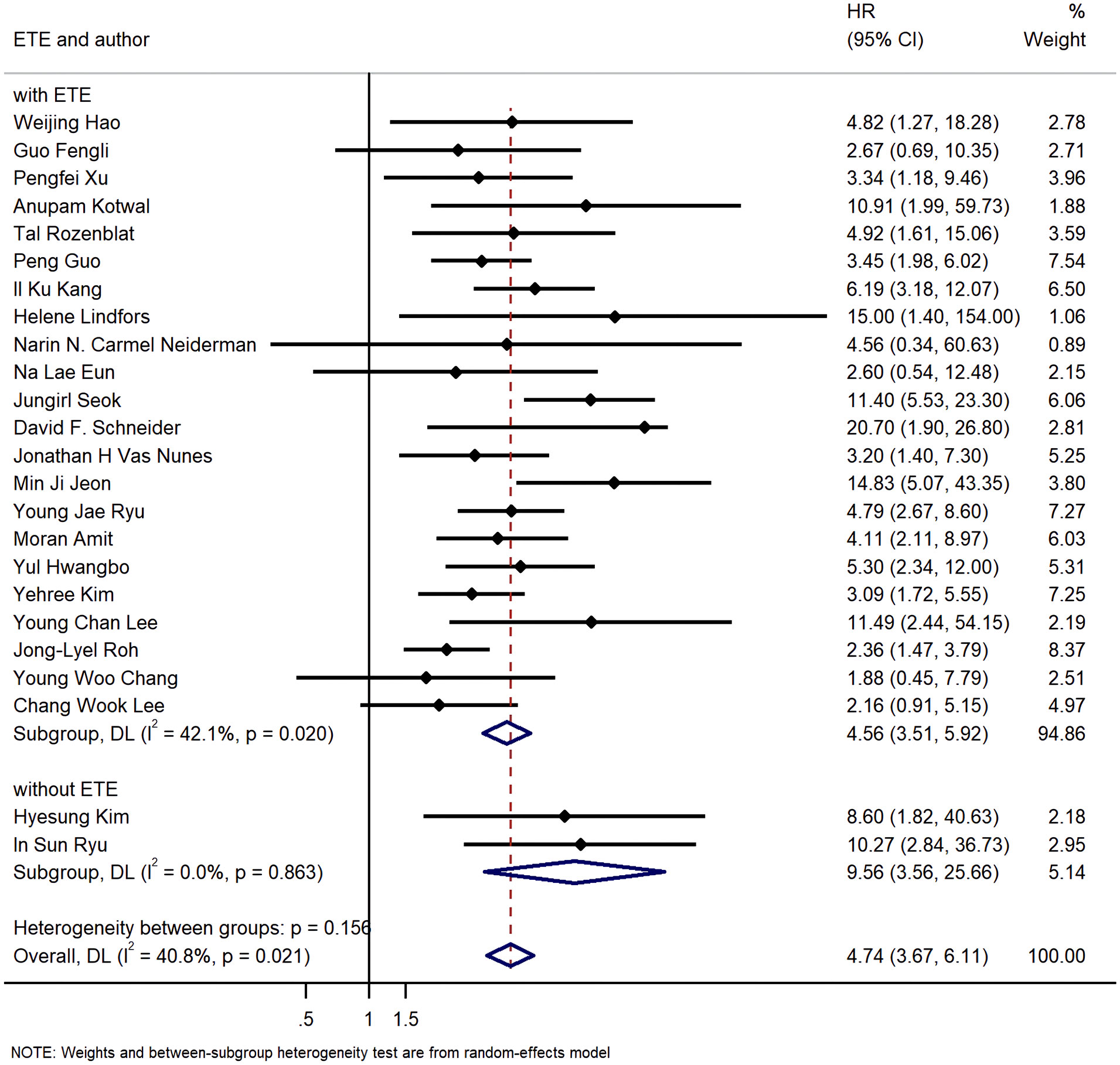
Figure 9 Forest plot for subgroup analysis by whether cases with extra thyroid extension were excluded.
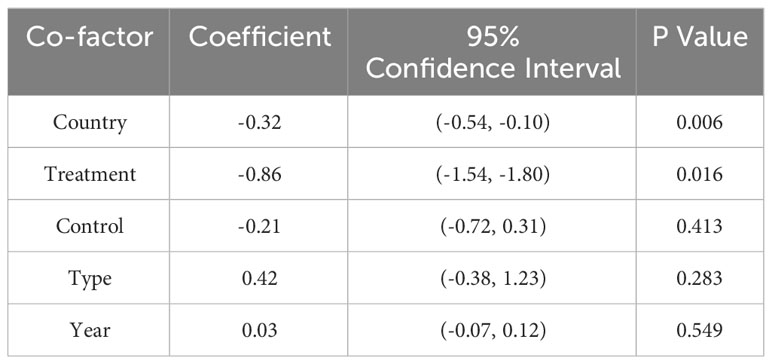
Besides, We performed a meta-regression to evaluate the influence of different factors including country, treatment, type of thyroid cancer, year and whether studies control factors in design or analysis to the pooled HR, and to clarify the origin of heterogeneity. Results suggested that the combination of these 5 factors could explain 73.01% of the variance among studies (P= 0.0444) (Table 4).
3.5 Publication bias
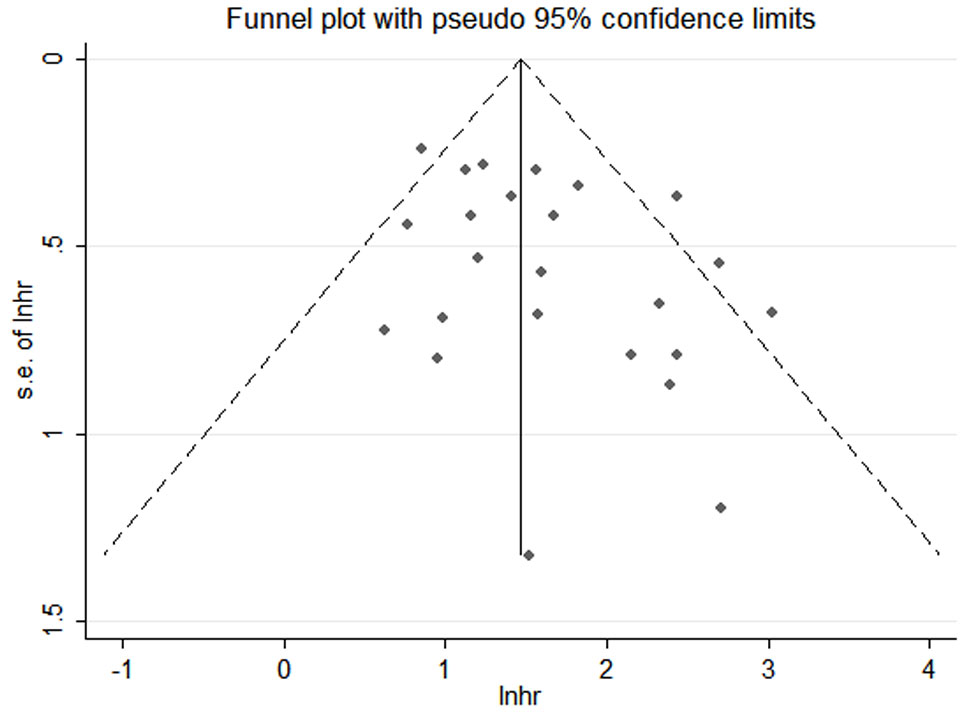
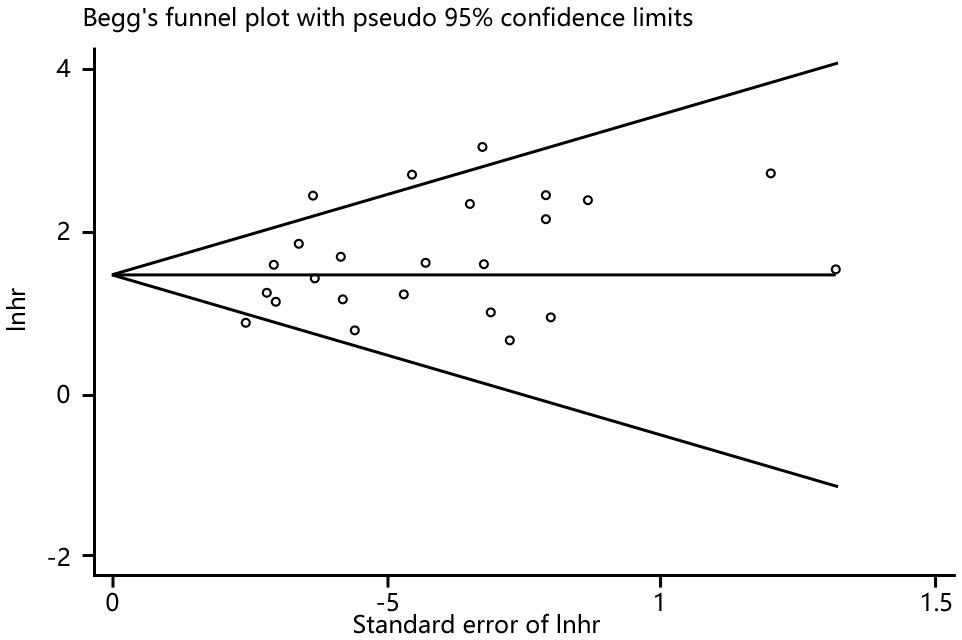
Funnel plots, Begg’s and Egger’s tests were done to evaluate publication bias, and results showed no significant publication bias (Begg’s test: P=0.286; Egger’s test: P=0.053) in the meta-analysis (Figures 10, 11).
4 Discussion
LNR is proved to be a significant prognostic factor for tumor recurrence and survival in multiple cancers. Several meta-analysis have suggested that high LNR is associated with low survival rates for patients with cancers (27–29, 33, 34). However, the value of LNR as prognostic variable has not been confirmed in thyroid cancer. 2015 ATA Management Guidelines has considered the number of involved lymph node to be a factor predicting recurrence, and the 2018 AJCC/TNM staging has only mentioned lymph node metastasis as the basis of thyroid cancer staging (35). It is should be noted that the number of involved lymph node metastasis are likely to be influenced by numerous factors, among which the number of lymph node examined is a major factor (25, 26). LNR, as a new indicator, takes both the number of lymph node involved and the number of checked lymph node into consideration (36). Therefore, many of studies have attempted to assess the potential of LNR to be a prognostic variable for thyroid cancer in order to provide more scientific guidance for staging,prognostic assessment, and treatment (37, 38). Unfortunately, no consensus currently exists because in addition to conflicting study results, the controversy of critical value of LNR is also a challenge on the evaluation of prognostic value of LNR for thyroid cancer.
Our study was the first formal meta-analysis to evaluate the prognostic value of LNR for thyroid cancer. In this meta-analysis,we found that high LNR was correlated with poor disease-free survival which meant LNR was a valuable potential prognostic factor for thyroid cancer. This result did not change in any type of thyroid cancer we analyzed including papillary and medullary thyroid cancer, while the prognostic efficacy was slightly higher in papillary thyroid cancer. Differences in prognostic value of LNR between Asian countries and non-Asia countries were also noteworthy. The cut-off values of LNR varied from 0.15 to 0.7 in different studies. Different prognostic values of LNR were shown based on different ranges of cut-off values. Significantly higher prognostic value was shown when the cut-off values of LNR ranging from 0.3 to 0.4 or greater than 0.5, although this possibly resulted from the small quantity of studies we could analyze in these 2 subgroups.
Some limitations of this meta-analysis should be mentioned. First, all included studies were retrospective studies which meant significant differences in patient characteristics, surgical plans, and other aspects might exist, leading to relatively low quality of data. Therefore, more prospective of large scale were expected to provide clinical data of ideal quality. Second, absence of data in tumor size, pathological stages, number of examined lymph node, number of metastasized lymph node and surgical methods limited further subgroup analysis. Third, the LNR cut-off values of some included articles were absent, which was likely to affect our exploration of the optimal range of LNR cut-off value in our subgroup analysis. Forth, the critical values of LNR in different studies were inconsistent. Although we grouped these studies by different ranges of critical value to analyze the cut-off value that best predicted recurrence, an exact optimal cut-off value was still left to be defined. Furthermore, moderate heterogeneity existed in this meta-analysis. However, we failed to seek single factor that caused heterogeneity through subgroup analysis and meta-regression with single covariate. We could only clarify that a combination of 5 factors including country, treatment, type of thyroid cancer, year and whether studies control factors in design or analysis influenced the heterogeneity of this analysis. Finally, we only included patients with papillary and medullary thyroid cancer, more studies discussing the prognostic role of LNR in other types of thyroid cancer were expected.
We should also point out the strength of our study. First, this was the first complete and formal meta-analysis to quantify the role of LNR in the prognosis of thyroid cancer. Second, 24 studies and a total of 15,698 patients were included in our meta-analysis, which allowed a relatively reliable statistical results. Thus, according to our analysis, the prognostic value of LNR was conspicuous.
In addition to the significant prognostic value, the potential of LNR to be included in the future thyroid cancer staging system may also be marked. Xu et al (39). proposed a new grading system combining the Ki67 index and LNR as a predictor of prognosis in medullary thyroid cancer. The prognostic performance of the new grading scheme was better than the Ki67, LNR and N staging alone. Lee et al (40). reported better predictive power of the new risk stratification systems combining metastasized lymph node factors including LNR, maximum diameter of metastatic focus and presence of extranodal extension with ATA risk stratification in N1 stage papillary thyroid cancer. In a word, more related studies are expected to establish a new risk stratification system and to assess the potential of LNR to be included in the system.
5 Conclusion
To conclude, higher LNR was correlated to poorer disease free survival in thyroid cancer and this result didn’t change in every subgroup we set. This study suggested that LNR could be a potential prognostic indicator for thyroid cancer. More effort should be made to assess the potential of LNR to be included in the future risk stratification systems for thyroid cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YH: Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. ZW: Data curation, Writing – review & editing. LD: Writing – review & editing. LZ: Writing – review & editing. LX: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine [grant numbers ZYYCXTD-D-202001], Scientific and Technological innovation project of China Academy of Chinese Medical Sciences [grant numbers CI2021A01602], and Special Project for the Construction of Clinical Medical Research Centre of Guang’anmen Hospital, China Academy of Traditional Chinese Medicine[grant numbers 2022LYJSZX08].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: globocan estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol (2022) 10(4):264–72. doi: 10.1016/S2213-8587(22)00035-3
3. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (2016) 388(10061):2783–95. doi: 10.1016/S0140-6736(16)30172-6
4. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol (2016) 12(11):646–53. doi: 10.1038/nrendo.2016.110
5. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res (2014) 74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155
6. LeClair K, Bell K, Furuya-Kanamori L, Doi SA, Francis DO, Davies L. Evaluation of gender inequity in thyroid cancer diagnosis: differences by sex in us thyroid cancer incidence compared with a meta-analysis of subclinical thyroid cancer rates at autopsy. JAMA Intern Med (2021) 181(10):1351–58. doi: 10.1001/jamainternmed.2021.4804
7. Fallahi P, Ferrari SM, Santini F, Corrado A, Materazzi G, Ulisse S, et al. Sorafenib and thyroid cancer. Biodrugs (2013) 27(6):615–28. doi: 10.1007/s40259-013-0049-y
8. Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid (2011) 21(2):125–34. doi: 10.1089/thy.2010.0021
9. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
10. Dralle H, Machens A, Basa J, Fatourechi V, Franceschi S, Hay ID, et al. Follicular cell-derived thyroid cancer. Nat Rev Dis Primers (2015) 1:15077. doi: 10.1038/nrdp.2015.77
11. Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat Rev Endocrinol (2018) 14(11):670–83. doi: 10.1038/s41574-018-0080-7
12. Zhang Y, Xing Z, Liu T, Tang M, Mi L, Zhu J, et al. Targeted therapy and drug resistance in thyroid cancer. Eur J Med Chem (2022) 238:114500. doi: 10.1016/j.ejmech.2022.114500
13. Tuttle RM. Controversial issues in thyroid cancer management. J Nucl Med (2018) 59(8):1187–94. doi: 10.2967/jnumed.117.192559
14. Tahara M, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, et al. Lenvatinib for anaplastic thyroid cancer. Front Oncol (2017) 7:25. doi: 10.3389/fonc.2017.00025
15. Chen DW, Lang B, McLeod D, Newbold K, Haymart MR. Thyroid cancer. Lancet (2023) 401(10387):1531–44. doi: 10.1016/S0140-6736(23)00020-X
16. Mitchell AL, Gandhi A, Scott-Coombes D, Perros P. Management of thyroid cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol (2016) 130(S2):S150–60. doi: 10.1017/S0022215116000578
17. Papaleontiou M, Reyes-Gastelum D, Gay BL, Ward KC, Hamilton AS, Hawley ST, et al. Worry in thyroid cancer survivors with a favorable prognosis. Thyroid (2019) 29(8):1080–88. doi: 10.1089/thy.2019.0163
18. Papachristos AJ, Nicholls LE, Mechera R, Aniss AM, Robinson B, Clifton-Bligh R, et al. Management of medullary thyroid cancer: patterns of recurrence and outcomes of reoperative surgery. Oncologist (2023) 28(12):1064–71. doi: 10.1093/oncolo/oyad232
19. Grønlund MP, Jensen JS, Hahn CH, Grønhøj C, Buchwald CV. Risk factors for recurrence of follicular thyroid cancer: a systematic review. Thyroid (2021) 31(10):1523–30. doi: 10.1089/thy.2020.0921
20. Isaacs JD, Lundgren CI, Sidhu SB, Sywak MS, Edhouse PJ, Delbridge LW. The delphian lymph node in thyroid cancer. Ann Surg (2008) 247(3):477–82. doi: 10.1097/SLA.0b013e31815efdc4
21. Kim J, Park J, Park H, Choi MS, Jang HW, Kim TH, et al. Metastatic lymph node ratio for predicting recurrence in medullary thyroid cancer. Cancers (Basel) (2021) 13(22):5842. doi: 10.3390/cancers13225842
22. Zhang J, Cheng X, Shen L, Wang X, Wang L, Sun X, et al. The association between lymph node stage and clinical prognosis in thyroid cancer. Front Endocrinol (Lausanne) (2020) 11:90. doi: 10.3389/fendo.2020.00090
23. Robinson TJ, Thomas S, Dinan MA, Roman S, Sosa JA, Hyslop T. How many lymph nodes are enough? Assessing the adequacy of lymph node yield for papillary thyroid cancer. J Clin Oncol (2016) 34(28):3434–39. doi: 10.1200/JCO.2016.67.6437
24. Ruel E, Thomas S, Perkins JM, Roman SA, Sosa JA. The impact of pathologically positive lymph nodes in the clinically negative neck: an analysis of 39,301 patients with papillary thyroid cancer. Ann Surg Oncol (2017) 24(7):1935–42. doi: 10.1245/s10434-016-5719-9
25. Yu ST, Ge JN, Sun BH, Wei ZG, Xiao ZZ, Zhang ZC, et al. Lymph node yield in the initial central neck dissection (cnd) associated with the risk of recurrence in papillary thyroid cancer: a reoperative cnd cohort study. Oral Oncol (2021) 123:105567. doi: 10.1016/j.oraloncology.2021.105567
26. Zhao H, Huang T, Li H. Risk factors for skip metastasis and lateral lymph node metastasis of papillary thyroid cancer. Surgery (2019) 166(1):55–60. doi: 10.1016/j.surg.2019.01.025
27. Zhu J, Xue Z, Zhang S, Guo X, Zhai L, Shang S, et al. Integrated analysis of the prognostic role of the lymph node ratio in node-positive gastric cancer: a meta-analysis. Int J Surg (2018) 57:76–83. doi: 10.1016/j.ijsu.2018.08.002
28. Zhang MR, Xie TH, Chi JL, Li Y, Yang L, Yu YY, et al. Prognostic role of the lymph node ratio in node positive colorectal cancer: a meta-analysis. Oncotarget (2016) 7(45):72898–907. doi: 10.18632/oncotarget.12131
29. Liu D, Chen Y, Deng M, Xie G, Wang J, Zhang L, et al. Lymph node ratio and breast cancer prognosis: a meta-analysis. Breast Cancer (2014) 21(1):1–09. doi: 10.1007/s12282-013-0497-8
30. Mansour J, Sagiv D, Alon E, Talmi Y. Prognostic value of lymph node ratio in metastatic papillary thyroid carcinoma. J Laryngol Otol (2018) 132(1):8–13. doi: 10.1017/S0022215117002250
31. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published kaplan-meier survival curves. BMC Med Res Methodol (2012) 12:9. doi: 10.1186/1471-2288-12-9
32. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
33. Zhou J, Lin Z, Lyu M, Chen N, Liao H, Wang Z, et al. Prognostic value of lymph node ratio in non-small-cell lung cancer: a meta-analysis. Jpn J Clin Oncol (2020) 50(1):44–57. doi: 10.1093/jjco/hyz120
34. Huang TH, Li KY, Choi WS. Lymph node ratio as prognostic variable in oral squamous cell carcinomas: systematic review and meta-analysis. Oral Oncol (2019) 89:133–43. doi: 10.1016/j.oraloncology.2018.12.032
35. Lamartina L, Grani G, Arvat E, Nervo A, Zatelli MC, Rossi R, et al. 8th edition of the ajcc/tnm staging system of thyroid cancer: what to expect (itco#2). Endocr Relat Cancer (2018) 25(3):L7–11. doi: 10.1530/ERC-17-0453
36. Zhao Y, Li G, Zheng D, Jia M, Dai W, Sun Y, et al. The prognostic value of lymph node ratio and log odds of positive lymph nodes in patients with lung adenocarcinoma. J Thorac Cardiovasc Surg (2017) 153(3):702–09. doi: 10.1016/j.jtcvs.2016.11.053
37. Gao B, Zhou D, Qian X, Jiang Y, Liu Z, Zhang W, et al. Number of positive lymph nodes is superior to lnr and lodds for predicting the prognosis of pancreatic neuroendocrine neoplasms. Front Endocrinol (Lausanne) (2021) 12:613755. doi: 10.3389/fendo.2021.613755
38. Teng J, Abdygametova A, Du J, Ma B, Zhou R, Shyr Y, et al. Bayesian inference of lymph node ratio estimation and survival prognosis for breast cancer patients. IEEE J BioMed Health Inform (2020) 24(2):354–64. doi: 10.1109/JBHI.2019.2943401
39. Xu P, Wu D, Liu X. A proposed grading scheme for predicting recurrence in medullary thyroid cancer based on the ki67 index and metastatic lymph node ratio. Endocrine (2023) 81(1):107–15. doi: 10.1007/s12020-023-03328-4
Keywords: thyroid cancer, meta-analysis, prognosis, lymph node ratio, disease-free survival
Citation: Hu Y, Wang Z, Dong L, Zhang L and Xiuyang L (2024) The prognostic value of lymph node ratio for thyroid cancer: a meta-analysis. Front. Oncol. 14:1333094. doi: 10.3389/fonc.2024.1333094
Received: 04 November 2023; Accepted: 25 January 2024;
Published: 07 February 2024.
Edited by:
Nerina Denaro, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Ziyu Luo, Shaanxi Provincial People’s Hospital, ChinaAnupam Kotwal, University of Nebraska Medical Center, United States
Copyright © 2024 Hu, Wang, Dong, Zhang and Xiuyang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Xiuyang, bGVleGl1eWFuZ0AxMjYuY29t
 Yue Hu
Yue Hu Zhiyi Wang
Zhiyi Wang Lishuo Dong
Lishuo Dong Lu Zhang
Lu Zhang Li Xiuyang
Li Xiuyang