- Department of Musculoskeletal Oncology, Center for Orthopaedic Surgery, The Third Affiliated Hospital of Southern Medical University, Guangzhou, China
Purpose: Periprosthetic fracture (PPF) is one of the severe complications in patients with osteosarcoma and carries the risk of limb loss. This study describes the characteristics, treatment strategies, and outcomes of this complication.
Methods: Patients were consecutively included who were treated at our institution between 2016 and 2020 with a PPF of distal femur. The treatment strategies included two types: 1) open reduction and internal fixation with plates and screws and 2) replacement with long-stem endoprosthesis and reinforcement with wire rope if necessary.
Results: A total of 11 patients (mean age 12.2 years (9–14)) were included, and the mean follow-up period was 36.5 (21–54) months. Most fractures were caused by direct or indirect trauma (n = 8), and others (n = 3) underwent PPF without obvious cause. The first type of treatment was performed on four patients, and the second type was performed on seven patients. The mean Musculoskeletal Tumor Society (MSTS) score was 20 (17–23). All patients recovered from the complication, and limb preservation could be achieved.
Conclusion: PPF is a big challenge for musculoskeletal oncologists, particularly in younger patients. Additionally, PPF poses a challenge for orthopedic surgeons, as limb preservation should be an important goal. Hence, internal fixation with plates and endoprosthetic replacement are optional treatment strategies based on fracture type and patient needs.
Introduction
Osteosarcoma (OS) is the most common primary malignancy of bone in children and adolescents, while primary bone neoplasms account for merely less than 0.2% of all cancers (1). Since the 1980s, there has been a consensus in osteosarcoma treatment that a combination of surgery and intensive, multi-agent chemotherapy dramatically improves osteosarcoma patients’ prognosis and maintains a high survival rate and long-term disease-free survival (2–4).
Various techniques have been reported for reconstruction after malignant tumor resection around the knee in children and adolescents (5, 6). However, revision of endoprosthetic reconstructions occurs frequently because of infection and mechanical complications (5, 7–10). In the context of endoprosthetic reconstruction for distal femoral tumors, the respective proportions of complications according to systematic classification are soft-tissue failure (4.6%), aseptic loosening (24.9%), structural failure (23.0%), infection (30.3%), and tumor recurrence (17.2%) (11). With the survival of osteosarcoma patients being improved, combined with most patients being young and active and with poor bone quality, the rate of periprosthetic fracture (PPF) is increasing among mechanical complications. PPF is one of the most common complications experienced by patients with malignant tumors around the knee joint (12, 13). Furthermore, in a study focusing on adolescent patients with bone tumors under the age of 18, the rates of periprosthetic fractures at 5 and 8 years were higher than those of infection and aseptic loosening (13).
The risk factors for PPF can be broadly categorized into three main groups. Patient-specific factors include diabetes, osteoporosis, age, or undergoing certain specific treatments. Implant-related factors encompass the design of implant components, such as the presence of collars. Lastly, surgical factors will involve considerations like notching at the anterior femoral cortex, surgical volume, and intraoperative mishandling. However, there is still controversy surrounding many of these factors (14, 15). Currently, various classification systems can be adopted for the treatment of periprosthetic fractures, depending on different focal points, including conservative treatment, open reduction internal fixation, and revision reconstruction. However, most patients with PPF need surgical treatment finally. At present, the Unified Classification System (UCS) classification system serves as the mainstream basis for selecting treatment approaches for periprosthetic fractures (16, 17). However, there are no widely accepted guidelines or consensus to directly treat PPF patients after limb salvage surgery caused by OS of the knee joint yet.
To our knowledge, very rare reports or recommendations have demonstrated treatment strategies for PPF with patients who experienced osteosarcoma around the knee joint (18–20). In this study, we aimed to produce different types of PPF and describe 13 cases with successful treatment of PPF with limb-salvaging strategies.
Patients and methods
Study design
Patients were consecutively included who were treated at our institution between 2016 and 2020 with a PPF of distal femur. A total of 11 patients have been included who 1) were treated at our institution with the diagnosis of PPF, 2) were treated between 2016 and 2020, 3) have undertaken prosthetic reconstruction surgery for osteosarcoma of the knee joint, 4) were younger than 14 years, and 5) were with complete clinical, radiological, and pathological data. The exclusion criteria included the following: 1) evidence of peri-prosthetic infection, 2) evidence of local tumor recurrence, 3) patients who had unfinished treatment of initial osteosarcoma, 4) patients who received specific treatment in other case–control studies, and 5) patients with a Karnofsky performance score <70. As for the types of PPF, the UCS was used for the classification (17). Additionally, PPF was classified according to the location and stability of the prosthesis: type I, around the tip of the prosthesis stem, or the prosthesis is unstable; and type II, away from the tip of the prosthesis, and the prosthesis is still well fixed, too. The follow-up interval for primary osteosarcoma followed National Comprehensive Cancer Network (NCCN) guidelines (1). The study was approved by the institutional review board (IRB) of our institutions and was conducted in accordance with the Declaration of Helsinki.
Treatment procedures
Internal fixation with plates and screws
X-ray positioning was used during surgery to determine the surgical approach. The fractured part was exposed, and the surrounding hematoma, osteocytes, and tissues in the medullary cavity were cleaned. Meanwhile, rapid pathological examination of intramedullary tissues was performed to eliminate the recurrence of the tumor. Then, the fracture was reduced by traction and fixed using locking plates (shaped according to bone shape) and screws. Locking-nail channels blocked by the prosthesis stem were fixed with a single cortical screw and wire rope. After fixation and defining reduction with X-ray, allogeneic/autologous bone was implanted around the fracture line of the femur.
Replacement of endoprosthesis
The fractured part was exposed, and the prosthesis of the knee joint was displaced. The affected part of the prosthesis was removed from the medullary cavity, and the stability of the unaffected part of the prosthesis was checked. The hyperplastic granulation tissue and bone cement in the medullary cavity were removed and flushed for 3 cycles. The antibiotic bone cement was injected, and a new custom-made prosthesis with a longer stem was installed. The position of the prosthesis was confirmed by an X-ray, and the fractured part was reinforced with wire rope if necessary.
Follow-up
The patients were followed every 3 months for the first and second years postoperatively and then every 6 months for three more years. After that, follow-up was performed once a year. Each follow-up visit included history taking, physical examination, and radiological examination, such as X-ray and CT scan for mechanical complication and tumor recurrence. PET-CT was used to assess the tumor’s metastatic status. The postoperative functional assessment was performed via the Musculoskeletal Tumor Society (MSTS) Functional Scoring System (21).
Results
Patients’ characteristics
Overall, 11 patients (11/313, 3.5%) with PPF were included in this study. The mean follow-up time was 36.5 (21–54) months. According to the UCS classification (17), these patients were classified as type B1 in five (45.5%) and type B2 in six (54.5%). According to the classification of our institution, seven (63.6%) patients were type I, and four patients (36.4%) were type II. Eight (72.7%) fractures were caused by direct or indirect trauma, while three (27.3%) fractures underwent PPF without obvious cause. Four (36.4%) patients with type II PPF underwent fixation with plates and screws, and seven (63.6%) patients with type I PPF underwent replacement of endoprosthesis with further reinforcement (Table 1). The mean MSTS score was 20 (17–23). Until the last follow-up, none experienced any complications such as infection, delayed wound healing, re-fracture, or non-union. No re-revision was necessary in all cases.
Special cases
Case 1
A 14-year-old girl suffered from a PPF at the left femur after falling. This patient complained of swelling and pain in the left leg. From the X-ray image, PPF in the middle part of the left femur with partial displacement (Type I) was observed (Figures 1A, B). Revision surgery with the replacement of a custom-made prosthesis stem was performed (Figure 1C). According to the last follow-up data, the patient was alive, and no postoperative complication happened.
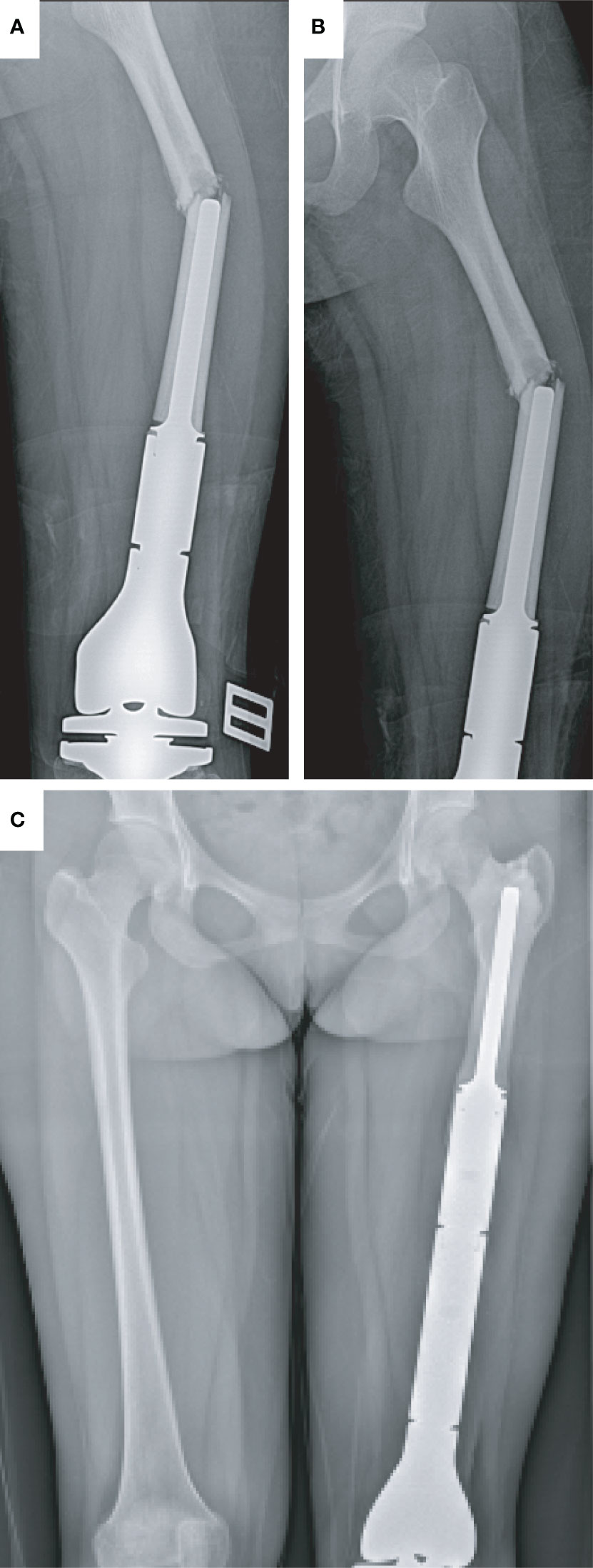
Figure 1 Type I periprosthetic fracture and corresponding surgical strategy (open reduction and replacement of endoprosthesis). (A, B) Preoperative X-ray image of the left femoral fracture and (C) postoperative X-ray image of the bilateral femur.
Case 2
A 14-year-old boy with right total knee prosthetic reconstruction after resection of tibial osteosarcoma had a sprain and suffered from a prosthetic femoral fracture at the middle part of the right femur (Type II) (Figures 2A, B). At the first visit, the affected limb was swelling and deformed with excruciating pain in the right thigh. After taking a careful physical examination and X-ray imaging of the affected limb and confirming the fracture site, type, and condition of the bone cortex, revision surgery with plate osteosynthesis was performed. At the last follow-up, no further complication happened, and the patient was fully mobilized (Figures 2C, D).
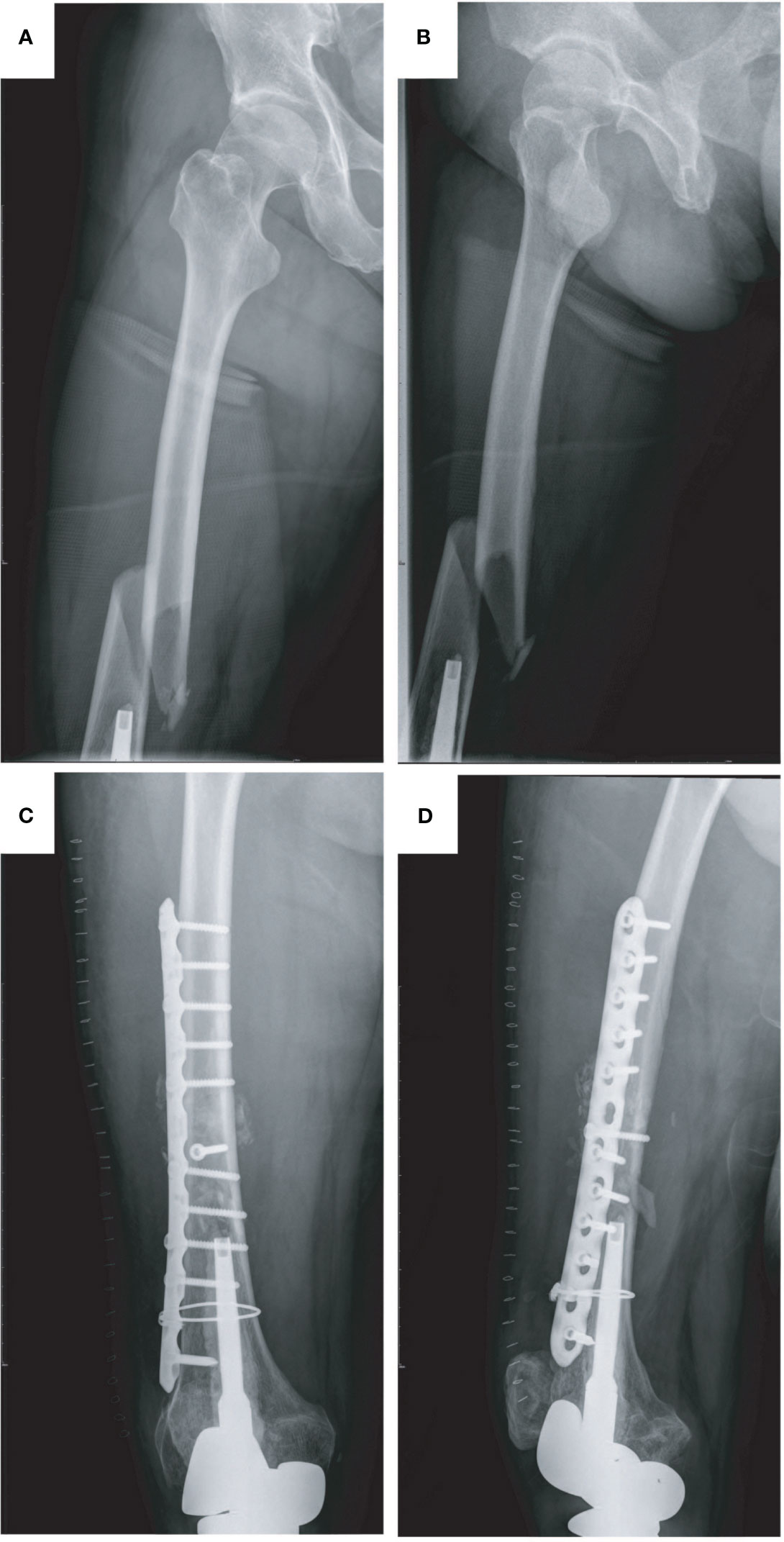
Figure 2 Type II periprosthetic fracture and corresponding surgical strategy (open reduction and internal fixation with plates and screws, reinforcement with wire rope). (A, B) Preoperative X-ray image of the right femoral fracture and (C, D) postoperative X-ray image of the right femur.
Discussion
Artificial prostheses are frequently used to reconstruct bone defects caused by resection of a bone malignancy from the distal femur. However, substantial mechanistic complication rates should be considered because of the severe situation, most notably for aseptic loosening and periprosthetic fracture (9, 20, 22–24). Henderson et al. reported that periprosthetic fracture was the most frequent type of mechanical failure with a rate of 17% followed by aseptic loosening (24). D. Andreou reported that structural complication including PPF and implant fracture is the most common complication experienced by patients with malignant tumors around the knee joint (12). From the database of our institution, nearly 17.7% (11/62) of patients experienced PPF among all patients of mechanical failure after prosthesis reconstruction. Notably, we should focus on PPF since it is one of the most common complications.
For traditional non-tumoral implants, the mean interval between implantation and PPF was 4.4 to 6.4 years (25–29). Periprosthetic fractures are often directly caused by trauma, and the risk factors for their occurrence are closely related to the patient’s own conditions, such as diabetes, osteoporosis, or severe bone loss, all of which contribute to decreased bone quality. The differences in components of the initial reconstruction implant, the width ratio of bone to implant, and the elastic modulus disparity between components and the recipient bone bed are also influencing factors (14, 15). However, the choice of fixation methods and hinge mechanisms for implants has no statistically significant impact on the occurrence of periprosthetic fractures following initial distal femoral reconstruction (30).
For patients who underwent total knee reconstruction after malignant tumor resection, the mean interval between first implantation and revision caused by PPF was significantly shorter than in those patients with non-tumoral disease (18, 19, 23). Andreou et al. (12) reported that the mean time to first mechanical complication for patients with bone tumors was 16 months. In this study, the mean age at the time of fracture was 12.2 years (range, 9–14), and the mean time from revision to implantation was 31 months (range, 6–65). This difference is owing to the primary diagnosis that led to the reconstruction. PPFs around tumor endoprosthesis are very different from those of standard implants. In this study, PPFs were classified as B1 in five (45.5%) cases and B2 in six (54.5%) cases according to UCS classification. Spina et al. (27), in a series of 61 periprosthetic femoral fractures, reported that nearly 80% of patients were classified as type B fractures, while only 2% were classified as type A. For a series of cases with tumor endoprosthesis, Andreou et al. (12) showed that over 65% of patients with PPF were within UCS type C fracture, and only 17% of patients were classified in type B fracture. There is not enough evidence to prove any difference between tumors and conventional prostheses. It is clear that trauma is a direct cause of PPF. In this study, which focuses on adolescent osteosarcoma patients, standardized chemotherapy during the preoperative and postoperative periods in the presence of immature bone can lead to decreased bone healing and poorer bone mass. In addition, younger patients are at increased risk of fracture due to being more physically active (13, 31). At the same time, the mismatch between the physiological curvature of the bone and the lateral position of the tumor prosthesis, the angle of curvature, and the actual length of these endoprostheses concentrate on stresses topically, leading to progressive destruction of the femoral cortex. Moreover, the shorter length of the remaining stable bone during the initial reconstruction to achieve complete tumor resection, as well as the soft-tissue stripping and diminished protection of the normal adjacent musculoskeletal tissues, significantly elevates the chances of fracture (32, 33).
PPF is a big challenge for patients and oncologists. The main goals of the treatment are limb preservation, preservation of function, and reduction of the re-revision rate. Most importantly, PPF accounted for the vast majority of the second revision followed by infection (10, 12, 13). The presented 11 cases demonstrated that even in such complex situations, successful fracture treatment and reducing the rate of complication were possible and critical for patients, especially young adolescent patients.
In this study, we classified the PPF into two types: type I, around the tip of the prosthesis stem, or the prosthesis is unstable (unable to fix the bone and prosthesis firmly via plates and screws); type II, away from the tip of the prosthesis, and the prosthesis is still well fixed (there was enough space for fixing the fractured bone firmly with plates and screws). Meanwhile, we demonstrated two ways of revision surgery according to the types of PPF: 1) P&S (open reduction and internal fixation with plates and screws) and 2) replacement of prosthesis with longer stem for different types of PPF. Revision surgery is recommended for the anatomical reduction of the fracture and the stability of the prosthesis, which could lead to early limb movement and the recovery of affected limbs. For patients with type I PPF, we recommended open reduction and replacement of the prosthesis with a longer stem. For several patients, we found that the prosthesis stem was unstable even though the fractured part was away from the tip of the stem. Therefore, we recommended prosthesis replacement instead of fixation with plates and screws. For patients with type II PPF, we recommended open reduction and internal fixation with plates. On the one hand, the residual bone was closely connected to the prosthesis stem and stable. On the other hand, there is enough bone mass and space around the fracture site for fixation with plates and screws. Hence, fixation with plates and screws was recommended considering the economic condition of patients. Up to now, those patients recovered successfully without any other complications from the first revision surgery. Fortunately, the mean MSTS score of this study was 20 points, and all patients were satisfied with the therapeutic effect of our revision surgery.
One of the main limitations is the low number of cases. In this study, we proposed two different types of surgical procedures to treat PPF in different types and achieve the goal of individualized treatment. Another limitation is that the time of follow-up of several cases is too short, and further follow-up was needed. Therefore, more clinical practice is needed to validate our proposed treatment strategy for different types of PPF.
Conclusion
PPF is a significant concern for musculoskeletal oncologists, particularly in younger patients. Additionally, PPF poses a challenge for orthopedic surgeons, as limb preservation should be an important goal. The difficulty lies in different types and severity and needs different means to work out. Minimizing complications and improving stability are the key to success. We demonstrate two types of procedures. The preservation of the extremity should always be the primary goal.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Third Affiliated Hospital of Southern Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
Q-LJ: Investigation, Methodology, Writing – original draft, Writing – review & editing. H-BS: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. S-HD: Investigation. C-HH: Data curation. ML: Data curation. S-WD: Writing – review & editing. Z-XL: Writing – review & editing. WC: Project administration, Writing – review & editing. H-ML: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported financially by the Clinical High-tech, Major and Special Technology Research of Guangzhou (No. 2023P-TS08) and the Basic and Applied Basic Research of Guangzhou (No. 2024A04J4646).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Biermann JS, Chow W, Reed DR, Lucas D, Adkins DR, Agulnik M, et al. NCCN guidelines insights: bone cancer, version 2.2017. J Natl Compr Canc Netw (2017) 15:155–67. doi: 10.6004/jnccn.2017.0017
2. Bacci G, Ferrari S, Bertoni F, Ruggieri P, Picci P, Longhi A, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol (2000) 18:4016–27. doi: 10.1200/JCO.2000.18.24.4016
3. Bielack S, Carrle D, Casali PG, ESMO Guidelines Working Group. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol (2009) 20(Suppl 4):137–9. doi: 10.1093/annonc/mdp154
4. Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res (2009) 152:239–62. doi: 10.1007/978-1-4419-0284-9_12
5. Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res (2006) 450:164–71. doi: 10.1097/01.blo.0000223978.36831.39
6. Mo S, Ding Z-Q, Kang L-Q, Zhai W-L, Liu H. Modified technique using allograft-prosthetic composite in the distal femur after bone tumor resection. J Surg Res (2013) 182:68–74. doi: 10.1016/j.jss.2012.08.012
7. Biau D, Faure F, Katsahian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am (2006) 88:1285–93. doi: 10.2106/JBJS.E.00553
8. Henderson ER, O’Connor MI, Ruggieri P, Windhager R, Funovics PT, Gibbons CL, et al. Classification of failure of limb salvage after reconstructive surgery for bone tumours: a modified system Including biological and expandable reconstructions. Bone Joint J (2014) 96-B:1436–40. doi: 10.1302/0301-620X.96B11.34747
9. Myers GJC, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br (2007) 89:521–6. doi: 10.1302/0301-620X.89B4.18631
10. Pala E, Trovarelli G, Calabrò T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clin Orthop Relat Res (2015) 473:891–9. doi: 10.1007/s11999-014-3699-2
11. Zan P, Wang H, Cai Z, Shen J, Sun W. Revision surgeries for tumor endoprostheses around the knee joint: a mid-long-term follow-up of 20 cases. World J Surg Onc (2022) 20:76. doi: 10.1186/s12957-022-02542-0
12. Theil C, Röder J, Gosheger G, Deventer N, Dieckmann R, Schorn D, et al. What is the likelihood that tumor endoprostheses will experience a second complication after first revision in patients with primary Malignant bone tumors and what are potential risk factors? Clin Orthop Relat Res (2019) 477:2705–14. doi: 10.1097/CORR.0000000000000955
13. El Ghoneimy AM, Shehab AM, Farid N. What is the cumulative incidence of revision surgery and what are the complications associated with stemmed cementless nonextendable endoprostheses in patients 18 years or younger with primary bone sarcomas about the knee. Clin Orthop Relat Res (2022) 480:1329–38. doi: 10.1097/CORR.0000000000002150
14. Al-Jabri T, Ridha M, McCulloch RA, Jayadev C, Kayani B, Giannoudis PV. Periprosthetic distal femur fractures around total knee replacements: A comprehensive review. Injury (2023) 54:1030–8. doi: 10.1016/j.injury.2023.02.037
15. Konow T, Baetz J, Melsheimer O, Grimberg A, Morlock M. Factors influencing periprosthetic femoral fracture risk: a German registry study. Bone Joint J (2021) 103-B:650–8. doi: 10.1302/0301-620X.103B4.BJJ-2020-1046.R2
16. Konan S, Sandiford N, Unno F, Masri BS, Garbuz DS, Duncan CP. Periprosthetic fractures associated with total knee arthroplasty: an update. Bone Joint J (2016) 98-B:1489–96. doi: 10.1302/0301-620X.98B11.BJJ-2016-0029.R1
17. Duncan CP, Haddad FS. The Unified Classification System (UCS): improving our understanding of periprosthetic fractures. Bone Joint J (2014) 96-B:713–6. doi: 10.1302/0301-620X.96B6.34040
18. Wu J, Zhu D, Wang J, Wang J, Liu Y, Lei J. Periprosthetic femoral fractures around tumor endoprostheses treated with limited revision surgery combined with allograft: A case report. Med (Baltimore) (2019) 98:e15018. doi: 10.1097/MD.0000000000015018
19. Han Q, Zhao X, Wang C, Chen B, Wang X, Zhang Z, et al. Individualized reconstruction for severe periprosthetic fractures around the tumor prosthesis of knee under assistance of 3D printing technology: A case report. Med (Baltimore) (2018) 97:e12726. doi: 10.1097/MD.0000000000012726
20. Piccioli A, Rossi B, Sacchetti FM, Spinelli MS, Di Martino A. Fractures in bone tumour prosthesis. Int Orthop (2015) 39:1981–7. doi: 10.1007/s00264-015-2956-7
21. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res (1993) 286:241–6. doi: 10.1097/00003086-199301000-00035
22. Bus MPA, Bramer J a. M, Schaap GR, Schreuder HWB, Jutte PC, van der Geest ICM, et al. Hemicortical resection and inlay allograft reconstruction for primary bone tumors: a retrospective evaluation in the Netherlands and review of the literature. J Bone Joint Surg Am (2015) 97:738–50. doi: 10.2106/JBJS.N.00948
23. Barut N, Anract P, Babinet A, Biau D. Peri-prosthetic fractures around tumor endoprostheses: a retrospective analysis of eighteen cases. Int Orthop (2015) 39:1851–6. doi: 10.1007/s00264-015-2915-3
24. Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am (2011) 93:418–29. doi: 10.2106/JBJS.J.00834
25. Lunebourg A, Mouhsine E, Cherix S, Ollivier M, Chevalley F, Wettstein M. Treatment of type B periprosthetic femur fractures with curved non-locking plate with eccentric holes: Retrospective study of 43 patients with minimum 1-year follow-up. Orthop Traumatol Surg Res (2015) 101:277–82. doi: 10.1016/j.otsr.2015.01.015
26. Apivatthakakul T, Phornphutkul C, Bunmaprasert T, Sananpanich K, Fernandez Dell’Oca A. Percutaneous cerclage wiring and minimally invasive plate osteosynthesis (MIPO): a percutaneous reduction technique in the treatment of Vancouver type B1 periprosthetic femoral shaft fractures. Arch Orthop Trauma Surg (2012) 132:813–22. doi: 10.1007/s00402-012-1489-4
27. Spina M, Rocca G, Canella A, Scalvi A. Causes of failure in periprosthetic fractures of the hip at 1- to 14-year follow-up. Injury (2014) 45(Suppl 6):S85–92. doi: 10.1016/j.injury.2014.10.029
28. Zuurmond RG, van Wijhe W, van Raay JJ a. M, Bulstra SK. High incidence of complications and poor clinical outcome in the operative treatment of periprosthetic femoral fractures: An analysis of 71 cases. Injury (2010) 41:629–33. doi: 10.1016/j.injury.2010.01.102
29. Verma N, Jain A, Pal C, Thomas S, Agarwal S, Garg P. Management of periprosthetic fracture following total knee arthroplasty- a retrospective study to decide when to fix or when to revise? J Clin Orthop Trauma (2020) 11:S246–54. doi: 10.1016/j.jcot.2019.10.005
30. Haijie L, Dasen L, Tao J, Yi Y, Xiaodong T, Wei G. Implant survival and complication profiles of endoprostheses for treating tumor around the knee in adults: A systematic review of the literature over the past 30 years. J Arthroplasty (2018) 33:1275–1287.e3. doi: 10.1016/j.arth.2017.10.051
31. Avedian RS, Goldsby RE, Kramer MJ, O’Donnell RJ. Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clin Orthopaedics Related Res (2007) 459:48–53. doi: 10.1097/BLO.0b013e3180514c66
32. Giurea A, Paternostro T, Heinz-Peer G, Kaider A, Gottsauner-Wolf F. Function of reinserted abductor muscles after femoral replacement. J Bone Joint Surg Br (1998) 80(2):284-7. doi: 10.1302/0301-620x.80b2.8179
Keywords: osteosarcoma, periprosthetic fracture, distal femur, limb salvage, revision surgery
Citation: Jin Q-l, Su H-b, Du S-h, Hou C-h, Lu M, Dai S-w, Lei Z-x, Chen W and Li H-m (2024) Revision surgery for periprosthetic fracture of distal femur after endoprosthetic replacement of knee joint following resection of osteosarcoma. Front. Oncol. 14:1328703. doi: 10.3389/fonc.2024.1328703
Received: 27 October 2023; Accepted: 12 January 2024;
Published: 12 February 2024.
Edited by:
Tao Ji, Peking University People’s Hospital, ChinaReviewed by:
Xuanhong He, Sichuan University, ChinaPaul Simon Unwin, Independent Researcher, Ludlow, United Kingdom
Copyright © 2024 Jin, Su, Du, Hou, Lu, Dai, Lei, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao-miao Li, bGloYW9taWFvMTk3N0Bob3RtYWlsLmNvbQ==; Wei Chen, NTEwMTI4NTg0QHFxLmNvbQ==
†These authors have contributed equally to this work
 Qing-lin Jin
Qing-lin Jin Hao-bin Su
Hao-bin Su Shao-hua Du
Shao-hua Du Ming Lu
Ming Lu Wei Chen
Wei Chen Hao-miao Li
Hao-miao Li