
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 May 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1326385
This article is part of the Research Topic Best Surgical Treatment of Breast Cancer Managed Primarily with Neoadjuvant Medical Therapy View all 9 articles
 Amina Maimaitiaili1†
Amina Maimaitiaili1† Yijun Li1†
Yijun Li1† Na Chai1
Na Chai1 Zhenzhen Liu2
Zhenzhen Liu2 Rui Ling3
Rui Ling3 Yi Zhao4
Yi Zhao4 Hongjian Yang5
Hongjian Yang5 Yunjiang Liu6
Yunjiang Liu6 Ke Liu7
Ke Liu7 Jianguo Zhang8
Jianguo Zhang8 Dahua Mao9
Dahua Mao9 Zhigang Yu10
Zhigang Yu10 Yinhua Liu11
Yinhua Liu11 Peifen Fu12
Peifen Fu12 Jiandong Wang13
Jiandong Wang13 Hongchuan Jiang14
Hongchuan Jiang14 Zuowei Zhao15
Zuowei Zhao15 Xingsong Tian16
Xingsong Tian16 Zhongwei Cao17
Zhongwei Cao17 Kejin Wu18
Kejin Wu18 Ailin Song19
Ailin Song19 Feng Jin20
Feng Jin20 Puzhao Wu21
Puzhao Wu21 Jianjun He1*
Jianjun He1* Zhimin Fan22*
Zhimin Fan22* Huimin Zhang1*
Huimin Zhang1*Purpose: This study aimed to investigate the factors associated with pathologic node-negativity (ypN0) in patients who received neoadjuvant chemotherapy (NAC) to develop and validate an accurate prediction nomogram.
Methods: The CSBrS-012 study (2010–2020) included female patients with primary breast cancer treated with NAC followed by breast and axillary surgery in 20 hospitals across China. In the present study, 7,711 eligible patients were included, comprising 6,428 patients in the primary cohort from 15 hospitals and 1,283 patients in the external validation cohort from five hospitals. The hospitals were randomly assigned. The primary cohort was randomized at a 3:1 ratio and divided into a training set and an internal validation set. Univariate and multivariate logistic regression analyses were performed on the training set, after which a nomogram was constructed and validated both internally and externally.
Results: In total, 3,560 patients (46.2%) achieved ypN0, and 1,558 patients (20.3%) achieved pathologic complete response in the breast (bpCR). A nomogram was constructed based on the clinical nodal stage before NAC (cN), ER, PR, HER2, Ki67, NAC treatment cycle, and bpCR, which were independently associated with ypN0. The area under the receiver operating characteristic curve (AUC) for the training set was 0.80. The internal and external validation demonstrated good discrimination, with AUCs of 0.79 and 0.76, respectively.
Conclusion: We present a real-world study based on nationwide large-sample data that can be used to effectively screen for ypN0 to provide better advice for the management of residual axillary disease in breast cancer patients undergoing NAC.
With the recognition of the importance of biology and systematic therapy in local control, we gradually agree that larger surgery does not cure bad biology in breast cancer (1). The adoption of a true multidisciplinary treatment approach, rather than the sequential use of different therapies, decreases the extent of surgery and its associated morbidity (2, 3).
Axillary lymph node dissection (ALND) has traditionally been used as routine axillary surgical management for breast cancer patients (4). Multiple prospective, randomized trials led by the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial (5) demonstrated that sentinel lymph node biopsy (SLNB) can replace ALND in patients with low nodal burden disease because of noninferior local control and survival, but with lower surgical morbidity. Neoadjuvant chemotherapy (NAC) results in frequent downstaging of tumors in both the breast and axilla, which can lead to fewer surgeries in patients with larger tumors at diagnosis. The implementation of NAC has enabled selected women to undergo breast-conserving surgery (BCS) in the last two decades; however, for patients who received NAC, the chance of de-escalated axillary surgery has not improved (6). The National Comprehensive Cancer Network (NCCN) breast cancer guidelines recommend SLNB for patients with cN0 to ycN0 disease after NAC, but ALND is still recommended for patients who are converted from cN+ to cN0, and SLNB is usually considered a relative contraindication due to its low identification rate and high false-negative rate (FNR) (7, 8). In the SENTinel NeoAdjuvant (SENTINA) study (9), the detection rate of SLNB after NAC in patients with cN+ to cN0 disease was 80.1% (95% CI 76.6–83.2), and the false-negative rate was 14.2% (95% CI 9.9–19.4). However, approximately 74% of breast cancer patients with cN0 disease are sentinel lymph node-negative, and postoperative complications still occur even after SLNB (10, 11).
Patients with a low risk of residual axillary involvement after NAC could benefit from omitting axillary surgical intervention if there are accurate tools for nodal response prediction (12). Currently, the commonly used clinical imaging methods for evaluating the axillary region include ultrasound, mammography, magnetic resonance imaging (MRI), and positron emission tomography CT (PET-CT) (13–15). Nevertheless, the accuracy of these techniques remains low, and there are no unified guidelines for axillary imaging evaluation of NAC response (16). The ACOSOG Z1071 (Alliance) trial reported that axillary ultrasound (AUS) after NAC can identify abnormal nodes, guide patient selection for SLN surgery instead of ALND, and reduce the FNR of SLNB to less than 10%. However, the accuracy of AUS after NAC was low; only 43.2% of patients who were negative for AUS were confirmed to have nodal pCR by ALND (16). Investigators also attempted to decrease the FNR by marking positive lymph nodes at diagnosis before NAC. However, clips can be found during surgery in only half of the patients (17). Imaging-guided localization (IGL) of the clipped node was introduced to increase the likelihood of clip removal. The lowest FNR was achieved when IGL was added to SLN biopsy, a procedure called targeted axillary dissection (TAD) (18, 19). However, it did not significantly change the performance of tailored axillary surgery, which left ≥2 positive nodes behind in 47.6% of the patients (20, 21). In all these explorations, the prediction model based on clinical and pathological factors still has clinical value and application prospects. The present study aimed to identify factors that are predictive of ypN0 and construct a novel nomogram that can effectively predict nodal negativity and thus potentially avoid axillary surgery, which can reduce women’s loss of function and lymphedema.
The Chinese Society of Breast Surgery (CSBrS-012) is a nationwide, multicenter, 10-year retrospective clinical epidemiological study conducted across 20 hospitals in China. The CSBrS-012 study included female primary breast cancer patients who received NAC and underwent standard breast and axillary surgery after NAC between January 2010 and December 2020. The 20 hospitals are located in central, northern, eastern, northwestern, northeastern, and southwestern China, and represent different levels of breast cancer burden. After excluding patients with incomplete data, 7,711 patients were enrolled in the study. Hospitals were randomly assigned to two groups comprising 6,428 patients in the primary cohort from 15 hospitals and 1,283 patients in the external validation cohort from five hospitals. We then randomized the patients in the primary cohort at a 3:1 ratio into the training and internal validation sets (Figure 1). The study was performed in accordance with the Declaration of Helsinki and approved by the Ethical Review Committee of the First Hospital of Jilin University (No. 2021–066). As this was a retrospective study and all data analyses were performed anonymously, the requirement for informed consent from the patients was waived.
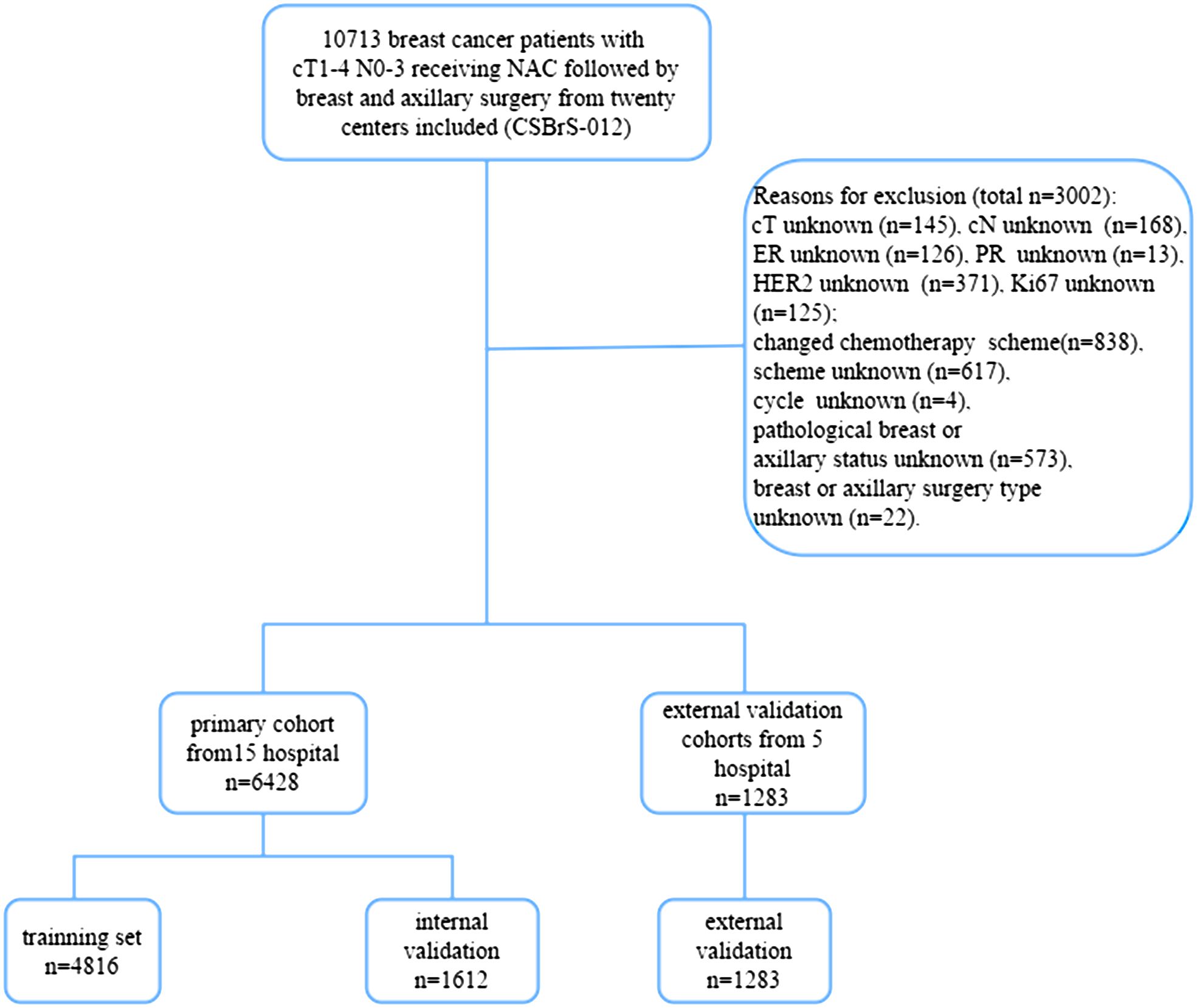
Figure 1 Flow diagram of the study. NAC, neoadjuvant chemotherapy; CSBrS-012: The Chinese Society of Breast Surgery study (2010–2020).
Variables included age, clinical tumor (cT) and clinical nodal (cN) stages before NAC, tumor histology, ER, PR, HR, HER2, Ki-67, biological subtypes, NAC regimen, NAC treatment cycle, and pCR status. Immunohistochemistry (IHC) was used to detect the expression of ER, PR, HER2, and Ki-67. ER and PR were defined as positive if ≥1% of cells were positive. HR was defined as positive if the ER and/or PR were positive. HER2 expression was defined as positive if 3+ by IHC or 2+ by IHC and positive by in situ hybridization. Tumor subtypes were categorized according to St. Gallen criteria (22): HR+/HER2−, HR+/HER2+, HR−/HER2+, and TNBC. The T and N stages were defined according to the 8th edition of the American Joint Committee on Cancer (AJCC) (23). cN0 was defined as no suspicious lymph nodes on axillary ultrasound or suspicious lymph nodes on axillary ultrasound but negative on either fine needle aspiration cytology or core needle biopsy or negative on SLNB prior to NAC. Suspicious lymph nodes were considered in cases of a hypoechoic round shape, focally thickened cortex, or absent fatty hilum. pCR was defined as the absence of residual invasive or in situ carcinoma in the breast or axillary lymph nodes (ypT0/ypN0). NAC and surgery were performed in accordance with the Chinese Society of Clinical Oncology (CSCO) Breast Cancer Guidelines and the National Comprehensive Cancer Network (NCCN) Breast Cancer Guidelines. In our study, we divided NAC regimens into three categories: (1) anthracycline combined with taxane, (2) taxane combined with platinum, and (3) other regimens.
Statistical analyses were performed using SPSS version 21.0 (Inc., Chicago, IL, USA) and R 4.2.2 (R Project for Statistical Computing) software. The differences in clinicopathological parameters between the training and internal validation sets were evaluated using Pearson’s χ2 test. Univariate logistic regression and backward stepwise selection were used for the final multivariate model. A predictive nomogram for ypN0 was established based on independent risk factors identified via multivariate analysis. The predictive value of the model was appraised using receiver operating characteristic (ROC) curves and calibration curves. The AUC (area under the receiver operating characteristic curve) was calculated.
A total of 7,711 female breast cancer patients, with a median age of 49 years, were enrolled. The baseline characteristics of the patients are summarized in Table 1. The proportion of patients with initial stage cT1–2 tumors was 79.8%, and that with initial stage cT3–4 tumors was 20.2%. The proportion of patients with cN0–1 stage disease in the study population was greater than that of patients with cN2–3 stage disease (81.5% vs. 18.5%). Most patients had invasive ductal cancer (6,971 [90.4%]). Anthracyclines and taxanes were the most common NAC treatments (76.3%). Approximately half of the HER2+patients received targeted therapy, among which single-agent HER2 blockade was more than twice as frequent as dual HER2 blockade.
As shown in Table 2, 3,560 patients (46.2%) achieved ypN0, and 1,558 patients (20.3%) achieved bpCR. Among the patients who achieved bpCR, 75.3% had ypN0, whereas 38.7% did not (p <0.001). The pathological responses of the breast and axillary lymph nodes according to the biological subtype are summarized in Table 3. Responses to NAC in the different subgroups were generally consistent between the breast and axillary regions. In both the breast and axilla, HR-negative patients showed a better response to NAC than HR-positive patients (p <0.001). In both the breast and axilla, HR+/HER2− subtypes exhibited relatively poor responses to NAC compared to the other subtypes (p <0.001). The ypN0 rate for all subtypes was significantly higher than the bpCR rate (p <0.05).
According to the univariate logistic regression analyses of the training set, cN stage, ER, PR, HER2, Ki67, NAC treatment cycle, and bpCR were associated with ypN0. All of the above parameters were subjected to multivariate logistic regression using backward selection analysis, and a lower cN stage, ER-negative status, PR-negative status, HER2-positive status with targeted therapy, Ki67 level ≥20, more NAC treatment cycles, and bpCR were confirmed to be independent predictors of ypN0 (Table 4).
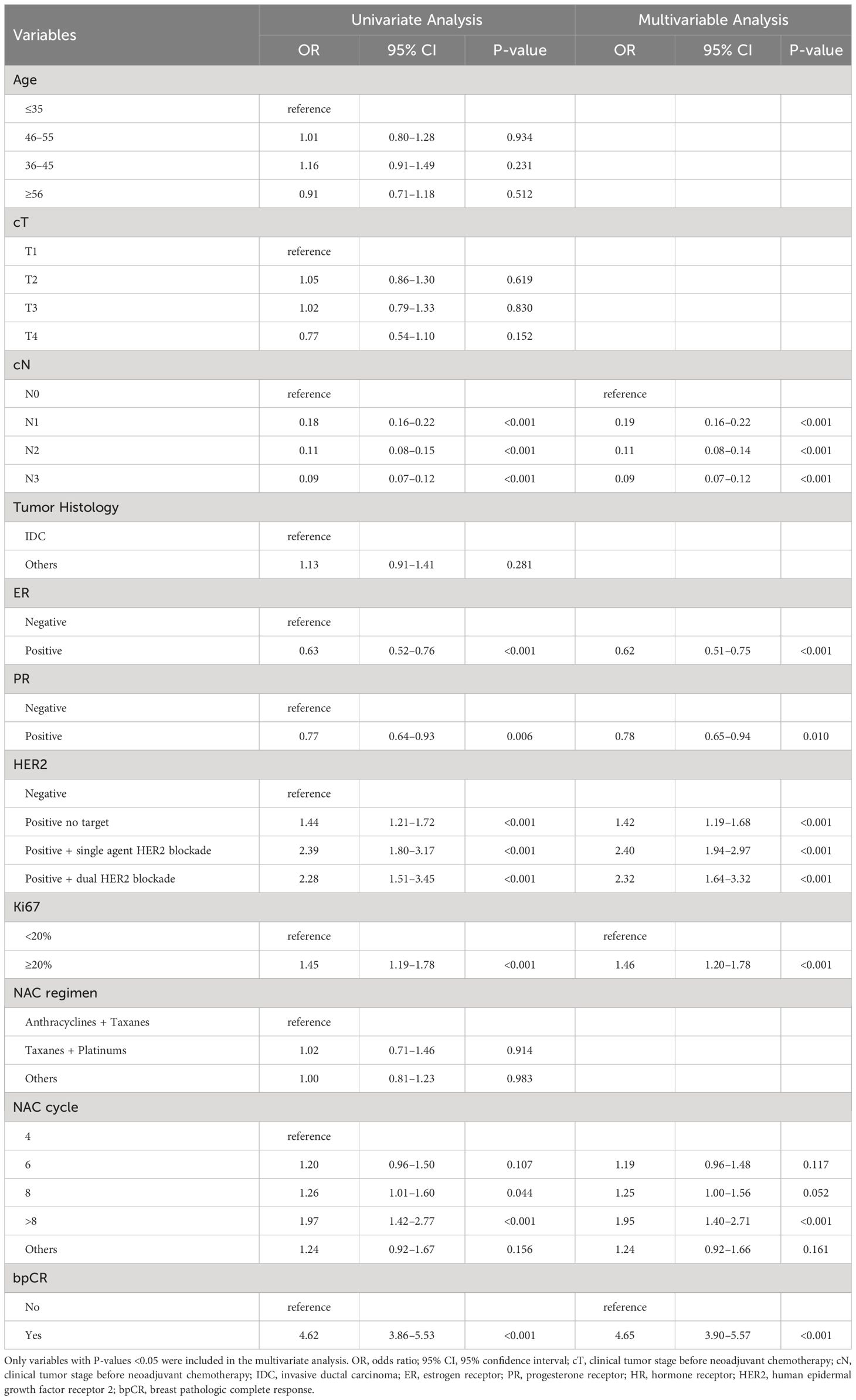
Table 4 Univariate and multivariate logistic analysis of factors predict the lymph node positivity after NAC in the training set.
A nomogram to predict ypN0 was developed based on multivariate logistic regression results. Points were assigned to each variable and summed to obtain the total number of points. Finally, the probability of ypN0 was determined by drawing a vertical line from the total score to the bottom row (Figure 2A). For example, a patient with HER2-amplified breast cancer with cN1 and Ki67 >20 who received eight cycles of NAC with single targeted therapy and achieved bpCR had a total of 188 points, so the possibility of ypN0 after NAC for this patient was 88% (Figure 2B), and a patient with triple-negative breast cancer with cN1 and Ki67 >20 who received eight cycles of NAC and did not achieve bpCR had a total of 88 points, so the possibility of ypN0 after NAC for this patient was 40% (Figure 2C).
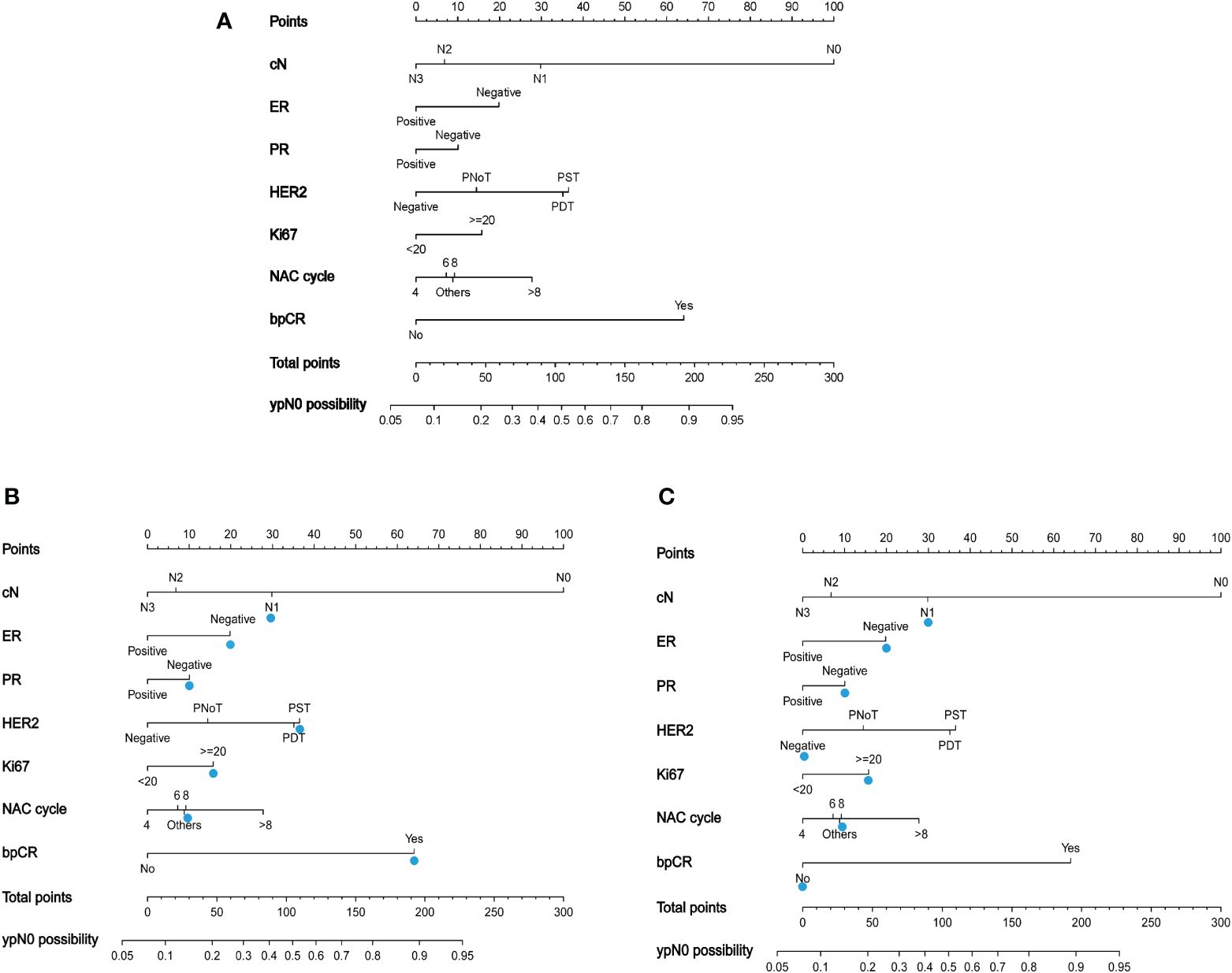
Figure 2 (A) A nomogram to predict the probability of ypN0 in breast cancer patients receiving neoadjuvant chemotherapy. PNoT, HER2 positive without targeted therapy; PST, HER2 positive with single agent HER2 blockade; PDT, HER2 positive with dual HER2 blockade. (B) The blue triangle demonstrates usage of the model: a patient with HER2-amplified breast cancer with cN1 and Ki67 >20 who received eight cycles of NAC with single-targeted therapy and achieved bpCR had a total of 188 points, and the possibility of ypN0 after NAC for this patient was 88%; (C) a patient with triple-negative breast cancer with cN1 and Ki67 >20 who received eight cycles of NAC and did not achieve bpCR had a total of 88 points. The possibility of ypN0 after NAC was 40% for this patient.
The discriminatory ability of the nomogram to predict ypN0 status was investigated using receiver operating characteristic (ROC) curve analysis. The AUCs of the training, internal validation, and external validation sets were 0.80, 0.79, and 0.76, respectively, indicating that the nomogram had potentially promising predictive power. The calibration plots presented excellent agreement between the training and validation sets and showed no significant difference between the predicted and actual probabilities of ypN0 (P = 1.000) (Figures 3–5).
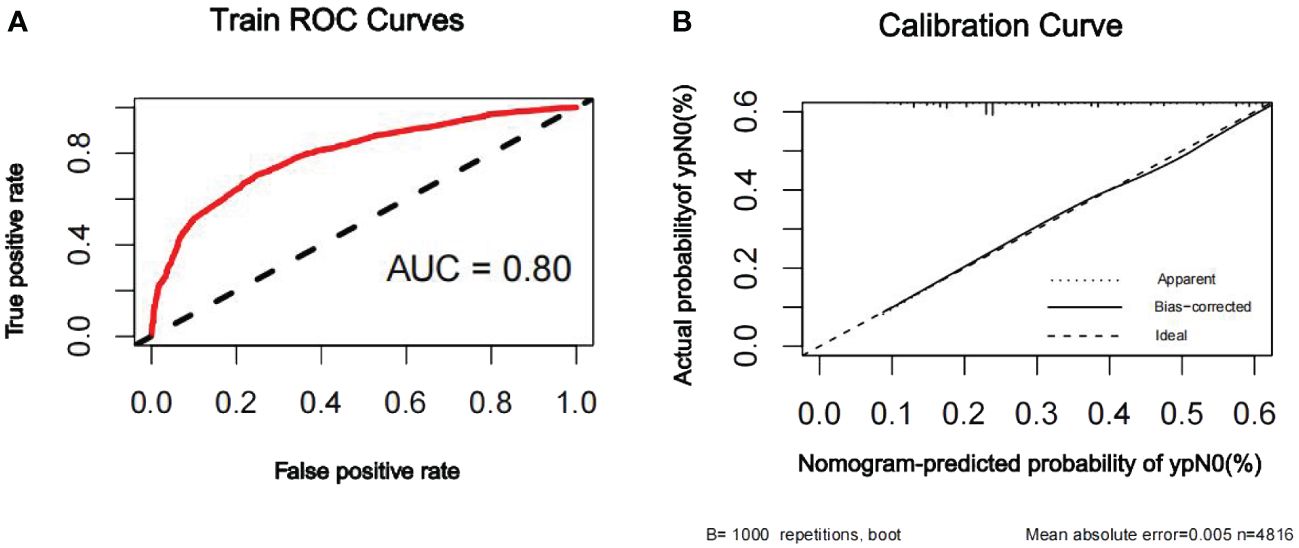
Figure 3 ROC curve (A) and calibration curve (B) are shown for the prediction model of ypN0 in the training cohort. The ROC curve for the training set indicated an AUC of 0.80. ROC, receiver operating characteristic curve; AUC, area under the curve.
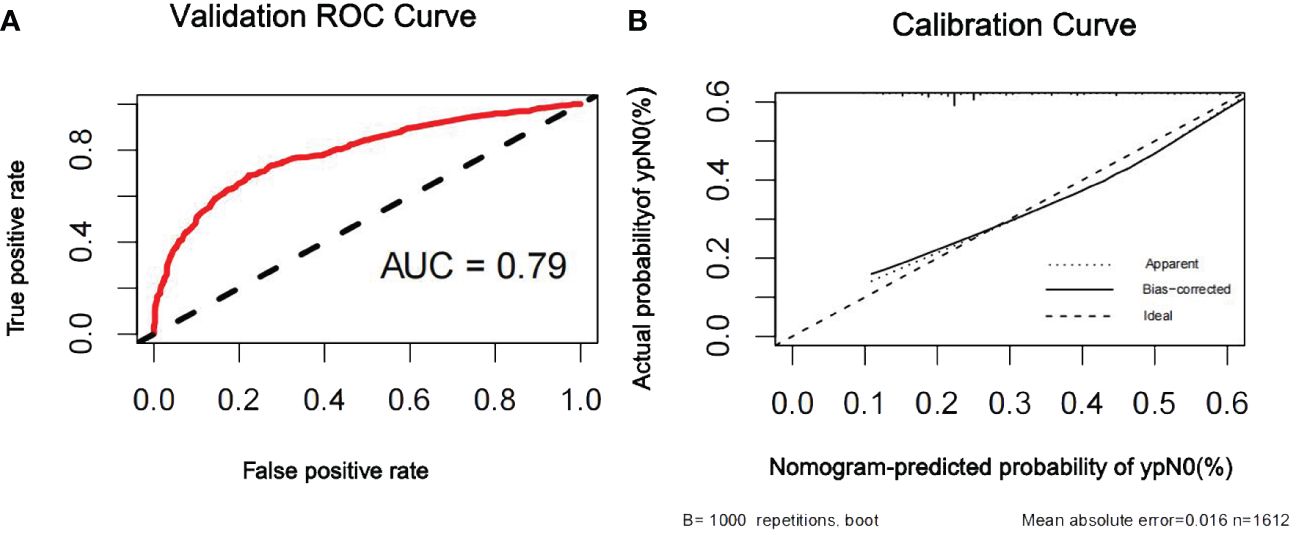
Figure 4 ROC curve (A) and calibration curve (B) are shown for the prediction model of ypN0 in the internal validation cohort. For discrimination in the internal validation set, the ROC curve indicated an AUC of 0.79. ROC, receiver operating characteristic curve; AUC, area under the curve.
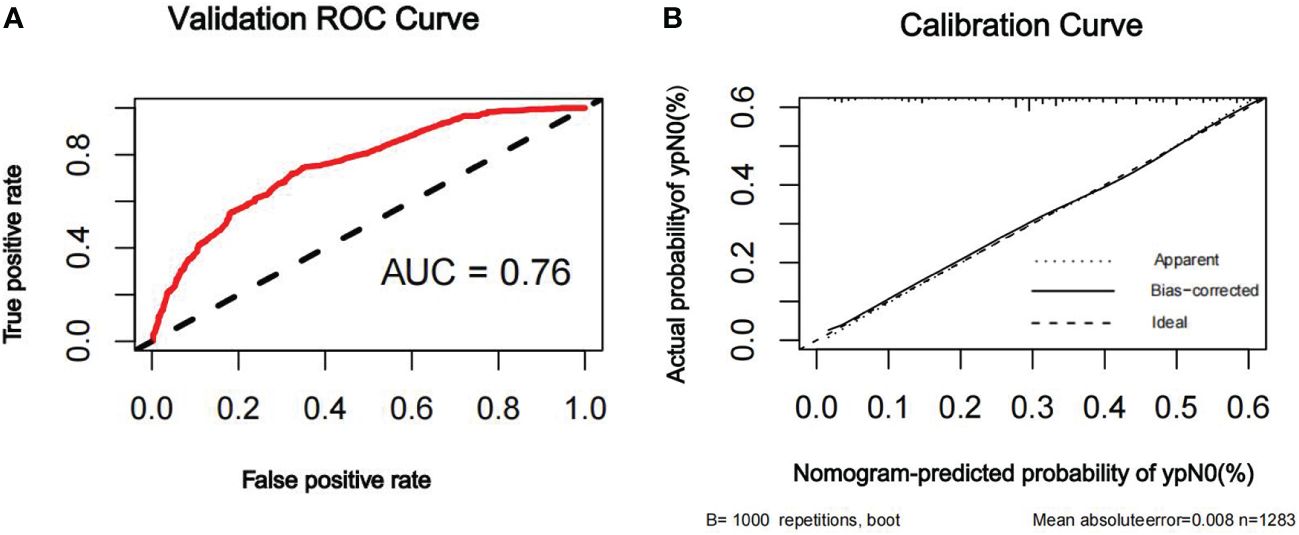
Figure 5 ROC curve (A) and calibration curve (B) are shown for the prediction model of ypN0 in the external validation cohort. For discrimination in the external validation set, the ROC curve indicated an AUC of 0.76. ROC, receiver operating characteristic curve; AUC, area under the curve.
Among patients with cN0 breast cancer, approximately 74% do not have axillary lymph node metastasis (24). This means that even SLNB represents overtreatment and causes unnecessary complications, with few advantages for many patients. However, the St. Gallen Consensus Panel in 2017 (25) and the German AGO recommendation in 2022 (26) recommend SLNB as the standard surgical procedure for patients who present with cN0 before and after NAC. In patients who are cN+ and achieved nodal pCR after NAC, ALND is still performed in clinical practice in some cases because of the unacceptable identification rate and FNR of SLNB (7–9, 27).
Recently, the 5-year survival results of the SOUND trial were published (28). This was a prospective non-inferiority phase III randomized clinical trial that enrolled 1,463 patients with small breast tumors (<2 cm) and a cN0 stage. Patients were randomized in a 1:1 ratio to either the SLNB group or the no axillary surgery group. Interestingly, omission of axillary surgery was not inferior to SLNB in terms of the 5-year DFS and OS. This was a study of patients who underwent upfront surgery. For patients who receive NAC, multiple prospective trials investigating whether axillary surgery can be safely abandoned in selected patients are underway. The European Breast Cancer Research Association of Surgical Trialists (EUBREAST)-01 is a prospective clinical trial in which axillary surgery will be eliminated completely (no SLNB) for initially cN0 patients with radiological complete remission and breast pCR in the lumpectomy specimen (29). The ASICS trial is a non-inferiority, single-arm trial open to both breast-conserving and mastectomy patients in which no SLNB is performed in cN0, triple-negative, or HER2-positive breast cancer patients with a radiological complete response on MRI (30). The results of these trials are expected in to continue for the next 5 years. Meanwhile, the prediction model for axillary nodal burden based on clinical and pathological factors has clinical value and application prospects. In the present study, we presented and validated a model based on nationwide multicenter data of breast cancer patients to predict the possibility of ypN0 disease after NAC. Moreover, to prove its universality, we externally validated the nomogram using patient information from different hospitals.
Researchers at the MD Anderson Cancer Center first proposed that breast pCR is strongly correlated with nodal status after NCT (31). In the present study, 46.2% of patients achieved ypN0 and 20.3% of patients achieved bpCR, and the rate of ypN0 was greater on patients who achieved bpCR than in the nonbpCR group (75.3% vs. 38.7%). Tumor response to NAC was significantly related to tumor subtype. Barron et al. (32) reported 30,821 patients with cT1/cT2 cN0/cN1 breast cancer treated with NAC from the American National Cancer Database and reported breast pCR rates of 37.2%, 58.2%, 37.2%, and 13.1%, respectively. The ypN0 rates were 78.6%, 84.5%, 75.3%, and 47.0% for TNBC, HR−/HER2+, HR+/HER2+, and HR+/HER2− subtypes, respectively. In our study, the distribution of tumor subtypes was consistent with that in the above study; however, the rates of bpCR and ypN0 were low because we included cT3–4 and cN2–3 patients. In addition, the pCR and ypN0 rates of HER2+ patients were not significantly high in the current study, possibly because only approximately half of the patients with HER2+ status (50.5%, 1,335/2,642) received molecular-targeted therapy because the targeted drugs were not covered by medical insurance in the early years.
As expected, clinical nodal stage and breast tumor response strongly predicted ypN0. To avoid the influence of receptors on molecular subtypes, we did not include subtypes in the analysis. We found that patients with ER-negative, PR-negative, and HER2-positive disease had a higher ypN0. Ki67 is a proliferation marker, and patients with higher Ki67 levels showed greater sensitivity to chemotherapy in previous studies (33, 34), which was consistent with our study. In the present study, multivariate analysis revealed that more treatment cycles were associated with ypN0, independent of the tumor histology and treatment regimen. Clinical tumor size has been shown to be a predictor of lymph node status in operable breast cancer patients in several previous studies (35–37); however, in the context of NAC, the relationship between cT and ypN0 was not significant in our study.
We developed a nomogram based on multivariate logistic regression results. In contrast to previous nomograms that predicted axillary pCR in initially cN+ patients (38–40) or in specific subtypes (41, 42), the current nomogram predicted ypN0 in all patients with stage cT1–4N0–3 disease. The AUC of the nomogram in the ROC curve analysis was 0.80, 0.79, and 0.76 in the training, internal, and external validation cohorts, respectively, and showed good discrimination in the prediction of ypN0. The advantage of our prediction nomogram is that most breast cancer patients who receive NAC can be assessed, and the indicators for building the nomogram can be easily acquired by surgeons. Moreover, as mentioned above, our findings are consistent with those of previous studies in a global context in terms of pCR for different subtypes, which indicates that our nomogram can also be applied to patients in different countries.
There are several limitations in our study. First, histological grade was found to be an independent prognostic factor for pCR in patients with breast cancer in previous studies (43, 44). In our study, we could not analyze this factor because it was not included in the initial database. Second, if we put this nomogram into practice for the omission of any axillary surgery, it should be determined before surgery, but bpCR is available after surgery. However, multiple studies have explored methods to detect bpCR without surgery (45–48). A prospective trial showed that image-guided vacuum-assisted core biopsy (VACB) of the primary breast tumor bed following NAC can identify patients who are very likely to have a bpCR with an FNR of <5% (49). Another potential limitation of this study was its retrospective nature. Our study, which reflects the current clinical practices across the country, will facilitate the design of prospective clinical trials in the future.
Future Directions: The developed nomogram may help clinicians weigh the lymph node tumor burden after NAC more appropriately. However, if our research conclusions are extended to clinical work, further clinical trials using this nomogram are required to determine the survival and local recurrence rates of patients who avoid axillary surgery following NAC. The authors expected that related studies of the nomogram could lead to more feasible progress, and that the nomogram could be well connected with targeted axillary dissection, including the clipped node.
We present a real-world study based on nationwide large sample data and construct a nomogram model that can effectively screen ypN0 to provide better advice for the management of residual axillary disease in breast cancer patients receiving NAC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethical Review Committee of the First Hospital of Jilin University (No. 2021-066). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because as this was a retrospective study and all data analyses were performed anonymously.
AM: Conceptualization, Formal Analysis, Methodology, Software, Validation, Writing – original draft, Investigation. YiJL: Data curation, Formal Analysis, Methodology, Software, Validation, Writing – review & editing. NC: Data curation, Writing – review & editing. ZL: Data curation, Writing – review & editing. RL: Data curation, Writing – review & editing. YZ: Data curation, Writing – review & editing. HY: Data curation, Writing – review & editing. YuJL: Data curation, Writing – review & editing. KL: Data curation, Writing – review & editing. JZ: Data curation, Writing – review & editing. DM: Data curation, Writing – review & editing. ZY: Data curation, Writing – review & editing. YHL: Data curation, Writing – review & editing. PF: Data curation, Writing – review & editing. JW: Data curation, Writing – review & editing. HJ: Data curation, Writing – review & editing. ZZ: Data curation, Writing – review & editing. XT: Data curation, Writing – review & editing. ZC: Data curation, Writing – review & editing. KW: Data curation, Writing – review & editing. AS: Data curation, Writing – review & editing. FJ: Data curation, Writing – review & editing. PW: Formal Analysis, Writing – review & editing. JH: Conceptualization, Project administration, Resources, Supervision, Visualization, Writing – review & editing. ZF: Conceptualization, Data curation, Resources, Supervision, Writing – review & editing. HZ: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Innovation Capability Support Project of Shaanxi Province (2023KJXX-032) and Xin Rui Cancer Supported Therapy Research Project (cphcf-2023-082).
We would like to thank the member units of the CSBrS for data collection and for supporting this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Morrow M. Leveraging the benefits of systemic therapy to tailor surgery. JAMA Surg. (2017) 152:671. doi: 10.1001/jamasurg.2017.0565
2. Pilewskie M, Sevilimedu V, Eroglu I, Le T, Wang R, Morrow M, et al. How often do sentinel lymph node biopsy results affect adjuvant therapy decisions among postmenopausal women with early-stage HR(+)/HER2(-) breast cancer in the post-rxPONDER era? Ann Surg Oncol. (2022) 29:6267–73. doi: 10.1245/s10434-022-12193-w
3. Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. (2019) 30:1541–57. doi: 10.1093/annonc/mdz235
4. Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. (2018) 29:2153. doi: 10.1093/annonc/mdx806
5. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. Jama. (2017) 318:918–26. doi: 10.1001/jama.2017.11470
6. Petruolo O, Sevilimedu V, Montagna G, Le T, Morrow M, Barrio AV. How often does modern neoadjuvant chemotherapy downstage patients to breast-conserving surgery? Ann Surg Oncol. (2021) 28:287–94. doi: 10.1245/s10434-020-08593-5
7. Vázquez JC, Piñero A, de Castro FJ, Lluch A, Martín M, Barnadas A, et al. The value of sentinel lymph-node biopsy after neoadjuvant therapy: an overview. Clin Transl Oncol. (2022) 24:1744–54. doi: 10.1007/s12094-022-02824-9
8. Lin SQ, Vo NP, Yen YC, Tam KW. Outcomes of sentinel node biopsy for women with breast cancer after neoadjuvant therapy: systematic review and meta-analysis of real-world data. Ann Surg Oncol. (2022) 29:3038–49. doi: 10.1245/s10434-021-11297-z
9. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. (2013) 14:609–18. doi: 10.1016/S1470-2045(13)70166-9
10. Bromham N, Schmidt-Hansen M, Astin M, Hasler E, Reed MW. Axillary treatment for operable primary breast cancer. Cochrane Database Syst Rev. (2017) 1:Cd004561. doi: 10.1002/14651858.CD004561.pub3
11. Voogd AC, Coebergh JW, Repelaer van Driel OJ, Roumen RM, van Beek MW, Vreugdenhil A, et al. The risk of nodal metastases in breast cancer patients with clinically negative lymph nodes: a population-based analysis. Breast Cancer Res Treat. (2000) 62:63–9. doi: 10.1023/A:1006447825160
12. Reimer T. Omission of axillary sentinel lymph node biopsy in early invasive breast cancer. Breast. (2023) 67:124–128. doi: 10.1016/j.breast.2023.01.002
13. Kong X, Zhang Q, Wu X, Zou T, Duan J, Song S, et al. Advances in imaging in evaluating the efficacy of neoadjuvant chemotherapy for breast cancer. Front Oncol. (2022) 12:816297. doi: 10.3389/fonc.2022.816297
14. Sutton EJ, Onishi N, Fehr DA, Dashevsky BZ, Sadinski M, Pinker K, et al. A machine learning model that classifies breast cancer pathologic complete response on MRI post-neoadjuvant chemotherapy. Breast Cancer Res. (2020) 22:57. doi: 10.1186/s13058-020-01291-w
15. Abd El-Gaid S, AbdelHafez MN, Mohamed G, Elazab MSS, Elahmadawy MA. Prediction of pathological response using 18F FDG PET/CT derived metabolic parameters in locally advanced breast cancer patients. Nucl Med Commun. (2022) 43:292–303. doi: 10.1097/MNM.0000000000001515
16. Boughey JC, Ballman KV, Hunt KK, McCall LM, Mittendorf EA, Ahrendt GM, et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American college of surgeons oncology group Z1071 trial (Alliance). J Clin Oncol. (2015) 33:3386–93. doi: 10.1200/JCO.2014.57.8401
17. Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. (2016) 263:802–7. doi: 10.1097/SLA.0000000000001375
18. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. (2016) 34:1072–8. doi: 10.1200/JCO.2015.64.0094
19. Swarnkar PK, Tayeh S, Michell MJ, Mokbel K. The evolving role of marked lymph node biopsy (MLNB) and targeted axillary dissection (TAD) after neoadjuvant chemotherapy (NACT) for node-positive breast cancer: systematic review and pooled analysis. Cancers (Basel). (2021) 13(7):1539. doi: 10.3390/cancers13071539
20. Heidinger M, Weber WP. ASO author reflections: imaging-guided localization does not improve the performance of tailored axillary surgery-preplanned OPBC-03/TAXIS substudy. Ann Surg Oncol. (2024) 31:1001–2. doi: 10.1245/s10434-023-14437-9
21. Weber WP, Heidinger M, Hayoz S, Matrai Z, Tausch C, Henke G, et al. Impact of imaging-guided localization on performance of tailored axillary surgery in patients with clinically node-positive breast cancer: prospective cohort study within TAXIS (OPBC-03, SAKK 23/16, IBCSG 57–18, ABCSG-53, GBG 101). Ann Surg Oncol. (2024) 31:344–55. doi: 10.1245/s10434-023-14404-4
22. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. (2013) 24:2206–23. doi: 10.1093/annonc/mdt303
23. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
24. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. (2007) 8:881–8. doi: 10.1016/S1470-2045(07)70278-4
25. Gnant M, Harbeck N, Thomssen C. St. Gallen/vienna 2017: A brief summary of the consensus discussion about escalation and de-escalation of primary breast cancer treatment. Breast Care (Basel). (2017) 12:102–7. doi: 10.1159/000475698
26. Banys-Paluchowski M, Thill M, Kühn T, Ditsch N, Heil J, Wöckel A, et al. AGO recommendations for the surgical therapy of breast cancer: update 2022. Geburtshilfe Frauenheilkd. (2022) 82:1031–43. doi: 10.1055/a-1904-6231
27. van Deurzen CH, Vriens BE, Tjan-Heijnen VC, van der Wall E, Albregts M, van Hilligersberg R, et al. Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: a systematic review. Eur J Cancer. (2009) 45:3124–30. doi: 10.1016/j.ejca.2009.08.001
28. Gentilini OD, Botteri E, Sangalli C, Galimberti V, Porpiglia M, Agresti R, et al. Sentinel lymph node biopsy vs no axillary surgery in patients with small breast cancer and negative results on ultrasonography of axillary lymph nodes: the SOUND randomized clinical trial. JAMA Oncol. (2023) 9(11):1557–64. doi: 10.1001/jamaoncol.2023.3759
29. Reimer T, Glass A, Botteri E, Loibl S, O DG. Avoiding axillary sentinel lymph node biopsy after neoadjuvant systemic therapy in breast cancer: rationale for the prospective, multicentric EUBREAST-01 trial. Cancers (Basel). (2020) 12(12):3698. doi: 10.3390/cancers12123698
30. Hersh EH, King TA. De-escalating axillary surgery in early-stage breast cancer. Breast. (2022) 62 Suppl 1:S43–s49. doi: 10.1016/j.breast.2021.11.018
31. Tadros AB, Yang WT, Krishnamurthy S, Rauch GM, Smith BD, Valero V, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. (2017) 152:665–70. doi: 10.1001/jamasurg.2017.0562
32. Barron AU, Hoskin TL, Day CN, Hwang ES, Kuerer HM, Boughey JC. Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg. (2018) 153:1120–6. doi: 10.1001/jamasurg.2018.2696
33. Potemski P, Pluciennik E, Bednarek AK, Kusinska R, Kubiak R, Jesionek-Kupnicka D, et al. Ki-67 expression in operable breast cancer: a comparative study of immunostaining and a real-time RT-PCR assay. Pathol Res Pract. (2006) 202:491–5. doi: 10.1016/j.prp.2006.02.005
34. Sinn HP, Schneeweiss A, Keller M, Schlombs K, Laible M, Seitz J, et al. Comparison of immunohistochemistry with PCR for assessment of ER, PR, and Ki-67 and prediction of pathological complete response in breast cancer. BMC Cancer. (2017) 17:124. doi: 10.1186/s12885-017-3111-1
35. Moorman AM, Rutgers EJT, Kouwenhoven EA. Omitting SLNB in breast cancer: is a nomogram the answer? Ann Surg Oncol. (2022) 29:2210–8. doi: 10.1245/s10434-021-11007-9
36. Alsumai TS, Alhazzaa N, Alshamrani A, Assiri S, Alhefdhi A. Factors predicting positive sentinel lymph node biopsy in clinically node-negative breast cancer. Breast Cancer (Dove Med Press). (2022) 14:323–34. doi: 10.2147/BCTT.S373005
37. Sanders SB, Hoskin TL, Stafford AP, Boughey JC. Factors influencing non-sentinel lymph node involvement in patients with positive sentinel lymph node(s) after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. (2022) 29(12):7769–78. doi: 10.1245/s10434-022-12064-4
38. Weiss A, Campbell J, Ballman KV, Sikov WM, Carey LA, Hwang ES, et al. Factors associated with nodal pathologic complete response among breast cancer patients treated with neoadjuvant chemotherapy: results of CALGB 40601 (HER2+) and 40603 (Triple-negative) (Alliance). Ann Surg Oncol. (2021) 28:5960–71. doi: 10.1245/s10434-021-09897-w
39. Murata T, Watase C, Shiino S, Kurita A, Ogawa A, Jimbo K, et al. Development and validation of a pre- and intra-operative scoring system that distinguishes between non-advanced and advanced axillary lymph node metastasis in breast cancer with positive sentinel lymph nodes: a retrospective study. World J Surg Oncol. (2022) 20:314. doi: 10.1186/s12957-022-02779-9
40. Corsi F, Albasini S, Sorrentino L, Armatura G, Carolla C, Chiappa C, et al. Development of a novel nomogram-based online tool to predict axillary status after neoadjuvant chemotherapy in cN+ breast cancer: A multicentre study on 1,950 patients. Breast. (2021) 60:131–7. doi: 10.1016/j.breast.2021.09.013
41. Guo R, Su Y, Si J, Xue J, Yang B, Zhang Q, et al. A nomogram for predicting axillary pathologic complete response in hormone receptor-positive breast cancer with cytologically proven axillary lymph node metastases. Cancer. (2020) 126 Suppl 16:3819–29. doi: 10.1002/cncr.32830
42. Xiao Y, Ding J, Ma D, Chen S, Li X, Yu K. Predicting pathological complete response in neoadjuvant dual blockade with trastuzumab and pertuzumab in HER2 gene amplified breast cancer. Front Immunol. (2022) 13:877825. doi: 10.3389/fimmu.2022.877825
43. Katayama A, Miligy IM, Shiino S, Toss MS, Eldib K, Kurozumi S, et al. Predictors of pathological complete response to neoadjuvant treatment and changes to post-neoadjuvant HER2 status in HER2-positive invasive breast cancer. Mod Pathol. (2021) 34:1271–81. doi: 10.1038/s41379-021-00738-5
44. Shinde AM, Zhai J, Yu KW, Frankel P, Yim JH, Luu T, et al. Pathologic complete response rates in triple-negative, HER2-positive, and hormone receptor-positive breast cancers after anthracycline-free neoadjuvant chemotherapy with carboplatin and paclitaxel with or without trastuzumab. Breast. (2015) 24:18–23. doi: 10.1016/j.breast.2014.10.008
45. Tasoulis MK, Lee HB, Yang W, Pope R, Krishnamurthy S, Kim SY, et al. Accuracy of post-neoadjuvant chemotherapy image-guided breast biopsy to predict residual cancer. JAMA Surg. (2020) 155:e204103. doi: 10.1001/jamasurg.2020.4103
46. van Loevezijn AA, van der Noordaa MEM, van Werkhoven ED, Loo CE, Winter-Warnars GAO, Wiersma T, et al. Minimally invasive complete response assessment of the breast after neoadjuvant systemic therapy for early breast cancer (MICRA trial): interim analysis of a multicenter observational cohort study. Ann Surg Oncol. (2021) 28:3243–53. doi: 10.1245/s10434-020-09273-0
47. Lee HB, Han W, Kim SY, Cho N, Kim KE, Park JH, et al. Prediction of pathologic complete response using image-guided biopsy after neoadjuvant chemotherapy in breast cancer patients selected based on MRI findings: a prospective feasibility trial. Breast Cancer Res Treat. (2020) 182:97–105. doi: 10.1007/s10549-020-05678-3
48. Heil J, Pfob A, Sinn HP, Rauch G, Bach P, Thomas B, et al. Diagnosing pathologic complete response in the breast after neoadjuvant systemic treatment of breast cancer patients by minimal invasive biopsy: oral presentation at the san antonio breast cancer symposium on Friday, December 13, 2019, program number GS5–03. Ann Surg. (2022) 275:576–81. doi: 10.1097/SLA.0000000000004246
Keywords: breast cancer, neoadjuvant chemotherapy, pathologic nodal response, prediction nomogram, pathologic complete response
Citation: Maimaitiaili A, Li Y, Chai N, Liu Z, Ling R, Zhao Y, Yang H, Liu Y, Liu K, Zhang J, Mao D, Yu Z, Liu Y, Fu P, Wang J, Jiang H, Zhao Z, Tian X, Cao Z, Wu K, Song A, Jin F, Wu P, He J, Fan Z and Zhang H (2024) A nomogram for predicting pathologic node negativity after neoadjuvant chemotherapy in breast cancer patients: a nationwide, multicenter retrospective cohort study (CSBrS-012). Front. Oncol. 14:1326385. doi: 10.3389/fonc.2024.1326385
Received: 26 December 2023; Accepted: 24 April 2024;
Published: 10 May 2024.
Edited by:
Riccardo Bonomi, Sussex Partnership NHS Foundation Trust, United KingdomReviewed by:
Ali-Farid Safi, Craniologicum—Center for Craniomaxillofacial Surgery, SwitzerlandCopyright © 2024 Maimaitiaili, Li, Chai, Liu, Ling, Zhao, Yang, Liu, Liu, Zhang, Mao, Yu, Liu, Fu, Wang, Jiang, Zhao, Tian, Cao, Wu, Song, Jin, Wu, He, Fan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huimin Zhang, aHVpbWluLnpoYW5nQHhqdHUuZWR1LmNu; Zhimin Fan, ZmFuem1Aamx1LmVkdS5jbg==; Jianjun He, Y2hpbmFoampAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.