
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 June 2024
Sec. Radiation Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1325987
Objective: To investigate the feasibility and evaluate the safety and effectiveness of Computed Tomography (CT) guided125I radioactive particle implantation for treating lymph node metastases in radioiodine-refractory differentiated thyroid cancer (RAIR-DTC). To verify the accuracy of the computerized three-dimensional treatment planning system (TPS) in treating lymph node metastasis using125I particle implantation at the dosimetric level.
Methods: A retrospective analysis was conducted on 42 patients with RAIR-DTC and lymph node metastases who were admitted to the General Hospital of the Northern Theater Command between December 2016 and January 2019. During this analysis, physicians utilized preoperative CT images to design an intraoperative plan using TPS. The dosimetric parameters of the postoperative plan were then compared to the preoperative plan. Additionally, this study examined the changes in tumor size and tumor-related marker Thyroglobulin (Tg) values in patients at 2, 6, and 12 months after the operation.
Results: The number of125I radioactive particles implanted in 42 patients was 226, with an average of 14.5 (range 2.0–30.0) particles implanted per lesion. The local remission rates were 97.62% (41/42), 88.10% (37/42), and 85.71% (36/42) at 2, 6, and 12 months postoperatively, respectively. The volume of the lesions was (4.44 ± 1.57) cm3, (4.20 ± 1.70) cm3, and (4.23 ± 1.77) cm3at 2, 6, and 12 months after treatment, respectively, which significantly decreased from the preoperative baseline level of (6.87 ± 1.67) cm3(t-values: 9.466, 9.923, 7.566, all P<0.05). The Tg levels were 15.95 (5.45, 73.93) μg/L, 8.90 (2.20, 39.21) μg/L, and 6.00 (1.93, 14.18) μg/L at 2, 6, and 12 months after treatment, respectively, which were significantly lower than the preoperative baseline levels of 53.50 (20.94, 222.92) μg/L (Z values: -5.258, -5.009, -4.987, all P < 0.001). Postoperatively, Delivered to 90% of the GTV(D90) was slightly lower than the prescribed dose in 95.23% (40/42) of patients, but the difference was not statistically significant [(12,378.8 ± 3,182.0), (12,497.8 ± 1,686.4) cGy; t=0.251, P>0.05], and postoperative dose parameters delivered to 100% of the gross tumor volume (GTV)(D100) (6,881.5 ± 1,381.8) cGy, the volume percentages of GTV receiving 150% of the prescribed dose(V150) (58.5 ± 18.40)%) were lower than the preoperative plan D100 (8,085.8 ± 2,330.0) cGy, V150 (66.5 ± 17.70)%; t-value=8.913 and 3.032, both P<0.05; the remaining indicators were not significantly different from the preoperative plan (the differences in the number of implanted particles, Planning Target Volume(PTV), the volume percentages of GTV receiving 100% of the prescribed dose(V100), Homogeneity Index(HI)were not statistically significant (t/Z = -0.593, -1.604, 1.493, -0.663, all P>0.05).
Conclusion: Referring to the TPS preoperative plan, the125I particle implantation therapy for RAIR-DTC lymph node metastasis can achieve the expected dose distribution, ensuring precise short-term local tumor control efficacy.
Thyroid cancer is a common malignancy of the endocrine system, with differentiated thyroid carcinoma (DTC) accounting for about 90% of cases (1). Most DTCs have a positive prognosis after surgery, supplemented with postoperative thyroid stimulating hormone (TSH) suppression or radioactive iodine (RAI) therapy. However, some tumor cells lose their ability to absorb iodine or have reduced TSH receptor expression, resulting in RAIR-DTC, with a 10-year survival rate of less than 10% (2). Finding effective treatments for RAIR-DTC is a major focus in the field of thyroid cancer. Currently, commonly used methods to treat RAIR-DTC include targeted drug therapy, local re-operation, chemotherapy, external irradiation therapy, immunotherapy, and gene therapy (3). In some cases, lymph node metastases are difficult to detect or are located near vital anatomical structures (such as the main trachea or large blood vessels), making surgery impossible. In recent years, radioactive particle implantation has become a widely used method for locally treating malignant tumors, such as Prostate Cancer (4);Lung Cancer (5); Breast Cancer (6). The distribution of the radiation dose is the most crucial factor in determining the effectiveness of particle implantation (7). By utilizing a TPS, a reasonable preoperative implantation plan can be developed to achieve the desired dose distribution. In this study, we investigated the short-term effectiveness and adverse effects of 125I particle implantation for treating lymph node metastases in RAIR-DTC. We compared the preoperative and postoperative dosimetric results of computerized 3D TPS-assisted particle implantation to evaluate the accuracy of this technique in guiding the dose distribution for particle implantation treatment.
Forty-two patients with RAIR-DTC lymph node metastases, who were admitted and treated with 125I particle implantation at the General Hospital of the Northern Theater Command between December 2016 and January 2021, were selected for the study. One patient with incomplete medical records, two patients without 125I particle implantation, and one patient with rectal cancer were excluded. Ultimately, a total of 42 patients (21 males and 21 females) were enrolled, with an average age of 50.82 ± 12.79 years. Among them, two cases had lymph nodes combined with single bone metastasis (left 7th rib and right iliac bone), two cases had lymph nodes combined with lung metastasis (both single lesions), and two cases had bone metastasis (sternum and right iliac bone). Each patient received a cumulative dose of 131I >22.2 GBq, and the 125I particle implantation was performed at least six months after the 131I treatment. The study was reviewed and approved by the Medical Ethics Committee of our hospital [No: YLS No. (2019) 69], and all patients provided informed consent. The patient data collection process is shown in Figure 1.
Inclusion criteria: (1) All patients who underwent total or near-total thyroidectomy (including extensive removal of cervical lymph nodes) before surgery, received postoperative 131I treatment, underwent biopsy of abnormal lymph nodes, and had confirmed metastasis of DTC through pathology. Metastatic lesions were confirmed using at least one imaging modality [such as 131I whole-body imaging, CT, Magnetic Resonance Imaging (MRI)] without iodine uptake. These patients were identified as having RAIR-DTC according to the 2015 American Thyroid Association (ATA) guidelines (8). (2) Patients with a white blood cell count (WBC) ≥3.5×109/L (normal range: 3.5 to 9.5×109/L), hemoglobin (Hb) ≥90 g/L (normal range: 130 to 175 g/L), prothrombin activity >40% (normal range: 70%~100%), platelet count (PLT) >100×109/L (normal range: 125~350×109/L), normal electrocardiogram (ECG), and liver function tests showing serum alanine aminotransferase and serum aspartate aminotransferase values not exceeding 3 times the upper limit of normal. (3) Patients with a Karnofsky performance status (KPS) score ≥70, detailed clinicopathological data, follow-up data, and a survival period of more than 1 year.
Exclusion criteria: (1) Patients with positive thyroglobulin antibody (TgAb). (2) Patients with severe cardiopulmonary, hepatic, or renal insufficiency. (3) Patients with severe coagulation dysfunction. (4) Patients with very poor general condition or cachexia.(5) Patients undergoing radiation and chemotherapy.(6)Patients undergoing immunotherapy.
①A Neu Viz 128 CT scanner from Neusoft Medical Group in Shenyang, China, was used to inject 0.1L of non-ionic contrast agent iodophoresol (Yangtze River Pharmaceutical Group, China; H10970358) at a flow rate of 2.5×10–3L/s through the patient’s elbow vein. The scan was delayed for 2 minutes, and the images were transmitted to the AW4.5 workstation provided by GE in the USA. The images were then transferred to the AW4.5 workstation for processing. ②The Discovery VCT PET/CT from GE in the USA was configured as a 64-row CT scanner. ③The Radioactive Particle TPS version 2.3 from Guangzhou Air Vision in China was used. ④The 125I radioactive particle source was produced by Beijing Atomic High Tech Co., Ltd. in China. It has a particle length of 4.5 mm, diameter of 0.8 mm, fully enclosed titanium shell, particle activity of 18.5~29.6 MBq, half-life of 59.6 days, and an average tissue penetration distance of 17 mm. The whole-body 131I imaging was performed using the Symbia T16 single photon emission computed tomography (SPECT) instrument from Siemens in Germany. Serum Tg was measured using a LIAISON chemiluminescence instrument from Sorin in Italy.
The CT scan images taken one week prior to surgery are imported into the TPS system for design, with the aim of avoiding important tissues and organs (such as large blood vessels, heart, spinal cord, etc.) as much as possible. The GTV and the PTV are outlined, with the PTV range including the tumor itself and the surrounding tissues that may be infiltrated (e.g. peritumor burr sign). The appropriate prescription dose of 110–140 Gy and particle activity (14.8–25.9 MBq) were set (9), following the principles of particle spacing of 0.5–1.0 cm, needle tract spacing of 1 cm, and a “sparse in the center, dense around” approach. By optimizing the prescription dose D90 > matched peripheral dose (MPD), the optimal needle route was simulated by calculating the number of particles and particle distribution source. The preoperative planning parameters D90 and D100, the volume percentages of GTV receiving 100% and 150% of the prescribed dose (V100 and V150), and the dose-volume histogram (DVH) were obtained. As shown in Figure 2.
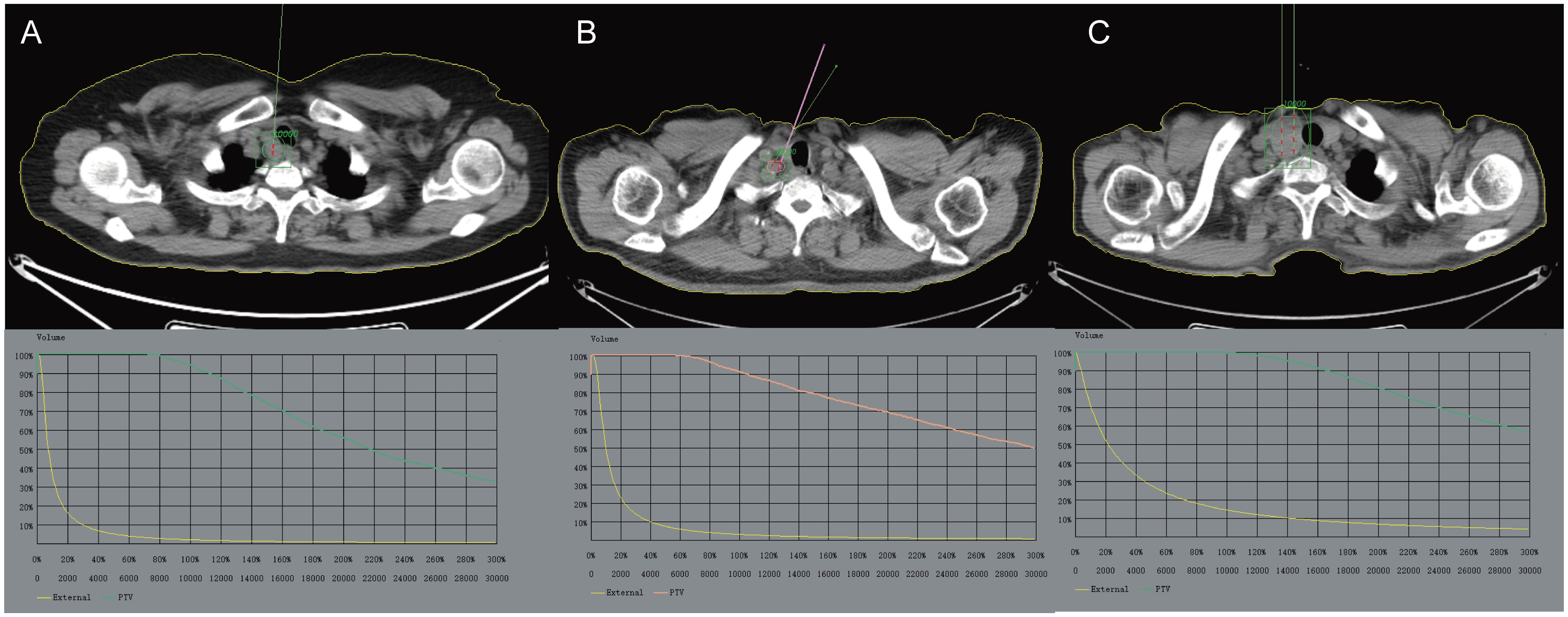
Figure 2 The preoperative CT scan images are imported into the TPS system for design, with the aim of avoiding important tissues and organs (such as large blood vessels, heart, spinal cord, etc.) as much as possible. (A) Enlarged right cervical lymph nodes with increased metabolism, indicating possible lymph node metastases. (B) Multiple lymph nodes in the right neck are enlarged and some of them have increased metabolism. (C) Occupation of the right lobe of the thyroid gland with increased metabolism is considered a local recurrence of thyroid cancer after surgery. The GTV and PTV are outlined. TPS, Treatment planning system; GTV, Gross Tumor Volume; PTV, Planning Target Volume.
Different positions were chosen based on the location of the lesion. An extracorporeal positioning grating was placed on the skin area corresponding to the lesion. The puncture site was determined using a CT scan. Local infiltration anesthesia was administered by injecting lidocaine with a mass fraction of 2%. The depth and angle of the puncture path were determined, and the needle was inserted layer by layer according to the TPS plan. Subsequently, one particle was implanted every 0.5–1.0 cm in a backward manner, replenishing the particles from the periphery to the center. The needle was then repositioned based on the position of the implanted needle. Care was taken to avoid important blood vessels and nerves during the implantation process. Throughout the procedure, the patient’s heart rate, blood pressure, respiration, and oxygen saturation were monitored. Additionally, the patient’s consciousness, pain, respiration, cough, and hemoptysis were observed, and timely symptomatic treatment was provided. After the surgery, the puncture site should be pressed for 10–20 minutes.
Immediately after the surgery, the CT images were sent to the TPS for postoperative verification. This involved identifying particles and gathering statistics on D90, D100, V100, V150, DVH, and the HI. A higher HI indicates a more even distribution of the dose in the target area (10).
(1) Determination of efficacy: The efficacy of 125I particle implantation treatment was assessed by reviewing neck ultrasound and neck CT scans before and 6 months after treatment. The evaluation was based on the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (11). (1) Complete remission (CR) was defined as the complete disappearance of lesions, with no imaging indicating recurrence, tumor remnants, or metastases, except for the presence of 125I particle metallic shadowing. (2) Partial remission (PR) was defined as a minimum 30% reduction in the sum of short diameters of target lesions compared to the sum of minimum diameters measured before treatment. (3) Stable disease (SD) was defined as a minimum 30% reduction in the sum of short diameters of target lesions compared to the sum of minimum diameters measured before treatment. (4) PD was defined as a ≥20% increase in the sum of the diameters of the target lesions compared to the minimum sum of the short diameters of the target lesions during the entire study, or an absolute increase of ≥5 mm in the sum of the diameters (the appearance of one or more new lesions was also considered as PD).
Adverse effects: Patients were closely monitored for adverse reactions such as bleeding, hematoma, particle displacement, and needle tract implantation after surgery. The Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) criteria were used to evaluate skin radiation injury caused by radioactive particle implantation, and complications during the intraoperative, postoperative, and follow-up periods were observed and assessed (12).
SPSS 21.1 statistical software was utilized for data analysis. Quantitative data were assessed for normal distribution using the Kolmogorov-Smirnov test. Normal data were presented as (mean ± s), while paired samples t-test was employed for comparing two groups. Skewed data were expressed as median and range of values [M(P25, P75)]. The rank sum test was used for comparing two groups. Statistical data were presented as n(%). The χ2 test was utilized for comparing two groups. All tests were considered statistically significant at P < 0.05.
All 42 patients underwent successful implantation, with a range of single particle activity from 14.8 to 25.9 MBq, with an average of 14.5 (2.0~30.0) particles implanted per lesion. A total of 226 particles were implanted, and the number of particles implanted in all lesions was consistent with the distribution of the treatment plan, resulting in a compliance rate of 95.23% (40/42). Two lesions were not implanted as planned due to patient movement and the proximity of the tumor to large blood vessels in the neck. Follow-up evaluations were conducted at 2, 6, and 12 months after surgery to assess the efficacy of 125I particle implantation, as shown in Table 1. Further analysis revealed that the volume of the lesions significantly decreased at 2, 6, and 12 months after surgery compared to the pre-surgery volume of (6.87 ± 1.67) cm3 (P<0.05). Additionally, the Tg value was significantly lower at 2, 6, and 12 months after surgery compared to the pre-surgery value of [53.50(20.94, 222.92) μg/L] (P<0.05), as shown in Table 2 (some patients had Tg values over 1000 μg/L or less than 0.2 μg/L). It is worth noting that the TgAb was negative in all cases. Figures 3–6 display typical case images.

Table 1 Efficacy analysis of 125 I seed implantation at different time points before and after treatment.
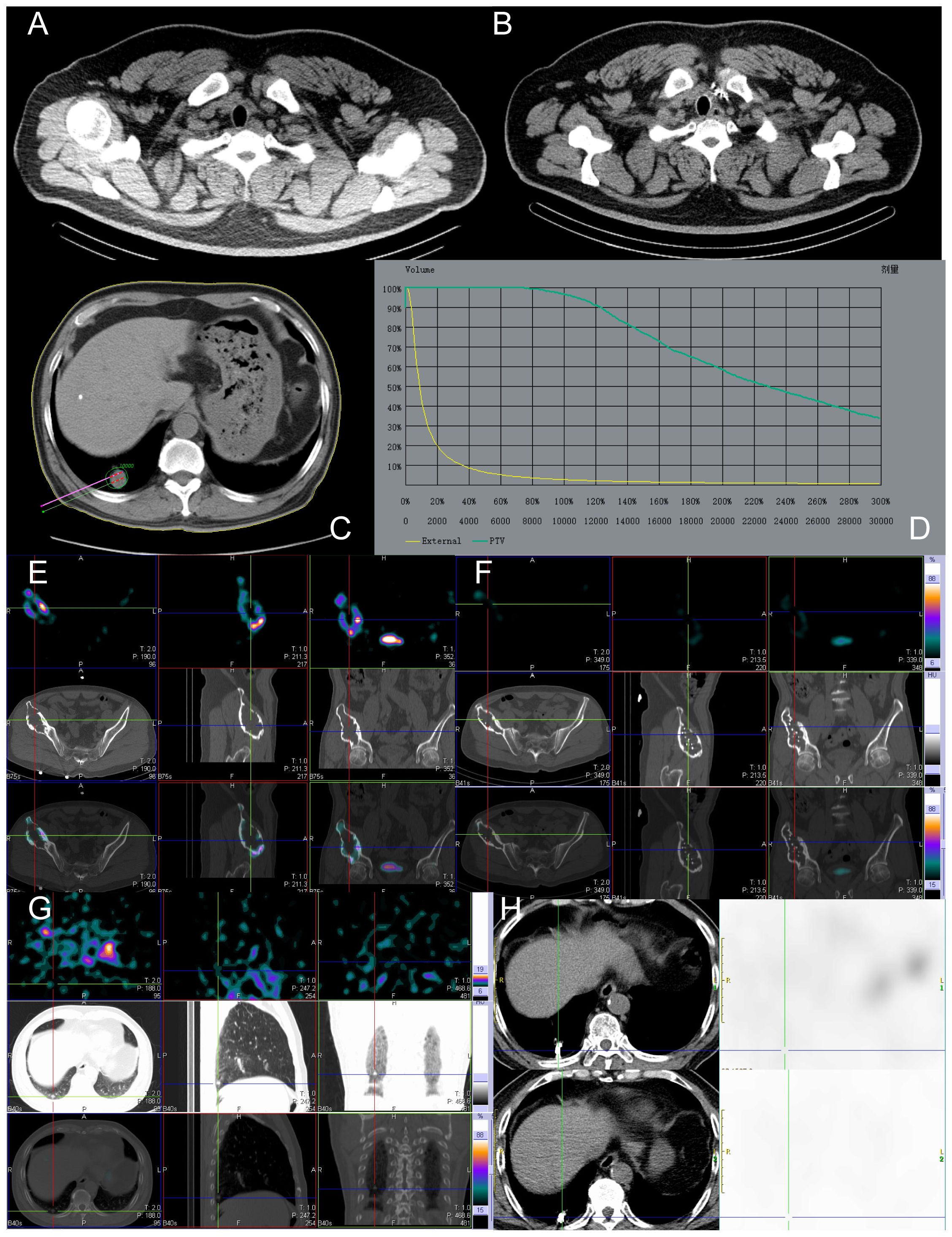
Figure 3 Before and after implantation of left supraclavicular lymph node, lung, and right ilium lesions with 125 I seeds. The patient, a 49-year-old male with RAIR-DTC, had postoperative pathologic findings of papillary thyroid carcinoma. (A) Images of 125 I seeds before implantation. (B) CT reexamination 12 months after the implantation of 125 I seeds showed only the shadow of metal seeds. (C) Preoperative needle placement of TPS system. (D) After the dose verification, the planned target DVH showed D90 > 100 Gy (prescription dose) and V200 < 60%, indicating satisfactory dose distribution of particle implantation with no “cold area” or “hot area” in dosimetry. (E) Single Photon Emission Computed Tomography/Computed Tomography (SPECT/CT) revealed iodine uptake in the right ilium metastatic lesion. (F) After 12 months of treatment, compared to before treatment, the radiation uptake significantly decreased, indicating effective local treatment, partial inhibition of tumor activity, and significant improvement in the patient’s bone pain. (G) No iodine uptake was found in the right lower lobe lung metastasis by SPECT/CT. (H) After 12 months of treatment, CT images showed a significant reduction in the mass and partial relief in the local effect. Tg, Thyroglobulin; RAIR-DTC:Radioiodine-refractory differentiated thyroid cancer; CT, Computed Tomography; TPS, Treatment planning system; DVH, Dose-volume histogram; SPECT/CT, Single Photon Emission Computed Tomography/Computed Tomography.
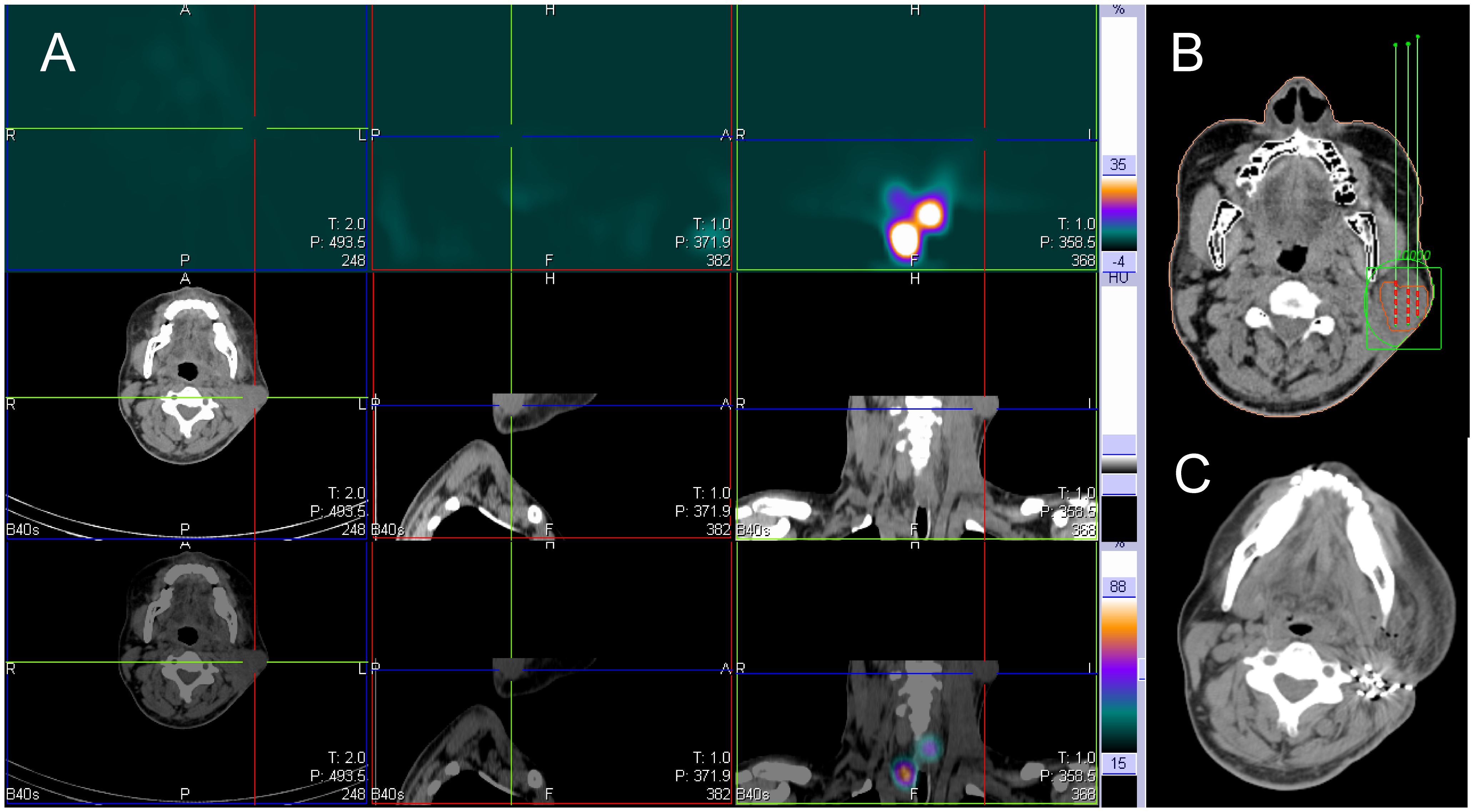
Figure 4 131I SPECT/CT imaging was performed on a 37-year-old male patient with 131I refractory differentiated thyroid cancer. The imaging revealed metastasis to the left neck lymph node, as indicated by the cross and arrow (shown in the image). (A) The 131I SPECT/CT neck fusion imaging showed no iodine uptake in the left cervical lymph node metastases. (B) The TPS was used for preoperative needle placement. (C) After 6 months, reexamination of the left cervical lymph node showed significant shrinkage, particle aggregation. SPECT/CT, Single Photon Emission Computed Tomography/Computed Tomography; TPS, Treatment planning system.
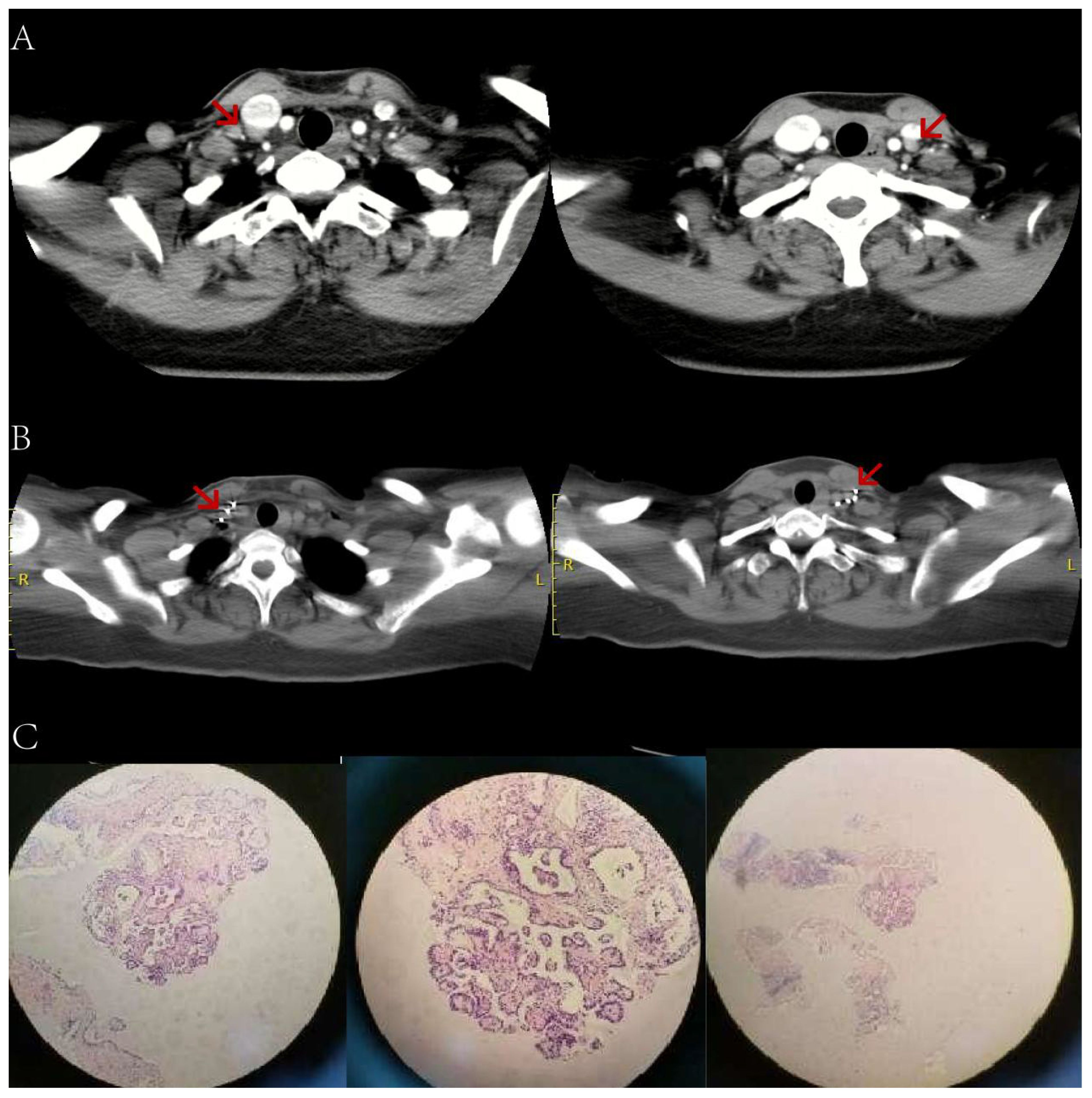
Figure 5 A female patient, aged 55, who underwent thyroid cancer surgery more than 2 years ago. (A) An enhanced CT scan of the neck reveals additional bilateral cervical lymph node metastases (indicated by arrows). The preoperative Tg level was 63.70ng/ml (in a TSH suppressed state). (B) 6 months after receiving 125 iodine radioactive particle implantation treatment, the lesion has significantly reduced in size compared to before. The Tg level is now 3.50ng/ml. (C) Pathology examination shows a small amount of epithelial cells in the tissue, indicating papillary severe heterogeneous hyperplasia. CT, Computed Tomography; Tg, Thyroglobulin; TSH, Thyroid stimulating hormone.
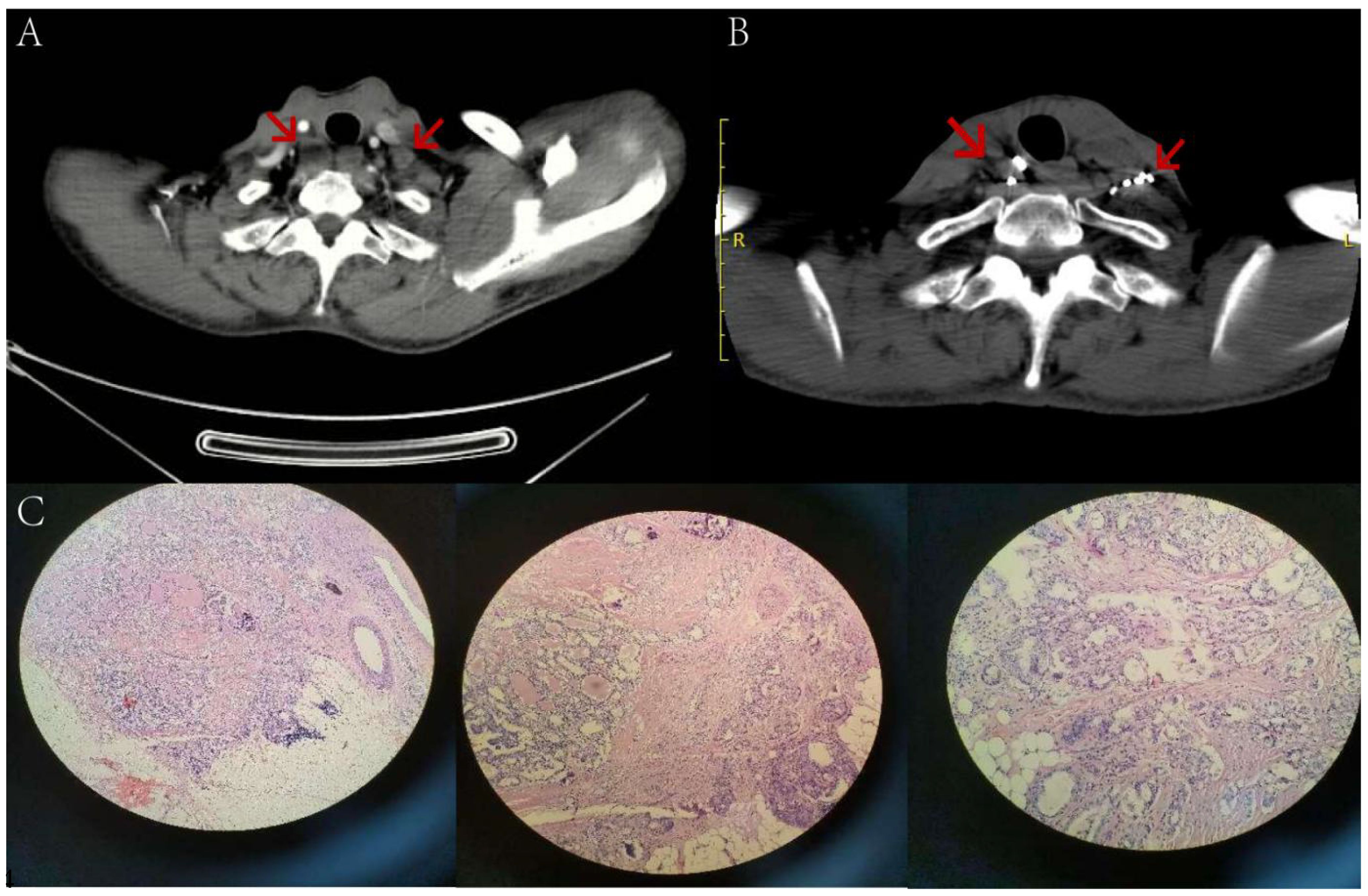
Figure 6 A 52-year-old male patient, who underwent thyroid cancer surgery over two years ago, has multiple lymph node metastases in both necks (indicated by arrows) as seen in image (A) Before the surgery, the patient had a preoperative Tg level of 56.50 ng/ml while in a TSH suppressed state. After 5 months of 125 iodine radioactive particle implantation treatment, the lesions have significantly reduced in size compared to before, as shown in image (B) The Tg level has also decreased to 8.21 ng/ml. Pathology examination reveals severe atypical hyperplasia of adenoid structures in the lymph nodes, which, when combined with immunohistochemical staining results, suggests metastatic adenocarcinoma of thyroid origin. Tg, Thyroglobulin; TSH, Thyroid stimulating hormone.
At 12 months post-surgery, 36 cases were categorized into the effective local control group (CR + PR + SD), while 6 cases were placed in the ineffective group (PD). This categorization was based on the evaluation criteria of RECIST 1.1 combined with MDA bone metastases. The volume and degree of enhancement of the lesions prior to treatment, as well as the presence of distant metastases, were found to be associated with recent efficacy (all P < 0.05). However, factors such as gender, pathological type (papillary, follicular), age (≤45 years, >45 years), and preoperative Tg values did not significantly impact the outcome, as indicated in Table 3.
Referring to the TPS schedule, the 125I particle implantation achieved the expected dose distribution (Table 4). Postoperatively, D90 was slightly lower than the prescribed dose in 95.23% (40/42) of patients, but the difference was not statistically significant (t=0.251, P>0.05). In terms of dosimetric indices, both D100 and V150 were lower than the preoperative plan (t value: 8.913, 3.032, both P<0.05); however, there were no statistically significant differences in the number of implanted particles, PTV, V100, and HI (t/Z =-0.593, -1.604, 1.493, -0.663, all P>0.05).
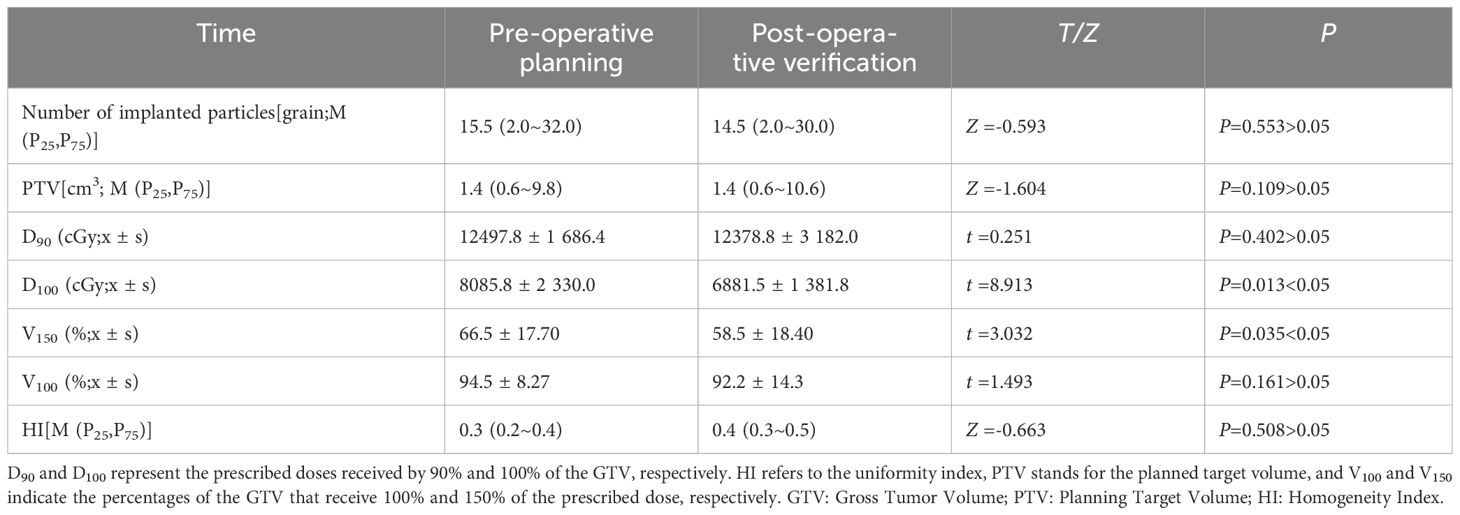
Table 4 Dose validation results were obtained before and after lesion particle implantation procedures in 42 patients diagnosed with iodine-refractory differentiated thyroid cancer.
Intraoperatively, there were no occurrences of serious bleeding or vascular embolism. One case experienced a small amount of intraoperative bleeding at the surgical site, which was successfully treated with intravenous injection of hemostatic drugs and compression to stop the bleeding. Additionally, two cases had grade II skin reactions, which improved after the administration of anti-inflammatory and topical drugs. Importantly, there was no migration of radioactive particles to other tissues or organs. Referring to the TPS preoperative plan, the 125I particle implantation therapy for RAIR-DTC lymph node metastasis can achieve the expected dose distribution with few complications, making it a safe and effective treatment method.
Cervical lymph node metastasis is the most common occurrence after Papillary Thyroid Carcinoma(PTC). The majority of these metastasis lesions are found near the large blood vessels in the neck, the trachea, and the area where important nerves run, and some tumor growth may invade surrounding tissues (13). The tumor focus grows rapidly and invades the surrounding tissues, leading to clinical symptoms such as facial edema, pain, hoarseness, and limb numbness. After multiple treatments with 131I, some patients develop RAIR-DTC. RDT may restore RAI avidity and induce RECIST response, particularly in those with RAS-driven ‘follicular’ phenotype, providing effective treatment for patients (14). Targeted drugs such as sorafenib and lenvatinib are also used as first-line treatments for RAIR-DTC, significantly prolonging progression-free survival but having minimal impact on overall survival. However, both treatment modalities exhibit significant drug resistance and adverse reactions, making them challenging to meet clinical needs (15). In recent years, the benefits of 125I seed therapy have been gradually explored in clinical settings. 125I continuously releases 27.4~31.4keV X-rays and 35.5keV γ-rays between tissues, with γ-rays directly causing breaks in the single-strand or double-strand DNA of the tumor, and X-rays inactivating tumor cells through the production of oxygen free radicals by indirect ionization (16).
Additionally, 125I particles act on some cancer cells in the quiescent phase (G0 phase), transforming them into proliferative phase (G2 and M phase) cells that are more susceptible to being killed after entering the mitotic phase (17, 18). Recently, ChenW (19) treated 15 patients with cervical recurrence after DTC using 125I seed therapy. The volume reduction rate was (83.5 ± 16.9)% 12 months after the procedure, and the postoperative serum Tg level was 4.9 (0.7,13.2) μg/L, significantly lower than the preoperative level of [57.0 (8.6,114.8) μg/L]. Furthermore, 22 high-enhancement foci transformed into low or no enhancement foci. In this study, among 17 patients who underwent 125I particle implantation therapy, there was 1 case of complete response (CR), 10 cases of partial response (PR), and 4 cases of stable disease (SD).The size of the lesions and the Tg levels after treatment were significantly lower than those before the procedure, consistent with the results of previous studies (19). After treatment, the symptoms of 3 cases of hoarseness and 5 cases of local tenderness were significantly relieved. In this study, fixed head support or human fixed body membrane was used to avoid and reduce patient displacement during implantation. The results of this study show that 125I radioactive seed therapy is minimally invasive, safe and can significantly improve the quality of life of patients in the short term. The results of this study show that 125I radioactive particle therapy is minimally invasive, safe, and can significantly improve the compression symptoms caused by cervical lymphadenopathy in the short term.
Dose determination is a crucial aspect of 125I seed implantation. The optimal dose distribution was automatically designed based on the tumor boundary, location of important tissues, prescription dose, and particle activity prior to TPS. After the procedure, the quality of radioactive particle implantation was assessed by identifying particles and verifying the discrepancy between the target dose and prescription dose. According to the Code for Clinical Application of radioactive particles in tumor treatment, the prescribed radiotherapy dose is set between 110 to 140Gy (20). If the dose is too low, it will fail to achieve the objective of tumor eradication; if the dose is too high, it will harm the normal tissues and organs surrounding the target area. In this study, there was no significant difference in dosimetric parameters such as D90, V100, and HI before and after the procedure. The postoperative D90 was (12378.8 ± 3182.0) cGy and V100 was (92.2 ± 14.3)%, indicating that the radioactive particle implantation essentially achieved the intended distribution and fulfilled the dosimetry requirements as per the preoperative TPS plan. However, in this study, the HI before and after the procedure is low, and the postoperative verification V150 exceeds 50%, suggesting an uneven dose distribution in the target area and an excessive volume of high-dose region. The possible causes of the errors were analyzed: (1) The change in focus size and the rapid reduction of perifocal edema accelerated the dispersion and regression of radiation dose, resulting in a decrease in the conformal index of dose distribution within the focus (21–27). Additionally, the postoperative verification dose does not reflect the actual absorbed dose of the tumor. In this study, the postoperative verification was conducted within 24 hours, and the local unsubsided tissue edema shortly after the operation affected the evaluation of the biological effect dose (25). (2) The accurate location identification of particles is challenging due to postoperative CT artifact, with one particle imaging at two levels and partial particle overlap, leading to dose deviation in postoperative verification measurements. (3) The density of the tumor varies due to bleeding or necrosis caused by the radiation effect of particles, resulting in changes in particle position. Furthermore, postoperative verification revealed disease progression in two patients approximately four months after implantation. Further investigation found that the postoperative D90 was significantly lower than the planned prescription dose before the operation. The main reasons for this are as follows: (1) The operation area structure in these two patients is disordered, their age is advanced, and the neck tissue is soft, which may have caused implantation deviation during the operation. (2) The shape of the target volume changed due to local muscle contraction caused by body position examination, intraoperative anesthesia, and puncture stimulation before and after the operation.
Most of the lesions in RAIR-DTC lymph node metastases are located near the body surface. The release of γ-rays by 125I seeds can lead to DNA double-strand breaks and impact the metabolism of skin cells. In this study, two patients experienced painful erythema and pigmentation, but no skin rupture occurred after one week of anti-inflammatory treatment. This was because many lesions were superficial and in close proximity to each other, resulting in the formation of local “hot spots” due to particle aggregation. It is recommended that when implanting particles, the distance between the particles and the skin’s surface layer should be at least 1cm greater, and the activity and number of particles should be adjusted based on the anatomical location of the lesions.
In summary, the treatment of RAIR-DTC lymph node metastasis using 125I seed implantation has the advantages of good efficacy and few complications. The use of TPS can improve the dose conformability of the target area, effectively killing the tumor, and better protecting the surrounding normal tissue. However, it is important to note that this study is a single-center retrospective study with a small sample size and a short follow-up cycle. Therefore, it is necessary to expand the sample size in order to analyze the effects of dose, tumor size, and other factors on the curative effect. This will provide better guidance for the standardized diagnosis and treatment of 125I seeds.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was reviewed and approved by the Medical Ethics Committee of our hospital [No: YLS No. (2019) 69]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WZ: Writing – original draft, Funding acquisition. SH: Writing – original draft, Formal analysis, Methodology. ZW: Writing – original draft, Conceptualization, Project administration, Software, Validation. TD: Conceptualization, Project administration, Software, Validation, Writing – original draft, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Visualization. GZ: Resources, Supervision, Writing – original draft, Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Liaoning Provincial People’s Livelihood Science and Technology Joint Plan 2022JH2/101500021.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fahiminiya S, de Kock L, Foulkes WD. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. (2016) 375:2306–7. doi: 10.1056/NEJMc1613118
2. Jin Y, Van Nostrand D, Cheng L, Liu M, Chen L. Radioiodine refractory differentiated thyroid cancer. Crit Rev Oncol Hematol. (2018) 125:111–20. doi: 10.1016/j.critrevonc.2018.03.012
3. Han JM, Bae JC, Kim HI, Kwon S, Jeon MJ, Kim WG, et al. Clinical outcomes of differentiated thyroid cancer patients with local recurrence or distant metastasis detected in old age. Endocrinol Metab (Seoul). (2018) 33:459–65. doi: 10.3803/EnM.2018.33.4.459
4. Song Y, Chan MF, Burman C, Cann D. Comparison of two treatment approaches for prostate cancer: intensity-modulated radiation therapy combined with 125I seed-implant brachytherapy or 125I seed-implant brachytherapy alone. J Appl Clin Med Phys. (2008) 9,2:1–14. doi: 10.1120/jacmp.v9i2.2283
5. Song M, Zhou X, Hou R, Sigdel M, Liu Y, Zhang C, et al. CT-guided radioactive 125I seeds brachytherapy for lung oligometastases from colorectal cancer: initial results. BMC Cancer. (2024) 24,1:265. doi: 10.1186/s12885–024-12013–2
6. Wang J, Chang X, Xu K, Liang Y, Zhao J, Liu Z, et al. CT-guided iodine-125 brachytherapy as salvage therapy for local-regional recurrent breast cancer. Front Oncol. (2023) 13:1171813. doi: 10.3389/fonc.2023.1171813
7. Chasseray M, Dissaux G, Bourbonne V, Boussion N, Goasduff G, Malloreau J, et al. Dose to the penile bulb and individual patient anatomy are predictive of erectile dysfunction in men treated with 125I low dose rate brachytherapy for localized prostate cancer. Acta Oncol. (2019) 58:1029–35. doi: 10.1080/0284186X.2019.1574981
8. Schmidt A, Iglesias L, Klain M, et al. Radioactive iodine-refractory differentiated thyroid cancer: an uncommon but challenging situation. Arch Endocrinol Metab. (2017) 61:81–9. doi: 10.1590/2359–3997000000245
9. Wang JJ. Standard for clinical application of radioactive particles in the treatment of tumor Vol. 24. Beijing: Peking University Medical Press (2011).
10. Shiraishi Y, Yorozu A, Ohashi T, Toya K, Saito S, Nishiyama T, et al. A dose-response analysis of biochemical control outcomes after 125I monotherapy for patients with favorable-risk prostate cancer. Int J Radiat Oncol Biol Phys. (2014) 90:1069–75. doi: 10.1016/j.ijrobp.2014.08.340
11. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
12. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the european organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. (1995) 31,5:1341–6. doi: 10.1016/0360–3016(95)00060-C
13. Robinson TJ, Thomas S, Dinan MA, Roman S, Sosa JA, Hyslop T. How many lymph nodes are enough? Assessing the adequacy of lymph node yield for papillary thyroid cancer. J Clin Oncol. (2016) 34:3434–9. doi: 10.1200/JCO.2016.67.6437
14. Toro-Tobon D, Morris JC, Hilger C, Peskey C, Durski JM, Ryder M. Clinical outcomes of radioactive iodine redifferentiation therapy in previously iodine refractory differentiated thyroid cancers. Thyroid: Off J Am Thyroid Assoc. (2024) 34,1:70–81. doi: 10.1089/thy.2023.0456
15. Su Y, Wang J, Huang L, Xie L, Yu X, Zha J. Clinical efficacy of iodine-125 (125I) seed implantation in patients with iodine-refractory differentiated thyroid cancer. Am J Cancer Res. (2023) 13,10:4794–802.
16. Yue TH, Xing W. 125I seed brachytherapy combined with single-agent chemotherapy in the treatment of non-small-cell lung cancer in the elderly: A valuable solution. Onco Targets Ther. (2020) 13:10581–91. doi: 10.2147/OTT.S272898
17. Zhang J, Zhu Y, Dong M, Yang J, Weng W, Teng L. Iodine-125 interstitial brachytherapy reduces tumor growth via Warburg effect inhibition in non-small cell lung cancer A549 xenografts. Oncol Lett. (2018) 16:5969–77. doi: 10.3892/ol.2018.9346
18. Zhu Y, Dong M, Yang J, Zhang J. Evaluation of iodine-125 interstitial brachytherapy using micro-positron emission tomography/computed tomography with 18F-fluorodeoxyglucose in hepatocellular carcinoma HepG2 xenografts. Med Sci Monit. (2019) 25:371–80. doi: 10.12659/MSM.912590
19. Chen W, Xie F, Zhang MB. Ultrasound guided 125I brachytherapy in iodine refractory differentiated thyroid cancer. Chin J Med Imaging. (2020) 28:26–30. doi: 10.3969/j.issn.1005–5185.2020.01.006
20. Xing C, Zhang KX, Yuan QQ. Dosimetry verification of radioactive seed implantation for Malignant tumor assisted by 3D-printing coplanar template. Chin J Radiol Med Prot. (2017) 37:514–7. doi: 10.3760/cma.j.issn.0254–5098.2017.07.008
21. Du P, Xiao Y, Lu W. Modified fan-shaped distribution technology for computed tomography (CT)-guided radioactive seed implantation in lung cancer patients with lung dysfunction. Med Sci Monit. (2017) 23:4366–75. doi: 10.12659/msm.902105
22. Mukai Y, Hayashi N, Koike I, Kaizu H, Takano S, Sugiura M, et al. Acute and late toxicities in localized prostate cancer patients treated with low-dose 125I brachytherapy (110 Gy) in combination with external beam radiation therapy versus brachytherapy alone (160 Gy). J Contemp Brachytherapy. (2018) 10:397–404. doi: 10.5114/jcb.2018.79379
23. Wu J, Wang J, Sui AX. Effect of tumor target volume reduction on dose after 125I seeds implantation. Chin J Exp Surg. (2015) 32:309. doi: 10.3760/cma.j.issn.1001–9030.2015.02.033
24. Wang JJ. Radioactive seeds implantation therapy in the era of precision medicine. Chin J Nucl Med Mol Imaging. (2018) 38:1–3. doi: 10.3760/cma.j.issn.2095–2848.2018.01.001
25. Zhao XZ, Zhang HT, Di XM. Relationship between the peripheral dose and radioactive counts of 125I seeds detected by SPECT/CT. Chin J Nucl Med Mol Imaging. (2017) 37:351–4. doi: 10.3760/cma.j.issn.2095–2848.2017.06.007
26. Lin Q, Zhang Y, Dai JJ. CT-guided 125I seed brachytherapy for superficial lymph node metastases: a report of 23 cases. J Shandong Univ (Health Sci). (2017) 55:38–44. doi: 10.6040/j.issn.1671–7554.0.2016.1350
Keywords: thyroid tumor, tumor metastasis, lymph nodes, brachytherapy, iodine radioisotope, radiation dose
Citation: Zhang W, Hao S, Wang Z, Ding T and Zhang G (2024) 125I seed implantation for lymph node metastasis from radioactive iodine-refractory differentiated thyroid carcinoma: a study on short-term efficacy and dosimetry. Front. Oncol. 14:1325987. doi: 10.3389/fonc.2024.1325987
Received: 22 October 2023; Accepted: 11 June 2024;
Published: 26 June 2024.
Edited by:
Timothy James Kinsella, Brown University, United StatesReviewed by:
Yan-Song Lin, Peking Union Medical College Hospital, ChinaCopyright © 2024 Zhang, Hao, Wang, Ding and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoxu Zhang, emhhbmdndW94dTUwMkBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.