
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 31 January 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1325249
 Patrizia Ciammella1*
Patrizia Ciammella1* Salvatore Cozzi2
Salvatore Cozzi2 Paolo Borghetti3
Paolo Borghetti3 Marco Galaverni4
Marco Galaverni4 Valerio Nardone5
Valerio Nardone5 Maria Paola Ruggieri1
Maria Paola Ruggieri1 Matteo Sepulcri6
Matteo Sepulcri6 Vieri Scotti7
Vieri Scotti7 Alessio Bruni8
Alessio Bruni8 Francesca Zanelli9
Francesca Zanelli9 Roberto Piro10
Roberto Piro10 Elena Tagliavini11
Elena Tagliavini11 Andrea Botti12
Andrea Botti12 Federico Iori1,13
Federico Iori1,13 Emanuele Alì1
Emanuele Alì1 Chiara Bennati14
Chiara Bennati14 Marcello Tiseo15
Marcello Tiseo15Background: Chemoradiation therapy (CRT) is the treatment of choice for locally advanced non-small cell lung cancer (LA-NSCLC). Several clinical trials that combine programmed cell death 1 (PD1) axis inhibitors with radiotherapy are in development for patients with LA-NSCLC. However, the effect of CRT on tumor cells programmed cell death ligand-1 (PD-L1) expression is unknown.
Methods: In this multicentric retrospective study, we analyzed paired NSCLC specimens that had been obtained pre- and post-CRT. PD-L1 expression on tumor cells was studied by immunohistochemistry. The purpose of this study was to evaluate the feasibility, risk of complications, and clinical relevance of performing re-biopsy after CRT in patients with PD-L1 negative LA-NSCLC.
Results: Overall, 31 patients from 6 centers with PD-L1 negative LA-NSCLC were analyzed. The percentage of tumor cells with PD-L1 expression significantly increased between pre- and post-CRT specimens in 14 patients (45%). Nine patients had unchanged PD-L1 expression after CRT, in five patients the rebiopsy material was insufficient for PD-L1 analysis and in two patients no tumor cells at rebiopsy were found. The post-rebiopsy complication rate was very low (6%). All patients with positive PD-L1 re-biopsy received Durvalumab maintenance after CRT, except one patient who had a long hospitalization for tuberculosis reactivation. Median PFS of patients with unchanged or increased PD-L1 expression was 10 and 16.9 months, respectively.
Conclusion: CRT administration can induce PD-L1 expression in a considerable fraction of PD-L1 negative patients at baseline, allowing them receiving the maintenance Durvalumab in Europe. Hence, after a definitive CRT, PD-L1 redetermination should be considered in patients with LA-NSCLC PD-L1 negative, to have a better selection of maintenance Durvalumab candidates.
Non-small cell lung cancer (NSCLC) is the second most common cancer and a leading cause of cancer-related death worldwide, with approximately 25% of patients diagnosed with a locally advanced disease (1). Historically the standard of care for patients with a good performance status and unresectable locally advanced NSCLC (LA-NSCLC) has been a platinum-based doublet chemotherapy in combination with a radiation treatment (namely, chemoradiotherapy: CRT). However, the median progression-free survival among patients who have received CRT was poor (approximately 8 months), and only 15% of patients were alive at 5 years (2–4). Although the attempts to improve patient’s survival by associating different new drugs with CRT, the results were disappointing until the results of Pacific trial (5–11). In fact, the randomized phase 3 PACIFIC trial established a new standard for unresectable LA-NSCLC, introducing the concept of immunotherapy maintenance with the anti-programmed-death ligand 1 (anti-PD-L1) agent Durvalumab, for patients without progressive disease after CRT. The maintenance with Durvalumab, administered for up to 12 months after CRT, increase both Overall Survival (OS: 43.5 vs 29.1 months at 3 years) and Progression-Free Survival (PFS) compared to placebo, with a low immune-related side events of any grade (25%) (10, 11).
PD-L1 is expressed by cells in the tumor microenvironment, and it engages PD-1 on T cells. It triggers inhibitory signaling of the T cell receptor, reducing T-cell killing capacity and blocking effector functions (12). However, the PD-L1 expression on tumor cells is dynamic and it can be induced by the administration of oncological treatments, such as CRT. There is evidence that in patients with NSCLC, who underwent neoadjuvant CRT followed by surgery, a significant increase in PD-L1 expression was determined (13). Considering this, re-biopsy after CRT may better select PD-L1 positive patients. Arguments against re-biopsy include the risk of complications with the likelihood of getting an insufficient amount of tumor tissue for analyses. Notwithstanding this, performing PD-L1 re-determination after CRT in patient resulted PDL1 negative at the beginning, can allow offering maintenance Durvalumab to patients who otherwise could have not benefit from it, as the European Medicines Agency (EMA) has recommended Durvalumab exclusively in patients with a tumor proportion score PDL1 (TPS)‗ 1%. Thus, although the Food and Drug Administration (FDA) and other pharmaceutical agencies have approved the use of Durvalumab in all patients, regardless of PD-L1 expression, PD-L1 negative patients cannot receive it in Europe. The EMA based this on a post-hoc analysis, which showed that patients with tumors that did not express PD-L1 had no survival advantage over control.
Thus, the aim of this retrospective multicentric study was to analyze paired NSCLC specimens pre- and post-CRT, in patients with inoperable LA-NCSLC, PD-L1 negative at diagnosis, to explore the impact of CRT on PD-L1. Additionally, this work aims to evaluate the feasibility, the risk of complications, and the clinical relevance for performing re-biopsy systematically after CRT, in patients with PD-L1 negative LA-NSCLC.
We have retrospectively evaluated patients with PD-L1 negative unresectable LA-NCSLC who undergoing CRT and subsequently re-biopsy for the re-determination of PD-L1 in 10 Italian centers.
Inclusion criteria were: 1) patients over 18 years of age; 2) histological diagnosis of unresectable LA-NSCLC; 3) negative PD-L1 expression tested before the start of CRT; 4) concurrent or sequential CRT; 5) no progressive disease at early evaluation after CRT; 6) re-biopsy of the tumor (primary tumor or mediastinal lymphadenopathies); 6) signature of informed consent. Exclusion criteria were following: 1) patients with stage IV NSCLC; 2) patients with Small Cell Lung Cancer; 3) absence of pre-treatment PD-L1 expression determination; 4) progressive disease after CRT; 5) previous thoracic irradiation; 6) diagnosis of other concurrent cancer except for non-melanomatous skin cancers; A diagnostic biopsy was performed at baseline (at diagnosis) and repeated after CRT.
All patient’s clinical characteristics (age, sex, smocking habitus), disease data (histological type, TNM stage, genomic aberrations, PD-L1 expression before and after CRT), treatment details (type of concurrent drugs, radiotherapy doses and fractionation) and clinical outcomes data (overall survival, progression free survival) were collected in anonymous database. Additionally, the re-biopsy modalities and complications were recorded. Complications were defined as severe if required hospitalization.
The primary endpoint was to evaluate the variation in PD-L1 expression before and after CRT in patients with LA-NSCLC. Secondary objectives were clinical relevance, defined as a potential of changing treatment, due to new histological evidence, specifically a change in PD-L1 TPS from negative (<1%) to positive (>1%) (possibility of administering maintenance Durvalumab), acute complication rate to re-biopsy, rate of non-diagnostic procedure (including negative ones or insufficient material to test PDL1), progression free survival (PFS) and overall survival (OS).
PD-L1 expression was examined by staining on the Dako Autostainer 48 (Dako Omnis platform) using the PD-L1 IHC 22C3 pharmDx kit. The percentage of PD-L1 positive tumor cells were evaluated by expert pathologists, blinded to clinical outcome. TPS was calculated as the percentage of tumor cells showing partial or complete membrane staining relative to all viable tumor cells in the sample. Based on staining intensity, a division into four main groups was performed: negative (<1%), weak (≥1% or <5%), moderate (≥5% or <50%) and strong (≥50%). No PD-L1 TPS was performed if no malignant cells or only suspected malignant cells were found. The present study received final approval by the Institutional Ethical Committee and was performed in accordance with the principles of Good Clinical Practice (GCP) in respect of the ICH GCP guidelines and the ethical principles contained in the Helsinki declaration and its subsequent updates. A written consent form was obtained from each patient.
For clinical and pathological characteristics, descriptive statistics were applied and presented as frequencies, percentages, and median (range). Time to re-biopsy was calculated as the interval from end of CRT to the performance of biopsy (days). PFS was defined as the time from CRT initiation to radiologically verified progression. Patients with no progression by the cut-off date of August 30, 2022 were listed. Overall survival was calculated from date of first-line treatment initiation to date of death or until a cut-off date of August 30th, 2022. The survival curves were calculated by the Kaplan-Meier method and differences in survival were tested by the log-rank test. Univariate analyses were performed with a log rank test. Statistics were performed using SPSS 20.0 software (Chicago, IL) and the significance level was set at P-value (2-sided) <0.05.
From January 2019 to January 2022, 31 consecutive patients, from 6 Italian centers, met the inclusion criteria and were enrolled in present study. All enrolled patients were PD-L1-negative LA-NSCLC patients at diagnosis and all were treated with up-front CRT (concurrent or sequential). With the exception of one patient, whose rebiopsy was postponed due to an acute cardiac event, all other 30 patients (97%) were investigated after up-front CRT.
Patient characteristics are summarized in Table 1. Among them, most patients were male (81%) and smokers (90%); 13 patients had stage IIIA disease, while 12 patients had stage IIIB and 6 pts IIIC disease, respectively. Nine patients had squamous histology, while 22 had non squamous NSCLC. Most patients (48%) received taxolo plus carboplatin as the CRT regimen.
Re-biopsy characteristics and results are reported in Table 2. The median time between the last day of RT and re-biopsy was 31 days (15–56). Among the 24 patients with sufficient material after CRT, 14 (58%) had positive PD-L1 expression on tumor cells in the post-CRT specimens. The positivization rate was 45% (95%CI: 35-55). In 9 patients PD-L1 expression was negative in both the pre- and post-CRT specimens. In five patients the rebiopsy material was insufficient for PD-L1 analysis and in two patients no tumor cells at rebiopsy were found.
The location of re-biopsy was primary lung tumor, mediastinal lymphadenopathies or both in 15 (48%), 12 (39%) and 4 (13%) patients, respectively. The location of the re-biopsy corresponded to the site of the original biopsy (same anatomic sites) in 28 patients. In 3 patients (9.6%), the re-biopsy was taken from another location than the diagnostic biopsy. In 26 patients, re-biopsies were performed at the “pulmonary endoscopy unit” with bronchoscopy (lung lesions), endoscopic ultrasound of peri-bronchial/-tracheal (endobronchial ultrasound, EBUS) or para-gastroesophageal (endoscopic ultrasound, EUS - either with the EBUS endoscope (EUS-B) or conventional EUS-scope) structures. Transthoracic sampling from lung lesions is performed in 5 patients. Re-biopsy modalities are reported in Table 2.
No severe acute complications occurred during the re-biopsy in any of the patients. The rate of overall complications to rebiopsy after CRT was 6% (n = 2, 1 pneumothorax and 1 bronchial hemorrhage). No severe late complications or subsequent sequelae occurred in any of the patient cases.
A potential clinical relevance of re-biopsy was obtained in 14 out of the re-biopsied 31 patients (45%); in fact, in the 14 patients in which a change from PD-L1 TPS negative to positive was highlighted, it was possible to administer Durvalumab maintenance (one patient was unable to take Durvalumab due to concurrent reactivated tuberculosis).
We also investigated the association between the change in PD-L1 expression and survival time. These survival curves are shown in Figure 1. Among the 24 patients with sufficient post-CRT material, PD-L1 expression in post-CRT material was associated with moderate increase of PFS (PD-L1-positive group versus PD-L1-negative group, median 16.9 versus 10 months respectively, p = 0.6) without difference in median OS (PD-L1-positive group versus PD-L1-negative group, median 20 versus 14 months, p >0.5, respectively). 13 patients out of 14 with positive PD-L1 post-CRT (93%) received Durvalumab maintenance during the observation period. In these patients the median PFS and OS were 17.7 and 21.3 months, respectively. No patient was positive for EGF-R while the ALK rearrangement and the KRAS mutation were present in one patient each, only.
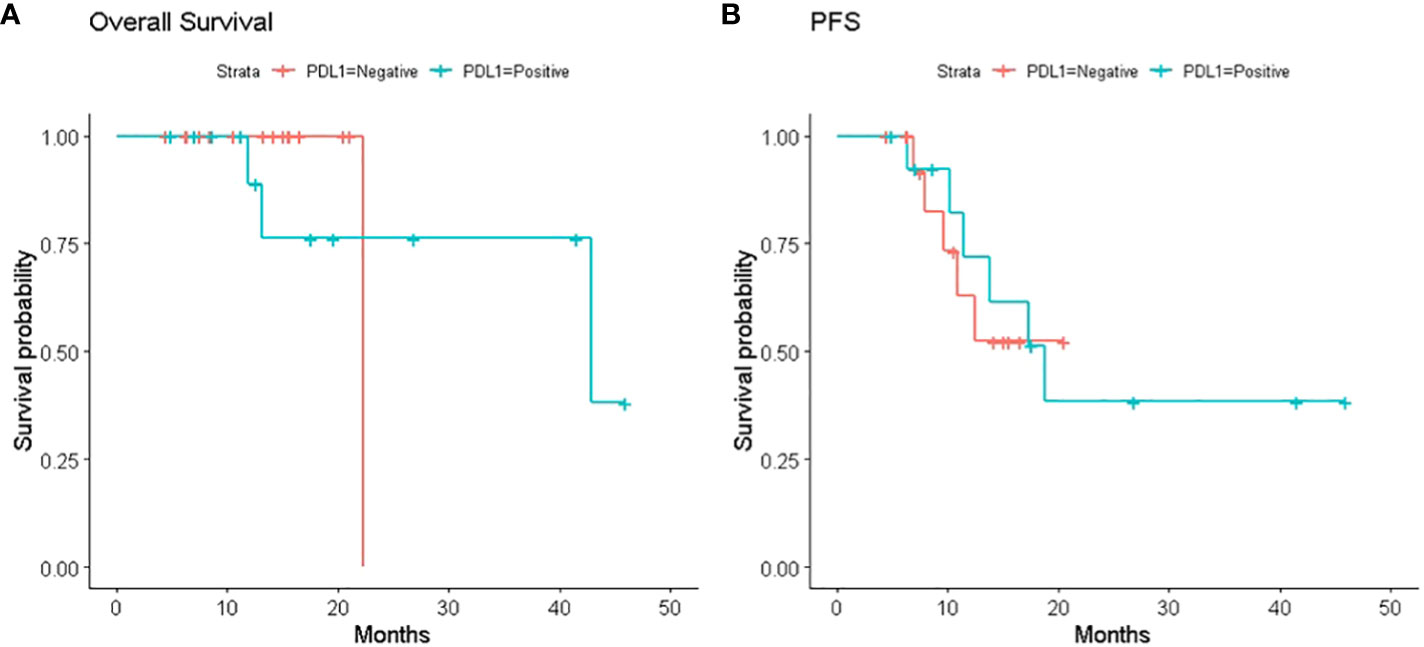
Figure 1 (A) Kaplan–Meier survival curves. Kaplan–Meier survival curves of overall survival (OS) in patients with or without PDL1 expression on tumor cells in the post-CRT specimens; (B) Kaplan–Meier survival curves of progression free survival (PFS) in patients with or without PDL1 expression on tumor cells in the post-CCRT specimens.
In the PACIFIC trial of unresectable LA-NSCLC patients whose disease had responded or stabilized after CRT, Durvalumab significantly improved PFS and OS (10). These results have led to the growing recognition of the ‘PACIFIC regimen’ (Durvalumab after CRT) as the standard of care in this setting, and to global approvals of Durvalumab for treatment of patients with unresectable, LA-NSCLC in the absence of disease progression following platinum-based CRT (14–16). However, in Europe, based on the results of post hoc analyses requested by the European Medicines Agency (EMA), patients must also have tumors that express PD-L1 on >1% of tumor cells (TCs) (17).
The field of research in PD-L1 TPS changes in NSCLC is mainly dominated by retrospective studies including patients with localized/resectable disease, having received neo-adjuvant or adjuvant chemotherapy (18–24), but the results remain conflicting and controversial. For example, Sheng et al. reported that the positivity of PD-L1 from 75% to 37.5% after neoadjuvant chemotherapy in NSCLC (18), while Rojkó revealed that PD-L1 expression showed no significant changes after neoadjuvant chemotherapy in patients with lung cancer (25). Song et al. demonstrated that the expression of PD-L1 could be upregulated by neoadjuvant chemotherapy in lung squamous cell carcinoma patients (19) as well as in Guo et al. ‘s study (26).
To our knowledge, however, there are no prospective studies that have investigated the role of rebiopsy after CRT in LA-NSCLC, either in terms of feasibility or clinical relevance. A retrospective study on 35 patients with LA-NSCLC, with paired NSCLC specimens that had been obtained pre- and post-CRT, showed that the percentage of PD-L1-positive tumor cells significantly decreased after CRT (27).
Our multicentric retrospective studies showed that PD-L1 TPS expression can be induced by CRT administration in approximately half of the PD-L1 TPS patients resulted negative at the baseline. Consequently, the execution of a re-biopsy in this group of patients may increase the number of candidates to maintenance Durvalumab according to EMA criteria, due to change in PD-L1 TPS expression from negative (<1%) to positive (>1%). Our data suggest that a re-biopsy is feasible, with the use of various biopsy-modalities, safe, as no severe complications were recorded, and with a high success-rate. This latter data is interesting as biopsies containing insufficient tumor tissue with none or too few tumor cells to perform molecular analysis was previously reported as a challenge by Chouaid and colleagues in 18% and 7% of cases, respectively (28).
Differences in PD-L1 TPS between tumor sites have been explored in several studies and a general consensus of both inter- and intra-tumoral heterogeneity have been established, above all in stage IV NSCLC (29–32). Almost all of these studies evaluated the concordance rate of PD-L1 expression between primary and metastatic tumor sites in stage IV NSCLC and report a high concordance for tumors with a PD-L1 TPS of <1% or ≥50% (32). A recent work confirms these data, and the authors point out how, due to the known and widely explored heterogeneity of PD-L1 expression, it could be questioned if the changes in PD-L1 TPS observed, could solely be explained by a different location of re-biopsy. These authors found a change in PD-L1 TPS with nearly the same incidence in patients who had a re-biopsy performed at the same or another location as the diagnostic biopsy (33). Our numbers are too small to determine whether a given biopsy procedure rather than the biopsied site is at greater risk of insufficient material; but it certainly makes sense that more biopsies can increase the success rate of the procedure.
This data is interesting considering the fear of performing biopsies in previously irradiated areas and that up to 40% of patients do not have tumor biopsies suitable for histological PD-L1 assessment at baseline (e.g., due to inadequate tissue collection using fine needle aspiration), and cytological assessment of PD-L1 expression, while feasible, is not yet widely standardized in routine clinical practice (34). Without considering patients who have negative PD-L1 and are denied access to maintenance Durvalumab after CRT.
The current study has some limitations. First, it is a retrospective study with a relatively small sample. Second, considering the great variability and the several problems in assessing PD-L1 expression, it is important to underline that no centralized review of either primary biopsies or re-biopsies was performed. Third, we did not investigate PD-L1 expression with the fluorescent in situ hybridization (FISH), generally considered more reliable.
Re-biopsy is often offered to patients with progressive stage IV NSCLC after the first-line therapy, as it can provide important biological information to guide the second-line treatment decisions. However, despite its potential clinical advantages, performing a re-biopsy is not mandatory or regularly incorporated into the daily clinical practice.
Our study showed that re-biopsy is feasible, with low risk of complications, and can be clinically relevant in patients with LA- NSCLC PD-L1 negative. Thus, PD-L1 redetermination should be considered after a definitive CRT, in patients with LA-NSCLC PD-L1 negative, as it may allow them receiving the maintenance Durvalumab. Certainly, future prospective studies are needed to validate our results.
The datasets presented in this study can be found in online repositories. Access to the raw data and names of the repositories should be directed to Patrizia Ciammella (cGF0cml6aWEuY2lhbW1lbGxhbEBhdXNsLnJlLml0).
Ethical approval was not required for the studies involving humans because this is a clinical practice activity with no management of single data and possible recognition of data and patient. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
PC: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. SC: Data curation, Software, Writing – review & editing. PB: Writing – review & editing. MG: Writing – review & editing. VN: Writing – review & editing. MR: Data curation, Writing – review & editing. MS: Writing – review & editing. VS: Writing – review & editing. ABr: Data curation, Formal analysis, Software, Writing – review & editing. FZ: Writing – review & editing. RP: Writing – review & editing. ET: Writing – review & editing. ABo: Data curation, Formal analysis, Software, Writing – review & editing. FI: Data curation, Writing – review & editing. EA: Data curation, Writing – review & editing. CB: Writing – review & editing. MT: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Editorial assistance in manuscript preparation were funded by AstraZeneca, Italy. Editorial assistance in the preparation of this article was provided by Edra S.p.A.
MT received speakers’ and consultants’ fee from Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre, Amgen, Merck, Sanofi. MT received institutional research grants from Astra-Zeneca, Boehringer Ingelheim.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung (2020) 198(6):897–907. doi: 10.1007/s00408-020-00407-5
2. Vansteenkiste J, De Ruysscher D, Eberhardt WE, Lim E, Senan S, Felip E, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2013) 24 Suppl 6:vi89–98. doi: 10.1093/annonc/mdt241
3. Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol (2017) 8(1):1–20. doi: 10.5306/wjco.v8.i1.1
4. Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using Docetaxel and Cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol (2015) 33(24):2660–6. doi: 10.1200/JCO.2014.60.0130
5. Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol (2008) 26(15):2450–6. doi: 10.1200/JCO.2007.14.4824
6. Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol (2008) 26(35):5755–60. doi: 10.1200/JCO.2008.17.7840
7. Wozniak AJ, Moon J, Thomas CR Jr, Kelly K, Mack PC, Gaspar LE, et al. A pilot trial of cisplatin/etoposide/radiotherapy followed by consolidation docetaxel and the combination of bevacizumab (NSC-704865) in patients with inoperable locally advanced stage III non-small-cell lung cancer: SWOG S0533. Clin Lung Cancer (2015) 16(5):340–7. doi: 10.1016/j.cllc.2014.12.014
8. Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol (2015) 16(2):187–99. doi: 10.1016/S1470-2045(14)71207-0
9. Hoang T, Dahlberg SE, Schiller JH, Mehta MP, Fitzgerald TJ, Belinsky SA, et al. Randomized phase III study of thoracic radiation in combination with paclitaxel and carboplatin with or without thalidomide in patients with stage III non-small-cell lung cancer: the ECOG 3598 study. J Clin Oncol (2012) 30(6):616–22. doi: 10.1200/JCO.2011.36.9116
10. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
11. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med (2018) 379(24):2342–50. doi: 10.1056/NEJMoa1809697
12. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
13. Yoneda K, Kuwata T, Kanayama M, Mori M, Kawanami T, Yatera K, et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer (2019) 121(6):490–6. doi: 10.1038/s41416-019-0541-3
14. Hui R, Özgüroğlu M, Villegas A, Daniel D, Vicente D, Murakami S, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol (2019) 20(12):1670–80. doi: 10.1016/S1470-2045(19)30519-4
15. European Medicines Agency. Durvalumab (Imfinzi). Summary of product characteristics 2018. Available at: https://www.ema.europa.eu/en/documents/product-information/imfizi-epar-product-information_en.pdf (Accessed January 13, 2020).
16. US Food and Drug Safety Administration. IMFINZI (Durvalumab) label 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761069s002lbl.pdf (Accessed October 15, 2019).
17. Pharmaceuticals and Medical Devices Agency (PMDA). List of Approved Products Financial Year 2018. Available at: https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html (Accessed October 15, 2019).
18. Sheng J, Fang W, Yu J, Chen N, Zhan J, Ma Y, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep (2016) 6:20090. doi: 10.1038/srep20090
19. Song Z, Yu X, Zhang Y. Altered expression of programmed death-ligand 1 after neo-adjuvant chemotherapy in patients with lung squamous cell carcinoma. Lung Cancer (2016) 99:166–71. doi: 10.1016/j.lungcan.2016.07.013
20. Sakai H, Takeda M, Sakai K, Nakamura Y, Ito A, Hayashi H, et al. Impact of cytotoxic chemotherapy on PD-L1 expression in patients with non-small cell lung cancer negative for EGFR mutation and ALK fusion. Lung Cancer (2019) 127:59–65. doi: 10.1016/j.lungcan.2018.11.025
21. Shin J, Chung JH, Kim SH, Lee KS, Suh KJ, Lee JY, et al. Effect of platinum-based chemotherapy on PD-L1 expression on tumor cells in non-small cell lung cancer. Cancer Res Treat (2019) 51(3):1086–97. doi: 10.4143/crt.2018.537
22. Lacour M, Hiltbrunner S, Lee SY, Soltermann A, Rushing EJ, Soldini D, et al. Adjuvant chemotherapy increases programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer recurrence. Clin Lung Cancer (2019) 20(5):391–6. doi: 10.1016/j.cllc.2019.05.013
23. Zhang P, Ma Y, Lv C, Huang M, Li M, Dong B, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci (2016) 107(11):1563–71. doi: 10.1111/cas.13072
24. Cho JH, Sorensen SF, Choi YL, Feng Y, Kim TE, Choi H, et al. Programmed death ligand 1 expression in paired non-small cell lung cancer tumor samples. Clin Lung Cancer (2017) 18(6):e473–9. doi: 10.1016/j.cllc.2017.04.008
25. Rojkó L, Reiniger L, Téglási V, Fábián K, Pipek O, Vágvölgyi A, et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J Cancer Res Clin Oncol (2018) 144(7):1219–26. doi: 10.1007/s00432-018-2642-4
26. Guo L, Song P, Xue X, Guo C, Han L, Fang Q, et al. Variation of programmed death ligand 1 expression after platinum-based neoadjuvant chemotherapy in lung cancer. J Immunother (2019) 42(6):215–20. doi: 10.1097/CJI.0000000000000275
27. Fujimoto D, Uehara K, Sato Y, Sakanoue I, Ito M, Teraoka S, et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci Rep (2017) 7(1):11373. doi: 10.1038/s41598-017-11949-9
28. Chouaid C, Dujon C, Do P, Monnet I, Madroszyk A, Le Caer H, et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01). Lung Cancer (2014) 86(2):170–3. doi: 10.1016/j.lungcan.2014.08.016
29. Haragan A, Field JK, Davies MPA, Escriu C, Gruver A, Gosney JR. Heterogeneity of PD-L1 expression in non-small cell lung cancer: Implications for specimen sampling in predicting treatment response. Lung Cancer (2019) 134:79–84. doi: 10.1016/j.lungcan.2019.06.005
30. Zhou J, Gong Z, Jia Q, Wu Y, Yang ZZ, Zhu B. Programmed death ligand 1 expression and CD8+ tumor-infiltrating lymphocyte density differences between paired primary and brain metastatic lesions in non-small cell lung cancer. Biochem Biophys Res Commun (2018) 498(4):751–7. doi: 10.1016/j.bbrc.2018.03.053
31. Pinato DJ, Shiner RJ, White SD, Black JR, Trivedi P, Stebbing J, et al. Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: Implications for immunotherapy. Oncoimmunology (2016) 5(9):e1213934. doi: 10.1080/2162402X.2016.1213934
32. Kim S, Koh J, Kwon D, Keam B, Go H, Kim YA, et al. Comparative analysis of PD-L1 expression between primary and metastatic pulmonary adenocarcinomas. Eur J Cancer (2017) 75:141–9. doi: 10.1016/j.ejca.2017.01.004
33. Frank MS, Bødtger U, Høegholm A, Stamp IM, Gehl J. Re-biopsy after first line treatment in advanced NSCLC can reveal changes in PD-L1 expression. Lung Cancer (2020) 149:23–32. doi: 10.1016/j.lungcan.2020.08.020
Keywords: locally advanced non-small cell lung cancer, chemo-radiation, PD-L1 expression, PD-L1 negative patients, re-biopsy, durvalumab
Citation: Ciammella P, Cozzi S, Borghetti P, Galaverni M, Nardone V, Ruggieri MP, Sepulcri M, Scotti V, Bruni A, Zanelli F, Piro R, Tagliavini E, Botti A, Iori F, Alì E, Bennati C and Tiseo M (2024) Redetermination of PD-L1 expression after chemio-radiation in locally advanced PDL1 negative NSCLC patients: retrospective multicentric analysis. Front. Oncol. 14:1325249. doi: 10.3389/fonc.2024.1325249
Received: 20 October 2023; Accepted: 02 January 2024;
Published: 31 January 2024.
Edited by:
Alessandro Morabito, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyReviewed by:
Jian Wang, Huazhong University of Science and Technology, ChinaCopyright © 2024 Ciammella, Cozzi, Borghetti, Galaverni, Nardone, Ruggieri, Sepulcri, Scotti, Bruni, Zanelli, Piro, Tagliavini, Botti, Iori, Alì, Bennati and Tiseo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrizia Ciammella, cGF0cml6aWEuY2lhbW1lbGxhbEBhdXNsLnJlLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.