- 1Department of Cutaneous Oncology, Moffitt Cancer Center, Tampa, FL, United States
- 2Department of Oncologic Sciences, University of South Florida Morsani College of Medicine, Tampa, FL, United States
Acral lentiginous melanoma is a rare subtype of melanoma generally associated with poor outcomes, even when diagnosed at an early stage. The tumor genetic profile remains poorly understood, but it is known to have a suppressed immune environment compared to that of non-acral cutaneous melanomas, which limits therapy options. There is significant attention on the development of novel therapeutic approaches, although studies are limited due to disease rarity. For local disease, wide local excision remains the standard of care. Due to frequent under-staging on preoperative biopsy, wider margins and routine sentinel lymph node biopsy may be considered if morbidity would not be increased. For advanced disease, anti-PD1 monotherapy or combination therapy with anti-PD1 and anti-CTLA4 agents have been used as first-line treatment modalities. Anti-PD1 and anti-CTLA4 combination therapies have been shown to be particularly beneficial for patients with BRAF-mutant acral lentiginous melanoma. Other systemic combination regimens and targeted therapy options may be considered, although large studies with consistent results are lacking. Regional and intralesional therapies have shown promise for cutaneous melanomas, but studies generally have not reported results for specific histologic subtypes, especially for acral melanoma. Overall, the unique histologic and genetic characteristics of acral lentiginous melanoma make therapy options significantly more challenging. Furthermore, studies are limited, and data reporting has been inconsistent. However, more prospective studies are emerging, and alternative therapy pathways specific to acral lentiginous melanoma are being investigated. As further evidence is discovered, reliable treatment guidelines may be developed.
1 Introduction
Acral lentiginous melanoma (ALM) is the rarest of the four major subtypes of cutaneous melanoma, accounting for 2-3% of all melanomas (1–3). ALM occurs predominantly in non-hair-bearing skin of the distal extremities, such as the palms of the hands, soles of the feet, and nailbeds (1, 3–6). This unique histologic subtype was first described by RJ Reed in 1976, as pigmented lesions with a radial (lentiginous) growth phase of melanocytes, which evolves into a dermal (vertical) invasive stage (4, 5, 7).
In addition to its distinctive growth pattern, ALM has additional characteristics separating it from non-ALM cutaneous melanoma. ALM has a much lower mutational burden than non-ALM cutaneous melanomas, including a lower incidence of activating mutations in BRAF and NRAS, variable KIT mutations, and a lack of ultraviolet (UV)-related mutational signatures (3, 7–9). A study by Li et al., utilizing single-cell RNA-sequencing to map the transcriptional landscape of ALM, found a lower overall immune infiltrate, fewer effector CD8 T cells and NK cells, and a near-complete absence of γδ T cells, compared to non-ALM cutaneous melanoma. This study also discovered that ALM and non-ALM cutaneous melanoma cells have different patterns, and overall reduced density, of cell-to-cell communications (8).
Due to disease rarity, poorly understood carcinogenic and immunogenic processes, and underrepresentation in the literature, there is a paucity of level 1 evidence-based guidance (evidence from a systematic review(s) of homogeneous randomized control trials) for managing ALM. Contemporary therapeutic techniques have been generally extrapolated from large, prospective randomized controlled trials on non-ALM cutaneous melanomas, and/or from small retrospective studies on ALM.
1.1 Epidemiology
ALM typically presents among patients at an older age, with a mean age at diagnosis of approximately 63 years for ALM compared to approximately 59 years for non-ALM cutaneous melanoma, according to large population-based studies (1, 2, 6, 10). There is a slight female predominance in ALM, and the majority of tumors are found on skin of the lower extremities (1, 6, 7, 10). Pathogenic mutations are unrelated to sun exposure and, consequently, ALM is the most frequent melanoma subtype found in individuals of Asian, African, and Hispanic or Latino descent, with the highest proportion of cases found among those of African descent (1, 3, 7, 9, 11). Geographic distribution is poorly understood due to the scarcity of reported cases, but it is overall reflective of the ethnic origin of the population in that region. For example, studies based in Asia found that ALM represented 55-58% of melanomas in Taiwan and Korea, compared to a population study in the United States which found that ALM represented approximately 2% of melanomas (2, 12, 13). Population-based studies within the United States have found no statistically significant differences in ALM distribution based solely on geographic location (2).
ALM has a significantly lower mutation rate compared to other cutaneous melanomas. Overall, studies worldwide have reported ALM mutation rates in BRAF of 4% to 34%, KIT of 11% to 36%, RAS of 32%, NRAS of 22%, NF1 of 17%, GNAQ of 17% (13–17). The mutation profile of ALM varies between ethnicities as well. Studies based on Asian populations have found lower KIT mutation rates (11% to 12%) compared to those from the United States (12% to 36%) (13–17).
Interestingly, pre-existing benign melanocytic acral nevi are not a risk factor for development of ALM (18). Benign acral nevi have been found to have a significantly higher BRAF mutation rate than in ALM, suggesting that they are not precursor lesions (19). Mechanical stress such as pressure and trauma may play a role in the development of advanced ALM, especially in the lower extremities, but studies have reported conflicting evidence of this potential association (4, 20–22).
1.2 Clinical presentation and diagnosis
ALM typically presents among older patients as an asymmetric pigmented lesion on the palms, soles, or nailbeds (4). These lesions have a higher tendency than non-ALM cutaneous melanoma to be ulcerated (7).
Diagnosing ALM can be clinically challenging, as ALM can mimic benign conditions such as ulcers related to vascular disease, diabetes, mechanical pressure, warts, or trauma. Furthermore, since ALM is more common in patients with dark skin pigmentation, lesions may not appear as visually prominent as they would be in patients with light skin tones. Dermoscopy serves as a useful adjunct to differentiate ALM from benign acral nevi, as palmoplantar ALM demonstrates a characteristic parallel ridge pattern and irregular diffuse pigmentation (18, 23). Characteristic dermoscopy findings for subungual ALM include longitudinal brown/black lines, irregular in their coloration, spacing, thickness, and parallelism (24). In the setting of a suspicious pigmented lesion on an acral site that does not respond to a short course of treatment for a suspected benign condition, a prompt biopsy should be undertaken (4).
An excisional biopsy, typically the gold standard for diagnosing melanocytic lesions, can be technically challenging in ALM, since lesion locations typically include sites with a restricted skin reservoir which would require amputation for complete removal of the lesion (4, 18). Therefore, initial biopsy technique is more commonly a punch, shave, or incisional biopsy (18, 25). However, due to the inability to completely evaluate the lesion pathologically, studies have found that a high proportion of patients with ALM had their lesions under-staged on biopsy (18, 26).
Once a biopsy specimen is obtained, histologic findings suggestive of ALM include acanthosis, spindle-cell makeup of the dermal component, poor circumscription, tendency of melanocytes to proliferate singly or in nests, lentiginous growth pattern into the upper epidermis, and inflammatory changes in the papillary dermis (7, 18). Pathologic characteristics must be incorporated into the clinical setting to aid in determining an accurate diagnosis.
For many reasons, often including misdiagnosis for benign conditions, patients with ALM tend to have a delay in diagnosis and are found to have a significantly higher disease stage at diagnosis than patients with non-ALM cutaneous melanoma (1, 7, 10, 18). To overcome these diagnostic challenges, it is critical to have a high level of suspicion for ALM, and to integrate the clinical, dermoscopic, histopathologic, and molecular findings.
1.3 Terminology
It is important to note that the term “acral lentiginous melanoma” is not equivalent to “acral melanoma”. The term “acral” refers to a lesion at a peripheral site, such as on the distal extremities. The “lentiginous” designation is reserved for cases with the characteristic radial growth pattern, as described by RJ Reed, and histologic characteristics (1, 4, 5, 7). Patients may have a lesion on the distal extremity appropriately classified as an “acral melanoma”, but without distinguishing features to classify as “lentiginous”, especially if the area has had high levels of ultraviolet and sun exposure. This distinction is frequently not made in the literature, with less than 40% of reports specifying “acral lentiginous melanoma” as a histologic subtype as well as the corresponding appropriate acral anatomic site (27). All efforts were made to focus this review specifically on ALM, with the understanding that some of the literature reviewed may have included non-ALM cutaneous melanoma on acral sites (4, 27).
1.4 Clinical outcomes
Large contemporary population-based outcome studies are limited for ALM, with most studies capturing time periods before 2016 (1, 2, 6, 7, 10). Based on currently available literature, ALM is associated with a worse prognosis than non-ALM cutaneous melanoma (1, 2, 7, 10, 11). This may be partially attributed to the tendency of ALM to be associated with a delayed diagnosis, older age, deeper thickness, more frequent ulceration, lymphovascular invasion, lymph node positivity, and higher stage at presentation (1, 6, 7, 28–30). Studies have shown approximately 64% to 68% of non-ALM cutaneous melanoma presenting at stage I, compared to 38% to 45% of ALM presenting at stage I (1, 7). Some studies suggest that ALM has a worse prognosis even despite controlling for variables such as stage and tumor thickness (1, 2, 7, 28). One study found race as a significant prognostic indicator, with significantly lower survival rates among Black patients, after controlling for stage; these patients were found to be older, predominantly men, and with thicker, more ulcerated disease (2). Other prognostic indicators identified include older age, male sex, positive lymph node status, pathologic stage, tumor thickness, ulceration, and socioeconomic status (2, 6, 7, 29, 31).
Population-based studies utilizing the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute database found 5-year melanoma-specific survival rates for ALM of approximately 80% to 81%, compared to 91% to 93% for non-ALM cutaneous melanoma (1, 2). Ten-year melanoma-specific survival rates were 67.5% for ALM, compared to 87.5% for non-ALM cutaneous melanoma (1). Slightly different results were found in a population-based study using the National Cancer Database, which found an unadjusted 5-year overall survival (OS) rate of 67.3% for ALM and 75.8% for non-ALM cutaneous melanoma (P<.001) (7). With further stratification by stage, 5-year OS in ALM vs non-ALM cutaneous melanoma was 84.6% vs 88.6% for stage I (P<.001); 62.1% vs 64% for stage II (P=.7); 47.5% vs 56.7% for stage III (P<.001); and 16.2% vs 16.4% for stage IV (P=.02) (7).
2 Management
Given the rarity of the disease and inconsistent reporting of ALM in the literature, management techniques have been generally extrapolated from studies on non-ALM cutaneous melanoma, and from fairly small, retrospective studies on ALM. This can be problematic given the inherent distinctions between ALM and non-ALM cutaneous melanoma, including different mutation profiles and tumor microenvironments (3, 7–9). Further research and prospective randomized trials are needed to develop reliable treatment guidelines specific to ALM.
2.1 Primary lesion
For localized, resectable disease, upfront surgical resection is standard of care. Margin guidelines have been generally extrapolated from those established for non-ALM cutaneous melanoma. However, given the high frequency of preoperative biopsy under-staging ALM lesions, wider margins and sentinel lymph node biopsy may be considered in patients with lower stage ALM but with residual pigmentation on exam (26). Reflectance confocal microscopy can be useful in the setting of positive margins, as it assists with targeted resection of the area of concern (32).
For subungual ALM, surgical resection with adequate margins typically involves amputation. However, universal amputation has been challenged in recent years. Nakamura et al. found that wide local excision with 0.5 cm to 1 cm peripheral margins and deep margins to underlying bone provided acceptable local control for in situ and intermediate thickness invasive disease. Four out of 50 patients with in situ disease experienced local recurrence at the lateral margin requiring re-excision, and one out of 12 patients with invasive disease experienced nodal metastasis over 7 years later requiring regional lymph node dissection. No patients with invasive disease experienced local recurrence, no patients required amputation, and all patients survived the follow-up period (24-207 months) (33). A systematic review with meta-analysis comparing Mohs micrographic surgery versus nail unit excision versus amputation for melanoma in situ of the nail apparatus found a local recurrence rate of 8.7% (2 of 23 patients) with Mohs micrographic surgery, 4.7% (12 of 254 patients) with nail unit excision, and 2.9% (1 of 34 patients) with amputation. There was no statistically significant difference in local recurrence rates between modalities (34). However, a difference of nearly 6% is likely to be clinically relevant, and the lack of statistical significance is likely related to the small sample sizes. Overall, definitive conclusions cannot yet be drawn based on currently available literature which is significantly limited in study design and power. Further research, including randomized controlled trials, in optimal excision technique for subungual ALM is warranted.
2.2 Adjuvant and neoadjuvant therapy
There is limited data regarding the role of systemic therapy in the adjuvant and/or neoadjuvant setting specifically for ALM. While these therapies have been studied extensively for cutaneous melanoma, ALM has served as only a small minority of those patients.
2.2.1 Adjuvant therapy
For stage IIB and IIC cutaneous melanoma, adjuvant therapy with pembrolizumab has been supported by the KEYNOTE-716 trial, and adjuvant therapy with nivolumab has been supported by the CheckMate 76K trial (35, 36). The KEYNOTE-716 trial found that patients with completely resected stage IIB or IIC cutaneous melanoma treated with adjuvant pembrolizumab had a significantly reduced risk of disease progression and death compared to placebo. This study did not report histologic subtype analysis (36). The CheckMate 76K trial treated patients with completely resected stage IIB or IIC cutaneous melanoma with adjuvant nivolumab or placebo. Patients treated with nivolumab had a 58% lower risk of recurrence or death compared to placebo. This study reported 43 patients with ALM, representing 5.4% of the total patient population (35).
For stage III-IV cutaneous melanoma, studies have also supported adjuvant pembrolizumab and adjuvant nivolumab. The EORTC 1325/KEYNOTE-054 phase III trial found a significantly improved recurrence-free survival (RFS) with adjuvant pembrolizumab compared to placebo for resected high-risk stage III melanoma, which led to its approval in the USA and Europe (37). The CheckMate 238 phase III trial compared adjuvant therapy with either nivolumab or ipilimumab for patients with resected stage IIIB-C or IV cutaneous melanoma, and found a significantly improved RFS with nivolumab (38). A 5-year follow-up study of the CheckMate 238 trial confirmed a sustained, long-term improvement in RFS with nivolumab compared to ipilimumab (5-year RFS rates of 50% vs 39%). Distant metastasis-free survival rates were 58% and 51%, and 5-year OS rates were 76% and 72% with nivolumab and ipilimumab, respectively (39). None of these studies reported histologic subtype analysis to determine the proportion of ALM within the study population.
The CheckMate 915 trial reaffirmed use of adjuvant nivolumab for resected stage IIIB-D or stage IV cutaneous melanoma. This study found that adjuvant nivolumab monotherapy was superior to combination therapy with ipilimumab (no difference in RFS, and higher rate of treatment adverse events in the combination group). Patients with ALM represented 2.9% of this total study population (n = 54) (40).
A small study by Maeda et al. evaluated 27 patients with ALM treated in the adjuvant setting; 5 patients were treated with nivolumab and 22 patients were treated with either chemotherapy (n = 4), interferon beta (n = 12), or observation (n = 6). This study found no difference in disease-free survival between the groups, although the small sample size limits any clinically significant conclusions (41).
2.2.2 Neoadjuvant therapy
The SWOG S1801 phase II trial studied patients with resectable stage IIIB-IVC cutaneous melanoma, with ALM representing 6% of the total study population; 154 patients were treated with neoadjuvant and adjuvant pembrolizumab, and 159 patients were treated with adjuvant-only pembrolizumab. This study found an event-free survival benefit in patients who received both neoadjuvant and adjuvant pembrolizumab (42).
Neoadjuvant therapy allows for monitoring of in-vivo tumor response to treatment, which is particularly useful for ALM, since treatment response is less certain. A large, pooled analysis from the International Neoadjuvant Melanoma Consortium found a significant correlation between a pathologic complete response to neoadjuvant therapy and improved RFS (89% vs 50% at 2 years) and OS (95% vs 83% at 2 years). In this study, neoadjuvant therapies included ipilimumab and nivolumab combination therapy (n = 104), anti-PD1 monotherapy (n = 37), and targeted therapy (n = 51). The pathologic complete response rates were 47% for targeted therapy, 43% with combination therapy, and 20% with anti-PD1 monotherapy. Histological subtype analysis was not reported in this study (43). This concept was further emphasized in a study by Huang et al., where patients with stage III/IV cutaneous melanoma who developed a “response signature”, a specific immune response measured on a blood sample at three weeks after one dose of neoadjuvant anti-PD1 therapy, had a lower disease recurrence than those who displayed mechanisms of resistance. This study did not report histologic subtype analysis (44).
The OpACIN-neo phase II trial studied three different dosing schedules for ipilimumab and nivolumab combination neoadjuvant therapy for patients with stage III melanoma. The radiological objective response rates ranged from 35-65% and the pathological response rates ranged from 65-80%, depending on the dosing schedule. The highest radiological and pathological response rates were seen in group A, with a regimen of two cycles of ipilimumab (3 mg/kg) plus nivolumab (1 mg/kg) once every three weeks intravenously. This study did not report histologic subtype analysis (45).
The PRADO trial, an extension cohort of the OpACIN-neo trial, 99 patients with stage IIIB-D nodal cutaneous melanoma were treated with neoadjuvant ipilimumab and nivolumab. The pathologic response rate was 72%, and the major pathologic response (¾ 10% viable tumor in their index lymph node) rate was 61%. Patients with a major pathologic response underwent no additional therapy. Patients with a pathologic partial response (10-50% viable tumor) underwent therapeutic lymph node dissection. Patients with pathologic non-response (>50% viable tumor) underwent therapeutic lymph node dissection, adjuvant systemic therapy, and possibly synchronous radiotherapy. Based on two-year relapse-free survival rates and distant metastasis-free survival rates, patients with major pathologic response could safely omit therapeutic lymph node dissection and adjuvant therapy. This study did not report histologic subtype analysis (46).
A study by Amaria et al. analyzed a combination therapy regimen with relatlimab (a LAG-3 inhibitor) and nivolumab in the neoadjuvant setting for patients with resectable stage III or oligometastatic stage IV cutaneous melanoma. This study found a pathologic complete response rate of 57% and an overall pathologic response rate of 70%. This study did not report histologic subtype analysis (47).
Ultimately, this area of interest requires more data from ALM-focused research trials to more fully evaluate the role of systemic therapy in the adjuvant and/or neoadjuvant setting.
2.3 Advanced disease
For unresectable, locally advanced, or metastatic ALM, systemic therapy options should be considered. Unfortunately, the immune microenvironment in ALM remains poorly understood, and studies have shown lower response rates and shorter response durations with ALM to systemic therapy, compared to non-ALM cutaneous melanoma (8, 48).
2.4 Immune checkpoint inhibitor therapy
2.4.1 Anti-CTLA4 monotherapy
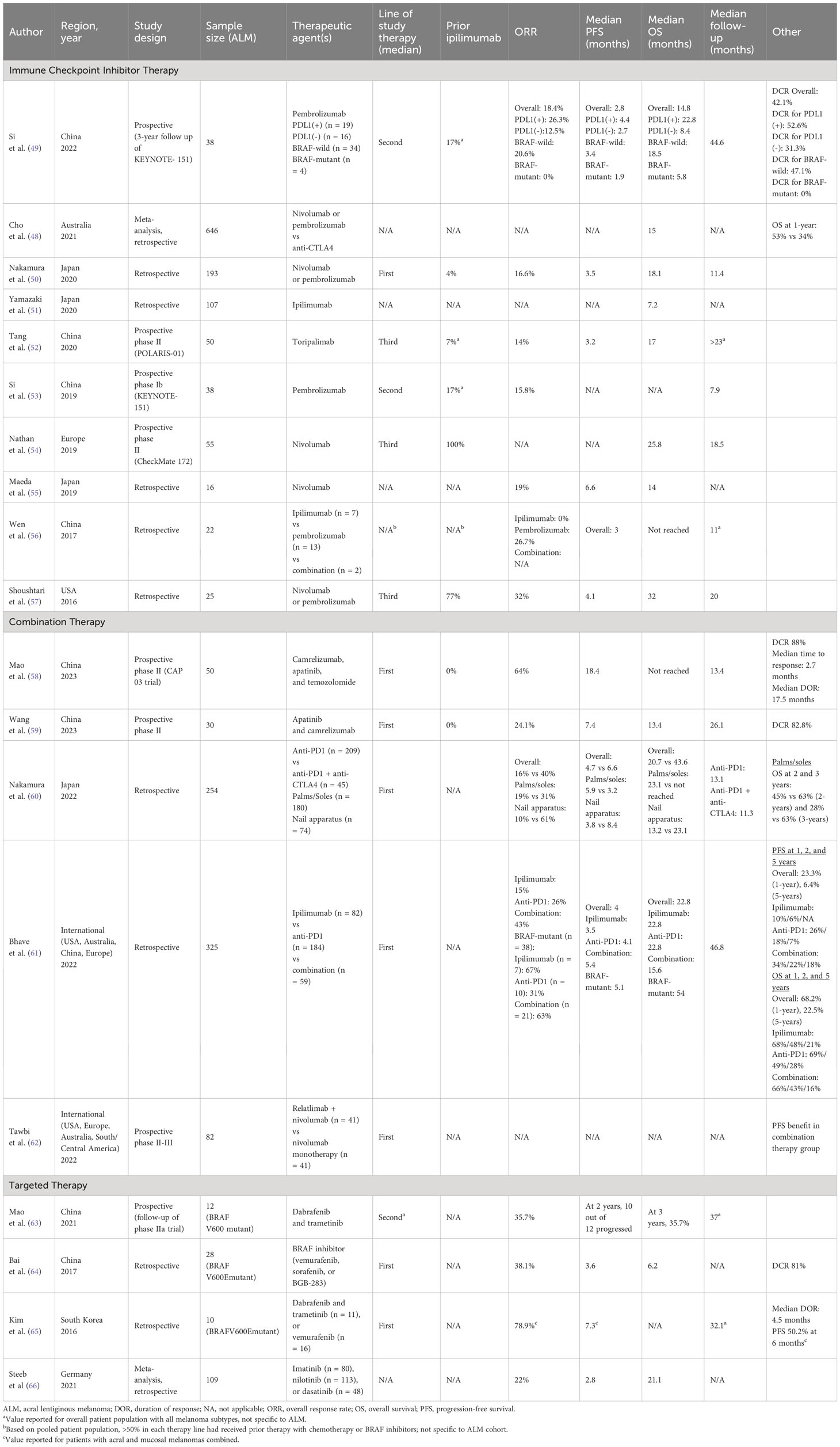
Studies have found that anti-CTLA4 agents, such as ipilimumab, used as monotherapy are not as effective as anti-PD1 monotherapy or a combination of anti-PD1 and anti-CTLA4 therapy (Table 1). In a large Australian meta-analysis by Cho et al., evaluating 646 patients with ALM, patients who underwent anti-PD1 monotherapy had a significantly higher OS at 1-year compared to those who underwent anti-CTLA4 monotherapy (53% vs 34%, P<.001) (48). A smaller study by Wen et al., which studied 22 patients with ALM, treated 7 patients with ipilimumab and 13 patients with pembrolizumab. The ORR among patients treated with ipilimumab was 0%, compared to an ORR of 26.7% for patients treated with pembrolizumab (56). A multi-institutional study by Bhave et al. analyzed 325 patients with ALM who received either ipilimumab monotherapy (n = 82), anti-PD1 monotherapy (n = 184), or ipilimumab/anti-PD1 combination therapy (n = 59). Patients who received ipilimumab monotherapy had a significantly lower ORR of 15% compared to the other cohorts (26% for anti-PD1 and 43% for combination therapy) and lower PFS at 1-year of 10% and 2-years of 6% (compared to 1-year and 2-year PFS of 26% and 18% for anti-PD1, and 34% and 22% for combination therapy) (61). A study by Yamazaki et al. treated 107 patients with ALM with ipilimumab monotherapy, and found a notably low median OS of 7.2 months (51).
2.4.2 Anti-PD1 monotherapy
Use of anti-PD1 therapeutic agents, such as pembrolizumab and nivolumab, for ALM was first adopted due to landmark trials mostly focused on non-ALM cutaneous melanoma. Studies have shown that ALM has a lower frequency of PD-L1 expression compared to non-ALM cutaneous melanoma (33% vs 62%) (67). Despite this, subsequent trials specific to ALM patients have shown promising results with anti-PD1 agents (Table 1).
In general, ORR, median PFS, and median OS with anti-PD1 therapy have been found to be lower in studies from China and Japan compared to those from Europe and the United States. Studies from China and Japan using nivolumab or pembrolizumab for ALM have found an ORR generally between 14% to 19% (one small study with 26.7%), median PFS between 2.8 and 6.6 months, and median OS between 14 and 18.1 months (49, 50, 52, 53, 55, 56). This is compared to an ORR of 32% and median PFS of 4.1 months from a United States-based study, and a median OS of 25.8 to 32 months from United States and European-based studies (54, 57).
The KEYNOTE-151 trial, a phase 1b study in China evaluating pembrolizumab as second-line therapy for advanced melanoma, with ALM representing 37.9% of their total study population, found an ORR of 15.8% for ALM (n = 38) (53). In a three-year follow-up study, the ORR for ALM was 18.4% (n = 38), median PFS was 2.8 months, median OS was 14.8 months, and disease control rate (DCR) was 41.2%. Patients were further stratified by PD-L1 and BRAF status. Patients with ALM and PD-L1-positive disease vs PD-L1-negative disease had an ORR of 26.3% vs 12.5%, a median PFS of 4.4 months vs 2.7 months, median OS of 22.8 months vs 8.4 months, and DCR of 52.6% vs 31.3%. Patients with ALM and BRAF wild-type disease vs BRAF-mutant disease had an ORR of 20.6% vs 0%, a median PFS of 3.4 months vs 1.9 months, median OS of 18.5 months vs 5.8 months, and DCR of 47.1% vs 0% (49).
Similar findings were reported in a Japanese study by Nakamura et al., which retrospectively analyzed 193 patients with ALM who received nivolumab or pembrolizumab as first line therapy, with an ORR of 16.6%, a median PFS of 3.5 months, and median OS of 18.1 months (50). A smaller Japanese study by Maeda et al., which retrospectively studied 16 patients with ALM who received nivolumab therapy, found an ORR of 19%, a median PFS of 6.6 months, and median OS of 14 months (55). A Chinese study by Wen et al. retrospectively studied 13 patients with ALM who underwent therapy with pembrolizumab, with a uniquely high ORR of 26.7% (56). A prospective phase II study in China by Tang et al. analyzed 50 patients with ALM who received a less commonly used anti-PD1 therapy drug, toripalimab, as a later line therapy, with an ORR of 14%, a median PFS of 3.2 months, and median OS of 17 months (52).
The CheckMate 172 trial, a phase II study based in Europe, with ALM representing 5.5% of their total study population, evaluated the use of nivolumab for advanced melanoma with progression on or after ipilimumab treatment. Median OS for ALM was 25.8 months (n = 55) (54). A United States-based multi-institutional retrospective cohort analysis by Shoushtari et al. showed similar findings, in which 25 patients with ALM were treated with nivolumab or pembrolizumab (85% treated with prior therapy, 77% with prior ipilimumab), with an ORR of 32%, a median PFS of 4.1 months, and median OS of 32 months (57).
2.4.3 Combination therapy versus monotherapy
Overall, combination therapy for advanced ALM has shown better efficacy than monotherapy, and is therefore the current standard of care (Table 1). Some combinations have included anti-PD1 and anti-CTLA4 agents, but others have incorporated newer mechanisms such as apatinib (a tyrosine kinase inhibitor that selectively inhibits vascular endothelial growth factor receptor-2), temozolomide (DNA-alkylating agent), and relatlimab (a LAG-3 inhibitor).
A large international study by Bhave et al., based on institutions from the United States, Australia, and Europe, retrospectively studied 325 patients with ALM who received either ipilimumab monotherapy, anti-PD1 monotherapy, or ipilimumab/anti-PD1 combination therapy. This study found a significantly higher ORR of 43% for the combination therapy group, vs 26% for the anti-PD1 monotherapy group, and 15% for the ipilimumab monotherapy group. PFS followed a similar trend, with a median PFS of 5.4 months, 4.1 months, and 3.5 months for the combination therapy group, anti-PD1 monotherapy group, and ipilimumab monotherapy group, respectively. With combination therapy, PFS was 34% at 1-year, 22% at 3-years, and 18% at 5-years. With anti-PD1 monotherapy, PFS was 26% at 1-year, 18% at 3-years, and 7% at 5-years. With ipilimumab monotherapy, PFS was 10% at 1-year, 6% at 3-years, and not evaluable at 5-years. This trend did not correlate with an OS advantage. Notably, patients with BRAF-mutant disease had better responses to all lines of therapy (ORR of 63% to combination, 31% to anti-PD1, and 67% to ipilimumab therapy vs 43% to combination, 26% to anti-PD1, and 15% to ipilimumab for all patients) and longer median OS, 4.5 years vs 1.9 years for all patients (61).
A large Japanese study by Nakamura et al. analyzed 254 patients with ALM who received either anti-PD1 monotherapy or anti-PD1/anti-CTLA4 combination therapy. Patients in the combination therapy group had a significantly higher ORR (40%) compared to patients in the monotherapy group (16%), P=.01. Patients in the combination therapy group also had a higher median PFS (6.6 vs 4.7 months) and median OS (43.6 vs 20.7 months), although neither was statistically significant. Of note, this study found that ALM of the nail apparatus responded particularly well to combination therapy compared to monotherapy (ORR 61% vs 10%, P<.001), although this did not correspond with statistically significant differences in PFS or OS. This ORR difference was not seen among patients with ALM of the palms or soles (31% vs 19%, P=.4) (60).
A recent Chinese prospective phase II trial by Mao et al. studied combination therapy with camrelizumab, apatinib, and temozolomide as first-line therapy for 50 patients with ALM. This study found a particularly high ORR of 64%, median PFS of 18.4 months, DCR of 88%, median time to response of 2.7 months, and median duration of response of 17.5 months (58). A similar recent prospective phase II study by Wang et al., also out of China, studied 30 patients with ALM treated with first-line combination therapy with apatinib and camrelizumab. This study found an ORR of 24.1%, a median PFS of 7.4 months, median OS of 13.4 months, and DCR of 82.8% (59).
A prospective phase II/III study by Tawbi et al. treated patients with advanced melanoma with either relatlimab and nivolumab combination therapy or nivolumab monotherapy. This study found a PFS benefit in the combination therapy group for all patients (n = 714) as well as for subgroups such as ALM (n = 82) (62).
2.5 Targeted molecular therapy
Like most therapy options used for ALM, targeted therapy models were based on those established for cutaneous melanoma. However, ALM has a lower somatic mutation rate than non-ALM cutaneous melanoma, so these therapies generally play a more limited role (68). Furthermore, ALM harbors heterogeneity in BRAF mutations, which can be distinct from V600E/V600K, commonly found in non-ALM cutaneous melanoma (69). In the relatively infrequent cases of ALM harboring BRAF mutations, the level of response to BRAF and BRAF-MEK inhibition is similar to BRAF-mutant non-ALM cutaneous melanoma, although the length of response tends to be shorter for ALM than non-ALM cutaneous melanoma (8).
In a prospective follow-up of a phase II Chinese study by Mao et al., 12 patients with BRAF-V600-mutant ALM were treated with a combination therapy of dabrafenib and trametinib, with an ORR of 35.7%, a progression of 83% at 2-years, and OS of 35.7% at 3-years (63). Similar results were found in another Chinese study by Bai et al., retrospectively analyzing 28 patients with BRAF-V600E-mutant ALM who were treated with either vemurafenib, sorafenib, or BGB-283. This study found an ORR of 38.1%, a median PFS of 3.6 months, median OS of 6.2 months, and DCR of 81% (64).
A retrospective study by Kim et al. based in South Korea analyzed 10 patients with BRAF-V600E-mutant ALM, treated first-line with either dabrafenib and trametinib or vemurafenib. This study reported results combining acral and mucosal melanomas (n = 19), with an ORR of 78.9%, a median PFS of 7.3 months, PFS at 6 months of 50.2%, and median duration of response of 4.5 months (65).
Genomic alterations in the receptor tyrosine kinase KIT have also been identified in ALM. In a systematic review by Steeb et al., which included studies investigating c-KIT inhibitor targeted therapy agents such as imatinib, nilotinib, and dasatinib, found an ORR of 22% (based on eight studies, n = 109 patients with ALM), a median PFS of 2.8 months (based on one study, n = 21 patients with ALM), and median OS of 21.1 months (based on one study, n = 21 patients with ALM) for patients with ALM. For ALM, imatinib showed a slightly higher ORR (27%) than nilotinib (22%). Objective responses were almost exclusively achieved by patients with KIT mutations in exons 11 and 13 (66).
2.6 Regional and intralesional therapy
The nature of ALM and its recurrence patterns in the distal extremities make it particularly amenable to regional therapy approaches (4). However, few studies have evaluated regional therapies specifically for ALM, and data are limited.
2.6.1 Isolated limb infusion
In a large study based in China by Li et al., 150 patients with cutaneous melanoma with in-transit metastases (with ALM representing 79% of the total study population) received an isolated limb infusion (ILI), with a 6% complete response (CR) rate and a 35% partial response (PR) rate. Patients with CR or PR to ILI had better in-field PFS and OS. Stage IV disease and higher burden of disease were associated with worse in-field PFS and OS (70). Compared to this study, where the vast majority of patients had ALM, studies focused on ILI for all cutaneous melanomas with in-transit metastases have found much higher CR and PR rates. Miura et al. studied 687 patients who underwent an ILI for cutaneous melanoma with in-transit metastases, and found a CR rate of 28.9% and a PR rate of 35.2%, with an ORR of 64.1% (71). Carr et al. reported on patients who underwent an ILI for cutaneous melanoma with in-transit metastases in the USA (n = 276) and Australia (n = 411), with a 29% CR, 24% PR, and 53% ORR in the USA, and a 30% CR, 43% PR, and 73% ORR in Australia (72). This indicates that ALM may be less responsive to ILI therapy than non-ALM cutaneous melanomas, although further studies are needed to explore this.
2.6.2 Intralesional talimogene laherparepvec
Intralesional talimogene laherparepvec (T-VEC), an oncolytic virus immunotherapy, has also been studied, both as monotherapy and in combination with systemic therapy. T-VEC was approved by the FDA after the landmark OPTiM trial, out of the United States, which found that T-VEC was well tolerated among 436 patients, and resulted in a higher DRR and longer median OS when compared to GM-CSF (73). However, this study did not account for histologic subtype, to draw conclusions specific to ALM. In The Netherlands, Franke and colleagues reported a case study of a patient with ALM who achieved a histopathologically confirmed complete response to T-VEC as first-line therapy (74).
A multi-institutional phase II study based in the United States and Europe, by Chesney et al., found that combining T-VEC with ipilimumab had a significantly higher ORR than treatment with ipilimumab alone (39% vs 18%, P=.002) (75). A more recent phase III trial, however, found that combination T-VEC with pembrolizumab did not significantly improve PFS or OS compared to placebo with pembrolizumab (76). Ultimately, though, neither of these studies reported on histologic subtypes, so it is unclear what proportion of ALM these studies represent.
2.6.3 Radiation therapy
Literature is severely lacking regarding the role of radiation therapy specifically in ALM. One case series from 1999 described four patients with unresectable ALM of the foot, and reports excellent responses to palliative radiation therapy (77). Currently, radiation therapy is most commonly used as adjuvant therapy for recurrent or metastatic melanoma, or for symptom palliation of metastatic disease. Further studies are needed to determine the potential role of upfront radiation therapy in unresectable ALM.
2.6.4 Electrochemotherapy
Electrochemotherapy was designed to increase cell permeability by applying an electrical current to tissues, thereby enhancing cytotoxicity of the locally administered chemotherapy agent. A case series reported two patients with advanced melanoma of the lower extremity, previously non-responsive to immunotherapy and isolated limb perfusion, who had a positive clinical response to bleomycin electrochemotherapy (78). Another case series of 31 patients with unresectable locoregional recurrent or metastatic melanoma, treated with bleomycin electrochemotherapy, found a PR rate of 49%, CR rate of 23%, and disease progression in 28%. ALM represented 13% of this study’s patient population (n = 4), of those, one patient had progression, two had PR, and one had CR (79). Overall, literature specifically related to electrochemotherapy for ALM is currently lacking, and further studies are needed.
3 Discussion
In general, ALM seems to respond to anti-PD1 therapy, more specifically with combination therapy regimens, typically including anti-PD1 therapy with anti-CTLA4 therapy but at a lower frequency than when used for cutaneous melanomas (49, 50, 52, 53, 55–57, 60–62). Patients with PD-L1 positive and/or BRAF-wild-type tumors have been found to have particular benefits with anti-PD1 therapy, including higher ORR, longer median PFS, longer median OS, and higher DCR when compared to patients with PD-L1 negative or BRAF-mutant tumors (49).
Results from large, recent studies have found significantly higher ORR with anti-PD1 and anti-CTLA4 combination therapy, compared to monotherapy (40%-43% vs 15%-26%), and longer PFS, although this did not consistently result in a median OS benefit (60–62). Of note, one large international study by Bhave et al. found that patients with BRAF-mutant tumors had superior response rates and a far superior median OS (54 months vs 22.8 months) for all lines of therapy (ipilimumab monotherapy, anti-PD1 monotherapy, and ipilimumab/anti-PD1 combination therapy) as well as overall results (61). This is in contrast to a smaller study analyzing BRAF-mutant tumors treated with anti-PD1 therapy alone, which found worse outcomes in BRAF-mutant disease (although sample size was prohibitively small for BRAF-mutant disease, with n = 4) (49). Therefore, the combination regimen with anti-CTLA4 agent may be particularly important for patients with BRAF-mutant disease. Bhave et al. found that the ORR for ipilimumab monotherapy was 67% in BRAF-mutant tumors, compared to 15% in the entire patient cohort. Furthermore, the ORR for combination therapy was 63% in BRAF-mutant disease compared to 43% for the entire patient cohort (61).
Other combinations including medications such as camrelizumab, apatinib, and temozolomide have also shown promising results (58, 59). A prospective study treating patients with a combination of camrelizumab, apatinib, and temozolomide found a particularly high ORR of 64% and DCR of 88% (58). A similar DCR was found in a study using a regimen of apatinib and camrelizumab, but the ORR was found to be lower, at 24.1% (59). Overall, studies are currently limited and further evidence is needed to analyze these therapy regimens.
Patients with BRAF V600E-mutant ALM might be good candidates for targeted BRAF inhibitor therapy. The ORR found among studies ranges from 35.7% to 78.9%, with one DCR of 81%, but a median OS of 6.2 months (63–65). These studies have small sample sizes and are mostly retrospective, so further data are necessary to determine the true significance of these findings.
3.1 Future directions
New targeted therapies with actionable targets specific to ALM are currently being investigated. Cellular pathways associated with a pathogenic role in ALM include MAPK, PI3K/AKT/PTEN, JAK/STAT3, TERT, WNT, CDK4/CDKN2A, MDM2/TP53, and MCR1-MITF (80). PI3K/AKT/mTOR inhibitors, CDK inhibitors, and MDM/p53 inhibitors, are also being studied (81–83). New immune checkpoint inhibitors could also be adapted to target specific checkpoints found in ALM (other than PD1 and CTLA4). Li et al. used single-cell RNA-sequencing to discover that ALM immune cells expressed additional therapeutically tractable checkpoints, including LAG-3, VISTA, TIGIT, and ADORA2. This study found that VISTA was expressed in 58.3% of myeloid cells, TIGIT was expressed in 22.3% of T/NK cells, and LAG-3 was expressed in 12.9% of T/NK cells (8). Ultimately, however, these may have limited use given the high rate of tumor heterogeneity, with different mutation profiles in various regions even within the same tumor (8, 69, 84).
There are notable differences in response rates to PD1 blockade that could be based on ethnicity and geographic location (85). It is critical to incorporate global inclusivity in future studies to fully evaluate these differences and tailor individualized treatments.
4 Conclusion
ALM is a rare melanoma subtype with a traditionally poor prognosis. In general, ALM seems to respond to anti-PD1 therapy, more specifically with combination therapy regimens, typically including anti-PD1 therapy with anti-CTLA4 therapy (49, 50, 52, 53, 55–57, 60–62). Other combinations including medications such as camrelizumab, apatinib, and temozolomide have also showed particularly promising results, but need further analysis (58, 59). Patients with BRAF V600E-mutant ALM might be good candidates for targeted BRAF inhibitor therapy, although more studies are needed to support this (63–65).
Overall, current prospective data for ALM are limited. To gain a deeper knowledge of this disease process and treatment response, it is critical to develop more randomized trials specific to ALM. It is also important that future studies incorporate global inclusivity to fully evaluate potential differences in response rates across different geographic regions and ethnic backgrounds.
Author contributions
MD: Conceptualization, Methodology, Project administration, Writing – original draft. MP: Conceptualization, Methodology, Project administration, Writing – review & editing. LK: Conceptualization, Methodology, Project administration, Writing – review & editing. JZ: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Editorial assistance was provided by Moffitt Cancer Center’s Office of Scientific Publishing by Daley White and Gerard Hebert; no compensation was given beyond their regular salaries.
Conflict of interest
Dr. JZ has received Honoraria from the Advisory Board of Delcath Systems. He has consulting agreements with Philogen, Merrit Medical, Castle Biosciences, and Merck. His institutional department receives research funding from Philogen, SWOG, Delcath Systems, and Provectus.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SP declared a shared affiliation with the authors to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: Incidence and survival patterns in the United States, 1986-2005. Arch Dermatol (2009) 145:427–34. doi: 10.1001/archdermatol.2008.609
2. Huang K, Fan J, Misra S. Acral lentiginous melanoma: Incidence and survival in the United States, 2006-2015, an analysis of the seer registry. J Surg Res (2020) 251:329–39. doi: 10.1016/j.jss.2020.02.010
3. Chen YA, Teer JK, Eroglu Z, Wu JY, Koomen JM, Karreth FA, et al. Translational pathology, genomics and the development of systemic therapies for acral melanoma. Semin Cancer Biol (2020) 61:149–57. doi: 10.1016/j.semcancer.2019.10.017
4. Perez MC, Messina JL, Karapetyan L, Neves RI, Sondak VK. Acral melanoma: Clinical advances and hope for the future. Clin Adv Hematol Oncol (2023) 21:400–9.
6. Behbahani S, Malerba S, Samie FH. Acral lentiginous melanoma: Clinicopathological characteristics and survival outcomes in the us national cancer database 2004-2016. Br J Dermatol (2020) 183:952–4. doi: 10.1111/bjd.19211
7. Bian SX, Hwang L, Hwang J, Ragab O, In GK, Peng D, et al. Acral lentiginous melanoma-population, treatment, and survival using the ncdb from 2004 to 2015. Pigment Cell Melanoma Res (2021) 34:1049–61. doi: 10.1111/pcmr.12999
8. Li J, Smalley I, Chen Z, Wu JY, Phadke MS, Teer JK, et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res (2022) 28:2131–46. doi: 10.1158/1078-0432.CCR-21-3145
9. Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature (2017) 545:175–80. doi: 10.1038/nature22071
10. Huayllani MT, Restrepo DJ, Boczar D, Avila FR, Bagaria SP, Spaulding AC, et al. National comprehensive analysis of characteristics of acral lentiginous melanoma. Anticancer Res (2020) 40:3411–5. doi: 10.21873/anticanres.14325
11. Arrington JH 3rd, Reed RJ, Ichinose H, Krementz ET. Plantar lentiginous melanoma: A distinctive variant of human cutaneous Malignant melanoma. Am J Surg Pathol (1977) 1:131–43.
12. Chang JW, Yeh KY, Wang CH, Yang TS, Chiang HF, Wei FC, et al. Malignant melanoma in Taiwan: A prognostic study of 181 cases. Melanoma Res (2004) 14:537–41. doi: 10.1097/00008390-200412000-00016
13. Jin SA, Chun SM, Choi YD, Kweon SS, Jung ST, Shim HJ, et al. Braf mutations and kit aberrations and their clinicopathological correlation in 202 korean melanomas. J Invest Dermatol (2013) 133:579–82. doi: 10.1038/jid.2012.338
14. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of kit in distinct subtypes of melanoma. J Clin Oncol (2006) 24:4340–6. doi: 10.1200/JCO.2006.06.2984
15. Kong Y, Si L, Zhu Y, Xu X, Corless CL, Flaherty KT, et al. Large-scale analysis of kit aberrations in chinese patients with melanoma. Clin Cancer Res (2011) 17:1684–91. doi: 10.1158/1078-0432.CCR-10-2346
16. Moon KR, Choi YD, Kim JM, Jin S, Shin MH, Shim HJ, et al. Genetic alterations in primary acral melanoma and acral melanocytic nevus in korea: Common mutated genes show distinct cytomorphological features. J Invest Dermatol (2018) 138:933–45. doi: 10.1016/j.jid.2017.11.017
17. Yeh I, Jorgenson E, Shen L, Xu M, North JP, Shain AH, et al. Targeted genomic profiling of acral melanoma. J Natl Cancer Inst (2019) 111:1068–77. doi: 10.1093/jnci/djz005
18. Darmawan CC, Jo G, Montenegro SE, Kwak Y, Cheol L, Cho KH, et al. Early detection of acral melanoma: A review of clinical, dermoscopic, histopathologic, and molecular characteristics. J Am Acad Dermatol (2019) 81:805–12. doi: 10.1016/j.jaad.2019.01.081
19. Smalley KSM, Teer JK, Chen YA, Wu JY, Yao J, Koomen JM, et al. A mutational survey of acral nevi. JAMA Dermatol (2021) 157:831–5. doi: 10.1001/jamadermatol.2021.0793
20. Zhang N, Wang L, Zhu GN, Sun DJ, He H, Luan Q, et al. The association between trauma and melanoma in the chinese population: A retrospective study. J Eur Acad Dermatol Venereol (2014) 28:597–603. doi: 10.1111/jdv.12141
21. Costello CM, Pittelkow MR, Mangold AR. Acral melanoma and mechanical stress on the plantar surface of the foot. N Engl J Med (2017) 377:395–6. doi: 10.1056/NEJMc1706162
22. Sheen YS, Liao YH, Lin MH, Chen JS, Liau JY, Tseng YJ, et al. A clinicopathological analysis of 153 acral melanomas and the relevance of mechanical stress. Sci Rep (2017) 7:5564. doi: 10.1038/s41598-017-05809-9
23. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol (2011) 38:25–34. doi: 10.1111/j.1346-8138.2010.01174.x
24. Ronger S, Touzet S, Ligeron C, Balme B, Viallard AM, Barrut D, et al. Dermoscopic examination of nail pigmentation. Arch Dermatol (2002) 138:1327–33. doi: 10.1001/archderm.138.10.1327
25. Jellinek N. Nail matrix biopsy of longitudinal melanonychia: Diagnostic algorithm including the matrix shave biopsy. J Am Acad Dermatol (2007) 56:803–10. doi: 10.1016/j.jaad.2006.12.001
26. Lee AY, Friedman EB, Sun J, Potdar A, Daou H, Farrow NE, et al. The devil's in the details: Discrepancy between biopsy thickness and final pathology in acral melanoma. Ann Surg Oncol (2020) 27:5259–66. doi: 10.1245/s10434-020-08708-y
27. Bernardes SS, Ferreira I, Elder DE, Nobre AB, Martinez-Said H, Adams DJ, et al. More than just acral melanoma: The controversies of defining the disease. J Pathol Clin Res (2021) 7:531–41. doi: 10.1002/cjp2.233
28. Teramoto Y, Keim U, Gesierich A, Schuler G, Fiedler E, Tuting T, et al. Acral lentiginous melanoma: A skin cancer with unfavourable prognostic features. A study of the german central Malignant melanoma registry (cmmr) in 2050 patients. Br J Dermatol (2018) 178:443–51. doi: 10.1111/bjd.15803
29. Nunes LF, Quintella Mendes GL, Koifman RJ. Acral melanoma: A retrospective cohort from the Brazilian national cancer institute (inca). Melanoma Res (2018) 28:458–64. doi: 10.1097/CMR.0000000000000476
30. Straker RJ 3rd, Thaler AS, Shannon AB, Miura JT, Chu EY, Karakousis GC, et al. Acral lentiginous melanoma in the era of immune checkpoint blockade and targeted therapy: A national cancer database analysis. J Am Acad Dermatol (2022) 87:169–72. doi: 10.1016/j.jaad.2021.06.887
31. Zemelman VB, Valenzuela CY, Sazunic I, Araya I. Malignant melanoma in Chile: Different site distribution between private and state patients. Biol Res (2014) 47:34. doi: 10.1186/0717-6287-47-34
32. Natarelli N, Hanlon K, Chen WS, Grichnik JM, Zager JS, Correa-Selm L. Reflectance confocal microscopic visualization of melanocytic bodies in the stratum corneum overlying acral lentiginous melanoma. Lasers Surg Med (2023) 55:253–6. doi: 10.1002/lsm.23647
33. Nakamura Y, Ohara K, Kishi A, Teramoto Y, Sato S, Fujisawa Y, et al. Effects of non-amputative wide local excision on the local control and prognosis of in situ and invasive subungual melanoma. J Dermatol (2015) 42:861–6. doi: 10.1111/1346-8138.12923
34. Le M, Gabrielli S, Zloty D. Mohs micrographic surgery is equivalent to nail unit excision or amputation for melanoma in situ of the nail unit: A systematic review and meta-analysis. Dermatol Surg (2023) 49:755–8. doi: 10.1097/DSS.0000000000003840
35. Long GV, Del Vecchio M, Weber J, Hoeller C, Grob J-J, Mohr P, et al. Adjuvant therapy with nivolumab versus placebo in patients with resected stage iib/c melanoma (checkmate 76k). SKIN J Cutaneous Med (2023) 7(2):163. doi: 10.25251/skin.7.supp.163
36. Luke JJ, Rutkowski P, Queirolo P, Del Vecchio M, Mackiewicz J, Chiarion-Sileni V, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage iib or iic melanoma (keynote-716): A randomised, double-blind, phase 3 trial. Lancet (2022) 399:1718–29. doi: 10.1016/S0140-6736(22)00562-1
37. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage iii melanoma (eortc 1325-mg/keynote-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol (2021) 22:643–54. doi: 10.1016/S1470-2045(21)00065-6
38. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage iii or iv melanoma. N Engl J Med (2017) 377:1824–35. doi: 10.1056/NEJMoa1709030
39. Larkin J, Del Vecchio M, Mandala M, Gogas H, Arance Fernandez AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage iii/iv melanoma: 5-year efficacy and biomarker results from checkmate 238. Clin Cancer Res (2023) 29:3352–61. doi: 10.1158/1078-0432.CCR-22-3145
40. Weber JS, SChadendorf D, Del Vecchio M, Larkin J, Atkinson V, Schenker M, et al. Adjuvant therapy of nivolumab combined with ipilimumab versus nivolumab alone in patients with resected stage iiib-d or stage iv melanoma (checkmate 915). J Clin Oncol (2023) 41:517–27. doi: 10.1200/JCO.22.00533
41. Maeda T, Yanagi T, Miyamoto K, Tokuchi K, Kitamura S, Ujiie H. Adjuvant nivolumab therapy may not improve disease-free survival in resected acral lentiginous melanoma patients: A retrospective case series. Dermatol Ther (2022) 35:e15817. doi: 10.1111/dth.15817
42. Patel SP, Othus M, Chen Y, Wright GP Jr, Yost KJ, Hyngstrom JR, et al. Neoadjuvant-adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med (2023) 388:813–23. doi: 10.1056/NEJMoa2211437
43. Menzies AM, Amaria RN, Rozeman EA, Huang AC, Tetzlaff MT, van de Wiel BA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the international neoadjuvant melanoma consortium (inmc). Nat Med (2021) 27:301–9. doi: 10.1038/s41591-020-01188-3
44. Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, et al. A single dose of neoadjuvant pd-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med (2019) 25:454–61. doi: 10.1038/s41591-019-0357-y
45. Rozeman EA, Menzies AM, van Akkooi ACJ, Adhikari C, Bierman C, van de Wiel BA, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage iii melanoma (opacin-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol (2019) 20:948–60. doi: 10.1016/S1470-2045(19)30151-2
46. Reijers ILM, Menzies AM, van Akkooi ACJ, Versluis JM, van den Heuvel NMJ, Saw RPM, et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage iii melanoma: The prado trial. Nat Med (2022) 28:1178–88. doi: 10.1038/s41591-022-01851-x
47. Amaria RN, Postow M, Burton EM, Tetzlaff MT, Ross MI, Torres-Cabala C, et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature (2022) 611:155–60. doi: 10.1038/s41586-022-05368-8
48. Cho KK, Cust AE, Foo YM, Long GV, Menzies AM, Eslick GD. Metastatic acral melanoma treatment outcomes: A systematic review and meta-analysis. Melanoma Res (2021) 31:482–6. doi: 10.1097/CMR.0000000000000764
49. Si L, Zhang X, Shu Y, Pan H, Wu D, Liu J, et al. Pembrolizumab in chinese patients with advanced melanoma: 3-year follow-up of the keynote-151 study. Front Immunol (2022) 13:882471. doi: 10.3389/fimmu.2022.882471
50. Nakamura Y, Namikawa K, Yoshino K, Yoshikawa S, Uchi H, Goto K, et al. Anti-pd1 checkpoint inhibitor therapy in acral melanoma: A multicenter study of 193 Japanese patients. Ann Oncol (2020) 31:1198–206. doi: 10.1016/j.annonc.2020.05.031
51. Yamazaki N, Kiyohara Y, Uhara H, Tsuchida T, Maruyama K, Shakunaga N, et al. Real-world safety and efficacy data of ipilimumab in Japanese radically unresectable Malignant melanoma patients: A postmarketing surveillance. J Dermatol (2020) 47:834–48. doi: 10.1111/1346-8138.15388
52. Tang B, Chi Z, Chen Y, Liu X, Wu D, Chen J, et al. Safety, efficacy, and biomarker analysis of toripalimab in previously treated advanced melanoma: Results of the polaris-01 multicenter phase ii trial. Clin Cancer Res (2020) 26:4250–9. doi: 10.1158/1078-0432.CCR-19-3922
53. Si L, Zhang X, Shu Y, Pan H, Wu D, Liu J, et al. A phase ib study of pembrolizumab as second-line therapy for chinese patients with advanced or metastatic melanoma (keynote-151). Transl Oncol (2019) 12:828–35. doi: 10.1016/j.tranon.2019.02.007
54. Nathan P, Ascierto PA, Haanen J, Espinosa E, Demidov L, Garbe C, et al. Safety and efficacy of nivolumab in patients with rare melanoma subtypes who progressed on or after ipilimumab treatment: A single-arm, open-label, phase ii study (checkmate 172). Eur J Cancer (2019) 119:168–78. doi: 10.1016/j.ejca.2019.07.010
55. Maeda T, Yoshino K, Nagai K, Oaku S, Kato M, Hiura A, et al. Efficacy of nivolumab monotherapy against acral lentiginous melanoma and mucosal melanoma in asian patients. Br J Dermatol (2019) 180:1230–1. doi: 10.1111/bjd.17434
56. Wen X, Ding Y, Li J, Zhao J, Peng R, Li D, et al. The experience of immune checkpoint inhibitors in chinese patients with metastatic melanoma: A retrospective case series. Cancer Immunol Immunother (2017) 66:1153–62. doi: 10.1007/s00262-017-1989-8
57. Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, et al. The efficacy of anti-pd-1 agents in acral and mucosal melanoma. Cancer (2016) 122:3354–62. doi: 10.1002/cncr.30259
58. Mao L, Lian B, Li C, Bai X, Zhou L, Cui C, et al. Camrelizumab plus apatinib and temozolomide as first-line treatment in patients with advanced acral melanoma: The cap 03 phase 2 nonrandomized clinical trial. JAMA Oncol (2023) 9:1099–107. doi: 10.1001/jamaoncol.2023.1363
59. Wang X, Wu X, Yang Y, Xu W, Tian H, Lian B, et al. Apatinib combined with camrelizumab in advanced acral melanoma patients: An open-label, single-arm phase 2 trial. Eur J Cancer (2023) 182:57–65. doi: 10.1016/j.ejca.2022.12.027
60. Nakamura Y, Namikawa K, Kiniwa Y, Kato H, Yamasaki O, Yoshikawa S, et al. Efficacy comparison between anti-pd-1 antibody monotherapy and anti-pd-1 plus anti-ctla-4 combination therapy as first-line immunotherapy for advanced acral melanoma: A retrospective, multicenter study of 254 Japanese patients. Eur J Cancer (2022) 176:78–87. doi: 10.1016/j.ejca.2022.08.030
61. Bhave P, Ahmed T, Lo SN, Shoushtari A, Zaremba A, Versluis JM, et al. Efficacy of anti-pd-1 and ipilimumab alone or in combination in acral melanoma. J Immunother Cancer (2022) 9(8):1099–107. doi: 10.1136/jitc-2022-004668
62. Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutierrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med (2022) 386:24–34. doi: 10.1056/NEJMoa2109970
63. Mao L, Ding Y, Bai X, Sheng X, Dai J, Chi Z, et al. Overall survival of patients with unresectable or metastatic braf v600-mutant acral/cutaneous melanoma administered dabrafenib plus trametinib: Long-term follow-up of a multicenter, single-arm phase iia trial. Front Oncol (2021) 11:720044. doi: 10.3389/fonc.2021.720044
64. Bai X, Mao LL, Chi ZH, Sheng XN, Cui CL, Kong Y, et al. Braf inhibitors: Efficacious and tolerable in braf-mutant acral and mucosal melanoma. Neoplasma (2017) 64:626–32. doi: 10.4149/neo_2017_419
65. Kim HK, Lee S, Kim K, Heo MH, Lee H, Cho J, et al. Efficacy of braf inhibitors in asian metastatic melanoma patients: Potential implications of genomic sequencing in braf-mutated melanoma. Transl Oncol (2016) 9:557–64. doi: 10.1016/j.tranon.2016.09.004
66. Steeb T, Wessely A, Petzold A, Kohl C, Erdmann M, Berking C, et al. C-kit inhibitors for unresectable or metastatic mucosal, acral or chronically sun-damaged melanoma: A systematic review and one-arm meta-analysis. Eur J Cancer (2021) 157:348–57. doi: 10.1016/j.ejca.2021.08.015
67. Kaunitz GJ, Cottrell TR, Lilo M, Muthappan V, Esandrio J, Berry S, et al. Melanoma subtypes demonstrate distinct pd-l1 expression profiles. Lab Invest (2017) 97:1063–71. doi: 10.1038/labinvest.2017.64
68. Furney SJ, Turajlic S, Stamp G, Thomas JM, Hayes A, Strauss D, et al. The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell Melanoma Res (2014) 27:835–8. doi: 10.1111/pcmr.12279
69. Fernandes M, Barcelos D, Comodo AN, Guimaraes DP, Lopes Carapeto FC, Cardili L, et al. Acral lentiginous melanomas harbour intratumor heterogeneity in braf exon 15, with mutations distinct from v600e/v600k. Am J Dermatopathol (2019) 41:733–40. doi: 10.1097/DAD.0000000000001418
70. Li S, Sheng X, Si L, Cui C, Kong Y, Mao L, et al. Outcomes and predictive factors of isolated limb infusion for patients with in-transit melanoma in China. Ann Surg Oncol (2018) 25:885–93. doi: 10.1245/s10434-017-6256-x
71. Miura JT, Kroon HM, Beasley GM, Mullen D, Farrow NE, Mosca PJ, et al. Long-term oncologic outcomes after isolated limb infusion for locoregionally metastatic melanoma: An international multicenter analysis. Ann Surg Oncol (2019) 26:2486–94. doi: 10.1245/s10434-019-07288-w
72. Carr MJ, Sun J, Kroon HM, Miura JT, Beasley GM, Farrow NE, et al. Oncologic outcomes after isolated limb infusion for advanced melanoma: An international comparison of the procedure and outcomes between the United States and Australia. Ann Surg Oncol (2020) 27:5107–18. doi: 10.1245/s10434-020-09051-y
73. Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol (2015) 33:2780–8. doi: 10.1200/JCO.2014.58.3377
74. Franke V, Smeets PMG, van der Wal JE, van Akkooi ACJ. Complete response to talimogene laherparepvec in a primary acral lentiginous melanoma. Melanoma Res (2020) 30:548–51. doi: 10.1097/CMR.0000000000000673
75. Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, et al. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol (2018) 36:1658–67. doi: 10.1200/JCO.2017.73.7379
76. Chesney JA, Ribas A, Long GV, Kirkwood JM, Dummer R, Puzanov I, et al. Randomized, double-blind, placebo-controlled, global phase iii trial of talimogene laherparepvec combined with pembrolizumab for advanced melanoma. J Clin Oncol (2023) 41:528–40. doi: 10.1200/JCO.22.00343
77. Harwood AR. Radiotherapy of acral lentiginous melanoma of the foot. J La State Med Soc (1999) 151:373–6.
78. Gallagher M, Chin KY, MacKenzie-Ross A. Bleomycin electrochemotherapy for the management of locally advanced metastatic melanoma: Two notable clinical cases potentially indicating a greater therapeutic role in the era of targeted and immuno-therapy. JPRAS Open (2020) 26:43–8. doi: 10.1016/j.jpra.2020.09.007
79. Mir-Bonafe JM, Vilalta A, Alarcon I, Carrera C, Puig S, Malvehy J, et al. Electrochemotherapy in the treatment of melanoma skin metastases: A report on 31 cases. Actas Dermosifiliogr (2015) 106:285–91. doi: 10.1016/j.ad.2014.10.007
80. Tod BM, Schneider JW, Bowcock AM, Visser WI, Kotze MJ. The tumor genetics of acral melanoma: What should a dermatologist know? JAAD Int (2020) 1:135–47. doi: 10.1016/j.jdin.2020.07.004
81. Lee B, McArthur GA. Cdk4 inhibitors an emerging strategy for the treatment of melanoma. Melanoma Manage (2015) 2:255–66. doi: 10.2217/mmt.15.14
82. Sanz G, Singh M, Peuget S, Selivanova G. Inhibition of p53 inhibitors: Progress, challenges and perspectives. J Mol Cell Biol (2019) 11:586–99. doi: 10.1093/jmcb/mjz075
83. Wu X, Yu J, Yan J, Dai J, Si L, Chi Z, et al. Pi3k/akt/mtor pathway inhibitors inhibit the growth of melanoma cells with mtor h2189y mutations in vitro. Cancer Biol Ther (2018) 19:584–9. doi: 10.1080/15384047.2018.1435221
84. Comodo-Navarro AN, Fernandes M, Barcelos D, Carapeto FCL, Guimaraes DP, de Sousa Moraes L, et al. Intratumor heterogeneity of kit gene mutations in acral lentiginous melanoma. Am J Dermatopathol (2020) 42:265–71. doi: 10.1097/DAD.0000000000001475
Keywords: melanoma, acral melanoma, acral melanoma management, immunotherapy, targeted therapy, regional therapy
Citation: Dugan MM, Perez MC, Karapetyan L and Zager JS (2024) Management of acral lentiginous melanoma: current updates and future directions. Front. Oncol. 14:1323933. doi: 10.3389/fonc.2024.1323933
Received: 18 October 2023; Accepted: 22 January 2024;
Published: 08 February 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Zsuzsanna Lengyel, University of Pécs, HungarySanjay Premi, Moffitt Cancer Center & Research Institute, United States
Siming Li, Peking University, China
Copyright © 2024 Dugan, Perez, Karapetyan and Zager. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan S. Zager, am9uYXRoYW4uemFnZXJAbW9mZml0dC5vcmc=
 Michelle M. Dugan
Michelle M. Dugan Matthew C. Perez1
Matthew C. Perez1 Lilit Karapetyan
Lilit Karapetyan